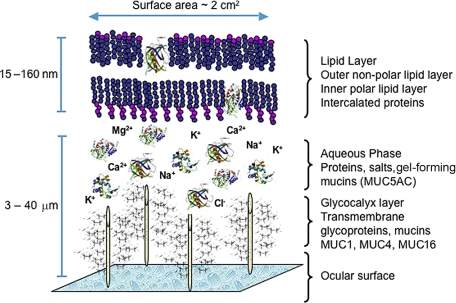
Understanding the molecular composition (e.g., proteins and lipids) of the tear film (TF) and the contribution of the meibomian gland to the TF is critical in gaining knowledge about TF instabilities, dry eye syndromes, contact lens (CL) incompatibilities, and other eye diseases. Among its functions, the lipid layer of the TF slows evaporation of the aqueous component, preserves a clear optical surface, and forms a barrier to protect the eye from microbial agents and organic matter, such as dust and pollen.1 The TF contains a complex mixture of proteins, enzymes, lipids, mucins, and salts that allows the TF to perform its functions (Fig. 1). Researchers believe the outer lipid layer is 5 to 10 molecules thick and is composed primarily of wax and sterol esters, possibly intercalated with each other and with proteins rather than forming distinct repeating layers of molecules.2,3 Evidence from interferometric studies indicate that the TF lipid layer thickness ranges from 20 to 160 nm.4 If the size of a lipid molecule is approximately 2.2 nm (22 Å), then the calculated thickness for one layer would be 11 to 44 nm. The addition of polar and nonpolar layers would add to the lipid thickness, which indicates that the lipid component of the TF may be multiple layers thick or have other contributing sources to correspond with reported thickness measurements.5
Figure 1.
A proposed model of the precorneal tear film showing the relationship and interaction of lipid-binding proteins and the outer lipid layer.
While the signs and symptoms of TF instability are reasonably well characterized, we are only beginning to understand the specific molecular components of the TF and their relationship with disease and TF stability. The purpose of this review is to examine the meibomian gland's contribution to TF lipids and lipid–protein interactions in health and disease.
Review of the Tear Film Lipid Layer
The meibomian glands are the main source of lipids for the human TF. The meibomian gland secretions consist of an extremely complex mixture of various polar and nonpolar lipids containing cholesteryl esters (CEs), triacylglycerol, free cholesterol, free fatty acids (FFAs), phospholipids, wax esters (WEs), and diesters.6–9 It was not until 1981 that the term meibum entered the lexicon, to describe these secretions.10
Current models of the TF originated in the 1950s11 and include three major, well-defined layers: the glycocalyx layer, the intermediate aqueous layer, and the outermost tear film lipid layer (TFLL). The glycocalyx layer, which covers the corneal epithelium, is believed to be relatively viscous because of the large amount of membrane-bound and secreted mucins. The aqueous layer is enriched in water-soluble proteins, mucins, and salts, whereas the TFLL is formed almost exclusively from lipids and attached and/or intercalated proteins.2 The TFLL is usually depicted as a two-layer structure: polar lipids form the lower sublayer and nonpolar lipids form the upper portion that is in contact with the air.11 This concept was first proposed by Holly12; Shine and McCulley later elaborated.13 Each sublayer is distinct in its responsibilities: The upper sublayer forms a thick blanket that seals the underlying aqueous portion of the TF. The outermost lipid component is believed to retard water evaporation, as lipid films have low water vapor transmissivity, depending on the lipid film thickness and composition.13 Nonpolar lipids are thermodynamically unstable when they are spread over an aqueous subphase; this allows them to collapse easily and form lipid droplets. When that happens, the aqueous portion of the TF is left unprotected and prone to rapid evaporation.13 Interestingly, the lower lipid sublayer is thought to create an interface that helps stabilize this upper portion. In this interface, the polar lipids are thought to be oriented perpendicularly, with their hydrophobic tails immersed in the nonpolar lipid sublayer, and their polar heads exposed to the aqueous layer. Shine and McCulley13 suggested that this polar lipid sublayer was one to three molecules thick. They further suggested that it is formed from phospholipids and other polar lipids, including phosphatidylcholine, phosphatidylethanolamine, sphingomyelin (SM), ceramides, and cerebrosides.13 More recently, another group of amphiphilic lipids—namely, very long chain (O-acyl)-ω-hydroxy fatty acids, have been described as meibum components and are theorized to contribute to the polar lipid sublayer.14 Polar-to-nonpolar lipid layer thickness measurements have not been performed; however, it has been suggested that the polar lipid is 5% to 15%2,6 of the total lipid fraction. Therefore, this polar surfactant layer is hypothesized to be between 7 and 20 molecules thick and consists of more hydrophobic lipids above the polar lipid layer.6,15 This estimate does not consider a (quite possible) redistribution of the lipids of different classes between the sublayers and is based on an assumption that the overall lipid composition of TFLL is identical with that of meibum.2
It is likely that lipids are not the only class of molecules from which the TFLL is assembled. For instance, proteins are now being considered an intrinsic part of TFLL.2 Many proteins are surface active compounds, meaning they will spontaneously populate the air–liquid interface, lowering the surface tension of water and creating a surface protein layer. This translocation of proteins from the bulk aqueous phase to the air–water interface is typically accompanied by protein denaturing (i.e., irreversible conformational changes—typically, unfolding—that, in the end, prevent proteins from submerging back into the depth of the aqueous layer). This results in the formation of a protein layer.2
In the presence of meibomian lipids, proteins have to compete for the available surface space. This competition results in either protein penetration (intercalation) in the lipid layer or protein attachment to (or association with) the lipid layer. Both result in surface property alterations of the TF and TFLL. Indeed, Saaren-Seppälä et al.16 demonstrated that TF lipocalin (Tlc) could actively interact with various artificial lipid membranes, regardless of overall charge and composition, and others have shown human Tlc binding to meibomian lipids organized in thin films. Similar experiments were conducted earlier to demonstrate that other tear proteins (such as lysozyme17 and mucins3) could also penetrate the lipid layers.
Thus, an update to the classic three-layer model (Fig. 1) of the TF and two sublayers of TFLL is warranted. This new model should incorporate proteins (lipocalin, lysozyme, mucins, and others) intercalated in and/or adsorbed to the TFLL, and an addition of a novel class of lipids recently identified in human meibum, very long chain (O-acyl)-ω-hydroxy fatty acids, which may act in the formation of an intermediate surfactant lipid sublayer between the thick outermost nonpolar lipid sublayer and the aqueous layer of the TF.8.15,18
Analytical Methods for Lipid Evaluation
With the advancement in the lipid detection and identification technology, sample quality is critical. Modern instrumentation can detect a wide variety of compounds and provide accurate information on their structures. Thus, many of the analytes that previously had been impossible to detect and/or identify in meibomian lipids can now be discovered and categorized, even if present in minute quantities.
Methods of Sampling and Storing Meibum and Tear Film Lipids
Handling and storage of lipid samples generally follows the recommendations provided in lipid chemistry textbooks and protocols available online from lipid chemical companies (see, for example, http://www.avantilipids.com, technical support, lipid storage, and handling).19 Care should be taken to minimize (or prevent) sample exposure or contact with any products made of plastic and silicone. Glass, stainless steel, noble metals, and Teflon (E.I. du Pont de Nemours and Co., Wilmington, DE) are the recommended collection and storage materials. The preferred conditions for storing lipid samples regardless of their origin are below temperature (−80°C), in an inert atmosphere (argon or nitrogen), and in a dark and dry state.
Meibum and TF samples can be collected using three main types of procedures: (1) soft or hard expression of meibum from the meibomian gland orifices10,20–22; (2) microcapillary collection of aqueous TF samples and meibum directly from the meibomian gland orifices23 and (3) Schirmer test strips or similar tools to collect aqueous TF samples.2,24,25 Another technique, the surgical removal of eyelids and/or meibomian glands, has been implemented in animal and human cadaver studies,26–28 but is unrealistic with living human volunteers.
Soft expression, or expression/pressure from the outside only of the eyelid, is possibly less likely to contaminate the samples with surrounding tissues due to the gentler handling of the eyelids. The hard expression technique is often described as a squeeze technique in which a conformer or device is used behind the lid while pressure is applied to the front of the eyelid. This technique yields a greater sample volume, yet may be more uncomfortable to subjects. Both techniques can yield samples contaminated by the tears as well as surrounding tissues (cells and debris). The contamination may vary by patient and/or examiner.
The use of powder- and latex-free gloves worn by the examiner is highly recommended to avoid contamination of the samples by examiner skin secretions. The use of a slit lamp during sampling allows a better visualization of the meibomian gland orifices and reduces the chances of contamination with lid margin tears and debris.
TF collection via a microcapillary technique is virtually painless and allows samples of aqueous tears to be collected directly from the tear menisci of the lower eyelids. The nonstimulated samples collected this way are typically between 2 and 5 μL, or a few micrograms, of wet sample.18 The microcapillary technique has also been used for collecting meibum directly from the individual meibomian gland orifices.22 The procedure is well tolerated by patients and is less invasive than hard expression, although the technique produces smaller sample volumes. The meibum sample solidifies at room temperature.18,29 The solid meibum lipid is removed from the microcapillary by pushing out with a thin wire or simply by soaking in chloroform/methanol. If a spatula is used for meibum collection, the sample is immediately stored in a solvent. On average, approximately 15 mg of meibum is collected per eye by microcapillary collection, which is generally enough for downstream mass spectrometry analytical analysis, although protein extraction and/ or additional analyses may require pooling.
Patients accept the Schirmer test strip technique as a general component of an ocular surface examination. However, the Schirmer test may be less comfortable for patients than the microcapillary technique. This is more than offset by the test's high safety profile, aided by the lack of sharp or hard objects used in sample collection. The Schirmer test cannot be used for collecting a pure meibum sample, however, because it is virtually impossible to avoid wetting the strips with aqueous tears. Consequently, it seems that expression and microcapillary tube collection or spatula collection is well suited for collecting samples of pure meibum, whereas microcapillary tube and Schirmer test strips are better suited for collecting aqueous tears. Additional research evaluating alternate and/or optimal collection and storage of lipid samples is warranted.
Thin-Layer Chromatography
Thin-layer chromatography (TLC) is an established method of quantifying global classes of lipids; however, TLC requires large amounts of sample that can make it difficult to study tear and meibum samples. Detection is visual, via a stain (bromothymol blue) or charring.30 To identify individual lipid species, the region of interest is removed from the TLC plate, and the lipids are extracted and either chemically derivatized to make volatile for gas chromatography or labeled with a chromophore or fluorophore for high-performance liquid chromatography (HPLC) detection. Bovine meibomian secretions were first analyzed by using this technique in 1976.31 McCulley and Shine32 used TLC followed by HPLC with ultraviolet detection and gas chromatography-mass spectrometry (GC-MS). While TLC still has many uses for quantifying lipids, newer techniques such as matrix assisted-laser desorption ionization (MALDI) and electrospray are gaining rapid acceptance and being used on their own or in conjunction with TLC. Lipid samples can be analyzed via MALDI and electrospray without derivitization or labeling.
Mass Spectrometry
Mass spectrometry is a very sensitive analytical method that allows for both detection and structural determination from very small sample amounts. Many mass spectrometry-based meibum studies have been published using analysis techniques including GC-MS,10,33 liquid chromatography-mass spectrometry,7,9,18,20,34 electrospray,8,9,23,35,36 atmospheric pressure chemical ionization,7,18,20 and MALDI.37,38 Phosphorylated lipids from rabbit and human tears have been detected and analyzed using a unique extraction procedure and sample preparation for MALDI-TOF (time of flight) analysis.38 No papers have been published on the detection of human meibum lipids using MALDI-TOF.
Nuclear Magnetic Resonance Spectroscopy
Nuclear magnetic resonance (NMR) measures quantum mechanical magnetic properties of an atomic nucleus that possess a spin such as 1H, 13C, 31P, 17O, and 15N.39,40 As NMR spectroscopy does not destroy the sample, it could be a promising technique to quantify major lipid classes before using more complex HPLC or mass spectroscopic measurements. The NMR technique must be validated and the band assignments should be carefully confirmed. A disadvantage of NMR is that milligram quantities of sample are needed, and phospholipids are not soluble in deuterated cyclohexane, which is the solvent of choice for NMR analysis of waxes. Therefore, pooled meibum samples would be necessary for NMR analysis.
Infrared and Raman Spectroscopy
The basic principle of infrared spectroscopy is that when a bond with an electric dipole is changing at the same frequency as the incident radiation (infrared light), light is absorbed. From the intensity and frequency of the absorbed radiation, vibrational transitions of molecules are measured that provide compositional, environmental, and conformational information at the molecular level. Infrared spectroscopy and Raman spectroscopy are complimentary techniques; they both measure vibrational modes. However, symmetric bands are generally more intense in Raman spectra and asymmetric bands more intense in infrared absorption spectra. Fluorescence does not interfere with infrared spectra like it does with Raman spectra, but water bands, associated with most biological tissues, can overwhelm and interfere with infrared spectral features. Infrared bands are inherently broader and less resolved than Raman bands.39,40
Raman and infrared spectroscopy has been applied to characterize the conformation of human meibum and human tear lipids.24,41–44 Meibum differs in composition from tear lipids in that it contains more C C and CH3 moieties than tear lipids. The conformation between meibum and tear lipids is also different.24
C and CH3 moieties than tear lipids. The conformation between meibum and tear lipids is also different.24
Chemical Properties of Lipids
Lipids are an extremely complex group of molecules, both structurally and functionally. One method of chemical property classification groups lipids into polar, amphiphilic and nonpolar lipids.45 By definition, polar lipids are relatively water-soluble. They include short chain fatty acids, hydroxylated fatty acids, hydroxy-ceramides (OH-Cer), monoacyl glycerols (MAGs), glycosylated lipids, phospholipids, and others.45 These lipids tend to have relatively high hydrophilic-to-lipophilic balance (HLB), which is an objective physicochemical parameter used to describe partitioning of solubilized molecules between polar (aqueous) and nonpolar (oil) subphases. Thus, in a water-in-oil emulsion, polar lipids would concentrate in the aqueous subphase. Nonpolar lipids, on the other hand, do not dissolve in water. Typical members of this family are hydrocarbons, very-long-chain acyl-ceramide, WE, CE, and triacyl glycerols.45 Their hydrophilic-to-lipophilic balance is very low; thus they easily partition into the oil phase of a water-in-oil emulsion. The borders between these three groups are blurry, as there are many factors that influence the solubility of lipids, such as the length of their carbon chains; the number and type of hydrophilic (hydroxy, amino, and carboxy) groups in their structures; the degree of unsaturation; cis, trans- and iso- and anteisomerism, for example.45
The formation of complex lipids happens through condensation reactions—mainly esterification and amidation. The reactions of esterification are involved in the biosynthesis of virtually every major complex lipid group (WE, CE, acylglycerols, and liposaccharides), while amidation is involved in the formation of fewer lipids such as fatty acid amides (FAs), Cer, SMs, cerebrosides.45 These reactions are reversible, which means that in situ complex lipids may undergo enzymatic or nonenzymatic hydrolysis, in the course of which they will revert to a mixture of more simple lipids and other compounds such as glycerol (in the case of acylglycerols) and carbohydrates (in the case of liposaccharides).45
The second major type of lipid transformation relating to TF and TFLL is lipid oxidation. One of the major prerequisites for lipid oxidation is the presence of one or more double bonds in the lipid structure. The double bonds may undergo enzymatic or nonenzymatic oxidation. Many lipids can isomerize, either induced by enzymatic transformation or spontaneously. High temperature, UV light, and oxidation are typical causes of isomerization. These reactions inevitably change the physical and chemical properties of lipids and their mixtures because (1) hydrolysis products are typically more water soluble than the starting complex lipids; (2) lipid (per)oxidation products also become more hydrophilic because of the addition of oxygen and/or the formation of shorter, more hydrophilic scission products; and (3) isomerization influences lipid packing and the physical properties of lipids (e.g., melting points, boiling point, and density).45
For up-to-date, easily available information on lipids, the following web sites are recommended: lipidmaps.org, lipidlibrary.co.uk, cyberlipid.com, hplc-ms.byrdwell.com, and lipidbanks.jp, among others. An overview of lipidomics was also recently published and may elucidate the molecular composition of these biomolecules.46
Lipids of the Tear Film
Normal Meibum
Biochemical characterization of meibum began in 1897,47 when Orlando Pes confirmed its lipid nature and suggested that it was rich in fats, FFAs, and cholesterol. Several decades later Linton et al.,30 Andrews,48 and Ehler49 each demonstrated that meibum was rich in neutral fats, steryl, and WE. In subsequent studies, acyl glycerols, ceramides, phospholipids, and other polar lipids were reported to be present in meibum, and most meibum components are nonpolar lipids of different classes.28,31,50–52 The polar lipids are a minority, though they have been implicated in playing a critical role in the TF stabilization and disintegration. Several reviews on the topic has been published,53–56 including the most recent one by the International Dry Eye Workshop.57 Table 1 summarizes all the lipids identified by a variety of techniques to date.7–10,13–15,20,23,29,32–34,36,47–50,52,56,58–74
Table 1.
Lipid Composition of Human Meibum*
| Method of Analysis | Hydro-carbons | Monoacyl-glycerols | Diacygly-cerols | Triacyl-glycerols | Wax Esters | Sterol Esters | Cholesteryl Esters | Free Sterols | Cholesterol | Free Fatty Alcohol | Long Chain Alpha Omegas | Free Fatty Acids | Polar Lipids | Phospho-lipids | Sphingo-lipids | Squalene |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemical stains46 | Pos | Pos | Pos | |||||||||||||
| PC28 | Pos | Neg | Neg | Pos | ||||||||||||
| TLC48 | Pos (trace) | Pos | Pos | Pos | Pos | Pos (trace) | ||||||||||
| TLC57 | Pos | Pos | Pos | Pos | Pos | Pos | ||||||||||
| TLC/GC47 | 33 | Pos† | Pos (C18)‡ | Pos | ||||||||||||
| TLC49 | 17.6 | 2.1 | 68.6 | 1.6 | 5 | 10.4 | TLC | |||||||||
| TLC/GLC/MS51,55,58 | 25–36 | 0.6–1.5 | 11–43 | 13–23 | 8–34 | Pos | Pos | 0–2 | 0–24 | 0–5 | 0.8–5 | 0.7–7 | ||||
| TLC/GLC/MS10,59–62 | 7.54 | 3.7 | 32.32 | 27.28 | 1.63 | 2 | Pos | 1.98 | 14.83 | |||||||
| TLC/GC/MS63 | 3.1 | 45.2 | 39.4 | 1.2 | 2.8 | |||||||||||
| GLC/MS13,32,64–69 | Pos | Pos§ | Pos‖ | Pos | 1.87–14.42 | Pos (C14-18n)¶ | 0.21–1.3 | Pos | Pos# | Pos** | ||||||
| HPLC/ESI-MS9,33,35,70 | Pos | Pos | Pos | Pos | Pos | Pos | Pos | Pos†† | Pos | |||||||
| RP HPLC, MS, & TLC7,15,18,20,71,72 | Neg | Neg | Pos | Pos | Pos | 30–33 | <0.5 | Neg | Pos | Pos‡‡ | <0.05 | Neg | Pos | |||
| GC/MS73,74 | Pos (C16–18) | |||||||||||||||
| MS22 | 0.05 | 28 | Pos | 13 | Small | Small | 3 | Pos‡‡ | Neg |
Numbers indicate percentages; PC, paper chromatography; TLC, thin later chromatography; GC, gas chromatography; MS, mass spectrometry; ESI-MS, electrospray ionization mass spectrometry; RP, reverse phase chromatography; HPLC, high pressure liquid chromatography; Pos, positive; Neg, negative.
35.7% of alcohol.
C18 25% of fatty acids.
81.84% of all esters.
18% of all esters.
Normal chain accounts for 48–68% of fatty alcohols.
PC and PE 38% and 16% of phospholipids, respectively.
Ceramide and cerebroside 30 and 70% of sphingolipids, respectively.
PC > PE.
(O-acyl)-ω-hydroxy fatty acids (OAHFAs).
The most often detected classes of meibomian lipids are probably ubiquitous WEs and CEs. Together, they are believed to represent up to 60% of meibum lipids. WEs and CEs are among the most hydrophobic members of meibum, whose lipophilicity is rivaled only by hydrocarbons.
WEs from normal human meibum have been recently characterized using HPLC-MSn.7,14,18,20 Numerous WE species were detected, and the most prominent compounds were C18:1-fatty acid based esters of very-long-chain saturated fatty alcohols with C18 to C30 carbons.7,18,20 In a later study,17 additional types of WEs were described that included multiple structures of C18:2-, C18:3-, and C18:4-fatty acid families. Their fatty acids were esterified to the same very-long-chain fatty alcohols, as were the C18:1-fatty acids. Compared with the monounsaturated WEs, the polyunsaturated WEs were relatively minor, but still noticeable, components of meibum. A more detailed analysis of these polyunsaturated WEs revealed that many of the individual members of these families were present in several isomeric forms, which most likely differed in the cis, trans geometry of their double bonds.17 For example, a compound with m/z 641 (an ester of a C26:0 alcohol and a C18:4 stearidonic acid, FAl:FA, C26:0:C18:4) was present as four isoforms, C26:0:C18:3 as three, and C26:0:C18:2 as two, whereas C26:0:C18:1 was represented by just one isomer. These observations suggest that the overall number of individual WE species in human meibum exceeds 100. Using direct-infusion ESI, WEs were found to be mainly C18:1, but with a considerable amount of C16:1.8 Meibomian lipids from another group (namely, CEs) are equally complex.8,15 Unlike WEs whose major fatty acids are of modestly long C18:1 to C18:4 variety, CEs detected in meibum can have very long saturated and unsaturated fatty acids, with their chains ranging between C18 and C34. More than 20 individual CEs were observed in human meibum. The dominant species were CEs with C22 to C30 FAs. The molar ratio of the oleic acid–based C18:1 CE—one of the common CEs in other tissues and organisms—was less than 5% of all meibomian CE. Free cholesterol appears to be less than 0.5% of meibomian sterols and steryl esters.15
The relative amounts of phospholipids in human meibum are very controversial and unresolved, but the amount of phospholipids appears to be far less than previously thought.6,7,13,20,22,75,76 Discrepancies in phospholipid quantitation and identification may relate to (1) variations in sample collection techniques; (2) varying degrees of contamination of meibum samples with aqueous tears; and (3) differences in instrumentation and associated techniques. However, given that the meibomian gland secretes through a holocrine mechanism and that cell membranes are enriched in phospholipids, it would seem that phospholipids are, at least initially, secreted by the gland into meibum. The ability to detect such polar lipids, and to assess the nature and extent of possible variations between experimental groups, may depend on the methods of data analysis.9,77–79 There are factors that can affect the sensitivity of an individual phospholipid molecular species, such as the unsaturation of fatty acyl substituent and chain length; however, the most significant factor is ionization efficiency, which is dependent on the polar head group of the individual phospholipid classes. Ion-suppression effects make it difficult to observe minor components when a major class is also present in the ion source. Finally, some phospholipids are more readily detected in the negative-ion mode, whereas the opposite is true for other classes of phospholipids that are more readily detected in the positive ion mode. Those limitations complicate any direct approach to accurate quantitation of phospholipids by mass spectrometry.77
The lipid patterns of human meibum samples show many similarities among individuals.9,36,79 For example, Joffre et al.73 and Souchier et al.80 evaluated the changes in the FA composition of normal donors and subjects with meibomian gland dysfunction (MGD) or aqueous-deficient dry eye before and after minocycline treatment. The FA composition remained consistent in the normal subjects and did not differ much from those in subjects with aqueous dry eye. The MGD patients, however, produced a different, but a repeatable, pattern with a lower ratio of saturated to unsaturated FA, and a higher ratio of branched FA to straight-chain ones. Butovich et al.20,22 found that the composition of meibomian lipids collected from normal donors was reproducible intersubject from sample to sample when compared visually.
Normal Tears
Recently, researchers have reported that human meibum and aqueous tears differ somewhat in lipid compositions and the relative amounts of individual lipids. The most noticeable difference was an increase in the molar ratio of lower molecular weight WE-type compounds.7,9,20,79 Another difference is in the hydrocarbon chain ordering between lipids of meibum and aqueous tears: The former were less ordered at any tested temperature and had a lower phase transition temperature. This was explained by suggesting that both types of the samples had different lipid compositions.44 Of particular interest, aqueous tear samples show the spectrometric signals of organic phosphate esters, similar to that found in phosphatidylcholines and SMs.24,42,81
Animal Meibum and Tear Film Lipids
Animal studies on meibum lipids have predominantly focused on bovines (castrated bulls) and rabbits, although other species such as the mouse, hamster, rat, and gerbil have also been studied. The major constituents of animal meibum have been identified as sterol and WEs,10,28,31,82,83 although the mouse may contain predominantly CEs.84 Cholesterol appears to be the major sterol in all animals tested,28,31,59,61,82,85,86 apart from the rabbit, in which it has been reported that 24,25-dihydro-Δ8-lanosterol is the major sterol.83 Other sterols identified have been 5-α-cholest-7-en-3/β-ol in the meibum of cows,28 cholestanol in that of rabbits,82 3β-hydroxy-5α-cholestane and 3β-hydroxy-A7-cholestene in that of hamsters,85 lathosterol and perhaps methyl sterol in that of rats,58 and 3β-hydroxy-5α-cholestane in that of gerbils.86 Wax and sterol esters combined make up between 63% and 70% of the percent-weight of lipids in cow meibum30 and 78.5% of lipids in meibum of rabbit,82 with CE being 32% to 41% of cow meibum.10,31 Free cholesterol or other sterols make up only approximately 3% of cow or rabbit meibum.10,82
The fatty acids and fatty alcohols have also been examined in detail. Using an isolated meibomian gland model, Kalattukudy et al.,28 found that the glands of steers synthesize a high proportion of anteiso-branched chains of both the acids and alcohols. Some acids with very long carbon chains, as long as C36, have been found in the ω-hydroxy fraction. Anteiso-C25, -C27, and -C23 were the most highly labeled alcohols, confirming the findings of Baron and Blough,31 who detected that the fatty alcohol moieties of the WE in isolated meibum are branched chain C23:0 to C27:0. Nicolaides et al.10 reported that the fatty alcohols in total lipids and WEs of the steer range from C18 to C31. The major synthesized fatty acids in the WEs are anteiso-C15, n-C16, anteiso-C17, and n-C18:1, whereas anteiso-C25 and -C27 are the major labeled acids in the sterol esters. Steer fatty acids appear to be mostly composed of anteiso-branched and -saturated types.10 The triglyceride fraction which contained 8% of the total lipids is composed of labeled fatty acids similar to those found in both the sterol and the wax ester fractions.28 A group of ω-hydroxy fatty acids have been identified from the meibomian glands of steers and humans. These acids comprise approximately 10% of all the acids of the steer lipid, are primarily monoenoic, and constitute three homologous series with members ranging from C30 to C36 or C38.60,61
For the rabbit, all chains of the fatty acids and alcohols are saturated. Fatty acids C15 to C29 predominate which are anteiso-branched. Three fatty alcohols have been identified; anteiso-C25, -C27, and -C29.82 Only esters of dihydrolanosterol have been found that contain anteiso-C15 to -C19 fatty acids. The principal WE contain anteiso-branched C25 to C31 fatty alcohols and anteiso-branched C15 to C19 fatty acids in combination, making esters in the C40 to C46 range.83 FFAs makes up only 4.4% ± 0.2% of the total lipids.82
Harvey and Tiffany84 reported that the fatty alcohols in mouse meibum are predominantly iso-C26 and anteiso-C27. Monounsaturated fatty acids belong mainly to the ω-9 series, and saturated acids belong to the iso-, anteiso-, and n-series. Several 1,2-diols were also identified, with the most abundant of these being iso-C16 and iso-C20. GC-MS studies on the intact WEs showed them to be composed of the branched-chain alcohols and both branched-chain and unsaturated acids.84 Studies on the meibum of hamsters have shown fatty acids with chain lengths from 10 to 32 carbon atoms are found, the most common being C15 to C18 and C25 to C30.85 Chain types are predominantly iso- or anteiso-branched, monounsaturated (C16 and C18), and straight. Fatty alcohols are mainly from the iso or anteiso series and tend to have longer chain lengths; the major alcohols have anteiso-C25 and -C27 and iso-C26 chains.85 The tears of Golden hamsters contain considerable amounts of the unusual lipid, 1-alkyl-2,3-diacylglycerol.87 The rat has fatty acids in its meibum with chain lengths of between C12 and C34 and a biphasic distribution of maxima around C16 to C18 and C25 to C27. The chains are straight-chain iso, anteiso, and monounsaturated. The unsaturated acids have double bonds in the ω-7 and ω-9 positions. The alcohols have corresponding structures.59 The gerbil has fatty acids in its meibum with chain lengths from C12 to C27 with, again, a biphasic distribution with maxima at C15 to C18 and C25 to C27. Chains are predominantly iso- or anteiso-branched. Unsaturated fatty acids are mainly C16 and C18. Fatty alcohols are mainly branched, with chain lengths between C25 and C27, although there were also several fatty alcohols, both branched and unsaturated, with chains up to C33.86
The phospholipids of rabbits have been examined in a unique NMR study on animal tears and meibum, Greiner et al.27 reported that the phospholipid in meibum of rabbits is composed of 40% phosphatidyl choline, 18% phosphatidyl ethanolamine, 9% SM, 9% ethanolamine plasmalogen, 7% phosphatidyl serine, and 6% dihydrosphingomyelin. Ham et al.37 reported that species related to platelet-activating factor and/or lysophosphatidylcholine, phosphatidylcholine, and SM were found in the tears of normal rabbits and rabbits made to have dry eye through surgical procedures. The varieties and the concentrations of SM were greater in tears of rabbits with dry eye than in those of normal rabbits.37 In addition, the authors reported that they could not detect phosphatidylserine in the tears of normal rabbits, but this lipid was detectable in the tears of those with dry eye.37 The amount of polar lipids in the meibum of bovine (steers) has been reported to be 13.3% of the total lipids.10
Meibum Lipid Changes in Disease
McCulley et al.88 demonstrated that various forms of blepharitis are associated with changes to the lipid composition of the meibomian gland secretions. Also, meibomian secretions from patients with meibomian keratoconjunctivitis (MKC) have shown lower levels of unsaturated fatty acids and alcohols of the wax and cholesterol esters and occasionally differences in triglyceride profiles.33,68,69
There are generally low levels of phosphatidyl ethanolamine (PE) and SM in meibum of patients with blepharitis who also have dry eye symptoms.89 In vitro, SM can inhibit peroxidation of unsaturated fatty acids in phosphatidyl choline monolayers,90 and it has been shown that lipid peroxides can be significantly higher in tears of patients with severe-to-moderate dry eye (P < 0.05) or in those with good TF production but increased symptoms than healthy controls.91 Alternatively, the increase in oleic acid in the meibum of those with meibomian seborrhea may help explain the clinically significant burning symptoms that this group of people reports.92
Some of the differences seen in lipid types associated with different forms of blepharitis may be due to the presence of certain types of commensal lid bacteria that can hydrolyze lipids. People with meibomian seborrhea with a clinical appearance of Staphylococcus infection appear to harbor significantly more coagulase-negative Staphylococcus (CNS) strains capable of hydrolyzing cholesteryl oleate than do normal individuals.65
In addition, differences in subgroups have been demonstrated in people with androgen hormone deficiency, including males taking antiandrogen therapy, females with complete androgen insensitivity syndrome, and persons with Sjögren's syndrome, all of whom show changes in the polar and nonpolar lipid components.34,38,64,79
Obstructive MGD is characterized by an abnormal structure of the gland with characteristic changes in viscosity of the lipid expressed.64 An analysis of the lipid components in patients affected by MGD showed a significant decrease in triglycerides and cholesterol93 and a decrease in the amount of monounsaturated fatty acid, specifically in oleic acid.92 Decreased unsaturation of the nonpolar fatty acids tends to increase their melting point, leading to thickening of the meibum within the central duct. Infrared studies have shown that lipid order and phase transition temperatures are higher in meibum of donors with meibomian gland disease.44
Recently, Joffre et al.74 demonstrated that the fatty acid profile of the excreta collected by Schirmer test strips in people with blepharitis is significantly different from controls. Total saturated fatty acids were 9.3% in those with blepharitis versus 24.6% in controls, with lower quantities of palmitic (C16:0) and stearic (C18:0) acids. Branched-chain fatty acids were present in greater proportion in MGD patients. Interestingly, small differences were observed in fatty acid composition between those with blepharitis and those with dry eye, with 50% more linoleic acid in the dry eye group.74
The TF lipid layer changes secondary to MGD have a negative effect on the quality of vision measured as contrast sensitivity94 and on evaporation of tears from the ocular surface. Gilbard et al.95 demonstrated in rabbits that meibomian occlusion resulted in a rise of tear osmolarity, possibly as a consequence of an increased evaporation rate of water from the TF. Goto et al.96 showed an alteration of the lipid layer determines an increased tear evaporation rate in people with MGD, which could be responsible for the increase of hyperosmolarity, which, in turn, is able to produce an inflammatory response and damage of ocular surface epithelia. The biochemical changes in meibomian gland lipids may have a direct toxic effect on ocular tissues, since FFAs have been shown to be able to irritate the skin in acne vulgaris.76
The consistent finding of higher than normal levels of FFA in MGD offers a potential basis for symptoms associated with MGD. However, a study of the effect of branched-chain fatty acids on cultivated conjunctival human cells treated with iso-C16 and iso-C20 has shown no effects on the parameters of cytotoxicity. Only the mitochondrial dehydrogenase activity was significantly decreased in relation to the isoC20 concentration increase.74
Ocular rosacea has been associated with MGD.97 The concentration of triglycerides and FFA, especially the mono- and polyunsaturated forms, is increased and may be responsible for the activation of neutrophils and inflammatory mediators.69 Furthermore, the analysis of the ocular microbiota of patients with rosacea demonstrated bacterial growth in all patients,98 suggesting that rosacea may induce the production of lipases which in turn could disrupt the lipid layer of the TF and its protective role.
Lipids on Contact Lenses
Types of CLs
There are two major classes of CLs: the rigid lenses and the soft lenses. Rigid lenses were initially made from polymethyl methacrylate (PMMA). The newer generations of rigid CLs, (rigid gas-permeable [RGP] lenses), add fluorine and/or silicone to the base material to facilitate oxygen transport. The rigid CLs are classified into four groups: group I materials contain no silicone or fluorine; group II materials contain silicone but not fluorine; group III contain silicone and fluorine; and group IV contain fluorine but not silicone.99–101
Soft CLs have been traditionally made from hydroxyethyl methacrylate (HEMA) and may add vinyl pyrrolidone or methacrylic acid. The U.S. Food and Drug Administration classifies soft CLs into several groups (I to IV) based on their water content and overall ionic nature of the lens material. Table 2 summarizes the properties of the soft CLs.
Table 2.
Summary of Soft Contact Lens Groups, as Classified by the FDA
| Group | Water Content | Polymer Type | Lens Material |
|---|---|---|---|
| I | <50% H2O | Nonionic polymer | Tetrafilcon A, Polymacon |
| II | >50% H2O | Nonionic polymer | Lidofilcon A or B, Alfafilcon A, |
| Omafilcon A, Nelfilcon A, | |||
| Vasurfilcon A, Hioxifilcon A | |||
| III | <50% H2O | Anionic polymer | Bufilcon A, Phemfilcon A, Ocufilcon A |
| IV | >50% H2O | Anionic polymer | Etafilcon A, Vifilcon A |
Source: U.S. Food and Drug Administration.
Since 1999, so-called silicone hydrogel CLs have been commercially available. These lenses were designed to achieve the oxygen permeability given by silicone and fluorine but in a soft lens form (>10% water content material). These lenses are classified in the FDA soft lens classification system outlined above. However, due to their very different chemical natures compared to classic HEMA-based soft lenses, there are proposals to separately classify these lenses into their own group (group V) within the soft lens system (Table 3).101
Table 3.
Proposed Classification of Silicone Hydrogel Lenses
| Group | Basis of Categorization | Examples of Polymer Types |
|---|---|---|
| Va | Nonlinear relationship between Dk (oxygen permeability) and water content | Comfilcon A |
| Vb | Contain an ionic (anionic) component | Balafilcon A |
| Vc | Plasma or bonded surface modification | Lotrafilcon A and B; Asmofilcon A |
| Vd | “Released” wetting agent | Galyfilcon A; Senofilcon A |
Lipid Deposition
While lipids from the meibomian gland appear to be essential for ease of lens wear,102 investigators have analyzed the deposition of lipids onto the surface of CLs due to the possible clinical consequences. In in vitro experiments, RGP lenses lipid deposition has been shown to be dependent on the lens matrix hydrophobicity.103 For the polymers siloxanyl alkyl acrylate and fluorosiloxanyl alkyl acrylate (silafocon A and paflufocon B, respectively), lipid in an artificial tear solution enhanced protein deposition but that protein in the artificial tear solution decreased lipid deposition on only the siloxanyl alkyl acrylate lens.103
In addition, differential lipid deposition can be seen by group. Group IV hydrogel lenses bind more phosphatidylcholine (although at <1 μg/lens) than other lens groups, possibly reflecting an interaction between the positively charged choline residue and the negative surface of the lens.104
Hydrogel lenses made of poly(2-hydroxyethyl methacrylate), poly(methy1 methacrylate)-poly(vinyl alcohol) or poly(2hydroxyethyl methacrylate)- poly(viny1 pyrrolidone)-poly(methacrylic acid) can all adsorb lipids from solution in vitro, and lenses made from poly(methy1 methacrylate)-poly(vinyl alcohol) tend to adsorb slightly more lipid.105 Adsorption of cholesterol to poly(HEMA) lenses may collapse/condense the hydrogel lens material and expel water, whereas lipid binding to PMMA lenses was simply an adsorptive process.106
Cholesterol adsorbs in greater quantities than phosphatidylethanolamine for silicone hydrogel lenses or group IV lenses (Table 4).107
Table 4.
Deposition of Lipids onto Contact Lenses107
| Amount of Lipid Adsorbed In Vitro (μg/lens) |
|||||
|---|---|---|---|---|---|
| Soft Lens Type | Polymer Name | Cholesterol | Cholesterol Oleate | Phosphatidyl Ethanolamine | Dioleoyl Phosphatidylcholine |
| Group I | Polymacon | 0.5 | 0.1 | ||
| Group II | Lidofilcon A | 0.6 | 0.1 | ||
| Group III | Phemphilcon A | 0.7 | 0.3 | ||
| Group IV | Phemphilcon A | 0.9 | 0.3 | ||
| Group IV | Etafilcon A | 7.0 | 0.1 | ||
| Group Va | Balafilcon A | 24.1 | 3.2 | ||
| Group Vc | Lotrafilcon B | 3.0 | 1.5 | ||
| Group Vd | Senofilcon A | 23.2 | 4.9 | ||
In vitro, the polyvinylpyrrolidone in both galyfilcon A and senofilcon A may be responsible for the increased binding to cholesterol or phosphatidyl ethanolamine. Silicone hydrogels bind cholesterol in relatively high levels and also bind squalene, CE, and WE.108 Similarly, the level of lipid binding was greater for galyfilcon A (group Vd) and balafilcon A (group Vb) than for lenses from group Vc (again no group Va lens was tested).107 Indeed, levels of cholesterol, squalene, cholesterol esters or wax esters on the group Vb lenses (lotrafilcon A and lotrafilcon B) were similar to levels adsorbed to a group IV HEMA-based hydrogel lenses.108
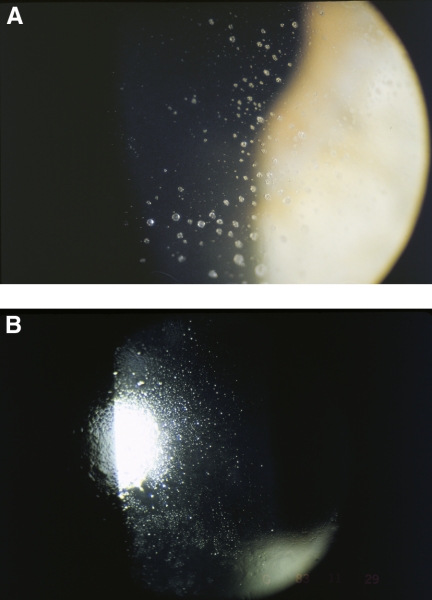
Initial in vivo studies demonstrated that lipid was present in deposits on CLs, with the principal lipid type being CE.109 A particular form of deposit on lenses, often called jelly bump deposits, was shown to be composed of long and intermediate sized CE, triglycerides, and WE.110 White spots, a similar particular type of deposits found on non-regularly replaced hydrogel lenses, are predominantly comprised of lipid.111,112 The lipid white spot deposits have a distinct structural stratification with a lipid layer providing the interface between the CL surface and the deposit superstructure. This initial interfacial layer has been shown to be made from cholesterol, cholesterol ester and unsaturated lipids.111,112 Of note, diet plays a part in formation of these white spots, and individuals who consumed larger amounts of alcohol, protein, and fat exhibited increased lipid deposition on their lenses.113 As hydrogel CLs tend to be replaced much more frequently today compared to 15 years ago (typically on a weekly, bi-weekly, or monthly basis today versus annually in the past), the incidence of these white spot deposits (Fig. 2) has reduced and they are now rarely seen.
Figure 2.
Soft contact lens lipid deposits seen on slit lamp examination (photos courtesy of the Brien Holden Vision Institute).
In vivo, RGP lenses deposit more lipid than many soft lens materials, probably due to the hydrophobicity of the lens.114 Silicone-based RGP lenses also deposit more lipid than fluorine-containing RGP lenses, probably because silicone increases the hydrophobicity of the CL, whereas fluorine decreases hydrophobicity and thus decreases lipid deposition.114 The level of lipid deposition on group 1 (polymacon and tetrafilcon A) and group III (Balafilcon A) appears to be related to characteristics of the wearer rather than lens material per se.115 FDA group II lenses deposit the most lipid, and FDA group III lenses deposit the least.103,114,116 On group II lenses (containing polyvinyl pyrrolidone) lipid deposition appears to increase over time (from 1–28 days of wear; P < 0.0001), whereas lipid deposition on the group IV lens reaches a maximum after 1 day and increases no further.117 Lipid levels on group II lenses containing polyvinyl pyrrolidone are approximately twice that on group IV lenses,118 and again, there was a significant intersubject variation in lipid deposition levels. Lipid deposition on lenses ex vivo is shown in Tables 4 and 5.
Table 5.
Amounts of Lipid Deposited on Lenses during Wear
| Soft Lens Polymer Type | Polymer Name | Amount of Lipid Adsorbed In Vivo (μg/lens, unless otherwise stated) |
||
|---|---|---|---|---|
| Total Lipid | Phospholipids | Cholesterol | ||
| Group I | Polymacon | 66.3,103 62113 | 2.1, 1180.01–0.05 (micromoles/lens)117 | |
| Group II | Alphafilcon | 427 (fluorescent units)111 | ||
| Group IV | Etafilcon A | 44.1,116 29 (fluorescent units)111 | 1.8118 | |
| Group Va | Balafilcon A | 19 (ng/lens SM), 19 (ng/lens PC)120 | 4.1–8.2,119 3.9120 | |
| Group Vb | Lotrafilcon B | 0.1–0.5119 | ||
| Group Vd | Senofilcon A | 59 (ng/lens SM), 195 (ng/lens PC)120 | 0.3–2.7,119 9.9120 | |
SM, sphingomyelin; PC, phosphatidylcholine.
Overall lipid deposition increases with longer replacement schedules (3 months vs. 1 month).121 These studies did not characterize the lipid types, but measured lipid adsorption using spectrophotometric methods, the sulfo-phospho-vanillin reaction or estimation of total phosphate (for phospholipids). The lipid deposits on worn hydrogel lenses were chemically analyzed, and detected WEs, fatty sterols, fatty alcohols, FFAs, and monoglycerides, whereas cholesterol, CEs, and triglycerides were not detectable.122 However, cholesteryl oleate, cholesterol, oleic acid, oleic acid methyl ester, and triolein were detected in extracts from worn hydrogel and RGP lenses.114 These discrepancies may be due to the types of lenses being investigated, with polar lipids depositing preferentially onto the more hydrophilic lenses compared to nonpolar lipids. Oleic acid methyl ester appears to adsorb less to group III and IV hydrogels and RGP than other lens types.114
For the silicone hydrogel lenses (groups Vb and Vc), the degree of lipid deposition in vivo appears to be substantially higher than that seen with conventional hydrogels.123 Cholesterol was the most commonly deposited lipid, although oleic acid and oleic acid methyl ester was also detected.123 Another study using balafilcon A, lotrafilcon A, and galyfilcon A lenses (groups Vb, Vd, and Vc, respectively) was also able to detect cholesterol in deposits,123 but found very low levels of oleic acid or its methyl ester (summarized in Table 6).
Table 6.
Lens Materials and Various Deposit Levels124
| Lens Material | Cholesterol (mg/Lens) | Oleic Acid (mg/Lens) | Oleic Methyl Ester (mg/Lens) |
|---|---|---|---|
| Balafilcon A | 15.6 ± 3.9 | 1.0 ± 0.4 | 0.2 ± 0.4 |
| Lotrafilcon A | 0.5 ± 0.3 | 0.7 ± 0.5 | 0.0 ± 0.1 |
| Galyfilcon A | 9.9 ± 5.7 | 0.7 ± 0.7 | 0.1 ± 0.2 |
Overall the levels of lipid deposition on the silicone hydrogels lenses were lotrafilcon A < galyfilcon A < balafilcon A, similar to the ranking and amounts seen during in vitro experiments.107,108 The differences in the cholesterol deposition between the two studies (300–600 μg/lens), are not easily explained.123,124 Another study examining the deposition of cholesterol onto various silicone hydrogel lenses had a ranking of lens types in their deposition of cholesterol; lotrafilcon B (group Vc) < senofilcon A (group Vd) < galyfilcon A (group Vd) < balafilcon A (group Vb), and the study identified the use of various cleaning/disinfecting solutions as a significant modulator of cholesterol deposition.120 Saville et al.114 found during silicone hydrogel lens wear (senofilcon A or balafilcon A) adsorption included a range of molecular type of both SM and PC, with SM C16:0 and PC C34:2.
Clinical Changes of Lipids and CL
The literature is unclear whether the deposition of lipid on CLs affects comfort, or whether clinical testing can be used to detect changes in lipid profiles on lenses. Clinically, galyfilcon A lenses (group Vd) tend to have more grade 3 to 4 lipid deposits than group IV lenses.120 Soaking silicone hydrogel lenses (group Vc) in lanolin reduces the drying time of tears over the lens surface and TF appears thinner over lens surface.125
There is an apparent decrease in cholesterol levels in tears for around 10 hours after lens insertion occurs.126 In addition, the phospholipid concentrations in tears of patients wearing polymacon (group I) or etafilcon A (group IV) lenses were 186 ± 39 μg/mL and 162 ± 33 μg/mL, respectively, with the latter concentration being significantly lower than that observed in the same subjects when not wearing CLs (220 ± 35 μg/mL; P = 0.0023).127 This may be of significance as concentrations of these phospholipids in the tears of patients with marginal and moderate dry eye have been reported to be significantly lower than those in subjects without dry eye128 and CL wear is well known to cause dryness and discomfort sensations in significant proportion of wearers.129,130 Furthermore, two studies131,132 reported that hydrogel lens wear altered the TF lipid composition by decreasing the levels of polar lipids and increasing levels of nonpolar lipids. These studies also found low levels of tear polar lipids (phospholipids) were associated with increased levels of tear instability during soft CL wear. It is possible that phospholipids in tears are degraded by group IIa secretory phospholipase A2 (sPLA2) deposited on CLs; etafilcon A (group IV) lenses deposit statistically significantly more group sPLA2 than polymacon (group I) lenses.133,134 These changes to the biochemistry of the TF may manifest as overt changes to the clinical picture of the lipid layer on the surface of the TF.
It has been established that wearing most CL types results in a disruption to the lipid layer appearance of the TF.135–137 Lipid layer thickness assessed via interference fringes is generally classified into six different types on the basis of the fringe patterns seen via a slit lamp system. These patterns—none, meshwork, wave, amorphous, colors, and other—increase in thickness layers form none to colors. During lens wear, the lipid layer does not uniformly coat RGP or soft hydrogel lenses.135–137 There is, however, an increase in lipid layer continuity over high-water-content soft lenses.138 Another study comparing two high-water-content hydrogel lenses, filcon 4A (67% water content) and lidofilcon (70% water content), found no difference between the two materials in terms of lipid layer appearance, but did demonstrate that the lipid layer over a lens just after waking was thicker than during normal open-eye conditions and that this correlated with more stable TF after waking than during open-eye.139
When the well-formed TF, including a healthy lipid layer over the CL, is missing or abnormal, evaporation of the TF during CL wear can occur, which may then lead to ocular discomfort. Thai et al.140 demonstrated that wearing either soft hydrogel lenses or silicone hydrogel lenses leads to increased evaporation of tears from the eye when compared to nonwear. While there were no statistically significant differences between evaporation rates between lens types, individuals did show significant differences with different lens types.140 Omafilcon A lenses have been noted in other studies to create thicker lipid layers.140,141 The ability of these lenses to sustain a thicker lipid layer may be due to the biomimetic nature of the phosphorylcholine in the lens. In vivo, the galyfilcon A lenses (silicone hydrogel) have a thicker lipid layer than the alphafilcon A (group II HEMA), which may give the former a more stable TF than the latter.141
In the absence of lens wear, no difference has been found in the lipid layer thickness (lipid layer pattern) or TF stability in asymptomatic or symptomatic lens wearers.142 However, TF stability is decreased in those intolerant of lens wear when compared with those who are tolerant, even though the lipid layer appearance was not different between the two groups.143 Further, people intolerant of CL wear (defined as being unable to wear CLs for more than 6 hours throughout the day) have increased levels of malondialdehyde and 4-hydroxy-2(E)-nonenal (degradation products of polyunsaturated fatty acid and related esters) in their TF. In addition, intolerant subjects had significantly more sPLA2 in their tears compared with tolerant subjects. No differences in the number of blocked meibomian glands were found between the two groups.144
Tear Lipid–Protein Interactions
The seminal paper on tear lipid–protein interactions was published in 1973.12 Holly showed that the spread of lipids is facilitated by mucins.12 With high surface pressure and low surface tension, the meibum lipid coalesces into a droplet and does not spread across the surface of water. When mucin is dissolved in water, such as with the mucin–aqueous gel gradient of the TF (Fig. 1), the surface tension is lowered allowing meibum lipids to spread across the aqueous surface.
Lipocalin
Cholesterol, fatty acids, fatty alcohols, glycolipids and phospholipids in the TF are bound by lipocalin and the binding remains after several levels of chromatographic separation.145 Additional binding studies of tear lipocalin revealed that apo tear lipocalin has a high affinity for phospholipids and stearic acid (Ki) of 1.2 and 1.3 μM, respectively, and much less affinity for cholesterol (Ki) of 15.9 μM. For fatty acids, binding affinity correlates with the length of the hydrocarbon chain. Tear lipocalin binds most strongly to the least soluble lipids permitting these lipids to exceed their maximum solubility in aqueous solution. These data implicate tear lipocalin in solubilizing and transporting lipids in the TF.145,146 Lipocalin is a major tear protein comprising 33% of total protein in a tear sample.147 It is secreted by the lacrimal gland and has also been detected in meibomian gland secretions.148 The structure, function, and molecular mechanisms of action of tear lipocalin have recently been reviewed.149–151
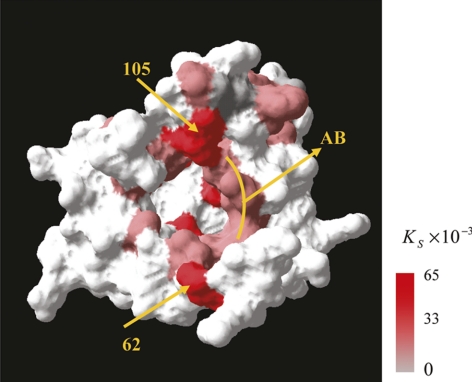
Conformational changes in tear lipocalin are evident when lipid binds the protein.152 It has been proposed that lipocalin scavenges lipid from the corneal surface and may enhance the transport and equilibration of lipid in the lipid surface layer.153 Recently, the solution structure of tear lipocalin bound to a native ligand was elucidated and the entire binding energy landscape was clarified by using a modification of site-directed tryptophan fluorescence.154 Gasymov et al.154 describe the process in which ligands explore multiple binding sites in nanoseconds before exiting the cavity of the protein. In Figure 3, the more intense red indicates greater static quenching or static binding. Subsequently, the tear lipocalin was crystallized in space group P21 with four protein molecules with bound artificial ligand 1,4-butanediol and its x-ray structure was solved at 0.026-nm (2.6 Å) resolution.155 Breustedt et al.155 showed that the loop region and adjoining areas of the β-barrel allow considerable conformational flexibility, which allows tear lipocalin to adapt to ligands that differ vastly in size and shape. This observed promiscuity in ligand recognition may be important in understanding the function of tear lipocalin and lipid–protein interactions on the TF.
Figure 3.
The solvent-accessible surface of TL. Residues are colored according to the static quenching constants observed with C12SL. Reprinted from Gasymov OK, Abduragimov AR, Glasgow BJ. Intracavitary ligand distribution in tear lipocalin by site-directed tryptophan fluorescence. Biochemistry. 2009;48:7219–7228 with permission from the American Chemical Society.
Tiffany and Gouveia156 found an interactive role of lipids and proteins in their tear viscosity study. It has been suggested that lipocalin forms dimers when lipid is bound to the protein. However, more recent work demonstrates that it is likely that tear lipocalin exists mainly as a monomer in the TF and that the dimeric form is minimal.153,157
Tear lipocalin deficiency is associated with meibomian gland dysfunction158 and the studies above show that lipocalin sequesters lipids. Whether the lipocalin/ lipid complex interacts with the lipid layer has been the focus of recent studies. When human meibum was used in a in vitro study by Millar et al.,159 lipocalin bound slowly to a human meibomian lipid film compared with lysozyme or lactoferrin. The adsorption of lipocalin to a human meibomian lipid film was very different from its adsorption to a bovine meibomian lipid film, indicating the nature of the lipids in the film is critical to the adsorption process.
Based on these studies, it seems likely that tear lipid–protein interactions occur in vivo and that these interactions change the physical properties of tears. There are several gaps in knowledge that when filled could facilitate the development of therapies to reduce dry eye and MGD symptoms.
Lysozyme
Lysozyme, a major protein found in tears, acts as a bacteriolytic protein that depolymerizes mucopolysaccharides. Lysozyme does not sequester lipids as lipocalin does,146 but it does interact with and binds in vitro to the phospholipids of membranes160 and meibum films.17,159,161,162 It is possible that lysozyme not only stabilizes the structure of the lipid layer but that loss of lysozyme in disease states disrupts this stability, causing an increase in the rate of evaporation.
Changes in the concentration of tear lysozyme by disease or drugs may disrupt the structure of the lipid layer of the TF. It would be useful to define interactions between lysozyme and tear lipids in vitro using spectroscopic approaches and to determine whether or not structural alterations in the lipid layer caused by a change in the concentration of lysozyme leads to increased rates of evaporation.
Apolipoprotein D
Apolipoprotein D (apoD) is a member of the lipocalin super family and has been shown to be produced in the lacrimal gland and has been found in the tears.163 Although the physiological function of apoD is currently unknown, it has the ability to bind phospholipids, cholesterol, and other lipids. The function of this protein in tears may be to interact with the meibomian lipids present in human tear fluid and perhaps contribute to the surface spreading of these lipids. Another possible function could be as a clearance factor, protecting the cornea from harmful lipophilic molecules.163
Phospholipid Transfer Protein
The presence of phospholipid transfer protein (PLTP) and cholesteryl ester transfer protein (CETP) in human tears was investigated using Western blot analysis and quantitated using ELISA.164 PLTP was found to be present in tear fluid, whereas CETP was not. ELISA indicated that the PLTP concentration in tear fluid, 10.9 ± 2.4 μg/mL, is approximately two times higher than that in human plasma. PLTP-facilitated phospholipid transfer activity in tears, 15.1 ± 1.8 micromoles mL−1 h−1, was also significantly higher than that measured in plasma. These results suggest that PLTP may be involved in the formation of the TF by mediating lipid transfer in tear fluid. However, the concentration of this protein is relatively small in tears compared to lipocalin and PLTP has not been specifically shown to bind with lipids in tears.
The studies by Millar et al.3,162 show that mucin binds to meibum in vitro; however, mucin–lipid interactions have never been studied on a molecular level. Currently, there is no evidence that certain mucins may interact with the lipid layer; conversely, there is no evidence mucin–lipid interactions do not exist, either.165
Although the amount of fatty acid and cholesterol bound to lipocalin has been quantified, the amount and type of phospholipid and lysophospholipid bound to lipocalin has not. In addition, it can be hypothesized that most phospholipids in tears could be bound to lipocalin. Although the interactions between various lipids have been studied extensively, those directly relating to the mucin–lipid relationship, if any, remain a mystery. Research has yet to quantify whether binding of lipocalin, lysozyme or tear fluid components to meibum lipid cause molecular–structural changes to the proteins or lipids. Nor do we yet know whether compositional changes in meibum with age or MGD alter the binding of proteins to meibomian lipids.
Influence of Bacteria on Tear Film Lipids
The normal microbiota of the eye, including S. aureus, Haemophilus influenza, CNS, Propionibacterium sp., and Corynebacterium sp.,166,167 produces enzymes that can degrade the lipids of the TF. CNS, Propionibacterium acnes, and coryneform bacteria from patients with chronic blepharitis, which along with S. aureus strains, produce lipolytic exoenzymes (cholesterol esterase, fatty wax esterase, and triglyceride lipase) that can hydrolyze cholesterol esters and WEs.88,168 A higher number of CNS, able to produce these enzymes, has been found on lids of patients with seborrheic blepharitis or meibomian keratoconjunctivitis.169 Changes in FFA of meibum secretions were also found in these patients. Of note, the ability of tetracycline to inhibit lipolytic enzymes before and during the inhibition of the growth of the bacteria66 may explain the effectiveness of such antibiotics in the treatment of conditions such as blepharitis, with or without meibomitis. Treatment of patients with blepharitis with minocycline decreased the concentration of diglyceride, FFA, and free cholesterol in the meibomian secretions, suggesting that this antibiotic had inhibited lipolytic exoenzymatic activity on the parent triglyceride, cholesterol ester, and fatty wax molecules.33 Clearly, the exact role of bacteria in changing TF lipids has yet to be fully resolved. There also should be further investigation into the molecular changes associated with bacterial colonization and use of antibiotics.
Surfactant Proteins of the Tear Film, Ocular Surface, and Lacrimal Apparatus
Superficially active substances, similar to the surfactant system of the lung, are of importance not only in TF but also in the auditory tube and on the skin.170 The presence of surfactant-associated protein D (SP-D) has been described in TF and lacrimal glands.171–173 Ni et al.174 were able to show that SP-D is also present in the cornea of mice and shows protective effects against keratitis caused by P. aeruginosa. It was recently demonstrated that not only SP-D, but also the surfactant-associated protein A (SP-A), along with SP-B and SP-C, is present at the ocular surface (conjunctiva, cornea) in the lacrimal apparatus (lacrimal glands, nasolacrimal ducts) and in tears.175,176 SP-A and SP-C in tear fluid, and SP-C in all examined tissues, show an expression pattern different from that of lung surfactant proteins, with the exception of lowering surface tension.166 This difference is probably due to tissue-specific posttranslational or posttranscriptional modifications of the proteins and may lead to differences in the activity spectrum of the surfactant proteins. SP-A, -B, -C, and -D are found in the acinar cells of the lacrimal gland and the accessory lacrimal glands of the lids, in the conjunctival epithelial cells and in columnar epithelial cells (particularly apically) as well as in serous portions of seromucous glands in the efferent tear ducts. Goblet cells do not produce any of the four SPs, and in contrast to tear fluid, the aqueous humor does not show the presence of SPs under physiological conditions.176 SP-B and SP-C are absent inside the cornea and on its surface.175 Thus, besides SP-D, which has already been reported as present in TF,172–174 SP-A, SP-B, and SP-C proteins are also present in the lacrimal fluid and on the ocular surface.175,176
Seeing that the hydrophobic surfactant proteins SP-B and SP-C exert an expanding influence on the surface tension of the air–liquid interface atop the alveolar lining layer, a similar effect could be discussed in relation to the TF and the tear fluid at the human ocular surface and efferent tear ducts. Within this context, a modified scheme of the TF could contain the surfactant proteins as evidenced and investigated with respect to their physicochemical properties and putative functions as discussed in tears, the lacrimal apparatus and at the ocular surface. This view demonstrates the possibility of small hydrophobic surfactant proteins B and C embedded into the lipid component of the TF, oriented according to their amphiphilic character. Furthermore, the water-soluble and polymerizeable collectin-like surfactant proteins A and D could be arranged within the aqueous component of the TF along with the various secreted and shed mucins that are already known. This hypothetical model supports possible functions of surfactant proteins in relation to severe diseases of the ocular surface (e.g., dry eye syndrome and as bacterial and viral infections). Hypothetically, absence of the small hydrophobic surfactant proteins B and C could result in alterations of TF stability and, as a consequence, interruption of the TF itself, leading to signs of dry eye syndrome (such as dry spots).
Further research is needed to establish a system of expression in which all four surfactant proteins can be manufactured by using recombinant methods, including open reading frames for mature forms and pre forms of the proteins. The recombinant surfactant proteins produced in this manner could be used in functional studies of the ocular surface and lacrimal system. Some recombinant surfactant proteins have already been manufactured.177,178 With the software programs and methods available today, it is possible to use comparative protein modeling or threading to create a reliable model of the 3-D structure of proteins and to identify the active sites. Blocking or modification of assumed active sites could alter the functionality of the proteins and lead to completely new proteins at the functional level.
Conclusions
Understanding the molecular composition (proteomics, lipidomics) of the TF and the contribution of the meibomian gland to the TF is critical to understand and describe TF instabilities, dry eye syndromes, CL incompatibilities and other eye diseases.
Elucidation of the compositional components of meibomian gland secretion and the TF has been challenging in the past because of limitations to analytical and biochemical techniques. Most analytical techniques had low sensitivity and low resolution, required large sample amounts (requiring pooling), and chemical derivitization for detection. This limitation caused possible degradation of the sample because of prolonged exposure time; low sample recovery due to derivation, isomerization and/or decomposition due to sustained high temperature analysis; long analysis time; lack of information on the actual molecular composition of the lipids; and contamination. Recently, new advances in analytical and biochemical techniques have allowed researchers better methods to examine the meibomian gland secretions and TF components with the ability to identify the actual specific molecular composition of lipids, proteins, posttranslational modifications and protein–lipid interactions. Although the signs and symptoms of TF instability are reasonably well characterized, we are only beginning to scratch the surface of understanding the specific molecular components of the TF and their relationship with MGD and TF stability.
Footnotes
Supported by the Tear Film and Ocular Surface Society (TFOS; http://www.tearfilm.org); individual author support is listed in the Appendix of the Introduction.
Disclosure: Each Workshop Participant's disclosure data can be found in the Appendix of the Introduction.
References
- 1. Holly FJ, Lemp MA. Tear physiology and dry eyes. Surv Ophthalmol. 1977;22:69–87 [DOI] [PubMed] [Google Scholar]
- 2. Butovich IA, Millar TJ, Ham BM. Understanding and analyzing meibomian lipids: a review. Curr Eye Res. 2008;33:405–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Millar TJ, Tragoulias ST, Anderton PJ, et al. The surface activity of purified ocular mucin at the air-liquid interface and interactions with meibomian lipids. Cornea. 2006;25:91–100 [DOI] [PubMed] [Google Scholar]
- 4. King-Smith PE, Hinel EA, Nichols JJ. Application of a novel interferometric method to investigate the relation between lipid layer thickness and tear film thinning. Invest Ophthalmol Vis Sci. 2010;51:2418–2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wojtowicz JC, Butovich IA, McCulley JP. Historical brief on composition of human meibum lipids. Ocul Surf. 2009;7:145–153 [DOI] [PubMed] [Google Scholar]
- 6. Butovich IA. The Meibomian puzzle: combining pieces together. Prog Retin Eye Res. 2009;28:483–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Butovich IA, Uchiyama E, Di Pascuale MA, McCulley JP. Liquid chromatography-mass spectrometric analysis of lipids present in human meibomian gland secretions. Lipids. 2007;42:765–776 [DOI] [PubMed] [Google Scholar]
- 8. Chen J, Green-Church KB, Nichols KK. Shotgun Lipidomic Analysis of Human Meibomian Gland Secretions with Electrospray Ionization Tandem Mass Spectrometry. Invest Ophthalmol Vis Sci. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sullivan BD, Evans JE, Dana MR, Sullivan DA. Influence of aging on the polar and neutral lipid profiles in human meibomian gland secretions. Arch Ophthalmol. 2006;124:1286–1292 [DOI] [PubMed] [Google Scholar]
- 10. Nicolaides N, Kaitaranta JK, Rawdah TN, Macy JI, Boswell FM, 3rd, Smith RE. Meibomian gland studies: comparison of steer and human lipids. Invest Ophthalmol Vis Sci. 1981;20:522–536 [PubMed] [Google Scholar]
- 11. Wolff E. ed. The Anatomy of the Eye and Orbit. 4th ed. London: H. K. Lewis and Co; 1954 [Google Scholar]
- 12. Holly FJ. Formation and rupture of the tear film. Exp Eye Res. 1973;15:515–525 [DOI] [PubMed] [Google Scholar]
- 13. Shine WE, McCulley JP. Polar lipids in human meibomian gland secretions. Curr Eye Res. 2003;26:89–94 [DOI] [PubMed] [Google Scholar]
- 14. Butovich IA, Wojtowicz JC, Molai M. Human tear film and meibum: very long chain wax esters and (O-acyl)-omega-hydroxy fatty acids of meibum. J Lipid Res. 2009;50:2471–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Butovich IA. Cholesteryl esters as a depot for very long chain fatty acids in human meibum. J Lipid Res. 2009;50:501–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saaren-Seppälä H, Jauhiainen M, Tervo TM, Redl B, Kinnunen PK, Holopainen JM. Interaction of purified tear lipocalin with lipid membranes. Invest Ophthalmol Vis Sci. 2005;46:3649–3656 [DOI] [PubMed] [Google Scholar]
- 17. Mudgil P, Torres M, Millar TJ. Adsorption of lysozyme to phospholipid and meibomian lipid monolayer films. Colloids Surf B Biointerfaces. 2006;48:128–137 [DOI] [PubMed] [Google Scholar]
- 18. Butovich IA. Lipidomic analysis of human meibum using HPLC-MSn. Methods Mol Biol. 2009;579:221–246 [DOI] [PubMed] [Google Scholar]
- 19. Avanti-Polar-Lipids-Inc Storage and handling of lipids. 2009 [Google Scholar]
- 20. Butovich IA, Uchiyama E, McCulley JP. Lipids of human meibum: mass-spectrometric analysis and structural elucidation. J Lipid Res. 2007;48:2220–2235 [DOI] [PubMed] [Google Scholar]
- 21. McCulley JP, Shine WE. Meibomian secretions in chronic blepharitis. Adv Exp Med Biol. 1998;438:319–326 [DOI] [PubMed] [Google Scholar]
- 22. Butovich IA. On the lipid composition of human meibum and tears: comparative analysis of nonpolar lipids. Invest Ophthalmol Vis Sci. 2008;49:3779–3789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nichols KK, Ham BM, Nichols JJ, Ziegler C, Green-Church KB. Identification of fatty acids and fatty acid amides in human meibomian gland secretions. Invest Ophthalmol Vis Sci. 2007;48:34–39 [DOI] [PubMed] [Google Scholar]
- 24. Borchman D, Foulks GN, Yappert MC, Ho DV. Temperature-induced conformational changes in human tearlipids hydrocarbon chains. Biopolymers. 2007;87:124–133 [DOI] [PubMed] [Google Scholar]
- 25. Yenice O, Onal S, Midi I, Ozcan E, Temel A, D IG. Visual field analysis in patients with Parkinson's disease. Parkinsonism Relat Disord. 2008;14:193–198 [DOI] [PubMed] [Google Scholar]
- 26. Greiner JV, Glonek T, Korb DR, Booth R, Leahy CD. Phospholipids in meibomian gland secretion. Ophthalmic Res. 1996;28:44–49 [DOI] [PubMed] [Google Scholar]
- 27. Greiner JV, Glonek T, Korb DR, Leahy CD. Meibomian gland phospholipids. Curr Eye Res. 1996;15:371–375 [DOI] [PubMed] [Google Scholar]
- 28. Kolattukudy PE, Rogers LM, Nicolaides N. Biosynthesis of lipids by bovine meibomian glands. Lipids. 1985;20:468–474 [DOI] [PubMed] [Google Scholar]
- 29. Linton RG, Curnow DH, Riley WJ. The Meibomian glands: an investigation into the secretion and some aspects of the physiology. Br J Ophthalmol. 1961;45:718–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jacob JT, Ham B. Compositional profiling and biomarker identification of the tear film. Ocul Surf. 2008;6:175–185 [DOI] [PubMed] [Google Scholar]
- 31. Baron C, Blough HA. Composition of the neutral lipids of bovine meibomian secretions. J Lipid Res. 1976;17:373–376 [PubMed] [Google Scholar]
- 32. McCulley JP, Shine W. A compositional based model for the tear film lipid layer. Trans Am Ophthalmol Soc. 1997;95:79–88; discussion 88–93 [PMC free article] [PubMed] [Google Scholar]
- 33. Shine WE, McCulley JP. The role of cholesterol in chronic blepharitis. Invest Ophthalmol Vis Sci. 1991;32:2272–2280 [PubMed] [Google Scholar]
- 34. Sullivan BD, Evans JE, Krenzer KL, Reza Dana M, Sullivan DA. Impact of antiandrogen treatment on the fatty acid profile of neutral lipids in human meibomian gland secretions. J Clin Endocrinol Metab. 2000;85:4866–4873 [DOI] [PubMed] [Google Scholar]
- 35. Ham BM, Jacob JT, Keese MM, Cole RB. Identification, quantification and comparison of major non-polar lipids in normal and dry eye tear lipidomes by electrospray tandem mass spectrometry. J Mass Spectrom. 2004;39:1321–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sullivan BD, Evans JE, Cermak JM, Krenzer KL, Dana MR, Sullivan DA. Complete androgen insensitivity syndrome: effect on human meibomian gland secretions. Arch Ophthalmol. 2002;120:1689–1699 [DOI] [PubMed] [Google Scholar]
- 37. Ham BM, Cole RB, Jacob JT. Identification and comparison of the polar phospholipids in normal and dry eye rabbit tears by MALDI-TOF mass spectrometry. Invest Ophthalmol Vis Sci. 2006;47:3330–3338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ham BM, Jacob JT, Cole RB. MALDI-TOF MS of phosphorylated lipids in biological fluids using immobilized metal affinity chromatography and a solid ionic crystal matrix. Anal Chem. 2005;77:4439–4447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Silverstein RM, Bassler GC, Morrill TC. Spectrometric Identification of Organic Compounds. 5th ed. New York: John Wiley and Sons; 1991 [Google Scholar]
- 40. Skoog DA, Leary JJ. Principles of Instrumental Analysis. 4th ed. New York: Saunders College Publishing; 1992 [Google Scholar]
- 41. Borchman D, Foulks GN, Yappert MC, et al. Physical changes in human meibum with age as measured by infrared spectroscopy. Ophthalmic Res. 2010;44:34–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Borchman D, Foulks GN, Yappert MC, Tang D, Ho DV. Spectroscopic evaluation of human tear lipids. Chem Phys Lipids. 2007;147:87–102 [DOI] [PubMed] [Google Scholar]
- 43. Oshima Y, Sato H, Zaghloul A, Foulks GN, Yappert MC, Borchman D. Characterization of human meibum lipid using raman spectroscopy. Curr Eye Res. 2009;34:824–835 [DOI] [PubMed] [Google Scholar]
- 44. Foulks GN, Borchman D, Yappert M, Kim SH, McKay JW. Topical azithromycin therapy for meibomian gland dysfunction: clinical response and lipid alterations. Cornea. 2010;29:781–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fahy E, Subramaniam S, Brown HA, et al. A comprehensive classification system for lipids. J Lipid Res. 2005;46:839–861 [DOI] [PubMed] [Google Scholar]
- 46. Shevchenko A, Simons K. Lipidomics: Coming to grips with lipid diversity. Nat Rev Mol Cell Biol. 2010;11:593–598 [DOI] [PubMed] [Google Scholar]
- 47. Pes O. Ricerche microchimiche sulla secrezione delle ghiandole sebacee palpebrali. Arch Ottal. 1897;5:82–91 [Google Scholar]
- 48. Andrews JS. Human tear film lipids. I. Composition of the principal non-polar component. Exp Eye Res. 1970;10:223–227 [DOI] [PubMed] [Google Scholar]
- 49. Ehlers N. The Precorneal Film. Biomicroscopical, Histological and Chemical Investigations. Acta Ophthalmol Suppl. 1965;(suppl)81:81–134 [PubMed] [Google Scholar]
- 50. Cory CC, Hinks W, Burton JL, Shuster S. Meibomian gland secretion in the red eyes of rosacea. Br J Dermatol. 1973;89:25–27 [DOI] [PubMed] [Google Scholar]
- 51. Nicolaides N. Skin Lipids. Ii. Lipid Class Composition of samples from various species and anatomical sites. J Am Oil Chem Soc. 1965;42:691–702 [DOI] [PubMed] [Google Scholar]
- 52. Tiffany JM. Individual variations in human meibomian lipid composition. Exp Eye Res. 1978;27:289–300 [DOI] [PubMed] [Google Scholar]
- 53. Bron AJ, Tiffany JM. The meibomian glands and tear film lipids; structure, function, and control. Adv Exp Med Biol. 1998;438:281–295 [DOI] [PubMed] [Google Scholar]
- 54. Bron AJ, Tiffany JM, Gouveia SM, Yokoi N, Voon LW. Functional aspects of the tear film lipid layer. Exp Eye Res. 2004;78:347–360 [DOI] [PubMed] [Google Scholar]
- 55. Ohashi Y, Dogru M, Tsubota K. Laboratory findings in tear fluid analysis. Clin Chim Acta. 2006;369:17–28 [DOI] [PubMed] [Google Scholar]
- 56. Tiffany JM. The lipid secretion of the meibomian glands. Adv Lipid Res. 1987;22:1–62 [DOI] [PubMed] [Google Scholar]
- 57. Research in dry eye: report of the Research Subcommittee of the International Dry Eye WorkShop. (2007) Ocul Surf. 2007;5:179–193 [DOI] [PubMed] [Google Scholar]
- 58. Keith CG. Seborrhoeic blepharo-kerato-conjunctivitis. Trans Ophthalmol Soc U K. 1967;87:85–103 [PubMed] [Google Scholar]
- 59. Harvey DJ, Tiffany JM, Duerden JM, Pandher KS, Mengher LS. Identification by combined gas chromatography-mass spectrometry of constituent long-chain fatty acids and alcohols from the meibomian glands of the rat and a comparison with human meibomian lipids. J Chromatogr. 1987;414:253–263 [DOI] [PubMed] [Google Scholar]
- 60. Nicolaides N, Ruth EC. Unusual fatty acids in the lipids of steer and human meibomian gland excreta. Curr Eye Res. 1982;2:93–98 [DOI] [PubMed] [Google Scholar]
- 61. Nicolaides N, Santos EC. The di- and triesters of the lipids of steer and human meibomian glands. Lipids. 1985;20:454–467 [DOI] [PubMed] [Google Scholar]
- 62. Nicolaides N, Santos EC, Papadakis K. Double-bond patterns of fatty acids and alcohols in steer and human meibomian gland lipids. Lipids. 1984;19:264–277 [DOI] [PubMed] [Google Scholar]
- 63. Nicolaides N, Santos EC, Papadakis K, Ruth EC, Muller L. The occurrence of long chain alpha, omega-diols in the lipids of steer and human meibomian glands. Lipids. 1984;19:990–993 [DOI] [PubMed] [Google Scholar]
- 64. Mathers WD, Lane JA. Meibomian gland lipids, evaporation, and tear film stability. Adv Exp Med Biol. 1998;438:349–360 [DOI] [PubMed] [Google Scholar]
- 65. Dougherty JM, McCulley JP. Analysis of the free fatty acid component of meibomian secretions in chronic blepharitis. Invest Ophthalmol Vis Sci. 1986;27:52–56 [PubMed] [Google Scholar]
- 66. Dougherty JM, McCulley JP, Silvany RE, Meyer DR. The role of tetracycline in chronic blepharitis. Inhibition of lipase production in staphylococci. Invest Ophthalmol Vis Sci. 1991;32:2970–2975 [PubMed] [Google Scholar]
- 67. Osgood JK, Dougherty JM, McCulley JP. The role of wax and sterol esters of meibomian secretions in chronic blepharitis. Invest Ophthalmol Vis Sci. 1989;30:1958–1961 [PubMed] [Google Scholar]
- 68. Shine WE, McCulley JP. Role of wax ester fatty alcohols in chronic blepharitis. Invest Ophthalmol Vis Sci. 1993;34:3515–3521 [PubMed] [Google Scholar]
- 69. Shine WE, McCulley JP. Meibomian gland triglyceride fatty acid differences in chronic blepharitis patients. Cornea. 1996;15:340–346 [DOI] [PubMed] [Google Scholar]
- 70. Shine WE, McCulley JP. Meibomianitis: polar lipid abnormalities. Cornea. 2004;23:781–783 [DOI] [PubMed] [Google Scholar]
- 71. Krenzer KL, Dana MR, Ullman MD, et al. Effect of androgen deficiency on the human meibomian gland and ocular surface. J Clin Endocrinol Metab. 2000;85:4874–4882 [DOI] [PubMed] [Google Scholar]
- 72. Butovich IA. Fatty acid composition of cholesteryl esters of human meibomian gland secretions. Steroids. 2010;75:726–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Joffre C, Souchier M, Gregoire S, et al. Differences in meibomian fatty acid composition in patients with meibomian gland dysfunction and aqueous-deficient dry eye. Br J Ophthalmol. 2008;92:116–119 [DOI] [PubMed] [Google Scholar]
- 74. Joffre C, Souchier M, Leclere L, et al. Branched-chain fatty acids, increased in tears of blepharitis patients, are not toxic for conjunctival cells. Br J Ophthalmol. 2009;93:1391–1395 [DOI] [PubMed] [Google Scholar]
- 75. McCulley JP, Shine WE. The lipid layer: the outer surface of the ocular surface tear film. Biosci Rep. 2001;21:407–418 [DOI] [PubMed] [Google Scholar]
- 76. McCulley JP, Shine WE. The lipid layer of tears: dependent on meibomian gland function. Exp Eye Res. 2004;78:361–365 [DOI] [PubMed] [Google Scholar]
- 77. Pulfer M, Murphy RC. Electrospray mass spectrometry of phospholipids. Mass Spectrom Rev. 2003;22:332–364 [DOI] [PubMed] [Google Scholar]
- 78. Sommer U, Herscovitz H, Welty FK, Costello CE. LC-MS-based method for the qualitative and quantitative analysis of complex lipid mixtures. J Lipid Res. 2006;47:804–814 [DOI] [PubMed] [Google Scholar]
- 79. Sullivan BD, Evans JE, Dana MR, Sullivan DA. Impact of androgen deficiency on the lipid profiles in human meibomian gland secretions. Adv Exp Med Biol. 2002;506:449–458 [DOI] [PubMed] [Google Scholar]
- 80. Souchier M, Joffre C, Gregoire S, et al. Changes in meibomian fatty acids and clinical signs in patients with meibomian gland dysfunction after minocycline treatment. Br J Ophthalmol. 2008;92:819–822 [DOI] [PubMed] [Google Scholar]
- 81. Borchman D, Foulks GN, Yappert MC. Human tear lipid compositional, structural and functional relationships using spectroscopy. The Pittsburgh Conference Chicago, IL; 2009 [Google Scholar]
- 82. Tiffany JM. The meibomian lipids of the rabbit, I: Overall composition. Exp Eye Res. 1979;29:195–202 [DOI] [PubMed] [Google Scholar]
- 83. Tiffany JM, Marsden RG. The meibomian lipids of the rabbit, II; detailed composition of the principal esters. Exp Eye Res. 1982;34:601–608 [DOI] [PubMed] [Google Scholar]
- 84. Harvey DJ, Tiffany JM. Identification of meibomian gland lipids by gas chromatography-mass spectrometry: application to the meibomian lipids of the mouse. J Chromatogr. 1984;301:173–187 [DOI] [PubMed] [Google Scholar]
- 85. Harvey DJ. Identification by gas chromatography/mass spectrometry of long-chain fatty acids and alcohols from hamster meibomian glands using picolinyl and nicotinate derivatives. Biomed Chromatogr. 1989;3:251–254 [DOI] [PubMed] [Google Scholar]
- 86. Harvey DJ. Long-chain fatty acids and alcohols from gerbil meibomian lipids. J Chromatogr. 1989;494:23–30 [DOI] [PubMed] [Google Scholar]
- 87. Seyama Y, Otsuka H, Ohashi K, Vivien-Roels B, Pevet P. Sexual dimorphism of lipids in Harderian glands of golden hamsters. J Biochem. 1995;117:661–670 [DOI] [PubMed] [Google Scholar]
- 88. McCulley JP, Dougherty JM, Deneau DG. Classification of chronic blepharitis. Ophthalmology. 1982;89:1173–1180 [DOI] [PubMed] [Google Scholar]
- 89. Shine WE, McCulley JP. Keratoconjunctivitis sicca associated with meibomian secretion polar lipid abnormality. Arch Ophthalmol. 1998;116:849–852 [DOI] [PubMed] [Google Scholar]
- 90. Subbaiah PV, Subramanian VS, Wang K. Novel physiological function of sphingomyelin in plasma: inhibition of lipid peroxidation in low density lipoproteins. J Biol Chem. 1999;274:36409–36414 [DOI] [PubMed] [Google Scholar]
- 91. Augustin AJ, Spitznas M, Kaviani N, et al. Oxidative reactions in the tear fluid of patients suffering from dry eyes. Graefes Arch Clin Exp Ophthalmol. 1995;233:694–698 [DOI] [PubMed] [Google Scholar]
- 92. Shine WE, McCulley JP. Association of meibum oleic acid with meibomian seborrhea. Cornea. 2000;19:72–74 [DOI] [PubMed] [Google Scholar]
- 93. Mathers WD, Stovall D, Lane JA, Zimmerman MB, Johnson S. Menopause and tear function: the influence of prolactin and sex hormones on human tear production. Cornea. 1998;17:353–358 [DOI] [PubMed] [Google Scholar]
- 94. Rolando M, Iester M, Macri A, Calabria G. Low spatial-contrast sensitivity in dry eyes. Cornea. 1998;17:376–379 [DOI] [PubMed] [Google Scholar]
- 95. Gilbard JP, Rossi SR, Heyda KG. Tear film and ocular surface changes after closure of the meibomian gland orifices in the rabbit. Ophthalmology. 1989;96:1180–1186 [DOI] [PubMed] [Google Scholar]
- 96. Goto E, Tseng SC. Differentiation of lipid tear deficiency dry eye by kinetic analysis of tear interference images. Arch Ophthalmol. 2003;121:173–180 [DOI] [PubMed] [Google Scholar]
- 97. Zengin N, Tol H, Gunduz K, Okudan S, Balevi S, Endogru H. Meibomian gland dysfunction and tear film abnormalities in rosacea. Cornea. 1995;14:144–146 [PubMed] [Google Scholar]
- 98. Ta CN, Shine WE, McCulley JP, Pandya A, Trattler W, Norbury JW. Effects of minocycline on the ocular flora of patients with acne rosacea or seborrheic blepharitis. Cornea. 2003;22:545–548 [DOI] [PubMed] [Google Scholar]
- 99. US FDA Draft Testing Guidance for Class III Soft Contact Lenses. Washington, DC: US Food and Drug Administration; 1988 [Google Scholar]
- 100. US FDA Premarket Notification (510.(k)) for Lens Care Products. Washington, DC: US Food and Drug Administration; 1997 [Google Scholar]
- 101. Stone RP. A new perspective for lens care classifying silicone hydrogels (Editorial). June 2007. Available at http://www.siliconehydrogels.org/editorials/jun_07.asp Accesssed February 22, 2011
- 102. Korb DR, Henriquez AS. Meibomian gland dysfunction and contact lens intolerance. J Am Optom Assoc. 1980;51:243–251 [PubMed] [Google Scholar]
- 103. Bontempo AR, Rapp J. Protein-lipid interaction on the surface of a hydrophilic contact lens in vitro. Curr Eye Res. 1997;16:776–781 [DOI] [PubMed] [Google Scholar]
- 104. Prager MD, Quintana RP. Radiochemical studies on contact lens soilation. II. Lens uptake of cholesteryl oleate and dioleoyl phosphatidylcholine. J Biomed Mater Res. 1997;37:207–211 [DOI] [PubMed] [Google Scholar]
- 105. Ho CH, Hlady V. Fluorescence assay for measuring lipid deposits on contact lens surfaces. Biomaterials. 1995;16:479–482 [DOI] [PubMed] [Google Scholar]
- 106. Lord MS, Stenzel MH, Simmons A, Milthorpe BK. The effect of charged groups on protein interactions with poly(HEMA) hydrogels. Biomaterials. 2006;27:567–575 [DOI] [PubMed] [Google Scholar]
- 107. Carney FP, Nash WL, Sentell KB. The adsorption of major tear film lipids in vitro to various silicone hydrogels over time. Invest Ophthalmol Vis Sci. 2008;49:120–124 [DOI] [PubMed] [Google Scholar]
- 108. Iwata M, Ohno S, Kawai T, Ichijima H, Cavanagh HD. In vitro evaluation of lipids adsorbed on silicone hydrogel contact lenses using a new gas chromatography/mass spectrometry analytical method. Eye Contact Lens. 2008;34:272–280 [DOI] [PubMed] [Google Scholar]
- 109. Hart DE. Lipid deposits which form on extended wear contact lenses. International Contact Lens Clinics. 1984;11:348–360 [Google Scholar]
- 110. Hart DE, Tidsale RR, Sack RA. Origin and composition of lipid deposits on soft contact lenses. Ophthalmology. 1986;93:495–503 [DOI] [PubMed] [Google Scholar]
- 111. Bowers RW, Tighe BJ. Studies of the ocular compatibility of hydrogels: white spot deposits—chemical composition and geological arrangement of components. Biomaterials. 1987;8:172–176 [DOI] [PubMed] [Google Scholar]
- 112. Bowers RW, Tighe BJ. Studies of the ocular compatibility of hydrogels; white spot deposits—incidence of occurrence, location and gross morphology. Biomaterials. 1987;8:89–93 [DOI] [PubMed] [Google Scholar]
- 113. Hart DE, Lane BC, Josephson JE, et al. Spoilage of hydrogel contact lenses by lipid deposits: tear-film potassium depression, fat, protein, and alcohol consumption. Ophthalmology. 1987;94:1315–1321 [DOI] [PubMed] [Google Scholar]
- 114. Bontempo AR, Rapp J. Lipid deposits on hydrophilic and rigid gas permeable contact lenses. CLAO J. 1994;20:242–245 [DOI] [PubMed] [Google Scholar]
- 115. Franklin V, Horne A, Jones L, Tighe B. Early deposition trends on group I (Polymacon and Tetrafilcon A) and group III (Bufilcon A) materials. CLAO J. 1991;17:244–248 [PubMed] [Google Scholar]
- 116. Bontempo AR, Rapp J. Protein and lipid deposition onto hydrophilic contact lenses in vivo. CLAO J. 2001;27:75–80 [PubMed] [Google Scholar]
- 117. Jones L, Fcoptom, Mann A, Evans K, Franklin V, Tighe B. An in vivo comparison of the kinetics of protein and lipid deposition on group II and group IV frequent-replacement contact lenses. Optom Vis Sci. 2000;77:503–510 [DOI] [PubMed] [Google Scholar]
- 118. Jones L, Evans K, Sariri R, Franklin V, Tighe B. Lipid and protein deposition of N-vinyl pyrrolidone-containing group II and group IV frequent replacement contact lenses. CLAO J. 1997;23:122–126 [PubMed] [Google Scholar]
- 119. Zhao Z, Carnt NA, Aliwarga Y, et al. Care regimen and lens material influence on silicone hydrogel contact lens deposition. Optom Vis Sci. 2009;86:251–259 [DOI] [PubMed] [Google Scholar]
- 120. Cheung SW, Cho P, Chan B, Choy C, Ng V. A comparative study of biweekly disposable contact lenses: silicone hydrogel versus hydrogel. Clin Exp Optom. 2007;90:124–131 [DOI] [PubMed] [Google Scholar]
- 121. Jones L, Franklin V, Evans K, Sariri R, Tighe B. Spoilation and clinical performance of monthly vs. three monthly group II disposable contact lenses. Optom Vis Sci. 1996;73:16–21 [DOI] [PubMed] [Google Scholar]
- 122. Rapp J, Broich JR. Lipid deposits on worn soft contact lenses. CLAO J. 1984;10:235–239 [PubMed] [Google Scholar]
- 123. Jones L, Senchyna M, Glasier MA, et al. Lysozyme and lipid deposition on silicone hydrogel contact lens materials. Eye Contact Lens. 2003;29:S75–79; discussion S83–S74, S192–S194 [DOI] [PubMed] [Google Scholar]
- 124. Maziarz EP, Stachowski MJ, Liu XM, et al. Lipid deposition on silicone hydrogel lenses, Part I: quantification of oleic acid, oleic acid methyl ester, and cholesterol. Eye Contact Lens. 2006;32:300–307 [DOI] [PubMed] [Google Scholar]
- 125. Morris CA, Holden BA, Papas E, et al. The ocular surface, the tear film, and the wettability of contact lenses. Adv Exp Med Biol. 1998;438:717–722 [DOI] [PubMed] [Google Scholar]
- 126. Young WH, Hill RM. Tear cholesterol levels and contact lens adaptation. Am J Optom Arch Am Acad Optom. 1973;50:12–16 [DOI] [PubMed] [Google Scholar]
- 127. Yamada M, Mochizuki H, Kawashima M, Hata S. Phospholipids and their degrading enzyme in the tears of soft contact lens wearers. Cornea. 2006;25:S68–72 [DOI] [PubMed] [Google Scholar]
- 128. Kawashima M, Yamada M, Arita R, Kawai M, Mashima Y. Measurement of phospholipids in human tears. Folia Ophthalmol Jpn. 2003;54:870–873 [Google Scholar]
- 129. Dumbleton KA, Woods CA, Jones LW, Fonn D. Comfort and adaptation to silicone hydrogel lenses for daily wear. Eye Contact Lens. 2008;34:215–223 [DOI] [PubMed] [Google Scholar]
- 130. Fonn D. Targeting contact lens induced dryness and discomfort: what properties will make lenses more comfortable. Optom Vis Sci. 2007;84:279–285 [DOI] [PubMed] [Google Scholar]
- 131. Guillon JP, Morris J, Hall B. Evaluation of the pre-lens tear film forming on three disposable contact lenses. Adv Exp Med Biol. 2002;506:901–915 [DOI] [PubMed] [Google Scholar]
- 132. Maissa C, Guillon M, Girard-Claudon K, Cooper P. Tear lipid composition of hydrogel contact lens wearers. Adv Exp Med Biol. 2002;506:935–938 [DOI] [PubMed] [Google Scholar]
- 133. Hume EB, Cole N, Parmar A, et al. Secretory phospholipase A2 deposition on contact lenses and its effect on bacterial adhesion. Invest Ophthalmol Vis Sci. 2004;45:3161–3164 [DOI] [PubMed] [Google Scholar]
- 134. Mochizuki H, Yamada M, Hatou S, Kawashima M, Hata S. Deposition of lipid, protein, and secretory phospholipase A2 on hydrophilic contact lenses. Eye Contact Lens. 2008;34:46–49 [DOI] [PubMed] [Google Scholar]
- 135. Guillon JP. Tear Film Structure and Contact Lenses. Lubbock, TX: Dry Eye Institute; 1986 [Google Scholar]
- 136. Korb DR. Tear film-contact lens interactions. Adv Exp Med Biol. 1994;350:403–410 [DOI] [PubMed] [Google Scholar]
- 137. Korb DR, Greiner JV, Glonek T. Tear film lipid layer formation: implications for contact lens wear. Optom Vis Sci. 1996;73:189–192 [DOI] [PubMed] [Google Scholar]
- 138. Young G, Efron N. Characteristics of the pre-lens tear film during hydrogel contact lens wear. Ophthalmic Physiol Opt. 1991;11:53–58 [PubMed] [Google Scholar]
- 139. Guillon M, Guillon JP. Hydrogel lens wettability during overnight wear. Ophthalmic Physiol Opt. 1989;9:355–359 [PubMed] [Google Scholar]
- 140. Thai LC, Tomlinson A, Doane MG. Effect of contact lens materials on tear physiology. Optom Vis Sci. 2004;81:194–204 [DOI] [PubMed] [Google Scholar]
- 141. Guillon M, Maissa C. Use of silicone hydrogel material for daily wear. Cont Lens Anterior Eye. 2007;30:5–10; quiz 71 [DOI] [PubMed] [Google Scholar]
- 142. Guillon M, Styles E, Guillon JP, Maissa C. Preocular tear film characteristics of nonwearers and soft contact lens wearers. Optom Vis Sci. 1997;74:273–279 [DOI] [PubMed] [Google Scholar]
- 143. Glasson MJ, Stapleton F, Keay L, Sweeney D, Willcox MD. Differences in clinical parameters and tear film of tolerant and intolerant contact lens wearers. Invest Ophthalmol Vis Sci. 2003;44:5116–5124 [DOI] [PubMed] [Google Scholar]
- 144. Glasson M, Stapleton F, Willcox M. Lipid, lipase and lipocalin differences between tolerant and intolerant contact lens wearers. Curr Eye Res. 2002;25:227–235 [DOI] [PubMed] [Google Scholar]
- 145. Gasymov OK, Abduragimov AR, Yusifov TN, Glasgow BJ. Binding studies of tear lipocalin: the role of the conserved tryptophan in maintaining structure, stability and ligand affinity. Biochim Biophys Acta. 1999;1433:307–320 [DOI] [PubMed] [Google Scholar]
- 146. Glasgow BJ, Abduragimov AR, Farahbakhsh ZT, Faull KF, Hubbell WL. Tear lipocalins bind a broad array of lipid ligands. Curr Eye Res. 1995;14:363–372 [DOI] [PubMed] [Google Scholar]
- 147. Fullard RJ. Identification of proteins in small tear volumes with and without size exclusion HPLC fractionation. Curr Eye Res. 1988;7:163–179 [DOI] [PubMed] [Google Scholar]
- 148. Tsai PS, Evans JE, Green KM, et al. Proteomic analysis of human meibomian gland secretions. Br J Ophthalmol. 2006;90:372–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Glasgow BJ, Abduragimov AR, Gasymov OK, Yusifov TN. Tear lipocalin: structure, function and molecular mechanisms of action. Adv Exp Med Biol. 2002;506:555–565 [DOI] [PubMed] [Google Scholar]
- 150. Redi B. Human tear lipocalin. Biochim Biophys Acta. 2000; 1482:241–248 [DOI] [PubMed] [Google Scholar]
- 151. Glasgow BJ, Gasymov OK. Focus on molecules: tear lipocalin. Exp Eye Res. 2010;August 21 [ePub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Gasymov OK, Abduragimov AR, Yusifov TN, Glasgow BJ. Structural changes in human tear lipocalins associated with lipid binding. Biochim Biophys Acta. 1998;1386:145–156 [DOI] [PubMed] [Google Scholar]
- 153. Glasgow BJ, Marshall G, Gasymov OK, Abduragimov AR, Yusifov TN, Knobler CM. Tear lipocalins: potential lipid scavengers for the corneal surface. Invest Ophthalmol Vis Sci. 1999;40:3100–3107 [PubMed] [Google Scholar]
- 154. Gasymov OK, Abduragimov AR, Glasgow BJ. Intracavitary ligand distribution in tear lipocalin by site-directed tryptophan fluorescence. Biochemistry. 2009;48:7219–7228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Breustedt DA, Chatwell L, Skerra A. A new crystal form of human tear lipocalin reveals high flexibility in the loop region and induced fit in the ligand cavity. Acta Crystallogr D Biol Crystallogr. 2009;65:1118–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Gouveia SM, Tiffany JM. Human tear viscosity: an interactive role for proteins and lipids. Biochim Biophys Acta. 2005;1753:155–163 [DOI] [PubMed] [Google Scholar]
- 157. Gasymov OK, Abduragimov AR, Glasgow BJ. Evidence for internal and external binding sites on human tear lipocalin. Arch Biochem Biophys. 2007;468:15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Yamada M, Mochizuki H, Kawai M, Tsubota K, Bryce TJ. Decreased tear lipocalin concentration in patients with meibomian gland dysfunction. Br J Ophthalmol. 2005;89:803–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Millar TJ, Mudgil P, Butovich IA, Palaniappan CK. Adsorption of human tear lipocalin to human meibomian lipid films. Invest Ophthalmol Vis Sci. 2009;50:140–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Mateu L, Caron F, Luzzati V, Billecocq A. The influence of protein-lipid interactions on the order-disorder conformational transitions of the hydrocarbon chain. Biochim Biophys Acta. 1978;508:109–121 [DOI] [PubMed] [Google Scholar]
- 161. Miano F, Calcara M, Millar TJ, Enea V. Insertion of tear proteins into a meibomian lipids film. Colloids Surf B Biointerfaces. 2005;44:49–55 [DOI] [PubMed] [Google Scholar]
- 162. Tragoulias ST, Anderton PJ, Dennis GR, Miano F, Millar TJ. Surface pressure measurements of human tears and individual tear film components indicate that proteins are major contributors to the surface pressure. Cornea. 2005;24:189–200 [DOI] [PubMed] [Google Scholar]
- 163. Holzfeind P, Merschak P, Dieplinger H, Redl B. The human lacrimal gland synthesizes apolipoprotein D mRNA in addition to tear prealbumin mRNA, both species encoding members of the lipocalin superfamily. Exp Eye Res. 1995;61:495–500 [DOI] [PubMed] [Google Scholar]
- 164. Jauhiainen M, Setala NL, Ehnholm C, et al. Phospholipid transfer protein is present in human tear fluid. Biochemistry. 2005;44:8111–8116 [DOI] [PubMed] [Google Scholar]
- 165. Tiffany JM. Tears in health and disease. Eye (Lond). 2003;17:923–926 [DOI] [PubMed] [Google Scholar]
- 166. Perkins RE, Kundsin RB, Pratt MV, Abrahamsen I, Leibowitz HM. Bacteriology of normal and infected conjunctiva. J Clin Microbiol. 1975;1:147–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Stapleton F, Willcox MD, Fleming CM, Hickson S, Sweeney DF, Holden BA. Changes to the ocular biota with time in extended- and daily-wear disposable contact lens use. Infect Immun. 1995;63:4501–4505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Dougherty JM, McCulley JP. Bacterial lipases and chronic blepharitis. Invest Ophthalmol Vis Sci. 1986;27:486–491 [PubMed] [Google Scholar]
- 169. McCulley JP, Dougherty JM. Bacterial aspects of chronic blepharitis. Trans Ophthalmol Soc U K. 1986;105(Pt 3):314–318 [PubMed] [Google Scholar]
- 170. Ridsdale RA, Palaniyar N, Possmayer F, Harauz G. Formation of folds and vesicles by dipalmitoylphosphatidylcholine monolayers spread in excess. J Membr Biol. 2001;180:21–32 [DOI] [PubMed] [Google Scholar]
- 171. Akiyama J, Hoffman A, Brown C, et al. Tissue distribution of surfactant proteins A and D in the mouse. J Histochem Cytochem. 2002;50:993–996 [DOI] [PubMed] [Google Scholar]
- 172. Madsen J, Kliem A, Tornoe I, Skjodt K, Koch C, Holmskov U. Localization of lung surfactant protein D on mucosal surfaces in human tissues. J Immunol. 2000;164:5866–5870 [DOI] [PubMed] [Google Scholar]
- 173. Stahlman MT, Gray ME, Hull WM, Whitsett JA. Immunolocalization of surfactant protein-D (SP-D) in human fetal, newborn, and adult tissues. J Histochem Cytochem. 2002;50:651–660 [DOI] [PubMed] [Google Scholar]
- 174. Ni M, Evans DJ, Hawgood S, Anders EM, Sack RA, Fleiszig SM. Surfactant protein D is present in human tear fluid and the cornea and inhibits epithelial cell invasion by Pseudomonas aeruginosa. Infect Immun. 2005;73:2147–2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Brauer L, Johl M, Borgermann J, Pleyer U, Tsokos M, Paulsen FP. Detection and localization of the hydrophobic surfactant proteins B and C in human tear fluid and the human lacrimal system. Curr Eye Res. 2007;32:931–938 [DOI] [PubMed] [Google Scholar]
- 176. Brauer L, Kindler C, Jager K, et al. Detection of surfactant proteins A and D in human tear fluid and the human lacrimal system. Invest Ophthalmol Vis Sci. 2007;48:3945–3953 [DOI] [PubMed] [Google Scholar]
- 177. Pfister RH, Soll RF. New synthetic surfactants: the next generation? Biol Neonate. 2005;87:338–344 [DOI] [PubMed] [Google Scholar]
- 178. Sorensen GL, Husby S, Holmskov U. Surfactant protein A and surfactant protein D variation in pulmonary disease. Immunobiology. 2007;212:381–416 [DOI] [PubMed] [Google Scholar]