Diagnostic tests of meibomian gland dysfunction (MGD) and of MGD-related disorders are based on the demonstration of abnormal anatomy and physiology of the glands and the detection of specific pathologic events. For this reason, this subcommittee report is divided into two sections. In part I, those aspects of meibomian anatomy and physiology that are relevant to currently available tests are described; a fuller account of the anatomy and physiology is provided in the report of the Anatomy Subcommittee of this workshop. In part II, each test and its performance is described in detail. In part III, the practical application of selected tests is summarized and recommendations for future approaches are made. Additional recommendations and a summary of pertinent literature and concepts are presented in Appendices 1 to 17.
I. Anatomy and Physiology of the Meibomian Glands: Clinical Implications
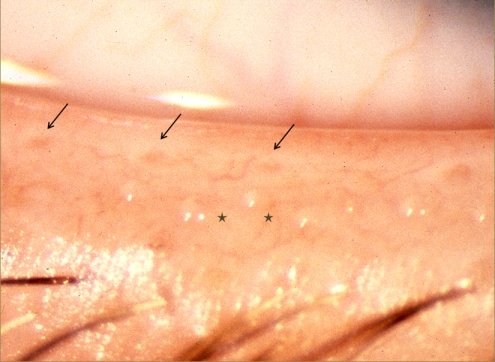
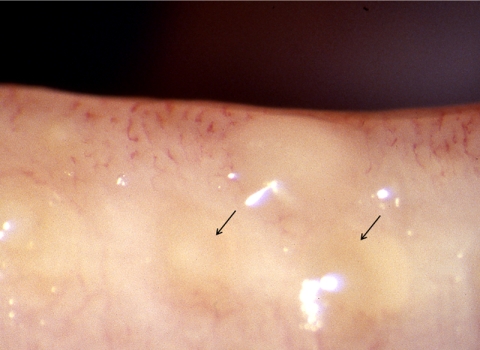
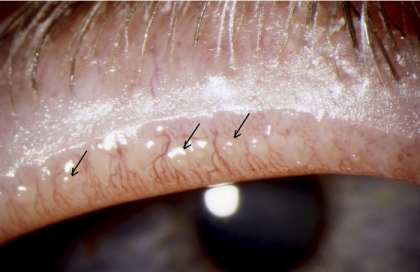
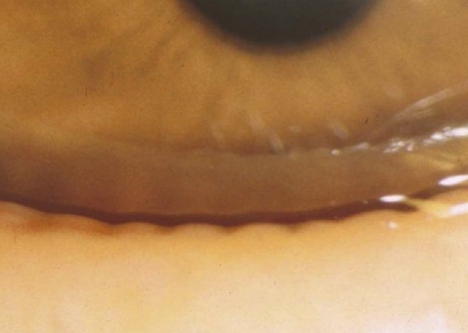
The superficial location of the meibomian glands in the tarsal plates permits their anatomic features to be quantified by meibography and confocal microscopy (Appendices 7, 8). In normal subjects, the meibomian orifices are disposed at regular intervals along the lid margins, just anterior to the mucocutaneous junction (MCJ). Biomicroscopically, they are surrounded by a characteristic ring-shaped architecture, reflecting the concentric arrangement of orifice, mucosa, distal acini, fibers of the muscle of Riolan, and the connective tissue sheath of the glands (Fig. 1).1,2 This configuration becomes less well-circumscribed in old age and is destroyed in advanced MGD.3 Loss of this architecture may be scored and is an important clinical sign of MGD.
Figure 1.
Normal lid margin, showing meibomian orifices (arrows) and clear, expressed oil (courtesy of A. Bron).
The lipid secretion of the meibomian glands is liquid at lid temperature and is delivered to the skin of the lid margin as a clear fluid termed meibum.4 Here, it forms shallow reservoirs on the upper and lower lid margins from which the tear film lipid layer (TFLL) is formed and replenished. The amount of lipid present in the normal, lower lid reservoir may be gauged by the technique of meibometry5–7 and used to infer the content of the total lid reservoir. In meibometry, a linear sample of meibum is blotted from the central third of the lower lid, onto a loop of plastic tape, and the amount of lipid present in the defined zone is gauged by the change in optical density (Appendix 9). In normal adults, the total amount of lipid contained in the upper and lower reservoirs has been estimated to be roughly 300 μg.5,8 This calculation was based on comparisons against a standard lipid with the assumption that the meibomian reservoir is shared equally between the upper and lower lids. However, a comparison of basal levels on the upper and lower lids has not yet been made. The technique may be used to quantify meibomian gland obstruction,9 but in the presence of MGD, the reading cannot be extrapolated to estimate the total extent of obstruction on both lids, because of the variability of the disease along the lid length.
As detailed in the Report on Tear Film Lipids, the meibomian secretion is a complex mixture of cholesterol, wax and cholesteryl esters, phospholipids with small amounts of triglycerides and triacylglycerols, and hydrocarbons.4,10–43
The phospholipid content has been promoted as the basis for the interaction between the TFLL and the aqueous subphase of the tear film, necessary for tear film spreading17,44; however, recent studies have reported negligible levels of phospholipids in meibomian lipid, so that it may be necessary to seek an alternative candidate for this interaction.20,22,24 This is currently debated. The presence of hydrocarbons and to a lesser extent, triglycerides, has been interpreted in part as due to contamination by sebum and environmental chemicals.
The lipid mixture has a melting range in the region of 19.5°C to 40°C, which ensures lipid mixture fluidity at the surface of the lid.45 The melting range of the lipid mixture also influences its stability in the TFLL, since the temperature of the cornea is cooler (approximately 33.5°C)46 than that of the lid margin. This temperature difference may also be the basis of the sustained integrity of the TFLL over a series of successive blinks (the pleating effect, described later), a normal feature of TFLL dynamics.47 The stability of the TFLL may be measured by static and dynamic interferometric techniques (see Appendix 10).
The manner of secretion and delivery of meibum has been examined by using meibometry to follow the recovery of lipid on the lower lid reservoir after total removal of lipid from the upper and lower lid margins with organic solvents.5 In normal adults undergoing surgery under general anesthesia, partial recovery occurred over periods of 3 to 40 minutes, indicating that secretion and delivery continues in the absence of blinking. Various studies have shown that, from time to time, aliquots of meibum are also jetted directly from some glands into the TFLL,6,27,48 and on this basis, it is generally accepted that blinking plays a role in the delivery of meibomian lipid to the TFLL.
Recent studies have followed the secretory recovery of single meibomian glands after drainage by compression.49,50 In these studies, a standardized device was used to apply a standard force to individual glands located at the center of the lower lid, to drain them of their meibomian lipid. The glands selected for study in 12 subjects aged 18 to 25 years, were optimally secreting in the sense that expression could be initiated within 2 seconds of the application of pressure. The mean time to effect drainage, was 12.1 ± 3.5 seconds (range, 8–20) and the time to partial recovery of secretion was 2.2 ± 0.5 hours. Repeat expression after partial recovery cleared the ducts of contained secretion in about half the time taken to drain them initially.49,50
Using meibometry, Chew5 found that the basal level of meibomian lipid in the lower reservoir was highest within the first hour after waking. This finding was interpreted to reflect a damming back of secreted lipid within the ducts during prolonged eye closure, in the absence of blinking, as in sleep, and the release of the accumulated lipid on eye opening. The latter hypothesis, however, neglected the potential influence of altered lipid excretion, which has been assumed to occur from the lid margin across the skin of the lids. It should be kept in mind that a reduced removal of meibomian lipid during prolonged eye closure would also lead to a rise in the recorded basal level shortly after waking. This question should be amenable to study using the compression and drainage approach.49,50
Meibomian Gland Activity
A few authors have addressed the question of gland activity in the waking state, using the term activity to mean expressibility of meibomian oil. Norn,51 staining with Sudan black or applying digital pressure along the full extent of the lower lid, concluded that approximately 45% of the adult glands were active at a given time. Here, it was assumed that, in the natural state, those ducts receiving lipid from actively secreting glands would be filled with liquid lipid and that would be reflected by the ability to express their contained oil.
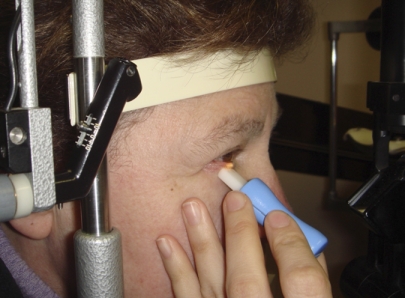
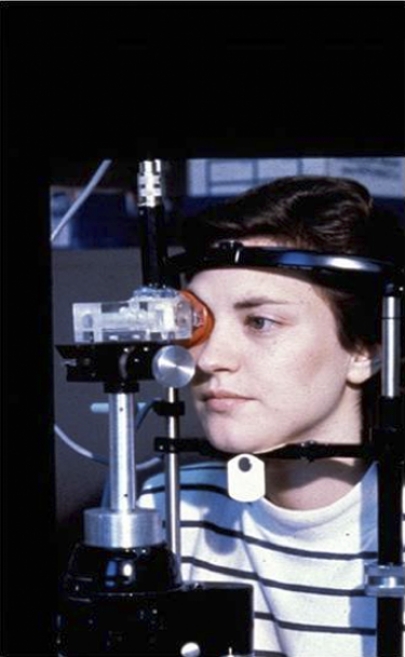
These findings have been supported by recent studies employing standardized meibomian gland expression. Korb and Blackie have developed a standardized technique for meibomian gland expression using a custom-made expression device49,50,52 (Appendix 6) that applies a standard force of 1.25 g/mm2 to the lid, over an area of approximately 40 mm2 (Fig. 2). This force was chosen to approximate between that applied by the lids to the globe during spontaneous blinking and that applied during deliberate, forced lid closure. In studies by Comberg and Stoewer,53 cited by Miller,54 a hard lid squeeze results in a rise of intraocular pressure in the region of 18 to 70 mm Hg, whereas the Korb expression device raises the pressure to between 30 and 40 mm Hg.49,50,52
Figure 2.
Standardized meibomian gland expression performed at the slit lamp using a diagnostic expression instrument (Korb and Blackie52). See text for further details (courtesy of D. Korb). Reprinted with permission from Korb DR, Blackie CA. Meibomian gland diagnostic expressibility: correlation with dry eye symptoms and gland location. Cornea. 2008;27(10):1142–1147.
The device achieves simultaneous expression from approximately eight glands (occupying approximately one third of the lid length, i.e., 8/24 glands). Gland expressibility is scored according to the number of the eight glands from which a fluid secretion can be expressed, regardless of its qualitative appearance. This is the Meibomian Glands Yielding Liquid Secretion (MGYLS) score. In a small group of normal young subjects, the average MGYLS score for the whole lower lid was 10.6 ± 2.6. The range was 6 to 15.5 (25%–65% were active, presuming there are 24 glands along the lower lid), suggesting that there is marked variation in activity between individuals. Also, these studies have shown consistently that the nasal glands are the most active, followed by the central glands, and finally, the temporal glands. In the normal sample, an MGYLS score of 0 was found in 86% of the temporal parts of the lid and in 6% of the nasal parts of the lids. The inference of these findings, already proposed by Norn using less sophisticated methods, is that only a proportion of the glands are actively secreting at any one time. Also, it appears that those glands on the nasal side are considerably more active than those on the temporal side. Pflugfelder et al.55 also evaluated meibomian gland expression in the upper lid and described a scale in which five glands were evaluated for expressibility, with the assumption that all glands are not continually expressing and that a reduction in expressivity is an indicator of disease.
In a more recent report by Blackie and Korb,56 the secretory activity of individual meibomian glands was studied in young healthy individuals without dry eye symptoms or signs. It was found that if a meibomian gland yielded liquid secretion at 8 AM, then, depending on its location along the lower lid, there was a high likelihood that it would continue to provide liquid secretion throughout a 9-hour day. For example, 70% of the nasal glands, 30% of the central glands, and 20% of the temporal glands provided liquid secretion throughout a 9-hour day. If a meibomian gland did not yield liquid secretion at 8 AM, it would provide liquid secretion sporadically during the course of the day or not at all. Assuming that meibomian glands on the upper lid function in a similar manner, it seems that the marginal lipid reservoirs are maintained by the activity of only a proportion of the total number of glands. It will be of future interest whether individual glands that are inactive at one time become active days or weeks later. Corroboration of these diurnal fluctuations in meibomian gland activity may lead to their use in future MGD diagnosis.
While these observations have not yet been confirmed by other groups, they have potentially important implications for those tests of meibomian function that depend on determining the expressibility of a set of glands.55 Based on the proportion of expressible glands alone (without reference to either quality of expressed secretion, state of the orifices or presence of local gland dropout), it may be difficult to differentiate between glands that are not expressible for physiological reasons or for pathologic reasons (i.e., due to the presence of MGD). Observation of orifice disease at the slit lamp could be helpful. Also, where an investigator selects expressibility as a measure of disease, it may be appropriate to specify location for consistency (e.g., the nasal third of the lid).
These studies also raise important questions about the temporal characteristics of meibomian gland secretion. It may be that the glands are engaged in a cycle of activity that changes from gland to gland over time across the length of the lids. This notion implies that each gland has periods of activity when secretions are released, followed by periods of quiescence, when their role is taken over by other glands. This hypothesis would fit in with the holocrine mode of meibomian lipid secretion. The studies cited earlier suggest that this does not occur in the short term (i.e., over a 24-hour period), but there may be a slower cycle in the long term, and this could be relevant to the conduct of clinical trials.
The Tear Film Lipid Layer
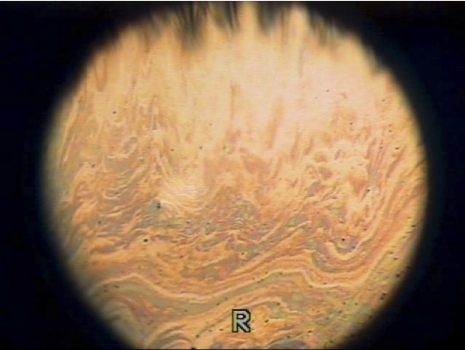
The reported thickness range of the normal TFLL is approximately 20 to 160 nm57,58 and occupies the most anterior part of the tear film where it performs a major role in reducing evaporative water loss from the exposed surface of the eye. The layer can be observed by interferometry in which the predominant spectral color represents the TFLL thickness (Fig. 3; Appendix 10). By interferometry (or by recording the movement of particles in the film) the lipid layer can be seen to spread upward in the upstroke of the blink and to become comparatively stable after approximately 1 to 2 seconds.59 Owens and Phillips60,61 give a value of 1.05 ± 0.39 seconds, whereas Goto and Tseng,62 using a different approach, report a value of 0.36 ± 0.22 seconds in healthy eyes, but 3.54 ±1.86 seconds in eyes with lipid tear deficiency. King-Smith et al.59 show a time constant associated with exponential decay of lipid drift in the upward direction of 0.564 second and total upward movement of 3.23 mm. Prolongation of the lipid spread time may be an indicator of aqueous tear deficiency, but this has not yet been converted into a formal test for general clinical use (Yokoi N, et al. IOVS 2010;51:ARVO E-Abstract 5201). The duration of the normal blink is approximately 200 to 300 ms.63,64 The direction of movement of the horizontal wavefront suggests that the TFLL is delivered to the tear film primarily from the lower reservoir. To explain the ability of the meibomian lipid to spread over the aqueous subphase of the tear film, it has been proposed that the TFLL has a lamellar structure with an internal polar, phospholipid layer that spreads over the aqueous phase of the tear film.17,44,65 As noted earlier, in view of current reports suggesting a low meibum phospholipid content,20 it may be necessary to seek an alternative lipid layer structure. The more superficial lipid layers are hypothesized to be composed of nonpolar lipids, such as cholesterol and sterol and wax esters, which spread over the polar phase. It should be emphasized that, when the spread of the TFLL is observed by interferometry, it is the full thickness of the TFLL that is visualized; the polar lipid layer, which is postulated to run in advance of the nonpolar layer, may be too thin to generate an interference pattern. Thinning of the TFLL has been noted in lipid tear deficiency.66
Figure 3.
Spreading of a normal tear film lipid layer image by interferometry (courtesy of N. Yokoi).
In normal subjects, the interferometric pattern of the TFLL is relatively constant in appearance over several blink cycles, implying that its architecture is conserved to some extent from blink to blink. This preservation occurs despite the expectation that, at the end of the downstroke of a complete blink, the lid margins will be apposed and the lipid reservoirs combined. To explain this phenomenon, it has been proposed that, over this period of stability, the TFLL folds up concertina-wise in the downstroke of the blink and is restored by unfolding during the upstroke.57 However, it should be noted that subtle or more marked changes in pattern can be observed from blink to blink, which implies some kind of molecular reorganization within the film, either by local movements of lipid within the layer or an exchange across the apposed folds of the lipid layer. At some point, after several blink cycles, an abrupt and complete change in the interferometric pattern occurs, implying a mixing of the TFLL with the combined meibomian reservoirs. This results in a complete restructuring of the TFLL and the cycle begins again. The stable pattern is likely to be influenced by the temperature of the surface of the open eye, influencing fluidity of the lipid mixture, and by the composition of the meibomian lipid, which will influence its melting range. The cycle of stability is shortened in the presence of MGD, and this has been proposed as a measure of MGD-related disease in the Dynamic Lipid Layer Interference Pattern (DLIP) test.67
With this background, the physiology of the meibomian glands may be summarized as follows: The glands are under neural and hormonal control and secrete their oil into shallow reservoirs on the lid margins. Secretion is intrinsic to the glands and delivery is aided by the blink. Only a fraction of the glands are active at a given time, with the possible inference that each gland goes through a cycle of activity followed by a period of quiescence, when acinar stores are replenished. There is an uneven distribution of gland activity along the length of the lid, with the least distribution temporally and the greatest distribution nasally. During sleep, it is hypothesized that secreted oil accumulates in the glands and that the excess is discharged on waking, with the resumption of blinking. The marginal lipid reservoir as well as direct expression from the meibomian gland68 are the sources of the TFLL. At the upstroke of the blink, lipid spreads from the lower reservoir onto the tear film to form the TFLL, with polar lipids, or some other surfactant component of the TFLL, interacting with the water phase of the tear film. Once formed, the TFLL maintains relative stability from blink to blink until it is reconstituted abruptly by a mixing of lipid from both reservoirs with that of the TFLL, and the cycle begins again.
Many details of this account have yet to be filled in, but this summary may serve for the selection and interpretation of diagnostic tests. Whether MGD occurs on its own, or is part of a wider constellation of diseases, diagnosis requires that its manifestations be distinguished from other, unrelated ocular surface disorders.
Ocular Surface Disorders
Several symptomatic disorders affecting the conjunctiva, cornea, and the lids may be conveniently grouped together in the category of ocular surface disorders (OSDs).69 They include lid and conjunctival disorders and those disorders responsible for aqueous-deficient and evaporative dry eye. There is a certain overlap, since a disorder in one category may be associated with a disorder in another category. MGD is a good example, since it may exist in its own right, give rise to ocular surface damage, or cause evaporative dry eye. These disorders correspond to those referred to as dysfunctional tear syndrome (DTS) by Berens et al.70 In that report, the term DTS was offered as an alternative to the term dry eye, where DTS may be reasonably considered to describe any cause of symptomatic ocular surface disease, including dry eye.
In attempting to differentiate a particular disorder from other members of this large group, diagnostic tests must discriminate, not only between that particular disease and the unaffected normal state, but also between that condition and other members of the wider group of OSDs. This report is focused on MGD, and as such a description of selected tests of lacrimal function is given, since, in relation to the diagnosis of dry eye, normal lacrimal function must be demonstrated as part of the diagnostic work-up of evaporative dry eye. Tests of meibomian and lacrimal function and of evaporative water loss considered by the diagnostic group are listed in Appendices 3 and 5 through 14.
Meibomian Gland Dysfunction
This report as a whole deals chiefly with MGD. Other diseases of the meibomian glands are listed in Table 1 and are also discussed in Report on Definition and Classification. The term MGD has been widely used in the literature, as if it were synonymous with posterior blepharitis, and has been used in contrast to the term anterior blepharitis.1,36,71–74 However, as discussed by the Definition and Classification Subcommittee, MGD is but one of several causes of posterior blepharitis. Therefore, for clarity, only the term MGD is used herein.
Table 1.
Classification of Diseases of the Meibomian Gland
| Reduced number of glands |
| Congenital deficiency |
| Replacement of glands |
| Distichiasis |
| Distichiasis lymphoedema syndrome |
| Metaplastic disease of the meibomian gland |
| Meibomian gland dysfunction |
| Hypersecretory* |
| Meibomian seborrhea |
| Hyposecretory† |
| Retinoid toxicity |
| Obstructive |
| Subclinical |
| Cicatricial or noncicatricial |
| Focal or diffuse |
| Primary, or secondary to: |
| Local disease |
| Anterior blepharitis; |
| Cicatricial conjunctivitis (e.g. Trachoma; Stevens-Johnson syndrome, pemphigoid; acne rosacea, atopy |
| Chemical burns |
| Systemic disease |
| Seborrheic dermatitis |
| Acne rosacea |
| Atopy |
| Ichthyosis |
| Psoriasis |
| Anhydrotic ectodermal dysplasia |
| Ectrodactyly |
| Fungal disease |
| Turner syndrome |
| Toxicity |
| PCB exposure; retinoids |
| Other (ocular) |
| Internal hordeolum |
| Chalazion |
| Concretions |
| Neoplasia |
Although there is evidence for an accumulation of meibomian oil within the glands, there is none yet for overproduction, as opposed to excessive release on expression.
Hypothetical: Evidence is not available for a condition of primary hyposecretion.
MGD can be an asymptomatic, subclinical condition detectable only by gland expression or meibography. Alternatively, it may be symptomatic and accompanied by specific clinical signs (Table 2). It may be primary and unassociated with other local or systemic disease, or it may be secondary to a range of systemic disorders, in particular, some common skin diseases, such as acne rosacea, atopic dermatitis, and seborrhea sicca and also, the cicatrizing conjunctival disorders (trachoma, Stevens-Johnson syndrome, and ocular pemphigoid).1,75 It may also be caused clinically by exposure to drugs and toxins. There are several experimental models for MGD.76
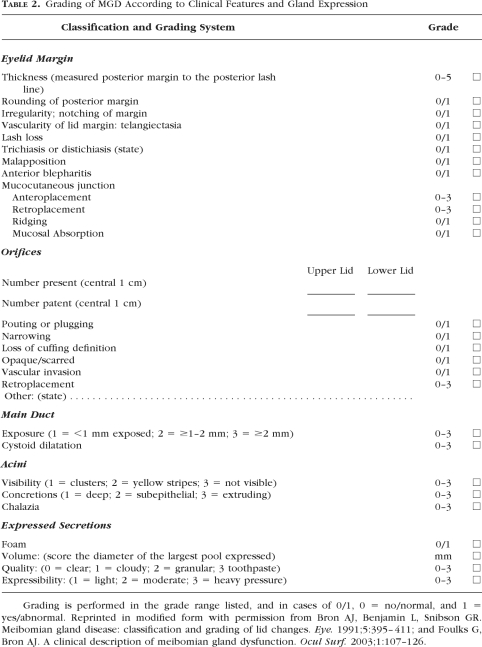
Table 2.
Grading of MGD According to Clinical Features and Gland Expression
MGD may be focal, when it affects scattered glands, or diffuse, when it affects all glands to some degree. Since the natural history of MGD has not been studied, it is not known whether focal disease is always a precursor of diffuse disease. It may also be cicatricial or noncicatricial (simple) and inflammatory (meibomitis) or noninflammatory. Characteristic signs of MGD include the release of cloudy meibum or more viscous material on expression of the glands or by an absence of expressible secretion. Occasionally, the meibomian orifices may be capped by a lipid globule covered by an intact skin (meibomiana), or cap, which is hypothesized to be oxidized lipid and epithelial material.
A diagnosis of MGD may be made by the demonstration of a single affected gland, but clinically relevant disease is due to the involvement of multiple glands. For this reason, diagnosis demands both a qualitative and a quantitative approach.
MGD may be symptomatic in its own right or give rise to symptoms through its contributions to ocular surface damage or to dry eye. The mechanism of primary MGD is not known, but the pathologic events of noncicatricial MGD include hyperkeratinization of the terminal ductules, accumulation of cellular and lipid material within duct lumina, duct obstruction; cystic dilatation of the ducts and acini and secondary, disuse atrophy of the meibomian acini73,77–79; and, at least in some instances, periglandular inflammatory changes.202
The clinical features of MGD may be intrinsic when they involve the meibomian glands alone or the lid tissues in their immediate vicinity, or extrinsic, when they affect neighboring lid structures. Intrinsic features include orifice plugging, duct obstruction, and dilatation, gland atrophy and dropout and qualitative changes in expressed secretions. Extrinsic features represent secondary changes caused by the presence of MGD, but are encountered in other forms of OSD. They include lid margin hyperemia and telangiectasia.
II. Diagnosis and Quantification of MGD
A. Clinical Subtypes and Associations with MGD
Clinically, MGD can be categorized into four subtypes, which are described in detail:
MGD alone Asymptomatic Symptomatic (noncicatricial, cicatricial)
MGD with associated with ocular surface damage
MGD-related evaporative dry eye
MGD associated with other ocular disorders.
Characterization of these subtypes requires diagnosis and quantification of MGD itself first, followed by the inclusion or exclusion of other OSDs. Diagnostic tests are referred to briefly in the following account. Details of each test are provided in the appendices.
MGD Alone.
Asymptomatic MGD (Preclinical).
Although MGD is a symptomatic disorder, it does, like other disorders, go through an asymptomatic preclinical stage, when its presence may not be obvious to the clinical observer.49,50,80–82 At this stage it may be diagnosed by meibomian gland expression, with the demonstration of an altered quality of expressed secretions and/or decreased or absent expression. With progression, MGD is likely to become symptomatic, and additional lid margin signs (e.g., hyperemia) may be detected with the slit lamp. At this point an MGD-related “posterior blepharitis” may be said to be present.
Korb and Henriquez80 studied meibomian gland expressibility in patients with or without contact lens intolerance, by using both gentle and forceful meibomian gland expression. In the asymptomatic group, they found that gentle expression generally released clear oil and rarely expressed inspissated material the consistency of toothpaste. There was a higher frequency of expressible glands in the asymptomatic group. With forceful expression, the number of expressible glands increased and in addition, more secretion was expressed from individual glands. An important observation was that in some asymptomatic subjects with apparently normal lids on simple clinical inspection, expression yielded either a creamy or an inspissated material from some glands, indicating the presence of MGD. Evidence of asymptomatic MGD was reported by Hykin et al.,83 who first documented an increase in clinical features of MGD with increasing age, but free of lid-related symptoms, and Mathers et al.43 also recorded meibomian gland dropout in historically normal subjects. The preclinical stages of MGD with apparently age-appropriate normal lid margins may require expression or meibography for clinical diagnosis.
It will be important to identify which preclinical features are likely to be predictive of progressive disease, as the question arises whether early treatment might delay progression or reverse pathologic events. Treatment for early-stage disease is relatively simple, and there may be good reason to offer treatment at an early, preclinical stage of the disease. This suggests the need to perform meibomian gland expression to detect the presence of asymptomatic MGD.
Symptomatic MGD.
Meibomian gland dysfunction has both subjective and objective features. Symptoms are a prominent feature of the disease.
Symptoms of MGD.
In the 1995 International Dry Eye Workshop, symptoms were included in a list of global features of dry eye, each of which was an essential component of the dry eye, but did not link the association of the feature to either aqueous-deficient or evaporative dry eye.84 Global features included symptoms, ocular surface damage, tear instability, and tear hyperosmolarity. This approach was reiterated in the 2007 DEWS report.85 No attempt was made to identify symptoms that distinguished aqueous-deficient dry eye from evaporative dry eye.
MGD is a common disorder1,47,57,86–89 and is associated with evaporative dry eye. It has also been suggested that evaporative dry eye is the most common form of dry eye disease (Castillanos E, et al. IOVS 2008;49:ARVO E-Abstract 2371), although the evidence is not strong. MGD is a symptomatic disorder in its own right, with symptoms generated by the lid disease and associated ocular surface consequences. Where MGD occurs as the basis of evaporative dry eye, it must be asked whether the associated symptoms are distinct from those of the dry eye itself. However, current dry eye symptom questionnaires are not designed either to distinguish the symptoms of MGD from those of dry eye (e.g., in a separate domain) or to differentiate between aqueous-deficient and evaporative dry eye (Appendix 1). This deficiency should be remedied, and it is possible that questions could be identified that would characterize MGD and distinguish it from aqueous-deficient dry eye.
While MGD is a symptomatic disease of the lids, distinct from MGD-based evaporative dry eye, the diagnostic watershed between them has not been explored. Nonetheless, in those reported studies in which evaporation rates have been compared between normal subjects who lack features of ocular surface disease and symptomatic patients with MGD,89–92 it may be presumed that MGD patients whose evaporative rates fell within the normal range (i.e., below the cutoff for evaporative dry eye) may represent patients with symptomatic MGD alone or MGD-associated ocular surface disease. The evidence from recent meta-analyses of dry eye disease in which evaporation (and tear turnover rate) was considered in groups subdivided by phenotypes of evaporative dry eye and aqueous-deficient dry eye suggest a generally mixed etiology for both.93 Individuals with a “pure” MGD phenotype represent an interesting group for further study, with the purpose of identifying an MGD-specific symptom set. It would be of particular interest to discriminate MGD from anterior blepharitis, another cause of lid-related symptoms. At the present time, no coherent effort has been made to identify symptoms that are specific to MGD itself.
With the use of currently available symptom questionnaires, one issue that arises is whether pure MGD, in the absence of dry eye, may masquerade as dry eye and therefore decrease the specificity of the test, when used as the sole identifier in dry eye diagnosis. A false-positive patient may be one with MGD, symptoms of discomfort, ocular surface staining, altered tear film lipid layer indices, but no tear hyperosmolarity. One hope would be that an MGD domain, consisting of a small number of selected questions, could be added to an existing questionnaire, which would allow the diagnosis of MGD (or at least of “blepharitis”) as a contributor to symptoms. An alternate hypothesis is that it is impossible to differentiate MGD from other ocular surface diseases on the basis of survey data alone; and therefore, combinations of subjective and objective measures may be necessary to fully differentiate the disease.
Some symptomatic features that might be anticipated to characterize MGD include personal habits related to the condition, such as lid rubbing to relieve itching and irritation; morphologic features, such as visible lid margin changes (e.g., redness and swelling) in the absence of crusts or flakes; and the presence of sensory symptoms referable to the lid margins (itching, irritation, and soreness).
Clinical signs of MGD.
The key signs of MGD are as follows: meibomian gland dropout, altered meibomian gland secretion, and changes in lid morphology. Each is described in turn, including existing grading schemes for each parameter.
Meibomian gland dropout.
Meibomian gland dropout refers to the loss of acinar tissue detected by meibography43,94 (Figs. 4, 5). It implies the partial or total loss of acinar tissue. In the original technique, the meibomian glands are observed in silhouette, by transillumination through the everted lids. The light source is applied to the skin side of the lid, and the disposition of the glands is viewed and recorded from the everted mucosal side. The detailed architecture of the glands is seen well in younger people, but becomes less well demarcated with age. The scope of this technique has been greatly increased by the introduction of noninvasive meibography in which the glands are documented, after eversion of the lids, by infrared photography79 (Fig. 4; Appendices 7, 8).
Figure 4.
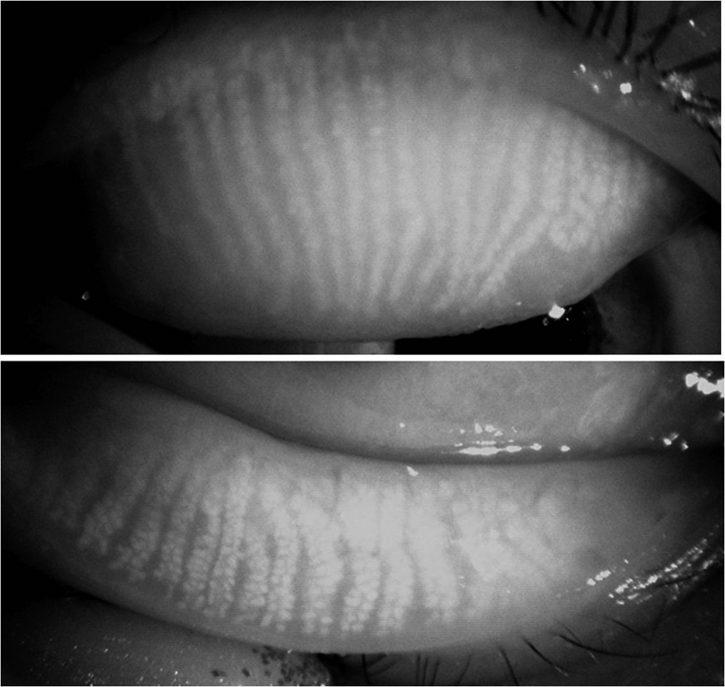
Normal meibomian glands of a 38-year-old woman, viewed by infrared meibography shows scattered gland absence or irregularity (courtesy of R. Arita).
Figure 5.
Photographic montage of the lower lid viewed by transillumination meibography. There is extensive meibomian gland dropout in a patient with meibomian gland dysfunction (courtesy of N. Yokoi).
Meibomian gland dropout increases with age in normal subjects,43 not necessarily in response to the presence of obstructive MGD. Obata has suggested that gland dropout also occurs as an age-related atrophic process.78 It is hypothesized that measurable dropout is a feature of MGD and increases with MGD severity. Loss may be proximal (at the attached border of the lid), central, or distal (at the free margin of the lid) or may involve the whole gland. Extensive dropout is associated with increasing evaporative water loss from the eye.86,87,90 It will be important in the future to identify whether total loss of meibomian gland mass and/or number of affected glands and/or site of dropout (e.g., proximal versus distal) has the greatest effect on the other meibomian indices, including clinical lid characteristics, size of the marginal lipid reservoir, spread and integrity of the lipid film, lipid composition, and the evaporation rate. No study to correlate the location of dropout with the presence of plugging or the expressibility or quality of expressed lipid has yet been conducted. It could be anticipated that distal dropout, close to the orifices, would have the most profound functional effect and may correlate most closely with a diagnosis of MGD. It is also unclear whether lipid composition would be altered in the gland with partial dropout.
Altered meibomian gland secretion: In young normal subjects, digital pressure applied to the tarsal plate expresses meiboian secretions resident in the ducts and possibly in proximal acini, as a pool of clear oil. The secretion is also referred to as meibum.4 In MGD both the quality and the expressibility of the expressed material is altered. This material, which is made up of a mixture of altered secretions and keratinized epithelial debris,95 is also referred to as meibomian excreta. It must be recognized that expressibility and secretory activity are not the same; it is merely assumed that where meibomian oil is freely expressible, secretion is “normal.”
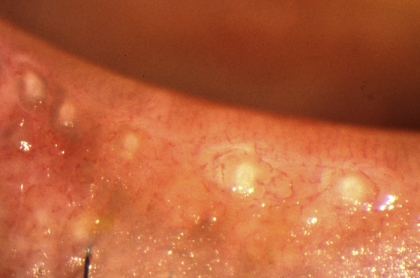
In MGD, the quality of expressed lipid varies in appearance from a clear fluid, to a cloudy fluid, to a viscous fluid containing particulate matter and a densely opaque, inspissated, toothpaste-like material (Figs. 6–8). These qualities have been incorporated into various grading schemes.75,96,97 Alternatively, the expressibility of glands during digital expression has been graded55,87,98 and expressibility from single or multiple glands, during the application of a standardized force, has also been measured by Korb and Blackie49,50,52 (Appendix 6).
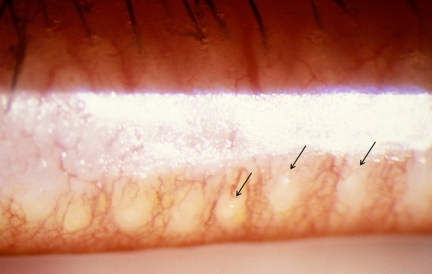
Figure 6.
Meibomian gland dysfunction. Cloudy expressed meibum (arrows) (courtesy of A. Bron).
Figure 7.
Meibomian gland dysfunction: expression of opaque meibum (courtesy of D. Korb).
Figure 8.
Meibomian gland dysfunction: strings of toothpaste-like opaque meibum expressed in response to forceful bimanual gland expression (courtesy of D. Korb).
Changes in lid morphology.
Several additional morphologic features occur and have been incorporated into grading schemes. These are summarized below and in Appendix 5.
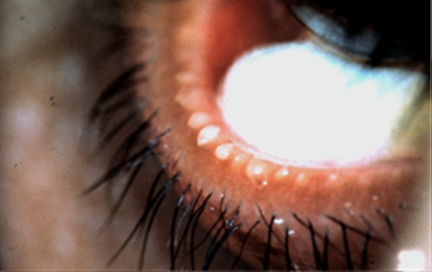
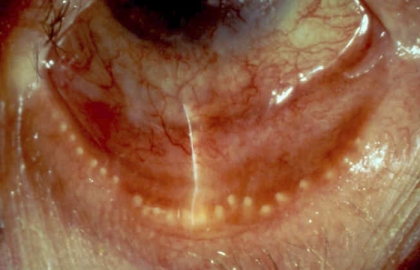
Plugging of the meibomian orifices.
The meibomian orifices may exhibit elevations above the surface level of the lid, referred to as plugging or pouting, which are due to obstruction of the terminal ducts and extrusion of a mixture of meibomian lipid and keratinized cell debris (meibomian excreta; Fig. 9). This is a pathognomonic clinical sign of MGD.
Figure 9.
Cicatricial meibomian gland dysfunction. Lid margin hyperemia with orifice opacity with plugging (arrows); (courtesy of A. Bron).
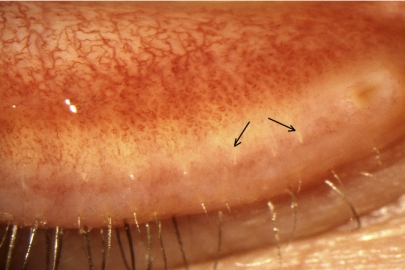
The meibomian orifices and the mucocutaneous junction.
Further important changes occur, affecting the location of the meibomian orifices in relation to the MCJ and the anteroposterior position of the MCJ itself. This junction is important because it forms the watershed between the lipid-wettable skin of the lid margin and the water-wettable mucosa.
Noncicatricial MGD, previously referred to as “simple MGD,”1,75 is a form in which, initially, the orifices retain their position anterior to the MCJ. In this situation, restoring the meibum delivery will allow oil be taken up once again into the TFLL.
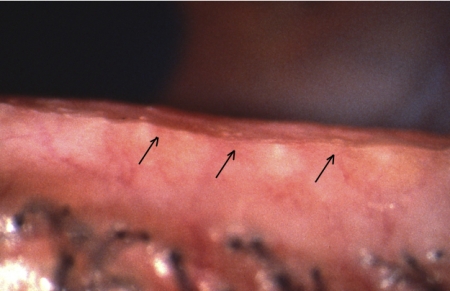
However, as Yamaguchi et al.99 observed in studying Marx's line, the MCJ migrates forward with age, causing the orifices to lie behind the junction, within the mucosa. This process has been called conjunctivalization.36 Marx's line is a line of conjunctival epithelial staining directly behind the MCJ, which is demonstrable with dyes such as rose bengal and lissamine green.100–103 It is present in all normal lids, in both sexes, and at all ages. Yamaguchi et al.99 demonstrated a forward movement of the line with age, at first encroaching on isolated meibomian orifices, then lying at the same level, and ultimately moving anterior to the gland orifices. As Marx's line indicates the location of the mucocutaneous junction, this report demonstrates an anterior migration of the line itself. This was found to correlate with the presence of MGD. This aging process contrasts with the process, which draws the orifices posteriorly across the MCJ and into the conjunctiva in cicatricial disease. Both events result in the orifice location to lie behind the MCJ, but the mechanisms are distinctly different.
With progression, noncicatricial MGD can proceed to cause orifice stenosis or obliteration and periductal fibrosis (Fig. 10), so that meibomian oil can no longer be expressed by tarsal pressure. At this point, it is clinically noted that the condition is irreversible.
Figure 10.
Advanced non-cicatricial meibomian gland dysfunction: dense orifice opacification with periductal fibrosis (courtesy of A. Bron).
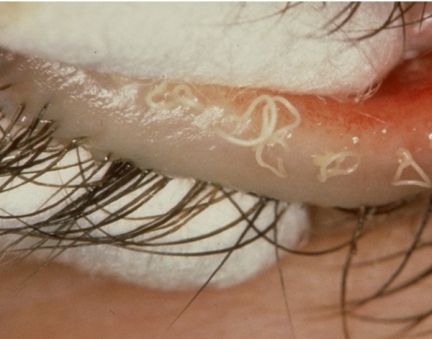
Cicatricial MGD may occur as an isolated, primary condition, in combination with noncicatricial MGD, but is most commonly found in association with the various forms of cicatricial conjunctivitis (e.g., trachoma, erythema multiforme, and pemphigoid). In this case, submucosal connective tissue scarring leads to a stretching and exposure of the terminal ducts of the glands and a thinning of the overlying conjunctival mucosa. This is termed ductal exposure and presents as a slightly elevated, riblike feature that is a telltale sign of the cicatricial process (Figs. 11, 12). Also, the affected orifices may be dragged posteriorly, across the MCJ, onto the tarsal plate, where they are ultimately lost to view or absorbed (Fig. 13). The affected ductules are frequently obstructed, but on occasion, pressure over the glands may express clear meibomian oil. Since affected orifices are located in the mucosa, any oil that they may deliver is released into the aqueous phase of the tear film and therefore is unlikely to contribute effectively to the tear film lipid layer (TFLL). The condition should be regarded as both structurally and functionally irreversible. Although therapy may suppress the inflammatory events, it cannot restore anatomic relationships. In this condition too, the MCJ may also be dragged posteriorly.
Figure 11.
Cicatricial meibomian gland dysfunction: All meibomian orifices open onto the marginal conjunctiva, with some exposure of terminal ducts (arrows) (courtesy of A. Bron).
Figure 12.
Cicatricial meibomian gland dysfunction: All meibomian orifices open onto the hyperemic marginal conjunctiva, with some exposure of terminal ducts (arrows) (courtesy of A. Bron).
Figure 13.
Advanced cicatricial meibomian gland dysfunction: orifice retroplacement and opacity (courtesy of G. Foulks).
Cicatricial and noncicatricial MGD may occur together on the same lid margin in the absence of a conjunctival scarring disease.
Additional features of MGD include rounding, notching, dimpling, telangiectasia, increased vascularity of the posterior lid margin, epithelial ridging between gland orifices (Figs. 14, 15), loss of orifice architecture, cystoid changes in the gland, formation of concretions within the acini and, possibly, the formation of chalazia (Appendix 5). The natural history of these changes and their clinical disease associations have not yet been explored.
Figure 14.
Dimpling or notching of the posterior lid margin due to tissue absorption in the region of the orifices (courtesy of J. Shimazaki).
Figure 15.
Advanced meibomian gland dysfunction: epithelial ridging extending between opacified meibomian gland orifices (courtesy of A. Bron).
B. Methods of Clinical Assessment of the Meibomian Glands: Grading Scales
Of those techniques described in the literature, the most consistently reported are those that quantify gland dropout and grade the quality or expressibility of meibum. Although the volume of expressed secretions has been proposed as an additional gradable parameter,90 the technique is not widely recommended, as this is a measurement of volume expressed, recorded as the diameter of expressed meibum, and is dependent both on the force applied and the duration of the force. Quantification of MGD is important, to assess its severity and monitor the response to therapy. It is also essential for application in clinical trials and in tracking its natural history. The diagnostic criteria for obstructive MGD proposed by the Japanese MGD Working Group can be seen in Appendix 17.
Meibomian Gland Dropout: Grading Scales.
Meibomian gland dropout implies partial or total gland loss or atrophy and can be quantified by meiboscopy, meibography, and confocal microscopy (Table 3).
Table 3.
Techniques for Imaging the Meibomian Glands
| Technique | Lid Region | Grading Scheme | Reference |
|---|---|---|---|
| Meiboscopy | LL | 0 = no dropout | Pflugfelder et al.55 |
| 1 = ≤33% | |||
| 2 = 34%–66% | |||
| 3 = ≥67 | |||
| Percent of partial or total gland dropout | |||
| Separate measurement over the nasal and temporal halves of the lower lid | |||
| Meibography (contact; retro-illumination) | LL | Total number of glands lost of eight central of the lower lid. Half gland loss was given a grade of 0.5 | Mathers et al.96,97 |
| 1 = normal | Jester et al.105 | ||
| 2 = gland visible w/decreased absorption | |||
| 3 = acini atrophic; duct visible | |||
| 4 = no structures visible | |||
| LL | 0 = no dropout | Shimazaki et al.86,87 | |
| 1 = ≤50% dropout | |||
| 2 = ≥51% dropout | |||
| LL | Dropout: (nasal half, lower eyelid) | de Paiva et al.106 | |
| 0 = no dropout | Composite score with lids signs and expressibility (0–11) | ||
| 1 = ≤25% | |||
| 2 = ≤50% | |||
| 3 = ≤75% | |||
| 4 = ≤100% | |||
| LL ≅ 15 glands | Gestalt method: | Nichols et al.107 | |
| 1 = no partial glands (PGs) | |||
| 2 = <25% PGs | |||
| 3 = 25%–75% PGs | |||
| 4 = >75% PGs | |||
| Noncontact | LL and UL | 0 = no loss, | Arita et al.3 |
| 1 = gland loss <33% of total area | |||
| 2 = loss, 33%–67% | |||
| 3 = >67% loss | |||
| Scores of upper and lower lid summed | |||
| Scale range 0–6 | |||
| Confocal microscopy | LL and/or UL | Acinar density: number of glands//mm2 (based on a 400 × 400 micrometer field) mean acinar diameter | Matsumoto et al.79 |
LL, lower lid; UL, upper lid.
Meiboscopy is the quantification of meibomian gland dropout by using lid transillumination94 and involves clinical observation alone. Meibography is the same technique, but using photodocumentation.104 Most current studies employ gland photography. Meibography is useful in providing a permanent record, which permits masking of scoring and therefore provides greater objectivity. Such records can be handled at a reading center, to provide improved standardization in clinical trials. The transillumination technique is relatively time-consuming, is challenging in patients with thickened tarsal plates, and may have limited general use. Arita et al.3 developed a noncontact method of meibography in which the glands of the everted upper or lower lids are imaged from the mucosal side with infrared photography. The technique is said to be more rapid and less disturbing for the patient than standard transillumination meibography. More recently, Matsumoto et al.79 have measured meibomian gland density per square millimeter and the diameter of intact glands, using in vivo confocal microscopy on the everted tarsal plate (Appendix 8). Table 3 summarizes studies of gland dropout in MGD, using the methods of meiboscopy and meibography, along with confocal microscopy.
Pflugfelder et al.55 used meiboscopy to estimate partial or total gland loss in the nasal and temporal halves of each of lid, using a 0 to 3 scale in which 0 was no gland dropout and 1 was 1% to 33%, 2 was 34% to 66%, and 3 was ≥67% dropout. Mathers et al.96,97 used meibography to examine the frequency and degree of MGD in patients with chronic blepharitis. The total number of glands lost in the central portion of the lower eyelid (of eight adjacent glands) was measured. A score of 0.5 was assigned for half gland loss. Shimazaki et al.86,87 adopted a relatively crude scale of 0 to 2, in which 0 is no gland dropout, 1 is gland loss involving up to half of the lower lid, and 2 is more than half the lower lid. de Paiva,106 also scoring the lower lid, used a 0 to 4 point scale, with 0 as no dropout and 1 as ≤25%, 2 as ≤50%, 3 as ≤75%, and 4 as ≤100% dropout.
The study by Nichols et al.107 has been particularly useful in validating the method of meibography (Table 4). Using a near infrared transillumination and capture system, imaging approximately 15 lower lid glands, the group reported the within- and between-observer reliability of two methods of grading. Image quality criteria were applied, and trained observers were used. In the gestalt system, they estimated the fractional, (partial or total) gland loss on a 1 to 4 scale, where 1 was no gland loss and 2 was 25%, 3 was 25% to 75%, and 4 was >75% gland loss in the image with partial glands. Alternatively, the number of intact glands in the region of interest was counted. It can be seen from Table 4, that for the gestalt system, using a weighted κ statistic, the method showed near perfect reliability within observers (κ = 0.91) and moderate reliability between observers (κ = 0.57). For the individual gland counting system, using the 95% limits of agreement method, reliability was judged to be moderate within observers and fair between observers. The two grading methods correlated highly (z = 15.15, P < 0.0001). The reader should consult the original article for details of the statistical treatment. However, overall this report appears to establish the method of meibography as a useful clinical tool.
Table 4.
Validation of Meibography107
| Test Reliability | ||
|---|---|---|
| Gestalt method: | Simple κ | Weighted κ |
| Within observer | κ = 0.78, 95% CI = 0.71–0.85 | κ = 0.91, 95% CI = 0.88–0.95 |
| Between observer | κ = 0.38, 95% CI = 0.30–0.46 | κ = 0.57, 95% CI = 0.47–0.85 |
| Intact gland counting | 95% limits of agreement | |
| Within observer | Moderate: 2.84–2.76 glands | |
| Between observer | Fair: 4.46–5.98 glands |
κstatistic scale: <0.00 poor reliability; 0.00–0.20, slight reliability; 0.21–0.4.0, fair reliability; 0.41–0.60, moderate reliability; 0.61–0.80 substantial reliability; and >0.80 near perfect reliability.
Arita et al.3 quantified glands from a montage of images (described above). The scores for the upper and lower lid were summed to give a scale range of 0 to 6 for the two lids. The result was termed the meiboscore.
A body of evidence is beginning to indicate that meibomian gland dropout correlates with the clinical features of MGD, such as altered quality of expressed secretions and the consequences of gland obstruction, such as altered tear film lipid layer stability, increased evaporative loss, and ocular surface damage.79 The grading of dropout at baseline and subsequent examinations may provide information about long-term progression.
Concerning the mechanism of noncicatricial MGD–related disease, it is assumed that duct obstruction and increasing acinar loss (particularly distal loss) results in reduced meibomian lipid delivery. This effect would be measured by gland loss from the upper and lower lids, and the combined dropout score from the upper and lower lids would be needed to reflect this most accurately.
Scale ranges must be considered, to demonstrate the relationship between dropout and other parameters. Currently, there is no consensus as to the number of discrete increments that should be used in clinical grading. Bailey et al.110 have addressed the effects of scaling on clinical grading and have demonstrated an improved ability to detect clinical change when fine rather than coarse scale increments are used. This approach has been used effectively for the grading of corneal staining on a 0.1-step scale increment within a 0 to 4 scale111,112 and in the quantification of cataract.113 The small increment approach could be useful if applied to meibography.
At present, sometimes for ease of performance or for operational reasons, measurements are made on a limited region of one lid and from either the upper or lower lid alone. This may be because it is convenient to perform expression on one set of lids and meibography on the other. In a recent study of meibomian gland function in blepharitis, a high correlation between measures, including gland dropout, was found between the upper and lower lids, with the lower lid offering the most effective single measure.114 However, in a disease that can involve a focal portion of the lid, such measurements cannot reliably reflect events affecting both lids of both eyes. There is a need to develop approaches that can assess the full extent of each tarsal plate, to produce an aggregate score. Noncontact meibography and confocal microscopy appear promising from this point of view.
Meibography is attractive because it offers a permanent record and permits masking of scoring. In the future, for clinical trials, it is likely that digital imaging techniques will be developed that will document gland dropout more accurately and permit a focus of attention on the terminal ductule, a region of strategic importance.
Meibomian Gland Expression: Grading Scales.
Meibomian gland expression is used in diagnosis and to obtain meibomian samples for lipid analysis (Appendix 6; Table 5). It is common to express the glands by applying digital pressure through the substance of the lids, but methods to standardize the application of force have also been developed. When the lids are normal, light expression may be expected to expel secretion contained in the ducts. It is possible that heavy expression releases presecretory lipids from the acini. Heavy expression is necessary to express the thicker grades of meibum associated with MGD or may be necessary therapeutically in the treatment of MGD. Expressibility is sometimes equated with functionality of the meibomian glands and they are likely to be closely related, but expression is not in itself a measurement of secretory activity, although it could be considered a surrogate measure of secretion.
Table 5.
Grading Meibomian Gland Expression
| Technique | Study Details | Lid Region | Grading Scheme | Reference |
|---|---|---|---|---|
| Meibum Characteristics | ||||
| Firm digital pressure | Volume of expressed meibum | Central eight glands of lower eyelid | 0 = Normal volume. Just covers orifice 1 = increased to 2 to 3 times normal 2 = increased more than 3 times 3 = increased more than 10 times |
Mathers et al.96,97 |
| Firm digital pressure | Viscosity of expressed meibum | Central eight glands of lower eyelid | 1 = normal, clear, may have a few particles 2 = opaque with normal viscosity 3 = opaque with increased viscosity 4 = severe thickening (toothpaste) |
Mathers et al.96,97 |
| Firm digital pressure | Volume and viscosity of expresssed meibum Clinic-based; referred for dry eye or blepharitis n = 513 total; n = 76 normal women (used to define aqueous deficiency) |
Central eight glands of lower eyelid | Obstructive: Viscosity ≥ 3 (1, clear; 2, slightly opaque; 3, thick, opaque; 4, toothpaste Avg. lipid volume: ≤0.3 mm (diameter of expressed lipid in millimeters) Dropout: >0 (presumably examined central eight glands; includes 1/2 and whole glands) Seborrheic: Viscosity: no criteria Avg. lipid volume: >0.7 mm |
Mathers et al.96,97 Mathers and Billborough108 |
| Meibum Quality and Expressibility | ||||
| Firm digital pressure | Quality of meibum | Number of glands not stated | 0 = clear fluid 1 = cloudy fluid |
Bron et al.75 |
| UL or LL | 2 = cloudy particulate fluid 3 = inspissated, like toothpaste |
|||
| Firm digital pressure | Expressility of meibum from five glands | UL or LL | 0 = all glands expressible 1 = 3–4 glands expressible 2 = 1–2 glands expressible 3 = no glands expressible |
Pflugfelder et al.55 |
| Standardized application of pressure | Expression applied to a set of about eight glands | Nasal, central and temporal lid | The MGYLS score is the number of Meibomian Glands out of 8, Yielding Liquid Secretion | Korb and Blackie52 Blackie and Korb81 |
| Meibum Expressibility | ||||
| Variable digital pressure | Gentle or forceful expression | LL | Analysis of expressed secretion | Henriquez and Korb98 |
| Variable digital pressure | Expressibility of meibum | LL | 0 = clear meibum, easily expressed | Shimazaki et al.86,87 |
| 1 = cloudy meibum, easily expressed | ||||
| 2 = cloudy meibum expressed with moderate pressure | ||||
| 3 = meibum not expressible, even with hard pressure | ||||
| Variable digital pressure using the Shimazaki schema | Measurement of lid morphology, expression and meibography | See grading box | Lid margin: Irregular Vascular engorgement Plugged orifices Displacement of MCJ, score “1” for each present |
Arita et al.109 |
| Clinic based n = 53 obstructive MGD subjects n = 60 age-matched controls |
Expressed meibum (upper eyelid): 0 = clear, easily expressed 1 = cloudy, mild pressure 2 = cloudy, > moderate pressure 3 = meibum not expressed, with hard pressure |
|||
| Meibography: upper and lower eyelids, meiboscore summed (0, no loss; 1, gland loss < 33% of total area; 2, loss = 33–67%; 3, ≥67% loss) | ||||
In MGD, the quality of expressed oil varies in appearance between that of a cloudy fluid, a viscous fluid containing particulate matter and a densely opaque, toothpaste-like material. These qualities have been incorporated into various ordinal grading schemes75,96,97 (Table 5). The scores in these four-point systems are 0, clear (normal); 1, cloudy; 2, cloudy with particles; and 3, inspissated (like toothpaste).75 Similarly, in the Mathers scheme, 1 is clear, 2 and 3 are liquid but of decreasing transparency, and 4 is like toothpaste). When the expression of a fixed number of glands is assessed, there are two ways of generating a score. One way is to record only the highest grade encountered from any of the expressed glands. In this case, for a single zone, the score range is 0 to 3. The other is to record the sum of scores for each gland expressed, to achieve a composite score. If eight glands are expressed, then the score range is 0 − (8 × 3) = 24. This approach is generally preferred and is recommended by this committee. However, a small caveat is that in long-term studies, inexpressibility encountered in normal lids is also a sign of total obstruction; an increase in the number of pathologically inexpressible glands with disease progression, would, paradoxically, lead to a fall in total score.
In addition, the expressibility of glands during digital expression has been graded,55,87,98 while expressibility from single or multiple glands, during the application of a standardized force, has been measured by Korb and Blackie (Table 5).49,50,81 For multiple glands, the standard force is applied for 10 to 15 seconds with a specially designed instrument.52 The Shimazaki approach grades expressibility according to the response to different levels of digitally applied pressure and therefore brings an additional subjective element into the grading process. The approaches of Pflugfelder and of Korb relate to a fixed number of expressed glands, and the latter system clearly instructs the investigator to score only those glands that yield a liquid secretion (the MGYLS score), regardless of its quality (Table 5). To increase the scale range and reflect the status of the full length of the upper and lower lids, an aggregate score can be created from the summed expression grades from the nasal, central, and temporal regions of each lid. As noted earlier, even in young normal subjects, the expressibility, in terms of the fraction of glands from which fluid meibum may be expressed, varies for different regions of the lid and reduces progressively from the nasal to the temporal side.49,50 However, it may be reasonable to generate a composite score for the upper and lower lid by summing the nasal and central scores from each lid, not attempting to score the temporal region.
Grading Morphologic Lid Changes: Grading Scales.
The approaches to grading (Appendix 5) other morphologic features of MGD were discussed earlier and are presented in Table 2. Grading scales may be expanded by dividing each lid into quarters and grading the highest level of change in each region.75 Quantifying selected features in this way offers an opportunity to generate an aggregate MGD score that may then be used in conjunction with measures of gland expressibility and dropout.1 This approach was adopted by de Paiva et al. in a comparison of normal subjects with those who had ocular irritation.106 An aggregate score with a scale range of 0 to 11 was created by combining a meibographic grading (see Table 2) with a grading of lid changes, as follows: Orifices: metaplasia present is 1; absent is 0; and brush marks (linear vascular features): present is 1, absent is 0; Expressibility using digital pressure applied over five lower lid glands: 0 is all five glands expressible and 1 is four, 2 is three, 3 is two, and 4 is 0 glands expressible. Similarly, Arita et al. scored for the presence or absence of lid abnormalities, as follows: irregularity of the lid margin, lid margin vascular engorgement, plugging of the meibomian orifices, and anterior or retroplacement of the MCJ, giving a score of 0 to 4.
C. The Utility of Current Grading Scales
These various tests have been used to explore the prevalence of MGD and its relation to ocular disorders. Age-related data are available in normal subjects concerning morphologic lid changes, lipid levels at the lid margin, meibomian gland dropout, and expressibility of meibomian secretion.
Mathers and et al.43,115 used meibography to examine 72 normal subjects without dry eye and found that gland dropout remained, on average, below one gland per eight assessed, up to about age 50 years. After that, it increased to approximately two glands per eight assessed (25%). Similarly, using noncontact meibography, Arita et al.3 found a significantly positive correlation between the meiboscore (implying dropout) and age (R = 0.428; P < 0.0001). They found meiboscores up to about grade 1 (i.e., gland loss under a third of the total gland area) at age 50 in normal subjects and then increasing scores and gland dropout with advancing age.
In a study of asymptomatic, normal subjects, Hykin and Bron,116 showed changes related to age, including increasing lid margin telangiectasia and cutaneous hyperkeratinization, increased narrowing and pouting (plugging) of meibomian gland orifices, and a decreased number of expressible glands. The quality (viscosity and degree of opacity) of expressed secretions did not change. In contradistinction, Mathers and Lane43 found that lipid viscosity increased with advancing age in normal subjects, a change that was highly significant for linear trend (P = 0.0006).
Chew et al.5 used meibometry in a large sample of normal subjects (n = 421) and found increasing lid margin levels of meibomian oil throughout life, with no differences found between the sexes after approximately age 50. These meibometry results seemingly contradict the finding that meibum is expressible from fewer orifices with advancing age.116 The paradox could be explained by a greater meibometry pickup from the lid margin with age.
Yamaguchi et al.99 assessed the disposition of Marx's line in normal subjects by using fluorescein and other dyes and the following grading system: 0, Marx's line runs entirely on the conjunctival side of the meibomian orifices; 1, parts of Marx's line touch the meibomian orifices; 2, Marx's line runs through the meibomian orifices; and 3, Marx's line lies on the skin side of the meibomian orifices. Grading was performed in the inner, central, and outer thirds of the lower lid, giving a range of scores for the whole lid of 0 to 9. It was found that grading was reasonably consistent between observers. With age, the grade score increased, implying that Marx's line (and the MCJ) moved anterior with time. The authors found a positive correlation between the regional meibography scores and quality of expressed meibum score (graded on a 0 to 4 basis), and the regional Marx's line scores.
Several investigators (using the various methods discussed herein) have shown decreasing functionality of the meibomian glands with aging. Norn51 found that a maximum of approximately 14.5 lower lid glands could be expressed by digital pressure in normal subjects at the at age of 20 years but that the number dropped to approximately seven glands beyond the age of 80 years. Hykin and Bron116 later confirmed these results.
Mathers et al.96,97 reported a prevalence of MGD of 20% in the normal population older than 20 years. In other studies the population prevalence of MGD has been reported to range between 3.5% and 68%. Arita et al.109 reported that positive meiboscores develop after the age of 20 years in men and after the age of 30 years in women. Meiboscores correlated with age in both sexes (R = 0.428: P < 0.001) and there was also a positive correlation between the lid margin score (based on a cluster of features) and age (R = 0.538: P < 0.0001) and between the meiboscore and lid margin score (R = −0.289; P = 0.0001).
Several investigators have concluded that meibomian gland dropout is a useful index of obstructive MGD.86,87,94,96,97 Using meibography, Mathers et al.96,97 found meibomian gland dropout in 76% of their patients with chronic blepharitis. The dropout score in their normal group was 0.18 ± 0.1 (per eight lower lid gland surveyed) compared to 1.97 ± 2.1 in their blepharitis group, which likely contained patients with non-MGD forms of blepharitis. On the basis of a cluster analysis, they concluded that only gland dropout was useful in classifying dysfunction. Obstructive MGD was associated with a high level of dropout (mean 3.67 ± 1.7 glands missing per eight glands surveyed; nearly 50%) versus normal subjects (mean 0.18 ± 0.1 glands missing; ∼2.2%). This study identified a group of patients with high levels of meibomian gland dropout, a high level of tear osmolarity, and high Schirmer values. This group would correspond well to that predicted by Bron et al.117 as an example of patients with evaporative dry eye during a phase of partial, reflex lacrimal gland compensation. A further group of patients was identified with high levels of meibomian gland dropout, tear hyperosmolarity, and low Schirmer values. This group would correspond to a more advanced stage of evaporative dry eye, in which it is predicted that lacrimal compensation has failed and evaporative dry eye is accompanied by a functional, aqueous-deficient dry eye. An average dropout of 5.5 ± 1.3 glands (∼69% of eight glands) was found in this sicca group. This contrasts with much lower levels of gland dropout for subjects with seborrheic MGD and those with low Schirmer scores alone. Taken together, these results suggest that quantitative assessment of gland dropout is a valuable indicator of obstructive MGD.
Pflugfelder et al.55 used clinical meiboscopy to assess gland dropout in several dry eye subtypes, albeit with modest sample sizes (n = 9–11 subjects per subtype). These authors found mean gland dropout scores (graded on a 0–3 scale based on percentage of gland dropout) of approximately grade 2 for inflammatory and noninflammatory MGD subjects compared to a grade <0.5 for the controls. A significant finding in their report was that the degree of acinar loss in inflammatory MGD and atrophic MGD was roughly equivalent. Thus, gland dropout alone may not adequately discriminate these two clinical conditions. Khanal et al.118 found gland dropout to be effective in differentiating the evaporative dry eye subtype from those without dry eye, but was not effective in differentiating aqueous-deficient dry eye.
Matsumoto et al.,79 using confocal microscopy, have shown a decrease in meibomian gland density in MGD patients (47.6 ± 26.6/ mm2, compared with 101.3 ± 33.8/ mm2 for a control group). This group also introduced the measurement of meibomian gland diameter as a new parameter reflecting the health of the glands (Fig. 16). In their study, MGD was associated with an increase in residual gland width (98.2 ± 53.3 μm in MGD and 41.6 ± 1.9 μm in controls) that was attributed to accumulated, inspissated debris within the acini. However, an alternative explanation may be that acinar enlargement is in part compensatory, due to the influence of a feedback loop.
Figure 16.
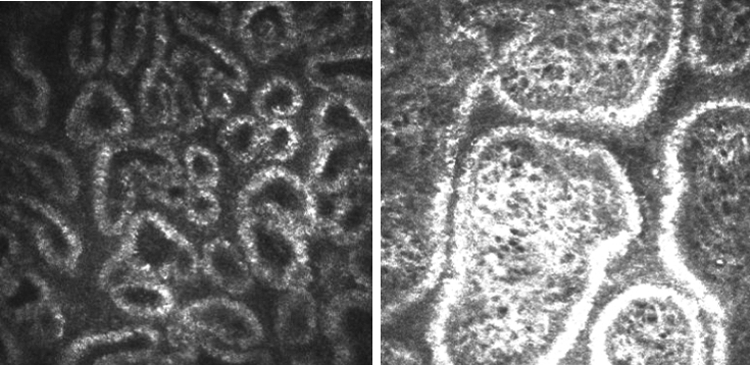
In vivo confocal microscopy of meibomian glands, showing the dilatation of acinar units in a patient with obstructive meibomian gland dysfunction (right) compared to that in a healthy control (left) (courtesy of M. Dogru).
This review of the current literature suggests that quantification of meibomian gland dropout provides a valuable baseline statement about the integrity of the meibomian glands. The dropout score appears to correlate with the presence of MGD diagnosed by other clinical criteria and to the effects of MGD on the surface of the eye.
MGD with Associated OSD.
OSD is encountered in association with MGD and is found in its most advanced form in MKC. Various etiologies have been proposed for such damage, including the release of inflammatory mediators into the tear film and the mechanisms of evaporative dry eye. One source of such mediators includes the breakdown products of meibomian lipid, altered by the lipases of microbial commensals. A possible relationship has been reported between meibomitis and phlyctenular keratitis, a keratitis that is sometimes encountered in young females. In a small group of patients with phlyctenular keratitis, 57% of whom had a history of chalazia, the location and severity of a meibomitis correlated well with the severity of the corneal nodules, and there was a possible association with specific HLA subtypes and with the presence of Propionibacterium acnes in expressed meibum.119
Ocular surface damage may be quantified by grading staining of the cornea and conjunctiva using selected dyes, by immunohistochemistry or flow cytometry on impression cytology specimens, and by the direct measurement of inflammatory mediators in the tears biochemically, with multiplex bead technology or using MALDI-TOF and proteomic techniques.120 These biochemical and clinical techniques have helped to describe the ocular surface phenotype in MGD and other OSDs and to monitor the severity of disease and response to treatment, but the events that they record are not specific to MGD, and they therefore have no unique role in its diagnosis. The precision of such tests was addressed in the 2007 DEWS Diagnosis report, and details of test sensitivity and specificity in the diagnosis of dry eye are summarized and incorporated both in the published templates and in additional materials available on the TFOS web site (www.tearfilm.org), Intrinsic glandular inflammatory events may be recorded directly by confocal microscopy (Appendix 8).
MGD-Related Evaporative Dry Eye.
In the presence of MGD, the amount of oil delivered to the reservoir is reduced, as a result of meibomian obstruction or gland atrophy or, in the case of cicatricial MGD, because the affected orifices are malpositioned, and the ducts are stretched and narrowed. A combination of mechanisms may often be at work when these forms of MGD occur together. With progression of MGD, it is assumed that a point is reached when the amount in the reservoir, or its distribution along the lid margins, is insufficient to maintain a normal TFLL, so that a functionally incompetent TFLL results. It is likely that compositional changes in meibum contribute to this disturbance, too. Abnormalities of the TFLL include abnormal (slow) spreading patterns,121 vertical interferometric patterning, and reduced TFLL stability. These are accompanied by an increased evaporative water loss (Fig. 17).
Figure 17.
Evaporimetry (courtesy of A. Tomlinson).
It is known that spreading of the TFLL is altered in the higher degrees of aqueous-deficient dry eye.62,66,122 This spreading has been attributed to thinning of the aqueous layer of the tear film.123 In a recent publication, it was suggested that this effect gives rise to a functional TFLL deficiency and a consequent increased evaporative water loss.117 Thus, it is proposed that a functional evaporative dry eye may occur in the presence of organic aqueous-deficient dry eye. This type of dry eye is predicted to occur in the absence of MGD, but would be compounded by it, if present. No TFLL spreading can be detected with a video-interferometer (DR-1; Kowa, Tokyo, Japan) in the severest form of aqueous-deficient dry eye, but recovery can be confirmed, after punctal occlusion.66,124
MGD Associated with Other Ocular Disorders.
There have been extensive reports of the association of MGD with other ocular and systemic disorders in the literature, including contact lens (CL) intolerance. The level of evidence associated with each ocular and systemic factor is discussed in detail in the report of the Epidemiology Subcommittee and is discussed briefly here for clinical significance.
Meibomian Keratoconjunctivitis.
McCulley and Sciallis72 described a condition of tear film instability, ocular inflammation, and ocular surface damage in a group of patients with chronic blepharitis, which they called MKC (Table 6).72,125 In the study, patients exhibited both anterior and posterior blepharitis and some form of associated skin disorder. The features of MKC are summarized in Table 6. Signs of obstructive MGD were associated with conjunctival injection and superficial punctate keratitis (SPK), preferentially affecting the lower interpalpebral globe and cornea. In all cases, MKC was associated with some form of skin disease, such as seborrhea sicca, (11.5%), acne rosacea (34.6%), or seborrheic dermatitis, on its own (38.5%) or in combination with atopy (15.4%).
Table 6.
Features of Meibomian Keratoconjunctivitis72
| Anterior blepharitis | Ocular surface damage |
| Crusting (61%) | SPK (100%) |
| Scales | Rose bengal staining (100%) |
| Lash loss (58%) | |
| Lid margin irregularity (46%) | |
| Posterior blepharitis | Ocular inflammation |
| Oil stagnation (100%) | Bulbar injection (100%) |
| Orifice abnormalities (23–53%) | Tarsal papillary change (100%) |
| Meibomian foam (62%) | |
| Reduced tear secretion | General signs |
| Schirmer test <10 mm (35%) | Concretions |
| An excess of tear debris | |
| Tear film instability | Clinical associations |
| Reduced BUT (100%) | Seborrhea sicca; seborrheic dermatitis with or without atopy; acne rosacea |
SPK, superficial punctate keratitis; BUT, break-up time.
MGD and CL Wear.
MGD is frequently associated with CL intolerance,80,98 and there are several clinical reports of an association between MGD and giant papillary conjunctivitis (GPC). Mathers and Billborough108 found significantly more gland dropout and greater viscosity of expressed secretions in CL wearers with GPC than without GPC, whereas Martin et al.126 found that the severity of GPC correlated with the severity of the MGD in a consecutive series of GPC patients. Although attention has been focused on the hypothesized role of MGD in CL intolerance and GPC, it is also possible that the conjunctivitis initiates changes in the meibomian gland by the release of inflammatory mediators. Ong and Larke127 found an increase in the frequency of MGD after 6 months of CL wear, and Arita et al.109 recently reported that CL wear is associated with a decrease in the number of functional meibomian glands, proportional to the duration of CL wear. Further research is necessary to determine the role of CL wear in the development of and/or progression of MGD.
Mixed Anterior Blepharitis and MGD.
Mixed anterior blepharitis associated with MGD is not uncommon and is often encountered clinically in seborrheic blepharitis,125 in atopic blepharitis,128,129 and as a specific complication of systemic retinoid therapy.130
Documenting MGD in Different Clinical Situations.
Quantification of MGD is important for diagnosis and treatment, but is also required in other clinical circumstances.
Recruitment of Patients for Clinical Trials.
Dry Eye.
Certain considerations apply in the recruitment of patients for trials of drugs to treat aqueous-deficient dry eye. Since extensive MGD may be associated with dry eye, there may be reasons to exclude patients exhibiting the higher grades of MGD, which may exacerbate the dry eye and influence interpretation of drug efficacy. On the other hand, particularly in recruitment of patients with severe dry eye, it is unrealistic to exclude all patients with MGD. A compromise is to permit recruitment of patients with a low degree of MGD, based on meibum quality or expressibility. The MGD grade can be used for stratification in data analysis.
MGD.
In clinical trials of drugs for the treatment of MGD or of MGD-related dry eye, a higher grade of MGD would be required at recruitment in order to demonstrate efficacy and permit responder analyses. The MGD grade, determined by one of the methods described above, would be recorded over the course of the study. An assessment of functionality, such as by the MGYLS score, would be an important inclusion.52 Measurement of gland dropout offers an objective way to stratify the baseline severity of the MGD. A detailed summary of existing trials is presented in the report of the Clinical Trials Subcommittee.
Monitoring for MGD as an Adverse Event.
MGD is a side-effect of systemic retinoid therapy, used in the treatment of acne vulgaris.97,131 In studies of the evolution of such changes it is necessary to recruit subjects with a low degree of MGD, in order that the development of pathological changes may be monitored carefully and detected quickly. This implies recruiting a relatively young, adult population and confining assessments to the nasal and possibly the central thirds of the lower lids, where normally, the percentage of active glands is relatively high.52
Natural History of MGD.
While the natural history of MGD is not yet known, clinicians and researchers have the tools to address this in the future. Such studies would allow the evolution of primary MGD to be elucidated and could identify the chain of events leading to secondary forms of MGD.
III. A Practical Approach to the Diagnosis of MGD and MGD-Related Diseases
Standardization and accessibility are the keys to successfully performing any test. Standardization can be achieved in any clinic by performing examinations within a standard environment and ensuring, when auxiliary staff are involved, that the staff are well trained. The diagnosis of MGD, whether in isolation or associated with ocular surface damage or dry eye, should be viewed in the context of diagnosing any ocular surface disease, and tests should be performed in an order that minimizes the extent to which one test influences the tests that follow.
The evidence base of tests used to define dry eye and its subtypes is summarized in Table 7. The effectiveness of these tests varies between 50% and 96%. However, the quality of evidence on which these statistics is based varies from study to study, dependent on the initial quality of the investigator's definition of the condition, the presence of selection bias in the study design, and the size and sample of the population studied. It can be seen from examining Table 7 that if a 70% level of sensitivity and specificity is accepted as appropriate for an effective test, several clinical and laboratory-based tests are effective in differentiating the normal from a generic dry eye. On the basis of the evidence in Table 7, however, when evaporation rate is used as the gold standard, only two types of tests, tear secretion measured by fluorophotometry and the fluorescein clearance rate, are able to differentiate evaporative- from aqueous-deficient dry eye, at the second stage of diagnosis. However, a diagnosis of evaporative dry eye can be reinforced by positive findings from meibography, meibometry, scoring the functional severity of the MGD, and measures of TFLL dynamics.
Table 7.
Diagnostic Efficacy of Tests for Evaporative and Aqueous-Deficient Dry Eye
| Test Measure | Normals vs. Dry Eye (Sens %/Spec %) | Normals vs. EDE (Sens %/Spec %) | EDE vs. ADDE (Sens %/Spec %) |
|---|---|---|---|
| Symptom questions | DE >14.5 (82/36; vs. RB, SCH, TBUT); McMonnies132 | ||
| DE >15; OSDI; (80/79 vs. Lissamine, Sch, Symp) (60/83, Dr. diagnosis); Johnson and Murphy133 | |||
| Tear stability | FBUT <10 seconds (82/86); Mengher et al.134 | ||
| Tear secretion: Schirmer I, Schirmer II | <5.5 mm/5 min (85/83); Khanal et al.92 | ||
| Index of tear volume, PRT | PRT <12 mm (56/69); Sakamoto et al.135 | ||
| PRT <20 mm (86/83); Patel et al.136 | |||
| Ocular surface damage | RB Stain >3.5; van Bijsterveld137 | ||
| RB Stain >4 (95% vs. 96%) (63/84); Vitali et al.138 | |||
| Lid (meibomian morphology) | NA | ||
| Meibomian gland expression | Expression grade >1.0* | ||
| Expressibility/volume/quality | 86/73 | ||
| Meibography | EDE ≥ 3 (83.0/90.0); Arita et al.3 | ||
| Confocal acinar unit density/diameter | Unit density <70/mm; Kobayashi et al.139; Matsumoto et al.140 (81/8) | ||
| Long diameter <65 μm (90/81) | |||
| Short diameter <25 μm (86/96) | |||
| Meibometry | NA | ||
| Interferometry | NA | ||
| Evaporation rate | DE <22 (51.1/89.9); Tomlinson et al.93 | EDE >22.3 (61.2/90.6) | EDE >27.5 (45.5/79.8) |
| Meibomian physicochemistry | |||
| Tear secretion: fluorometry, fluorescein clearance | DE <12.9 (74.5/73.6); Tomlinson et al.93 | EDE <15.1 (80.2/58.7) | ADDE <9.6 (69.5/96.8) |
| Tear volume: fluorimetry | NA | ||
| Tear meniscus height/radius/volume | DE <0.25 (74.5/73.6)-R; Yokoi and Komuro124 | ||
| DE <0.18 (72.8/66.6)-TMH; Farrell et al.141 | |||
| DE <9.6 (93.3/66.7); Mainstone et al.142 | |||
| Tear osmolarity | DE >31694 (69%/92.8%); Tomlinson et al.93 | EDE >315 (73%/72%); Khanal et al.118 | ADDE >325 (60%/39%); Khanal et al.118 |
| Tear dynamics/indices/evap/total flow | DE >15 (NA); Tomlinson et al.93 | EDE >15; Tomlinson et al.93 (na) | EDE >NA (NA) |
| Tear dynamics/indices/evaporation/TTR | DE >20 (NA); Tomlinson et al.93 | EDE >20 (NA); Tomlinson et al.93 | EDE >NA (NA) |
| Tear dynamics/indices/TFI | DE <96; Xu et al.143 | NA | |
| DE <240 (64.7/60) (83%/40%); McCann et al.144 |
The sensitivity and specificity of tests discriminating normals from dry eye and its subtypes are reported. DE, dry eye; EDE, evaporative dry eye; ADDE, aqueous-deficient dry eye; TFI, tear function index; FBUT, fluorescein break-up time; PRT, phenol red thread; RB, rose bengal; SCH, Schirmer; TBUT, tear breakup time; TTR, tear turnover rate; SENS, sensitivity; SPEC, specificity.
McCann LC, et al. IOVS 2008;49:ARVO E-Abstract 1532.
With this background in mind, a series of recommended tests to be used in the diagnosis of MGD and in MGD-related disorders, including evaporative dry eye, is presented as follows (Table 8).
- Tests for the Diagnosis of MGD
- In asymptomatic adults it is appropriate to include gland expression (e.g., by the application of moderate digital pressure to the central lower lid) to the routine work-up of the patient, to detect asymptomatic, nonobvious MGD.
- A diagnosis of MGD may require that the patient be further assessed for ocular surface damage and dry eye, by using appropriate diagnostic techniques.
- In patients with ocular surface symptoms or morphologic lid signs of MGD (e.g., orifice plugging and other orifice or lid margin signs), meibomian gland functionality should be assessed by digital pressure over the central (±nasal) third of the lower/upper lids, to determine the extent and severity of the MGD (expressibility and secretion quality). This procedure should be performed using moderate digital pressure or a standardized technique, in the manner outlined in the previous sections. The patient should be further assessed for evidence of ocular surface damage and dry eye.
- Tests for the Diagnosis of MGD-Related Dry Eye The Committee recommends a two-tiered approach:
- The first step is one in which normal subjects are discriminated from patients with dry eye of any type (generic dry eye).
- The second step involves the differential diagnosis of MGD-related evaporative dry eye from aqueous-deficient dry eye.
Two approaches are proposed: one suitable for practitioners working in a general clinic and the other for investigators working in specialized units. The evidence base of the tests proposed varies according to the clinical setting needs.
Table 8.
Specialized and Nonspecialized Tests for MGD and MGD-Related Disease
| Testing Category | Specific Test(s) | Tests for a General Clinic | Tests for a Specialized Unit |
|---|---|---|---|
| Symptoms | Questionnaires | McMonnies; Schein; OSDI; DEQ; OCI; SPEED etc. | McMonnies; Schein; OSDI; DEQ; OCI; SPEED etc. |
| Signs | |||
| Meibomian function | Lid morphology | Slit-lamp microscopy | Slit lamp microscopy Confocal microscopy |
| Meibomian gland mass | — | Meibography | |
| Gland expressibility | Slit-lamp microscopy | Slit lamp microscopy | |
| Expressed oil: quality | |||
| Expressed oil: volume | |||
| Lid margin reservoir | — | Meibometry | |
| Tear Film Lipid Layer | |||
| Thickness | Interferometry | Interferometry | |
| Spread time | Slit-lamp | Slit-lamp | |
| Spread rate | — | Video interferometry | |
| Evaporation | Evaporimetry | — | Evaporimetry |
| Tears | |||
| Osmolarity | Osmolarity | TearLab device, other | TearLab device, other |
| Stability | Tear film | TFBUT; OPI | TFBUT; OPI |
| TFLL | Spread time | Interferometry; spread rate; pattern | |
| Indices of volume and secretion | Tear secretion Tear volume Tear volume Tear clearance |
Schirmer 1 Not available Meniscus height TFI |
Fluorophotometry/FCR Volume by fluorophotometry Meniscus radius of curvature; meniscometry TFI |
| Ocular surface inflammation | Ocular surface staining biomarkers | Oxford scheme; NEI/Industry scheme | Flow cytometry; bead arrays; microarrays; mass spectrometry: cytokines and other mediators; interleukins; MMPs |
Tests of meibomian gland function are presented first followed by those for related disorders such as dry eye. See text for a recommended sequence of performance. DEQ, Dry Eye Questionnaire; FCR, fluorescein clearance rate; MMPs, matrix metalloproteinases; OCI, Ocular Comfort Index; OPI, Ocular Protection Index; OSDI, Ocular Surface Disease Index; SPEED, Standard Patient Evaluation of Eye Dryness; TFBUT, tear film breakup time; TFI, tear film index; TFLL, tear film lipid layer.
A. Diagnosis of MGD-Related Disease within a General Clinic
A suitable sequence of tests to perform in a general clinic, in patients presenting with symptoms of ocular surface disease is as follows:
Administration of a symptom questionnaire.
Measurement of the blink rate and calculation the blink interval (BI).
Measurement of lower tear meniscus height.
Measurement of tear osmolarity (if available).
Instillation of fluorescein and measurement of the tear film breakup time (TFBUT). Measurement is facilitated by viewing with a blue exciter filter and a yellow barrier filter. The diagnostic cutoff value for dry eye will be influenced by the volume instilled. The Ocular Protection Index145 can be calculated as the ratio of TFBUT/BI (blink interval). A value of <1 is pathologic and implies that tear breakup is occurring in the waking state. The lower the value the greater the degree of tear film instability.
Immediately after measurement of the TFBUT, fluorescein staining can be graded on both the exposed cornea and conjunctiva. When a yellow barrier filter has not been used, it will be necessary to grade conjunctival staining independently by using lissamine green. This grading may be performed after the Schirmer test.
-
Schirmer test or alternate (phenol red thread test).
A positive result (abnormal) from tests described in 1, 4, 5, and 6 provides partial evidence of the presence of generic dry eye, without specifying whether it is aqueous-deficient or evaporative. Evidence of aqueous-deficient dry eye may be obtained by measuring tear flow or an assessment of aqueous volume on the basis of tear meniscus height or Schirmer test.
- If MGD has not been characterized (symptomatic/ asymptomatic) at a previous visit, then it can be assessed at the end of this sequence as follows:
- Quantification of morphologic lid features.
- Expression: quantification of meibum expressibility/quality.
- Meibography: quantification of dropout.
If testing suggest the diagnosis of a generic dry eye and tests of tear flow and volume are normal, then evaporative dry eye is implied, and quantification of MGD will indicate the meibomian gland's contribution.
This test sequence also permits a diagnosis of symptomatic MGD, with or without ocular surface staining and with or without dry eye, to be made. The graded scores for each test can be used to monitor the disease during treatment.
B. Diagnosis of MGD-Related Disease within a Specialized Unit
An “ideal” or comprehensive test series is proposed for corneal specialists or for investigators engaged in clinical trials, in which they have access to a wider range of diagnostic equipment. Some of the tests listed are alternatives. It is suggested again that the diagnosis be made in two steps: First, diagnose generic dry eye and then subtype it with the grade of MGD.
This test series consists of a symptom assessment (Appendix 1; e.g., the OSDI,146 DEQ,147), a measure of tear osmolarity (Appendix 15), a tear secretion test (fluorophotometry or fluorescein clearance rate; Appendix 13), a measure of the volume of the tears in the eye (by fluorophotometry and meniscometry; Appendix 14), a stability test (the TFBUT or noninvasive TBUT, Appendix 2; or interferometry, Appendix 10), and a measurement of tear evaporation (by evaporimetry, Appendix 11). Tests of ocular surface damage, such as corneal and conjunctival staining (Appendix 4), are also included in the test series (Table 9). Tests of inflammatory mediators and the presence of inflammatory cell markers and other proteomic and lipidomic mass spectrometry analyses (Appendix 12) can also be assessed to provide information regarding overall ocular surface inflammatory status, although the link to MGD specifically is not known at this time. Specific measures of tear production (Appendix 3) for the diagnosis of aqueous-deficient dry eye are also recommended.
Table 9.
Staging the Severity of MGD and Individual Clinical Parameters
| Severity Level |
||||||
|---|---|---|---|---|---|---|
| Level 0 Normal | Level 1 Subclinical | Level 2 Symptomatic Minimal | Level 3 Symptomatic Mild | Level 4 Symptomatic Moderate | Level 5 Symptomatic Severe | |
| Symptom frequency and severity | No symptoms | Asymptomatic or occasional symptoms | Some of the time; precipitated by environmental factors | Half of the time; some limitation of activity | Most of the time; frequent limitation of activity | All of the time Severe/disabling/constant |
| OSDI grade range (0–100) | 0 | 0–12 | 0–12 | 13–22 | 23–32 | 33–100 |
| MGD Grade | Clear | Subclinical, nonobvious MGD; altered quality, only on expression; no gland loss | Minimally altered quality of expressed meibum from scattered glands; None to minor gland loss | Mildly altered meibum quality; occasional lid margin signs; mild gland loss | Moderately increased opacity and viscosity of meibum; plugging; increased marginal vascularity; loss of orifice definition; moderate gland loss | Marked, diffuse MGD; cicatricial or noncicatricial; multiple lid margin signs; lid deformity and marked lid margin hyperaemia; Severe gland loss |
| Quality of expressed meibum grade range 0–3, LL, 8 glands, Range (0–24)* | 0 | 1–5 | 6–10 | 11–15 | 16–20 | 21–24 |
| Treatment of MGD based on symptoms and gland status | + General advice about MGD, the potential influence of diet, home and work environment | + Hygienic measures, heat and massage | ±Topical ATs | +Oral tetracycline derivatives | ± Anti-inflammatories | |
| ±Hygienic measures | ± Emollient lubricant or liposomal spray | |||||
| ± Topical azithromycin | ||||||
| ± Consider oral tetracycline derivatives | ||||||
This table should be read in conjunction with Table 11, which provides a staging scheme for MGD-related ocular surface disease. Severity levels for each parameter are graded 1–5. A subclinical severity level has been introduced to accommodate asymptomatic MGD with normal lid margin features (nonobvious MGD) diagnosed only after gland expression. Note that this MGD scoring system does not provide a score for totally obstructed glands. Alternative systems for grading MGD exist and should be considered (see Appendices 5–7). Arita et al.3 graded meibomian dropout in the combined upper and lower lids, using noninvasive meibography, with a scale range of 0–6 (Table 3). de Paiva et al.106 used a composite system combining dropout, lid signs and meibum expressibility, with a scale range of 0–11 (Table 3). Korb and Blackie52 record the number of glands in a zone of 8, which yield a liquid secretion after standardized expression (MGYLS score 0–8). General treatment concepts, summarized here, are adapted from the Report on Management and Therapy. Recommended treatments are additive. At each clinical assessment, lack of response to treatment at the previous level moves treatment to the next level. ±, the decision to use this treatment is based on clinical judgment; +, treatment is recommended at this level. LL, lower lid; OSDI, Ocular Surface Disease Index.
The increase in severity of MGD with increase in grade is denoted by a reduced quality of expressed meibum. Meibum quality (clarity and consistency) is assessed in eight glands of the central third of the lower lid on a 0–3 scale for each gland: 0 = clear; 1 = cloudy; 2 = cloudy with debris; 3 = thick, like toothpaste (total score range 0–24).75
A Severity Scale for MGD and MGD-Related Disease, Including Dry Eye
It is critical to understand the severity of any disease to assess its burden to the patient, the efficacy of therapy, and the prognostic implications (Appendices 5–7). Assigning severity levels to a disease is difficult, because the various elements that comprise the disease complex are of different weight and may not move in parallel as the disease progresses. The committee acknowledges that information of this kind is not yet available to inform the development of a severity rating for MGD and related disease. However, it was considered to be important to offer a provisional framework that could be assessed in the future as described below.
In the preamble to this section, we suggested that MGD may be a symptomatic disorder in its own right, a disorder that causes ocular surface damage and one that causes evaporative dry eye, which in turn may cause surface damage. Because these disease components may progress at different rates, separate severity levels have been generated for MGD and for MGD-associated disorders, using symptoms as a bridge between the two.
Severity levels for the parameters discussed above are presented in Tables 9 and 10. Treatment aspects are dealt with briefly, and a fuller account can be found in the report of the Management and Therapy Subcommittee.
Table 10.
Staging the Severity of MGD-Related Ocular Surface Disease
| Severity Level |
||||||
|---|---|---|---|---|---|---|
| Level 0 Normal | Level 1 Subclinical | Level 2 Minimally Symptomatic | Level 3 Mildly Symptomatic | Level 4 Moderately Symptomatic | Level 5 Severely Symptomatic | |
| Symptom frequency and severity | No symptoms | Asymptomatic or occasional symptoms | Some of the time. | Half the time | Most of the time | All the time |
| Precipitated by environmental factors | Some limitation of activity | Frequent limitation of activity | Severe/disabling/constant | |||
| OSDI range (0–100) | 0 | 0–12 | 0–12 | 13–22 | 23–32 | 33–100 |
| TFBUT, s | ≥10 | <10 to ≥7 | <7 to ≥5 | <5 to ≥3 | <3 to ≥1 | <1 or instant breakup |
| Tear osmolarity, mOsM | Normal <308 | Normal <308 | Normal <308 | Mildly increased >308 to ≤313 | Moderately increased >314 to ≤317 | Markedly increased >317 |
| Conjunctival hyperemia | Nil | Minimal | Mild | Moderate | Marked | |
| CCLRU | Nil | Nil | CCLRU 1 | CCLRU 2 | CCLRU 3 | CCLRU 4 |
| Ocular surface staining | 0 | Nil | Minimal | Mild | Moderate | Severe |
| Oxford scale (0–15) | 0 | Nil | 0–3 | 4–6 | 7–10 | 11–15 |
| NEI Industry scale (0–33) | 0 | Nil | 0–7 | 8–14 | 15–23 | 24–33 |
| Schirmer score, mm | ≥10 | ≥10 | <10 to ≥7 | <7 to ≥5 | <5 to ≥3 | <3 |
| Treatment of MGD-related ocular surface disease | No treatment | No treatment | + Artificial tear substitutes | + Alternative AT selection | + Alternative AT selection | + Alternative AT selection |
| + Simple viscosity agents (preservatives allowable at low frequency of use) | + Immune modulation | + Gels and ointments | + Autologous serum | |||
| ± Punctal plugs | + Conserving spectacles | |||||
| ± Moisture conserving spectacles | + Surgical procedures | |||||
This should be read in conjunction with Table 9 which provides a staging scheme for MGD. Increasing MGD severity is perceived to lead to impaired spreading and stability of the tear film lipid layer, increased evaporative water loss, increased tear osmolarity, and ocular surface damage, which leads to conjunctival hyperemia and symptoms. These events are accompanied by inflammatory responses in the lids and on the ocular surface. Each measured parameter scales from least to most severe disease in five levels of severity. The numerical divisions are literature based, but require further validation. In an individual patient, it is unlikely that stages will lie in register for each parameter, but a global score can be generated by summing grades within the levels. Extensive MGD can be a cause of evaporative dry eye rather than aqueous-deficient dry eye. However, the Schirmer test is included in the battery of tests, to allow for the coinciding occurrence of both conditions. Treatment is based on symptoms, ocular surface damage and disturbed tear dynamics. For details, see the Report on Management and Therapy. Recommended treatments are additive at each level. At each clinical assessment, lack of response to treatment at the previous level moves treatment to the next level. ±, the decision to use this treatment is based on clinical judgement; +, treatment is recommended at this level. MGD, meibomian gland dysfunction; AT, artificial tears; CCLRU, Cornea and Contact Lens Research Unit (School of Optometry and Vision Science, University of New South Wales, Sydney, Australia); NEI, National Eye Institute; OSDI, Ocular Surface Disease Index.
Overall Recommendations.
The recommendations of the Diagnostic Subcommittee are as follows and are summarized in Table 11:
Table 11.
Assessment of Meibomian Gland Function
| Based on Meibomian Gland Expression |
| Grades and comments |
| Methods: A plus Ba or Bb |
| A. Meibum quality: LL or UL, central 8 glands |
| 0 = clear fluid |
| 1 = cloudy fluid |
| 2 = cloudy particulate fluid |
| 3 = inspissated, like toothpaste. |
| Ba. Meibum expressibility: LL or UL, central 8 glands |
| 0 = all glands expressible |
| 1 = 3–4 glands expressible |
| 2 = 1–2 glands expressible |
| 3 = no glands expressible |
| Bb. Meibum expressibility: LL nasal or central eight glands |
| The MGYLS score is the number of Meibomian Glands of eight, Yielding Liquid Secretion. |
| Assessment by gland expression |
| ≤20 years: A score of greater than 1 for quality or expressibility (A; B) is abnormal. |
| >20 years: A score of 1 for either quality or expressibility is acceptable as normal; a score of 1 for both, or of >1 for either, is abnormal. |
| For research and some clinical trial purposes, the utility of this approach would be enhanced by generating a composite score derived from the expression of the LL, UL nasal and central zones. |
| Based on Meibomian Gland Dropout |
| Meibography: The technique of meibography offers an excellent opportunity to refine the quantification of gland loss by digitizing the images. It should be noted that estimates of 'gland loss' are based on an assumption of the original size of ea ch gland. Therefore estimates of residual gland area will be more accurate, although relevant to a particular individual. There is a need for detailed age/sex stratified information about gland area. |
| A precise description of any technique proposed must be given. For example, if the term 'partial gland loss' is used, this must be defined. An estimate of loss is based on the presumed, intact length of each gland. Training would be enhanced by the use of videos showing both the performance of the technique of method of scoring in use. |
| Either contact or noncontact meibography can be used. |
| Method C: LL 15 glands: A partial gland is one that is incomplete and present in clumps or clusters. |
| 1 = no partial glands (PGs) |
| 2 = <25% PGs |
| 3 = 25%–75% PGs |
| 4 = >75% PGs |
| An aggregate score from the combined LL/UL would expand the scale |
| Method D: (nasal half, lower eyelid)* |
| 0 = no dropout |
| 1 = ≤25% |
| 2 = ≤50% |
| 3 = ≤75% |
| 4 = ≤100% |
| Based on Meibography |
| ≤20 years. Method C. Normal is 0 |
| >20 years. Method C. 1 = ≤25% is acceptable as normal; >1 is abnormal |
| Diagnosis of MGD-related disease, including dry eye: |
| In the general clinic. See Table 8 |
| In a specialized clinic. See Table 8 |
MGD Criteria for Specific Purposes
|
de Paiva et al.106 have also devised a composite score including lids signs and expressibility, with a scale range of 0–11.
MGD is a common disorder that may be asymptomatic or give rise to symptoms, either confined to the affected lids or arising from MGD-related ocular surface disease, including evaporative dry eye. It can also exacerbate aqueous-deficient dry eye.
The natural history of MGD is not precisely known; for practical purposes it should be regarded as a progressive but treatable disease in which therapy may prevent irreversible changes. This approach is a safe one that can be modified as further information becomes available.
Therapy is based on diagnosis and a decision to treat depends on the severity of disease. While simple diagnosis is straightforward, quantification of the degree and severity of MGD, which is the basis for treatment, is more complex.
A two-step approach to diagnosis is recommended in symptomatic patients. The assessment of meibomian gland function is based on lid morphology, gland dropout, meibum expressibility, TFLL appearance, and tear evaporation. A diagnosis of dry eye is established from measures of tear production and clearance, tear osmolarity and tear film stability, and the presence of ocular surface changes by tissue staining and perhaps further characterized by the presence of inflammatory biomarkers. Patients with symptoms of ocular surface disease should be assessed for ocular surface damage and for abnormalities of tear dynamics characteristic of dry eye (Table 8).
Quantification of MGD is based on grading meibum quality and expressibility. When the presence of MGD is more than trivial and treatment is instituted, the score should be noted and repeated periodically at follow-up. An aggregate score derived from the expression of upper and lower, central, and nasal lid zones should be considered as a method of monitoring the response to treatment. Newer, quantitative methods of expression may make grading more accurate in the future.
Although such grading approaches have been used to differentiate mild from severe disease, their repeatability is unknown, and therefore their value in demonstrating small changes in disease severity is unknown. There is good evidence that meibomian gland dropout is closely associated with MGD severity. It is therefore recommended that, when possible, baseline measurements of gland dropout be made by using meibography. Baseline measurements can be used for stratification purposes in clinical trials, but when such trials are extended, or in natural history studies or where meibomian gland damage occurs as an adverse event, they may provide a record of change over time.
IV. Appendices
Method of Working
Each reviewer used the following format when analyzing the diagnostic tests:
Identify the test.
Provide rationale for use.
Describe each of the techniques used in full detail.
Wherever available, test values for normals, MGD and dry eye were identified, together with the sensitivity and specificity of the test and recommended or reported diagnostic cutoff values. In those cases in which published papers included the diagnostic effectiveness of the test, these values were included in the reviewer's report. Throughout the appendices, in the tables the following abbreviations are used: N, normal subject (i.e., no dry eye); DE, dry eye; EDE, evaporative dry eye; ADDE, aqueous-deficient dry eye.
Appendix 1
Test Identification: Symptom Questionnaires
A wide range of questionnaires have been used to assess the symptoms of ocular discomfort associated with dry eye conditions.148–152 Extensive reviews of the utility of these questionnaires have been published elsewhere.153,154 Despite the numerous questionnaires available, those most commonly used show good agreement.155
Rationale
Questionnaires allow the assessment of a range of symptoms associated with ocular discomfort. However, due to the commonality of symptoms across a range of disorders including dry eye and MGD,156 these questionnaires are unlikely to be able to differentiate between etiologically distinct disease entities. Despite this limitation, some studies have looked at the role of questionnaires in assessing symptoms in MGD.
Method and Description
Studies have shown that MGD (diagnosed by gland orifice plugging and lid margin telangiectasia) is present in 61.7% of symptomatic patients.157 This was in close agreement with other studies where 63.6%158 and 64.6%159 of symptomatic subjects were found to have signs of MGD. A higher proportion of MGD sufferers were found (74.3%) among symptomatic video display unit (VDU) users but this is likely to be due to the population studied. Interestingly, in this VDU population MGD sufferers did not exhibit more severe symptoms than subjects with no evidence of MGD.160 Further evidence for the role of MGD in producing symptoms comes from the observation that a statistically significant negative correlation was observed between the number of meibomian glands capable of yielding liquid secretion and symptom score.161
It is clear from these studies that MGD is present in excess of 60% of patients with ocular discomfort. Current questionnaires have not been optimized or tested in their ability to differentiate between MGD and other causes of ocular discomfort. Undoubtedly, further studies are needed, particularly to assess the sensitivity and specificity of symptom questionnaires in the diagnosis of robustly defined MGD patients. Because of the commonality of symptoms with other disorders and the lack of a pathognomonic symptom in MGD, it is likely that questionnaires, although useful, will have to be used in conjunction with other methods in the characterization and diagnosis of MGD.
Of interest, the Ocular Surface Disease Index (Allergan Inc., Irvine, CA) has been recently validated across ocular surface disease severity,162 and while the symptoms may be different in MGD, it can be hypothesized that the OSDI could be used to document disease progression. The ocular surface disease ratings are as follows across the scale of the questionnaire (0–100): normal, 0–12; mild, 13–22; moderate, 23–32; and severe, 33–100, with a seven-unit change noted as clinically significant.162
Appendix 2
Test Identification: Fluorescein/Noninvasive Breakup Time
Breakup time is thought to be a surrogate measure of tear stability.
Rationale
Tear breakup time (TBUT) is generally regarded as a test for diagnosis of evaporative dry eye; however, as discuused herein, TBUT testing is relevant in the diagnosis of MGD. Tear film instability is one of the core mechanisms of dry eye and may be the initiating event.163 It is dependent on many factors, including an adequate tear film lipid layer,164–171 which in turn is dependent on meibomian gland function.166,172,173 There is strong evidence to suggest that both lipid quantity and quality correlate with meibomian gland function and dry eye states.165–169,172–178 Hence, low TBUTs imply a possibility of lipid layer compromise and thus meibomian gland dysfunction, whereas high TBUTs suggest a normal lipid layer and adequate meibomian gland function.164,168,170,171 Thus, whenever TBUT is low, meibomian gland function and expressibility should be investigated for diagnosis and in considering treatment.
Description: Fluorescein Breakup Time
Tear film stability is measured by a test of fluorescein breakup time (FBUT), defined as the time to initial breakup of the tear film after a blink.179 It has been proposed that fluorescein breakup can be caused by quenching of fluorescence related to the increase in fluorescein concentration caused by evaporation.180 The classic and usual method to determine breakup time utilizes fluorescein to stain the tear film (FBUT).181–183 The fluorescein may be applied by wetting a commercially available fluorescein-impregnated strip with sterile saline and applying to the inferior fornix or to the bulbar conjunctiva. After instillation, the patient is asked to blink several times and to move the eyes, to mix the fluorescein in the tears. Observation is with the slit lamp, a cobalt blue filter, a beam width of approximately 4 mm, and full height, and the beam is slowly moved from side to side to cover the entire cornea.182,183 A yellow barrier filter enhances observation of the fluorescent tear film.179,184 The patient is instructed to blink naturally and then, once homogeneous tear film fluorescence is confirmed, to keep the eyes open while looking straight ahead. The time from upstroke of the last blink to the first tear film break or dry spot formation is recorded as the FBUT measurement. Either one or the average of three consecutive trials is the final value.178,179,182,184,185 Optionally, a video camera may be used to record TBUT with various methods used to automate timing and permit masking of the measurement.179,184 An alternative to the use of fluorescein-impregnated strips is the instillation of liquid unpreserved fluorescein onto the bulbar or conjunctival conjunctiva with a micropipette. Concentrations of 2% to 5% and volumes of 1 to 5 μL have been advocated179,186 (Welch D, et al. IOVS 2008;49:ARVO E-Abstract 2485). The observation procedure is the same as for the fluorescein-impregnated strip.
In performing a series of clinical tests for dry eye, measurement of the TFBUT is usually followed by measurement of fluorescein staining. There is a distinct advantage in completing the series in the right eye, before instilling dye and performing the series in the left eye, since this avoids dilution of dye in the tear film and diffusion of dye taken up into the ocular surface of the second eye (Appendix 4).
Variations in the Technique of Administering the FBUT Test.
Despite the acknowledgment of the value of quantification of tear film stability, FBUT has been criticized as being inaccurate and poorly reproducible186–190 (Welch D, et al. IOVS 2008;49:ARVO E-Abstract 2485). The inherent nature of a large fluorescein-impregnated strip and the lack of a standardized procedure for moistening and applying the strip to the tear film prevents control of the volume delivered to the tear film and must result in variability. There is no agreement as to whether the moistened strip should be shaken before instillation or whether the strip should be applied to the superior, inferior, temporal, or inferior temporal bulbar conjunctiva or to the tear meniscus.169,178,182,185–187
The greatest source of variability in FBUTs relates to the volume of fluorescein delivered. FBUT measurement reliability is increased when 2 μL or less of a 5% fluorescein solution is applied with a micropipette versus the conventional strip method.179,186 Although micropipettes offer a precise method of instillation of microliter quantities of fluorescein, the use of unpreserved fluorescein solutions in the clinical setting requires sterile procedures, and while these prodecures are applicable to research studies, they are not readily adaptable to clinical practice. Further, FBUTs are altered by reflex tearing, and the use of a pipette frequently causes apprehension in the patient and reflex tearing. A novel approach to both reducing the volume of fluorescein and eliminating sensation and reflex tearing during FBUT measurements, the Dry Eye Test (DET; Nomax, Inc., St. Louis, MO) was developed to deliver 1 μL of fluorescein solution to the tear film by application to the superior temporal bulbar conjunctiva.184 The DET is applicable in either research studies or clinical practice.179,184
Recommendations for Conduct of the FBUT Test.
Either the micropipette or the DET strip is applicable for research studies. The micropipette method should be standardized for volume and concentration of fluorescein. Recommendations for volume have varied from 5 to 1 μL and for concentration from 1% to 5%.179,186 The DET strip provides a standardized method to deliver 1 μL of volume.185
Estimated Values of FBUT in Normal, Dry, and MGD Eyes.
There are no reported estimated values for subjects with only MGD specifically.
With traditional volumes of fluorescein, FBUTs in normal subjects are >10 seconds versus ≤10 seconds in those with dry eye.179,191 With micro volumes of fluorescein, FBUTs in normal subjects are >5 seconds versus ≤5 seconds in dry eye.179,192
Sensitivity and Specificity.
The sensitivity and specificity of the FBUT test are reported to be 72% and 62%.169
Method and Description: Noninvasive Breakup Time Measurement
Noninvasive breakup time (NIBUT) measurement utilizes a grid or other pattern projected onto the precorneal tear film for the observation of distortion and/or abnormalities in the image.189 The patient is instructed to blink normally while looking straight ahead. The time interval in seconds from the upstroke of a blink to the first change of the image after a blink is defined as the NIBUT. The result of either one or the average of three consecutive trials is the final value.178,179,182,184,185 Optionally, a video camera may be used to record TBUT with various methods to automate timing179 (Welch D, et al. IOVS 2008;49:ARVO E-Abstract 2485).
The NIBUT test eliminates physical disturbance of the tear film from the instillation of fluorescein, along with the possibility of inducing tactile reflex tearing.169,179,188,189 NIBUT would therefore appear to be an ideal theoretical method of evaluating tear film stability, because it overcomes the objections to fluorescein invasive FBUT measurement and can provide more reliable and reproducible results. The TBUTs obtained with the NIBUT test are significantly greater than those values found with the FBUT,169,170,182,193–195 which has been attributed to the destabilizing effect of the instilled fluorescein. A study advocating the use of the NIBUT test for the diagnosis of mucous layer deficiencies and for distinguishing between aqueous tear deficiency and MGD nevertheless stated that the NIBUT test did not replace the FBUT test as the test of choice for the evaluation of tear film stability.169
Estimated Values of NIBUT in Normal, Dry, and MGD Eyes.
The normal range for NIBUT is typically 40 to 60 seconds193 compared with normal ranges of 10 to 34 seconds181,194for the FBUT. For dry eye NIBUT ≤10 seconds.190 Reported values of NIBUT vary significantly between investigators and equipment used, with the values of NIBUT remaining higher than those for FBUT.170,195 It has been suggested that the two methods measure different phenomena.169 There are no specifically reported estimated values for subjects with only MGD.
Sensitivity and Specificity.
The sensitivity and specificity of the NIBUT test are reported to be 82% and 86%.189
Appendix 3
Test Identification: Schirmer test (in the Diagnosis of MGD)
The Schirmer test is traditionally a measure of tear production when performed for the recommended 5 minutes, although some research indicates that the test, when administered for shorter durations, may be a measure of tear volume on the ocular surface.
Rationale
The Schirmer test may not be a direct test of MGD; however, it is useful in the differentiation of aqueous-deficient dry eye and evaporative dry eye, both of which may occur concurrently with MGD. Although MGD may be a causative factor in evaporative dry eye, aqueous-deficient dry eye can occur simultaneously.
Method and Description
The Schirmer test without anesthesia is a well-standardized test performed with the patient's eyes closed.196 There is wide intrasubject, temporal, and visit-to-visit variation, but the variation and the absolute decrease in aqueous deficiency are mostly due to the decreased reflex response with lacrimal failure. When the cutoff value is set at <5.5 mm/5 minutes, the sensitivity and specificity of the testing are 85% and 83%, respectively.197
The diagnostic cutoff used at present is <5.0 mm in 5 minutes, the reason for which is still unclear. Lowering the cutoff decreases the detection rate (sensitivity), but increases the specificity of the test.196 The repeatability of the Schirmer 1 test appears to be better with lower values (i.e., in more severe aqueous deficiency).198 A significant correlation was shown between Schirmer 1 test, tear stability and fluorescein staining in a recent study by Nichols et al.199 Meibomian gland disease greater than grade 1 (according to the method of Bron et al.196) did not appear to correlate with the Schirmer 1 result, tear meniscus height, phenol red test, and staining with vital dye in that study. Nichols et al.200 reported a poor correlation between Schirmer 1 test and dry eye symptoms. Many studies showed that no significant differences existed in tear quantity (Schirmer 1 test values) between patients with simple MGD and healthy control subjects, suggesting the difficulty of differentiating MGD patients from normal subjects based only on tear quantity.201–204 Shimazaki et al.205 and Goto et al.206 found no differences in Schirmer 1 test scores between patients with Sjögren's syndrome and those with non-Sjögren's syndrome dry eye. However, the presence of MGD in association resulted in much more severe ocular surface disease characterized by higher fluorescein and rose bengal vital staining scores or tear evaporation. Den et al.207 found that changes in the lid margin, including vascular engorgement, irregularity, plugging of MG orifices, and replacement of the MCJ were closely related to aging and that there was an age-related decrease in tear quantity scores assessed with Schirmer 1 test. Subjects with Schirmer test scores <5 mm had significantly decreased meibomian gland expressibility grades. Arita et al.208 found an age-related decrease in Schirmer 1 test scores and meibomian gland dropout grades in a recent study. Sterile Schirmer test strips were used to collect meibomian oil in healthy individuals (n = 20), dry eye patients (aqueous-deficient; n = 32) and MGD patients (n = 25) after gentle massage of the lid margin in another study.209 Meibomian fatty acids were directly transmethylated and analyzed by using gas chromatography (GC) and GC mass spectrometry. Meibomian fatty acids were similar in healthy individuals and in dry eye patients with aqueous deficiency, but were different in MGD patients, who showed significantly higher levels of branched-chain fatty acids (29.8% vs. 20.2%) and lower levels of saturated fatty acids (9.3 vs. 24.6%)–-in particular, lower levels of palmitic (C16) and stearic (C18) acids. The increase in branched-chain fatty acids may reflect greater quantities of wax and cholesterol esters and triglycerides in meibomian gland excreta. It was concluded that meibomian fatty acid composition and particularly the increase in branched chains evaluated with Schirmer strips could be a marker for meibomian gland dysfunction. The methodology also proved to reflect treatment effects by oral minocycline treatment suggesting iso-C20 (extracted from Schirmer strips) to be a useful biomarker for the diagnosis of MGD.210
Test Identification: Phenol Red (Cotton Thread) Test
The phenol red test (PRT) test has been developed as an alternative to the Schirmer test and is another method of analyzing a patient's lacrimal system.
Rationale
The PRT tear test represents another approach to the analysis of a patient's lacrimal system. It was developed to overcome the disadvantages of the Schirmer tear test, including variable results, poor repeatability, and failure to measure basal secretion, even when used with anesthesia.
Method and Description
Although the PRT method is quite similar to Schirmer, there are distinct differences. There is little or no sensation of the thread, making anesthesia unnecessary. A test time of only 15 seconds is required in comparison to the 5 minutes per eye needed for the Schirmer test. This test is performed with the patient's eyes open while blinking naturally. The length (in millimeters) of the wet portion of the thread is recorded as the result. Because of the short test time and minimal sensation of the thread, it is theorized that this test gives an indication of amount of residual tears located primarily in the inferior conjunctival sac of the eye.211 Using a cutoff value of 12 mm, the sensitivity and specificity of the PRT are 56% and 69%, respectively. Even if the agreement with the Schirmer 1 test is highly significant, 32% of patients have discordant results. These two methods of functional assessment of tear secretion seem to be complementary, and further studies remain necessary to better understand the correlation of both tests in clinical practice.212 A recent study found a weak agreement between Schirmer test and phenol red thread tests and between each test and symptoms of dry eyes.213 To determine the clinical viability of a phenol red–impregnated cotton thread in differentiating between normal, aqueous deficient, and non–aqueous-deficient dry eyes, Patel et al.214 recruited subjects on the basis of subjective symptoms, tear stability, rose bengal staining, Schirmer test, conjunctival hyperemia, patency and number of meibomian glands, presence of mucin strands, and appearance of lower tear meniscus. Based on the outcome of the tests, the subjects were categorized as having aqueous-deficient dry eye, non–aqueous-deficient dry eye, or normal eyes. Subjects were randomized, and a thread was applied by inserting it into the lower fornix of the right eye and leaving the thread in place for 120 seconds. The mean thread-wetting values were 15.5 ± 4.7 mm in aqueous-deficient dry eyes (n = 35), 22.7 ± 5 mm in non–aqueous-deficient eyes, and 19.4 ± 5 mm in the normal eyes (n = 38). For the aqueous-deficient and non–aqueous-deficient dry eyes only, when a cutoff value of 20 mm was used, the calculated sensitivity and specificity were 86% and 83%, respectively.196,214 PRT was found not to have any correlation with MGD (in patients with more than grade 1 MGD as classified by Bron's grading scheme) by Nichols et al.199 The test was also found to have poorer repeatability than the Schirmer test.198 The test was removed from Japanese dry eye diagnostic criteria on the founder's request due to low repeatability and wide variation in scores.214
Appendix 4
Test Identification: Ocular Surface Staining
Rationale.
Ocular surface damage is encountered in association with MGD and is found in its most advanced form in MKC. Various etiologies have been proposed for such damage, including the release of inflammatory mediators into the tear film215,216 and the mechanism of evaporative dry eye.217 One source of such mediators includes the breakdown products of meibomian lipids altered by the lipases of microbial commensals.218–221
Ocular surface damage may be quantified by grading staining of the cornea and conjunctiva by using selected dyes, by immunohistochemistry, or by flow cytometry on impression cytology specimens and the direct measurement of inflammatory mediators in the tears biochemically or with multiplex beads, matrix assisted-laser desorption ionization (MALDI)-TOF (time of flight), and proteomic techniques222–224 (Reinoso R, et al. IOVS 2009;50:ARVO E-Abstract 517; Topcu Yilmaz P, et al. IOVS 2009;50:ARVO E-Abstract 3592; Calonge M, et al. IOVS 2009;50:ARVO E-Abstract 2548); Nichols KK, et al. IOVS 2009;50:ARVO E-Abstract 541).
Additional approaches include confocal microscopy (Appendix 8). These techniques have helped to describe the ocular surface phenotype in MGD and other ocular surface diseases and to monitor the severity of disease and response to treatment, but the events that they record are not specific to MGD, and therefore they have no unique role in diagnosis. The precision of such tests was addressed in the 2007 DEWS report,196 where details of their sensitivity and specificity in the diagnosis of dry eye is summarized and incorporated, both in the published templates and in additional materials available on the TFOS web site. In addition to the DEWS Management and Therapy report,225 ocular surface staining was cited as an important diagnostic criterion by Behrens et al.226 in the clinical diagnosis of ocular surface disease.
Method and Description
Grading Ocular Surface Staining.
Several grading schemes have been reported and are discussed below.
van Bijsterveld.
One drop of rose bengal 1% is instilled. Staining is graded 0 to 3 on the cornea and for two exposed conjunctival segments (range: 0–9).
| Cut-off: ≤3.5 | N vs. DE | N vs. EDE | ADDE vs. EDE |
|---|---|---|---|
| Sensitivity/specificity | 95% vs. 96% | NA | NA |
In the European/American criteria for the diagnosis of Sjögren Syndrome231 a 2.5 μL solution of rose bengal is instilled in the lower fornix. Grading is according to van Bijsterveld 1969.227 The sensitivity/specificity of rose Bengal staining is as follows:
| Cut-off: ≤4 | N vs. DE | N vs. EDE | ADDE vs. EDE |
|---|---|---|---|
| Sensitivity/specificity in diagnosis of SS | 63% vs. 84% | NA | NA |
NEI/Industry Schema.
Nichols et al.232 used a modified version in dry eye diagnosis. The grading proforma presents five corneal and 2 × 3 conjunctival zones. Grades are 0 to 3 per zone, including 0.5 steps. Fluorescein or rose bengal is instilled from impregnated strips in control and dry eye subjects. There is strong agreement between corneal and conjunctival staining.
A revised version of this test, incorporating features of the NEI/Workshop grading system and the Oxford system (dubbed the Oxford version 2),230 was presented as a poster at a Tear Film and Ocular Surface meeting (Taormina, Italy 2006).The purpose was to provide a finer scale and to standardize the conduct of the test. The NEI/Industry system has been modified to (1) standardize the size and location of recording zones and (2) to create panels of random dots whose increasing density in numbers from panel to panel is mathematically defined. To do this (1) a series of 10 panels is generated, with the probability P that a pixel would be black given by P = exp(0.9 · a)/exp(0.9 · b) − k, where a is the number of the current panel, b is the number of the last panel + 1, and k = 0.00042. So that the dot size on the printed panels approximates that of a staining point on the ocular surface, the panels are scaled in a word processing program (MSWord; Microsoft, Redmond, WA), so that the dot size reflects the apparent size of an epithelial cell at the magnification used at the slit lamp. The grading range for each zone is from 1 to 10. Therefore, since the number of zones scored is 2 × 3 conjunctival and five corneal, the grade range is from 0 to 110. The system has not yet been validated.
Oxford Grading System.
In this system the cornea and two conjunctival zones are graded, with a grade score 0 to 5 per zone (total range, 0–15). In an intra-observer study, two trained ophthalmologists graded a series of standard slides, showing corneal and conjunctival fluorescein staining, on two separate occasions. In an inter-observer study the same two observers graded fluorescein (blue exciter; yellow filter) and rose bengal in 13 dry eye patients at an interval between 2 and 3 weeks. The κ values were in the good to excellent range in the assessment of the standard slides. In dry eye patients, the κ values were in the excellent category for for cornea, but in the fair category for conjunctiva.233
Additional studies have been undertaken to explore the utlility of batteries of tests, including symptom questionnaires, ocular surface staining, Schirmer test, fluorescein clearance test (FCT), and corneal sensitivity, in the diagnosis of dry eye. These are summarized under the heading of mixed tests in the dry eye templates published in the DEWS (2007) report. In a study by Afonso et al.234 in patients with irritative ocular surface symptoms, meibomian gland dropout or orifice metaplasia correlated significantly with reduced fluorescein clearance and inversely with the Schirmer result.
Appendix 5
Test Identification: Signs of MGD in Lid Morphology
Rationale.
The classification of MGD is based on clinical findings. Some recent classifications have used a terminology based on functional and microbiologic associations.235–239 McCulley et al.240 have suggested that the clinical spectrum of chronic blepharitis has changed and that the relative prevalence of Staphylococcus aureus, alone or in combination with seborrheic blepharitis, has decreased. In the literature and clinically, it has been hypothesized that the relative prevalence of seborrheic blepharitis has increased, with or without associated excess meibomian secretions (meibomian seborrhea) or inflammation (meibomitis). The terminology used clinically has been inconsistent, and the The Report on Definition and Classification has the specific purpose of unifying terminology. Nonetheless, clinically, primary meibomitis appears not to be a primarily infectious entity but to represent a facet of generalized sebaceous gland dysfunction found in association with seborrheic dermatitis or acne rosacea. These entities are recognized as chronic diseases requiring control for which there is no cure.
Additional attempts have been made to incorporate morphologic features of meibomian abnormality occurring in the gland acinus, duct, or orifice.241 Before meaningful classification of MGD morphology can be performed, it is important to define the normal anatomy of the lid and meibomian gland apparatus and its associated age-related changes.242 The appearance of the normal lid then can be used to provide a basis for a morphologic classification of posterior blepharitis, enabling better correlation with MGD and the earlier recognition of the diseased lid. Clinical descriptions of lid margin changes across age (children to elderly) are summarized below.235–246
Lid Margin.
The lid margin thickness has a normal range for adults and children. The lid margin in adults is 2 mm thick at its free edge and has lashes on its anterior aspect. Lid margin thickness in children ranges between 1.43 and 1.63 mm in the upper lid and 1.41 and 1.61 mm in the lower one. From adolescence onward, lid thickness increases to between 1.88 and 2.02 mm in the upper lid and 1.81 and 1.93 mm in the lower lid.242 The lid thickening that apparently occurs after childhood may be related to enlargement of the orbicularis muscle. Hormone-induced enlargement of sebaceous glands at puberty and could affect the meibomian glands.
Lid Vascularity.
The lids of children are typically less vascular, with no telangiectasia, cutaneous hyperkeratinization, or squamous blepharitis. The absence of lid margin vascularity in children is striking, and the increase from adolescence may be secondary to increased MGD. In the elderly, telangiectasia, and cutaneous hyperkeratinization are significantly more common in the lower lid. This perhaps reflects increased exposure of the lower lid to various insults, including ultraviolet radiation. The increased prevalence of upper lid margin rounding in the elderly has not been thought of as a physiological finding and is generally considered to be more common in the lower lid, in association with posterior blepharitis and subconjunctival fibrosis. Other factors, such as exposure at work to dust particles, urbanization, and cosmetics may be important.
Cilia.
The cilia count in the sagittal plane does not change significantly with age; however, it is a clinical impression that loss of cilia occurs in elderly patients, as does hair loss elsewhere in the body.
Mucocutaneous Junction.
The MCJ is constant in position immediately posterior to the meibomian gland orifices. The MCJ lies at the junction of the anterior two thirds and posterior one third of the lid, but may run an irregular course in normal elderly persons. No significant age-related changes in the position or form of the MCJ have been noted. Changes are typically seen in disease states, particularly MGD, acne rosacea, and severe atopic eye disease.
Orifices.
The meibomian gland orifices are situated just anterior to the MCJ. The orifices are round, are rarely narrowed or pouted, and no orifice obliteration or retroplacement occurs. They may be congenitally absent, in association with the underlying gland and have been described as plugged by keratin and desquamated epithelial cells, damaged, or patent in a nonsecretory, resting phase. Plugging may eventually lead to obliteration of orifices with atrophy of gland and duct. Meibomian gland orifice narrowing and pouting showed an age-related increase in frequency. Pouting is a feature previously reported in chronic blepharitis. Orifice narrowing and pouting probably represent hypertrophy and keratinization of duct epithelium. Pouting of orifices in asymptomatic individuals may be a feature of the aging lid as well as an early sign of MGD. Narrowing of the orifices increases with age, and the associated change in the shape of the surrounding epithelial cuff suggests that there is uneven distribution of tissue stress in the coronal and sagittal planes of the lid margin. Orifice obliteration is significantly increased with age in the upper lid. It has been reported in MGD, acne rosacea, and severe mucous membrane disease, such as trachoma and cicatricial pemphigoid, in which secondary MGD occurs, but not in normal subjects.
Main Duct.
The glands themselves can be seen as yellow streaks through the tarsal plate in young people. They have a main duct opening on the lid margin at a meibomian gland orifice and 50 to 60 lateral ductules leading to a single or composite acinus. They are modified sebaceous glands; the upper lid contains approximately 30 and the lower 20. The upper lid glands are longer (10 mm) than the lower (5 mm).
Acini.
These will be described in the section on meibography.
Tarsal Plate.
Lower lid conjunctival hyperemia occurs with increasing frequency in elderly patients.
Secretions.
A significant decrease in the quantity of secretion occurs with age, with fewer orifices freely expressing meibomian secretions. However, the decrease is usually not accompanied by an increased opacity or viscosity of the secretions, suggesting that these may represent markers of disease and result in the typical plugging of meibomian gland orifices in MGD.
Clinical Anatomy in MGD
Lid Margin.
Thickening of the lid is a common feature of meibomian gland disease, but is difficult to measure because of the rounded contour of the anterior margin. It is best measured from the posterior margin to the posterior lash line, which are relatively constant features of the lid. Rounding of the posterior lid margin is often associated with thickening and interferes with the normal apposition of lid to globe. Vascularization increases with age. In MGD, there is an exaggeration with invasion of the outer and then inner cuffs of the orifice. Hyperkeratinization is an eczematous appearance of the cutaneous margin, frequently encountered in atopes with facial eczema, but also in nonatopic subjects. Irregularity of the lid margin arises from absorption of tissue, often in the region of obliterated meibomian orifices, but will occur with more gross distortions of lid architecture in cicatricial and ulcerative lid disease.
Mucocutaneous Junction.
The MCJ location and morphology may be altered in MGD. The MCJ is best identified by its specular reflection. Although the position of the anterior edge of the tear meniscus may correspond with it in health, in disease it may not be an accurate guide.
Anteroplacement. The junction becomes irregular in MGD. The mucosa may spread forward, so that the orifices appear to lie in mucosal tissue.
Retroplacement. There is a posterior movement of the MCJ, with a spreading, keratinizing, squamous metaplasia of the posterior lid margin that extends onto the tarsal plate. The meibomian orifices may or may not move with the MCJ, which will determine whether the tear oil is delivered onto the surface of the tear film. Retroplaccment is more common than anteroplacement.
Mucosal absorption. This may occur without retroplacement of the MCJ so that the MCJ and orifices are still at the same distance from the lash line, but come to lie closer to a new posterior lid margin.
Ridging. There is a ridgelike elevation of the MCJ or of tissue between the orifices. It may also be a secondary effect of mucosal absorption.
Orifices.
Orifices demonstrate several presentations in MGD.
Number. The orifices may be reduplicated, or reduced in number, congenitally, sometimes as part of a syndrome, or as an acquired feature of MGD.
Capping. Scattered orifices may be capped by a dome of oil with a tough surface, but may be pierced by a needle tip to release the oil. The underlying orifice may be ulcerated and the cap epithelialized. Capping usually affects only occasional orifices and may be found in otherwise normal lids.
Pouting. An early sign of MGD is the elevation or pouting of the orifice, which is no longer flush with the surface. The term is probably equivalent to plugging. The meibomian orifice may be dilated, and expression may demonstrate the terminal ductule plugged with inspissated secretion or other material.
Retroplacement. This term is used to describe the result of a cicatricial process involving the posterior lid margin and may be associated with more extensive cicatricial changes within the tarsal mucous membrane near the marginal mucosa. The orifices may become ovally elongated at right angles to the plane of the lid margin, and posterior movement may be accompanied by duct exposure.
Obliteration narrowing. The punctum of the orifice may not be visible. The appearance of narrowing is accompanied by absent expressibility of lipid. Loss of definition of the cuffs of the orifices is a feature that is seen with age and in early MGD. Vascular invasion may accompany the process of loss of definition.
Opaque orifices. The degree of opacity of the inner cuff becomes accentuated. Opaque orifices are far more visible at the lid margin than normal. Scarring of the region of the orifices may occur, with tissue loss and depression of the surface. It is often accompanied by a range of degenerative changes at the lid margin.
Duct exposure. Exposure of the terminal duct of the gland in varying degrees is a common feature of MGD, suggesting the presence of an irreversible cicatricial process in the adjacent submucosa. The duct, as it forms the orifice at the lid margin, is seen to turn on its side anteroposteriorly, so that it becomes visible at the surface of the lid margin. The outer cuff becomes lost from view, whereas the inner cuff (the epithelial lining) and the translucent zone (the presumed dermal layer) are seen in profile. In the early stages, the duct may be patent and functional; later it is not. The changes may extend over the lid margin for several millimeters, which raises the question of whether it is associated with duct elongation or absorption of the distal part of the tarsal plate.
Cystoid dilatation of duct. Cystoid expansion may be seen anywhere along the course of the duct as a dark round or ovoid region along the course of a meibomian gland. Sometimes there are extended, cigar-shaped structures that seem to occupy the position of one or more meibomian glands, but it is not easy to distinguish dilatation of the duct from that of the gland acini by routine methods. Enlarged, distorted and also shortened glands may be distinguished by meibography and confocal microscopy.
Acini.
The acini are susceptible to age-related and disease-associated alterations.
Visibility. As mentioned earlier, congenitally absent or deficient glands are represented by deficient orifices. Although the presence of ascini may readily be judged in young, uninflamed lids, the visibility of the acini, when viewed by diffuse illumination of the tarsal plate, decreases with age as well as in the presence of chronic conjunctival inflammation. Observation can be improved by meibography. Enlargement or reduction in size of the glands may be recorded and concretions and chalazia may be present.
Concretions may follow the line of the meibomian glands and are believed to be deposits of lime salts within acini. The clinical features of chalazia are well known and start as a firm, circumscribed, painless elevation on the tarsal plate, visible and palpable through the skin, which evolves slowly with time. The lesion is in line with the tarsal gland that it replaces, and the corresponding ductile orifice is occluded, with no oil being expressible.
Chalazia occur more frequently under the upper than the lower lid and more commonly in adults than in the young. They may he single or multiple, and they may be confluent. The lid may be sufficiently thickened to prevent eversion. More than one lid may be affected. Multiple chalazia are said to be more frequent in young people, especially seborrheic subjects with a history of chronic blepharoconjunctivitis, but also occur in elderly people or those with rosacea.
Secretions Expressed.
The secretory functions of the meibomian glands are assessed indirectly by compressing the tarsal plate locally in relation to individual groups of orifices. This prodecure may be performed with finger pressure, a cotton tip, or a glass rod or with the Korb expression device, to produce, in normal lids, a dome of clear oil over the orifices. The quality of the expressed secretion that can be elicited in this way in MGD is as follows:
Clear (i.e., normal).
Cloudy: diffusely turbid fluid secretions.
Granular: usually turbid fluid secretions, but contains particulate matter. The color of these secretions varies from whitish-gray to yellow.
Inspissated: a semisolid plug or a substance of toothpaste-like consistency; may be extruded as a plug or curled thread. Expression is usually delayed or requires extra pressure. The material contains keratinized epithelial cells.
The classification scheme, while not complete, is comprehensive enough to permit a detailed assessment of meibomian and lid morphology for the purposes of natural history and therapeutic studies. The purpose of classifying the features of MGD is the opportunity it provides to quantify them.
Appendix 6
Test Identification: Meibomian Gland Expressibility
Rationale.
Meibomian gland expression can be performed as an indicator of meibomian gland function. In the normal patient, a clear to light yellow oil (meibum) is excreted from the glands when digital pressure is placed on the glands. Changes in meibomian gland expressibility may be a valuable indicator of disease.
Method and Description
The only method to determine whether a specific meibomian gland is functional and capable of providing secretion is to observe the secretion expressed from that gland. Since it is not possible to observe the flow of secretion from an individual gland during blinking or forced blinking, assessment requires expressing the meibomian gland with a physical force applied to the outer surface of the eyelid, while simultaneously observing the orifice of the gland with adequate magnification and conditions to detect the outflow of meibomian gland secretion.247–263 There are four types of expression:
Traditional diagnostic expression to determine habitual meibomian gland functionality, usually described without specifying the quantification of the physical force or time of expression. The description of the force applied has been limited to gentle or forceful.256,261,263 The usual procedure is to digitally express the central glands with a force that does not require a rigid surface on the inside surface of the lid. The finger is usually used for the expression, although a spatula, glass rod, or paddle may also be used.254,256,259,264 It is suggested that the expression should be maintained for 10 to 15 seconds.264
Standardized force diagnostic expression to determine meibomian gland functionality, using a newly developed handheld instrument to provide a force of approximately 1.25 g/mm2 (0.3 PSI) to simulate the forces of the eyelids on the meibomian gland(s) during deliberate or forced blinking, thus determining the functionality of individual meibomian glands.264 The instrument is designed to express one third of the lower lid margin, or approximately eight glands simultaneously. The time for application of this force is standardized at 10 to 15 seconds. It is informative to evaluate all three sections of the lower lid in that manner. Standardized force diagnostic expression allows us to determine “the minimum number of glands required to provide an adequate lipid layer for tear film function.”265
Diagnostic expression to determine the likelihood of successful treatment and gland rehabilitation. The forces required for this diagnosis are of a magnitude which requires the use of a rigid surface on the inner lid surface to prevent the transmission of force to the globe, allowing the potential expression of presecretory excreta (inspissated). This process is diagnostic since it is assumed that if presecretory excreta can be expressed, the gland has the potential to be treated. Q-tips, spatulas, and glass rods have been used for application to the inner lid surface, while the finger is usually used for the outer lid surface. The amount of force has only recently been defined; frequently the amount of force approaches the maximum that can be tolerated by the patient, usually in the range of 15 to 20 PSI.266
Therapeutic expression for treating obstruction and/or expressing undesirable secretion/excreta, such as hypersecretion or purulence. Therapeutic expression requires forces of a magnitude that require the use of a rigid surface on the inner lid surface to evaluate whether force can express the obstructive keratinized epithelial material and other excreta. Q-tips, spatula, and glass rods have been reported for the application to the inner lid surface, while the finger is usually used for the outer lid surface. The amount of force has not been defined; however, the amount of force is usually the maximum force that can be tolerated by the patient, usually in the range of 15 to 20 PSI, but if tolerated may be significantly greater.259,266,267
Obstructive MGD is now recognized to be the most common cause of evaporative dry eye.256,268–272 It is imperative to note that obstructive MGD may not be accompanied by obvious lid inflammation and other signs of lid pathology, and thus masquerade as nonobvious to the usual slit lamp examination. Thus, despite a wide prevalence in the general population, nonobvious obstructive MGD is usually overlooked due to minimally observable clinical signs associated with this type of MGD.56,249,272 It is therefore recommended that diagnostic expression be performed when dry eye symptoms are present, even when there is no obvious blepharitis, since the most prevalent form of MGD occurs in the absence of obvious blepharitis, and can only be detected by physical expression. After diagnostic expression, expression to determine the likelihood of successful meibomian gland treatment should follow. Therapeutic expression may be instituted as indicated.249,254,268–272
Estimated Values in Normal, Dry Eye, and MGD Eyes
There are only three studies in which the the number of meibomian glands yielding secretion was correlated to symptoms, and no studies correlating to other ocular surface findings.250,264,273 These three studies examined lower lids only. With digital expression, if four or more of the central six to eight glands are open, there is a low likelihood of dry eye symptoms. Using the instrument for standardized force expression, if three or more of the central six to eight glands are open, there is a low likelihood of dry eye symptoms. For the entire lid, with digital expression, if 10 or more of the approximate 24 glands yield secretion, there is a low likelihood of dry eye symptoms. Using the instrument for standardized force expression, for the entire lower lid, if 6 or more of the ∼24 glands yield secretion, there is a low likelihood of dry eye symptoms. Conversely if 4 or fewer of the ∼24 glands yield secretion, there is a high likelihood of dry eye symptoms.
Sensitivity and Specificity
There are no sensitivity or specificity data for nonobvious obstructive MGD. There is one study in which sensitivity and specificity were determined for meibomian gland function in blepharitis. The study reported the sensitivity/specificity data as follows: meibomian gland expression of the upper lid, 86%/73%, and for the lower lid, 53%/66% (McCann LC, et al. IOVS 2008;49:ARVO E-Abstract 1532).
Volume and Quality
Lipid Volume.
Lipid volume has been assessed semiquantitatively by measuring the average diameter of the dome of expressed lipid in millimeters, using the slit lamp after 5 seconds of digital pressure on the lower lid.263,270,274 However; this evaluation can only measure lipid secretion, which is of a viscosity permitting the formation of a dome. The desired lipid secretion is a clear fluid oil249,253,254 and cannot form a dome, limiting the use of this technique to abnormal or presecretory excreta. There are several references that provide information for the total volume of meibomian glands; however, the data do not provide clinically relevant information regarding meibomian gland expressibility and function.275–277 Further, indirect estimates of oil volume may be obtained by meibometry.287,279 Presently, virtually all the aspects of the volume of meibomian gland secretion require the development of metrics and methods for their determination.
Lipid Quality.
There are numerous studies analyzing the various components of meibomian oil,280–284 but this is a developing concept, since a defining study for determining precise characterization of an optimal lipid layer has not been published. Similarly, lipid viscosity has not been standardized, although viscosity qualifiers such as thick, toothpaste-like, or globular versus fluid can be useful clinically.249,253,254
Appendix 7
Test Identification: Meibography
Rationale.
Meibomian gland tissue can be visualized by using meibography. As such, gland atrophy can be assessed.
Method and Description
Meibography is a technique for observing and documenting the morphology of meibomian glands in vivo. In the first published report of meibography, white light from an illuminator was applied to the conjunctival side of the everted eyelid, and the images were documented on black-and-white film.285 In the most basic version, white light from a transilluminator is applied to the cutaneous side of the everted eyelid, which allows observation and documentation of morphologic changes in meibomian glands from the conjunctival side once the lid is everted. The images are documented on black-and-white film,285,286 infrared film,287–289 a near-infrared CCD video camera,290 or infrared CCD videocameras.291,292 In a recent variation of the technique a near infrared290 or infrared light source is used.291,292 In a recent study292 involving an infrared filter and an infrared CCD videocamera, meibomian glands were observed without a light source applied onto the cutaneous side of the everted eyelid, which made the meibography a patient-friendly examination.
The observable morphologic changes include gland loss and gland shortening, which is quantified using scoring systems. Different authors used different scoring scales as follows. Mathers and Billborough293 scored gland dropout by the number of whole or partial glands missing from the central two thirds of the lower lid. Shimazaki et al.294 scored loss of the meibomian glands in the lower eyelid according to the following scale: grade 0 (no loss of meibomian glands), grade 1 (lost area 50% or less than the observed area), and grade 2 (lost area more than 50% of the observed area). Pflugfelder et al.246 scored partial or complete loss of the meibomian glands in the lower eyelid by using the following scale: grade 0 (no loss of meibomian glands), grade 1 (lost area less than one third of the observed area), grade 2 (lost area between one third and two thirds of the observed area), and grade 3 (lost area more than two thirds of the observed area). Nichols et al.290 scored the gland dropout using the following scale: grade 1 (no partial glands), grade 2 (less than 25% of the image contains partial meibomian glands), grade 3 (between 25% and 75% of the image contains partial meibomian glands), and grade 4 (more than 75% of the image contains partial meibomian glands). Arita et al.246 scored partial or complete loss of the meibomian glands using the following grades for each eyelid (meiboscore): grade 0 (no loss of meibomian glands), grade 1 (lost area less than one third of the total area of meibomian glands), grade 2 (lost area between one third and two thirds of total area), grade 3 (lost area more than two thirds of the total area). Meiboscores for the upper and lower eyelids were summed to obtain a score from 0 through 6 for each eye.
As shown below, diagnostic cutoff values for the meiboscore offer promising sensitivity and specificity when normal eyes were compared with eyes with obstructive MGD in a recent study.292
| Normal vs. Obstructive MGD | |
|---|---|
| Cut-off | Aqueous-deficient dry eye ≥3 |
| (Sensitivity/specificity) | (83.0/90.0) |
Appendix 8
Test Identification: In Vivo Laser Scanning Confocal Microscopy
Rationale.
Scanning confocal microscopy allows for in vivo microscopy of ocular surface morphology in health and disease.
Method and Description
Confocal microscopy is a novel emerging noninvasive technology that is useful as a supplementary diagnostic tool for the in vivo assessment of the histopathology of many ocular surface diseases and anterior segment disorders associated with dry eye disease, including the in vivo examination of the bulbar and palpebral conjunctiva and the meibomian glands.296–305 In studies related to MGD, in vivo laser confocal microscopy was performed with a new-generation confocal microscope, the Rostock Corneal Software Version 1.2 of the HRTII-RCM (Heidelberg Retina Tomograph II-Rostock Cornea Module; Heidelberg Engineering GmbH, Dossenheim, Germany). Briefly, after the upper or the lower eyelid is everted, the center of the Tomo-Cap containing 2 mg carbomer gel preserved with cetrimide (Comfort Gel; Bausch & Lomb, Berlin, Germany) is applanated onto the palpebral conjunctiva, and the meibomian glands are scanned while moving the applanating lens from the lids margins toward the fornix with minute vertical movements. The meibomian glands are also scanned while the applanating lens is moved along the entire lid length with minute horizontal movements. It is recommendable to scan the temporal, central, and horizontal lid with the side camera attachment and to make notes of which sequences belong to which anatomic location in the lid margin, for ease in the later analysis. The examination time for each eyelid takes approximately 5 minutes. To reduce patient discomfort from touch, a drop of topical anesthetic is applied. No patient discomfort or any adverse effect related to this examination has been observed or reported.
In the examination of the MGD, the longest and shortest acinar unit diameter, periglandular inflammatory cell density, and acinar unit density have been recommended and found to be efficient parameters to evaluate the morphologic changes in the meibomian glands.304,305 The density of glandular acinar units and inflammatory cell density can be measured with an internal software. Clearly visible acinar units are all counted in a 400 × 400-μm frame, and the acinar density is described as the number of units per square millimeter. The longest and shortest diameters in micrometers can be calculated by using Image J software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; http://rsb.info.nih.gov/ij/index.html). Three randomized, nonoverlapping, high-quality digital images of the nasal, middle, and temporal lower eyelid (total of nine images per eyelid or more) can be used for calculation of the confocal microscopy parameters. A recent report concluded that acinar unit density and diameter seem to be two promising new confocal microscopy parameters, which are believed to aid in the diagnosis and evaluation of simple MGD.304
In vivo confocal microscopy has been reported to be useful in describing the phenotypic alterations in MGD, such as subepithelial fibrosis, obstruction of meibomian gland orifices, cystic dilatation of the ducts, and lipid/glandular secretory accumulations in the acinar units and the ducts.305,306 Inflammatory cell density also seems to serve as a new and promising diagnostic parameter of in vivo confocal microscopy for evaluation of treatment responses in advanced obstructive MGD as well. In another recent study, a few periglandular inflammatory cells were noted in the eyelids of healthy control subjects (20 eyes of 10 subjects; mean age, 66.4 ± 8.9 years; mean inflammatory cell density in in vivo confocal microscopy, 50 ± 30 cells/mm2). The number of inflammatory cells in the eyelids of patients with obstructive MGD before treatment was observed to be approximately 10 to 30 times higher than in those of healthy control subjects. These observations suggest the potential of this novel technology in differentiating inflammatory obstructive MGD from noninflammatory subtypes and the potential for evaluating the outcome of different treatment protocols.305 The caveat for this parameter is that the current resolution of in vivo confocal microscopy cannot differentiate between inflammatory cell subtypes, except for dendritic cells and polymorphs. In vivo and ex vivo observations made with this new technology have the potential to overcome this disadvantage. In testing the applicability of the aforementioned confocal microscopy–based parameters in the diagnosis of MGD with an expressibility grade ≥2 (Shimazaki grading) and a meibomian gland dropout grade of 2 (Shimazaki: loss of ≥50% of glands along the entire eyelid), the receiver operating characteristic curve technique has recently been used to delineate the sensitivity, specificity, and cutoff value for each parameter. In this study,307 20 right eyes of 20 patients with simple MGD (11 women and 9 men; mean age, 63.5 ± 16.5 years; range: 30–99) and 26 right eyes of 26 healthy control subjects (13 women and 13 men; mean age, 53.2 ± 15.7 years; range: 32–78) were analyzed. Individually, each confocal parameter was observed to have acceptable sensitivity and specificity for the diagnosis of MGD, which appears to be an important observation. Further studies looking into the sensitivity and specificity of these parameters for the diagnosis of mild stages of MGD will provide invaluable information. Moreover, the parameters seemed to correlate well with tear stability, vital staining scores, tear evaporation rate, and clinical grading of meibomian gland expressibility and glandular loss.
When the cutoff value of MG acinar unit density (MGAUD) is set at less than 70 units/mm2, the area under the curve (AUC) is 0.91, and the sensitivity and specificity of the parameter are 81% and 81%, respectively. The AUC is 1 when the cutoff value of inflammatory cell density is set at less than 300 cells/mm2; the sensitivity and specificity of the examination is 100% and 100%, respectively. The AUCs are 0.93, 0.97 when the cutoff values for MGLD (MG longest diameter), MGSD (MG shortest diameter) in the diagnosis of MGD are set at less than 65 and 25 μm, respectively. The sensitivity and specificity of these parameters under these cutoff values are 90% and 81% for MGLD and 86% and 96% for MGSD, respectively.
It seems that the combination of acinar unit diameter (MGAUD) with tear stability examination employing 1 μL of 1% fluorescein solution applied with a micropipette or fluorescein staining results in higher specificity without considerable change in sensitivity. Combination of MGAUD with tear stability or fluorescein vital staining examination also shows a higher specificity without considerable changes in sensitivity.
Appendix 9
Test Identification: Meibometry
Rationale.
Casual lid margin oil level can be measured via meibometry.
Methods and Description
Meibometry was first reported by Chew et al. in 1993308,309 as a method of indirect assessment of the steady state level of meibomian lipids at the lid margin (the casual level). In this examination, the meibomian lipids are blotted onto a loop of plastic tape from the central third of the lower lid margin, and the amount of lipids taken up is measured optically or scanned and measured by a computer equipped with commercially available densitometric software. In the first reports,308,309 it was shown that the casual lipid level at the lower lid increases with age, yet is lower in women in their 20s through 60s, and the lipid level is evaluated as highest in the first hour after waking, but settles to a constant level throughout the remainder of the day. In the original meibometry method, optical density was read with a clinical meibometer (MB 550; Courage & Khazaka Electronic GmbH, Cologne, Germany) that obtained a point reading at the center of the blot. After that, Yokoi et al.310 reported another method in which the sampled lipid is scanned and the increase in transparency is integrated over the length of the blot. A few years later, Komuro et al.311 reported an originally developed meibometer that included a laser device comprised of a laser diode (690 nm) and photodetection units (window size: 2.5 × 5 mm), and an ultrasonography probe was used as the mounting area for the plastic tape.311 The latter studies found that the casual lipid level of meibomian gland dysfunction (MGD) is significantly lower than that in aqueous-deficient dry eyes and normal eyes. The present limitation of meibometry is that in normal subjects, the lipid blot is uniform, and results can be extrapolated to the total lid margin. However, in cases of MGD, focal gland obstruction may vary along the lid length, so that central readings may not truly reflect the overall picture. In future studies, calibrations are needed to convert densitometry readings into equivalent values for the sampled meibomian lipid. Furthermore, development of a system to integrate along the full length of the lid would be ideal, and cutoff values for the diagnosis of MGD are needed. A detailed explanation of the examination method is detailed in the following text.
Correlation between Confocal Microscopy Parameters, Tear Functions, Meibomian Gland Status
| BUT | FS | RB | MG Expressibility Grade | MG Dropout Grade | TEROS | |
|---|---|---|---|---|---|---|
| Inflammatory cell density | −0.552‡ | 0.524‡ | 0.479† | 0.787‡ | 0.781‡ | 0.499† |
| MG acinar unit density | 0.557‡ | −0.507‡ | −0.460† | −0.706‡ | −0.678‡ | −0.530‡ |
| MG acinar shortest diameter | −0.408† | 0.451† | 0.374* | 0.731‡ | 0.813‡ | 0.346* |
| MG acinar longest diameter | −0.342* | 0.477‡ | 0.308* | 0.611‡ | 0.723‡ | 0.388* |
This table was published in Ophthalmology, Vol 117, Ibrahim OM, Matsumoto Y, Dogru M, et al., The efficacy, sensitivity, and specificity of in vivo laser confocal microscopy in the diagnosis of meibomian gland dysfunction. Page 669, © Elsevier (2010). Reprinted with permission.
BUT = break-up time; FS = fluorescein staining; RB = Rose Bengal staining; TEROS = tear evaporation rate measurements from the ocular surface.
Spearman's correlation coefficient by rank test.
P < 0.05, considered significant.
P < 0.005, considered very significant.
P < 0.0001, considered extremely significant.
Figure 18.
This figure was published in Ophthalmology, Vol 117, Ibrahim OM, Matsumoto Y, Dogru M et al., The efficacy, sensitivity, and specificity of in vivo laser confocal microscopy in the diagnosis of meibomian gland dysfunction. Page 670, ©Elsevier (2010). Reprinted with permission.
In the standard technique of meibometry, a preformed loop of meibometry tape (8 mm wide) is placed in the reading head of the meibometer, to establish the 0 reading. The loop is formed by heat-sealing the tape at a predetermined point to give a loop length of 20 mm. The handle is clipped to the prism housing of a Goldmann applanation tonometer or an ultrasonography probe holder mounted on the slit lamp biomicroscope. This arrangement permits controlled placement of the probe on the lid margin under direct vision. The tonometer or ultrasonography probe is set at 0 for each impression. With the subject looking upward without blinking, the lower lid is gently inverted (stretching should be avoided, as it might express oil), and the loop is then pressed onto the central third of the lid margin with sufficient pressure to obtain an imprint across the entire width of the tape, yet without bending the handle of the loop. A line of contact is seen across the full width of the tape, and contact is maintained for 3 seconds. After the blot is obtained, the tape is kept in the air for 3 minutes, to allow evaporation of any tears picked up from the lid. The loop is placed in the reading head of the clinical meibometer, and a reading is taken in the standard way. The casual lipid level (expressed as arbitrary optical density units) is calculated as (C − B), where C is the casual reading and B is the reading from the untouched tape (background). In the process of integrated meibometry, the tape loop is opened and attached to a strip of exposed 35-mm negative film to provide a black backing. The oil imprint is scanned with a handheld scanner into a computer for densitometric analysis. In the process of laser meibometry, the meibometry is performed with an originally developed laser device (laser diode with 5 mV and 690 nm in wavelength and silicon photo diode, window size: 2.5 × 5 mm) where the casual lipid level (arbitrary units) is obtained by (C − B)/A, where A is the reading without tape, B is the reading before blotting, and C is the reading after blotting.
Appendix 10
Test Identification: Interferometry
Rationale.
Interferometry utilizes optical principles to visualize the tear film lipid layer, which consists of the lipid secreted from the meibomian glands. At the time of eye opening, this lipid layer is repeatedly spread, by blinking, over the aqueous layer of the tear film.312 The layer is very thin, and thus the light reflected from the surface and back of the lipid layer produces interference images that can be observed as specular images.
Method and Description
Interferometers are instruments that allow visualization and analysis of the interference image from the lipid layer. Several types of interferometers have been developed to see the lipid layer.313–323 Among them, the DR-1 (Kowa) has successfully been able to give quantitative analysis.317–319,324 Even before the development of interferometers, the spreading of the lipid layer over the aqueous layer had been observed. Interferometers provided a clearer image, and thus a difference was noticed in the interference patterns between normal subjects and dry eyes. Based on these observed differences, grade classifications were made for precorneal tear film325 and precontact lens tear film.316,325 For example, for the DR-1 there are five grades ranging from normal (grade 1 or 2) to dry eye (grades 2 to 5, with a grade of 5 being the most severe).320 Despite the limitation of this classification system where grade 2 may be classified either as normal or dry eye, the noninvasive nature of this test makes it a valuable tool for screening dry eye and assessing severity. To determine the condition of the lipid layer, one may measure either the thickness or the spreading rate of the lipid layer over the ocular surface. Early methods of measuring the thickness of the lipid layer did not give a precise value for the thickness, since they relied on Newton's color scale which provides a relatively rough and semiquantitative value.315 However, over time, several advances have been made in the ability to determine the condition of the lipid layer by quantitative values. The most sophisticated of these was developed by a colorimetric approach,317 in which a new tear interference color chart was developed to describe the thickness of the tear film lipid layer.
A different approach, aimed at assessing kinetically the rate of spread of the lipid layer over the ocular surface, is based on two methodologies: One is measuring the spread time of the lipid layer (the time required to reach a stable lipid film after opening the eye).318,319 In a study conducted to evaluate tear lipid spread time and pattern, the lipid spread was found to be horizontal in healthy eyes but vertical in lipid tear deficiency (LTD); lipid spread time is greater in normal subjects than in those with LTD.318 The second methodology measures the lipid layer spread more directly by using the rheological model, and it was noticed that the rate of spreading of the lipid layer depends on the volume of the aqueous layer.320 Based on this method, one can assess the precorneal aqueous tear volume by measuring the spread rate of the lipid layer, and normal tear volume may be given to the higher rate of spreading. Even though these approaches are still under development, the techniques have promise in many clinical applications in the diagnosis and/or quantitative grading of the severity of dry eye.325
Appendix 11
Test Identification: Evaporimetry
Rationale.
Evaporimetry measures tear evaporation from the ocular surface. The evaporation is very effectively reduced by the lipid barrier of the tear film.326 In conditions of 52% humidity at temperatures of 22°C, the evaporation of water from an open bath is 100 × 10−7g/cm2/s.327 When measured under these conditions, the lipids of the ocular tear film reduce their evaporation by approximately 80% to 90% in the normal eye. Mishima and Maurice328 in 1961 were the first to establish that the lipid layer retarded evaporation in an animal model of the rabbit eye. Iwata et al.329 developed another in vitro rabbit model with a cornea covered with a chamber through which dry air was passed; from the weight of water collected, they determined the evaporative rate to be 10.1 × 10−7g/cm2/s. They found that a fourfold increase in evaporation occurred with the removal of the rabbit's tear film lipid layer. A similar proportional increase in human tear film evaporation was measured by Craig and Tomlinson330 in patients with an incomplete or absent lipid layer, a situation commonly found in MGD. Tear film evaporation depends on a variety of parameters, including ambient air flow and interaction of the numerous components in the tear film, including the lipid layer.
Evaporation Rate Derived from Capture of Fluid Loss from the Ocular Surface
Evaporation of fluid from the ocular tear film has been measured by numerous investigators since the first report in 1980 by Hamano et al.,331 and a range of different techniques have been used. Hamano et al.332 determined the evaporation from the corneal area enclosed in a capsule by a pressure gradient technique, Cedarstaff et al.333 measured the increase in electrical resistance of air passed over the eye with an increase in humidity measured with resistance hygrometry. Subsequently, they adopted the vapor pressure gradient technique, calculating relative humidity and temperature at two points above an evaporative surface.334 Others have measured the increases in humidity of the air in a sealed goggle over time.335–337 Recently a continuous recording device measured changes in the humidity of the air stream passing over the eye, by microbalance technology.338
Tear film evaporation rate has been reported in different units by various researchers,330,334,337,339; most use units of ×10−7g/cm2/s, but others report values in grams per square meter per hour (g/m2/h). This difference may be resolved and all values rendered to the same units (−10−7g/cm2/s) by dividing the values expressed in grams per square meter per hour by a factor of 3.6.340 Borchman et al.341 provide an alternate view in which they have proposed that the evaporation rate be expressed as an equivalent thinning rate of the tear film in micrometers per minute. These units are simpler and can be compared directly to measurements of tear thinning rates.342
Evaporation rates recorded by the measurement of fluid loss from the ocular surface for normal and dry eyes have been reported in the literature over the past 30 years (McCann LC, et al. IOVS 2008;49:ARVO E-Abstract 1542).335–339,343–351 The rate is also reported in units of microliters per minute by some researchers.338,339 The evaporation rate in microliters per minute is numerically equal to a hundreth of the value of the evaporation rate stated in units of 10−7g/cm2/s, when the area of the evaporating ocular surface is 167 mm2. The use of different techniques for measurement of tear film evaporation makes it difficult to compare evaporative findings in normal and dry eyes among different studies, because the absolute values recorded are technique dependent. However, there is a pattern to the observations reported in the literature, with significant increases from normal tear film evaporation seen in patients with both aqueous-deficient dry eye and MGD and evaporative dry eye (McCann LC, et al. IOVS 2008;49:ARVO E-Abstract 1542).335,339,344–347,352 Strictly, these comparative differences within individual studies are of diagnostic significance only where values in normal and dry eyes are recorded by the same technique in the same laboratory. However such evaluations as meta-analysis of evaporation studies are valid means of detecting the pattern of differences in normal and dry eyes; therefore, it is permissible that all values be included in such an analysis, irrespective of measurement technique.354 Tear film evaporation is raised in both aqueous-deficient dry eye and evaporative dry eye, compared with normal. In aqueous-deficient dry eye, the evaporation rises to an average of 17.91 ± 10.49 × 10−7g/cm2/s (from a level in the normal eye of 13.57 ± 6.52 × 10−7g/cm2/s) and is higher still in evaporative dry eye at 25.34 ± 13.80 × 10−7g/cm2/s.355
Evaporation measurements are important in the differential diagnosis of dry eye. Several studies report tear film evaporation in aqueous-deficient dry eye and MGD or patients with evaporative dry eye (McCann LC, et al. IOVS 2008;49:ARVO E-Abstract 1542).335,339,344–347,352,353 In most cases, the evaporation rate is greater in the dry eye than in the normal eye, as the increased water loss from the tear film contributes to the dry eye condition. In one study, almost 90% of the dry eye patients showed lower readings of tear film evaporation than did normal subjects.345 This discrepancy was explained by considering the relative contribution of tear evaporation to tear dynamics in the dry eye condition; the proportional loss through evaporation in the dry eye was greater than in normal eyes, although the actual water loss (in absolute terms) was decreased compared with normal values. A reduction in tear fluid volume, however, would not be reflected in a reduction in evaporation rate unless the surface area was reduced as well. Although this could occur if there were very large areas of breakup, it is unlikely, because a sudden drop in evaporation rate would occur as the large dry areas develop. A reduced rate of evaporation can be seen in dry eyes, but is more likely to be related to a change (increase) in the retardation effect of the lipid layer. Dry eyes often appear to have a more viscous, and perhaps thicker, lipid layer than normal.
Evaporation Rate Derived from Measures of Tear Film Thinning
A new paradigm has recently been introduced into the field of measurement of human tear film evaporation by King-Smith et al.342,356–358 who infer rates of evaporation from observations of tear film thinning. The notion of the actual fluid loss from the tear film has been thrown into confusion by their recent suggestion that the primary thinning of the tear film observed by their imaging interferometer is due to evaporation.356–358 The values for evaporation inferred from tear thinning are of a different order, a factor of approximately four to five times that reported in studies of direct measures of the capture of fluid loss from the ocular surface.355
In a recent meta-analysis, diagnostic cutoff values for evaporation rate were found to offer limited sensitivity but better specificity when normal eyes are compared with all dry eyes and within each of the dry eye subtypes.355 As a result of the potential extreme skewing of weighted averages for evaporation in this meta-analysis, the values obtained from tear thinning measures are not included.
| N vs. DE | N vs. EDE | ADDE vs. EDE | |
|---|---|---|---|
| Cutoff | DE < 22 | EDE > 22.3 | EDE > 27.5 |
| (Sensitivity/specificity) | (51.1/89.9) | (61.2/90.6) | (45.5/79.8) |
Appendix 12
Test Indentification: Tear Lipid Composition and the Diagnosis of MGD
Rationale.
Considerable work has been undertaken in investigating the composition of tear lipids. The research has been hindered by the small sample size, difficulty of collection, the danger of contamination with skin lipids and cosmetics, storage problems, and intrinsic complexity of the mixture of the lipids. The analytical techniques used therefore reflect this complexity. A review of the analysis and composition of human tear lipids in health and disease will be dealt with in detail elsewhere in this MGD report.
Methods and Description
Within the current state of knowledge several impediments hinder the adoption of lipid composition as part of the clinical diagnosis of MGD. As yet, no uniform method of sample collection has been adopted. Collection techniques have included meibum from forced expression359 collected with capillaries360 or spatula (Butovich IA, et al. IOVS 2007;48:ARVO E-Abstract 441), lipid extracted from whole tears361 or extracted from Schirmer strips.362 Once collection is achieved, analysis of the lipids involves complex multistep analytical techniques such as FTIR, NMR, GC-MS, TLC-GC-MS, and HPLC-MS.363 Mass spectroscopy often involves exotic ionization techniques such as MALDI, ESI, and API. The latest techniques make use of multistage fragmentation of ions (MSn), which does allow greater elucidation of structure. These techniques are inherently expensive and time-consuming and require a high level of expertise. Unfortunately, qualitative information is gained but quantitative analysis is not readily generated.
Controversy remains regarding the composition of tear lipids in healthy persons, such as the presence359 or absence (Butovich IA, et al. IOVS 2007;48:ARVO E-Abstract 441) of phospholipids and ceramides, and reports of high364 and low361 levels of the fatty acid amide oleamide. Changes in lipids as a result of disease have been detected in several studies,360,365–369 but as yet, the changes are not fully understood. It should also be noted that much of the work has been done in animal models360,366 rather than human subjects.
As each of these issues is addressed, it is anticipated that lipid analysis will start to play a role in the understanding of MGD, particularly in a research setting. Considerable further advances are necessary before lipid analysis can be used as a diagnostic tool in clinical practice.
Appendix 13
Test Identification: Fluorophotometry
Rationale.
Tear production, tear turnover, and tear volume are assessed with fluorophotometry. Several tests have been devised to measure the rate of disappearance of a dye marker placed in the tear film with the production of new tears and through tear elimination from the eye. In most studies, the disappearance of sodium fluorescein dye from the tear film has been used to record tear turnover (TTR) by the technique of fluorophotometry.370–376
Method and Description
Early studies used modified slit lamp fluorophotometers,371,373,377 but the development of a commercial instrument and analysis software376 helped standardize the procedure,372 (i.e., the Fluorotron Master; Coherent Radiation Inc., Buffalo, NY). The decay of fluorescein concentration in the tear film is measured by these techniques over a period of 30 minutes after instillation of 1 μL of 2% fluorescein sodium into the lower fornix with a measuring pipette, with scans being performed every 2 minutes. The change in rate of decay of fluorescence is then calculated for the total measurement period, and a biphasic decay in fluorescence is observed.372 The measurements for the first 5 minutes show a rapid decay, thought to be due to the initial reflex tearing produced by the instillation of the fluorescein drop. The later part of the curve (from 5 minutes outward) represents the measurement of tear turnover under basal conditions of secretion. It is this part of the curve that is fitted using appropriate software,376 and the decay in fluorescence is calculated from the log of the curve obtained from the following formula, to obtain the basal tear turnover rate:
 |
where Ct(t) is the fluorescein concentration in tear film at time t(min).
Assuming a monophasic decay of fluorescence from 5 minutes after instillation with a decay time constant b (min−1)
the following is obtained:
This calculation gives a measurement of the tear turnover recorded in percentage per minute (%/min). To express the turnover value in terms of microliters per minute (sometimes called flow), it is necessary to either assume a value for the tear volume (typically 7 μL377) or to measure the volume from the initial dilution of the instilled sodium fluorescein in the tears. Initial dilution is calculated by back extrapolation to time 0 of the initial fluorescence decay. In this technique, it is the monophasic decay of fluorescence in the first 5 minutes after instillation of the fluorescein that is determined.376,378
Tear volume is derived from the formula377:
where Cd is the fluorescein concentration in the drop, and Cm is the initial fluorescein concentration calculated by back extrapolation with the Fluorotron in nanograms per milliliter.
The turnover in microliters per minute is then calculated from the product of tear turnover in percent per minute and tear volume. Values have been reported for tear turnover (%/min) and tear flow (μl/min) in major studies in the literature for normal and dry eye subjects, obtained with a commercial fluorophotometer.375,376,379,380 The data reported for normal subjects in most studies ranges from 10% to 20%/min, which equates to a tear flow rate of just over 1 μL/min.375–377,379,381–383 In contrast, Mathers et al.384 found normal tear turnover on the order of 7%/min or 0.19 μL/min, values not dissimilar to those found in dry eyes. It is possible that the values of TTR published by Mathers are in error, and later reports by this group suggest a difference in their calculations, producing higher values in the range of 0.34 to 0.49 μL/min.385 TTR in normal subjects averages 1.03 ± 0.39 μL/min (16.19% ± 5.10%/min) when Mathers' values are excluded and for the dry eye in all its forms, the average is 0.54 ± 0.28 μL/min (9.26% ± 5.08%/min). In those cases subtyped as aqueous-deficiency dry eye, the mean TTR is 0.40 ± 0.1 μL/min (7.71% ± 1.02%/min) and in evaporative dry eye, the mean is 0.71 ± 0.25 μL/min (11.95 ± 4.25%/min). These results suggest that all dry eyes show a reduced production facility (TTR) relative to the normal by approximately 60% in aqueous-deficient dry eye and by 30% in evaporative dry eye. Diagnostic cutoffs for TTR offer promising sensitivity and specificity when normal eyes were compared with all dry eyes and with each of the dry eye subtypes in a recent meta-analysis.386 TTR has potential in differentiating both evaporative dry eye (resulting from MGD) from the normal, as well as from aqueous-deficient dry eye, being a sensitive measure in the former case and highly specific in the latter (i.e.; efficient in classifying evaporative dry eye).
| N vs. DE | N vs. EDE | ADDE vs. EDE | |
|---|---|---|---|
| Cutoff | DE < 12.9 | EDE < 15.1 | ADDE < 9.6 |
| (Sensitivity/specificity) | (74.5/73.6) | (80.2/58.7) | (69.5/96.8) |
Tear Volume by Fluorophotometry: Reported Values
Measurement of tear volume by the fluorometric technique has yielded little difference between the volume in normal or dry eyes (including MGD).379,380 This outcome is unlike the situation with measurement by meniscometry where differences have been found. Scherz et al.383 have also found correlations between tear meniscus height (TMH) and volume by fluorophotometry. Volume measures by fluorophotometry were not found to correlate with PRT by Tomlinson et al.386
Appendix 14
Test Identification: Meniscometry
Rationale.
Meniscometry provides a measure of tear meniscus height, radius, and volume.
Methods and Description
There are many ways to evaluate tear meniscus parameters, such as measuring the height, radius, width, and cross-sectional area, because 75% to 90% of total tear volume of the ocular surface is estimated to be kept in the tear meniscus.387 Among those parameters, measurement of tear meniscus height is the most popular assessment method, and there have been numerous reports that have attempted to use meniscus height in the diagnosis of tear deficiency. However, those previous methods require fluorescein instillation to obtain clear visualization, and this may induce reflex tearing due to some invasiveness. Yet in a report on slit-image photography388 that compared tear meniscus parameters, including height, radius, width, and cross-sectional area, the height and radius of the meniscus were found to be the best parameters for the diagnosis of dry eye.388 That method did employ fluorescein instillation, however, thus introducing some invasiveness that may cause reflex tearing and may add some aqueous to the original tear volume. Based on that background, meniscometry was developed.389,390 Today, there are two systems of meniscometry; one based on photography390 and one based on the use of video.391 In a newly developed video-meniscometer, a rotatable projection system with a target comprising a series of black and white stripes (four black and five white; each 4 mm wide) was introduced coaxially, using a half-silvered mirror. The coaxial alignment of the video-meniscometer permits the meniscus of either eye to be readily accessed and allows for real-time recording of meniscus behavior corresponding to a 1.1 × 1.5 mm rectangular area of the meniscus. For the purpose of calculating the radius of tear meniscus curvature, a selected meniscus image recorded on a digital video recorder is captured on the computer, and analyzing software is applied for the calculation of the radius according to the concave mirror formula:
where R is the radius of the tear meniscus, W is the working distance, I is the image size, and T is the target size.
Using meniscometry, the R values in normal eyes were calculated as 0.365 ± 0.153 mm (n = 36) by the photographic system. However, probably due to some invasiveness of the photographic system as it sought the image in a dim light, the calculated R values were larger than those obtained by the video system391 (0.30 ± 0.10 mm, n = 36), but smaller than those obtained by slit-image photography (0.55 ± 0.26, n = 15).388 Those differences are due to the effect of reflex tearing or the instillation of fluorescein into the aqueous. In a recent advancement in optical coherence tomography (OCT), the R values are reportedly the smallest yet obtained (0.239 ± 0.112 mm n = 40).393 It has also been reported that those normal R values were smaller than those in dry eyes (0.17 ± 0.05 mm,393 n = 38; 0.22 ± 0.09 mm,394 n = 29).
Through research using a video-meniscometer, the radius of the tear meniscus at the central lower lid margin of the left eye was measured in 36 healthy volunteers, 38 dry eye subjects (diagnoses based on the Japanese dry eye criteria), and seven dry eye patients with punctal plugs in both upper and lower puncta. Among those groups, the respective tear meniscus radii (R, in millimeters) were compared. The results showed a significantly smaller meniscus in dry eyes (R = 0.17 ± 0.05 [SD]) compared with that in normal eyes (0.30 ± 0.11 mm; P < 0.0001), whereas a significantly larger meniscus was found in dry eye patients with punctal plugs (0.57 ± 0.23) than in normal eyes (P < 0.0001) or dry eyes (P < 0.0001).393 Fourteen subjects from the normal group and 31 patients from the dry eye group had undergone the Schirmer 1 test, so the correlation between the radii and values of the Schirmer I test was investigated in those groups. It was found that there was an excellent agreement between the radius of tear meniscus and the Schirmer 1 test. If normal is determined by the fact that both the tear and ocular surface examinations are normal and the cutoff value of the radius is determined as 0.25 mm, then the sensitivity and specificity for the radius were calculated as 88.9% and 77.8%, respectively, which is compatible with the measurement of meniscus height.395
Considering that the radius measurement obtained by meniscometry is noninvasive and that there is a significantly good correlation between R and total tear volume over the ocular surface,396 the tear meniscus may be the expectable parameter for the screening of tear deficiency. For other applications, the video-meniscometer enables real-time monitoring of tear volume and also allows tear turnover to be evaluated after the instillation of eye drops, where not only the turnover of tear substitute at the ocular surface is evaluated but also the efficacy of drainage of the lacrimal pathway.
Appendix 15
Test Identification: Osmolarity
Rationale.
Tear film osmolarity indicates the balance of inputs and outputs of the lacrimal system.
Methods and Description
The osmolarity of a sample can be determined in several ways, both in situ and by sampling, using methods that measure the colligative properties of the tears. These properties, such as freezing-point depression and vapor pressure, depend on the number of dissolved particles in a solution but are not dependent on the identity of the particles. The freezing point depression nanoliter osmometer is at present the most commonly applied principle in osmolarity measurement.397,398 In this method, the temperature of the freezing point is directly proportional to the total number of dissolved particles in the solution. Therefore, the osmolarity can be calculated from the depression in the freezing point. The most frequently applied freezing point depression techniques in tear research use nanolitre samples,398–402 most commonly with the Clifton Nanolitre Osmometer (Clifton Technical Physics, Hartford, NY).400 Although used in the diagnosis of dry eye disease, this method requires significant expertise, takes considerable time, and is open to error due to evaporation of test samples.401 Other techniques using the freezing-point depression technique such as the Advanced Tear Osmometer (Advanced Instruments, Inc., Norwood, MA) and the Otago Osmometer (Otago Osmometers Ltd, Dunedin, New Zealand) are also available.
Vapor pressure techniques have also been used in the measurement of osmolarity.402 These work on the principle that the vapor pressure of a solution is lower than that of the pure solvent at the same temperature and pressure; the decrease in vapor pressure, like depression of freezing point is proportional to the number of dissolved particles in the solution. Thus, the osmolarity of a solute can be calculated from its vapor pressure. Original vapor pressure osmometers engaged a precision thermocouple hygrometer to measure dew point depression and required large sample volumes.402 This necessitated the collection of reflex tears which in turn could lower the osmolarity values obtained.403 More recently, vapor pressure osmometers, such as the Wescor (Wescor, Inc., Logan UT) have been used. However, although easier to operate and more streamlined than freezing-point depression osmometers, they are still not suitable for the quick, easy application required in clinical practice.
There is a need for a new instrument to facilitate clinical application and the adoption of osmolarity as a diagnostic test in dry eye disease. Recently the OcuSense system (OcuSense Inc., San Diego, CA) has been developed.400,404 This new osmometer is based on electrical impedance and “laboratory-on-a-chip” technology, which allows the calculation of osmolarity. This technique allows osmolarity testing of a very small volume (less than 20 nL), is a quick and accurate measurement of the osmolarity of the tear film in a clinical setting, and reduces the evaporation of the fluid. However, although the device measures charged particles, corrections or assumptions are made with regard to the contribution made by noncharged particles in the tear sample. The OcuSense system has recently been approved as a medical device by U.S. Food and Drug Administration. A recent study compared the new OcuSense osmometer with the Clifton Osmometer, to determine the comparability of results between the instruments. Osmolarity values for controls and dry eye were 308 ± 6 mOsm/L and 321 ± 16 mOsm/L, respectively (OcuSense) and 310 ± 7 mOsm/L and 323 ± 14 mOsm/L respectively (Clifton); the difference was signnificant. Significant correlation was found between OcuSense and Clifton measurements (r = 0.904; P = 0.006). Bland-Altman analysis revealed agreement between techniques; most of the points fell within the 95% confidence limits, and actual values differed by less than 1%.404
A previous meta-analysis was performed on published data for tear osmolarity in samples of normal subjects and various subtypes of dry eye and pooled estimates of the mean and standard deviations for normal and (all) dry eye subjects were determined.405 A diagnostic referent (cutoff) value was derived and tested for effectiveness of diagnosis on independent groups of normal and dry eye subjects. A referent value of 315.6 mOsm/L was derived from the intercept of the distribution curves, and 316 mOsm/L from the ROC curve. When applied to independent groups of normal and (all) dry eye subjects, a value of 316 mOsm/L was found to yield sensitivity of 59%, specificity of 94%, and overall predictive accuracy of 89% for the diagnosis of dry eye syndrome. Tear hyperosmolarity, defined by a referent value of 316 mOsm/L, was superior in overall accuracy to any other single test for dry eye diagnosis.
Osmolarity is use in differentiating evaporative dry eye from the normal, but is of limited ability in assigning the subtypes into categories of aqueous-deficient dry eye and evaporative dry eye; this outcome is not unexpected when osmolarity in the subtypes is reported as 330.01 ± 13.34 and 325.57 ± 14.76, respectively, and 308.39 ± 9.29 in the normal in a recent study.406 Utilization of easy-to-operate instrumentation with high levels of sensitivity alone or in addition to dry eye clinical testing will continue to provide valuable information about the fundamental underpinnings of osmolarity as it relates to ocular surface disease.407
Appendix 16
Test Identification: Indices of Tear Film Dynamics
Rationale.
It would be useful in the study of dry eye to be able to describe and quantify tear film dynamics–-the balance of inputs and outputs–-of the lacrimal system (the combination of production and evaporative loss) by a single index that describes the balance of input and output of the system.409
Methods and Description
Tear Function Index.
An early index for tear film dynamics was the Tear Function Index (TFI) devised by Xu et al.410 This index combined values obtained for tear secretion (from the Schirmer test with anesthesia) with measurements for drainage (turnover as measured by the fluorescein clearance test411) in the following formula:
 |
This index includes measures of two of the three main factors that determine tear dynamics410: secretion and drainage. It has been argued that tear secretion is the most important determinant of tear dynamics,410 but, as it could not be measured independently and directly, the Schirmer test result had to represent the production component of dynamics.410 The ability of the TFI to discriminate between normal and dry eye patients was found to be considerably better than the Schirmer test or the tear clearance rate values alone. A value of the log to the base 2 of the TFI below 96 gave a sensitivity and specificity in the diagnosis of dry eye of 67.4% and 60%, respectively. A value for TFI below 34, gave sensitivity and specificity calculations for Sjögren syndrome of 78.9% and 91.8%411 The major deficiency of the TFI as an index of tear dynamics is that it fails to take into account the elimination of tear fluid from the eye through evaporation. Evaporation is a key variable in differentiating some groups of dry eye.413 A recent study determined the effectiveness in dry eye diagnosis of another Tear Function Index (the Liverpool modification of the TFI) test.414 This report showed high sensitivity at 83% in the diagnosis of all dry eye from the normal but poor specificity (40%).
Total Tear Flow
Ideally, any index of tear dynamics should define the imbalance that leads to the condition of dry eye. Under basal conditions, the majority of the input, and output, of the lacrimal system can be determined through measurement of tear turnover and fluid loss by evaporation.409 Mathers415 has suggested “total tear flow” as an index that captures the principal sources of elimination of tear fluid from the eye. As the drainage facility is not necessarily affected in dry eye states,416 the tear flow is determined from tear turnover rate (effectively a measure of drainage) and, combined with evaporation, it gives an estimation of the tear production facility of the eye.415 Therefore, dry eye may result when tear flow (turnover) is reduced due to a deficiency of tear production deficiency (aqueous-deficient dry eye) or a high level of evaporation occurs (evaporative dry eye) in MGD or blepharitis. Mathers suggests that in the assessment of the balance of production and outflow from the eye, the proportion of elimination due to evaporation as a part of the total tear flow is essential. Tomlinson et al.,417 in a meta-analysis, analyzed the values for tear turnover and evaporation rates and the total tear flow from the literature (including Mathers' publications,413,415,418 even though the values recorded for tear turnover by Mathers et al. in some reports417,418 are considerably below those recorded by others and may be in error). Mathers415,419 has reported that approximately one third of the resting tear flow evaporates in the normal eye. This increases to 75% of the total tear flow in the dry eye,415 although the total tear flow in normal subjects and dry eye patients recorded by Mathers is similar, at around 0.5 μL/min. In a later paper, Mathers418 observed the percentage loss of tear fluid from the eye through evaporation to be approximately 55% in dry eyes.
The value for the comparison of evaporative loss to total tear flow (evaporation+TTR) in the meta-analysis417 indicates that the proportion of loss is about one eighth or 12% in the normal (14.4% if Mathers' data are included). In dry eye of all types, this increses to over a quarter or 28%. It is similar in both the dry eye subtypes, being 28.3% in aqueous-deficient dry eye and 26.8% in evaporative dry eye.
Evaporation and Tear Turnover
A similar but simpler index is derived from the proportion of the production, measured by tear turnover rate (TTR), lost through evaporation.417 For these analyses, TTR can be thought of as a measure of production, although TTR may also be considered a measure of drainage. A meta-analysis (with and without Mathers' values for TTR) show that for this simple ratio, close to one eighth (13.6%) of the tear production (TTR) is lost through evaporation in the normal eye. In (all) dry eye the level of evaporative loss rises to 38.9%. In the subtypes of dry eye, the evaporative loss for aqueous-deficient dry eye is 39.5% of TTR and for evaporative dry eye, the loss is similar, at 36.6% of the TTR.
The use of indices derived from evaporation and TTR measures, whether combined into “total tear flow” as a denominator414 or using a single turnover measure for production, makes little difference in the ability to distinguish dry eye states from the norm. In fact, the difference with the simpler index of evaporation/TTR is slightly larger than for evaporation/total tear flow index; 2.7 to 2.9 times compared with 2.2 to 2.4×. Both indices show that dry eye, in all its forms, has a greater loss of fluid by evaporation, compared with its production, than occurs in the normal eye. The dynamic imbalance of loss to production is slightly greater in aqueous-deficient dry eye than in evaporative dry eye, even though the eye with evaporative dry eye has a greater loss through evaporation. The combined, though moderate, increase in evaporation in the eye with aqueous-deficient dry eye, and the poorer production facility (approximately half that of evaporative dry eye) accounts for the greater imbalance in the eye with aqueous-deficient dry eye.417 It appears that a change in the balance between input (TTR) and output (evaporation) by a factor of more than two or three times (dependent on index) leads to dry eye. So that the output:input percentage changes from just over 12% in the normal to greater than 25% in dry eye states (26.8% in evaporative dry eye and 28.3% in aqueous-deficient dry eye with the total tear flow index; or 36.6% in evaporative dry eye and 39.5% in aqueous-deficient dry eye from the simple index).
| N vs. DE411 | N vs. DE414 | N vs. EDE414 | ADDE vs. EDE414 | |
|---|---|---|---|---|
| TFI Cutoff | DE < 96 | DE < 240 | EDE, NA | EDE, NA |
| (Sensitivity/specificity) | (64.7/60) | (83%/40%) | (NA) | (NA) |
The above analysis is based on considering one input to the tear film from the lacrimal gland, and two outputs: drainage and evaporation. Levin and Verkman,420 have emphasized the importance of a second input–-osmotic flow from the conjunctiva and cornea into the hyperosmolar tears. This osmotic flow helps to reduce the osmolarity increase caused by evaporation and could help to explain why the high evaporation rates proposed by King-Smith et al.421 may not necessarily lead to unreasonably high osmolarity.
Appendix 17
The following are the diagnostic criteria for obstructive MGD proposed by the Japanese MGD Working Group.
Obstructive Meibomian gland dysfunction is considered to be present when all of the following three signs/findings are present:
Chronic ocular discomfort.
- Anatomic abnormalities around the meibomian gland orifices (presence of one or more of the following is positive).
- Vascular engorgement.
- Anterior or posterior displacement of the MCJ.
- Irregularity of the lid margin.
- Obstruction of the meibomian glands (presence of both is considered positive).
- Obstructive findings of the gland orifices by slit lamp biomicroscopy (pouting, plugging, or ridge).
- Decreased meibum expression by moderate digital pressure.
These diagnostic criteria for obstructive MGD were published in Atarashii Ganka (Journal of the Eye) 2010;27:627–631, and are reprinted in this appendix with the permission from the publisher (Medical-Aoi Publications Inc.).
The Japanese MGD Working Group consists of the following members:
Shiro Amano, Reiko Arita (University of Tokyo), Shigeru Kinoshita, Norihiko Yokoi, Chie Sotozono, Aoi Komuro, Tomo Suzuki (Kyoto Prefectural University of Medecine), Jun Shimazaki, Seika Den (Tokyo Dental College), Kohji Nishida, Naoyuki Maeda, Shizuka Ko (Osaka University), Yukichi Hori (Toho University), Hisayo Kubota (Tohoku University), Eiki Goto (Tsurumi University), Masahiko Yamaguchi (Ehime University), Hiroto Obata (Jichi Medical University), Masakazu Yamada (Tokyo Medical Center), Dogru Murat, Yoko Ogawa, Yukihiro Matsumoto, Kazuo Tsubota (Keio University).
Footnotes
Supported by the Tear Film and Ocular Surface Society (TFOS; http://www.tearfilm.org); individual author support is listed in the Appendix of the Introduction.
Disclosure: Each Workshop Participant's disclosure data can be found in the Appendix of the Introduction.
References
- 1. Foulks G, Bron AJ. A clinical description of meibomian gland dysfunction. Ocul Surf. 2003;1:107–126 [DOI] [PubMed] [Google Scholar]
- 2. Kozak I, Bron AJ, Kucharova K, et al. Morphologic and volumetric studies of the meibomian glands in elderly human eyelids. Cornea. 2007;26:610–614 [DOI] [PubMed] [Google Scholar]
- 3. Arita R, Itoh K, Inoue K, Amano S. Noncontact infrared meibography to document age-related changes of the Meibomian glands in a normal population. Ophthalmology. 2008;115:911–915 [DOI] [PubMed] [Google Scholar]
- 4. Nicolaides N, Kaitaranta JK, Rawdah TN, Macy JI, Boswell FM, 3rd, Smith RE. Meibomian gland studies: comparison of steer and human lipids. Invest Ophthalmol Vis Sci. 1981;20:522–536 [PubMed] [Google Scholar]
- 5. Chew CKS, Hykin PG, Jansweijer C, Dikstein S, Tiffany JM, Bron AJ. The casual level of meibomian lipids in humans. Curr Eye Res. 1993;12:255–259 [DOI] [PubMed] [Google Scholar]
- 6. Yokoi N, Mossa F, Tiffany JM, Bron AJ. Assessment of meibomian gland function in dry eye using meibometry. Arch Ophthalmol. 1999;117:723–729 [DOI] [PubMed] [Google Scholar]
- 7. Yokoi N, Bron AJ, Tiffany J, et al. Reflective meniscometry: a non-invasive method to measure tear meniscus curvature. Br J Ophthalmol. 1999;83:92–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chew CKS. Quantifying meibomian lipid on the human lid margin. Thesis Oxford, UK: University of Oxford; 1993 [Google Scholar]
- 9. Yokoi N, Bron AJ, Tiffany JM, et al. Relationship between tear volume and tear meniscus curvature. Arch Ophthalmol. 2004;122:1265–1269 [DOI] [PubMed] [Google Scholar]
- 10. Andrews JS. Human tear film lipids. I. Composition of the principal non-polar component. Exp Eye Res. 1970;10:223–227 [DOI] [PubMed] [Google Scholar]
- 11. Nicolaides N, Ruth EC. Unusual fatty acids in the lipids of steer and human meibomian gland excreta. Curr Eye Res. 1982;2:93–98 [DOI] [PubMed] [Google Scholar]
- 12. Nicolaides N, Santos EC, Papadakis K, Ruth EC, Muller L. The occurrence of long chain alpha, omega-diols in the lipids of steer and human meibomian glands. Lipids. 1984;19:990–993 [DOI] [PubMed] [Google Scholar]
- 13. Nicolaides N, Santos EC, Papadakis K. Double-bond patterns of fatty acids and alcohols in steer and human meibomian gland lipids. Lipids. 1984;19:264–277 [DOI] [PubMed] [Google Scholar]
- 14. Nicolaides N, Santos EC. The di- and triesters of the lipids of steer and human meibomian glands. Lipids. 1985;20:454–467 [DOI] [PubMed] [Google Scholar]
- 15. Tiffany JM. Individual variations in human meibomian lipid composition. Exp Eye Res. 1978;27:289–300 [DOI] [PubMed] [Google Scholar]
- 16. Tiffany JM. The lipid secretion of the meibomian glands. Adv Lipid Res. 1987;22:1–62 [DOI] [PubMed] [Google Scholar]
- 17. McCulley JP, Shine W. A compositional based model for the tear film lipid layer. Trans Am Ophthalmol Soc. 1997;95:79–88 [PMC free article] [PubMed] [Google Scholar]
- 18. Sullivan BD, Evans JE, Dana MR, Sullivan DA. Influence of aging on the polar and neutral lipid profiles in human meibomian gland secretions. Arch Ophthalmol. 2006;124:1286–1292 [DOI] [PubMed] [Google Scholar]
- 19. Butovich IA. Fatty acid composition of cholesteryl esters of human meibomian gland secretions. Steroids. 2010;75:726–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Butovich IA. The meibomian puzzle: Combining pieces together. Prog Retin Eye Res. 2009;28(6):483–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Butovich IA. Cholesteryl esters as a depot for very long chain fatty acids in human meibum. J Lipid Res. 2009;50:501–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Butovich IA. On the lipid composition of human meibum and tears: comparative analysis of nonpolar lipids. Invest Ophthalmol Vis Sci. 2008;49:3779–3789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Butovich IA, Wojtowicz JC, Molai M. Human tear film and meibum: very long chain wax esters and (O-acyl)-omega-hydroxy fatty acids of meibum. J Lipid Res. 2009;50:2471–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Butovich IA, Millar TJ, Ham BM. Understanding and analyzing meibomian lipids: a review. Curr Eye Res. 2008;33:405–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Butovich IA, Uchiyama E, McCulley JP. Lipids of human meibum: mass-spectrometric analysis and structural elucidation. J Lipid Res. 2007;48:2220–2235 [DOI] [PubMed] [Google Scholar]
- 26. Nichols KK, Ham BM, Nichols JJ, Ziegler C, Green-Church KB. Identification of fatty acids and fatty acid amides in human meibomian gland secretions. Invest Ophthalmol Vis Sci. 2007;48:34–39 [DOI] [PubMed] [Google Scholar]
- 27. Linton RG, Curnow DH, Riley WJ. The Meibomian Glands: an investigation into the secretion and some aspects of the physiology. Br J Ophthalmol. 1961;45:718–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shine WE, McCulley JP. The role of cholesterol in chronic blepharitis. Invest Ophthalmol Vis Sci. 1991;32:2272–2280 [PubMed] [Google Scholar]
- 29. Sullivan BD, Evans JE, Krenzer KL, Dana MR, Sullivan DA. Impact of antiandrogen treatment on the fatty acid profile of neutral lipids in human meibomian gland secretions. J Clin Endocrinol Metab. 2000;85:4866–4873 [DOI] [PubMed] [Google Scholar]
- 30. Sullivan DA, Sullivan BD, Ullman MD. Androgen influence on the meibomian gland. Invest Ophthalmol Vis Sci. 2000;41:3732–3742 [PubMed] [Google Scholar]
- 31. Sullivan BD, Evans JE, Cermak JM, Krenzer KL, Dana MR, Sullivan DA. Complete androgen insensitivity syndrome: effect on human meibomian gland secretions. Arch Ophthalmol. 2002;120:1689–1699 [DOI] [PubMed] [Google Scholar]
- 32. Sullivan DA, Sullivan BD, Evans JE, et al. Androgen deficiency, meibomian gland dysfunction, and evaporative dry eye. Ann NY Acad Sci. 2002:966:211–222 [DOI] [PubMed] [Google Scholar]
- 33. Pes O. Ricerche microchimiche sulla secrezione delle ghiandole sebacee palpebrali. Arch Ottal 1897;5:82–91 [Google Scholar]
- 34. Ehlers N. The precorneal film. biomicroscopical, histological and chemical investigations. Acta Ophthalmol Suppl. 1965;(suppl)81:81–134 [PubMed] [Google Scholar]
- 35. Cory CC, Hinks W, Burton JL, Shuster S. Meibomian gland secretion in the red eyes of rosacea. Br J Dermatol. 1973;89:25–27 [DOI] [PubMed] [Google Scholar]
- 36. Keith CG. Seborrhoeic blepharo-kerato-conjunctivitis. Trans Ophthalmol Soc U K. 1967;87:85–103 [PubMed] [Google Scholar]
- 37. Harvey DJ, Tiffany JM, Duerden JM, Pandher KS, Mengher LS. Identification by combined gas chromatography-mass spectrometry of constituent long-chain fatty acids and alcohols from the meibomian glands of the rat and a comparison with human meibomian lipids. J Chromatogr. 1987;414:253–263 [DOI] [PubMed] [Google Scholar]
- 38. Dougherty JM, McCulley JP. Analysis of the free fatty acid component of meibomian secretions in chronic blepharitis. Invest Ophthalmol Vis Sci. 1986;27:52–56 [PubMed] [Google Scholar]
- 39. Dougherty JM, McCulley JP, Silvany RE, Meyer DR. The role of tetracycline in chronic blepharitis. Inhibition of lipase production in staphylococci. Invest Ophthalmol Vis Sci. 1991;32:2970–2975 [PubMed] [Google Scholar]
- 40. Joffre C, Souchier M, Gregoire S, et al. Differences in meibomian fatty acid composition in patients with meibomian gland dysfunction and aqueous-deficient dry eye. Br J Ophthalmol. 2008;92:116–119 [DOI] [PubMed] [Google Scholar]
- 41. Joffre C, Souchier M, Leclere L, et al. Branched-chain fatty acids, increased in tears of blepharitis patients, are not toxic for conjunctival cells. Br J Ophthalmol. 2009;93(10):1391–1395 [DOI] [PubMed] [Google Scholar]
- 42. Krenzer KL, Dana MR, Ullman MD, et al. Effect of androgen deficiency on the human meibomian gland and the ocular surface. J Clin Endocrinol Metab. 2000;85:4874–4882 [DOI] [PubMed] [Google Scholar]
- 43. Mathers WD, Lane JA. Meibomian gland lipids, evaporation, and tear film stability. Adv Exp Med Biol. 1998;438:349–360 [DOI] [PubMed] [Google Scholar]
- 44. Holly FJ. Formation and rupture of the tear film. Exp Eye Res. 1973;15:515–525 [DOI] [PubMed] [Google Scholar]
- 45. Tiffany JM. Refractive index of meibomian and other lipids. Curr Eye Res. 1986;5(11):887–889 [DOI] [PubMed] [Google Scholar]
- 46. Kocak I, Orgul S, Flammer J. Variability in the measurement of corneal temperature using a noncontact infrared thermometer. Ophthalmologica. 1999;213:345–349 [DOI] [PubMed] [Google Scholar]
- 47. Bron AJ, Tiffany JM. The contribution of meibomian disease to dry eye. Ocul Surf. 2004;2(2):149–165 [DOI] [PubMed] [Google Scholar]
- 48. Wolff E. Muco-cutaneous function of the lid margin and the distribution of the tear fluid. Trans Ophthalmol Soc UK. 1946;66:291–308 [Google Scholar]
- 49. Blackie CA, Korb DR. Recovery time of an optimally secreting meibomian gland. Cornea. 2009;28:293–297 [DOI] [PubMed] [Google Scholar]
- 50. Blackie CA, Korb DR. The diurnal secretory characteristics in individual meibomian glands. Cornea. 2009;29:34–38 [DOI] [PubMed] [Google Scholar]
- 51. Norn M. Expressibility of meibomian secretion: relation to age, lipid precorneal film, scales, foam, hair and pigmentation. Acta Ophthalmol. 1987;65:137–142 [DOI] [PubMed] [Google Scholar]
- 52. Korb DR, Blackie CA. Meibomian gland diagnostic expressibility: correlation with dry eye symptoms and gland location. Cornea. 2008;27(10):1142–1147 [DOI] [PubMed] [Google Scholar]
- 53. Comberg W, Stoewer E. Die Augendruck-steingende Wirkung vershiedener: Muskelaktionen und ihre Bedeutun. Z Augenheilk. 1923;58:617 [Google Scholar]
- 54. Miller D. Pressure of the lid on the eye. Arch Ophthalmol. 1967;78:328–330 [DOI] [PubMed] [Google Scholar]
- 55. Pflugfelder SC, Tseng S, Sanabria O, et al. Evaluation of subjective assessments and objective diagnostic tests for diagnosing tear-film disorders known to cause ocular irritation. Cornea. 1998;17:38–56 [DOI] [PubMed] [Google Scholar]
- 56. Blackie CA, Korb DR, Knop E, Bedi R, Knop N, Holland EJ. Nonobvious obstructive meibomian gland dysfunction. Cornea. 2010;29:1333–1345 [DOI] [PubMed] [Google Scholar]
- 57. Bron AJ, Tiffany JM, Gouveia SM, Yokoi N, Voon LW. Functional aspects of the tear film lipid layer. Exp Eye Res. 2004;78:347–360 [DOI] [PubMed] [Google Scholar]
- 58. King-Smith PE, Hinel EA, Nichols JJ. Application of a novel interferometric method to investigate the relation between lipid layer thickness and tear film thinning. Invest Ophthalmol Vis Sci. 2010;51(5):2418–2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. King-Smith PE, Fink BA, Nichols JJ, Nichols KK, Braun RJ, McFadden GB. The contribution of lipid layer movement to tear film thinning and breakup. Invest Ophthalmol Vis Sci. 2009;50(6):2747–2756 [DOI] [PubMed] [Google Scholar]
- 60. Owens H, Phillips JR. Spreading of tears after a blink: velocity and stabilization time in healthy eyes. Cornea. 2001;20:484–487 [DOI] [PubMed] [Google Scholar]
- 61. Owens H, Phillips JR. Tear spreading rates: post-blink. Adv Exp Med Biol. 2002;506:1201–1204 [DOI] [PubMed] [Google Scholar]
- 62. Goto E, Tseng SC. Differentiation of lipid tear deficiency dry eye by kinetic analysis of tear interference images. Arch Ophthalmol. 2003;121:173–180 [DOI] [PubMed] [Google Scholar]
- 63. Doane MG. Interactions of eyelids and tears in corneal wetting and dynamics of the normal human eyeblink. Am J Ophthalmol. 1980;89:507–516 [DOI] [PubMed] [Google Scholar]
- 64. Tsubota K. Tear dynamics and dry eye. Prog Retin Eye Res. 1998;17:565–596 [DOI] [PubMed] [Google Scholar]
- 65. McCulley JP, Shine WE. Meibomian gland and tear film lipids: structure, function and control. Adv Exp Med Biol. 2002;506:373–378 [DOI] [PubMed] [Google Scholar]
- 66. Goto E, Tseng SC. Kinetic analysis of tear interference images in aqueous tear deficiency dry eye before and after punctal occlusion. Invest Ophthalmol Vis Sci. 2003;44:1897–1905 [DOI] [PubMed] [Google Scholar]
- 67. Rolando M, Valente C, Barabino S. New test to quantify lipi layer behavior in healthy subjects and patients with keratoconjunctivitis sicca. Cornea. 2008;27(8):866–870 [DOI] [PubMed] [Google Scholar]
- 68. McDonald JE. Surface phenomena of the tear film. Am J Ophthalmol. 1969;67:56–64 [DOI] [PubMed] [Google Scholar]
- 69. DEWS Methodologies to Diagnose and Monitor Dry Eye Disease: Report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop. Ocul Surf. 2007;5:108–152 [DOI] [PubMed] [Google Scholar]
- 70. Behrens A, Doyle JJ, Stern L, et al. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea. 2006;25:900–907 [DOI] [PubMed] [Google Scholar]
- 71. Duke-Elder WS, MacFaul PA. The ocular adnexa, Part I: inflammations of the lid margins. Vol 13. In: System of Ophthalmology. London: H. Kimpton; 1974:205–250 [Google Scholar]
- 72. McCulley JP, Sciallis GF. Meibomian keratoconjunctivitis. Am J Ophthalmol. 1977;84(6):788–793 [DOI] [PubMed] [Google Scholar]
- 73. Gutgesell VJ, Stern GA, Hood CI. Histopathology of meibomian gland dysfunction. Am J Ophthalmol. 1982;94:383–387 [DOI] [PubMed] [Google Scholar]
- 74. Luchs J. Efficacy of topical azithromycin ophthalmic solution 1% in the treatment of posterior blepharitis. Adv Ther. 2008;25(9):858–870 [DOI] [PubMed] [Google Scholar]
- 75. Bron AJ, Benjamin L, Snibson GR. Meibomian gland disease. classification and grading of lid changes. Eye. 1991;5:395–411 [DOI] [PubMed] [Google Scholar]
- 76. Schrader S, Mircheff AK, Geerling G. Animal models of dry eye. Dev Ophthalmol. 2008;41:298–312 [DOI] [PubMed] [Google Scholar]
- 77. Bron AJ, Tiffany JM. The evolution of lid margin changes in blepharitis. In: Lass JH. ed. Advances in Corneal Research: Selected Transactions of the World Congress on the Cornea IV. New York: Plenum Press: 1997:3–18 [Google Scholar]
- 78. Obata H. Anatomy and histopathology of human meibomian gland. Cornea. 2002;21(7 Suppl):S70–S74 [DOI] [PubMed] [Google Scholar]
- 79. Matsumoto Y, Sato EA, Ibrahim OM, et al. The application of confocal microscopy to the diagnosis and evaluation of meibomian gland dysfunction. Mol Vis. 2008;14:1263–1271 [PMC free article] [PubMed] [Google Scholar]
- 80. Korb DR, Henriquez AS. Meibomian gland dysfunction and contact lens intolerance. J Am Optom Assoc. 1980;51(3):243–251 [PubMed] [Google Scholar]
- 81. Korb DR, Blackie CA. Diagnostic versus therapeutic meibomian gland expression (abstract). American Academy of Optometry annual meeting, Orlando, 2009. Available at http://www.aaopt.org/Submission/Search/SubmissionViewer.asp?SID=25745&BR=SP. Abstract 90745 [Google Scholar]
- 82. Goto E, Shimazaki J, Monden Y, et al. Low-concentration homogenized castor oil eye drops for noninflamed obstructive meibomian gland dysfunction. Ophthalmology. 2002;109(11):2030–2035 [DOI] [PubMed] [Google Scholar]
- 83. Hykin PG, Bron AJ. Age-related morphological changes in lid margin and meibomian gland anatomy. Cornea. 1992;11:334–342 [DOI] [PubMed] [Google Scholar]
- 84. Lemp MA. Report of the National Eye Institute/Industry Workshop on Clinical Trials in Dry Eyes. CLAO J. 1995;21:221–231 [PubMed] [Google Scholar]
- 85. DEWS The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop. Ocul Surf. 2007;5:75–92 [DOI] [PubMed] [Google Scholar]
- 86. Shimazaki J, Sakata M, Tsubota K. Ocular surface changes and discomfort in patients with meibomian gland dysfunction. Arch Ophthalmol. 1995;113:1266–1270 [DOI] [PubMed] [Google Scholar]
- 87. Shimazaki J, Goto E, Ono M, et al. Meibomian gland dysfunction in patients with Sjögren syndrome. Ophthalmology. 1998;105:1485–1488 [DOI] [PubMed] [Google Scholar]
- 88. Driver PJ, Lemp MA. Meibomian gland dysfunction. Surv Ophthalmol. 1996;40(5):343–367 [DOI] [PubMed] [Google Scholar]
- 89. Lemp MA, Nichols KK. Blepharitis in the United States 2009: a survey-based perspective on prevalence and treatment. Ocul Surf. 2009;7(suppl 2):S1–S14 [DOI] [PubMed] [Google Scholar]
- 90. Mathers WD. Ocular evaporation in meibomian gland dysfunction and dry eye. Ophthalmology. 1993;100:347–351 [DOI] [PubMed] [Google Scholar]
- 91. Mathers WD. Meibomian gland disease. In: Pflugfelder SC, Stern ME, Beuerman RW. eds. Dry Eye and the Ocular Surface: A Unified Approach. New York: Marcel Dekker; 2004:chap 22 [Google Scholar]
- 92. Khanal S, Tomlinson A, McFadyen A, Diaper C, Ramaesh K. Dry eye diagnosis. Invest Ophthalmol Vis Sci. 2008;49:1407–1414 [DOI] [PubMed] [Google Scholar]
- 93. Tomlinson A, Doane MG, McFadyen A. Inputs and outputs of the lacrimal system: review of production and evaporative loss. Ocul Surf. 2009;7(4):186–198 [DOI] [PubMed] [Google Scholar]
- 94. Robin JB, Jester JV, Nobe J, Nicolaides N, Smith RE. In vivo transillumination biomicroscopy and photography of meibomian gland dysfunction; a clinical study. Ophthalmology. 1985;92:1423–1426 [DOI] [PubMed] [Google Scholar]
- 95. Ong BL, Hodson SA, Wigham T, Miller F, Larke JB. Evidence for keratin proteins in normal and abnormal human meibomian fluids. Curr Eye Res. 1991;10:1113–1119 [DOI] [PubMed] [Google Scholar]
- 96. Mathers WD, Shields WJ, Sachdev MA, Petroll WM, Jester JV. Meibomian gland dysfunction in chronic blepharitis. Cornea. 1991;10:277–285 [DOI] [PubMed] [Google Scholar]
- 97. Mathers WD, Shields WJ, Sachdev MS, et al. Meibomian gland morphology and tear osmolarity: changes with Accutane therapy. Cornea. 1991;10:286–290 [DOI] [PubMed] [Google Scholar]
- 98. Henriquez AS, Korb DR. Meibomian glands and contact lens wear. Br J Ophthalmol. 1981;65:108–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Yamaguchi M, Kutsuna M, Uno T, et al. Marx line: fluorescein staining line on the inner lid as indicator of meibomian gland function. Am J Ophthalmol. 2006;141:669–675 [DOI] [PubMed] [Google Scholar]
- 100. Marx E. Uber vitale Farbung des Auges und der Augenlieder. I. Uber Anatomie, Physiologie und Pathologie des Augenlidrandes under der Tranenpunkte. Graefes Arch Clin Exp Ophthalmol. 1924;114:465–482 [Google Scholar]
- 101. Marx E. Uber vitale Farbung des Auges und der Augenlieder. I. Uber Anatomie, Physiologie und Pathologie des Augenlidrandes under der Tranenpunkte. Graefes Arch Clin Exp Ophthalmol. 1926;116:114–125 [Google Scholar]
- 102. Doughty MJ, Naase T, Donald C, Hamilton L, Button NF. Visualization of “Marx's line” along the marginal eyelid conjunctiva of human subjects with lissamine green dye. Ophthalmic Physiol Opt. 2004;24:1–7 [DOI] [PubMed] [Google Scholar]
- 103. Hughes C, Hamilton L, Doughty MJ. A quantitative assessment of the location and width of Marx's line along the marginal zone of the human eyelid. Optom Vis Sci. 2003;80:564–572 [DOI] [PubMed] [Google Scholar]
- 104. DEWS Meibography/Meiboscopy. Template 25. 2007c. Available online only at: http://www.tearfilm.org/dewsreport/pdfs/MeibographyMeiboscopy%20(Foulks).pdf Accessed Sept. 29, 2010
- 105. Jester JV, Nicolaides N, Smith RE. Meibomian gland dysfunction, I: keratin protein expression in normal human and rabbit meibomian glands. Invest Ophthalmol Vis Sci. 1989;30:927–935 [PubMed] [Google Scholar]
- 106. de Paiva CS, Lindsey JL, Pflugfelder SC. Assessing the severity of keratitis sicca with videokeratoscopic indices. Ophthalmology. 2003;110:1102–1109 [DOI] [PubMed] [Google Scholar]
- 107. Nichols JJ, Berntsen DA, Mitchell GL, Nichols KK. An assessment of grading scales for meibography images. Cornea. 2005;24:382–388 [DOI] [PubMed] [Google Scholar]
- 108. Mathers WD, Billborough M. Meibomian gland function and giant papillary conjunctivitis. Am J Ophthalmol. 1992;114:188–192 [DOI] [PubMed] [Google Scholar]
- 109. Arita R, Itoh K, Inoue K, Kuchiba A, Yamaguchi T, Amano S. Contact lens wear is associated with decrease of meibomian glands. Ophthalmology. 2009;116(3):379–384 [DOI] [PubMed] [Google Scholar]
- 110. Bailey IL, Bullimore MA, Raasch TW, Taylor HR. Clinical grading and the effects of scaling. Invest Ophthalmol Vis Sci. 1991;32:422–432 [PubMed] [Google Scholar]
- 111. Efron N, Morgan PB, Katsara SS. Validation of grading scales for contact lens complications. Ophthalmic Physiol Opt. 2001;21:17–29 [PubMed] [Google Scholar]
- 112. Paugh JR, Marsden HJ, Edrington TB, DeLand PN, Simmons PA, Vehige JG. A pre-application drop containing carboxymethylcellulose can reduce multipurpose solution-induced corneal staining. Optom Vis Sci. 2007;84:65–71 [DOI] [PubMed] [Google Scholar]
- 113. Sparrow NA, Frost NA, Pantelides EP, Laidlaw DA. Decimalization of The Oxford Clinical Cataract Classification and Grading System. Ophthalmic Epidemiol. 2000;7:49–60 [PubMed] [Google Scholar]
- 114. McCann LC, Tomlinson A, Pearce EI, Diaper C. Tear and meibomian gland function in blepharitis and normals. Eye Contact Lens. 2009;35(4):203–208 [DOI] [PubMed] [Google Scholar]
- 115. Mathers WD, Lane JA, Sutphin JE, et al. Model for ocular tear film function. Cornea. 1996;15:110–119 [DOI] [PubMed] [Google Scholar]
- 116. Hykin PG, Bron AJ. Age-related morphological changes in lid margin and meibomian gland anatomy. Cornea. 1992;11:334–342 [DOI] [PubMed] [Google Scholar]
- 117. Bron AJ, Yokoi N, Gaffney E, Tiffany JM. Predicted phenotypes of dry eye: proposed consequences of its natural history. Ocul Surf. 2009;7:78–92 [DOI] [PubMed] [Google Scholar]
- 118. Khanal S, Tomlinson A, Diaper CJ. Tear physiology of aqueous deficiency and evaporative dry eye. Optom Vis Sci. 2009;86(11):1235–1240 [DOI] [PubMed] [Google Scholar]
- 119. Suzuki T, Mitsuishi Y, Sano Y, Yokoi N, Kinoshita S. Phlyctenular keratitis associated with meibomitis in young patients. Am J Ophthalmol. 2005;140(1):77–82 [DOI] [PubMed] [Google Scholar]
- 120. Lam H, Bleiden L, de Paiva CS, Farley W, Stern ME, Pflugfelder SC. Tear cytokine profiles in dysfunctional tear syndrome. Am J Ophthalmol. 2009;147(2):198–205-e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Goto E, Endo K, Suzuki A, et al. Tear evaporation dynamics in normal subjects and subjects with obstructive meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2003;44:533–539 [DOI] [PubMed] [Google Scholar]
- 122. Yokoi N. [Tear dynamics and dry eye.] Nippon Ganka Gakkai Zasshi. 2004;108:275–276 [PubMed] [Google Scholar]
- 123. Yokoi N, Yamada H, Mizukusa Y, et al. Rheology of tear film lipid layer spread in normal and aqueous tear-deficient dry eyes. Invest Ophthalmol Vis Sci. 2008;49:5319–5324 [DOI] [PubMed] [Google Scholar]
- 124. Yokoi N, Komuro A. Non-invasive methods of assessing the tear film. Exp Eye Res. 2004;78(3):399–407 [DOI] [PubMed] [Google Scholar]
- 125. McCulley JP, Dougherty JM, Deneau DG. Classification of chronic blepharitis. Ophthalmology. 1982;89(10),1173–1180 [DOI] [PubMed] [Google Scholar]
- 126. Martin NF, Rubinfeld RS, Malley JD, Manzitti JD. Giant papillary conjunctivitis and meibomian gland dysfunction blepharitis. CLAO J. 1992;18:165–169 [PubMed] [Google Scholar]
- 127. Ong BL, Larke JR. Meibomian gland dysfunction: some clinical, biochemical and physical observations. Ophthalmic Physiol Opt. 1990;10:144–148 [DOI] [PubMed] [Google Scholar]
- 128. Huber-Spitzy V, Baumgartner I, Böhler-Sommeregger K, Grabner G. Blepharitis—a diagnostic and therapeutic challenge. A report on 407 consecutive cases. Graefes Arch Clin Exp Ophthalmol. 1991;229:224–227 [DOI] [PubMed] [Google Scholar]
- 129. Huber-Spitzy V, Böhler-Sommeregger K, Arocker-Mettinger E, Grabner G. Ulcerative blepharitis in atopic patients—is Candida species the causative agent? Br J Ophthalmol. 1992;76(5):272–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Blackman HJ, Recka GL, Olsen TG, Bergsma DR. Blepharoconjunctivitis: a side effect of 13-cis-retinoic acid therapy for dermatologic diseases. Ophthalmology. 1979;86:753–759 [DOI] [PubMed] [Google Scholar]
- 131. Fraunfelder FT, LaBraico JM, Meyer SM. Adverse ocular reactions possibly associated with isotretinoin. Am J Ophthalmol. 1985;100:534–537 [DOI] [PubMed] [Google Scholar]
- 132. McMonnies CW. Key questions in a dry eye history. J Am Optometric Assoc. 1986;57:512–517 [PubMed] [Google Scholar]
- 133. Johnson ME, Murphy PJ. Measurement of ocular surface irritation on a linear interval scale with the ocular comfort index. Invest Ophthalmol Vis Sci. 2007;48(10):4451–4458 [DOI] [PubMed] [Google Scholar]
- 134. Mengher LS, Bron AJ, Tonge SR, et al. Effect of fluorescein instillation on the precorneal tear film stability. Curr Eye Res. 1985;4:9–12 [DOI] [PubMed] [Google Scholar]
- 135. Sakamoto R, Bennett ES, Henry VA, et al. The phenol red thread tear test: a cross-cultural study. Invest Ophthalmol Vis Sci. 1993;34:3510–3514 [PubMed] [Google Scholar]
- 136. Patel S, Farrell J, Blades KJ, Grierson DJ. The value of a phenol red impregnated thread for differentiating between the aqueous and non-aqueous deficient dry eye. Ophthalmic Physiol Opt. 1998;18:471–476 [PubMed] [Google Scholar]
- 137. van Bijsterveld OP. Diagnostic tests in the Sicca syndrome. Arch Ophthalmol. 1969;82(1):10–14 [DOI] [PubMed] [Google Scholar]
- 138. Vitali C, Bombardieri S, Johsson R, et al. Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61(6):554–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Kobayashi A, Yoshita T, Sugiyama K. In vivo findings of the bulbar/palpebral conjunctiva and presumed meibomian glands by laser scanning confocal microscopy. Cornea. 2005;24(8):985–988 [DOI] [PubMed] [Google Scholar]
- 140. Matsumoto Y, Dogru M, Sato EA, et al. The application of in vivo confocal scanning laser microscopy in the management of Acanthamoeba keratitis. Mol Vis. 2007;13:1319–1326 [PubMed] [Google Scholar]
- 141. Farrell J, Patel S, Grierson DG, Sturrock RD. A clinical procedure to predict the value of temporary occlusion therapy in keratoconjunctivitis sicca. Ophthalmic Physiol Opt. 2003;23:1–8 [DOI] [PubMed] [Google Scholar]
- 142. Mainstone JC, Bruce AS, Golding TR. Tear meniscus measurement in the diagnosis of dry eye. Curr Eye Res. 1996;15:653–661 [DOI] [PubMed] [Google Scholar]
- 143. Xu KP, Yagi Y, Toda I, Tsubota K. Tear function index. a new measure of dry eye. Arch Ophthalmol. 1995;113:84–88 [DOI] [PubMed] [Google Scholar]
- 144. McCann LC, Tomlinson A, Pearce EI, Khanal S, Kaye SB, Fisher AC. A clinical alternative to fluorophotometry for measuring tear production in the diagnosis of dry eye. Cornea. 2010;29(7):745–750 [DOI] [PubMed] [Google Scholar]
- 145. Ousler GW, 3rd, Hagberg KW, Schindelar M, Welch D, Abelson MB. The Ocular Protection Index. Cornea. 2008;27(5):509–513 [DOI] [PubMed] [Google Scholar]
- 146. Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118(5):615–621 [DOI] [PubMed] [Google Scholar]
- 147. Begley CG, Caffery B, Nichols K, Mitchell GL, Chalmers R; DREI study group: results of a dry eye questionnaire from optometric practices in North America. Adv Exp Med Biol. 2002;506:1009–1016 [DOI] [PubMed] [Google Scholar]
- 148. McMonnies CW. Key questions in a dry eye history. J Am Optom Assoc, 1986:57;512–517 [PubMed] [Google Scholar]
- 149. Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118(5):615–621 [DOI] [PubMed] [Google Scholar]
- 150. Bandeen Roche R, Muñoz B, Teilsch JM, West SK, Schein OD. Self-reported assessment of dry eye in a population-based setting. Invest Ophthalmol Vis Sci., 1997;38(12):2469–2475 [PubMed] [Google Scholar]
- 151. Johnson ME, Murphy PJ. Measurement of ocular surface irritation on a linear interval scale with the ocular comfort index. Invest Ophthalmol Vis Sci., 2007;48(10):4451–4458 [DOI] [PubMed] [Google Scholar]
- 152. Begley CG, Caffery B, Chalmers RL, Mitchell GL; Dry Eye Investigation (DREI) Study Group Use of the dry eye questionnaire to measure symptoms of ocular irritation in patients with aqueous tear deficient dry eye. Cornea. 2002;21(7):664–670 [DOI] [PubMed] [Google Scholar]
- 153. Nichols KK. Pateint history, symptoms, and questionnaires for dry eye disease. In: Asbell PA, Lemp MA. eds. Dry Eye Diseases., New York: Thieme; 2006:24–32 [Google Scholar]
- 154. DEWS The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5(2):93–107 [DOI] [PubMed] [Google Scholar]
- 155. Simpson TL, Situ P, Jones LW, Fonn D. Dry eye symptoms assessed by four questionnaires. Optom Vis Sci. 2008;85(8):692–698 [DOI] [PubMed] [Google Scholar]
- 156. DEWS The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the international Dry Eye WorkShop (2007). Ocul Surf. 2007;5(2):75–92 [DOI] [PubMed] [Google Scholar]
- 157. Lin PY, Cheng CY, Hsu WM. Association between symptoms and signs of dry eye among an elderly chinese population in Taiwan: The Shihpai Eye Study. Invest Ophthalmol Vis Sci. 2005;46(5):1593–1598 [DOI] [PubMed] [Google Scholar]
- 158. Lekhanont K, Rojanaporn D, Chuck RS, Vongthongsri A. Prevalence of dry eye in Bangkok, Thailand. Cornea. 2006;25(10):1162–1167 [DOI] [PubMed] [Google Scholar]
- 159. Shimazaki J, Sakata M, Tsubota K. Ocular surface changes and discomfort in patients with meibomian gland dysfunction. Arch Ophthalmol. 1995;113(10):1266–1270 [DOI] [PubMed] [Google Scholar]
- 160. Fenga C, Aragona P, Cacciola A, et al. Meibomian gland dysfunction and ocular discomfort in video display terminal workers. Eye. 2008;22(1):91–95 [DOI] [PubMed] [Google Scholar]
- 161. Korb DR, Blackie CA. Meibomian gland diagnostic expressibility: correlation with dry eye symptoms and gland location. Cornea. 2008;27(10):1142–1147 [DOI] [PubMed] [Google Scholar]
- 162. Miller KL, Walt JG, Mink DR, et al. Minimal clinically important difference for the Ocular Surface Disease Index. Arch Ophthalmol. 2010;128(1):94–101 [DOI] [PubMed] [Google Scholar]
- 163. DEWS The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop. Ocul Surf. 2007;5:87. [DOI] [PubMed] [Google Scholar]
- 164. Craig JP, Tomlinson A. Importance of the lipid layer in human tear film stability and evaporation. Optom Vis Sci. 1997;74(1):8–13 [DOI] [PubMed] [Google Scholar]
- 165. Bron AJ, Tiffany JM. The contribution of meibomian disease to dry eye. Ocul Surf. 2004;2(2):149–165 [DOI] [PubMed] [Google Scholar]
- 166. Foulks GN. The correlation between the tear film lipid layer and dry eye disease. Surv Ophthalmol. 2007;52:369–74 [DOI] [PubMed] [Google Scholar]
- 167. Yokoi N, Mossa F, Tiffany JM, Bron AJ. Assessment of meibomian gland function in dry eye using meibometry. Arch Ophthalmol. 1999;117(6):723–729 [DOI] [PubMed] [Google Scholar]
- 168. Isreb MA, Greiner JV, Korb DR, et al. Correlation of lipid layer thickness measurements with fluorescein tear film break-up time and Schirmer's test. Eye. 2003;17(1):79–83 [DOI] [PubMed] [Google Scholar]
- 169. Pflugfelder SC, Tseng SC, Sanabria O, et al. Evaluation of subjective assessments and objective diagnostic tests for diagnosing tear-film disorders known to cause ocular irritation. Cornea. 1998;17:38–56 [DOI] [PubMed] [Google Scholar]
- 170. Nichols JJ, Nichols KK, Puent B, et al. Evaluation of tear film interference patterns and measures of tear break-up time. Optom Vis Sci. 2002;79(6):363–369 [DOI] [PubMed] [Google Scholar]
- 171. Goto E, Dogru M, Fukagawa K, et al. Successful tear lipid layer treatment for refractory dry eye in office workers by low-dose lipid application on the full-length eyelid margin. Am J Ophthalmol. 2006;142(2):264–270 [DOI] [PubMed] [Google Scholar]
- 172. Mishima S, Maurice DM. The oily layer of the tear film and evaporation from the corneal surface. Exp Eye Res. 1961;1:39–45 [DOI] [PubMed] [Google Scholar]
- 173. McCulley JP, Shine WE. Meibomian gland function and the tear lipid layer. Ocul Surf. 2003;1:97–106 [DOI] [PubMed] [Google Scholar]
- 174. Norn M. Expressibility of meibomian secretion. Relation to age, lipid precorneal film, scales, foam, hair and pigmentation. Acta Ophthalmol (Copenh). 1987;65(2):137–142 [DOI] [PubMed] [Google Scholar]
- 175. Korb DR, Greiner JV. Increase in tear film lipid layer thickness following treatment of meibomian gland dysfunction. Adv Exp Med Biol. 1994;350:293–298 [DOI] [PubMed] [Google Scholar]
- 176. Korb DR, Blackie CA. Meibomian gland diagnostic expressibility: correlation with dry eye symptoms and gland location. Cornea. 2008;27(10):1142–1147 [DOI] [PubMed] [Google Scholar]
- 177. Guillon JP, Guillon M. Tear film examination of the contact lens patient. Optician. 1993;206(5421):21–29 [Google Scholar]
- 178. Korb DR. The tear film: its role today and in the future. In: Korb DR, Craig J, Doughty M, Guillion J-P, Tomlinson A, Smith G. eds. The Tear Film: Structure, Function and Clinical Examination. Boston: Butterworth-Heinemann; 2002;9–13 [Google Scholar]
- 179. DEWS The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop. Ocul Surf. 2007;5:133. [DOI] [PubMed] [Google Scholar]
- 180. King-Smith PE, Fink BA, Nichols JJ, Nichols KK, Braun RJ, McFadden GB. The contribution of lipid layer movement to tear film thinning and breakup. Invest Ophthalmol Vis Sci. 2009;50(6):2747–2756 [DOI] [PubMed] [Google Scholar]
- 181. Korb DR. Survey of preferred tests for diagnosis of the tear film and dry eye. Cornea. 2000;19:483–486 [DOI] [PubMed] [Google Scholar]
- 182. Lemp MA, Hamill JR. Factors affecting tear film break-up in normal eyes. Arch Ophthalmol. 1973;89:103–105 [DOI] [PubMed] [Google Scholar]
- 183. Norn MS. Desiccantion of the precorneal tear film. I. Corneal wetting time. Acta Ophthalmol (Copenh) 1969;47:865–880 [DOI] [PubMed] [Google Scholar]
- 184. Abelson M, Ousler G, Nally L. Alternate reference values for tear film break-up time in normal and dry eye populations. Adv Exp Med Biol. 2002;506:1121–1125 [DOI] [PubMed] [Google Scholar]
- 185. Korb DR, Greiner JV, Herman J. Comparison of fluorescein break-up time measurement reproducibility using standard fluorescein strips versus the Dry Eye Test (DET) method. Cornea. 2001;20(8):811–815 [DOI] [PubMed] [Google Scholar]
- 186. Marquardt R, Stodmeister R, Christ T. Modification of tear film break-up time test for increased reliability. In: Holly FJ. ed. The Precorneal Tear Film in Health, Disease, and Contact Lens Wear. Lubbock, TX: Dry Eye Institute; 1986:57–63 [Google Scholar]
- 187. Vanley GT, Leopold IH, Greg TH. Interpretation of tear film breakup. Arch Ophthalmol. 1977;95:445–448 [DOI] [PubMed] [Google Scholar]
- 188. Mengher LS, Bron AJ, Tonge SR, et al. Effect of fluorescein instillation on the precorneal tear film stability. Curr Eye Res. 1985;4:9–12 [DOI] [PubMed] [Google Scholar]
- 189. Mengher LS, Bron AJ, Tonge SR, et al. A non-invasive instrument for clinical assessment of the pre-corneal tear film stability. Curr Eye Res. 1985;4:1–7 [DOI] [PubMed] [Google Scholar]
- 190. Larke JR. The Eye in Contact Lens Wear. 2nd ed. Oxford, UK: Butterworth-Heinemann; 1997:32–33 [Google Scholar]
- 191. Lemp MA. Report of the National Eye Institute/Industry Workshop on Clinical Trials in Dry Eye. CLAO J. 1995;23:221–232 [PubMed] [Google Scholar]
- 192. Vitali C, Moutsopoulos HM, Bombardieri S. The European Community Study Group on diagnostic criteria for Sjogren's syndrome. Sensitivity and specificity of tests for ocular and oral involvement in Sjogren's syndrome. Ann Rheum Dis. 1994;53(10): 637–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Tonge SR, Hunsaker J, Holly FJ. Noninvasive assessment of tear film break-up time in a group of normal subjects: implications for contact lens wear. J Br Cont Lens Assoc. 1991;14:201–205 [Google Scholar]
- 194. Rengstorff RH. The precorneal tear film: break-up time and location in normal subjects. Am J Optom Physiol Opt. 1974;51:765–759 [PubMed] [Google Scholar]
- 195. Cho P, Douthwaite W. The relation between invasive and noninvasive tear break-up time. Optom Vis Sci. 1995. January;72(1):17–27 [DOI] [PubMed] [Google Scholar]
- 196. DEWS Methodologies to diagnose and monitor dry eye disease: Report of the Diagnostic Methodology Subcommittee of the International Dry Eye Workshop. Ocul Surf. 2007;5:108–152 [DOI] [PubMed] [Google Scholar]
- 197. Khanal S, Tomlinson A, McFadyen A, Diaper C, Ramaesh K. Dry eye diagnosis. Invest Ophthalmol Vis Sci. 2008;49:1407–1414 [DOI] [PubMed] [Google Scholar]
- 198. Nichols K, Mitchell L, Zadnik K. The repeatability of clinical measurements of dry eye. Cornea. 2004;23:272–285 [DOI] [PubMed] [Google Scholar]
- 199. Nichols KK, Nichols JJ, Mitchell GL. The relation between tear film tests in patients with dry eye disease. Ophthal Physiol Opt. 2003;23:553–560 [DOI] [PubMed] [Google Scholar]
- 200. Nichols KK, Nichols JJ, Mitchell GL. The lack of association between signs and symptoms in patients with dry eye disease. Cornea. 2004;23:762–770 [DOI] [PubMed] [Google Scholar]
- 201. Matsumoto Y, Sato EA, Ibrahim OM, et al. The application of in vivo laser confocal microscopy to the diagnosis and evaluation of meibomian gland dysfunction. Mol Vis. 2008;14:1263–1271 [PMC free article] [PubMed] [Google Scholar]
- 202. Matsumoto Y, Shigeno Y, Sato EA, et al. The evaluation of the treatment response in obstructive meibomian gland disease by in vivo laser confocal microscopy. Graefes Arch Clin Exp Ophthalmol. 2009;247(6):821–829 [DOI] [PubMed] [Google Scholar]
- 203. Ishida R, Matsumoto Y, Onguchi T, et al. Tear film with Orgahexa eye masks in patients with meibomian gland dysfunction. Optom Vis Sci 2008;85:684–691 [DOI] [PubMed] [Google Scholar]
- 204. Matsumoto Y, Dogru M, Goto E, et al. Efficacy of a new warm moist air device on tear functions of patients with simple meibomian gland dysfunction. Cornea. 2006;25:644–650 [DOI] [PubMed] [Google Scholar]
- 205. Shimazaki J, Goto E, Ono M, Shimmura S, Tsubota K. Meibomian gland dysfunction in patients with Sjögren's syndrome. Ophthalmology. 1998;105:1485–1488 [DOI] [PubMed] [Google Scholar]
- 206. Goto E, Matsumoto Y, Kamoi M, et al. Tear evaporation rates in Sjogren's syndrome and non Sjogren dry eye patients. Am J Ophthalmol. 2007;144:81–85 [DOI] [PubMed] [Google Scholar]
- 207. Den S, Shimizu K, Ikeda T, Tsubota K, Shimmura S, Shimazaki J. Association between meibomian gland changes and aging, sex or tear function. Cornea. 2006;25:651–655 [DOI] [PubMed] [Google Scholar]
- 208. Arita R, Itoh K, Inoue K, Amano S. Noncontact infrared meibography to document age related changes of the meibomian glands in a normal population. Ophthalmology. 2008;115:911–915 [DOI] [PubMed] [Google Scholar]
- 209. Joffre C, Souchier M, Grégoire S, et al. Differences in meibomian fatty acid composition in patients with meibomian gland dysfunction and aqueous-deficient dry eye. Br J Ophthalmol. 2008;92:116–119 [DOI] [PubMed] [Google Scholar]
- 210. Souchier M, Joffre C, Grégoire S, et al. Changes in meibomian fatty acids and clinical signs in patients with meibomian gland dysfunction after minocycline treatment. Br J Ophthalmol. 2008;92:819–822 [DOI] [PubMed] [Google Scholar]
- 211. Sakamoto R, Bennett ES, Henry VA, et al. The phenol red thread tear test: a cross-cultural study. Invest Ophthalmol Vis Sci. 1993;34:3510–3514 [PubMed] [Google Scholar]
- 212. Labetouelle M, Mariette X, Joyeau L, et al. The phenol red thread first results for the assessment of of the cut off value in ocular sicca syndrome (in French). J Fr Ophtalmol. 2002;25:674–680 [PubMed] [Google Scholar]
- 213. Saleh TA, McDermott B, Bates AK, Ewings P. Phenol red thread test versus Schirmer's test: a comparative study. Eye. 2006;20:913–915 [DOI] [PubMed] [Google Scholar]
- 214. Patel S, Farrell J, Blades KJ, Grierson DJ. The value of a phenol red impregnated thread for differentiating between the aqueous and non-aqueous deficient dry eye. Ophthalmic Physiol Opt. 1998;18:471–476 [PubMed] [Google Scholar]
- 215. Pflugfelder SC, de Paiva CS, Li DQ, Stern ME. Epithelial-immune cell interaction in dry eye. Cornea. 2008;27(suppl 1):S9–S11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Luo L, Li DQ, Doshi A, et al. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004;45:4293–4301 [DOI] [PubMed] [Google Scholar]
- 217. Mathers WD. Ocular evaporation in meibomian gland dysfunction and dry eye. Ophthalmology. 1993;100:347–351 [DOI] [PubMed] [Google Scholar]
- 218. Shine WE, Silvany R, McCulley JP. Relation of cholesterol-stimulated Staphylococcus aureus growth to chronic blepharitis. Invest Ophthalmol Vis Sci. 199334:2291–2296 [PubMed] [Google Scholar]
- 219. McCulley JP, Sciallis GF. Meibomian keratoconjunctivitis. Am J Ophthalmol. 1977;84(6):788–793 [DOI] [PubMed] [Google Scholar]
- 220. McCulley JP. Meibomitis. In: Kaufman HE, Barron, McDonald MB. eds. The Cornea. London: Churchill Livingstone: 1988:125–137 [Google Scholar]
- 221. Dougherty JM, McCulley JP. Bacterial lipases and chronic blepharitis. Invest Ophthalmol Vis Sci. 1986;27(4):486–491 [PubMed] [Google Scholar]
- 222. Brignole F, Pisella PJ, Goldschild M, et al. Flow cytometric analysis of inflammatory markers in conjunctival epithelial cells of patients with dry eyes. Invest Ophthalmol Vis Sci. 2000;41:1356–1363 [PubMed] [Google Scholar]
- 223. Brignole F, Pisella PJ, De Saint Jean M, Goldschild M, Goguel A, Baudouin C. Flow cytometric analysis of inflammatory markers in KCS: 6-month treatment with topical cyclosporin A. Invest Ophthalmol Vis Sci. 2001;42:90–95 [PubMed] [Google Scholar]
- 224. Brignole F, Ott AC, Warnet JM, Baudouin C. Flow cytometry in conjunctival impression cytology: a new tool for exploring ocular surface pathologies. Exp Eye Res. 2004;78:473–481 [DOI] [PubMed] [Google Scholar]
- 225. DEWS Management and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5(2):163–178 [DOI] [PubMed] [Google Scholar]
- 226. Behrens A, Doyle JJ, Stern L, et al. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea. 2006;25(8):900–907 [DOI] [PubMed] [Google Scholar]
- 227. van Bijsterveld OP. Diagnostic tests in the sicca syndrome. Arch Ophthalmol. 1969;82(1):10–14 [DOI] [PubMed] [Google Scholar]
- 228. Lemp MA. Report of the National Eye Institute/Industry Workshop on Clinical Trials in Dry Eye. CLAO J. 1995;23:221–232 [PubMed] [Google Scholar]
- 229. Bron AJ., Evans VE, Smith JA, et al. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22(7):640–650 [DOI] [PubMed] [Google Scholar]
- 230. Bron AJ, Lang C, Foulks GN, Tiffany JM. A new system for grading staining at the surface of the eye (poster). TFOS Meeting, Taormina, Italy; 2006 [Google Scholar]
- 231. Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61(6): 554–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Nichols K, Mitchell L, Zadnik K, et al. The repeatability of clinical measurements of dry eye. Cornea. 2004;23(3): 272–285 [DOI] [PubMed] [Google Scholar]
- 233. Berntsen DA, Mitchell GL, Nichols JJ. Reliability of grading lissamine green conjunctival staining. Cornea. 2006;25:695–700 [DOI] [PubMed] [Google Scholar]
- 234. Afonso A, Monroy D, et al. Correlation of tear fluorescein clearance and Schirmer test scores with ocular irritation symptoms. Ophthalmology. 1999;106:803–810 [DOI] [PubMed] [Google Scholar]
- 235. Foulks GN, Bron AJ. Meibomian gland dysfunction: a clinical scheme for description, diagnosis, classification, and grading. Ocul Surf. 2003;1(3):107–126 [DOI] [PubMed] [Google Scholar]
- 236. Mathers WD, Choi D. Cluster analysis of patients with ocular surface disease. blepharitis, and dry eye. Arch Ophthalmol. 2004;122:1700–1704 [DOI] [PubMed] [Google Scholar]
- 237. McCulley JP, Shine WE. Eyelid disorders: the meibomian gland, blepharitis, and contact lenses. Eye Contact Lenses. 2003;29(15):93–95 [DOI] [PubMed] [Google Scholar]
- 238. Guillon JP. Abnormal lipid layers: observation, differential diagnosis, and classification Adv Exp Med Biol. 1998;438:309–313 [PubMed] [Google Scholar]
- 239. Bron AJ, Benjamin L, Snibson GR. Meibomian gland disease: classification and grading of lid changes. Eye. 1991;5:395–411 [DOI] [PubMed] [Google Scholar]
- 240. McCulley JP, Dougherty JM, Deneau DG. Classification of chronic blepharitis. Ophthalmology. 1982;89:1173–1180 [DOI] [PubMed] [Google Scholar]
- 241. Matsumoto Y, Sato EA, et al. The application of in vivo laser confocal microscopy to the diagnosis and evaluation of meibomian gland dysfunction. Mol Vis. 2008:14:1263–1271 [PMC free article] [PubMed] [Google Scholar]
- 242. Hykin PG, Bron AJ. Age-related morphologic changes in lid margin and meibomian gland anatomy. Cornea. 1992;11(4):334–342 [DOI] [PubMed] [Google Scholar]
- 243. Mathers WD, Billborough M. Meibomian gland function and giant papillary conjunctivitis. Am J Ophthalmol. 1992;114:188–192 [DOI] [PubMed] [Google Scholar]
- 244. Shimazaki J, Goto E, Ono M, et al. Meibomian gland dysfunction in patients with Sjögren syndrome. Ophthalmology. 1998;105:1485–1488 [DOI] [PubMed] [Google Scholar]
- 245. Pflugfelder SC, Tseng SC, Sanafina O, et al. Evaluation of subjective assessments and objective diagnostic tests for diagnosing tear-film disorders known to cause ocular irritation. Cornea. 1998;17:38–56 [DOI] [PubMed] [Google Scholar]
- 246. Arita R, Itoh K, Inoue K, Amano S. Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmology. 2008:115:911–915 [DOI] [PubMed] [Google Scholar]
- 247. Craig JP, Blades K, Patel S. Tear lipid layer structure and stability following expression of the meibomian glands. Ophthalmic Physiol Opt. 1995;15:569–574 [PubMed] [Google Scholar]
- 248. Gifford SR. Meibomian glands in chronic blepharoconjunctivitis. Am J Ophthalmol. 1921;4:489–494 [Google Scholar]
- 249. Korb DR, Henriquez AS. Meibomian gland dysfunction and contact lens intolerance. J Am Optom Assoc. 1980;51:243–251 [PubMed] [Google Scholar]
- 250. Norn MS. Expressibility of meibomian secretion: relation to age, lipid precorneal film, scales, foam, hair and pigmentation. Acta Ophthalmol 1987;65:137–142 [DOI] [PubMed] [Google Scholar]
- 251. Hom MM, Silverman MW. Displacement technique and meibomian gland expression. J Am Optom Assoc. 1987;58:223–226 [PubMed] [Google Scholar]
- 252. Hom MM, Martinson JR, Knapp LL, et al. Prevalence of meibomian gland dysfunction. Optom Vis Sci. 1990;67:710–712 [DOI] [PubMed] [Google Scholar]
- 253. Ong BL, Larke JR. Meibomian gland dysfunction: some clinical, biochemical and physical observations. Ophthalmic Physiol Opt. 1990;10:144–148 [DOI] [PubMed] [Google Scholar]
- 254. Bron AJ, Benjamin L, Snibson GR. Meibomian gland disease: classification and grading of eyelid changes. Eye. 1991;5:395–411 [DOI] [PubMed] [Google Scholar]
- 255. Driver PJ, Lemp MA. Meibomian gland dysfunction. Surv Ophthalmol. 1996;40:343–367 [DOI] [PubMed] [Google Scholar]
- 256. Goto E, Monden Y, Takano Y, et al. Treatment of non-inflamed obstructive meibomian gland dysfunction by an infrared warm compression device. Br J Ophthalmol. 2002;86:1403–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Goto E, Shimazaki J, Monden Y, et al. Low-concentration homogenized castor oil eye drops for noninflamed obstructive meibomian gland dysfunction. Ophthalmology. 2002;109:2030–2035 [DOI] [PubMed] [Google Scholar]
- 258. Romero JM, Biser SA, Perry HD, et al. Conservative treatment of meibomian gland dysfunction. Eye Contact Lens. 2004;30:14–19 [DOI] [PubMed] [Google Scholar]
- 259. Korb DR, Greiner JV. Increase in tear film lipid layer thickness following treatment of meibomian gland expression. In: Sullivan DA, Stern ME, Tsubota K, et al., eds. Lacrimal Gland, Tear Film, and Dry Eye Syndromes. New York: Plenum Press; 1994:293–298 [Google Scholar]
- 260. Foulks GN. Blepharitis: lid margin disease and the ocular surface. In: Holland EJ, Mannis MJ. eds. Ocular Surface Disease: Medical and Surgical Management.. New York: Springer; 2002:39–48 [Google Scholar]
- 261. Mastrota KM. The meibomian Mastrota paddle. Rosenberg, TX: Cynacon/Ocusoft; Available at: http://www.ocusoft.com/for-eye-care-professionals/surgical/misc-surgical-instruments/mastrota-meibomian-paddle.html Accessed September 2, 2009 [Google Scholar]
- 262. Romero JM, Biser SA, Perry HD, et al. Conservative treatment of meibomian gland dysfunction. Eye Contact Lens. 2004;30:14–19 [DOI] [PubMed] [Google Scholar]
- 263. Mathers WD, Shields WJ, Sachdev MS, et al. Meibomian gland dysfunction in chronic blepharitis. Cornea. 1991;10(4):277–285 [DOI] [PubMed] [Google Scholar]
- 264. Korb DR, Blackie CA. Meibomian gland diagnostic expressibility: correlation with dry eye symptoms and gland location. Cornea. 2008;27(10):1142–1147 [DOI] [PubMed] [Google Scholar]
- 265. DEWS Research in dry eye: report of the Research Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5(2):179–193 [DOI] [PubMed] [Google Scholar]
- 266. Korb DR, Blackie CA. Diagnostic versus therapeutic meibomian gland expression. American Academy of Optometry annual meeting, Orlando, 2009. Available at http://www.aaopt.org/Submission/Search/SubmissionViewer.asp?SID=25745&BR=SP. Abstract 90745
- 267. McCulley JP, Sciallis GF. Meibomian keratoconjunctivitis. Am J Ophthalmol. 1977;84(6):788–793 [DOI] [PubMed] [Google Scholar]
- 268. DEWS The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5(2):75–92 [DOI] [PubMed] [Google Scholar]
- 269. Bron AJ, Tiffany JM. The contribution of meibomian disease to dry eye. Ocul Surf. 2004;2(2):149–165 [DOI] [PubMed] [Google Scholar]
- 270. Foulks GN, Bron AJ. Meibomian gland dysfunction: a clinical scheme for description, diagnosis, classification, and grading. Ocul Surf. 2003;1(3):107–126 [DOI] [PubMed] [Google Scholar]
- 271. Bron AJ, Tiffany JM, Gouveia SM, et al. Functional aspects of the tear film lipid layer. Exp Eye Res 2004;78(3):347–360 [DOI] [PubMed] [Google Scholar]
- 272. Shimazaki J, Sakata M, Tsubota K. Ocular surface changes and discomfort in patients with meibomian gland dysfunction. Arch Ophthalmol. 1995;113(10):1266–1270 [DOI] [PubMed] [Google Scholar]
- 273. Blackie CA, Korb DR. Recovery time of an optimally secreting meibomian gland. Cornea. 2009;28(3):293–297 [DOI] [PubMed] [Google Scholar]
- 274. Mathers WD, Billborough M. Meibomian gland function and giant papillary conjunctivitis. Am J Ophthalmol. 1992;114:188–192 [DOI] [PubMed] [Google Scholar]
- 275. Nicolaides N, Kaitaranta JK, Rawdah TN, et al. Meibomian gland studies: comparison of steer and human lipids. Invest Ophthalmol Vis Sci. 1981;20:522–536 [PubMed] [Google Scholar]
- 276. Greiner JV, Glonek T, Korb DR, et al. Volume of the human and rabbit meibomian gland system. Adv Exp Med Biol. 1998;438:339–343 [DOI] [PubMed] [Google Scholar]
- 277. Kozak I, Bron AJ, Kucharova K, et al. Morphologic and volumetric studies of the meibomian glands in elderly human eyelids. Cornea. 2007;26(5):610–614 [DOI] [PubMed] [Google Scholar]
- 278. Chew CKS, Hykin PG, Jansweijer C, et al. The casual level of meibomian lipids in humans. Curr Eye Res. 1993;12:255–259 [DOI] [PubMed] [Google Scholar]
- 279. Chew CKS, Jansweijer C, Tiffany JM, et al. An instrument for quantifying meibomian lipid on the lid margin: the Meibometer. Curr Eye Res. 1993;12:247–254 [DOI] [PubMed] [Google Scholar]
- 280. Shine WE, McCulley JP. Meibomitis. Polar lipid abnormalities. Cornea. 2004;23:781–783 [DOI] [PubMed] [Google Scholar]
- 281. Tiffany JM. Individual variations in human meibomian lipid composition. Exp Eye Res 1978;27(3):289–300 [DOI] [PubMed] [Google Scholar]
- 282. Tiffany JM. The lipid secretion of the meibomian glands. Adv Lipid Res. 1987;22:1–62 [DOI] [PubMed] [Google Scholar]
- 283. Nicolaides N, Kaitaranta JK, Rawdah TN, et al. Meibomian gland studies: comparison of steer and human lipids. Invest Ophthalmol Vis Sci., 1981;20(4):522–536 [PubMed] [Google Scholar]
- 284. Nichols KK, Ham BM, Nichols JJ, et al. Identification of fatty acids and fatty acid amides in human meibomian gland secretions. Invest Ophthalmol Vis Sci. 2007;48(1):34–39 [DOI] [PubMed] [Google Scholar]
- 285. Tapie R. Etude biomicroscopique des glandes de meibomius. Ann Oculistique. 1977;210:637–648 [Google Scholar]
- 286. Jester JV, Rife L, Nii D, Luttrull JK, Wilson L, Smith RE. In vivo biomcroscopy and photography of meibomian glands in a rabbit model of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 1982;22:660–667 [PubMed] [Google Scholar]
- 287. Robin JB, Jester JV, Nobe J, Nicolaides N, Smith RE. In vivo transillumination biomicroscopy and photography of meibomian gland dysfunction. Ophthalmology. 1985;92:1423–1426 [DOI] [PubMed] [Google Scholar]
- 288. Mathers WD, Shields WJ, Sachdev MA, Petroll WM, Jester JV. Meibomian gland dysfunction in chronic blepharitis. Cornea. 1991;10:277–285 [DOI] [PubMed] [Google Scholar]
- 289. Mathers WD, Daley T, Verdick R. Video imaging of the meibomian gland. Arch Ophthalmol. 1994;112:448–449 [DOI] [PubMed] [Google Scholar]
- 290. Nichols JJ, Berntsen DA, Mitchell GL, Nichols KK. An assessment of grading scales for meibography images. Cornea. 2005;24:382–388 [DOI] [PubMed] [Google Scholar]
- 291. Yokoi N, Komuro A, Yamada H, et al. A newly developed video-meibography system featuring a newly designed probe. Jpn J Ophthalmol. 2007;51:53–56 [DOI] [PubMed] [Google Scholar]
- 292. Arita R, Itoh K, Maeda S, et al. Proposed diagnostic criteria for obstructive meibomian gland dysfunction. Ophthalmology. 2009;116:2058–2063 [DOI] [PubMed] [Google Scholar]
- 293. Mathers WD, Billborough M. Meibomian gland function and giant papillary conjunctivitis. Am J Ophthalmol. 1992;114:188–192 [DOI] [PubMed] [Google Scholar]
- 294. Shimazaki J, Sakata M, Tsubota K. Ocular surface changes and discomfort in patients with meibomian gland dysfunction. Arch Ophthalmol. 1995;113:1266–1270 [DOI] [PubMed] [Google Scholar]
- 295. Pflugfelder SC, Tseng, et al. Evaluation of subjective assessments and objective diagnostic tests for diagnosing tear-film disorders known to cause ocular irritation. Cornea. 1998;17:38–56 [DOI] [PubMed] [Google Scholar]
- 296. Benitez del Castillo JM, Wasfy MA, Fernandez C, Garcia-Sanchez J. An in vivo confocal masked study on corneal epithelium and subbasal nerves in patients with dry eye. Invest Ophthalmol Vis Sci. 2004;45(9):3030–3035 [DOI] [PubMed] [Google Scholar]
- 297. Kobayashi A, Yoshita T, Sugiyama K. In vivo findings of the bulbar/palpebral conjunctiva and presumed meibomian glands by laser scanning confocal microscopy. Cornea. 2005;24(8):985–988 [DOI] [PubMed] [Google Scholar]
- 298. Messmer EM, Mackert MJ, Zapp DM, Kampik A. In vivo confocal microscopy of normal conjunctiva and conjunctivitis. Cornea. 2006;25(7):781–788 [DOI] [PubMed] [Google Scholar]
- 299. Villani E, Galimberti D, Viola F, et al. The cornea in Sjogren's syndrome: an in vivo confocal study. Invest Ophthalmol Vis Sci 2007;48(5):2017–2022 [DOI] [PubMed] [Google Scholar]
- 300. Zhivov A, Stachs O, Kraak R, et al. In vivo confocal microscopy of the ocular surface. Ocul Surf. 2006;4(2):81–93 [DOI] [PubMed] [Google Scholar]
- 301. Zhang M, Chen J, Luo L, et al. Altered corneal nerves in aqueous tear deficiency viewed by in vivo confocal microscopy. Cornea. 2005;24(7):818–824 [DOI] [PubMed] [Google Scholar]
- 302. Hu Y, Adan ES, Matsumoto Y, et al. Conjunctival in vivo confocal scanning laser microscopy in patients with atopic keratoconjunctivitis. Mol Vis. 2007;13:1379–1389 [PubMed] [Google Scholar]
- 303. Matsumoto Y, Dogru M, Sato EA, et al. The application of in vivo confocal scanning laser microscopy in the management of Acanthamoeba keratitis. Mol Vis 2007;13:1319–1326 [PubMed] [Google Scholar]
- 304. Matsumoto Y, Sato EA, Ibrahim OM, et al. The application of in vivo laser confocal microscopy to the diagnosis and evaluation of meibomian gland dysfunction. Mol Vis. 2008;14:1263–271 [PMC free article] [PubMed] [Google Scholar]
- 305. Matsumoto Y, Shigeno Y, Sato EA, et al. The evaluation of the treatment response in obstructive meibomian gland disease by in vivo laser confocal microscopy. Graefes Arch Clin Exp Ophthalmol. 2009;247(6):821–829 [DOI] [PubMed] [Google Scholar]
- 306. Messmer EM, Torres SE, Mackert MI, Zapp DM, Kampik A. Konfokale In vivo mikroskopie bei blepharitis. Klin Monatsbl Augenheilk. 2005;222:894–900 [DOI] [PubMed] [Google Scholar]
- 307. Ibrahim OM, Matsumoto Y, Dogru M, et al. The efficacy, sensitivity, and specificity of in vivo laser confocal microscopy in the diagnosis of meibomian gland dysfunction. Ophthalmology. 2010;117(4):665–672 [DOI] [PubMed] [Google Scholar]
- 308. Chew CK, Jansweijer C, Tiffany JM, Dikstein S, Bron AJ. An instrument for quantifying meibomian lipid on the lid margin: the meibometer. Curr Eye Res. 1993;12:247–254 [DOI] [PubMed] [Google Scholar]
- 309. Chew CK, Hykin PG, Jansweijer C, Dikstein S, Tiffany JM, Bron AJ. The casual level of meibomian lipids in humans. Curr Eye Res. 1993;12:255–259 [DOI] [PubMed] [Google Scholar]
- 310. Yokoi N, Mossa F, Tiffany JM, Bron AJ. Assessment of meibomian gland function in dry eye using meibometry. Arch Ophthalmol. 1999;117:723–930 [DOI] [PubMed] [Google Scholar]
- 311. Komuro A, Yokoi N, Kinoshita S, Tiffany JM, Bron AJ, Suzuki T. Assessment of meibomian gland function by a newly-developed laser meibometer. Adv Exp Med Biol. 2002;506:517–520 [DOI] [PubMed] [Google Scholar]
- 312. Bron AJ, Tiffany JM, Gouveia SM, Yokoi N, Voon LW. Functional aspects of the tear film lipid layer. Exp Eye Res. 2004;78:347–360 [DOI] [PubMed] [Google Scholar]
- 313. Doane MG. An instrument for in vivo tear film interferometry. Optom Vis Sci. 1989;66:383–388 [DOI] [PubMed] [Google Scholar]
- 314. Danjo Y, Hamano T. Obeservation of precorneal tear film in patient with Sjogren's syndrome. Acta Ophthalmol Scand. 1995;73:501–505 [DOI] [PubMed] [Google Scholar]
- 315. Korb DR, Baron DF, Herman JP, et al. Tear film lipid layer thickness as a function of blinking. Cornea. 1994;13:354–359 [DOI] [PubMed] [Google Scholar]
- 316. Guillon JP. Tear film photography and contact lens wear. J Br Contact Lens Assoc. 1982;5:84–87 [Google Scholar]
- 317. Goto E, Dogru M, Kojima T, Tsubota K. Computer-synthsis of an interference color chart of human tear lipid layer by a colorimetric approach. Invest Ophthalmol Vis Sci. 2003;44:4693–4697 [DOI] [PubMed] [Google Scholar]
- 318. Goto E, Tseng SC. Differentiation of lipid tear deficiency dry eye by kinetic analysis of tear interference images. Arch Ophthalmol. 2003;121:173–180 [DOI] [PubMed] [Google Scholar]
- 319. Goto E, Tseng SC. Kinetic analysis of tear interference images in aqueous tear deficiency dry eye before and after punctual occlusion. Invest Ophthalmol Vis Sci. 2003;44:1897–1905 [DOI] [PubMed] [Google Scholar]
- 320. Yokoi N, Takehisa Y, Kinoshita S. Correlation of tear lipid layer interference patterns with the diagnosis and severity of dry eye. Am J Ophthalmol. 1996;122:818–824 [DOI] [PubMed] [Google Scholar]
- 321. King-Smith PE, Fink BA, Nichols JJ, Nichols KK, Hill RM. Interferometric imaging of the full thickness of the precorneal tear film. J Opt Soc Am A Opt Image Sci Vis. 2006;23(9):2097–2104 [DOI] [PubMed] [Google Scholar]
- 322. Kimball SH, King-Smith PE, Nichols JJ. Evidence for the major contribution of evaporation to tear film thinning between blinks. Invest Ophthalmol Vis Sci. 2010;51:6294–6297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. King-Smith PE, Hinel EA, Nichols JJ. Application of a novel interferometric method to investigate the relation between lipid layer thickness and tear film thinning. Invest Ophthalmol Vis Sci. 2010;51(5):2418–2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Yokoi N, Yamada H, Mizukusa Y, et al. Rheology of tear film lipid layer spread in normal and aqueous tear-deficient dry eyes. Invest Ophthalmol Vis Sci. 2008;49:5319–5324 [DOI] [PubMed] [Google Scholar]
- 325. Maruyama K, Yokoi N, Takamata A, Kinoshita S. Effect of environmental conditions on tear dynamics in soft contact lens wearers. 1: Invest Ophthalmol Vis Sci. 2004;45:2563–2568 [DOI] [PubMed] [Google Scholar]
- 326. McCulley JP, Shine WE. Changing concepts in the diagnosis and management of blepharitis. Cornea. 2000;19:650–658 [DOI] [PubMed] [Google Scholar]
- 327. Hisatake K, Tanaka S, Aizawa Y. Evaporation rate of water in a vessel. J Appl Phys. 1993; 11:7395–7401 [Google Scholar]
- 328. Mishima S, Maurice DM. The oily layer of the tear film and evaporation from the corneal surface. Exp Eye Res. 1961;1:39–45 [DOI] [PubMed] [Google Scholar]
- 329. Iwata S, Lemp M, Holly FJ, Dohlman CH. Evaporation rate of water from the pre-corneal tear film and cornea in the rabbit. Invest Ophthalmol. 1969;8:613–619 [PubMed] [Google Scholar]
- 330. Craig JP, Tomlinson A. Importance of the lipid layer in human tear film stability and evaporation. Optom Vis Sci. 1997;74:8–13 [DOI] [PubMed] [Google Scholar]
- 331. Hamano H, Hori M, Kawabe H, et al. Modification of the superficial layer of the tear film by the secretion of the meibomian glands. Folio Ophthalmol Japonica. 1980;31:353–360 [Google Scholar]
- 332. Hamano H, Hori M, Mitsunaga S. Measurement of evaporation rate of water from theprecorneal tear film and contact lenses. Contacto. 1981;25:7–14 [Google Scholar]
- 333. Cedarstaff TH, Tomlinson A. A comparative study of tear evaporation rates and water content of soft contact lenses. Am J Optom Physiol Opt. 1983;60(3):167–174 [DOI] [PubMed] [Google Scholar]
- 334. Trees G, Tomlinson A. Effect of artificial tear solutions and saline on tear film evaporation. Optom Vis Sci. 1990;67:886–890 [DOI] [PubMed] [Google Scholar]
- 335. Rolando M, Refojo MF. Tear evaporimeter for measuring water evaporation rate from the tear film under controlled conditions in humans. Exp Eye Res. 1983;36:25–33 [DOI] [PubMed] [Google Scholar]
- 336. Yamada M, Tsubota K. Measurement of tear evaporation from ocular surface (in Japanese). Nippon Ganka Zasshi. 1990;11:1061–1070 [PubMed] [Google Scholar]
- 337. Mathers WD. Ocular evaporation in meibomian gland dysfunction and dry eye. Ophthalmology. 1993;100:347–351 [DOI] [PubMed] [Google Scholar]
- 338. Goto E, Endo K, Suzuki A, et al. Tear evaporation dynamics in normal subjects and subjects with obstructive meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2003;44:533–539 [DOI] [PubMed] [Google Scholar]
- 339. Mathers W. Evaporation from the ocular surface. Exp Eye Res. 2004;78:389–394 [DOI] [PubMed] [Google Scholar]
- 340. Tomlinson A, Khanal S. Assessment of tear film dynamics: quantification approach. Ocul Surf. 2005;3:81–95 [DOI] [PubMed] [Google Scholar]
- 341. Borchman D, Foulks GN, Yappert MC, Mathews J, Leake K, Bell J. Factors affecting evaporation rates of tear film components measured in vitro. Eye Contact Lens. 2009;35(1):32–37 [DOI] [PubMed] [Google Scholar]
- 342. Nichols JJ, Mitchell GL., King-Smith PE. Thinning rate of the precorneal and prelens tear films. Invest Ophthalmol Vis Sci. 2005;46:2353–2361 [DOI] [PubMed] [Google Scholar]
- 343. Tomlinson A, Pearce EI, Simmons PA, et al. Effect of oral contraceptives on tear physiology. Ophthalmic Physiol Opt. 2001;21:9–16 [PubMed] [Google Scholar]
- 344. Mathers WD, Lane JA, Sutphin JE, Zimmerman MB. Model for ocular tear film function. Cornea. 1996;15:110–119 [DOI] [PubMed] [Google Scholar]
- 345. Tsubota K, Yamada M. Tear evaporation from the ocular surface. Invest Ophthalmol Vis Sci. 1992;33:2942–2950 [PubMed] [Google Scholar]
- 346. Shimazaki J, Sakata M, Tsubota K. Ocular surface changes and discomfort in patients with meibomian gland dysfunction. Arch Ophthalmol. 1995;113:1266–1270 [DOI] [PubMed] [Google Scholar]
- 347. McCulley JP, Shine WE, Aronowicz J, Oral D, Vargas J. Hyposecretory/hyperevaporative KCS: tear characteristics. Trans Am Ophthalmol Soc. 2003;101:141–154 [PMC free article] [PubMed] [Google Scholar]
- 348. Khanal S, Tomlinson A, Diaper CJ. Tear physiology of aqueous deficiency and evaporative dry eye. Optom Vis Sci. 2009;86(11):1235–1240 [DOI] [PubMed] [Google Scholar]
- 349. Khanal S. Dry eye: diagnosis and management. PhD Thesis, Glasgow Caledonian University, 2006:110 [Google Scholar]
- 350. DEWS Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5(2):108–152 [DOI] [PubMed] [Google Scholar]
- 351. Tomlinson A, Giesbrecht C. Effect of age on human tear film evaporation in normals. Adv Exp Med Biol. 1994;350;271–274 [DOI] [PubMed] [Google Scholar]
- 352. Mathers WD, Daley TE. Tear flow and evaporation in patients with and without dry eye. Ophthalmology. 1996;103:664–669 [DOI] [PubMed] [Google Scholar]
- 353. Mathers WD, Binarao G, Petroll M. Ocular water evaporation and the dry eye: a new measuring device. Cornea. 1993;12:335–340 [DOI] [PubMed] [Google Scholar]
- 354. Glass GV. Integrating findings: the meta-analysis of research. Rev Res Educ, 1977;5:351–379 [Google Scholar]
- 355. Tomlinson A, Doane MG, McFadyen A. Inputs and outputs of the lacrimal system: review of product and evaporative loss. Ocul Surf. 2009;7:186–198 [DOI] [PubMed] [Google Scholar]
- 356. King-Smith E, Nichols JJ, Nichols KK, Fink BA, Braun RJ. Contributions of evaporation and other mechanisms to tear film thinning and break-up. Optom Vis Sci. 2008;85:623–630 [DOI] [PubMed] [Google Scholar]
- 357. King-Smith E, Fink BA, Nichols JS, Nichols KK, Hill RM. Interferometric imaging of the full thickness of the precorneal tear film. J Opt Soc Am Opt Image Sci Vis 2006;23:2097–2104 [DOI] [PubMed] [Google Scholar]
- 358. Kimball S, King-Smith E, Nichols JJ. Evidence for the major contribution of evaporation to tear film thinning between blinks. Invest Ophthalmol Vis Sci. 2010;51:5294–6297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Shine WE, McCulley JR. Polar lipids in human meibomian gland secretions. Curr Eye Res, 2003;26(2):89–94 [DOI] [PubMed] [Google Scholar]
- 360. Ham BM, Jacob JT, Keese MM, Cole RB. Identification, quantification and comparison of major non-polar lipids in normal and dry eye tear lipidomes by electrospray tandem mass spectrometry. J Mass Spectrom. 2004;39(11):1321–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Butovich IA. On the lipid composition of human meibum and tears: Comparative analysis of nonpolar lipids. Invest Ophthalmol Vis Sci. 2008;49(9):3779–3789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Borchman D, Foulk GN, Yappert MC, Tang D, Ho DV. Spectroscopic evaluation of human tear lipids. Chem Phys Lipids. 2007;147(2):87–102 [DOI] [PubMed] [Google Scholar]
- 363. Butovich IA, Millar TJ, Ham BM. Understanding and analyzing meibomian lipids: a review. Curr Eye Res. 2008;33(5–6):405–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Nichols KK, Ham BM, Nichols JJ, Ziegler C, Green-Church KB. Identification of fatty acids and fatty acid amides in human meibomian gland secretions. Invest Ophthalmol Vis Sci. 2007;48(1):34–39 [DOI] [PubMed] [Google Scholar]
- 365. Shine WE, McCulley JP. Meibomian gland triglyceride fatty acid differences in chronic blepharitis patients. Cornea. 1996;15(4) 340–346 [DOI] [PubMed] [Google Scholar]
- 366. Ham BM, Cole RB, Jacob JT. Identification and comparison of the polar phospholipids in normal and dry eye rabbit tears by MALDI-TOF mass spectrometry. Invest Ophthalmol Vis Sci. 2006;47(8):3330–3338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367. Shine WE, McCulley JP. Meibomianitis: polar lipid abnormalities. Cornea. 2004;23(8):781–783 [DOI] [PubMed] [Google Scholar]
- 368. Shine WE, McCulley JP. Keratoconjunctivitis sicca associated with meibomian secretion polar lipid abnormality. Arch Ophthalmol. 1998;116(7):849–852 [DOI] [PubMed] [Google Scholar]
- 369. Joffre C, Souchier M, Grégoire S, et al. Differences in meibomian fatty acid composition in patients with meibomian gland dysfunction and aqueous-deficient dry eye. Br J Ophthalmol. 2008;92(1):116–119 [DOI] [PubMed] [Google Scholar]
- 370. Norn MS. Tear secretion in normal eyes: estimated by a new method–-the lacrimal streak dilution test. Acta Ophthalmol. 1965;43:567–573 [DOI] [PubMed] [Google Scholar]
- 371. Furukawa RE, Polse KA. Changes in tear flow accompanying ageing. Am J Optom Physiol Opt. 1978;55:69–74 [DOI] [PubMed] [Google Scholar]
- 372. van Best JA, Oosterhuis JA. Computer fluorophotometry. Doc Ophthalmol. 1983;56;89–97 [DOI] [PubMed] [Google Scholar]
- 373. Webber WRS, Jones DP, Wright P. Fluorophotometric measurements of tear turnover rate in normal healthy person: evidence for a circadian rhythm. Eye. 1987;1:615–620 [DOI] [PubMed] [Google Scholar]
- 374. Occhipinti JR, Mosier MA, La Motte J, Monji GT. Fluorophotometric measurement of human tear turnover rate. Curr Eye Res. 1988;7:995–1000 [DOI] [PubMed] [Google Scholar]
- 375. Gobbels M, Goebels G, Breitbach R, Spitznas M. Tear secretion in dry eyes as assessed by objective fluorophotometry. Ger J Ophthalmol. 1992;1:350–353 [PubMed] [Google Scholar]
- 376. van Best JA, Benilez Del Castillo JM, Coulangeon LM. Measurement of basal tear turnover using a standardized protocol: European Concerted Action on Ocular Fluorometry. Graefes Arch Clin Exp Ophthalmol. 1995;233:1–7 [DOI] [PubMed] [Google Scholar]
- 377. Mishima S. Some physiological aspects of the precorneal tear film. Arch Ophthalmol. 1965;73:233–241 [DOI] [PubMed] [Google Scholar]
- 378. Kuppens EV, Stolwijk TR, de Keizer RJW, aBest JA. Basal tear turnover and topical timolol in glaucoma patients and healthy controls by fluorophotometry. Invest Ophthalmol Vis Sci. 1992;33:3442–3448 [PubMed] [Google Scholar]
- 379. Khanal S, Tomlinson A, Diaper CJ. Tear physiology of aqueous deficiency and evaporative dry eye. Optom Vis Sci. 2009;86(11):1235–1240 [DOI] [PubMed] [Google Scholar]
- 380. McCann LC, Tomlinson A, Pearce EI, Diaper C. Tear and meibomian gland function in blepharitis. Eye Contact Lens. 2009;35(4):203–208 [DOI] [PubMed] [Google Scholar]
- 381. van Best JA, Benitez del Castillo JM, Coulangeon LM. Measurement of basal tear turnover using a standardized protocol. European concerted action on ocular fluorometry. Graefes Arch Clin Exp Ophthalmol. 1995;233(1):1–7 [DOI] [PubMed] [Google Scholar]
- 382. Scherz W, Doane MG, Dohlman CH. Tear volume in normal eyes and keratoconjunctivitis sicca. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1974;192(2):141–150 [DOI] [PubMed] [Google Scholar]
- 383. Keijser S, van Best JA, van der Lelij A, Jager MJ. Reflex and steady state tears in patients with latent stromal herpetic keratitis. Invest Ophthalmol Vis Sci. 2002;43:87–91 [PubMed] [Google Scholar]
- 384. Mathers WD, Lane JA, Sutphin JE, Zimmerman MB. Model for ocular tear film function. Cornea. 1996;15:110–119 [DOI] [PubMed] [Google Scholar]
- 385. Mathers WD, Daley TE. Tear flow and evaporation in patients with and without dry eye. Ophthalmology. 1996;103:664–669 [DOI] [PubMed] [Google Scholar]
- 386. Tomlinson A, Doane MG, McFadyen A. Inputs and outputs of the lacrimal system: Meta-analysis of product and evaporative loss. Ocul Surf. 2009:7:17–29 [DOI] [PubMed] [Google Scholar]
- 387. Holly FJ: Physical chemistry of the normal and disordered tear film. Trans Ophthalmol Soc UK. 1985;104:374–380 [PubMed] [Google Scholar]
- 388. Mainstone JC, Bruce AS, Golding TR. Tear meniscus measurement in the diagnosis of dry eye. Curr Eye Res. 1996;15:653–661 [DOI] [PubMed] [Google Scholar]
- 389. Bron AJ: The Doyne Lecture: reflections on the tears. Eye. 1997;11:583–602 [DOI] [PubMed] [Google Scholar]
- 390. Yokoi N, Bron AJ, Tiffany JM, Brown NAP, Hsuan JD, Fowler CW. Reflective meniscometry: a non-invasive method to measure tear meniscus curvature. Br J Ophthalmol. 1999;83:92–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391. Yokoi N, Bron AJ, Tiffany JM, Kinoshita S. Reflective meniscometry: a new field of dry eye assessment. Cornea. 2000;19:S37–S43 [DOI] [PubMed] [Google Scholar]
- 392. Wang J, Palakuru JR, Aquavella JV. Correlations among upper and lower tear menisci, non-invasive tear break-up time, and the Schirmer test. Am J Ophthalmol. 2008;145:795–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393. Yokoi N, Komuro A. Non-invasive methods of assessing the tear film. Exp Eye Res 2004;78:399–407 [DOI] [PubMed] [Google Scholar]
- 394. Oguz H, Yokoi N, Kinoshita S. The height and radius of the tear meniscus and methods for examining these parameters. Cornea. 2000;19:497–500 [DOI] [PubMed] [Google Scholar]
- 395. Farrell J, Patel S, Grierson DG, Sturrock RD. A clinical procedure to predict the value of temporary occlusion therapy in keratoconjunctivitis scicca. Ophthalmic Physiol Opt. 2003;23:1–8 [DOI] [PubMed] [Google Scholar]
- 396. Yokoi N, Bron AJ, Tiffany JM, Maruyama K, Komuro A, Kinoshita S. Relationship between tear volume and tear meniscus curvature. Arch Ophthalmol. 2004;122:1265–1269 [DOI] [PubMed] [Google Scholar]
- 397. Gilbard JP, Farris RL, Santamaria J., 2nd Osmolarity of tear microvolumes in keratoconjunctivitis sicca. Arch Ophthalmol. 1978;96(4):677–681 [DOI] [PubMed] [Google Scholar]
- 398. Farris RL. Tear osmolarity–a new gold standard? Adv Exp Med Biol. 1994;350:495–503 [DOI] [PubMed] [Google Scholar]
- 399. Gilbard JP, Dartt DA. Changes in rabbit lacrimal gland fluid osmolarity with flow rate. Invest Ophthalmol Vis Sci. 1982;23(6):804–806 [PubMed] [Google Scholar]
- 400. Sullivan BD. Clinical resorts of a first generation laboratory-on-chip nanolitre tear film osmometer. Ocul Surf. 2005;3:S31 [Google Scholar]
- 401. Nelson JD, Wright JC. Tear film osmolality determination: an evaluation of potential errors in measurement. Curr Eye Res. 1986;5(9):677–681 [DOI] [PubMed] [Google Scholar]
- 402. Terry JE, Hill RM. Human tear osmotic pressure: diurnal variations and the closed eye. Arch Ophthalmol. 1978;96(1):120–122 [DOI] [PubMed] [Google Scholar]
- 403. White KM, Benjamin WJ, Hill RM. Human basic tear fluid osmolality. I. Importance of sample collection strategy. Acta Ophthalmol (Copenh). 1993;71(4):524–529 [DOI] [PubMed] [Google Scholar]
- 404. Tomlinson A, McCann LC, Pearce EI. Comparison of human tear film osmolarity measured by electrical impedance and freezing point depression techniques. Cornea. 2010(9):1036–1041 [DOI] [PubMed] [Google Scholar]
- 405. Tomlinson A, Khanal S, Ramaesh K, et al. Tear film osmolarity: determination of a referent for dry eye diagnosis. Invest Ophthalmol Vis Sci. 2006;47(10):4309–4315 [DOI] [PubMed] [Google Scholar]
- 406. Khanal S, Tomlinson A, Diaper CJ. Tear physiology of aqueous deficiency and evaporative dry eye. Optom Vis Sci. 2009;86(11):1235–1240 [DOI] [PubMed] [Google Scholar]
- 407. Sullivan BD, Whitmer D, Nichols KK, et al. An objective approach to dry eye disease severity. Invest Ophthalmol Vis Sci. 2010;51:6125–6130 [DOI] [PubMed] [Google Scholar]
- 408. Tomlinson A, Doane MG, McFadyen A. Inputs and outputs of the lacrimal system: review of production and evaporative loss. Ocul Surf. 2009;(4):186–198 [DOI] [PubMed] [Google Scholar]
- 409. Tomlinson A, Khanal S. Assessment of tear film dynamics: quantification approach. Ocul Surf. 2005;3:81–95 [DOI] [PubMed] [Google Scholar]
- 410. Xu KP, Yagi Y, Toda I, Tsubota K. Tear function index: a new measure of dry eye. Arch Ophthalmol. 1995;113:84–88 [DOI] [PubMed] [Google Scholar]
- 411. Xu K, Tsubota K. Correlation of tear clearance rate and fluorophotometric assessment of tear turnover. Br J Ophthalmol. 1995;79:1042–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412. Tsubota K. Tear dynamics and dry eye. Prog Retin Eye Res. 1998;17:565–596 [DOI] [PubMed] [Google Scholar]
- 413. Mathers WD, Choi D. Cluster analysis of patients with ocular surface disease, blepharitis and dry eye. Arch Ophthalmol. 2004;122:1700–1704 [DOI] [PubMed] [Google Scholar]
- 414. McCann LC, Tomlinson A, Pearce EI, Kaye SB, Fisher AC. A clinical alternative to fluorophotometry for measuring tear production in the diagnosis of dry eye. Cornea. 2010;29:745–750 [DOI] [PubMed] [Google Scholar]
- 415. Mathers WD. Why the eye becomes dry: a cornea and lacrimal gland feedback model. CLAO J. 2000;26:159–165 [PubMed] [Google Scholar]
- 416. Sahlin S, Chen E. Evaluation of the lacrimal drainage function by the drop test. Am J Ophthalmol. 1996;122:701–708 [DOI] [PubMed] [Google Scholar]
- 417. Tomlinson A, Doane MG, McFadyen A. Inputs and outputs of the lacrimal system: review of production and evaporative loss. Ocul Surf. 2009(4):186–198 [DOI] [PubMed] [Google Scholar]
- 418. Mathers WD, Lane JA, Sutphin JE, Zimmerman MB. Model for ocular tear film function. Cornea. 1996;15:110–119 [DOI] [PubMed] [Google Scholar]
- 419. Craig JP, Tomlinson A. Importance of the lipid layer in human tear film stability and evaporation. Optom Vis Sci. 1997;74:8–13 [DOI] [PubMed] [Google Scholar]
- 420. Levin MH, Verkman AS. Aquaporin-dependent water permeation at the mouse ocular surface: in vivo microfluorimetric measurements in cornea and conjunctiva. Invest Ophthalmol Vis Sci. 2004;45(12):4423–4432 [DOI] [PubMed] [Google Scholar]
- 421. King-Smith PE, Fink BA, Nichols JJ, Nichols KK, Braun RJ, McFadden GB. The contribution of lipid layer movement to tear film thinning and breakup. Invest Ophthalmol Vis Sci. 2009;50(6):2747–2756 [DOI] [PubMed] [Google Scholar]