Abstract
Human stem cell-based therapeutic intervention strategies for treating HIV infection have recently undergone a renaissance as a major focus of investigation. Unlike most conventional antiviral therapies, genetically engineered hematopoietic stem cells possess the capacity for prolonged self-renewal that would continuously produce protected immune cells to fight against HIV. A successful strategy therefore has the potential to stably control and ultimately eradicate HIV from patients by a single or minimal treatment. Recent progress in the development of new technologies and clinical trials sets the stage for the current generation of gene therapy approaches to combat HIV infection. In this review, we will discuss two major approaches that are currently underway in the development of stem cell-based gene therapy to target HIV: One that focuses on the protection of cells from productive infection with HIV, and the other that focuses on targeting immune cells to directly combat HIV infection.
Introduction
As we enter the fourth decade since the HIV/AIDS pandemic was first recognized in 1981, there still exists a strong and pressing need for the development of novel therapeutic strategies to treat the disease. There is currently no effective, wide-scale vaccination strategy nor is there a practicable therapy that results in the eradication of the virus from infected individuals. However, based on the historically unprecedented research into this infectious disease, the development of antiretroviral drug therapy has radically changed the natural history of the disease throughout the world. These therapies have significant associated problems and ultimately fail to result in a functional “cure”(Volberding and Deeks, 2010). Thus, new approaches are required that can complement or replace existing therapies that enable full control of the virus and the restoration of the damaged immune system that HIV targets. Recent advances in the development of stem-cell based therapeutic approaches as well as the development of technologies that allow the genetic modification of these cells have provided impetus towards recent progress made in developing novel therapeutic strategies that target HIV infection. While many of these approaches are currently in the early stages of investigation, they provide a new avenue that, at the very least, will lend new insights into HIV infection and pathogenesis; and, at the very best, provide a viable therapy that successfully treats HIV infection and has an impact on what is a highly confounding disease.
Current HIV therapy and limitations
The current HIV therapy using combinations of antiretroviral drugs termed highly active antiretroviral therapy (HAART), has decreased the morbidity and mortality of HIV infected patients (Palella et al., 1998). Although, HAART has dramatically improved patient’s quality of life, HAART requires continuous drug administration to suppress virus production from HIV reservoirs (Chun et al., 1999). The life long treatment creates serious complications such as drug toxicities and side effects, adherence difficulties, and drug resistance. In addition, life long treatment costs can be expensive. Even under HAART, ongoing low level viremia is evident in some patients (Dinoso et al., 2009), potentially contributing to chronic inflammation, immune dysfunction and accelerated aging (Deeks, 2010). Long-term HIV control and elimination of latently infected cells have become major challenges in the HAART era (Richman et al., 2009). Despite extensive efforts to purge residential HIV from reservoirs, existing drug therapies do not eliminate HIV reservoirs even by drug intensification (Dinoso et al., 2009). In contrast, a hematopoietic stem/progenitor cell (HSC)-based gene therapy approach would offer continuous, long-term production of genetically engineered HIV resistant or HIV-targeted cells and a potential to provide stable control or eradication of HIV by a one time or minimal treatment.
Hematopoietic stem cell gene therapy approach to achieve long term HIV resistance
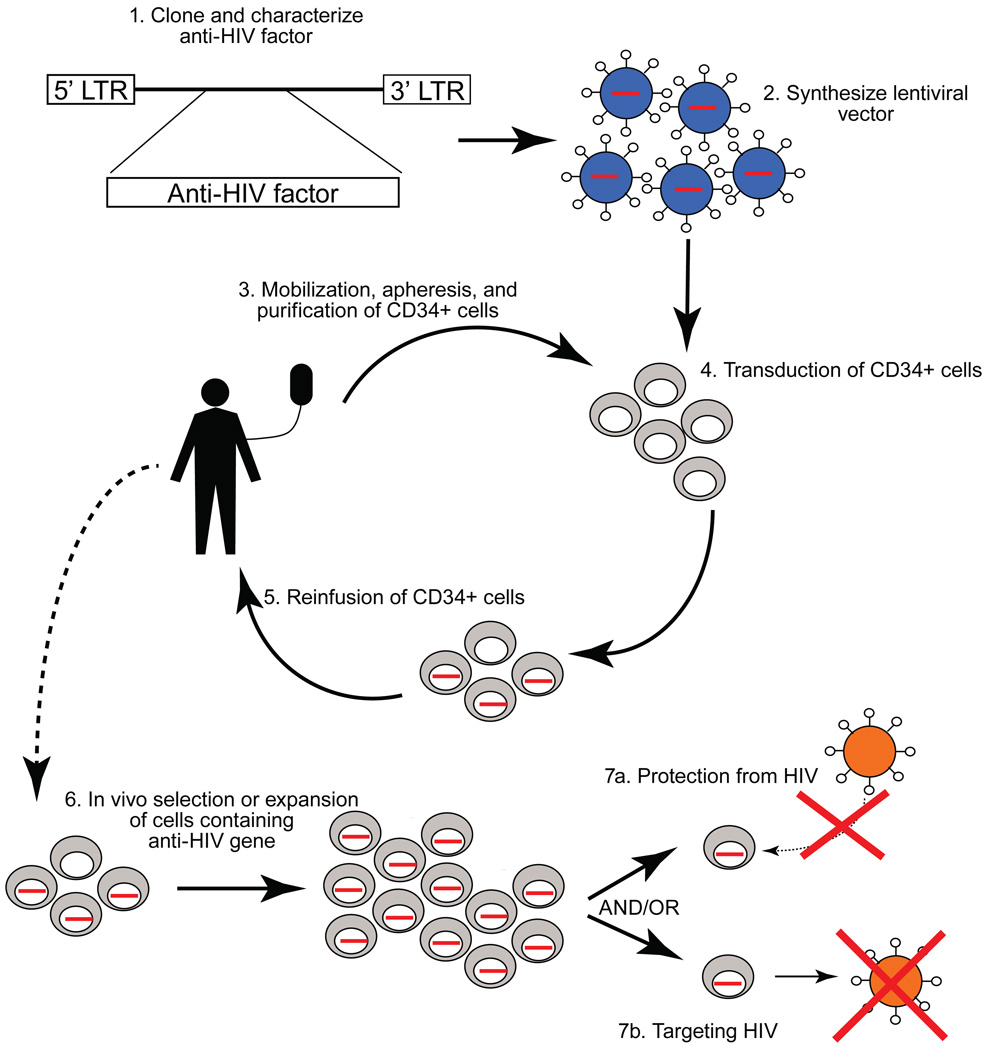
Substantial progress has been made in developing a new therapeutic approach using gene therapy through HSCs to attempt to confer long-term resistance against HIV (figure1). HSCs are capable of self-renewal and differentiation into all hematopoietic lineages. In theory, gene therapy approaches that introduce protective genes against HIV via HSCs can continuously produce their anti-HIV genes in all differentiated cells, including HIV target cells such as CD4+ T lymphocytes and macrophages. Successful replacement of a patient’s immune system by gene modified HIV protected cells may have the potential to minimize viral loads as well as reduce reservoirs of infected and latently infected cells. Newly differentiated protected cells may prevent viral production and spread from persistently infected cells and may allow the functional restoration of the damaged immune system. Currently, a significant clinical benefit by HSC-based gene therapy approaches for HIV diseases has not been achieved; however, this approach has the potential to provide long-term control of HIV through a single treatment. If successful, gene therapy through stem cells could free patients from lifelong daily medications and significantly impact their quality of life.
Figure 1.
Schematic illustrating non-myeloablative HSC-based approaches to treat HIV infection. The anti-HIV factor (such as a siRNA to CCR5 or a molecularly cloned TCR) is cloned and characterized (1) and made into lentiviral vector (or other form enabling genetic transduction of target cells) (2). Stem cells are mobilized from the bone marrow of HIV infected individuals and peripheral blood CD34+ HSCs are obtained by apheresis and cell sorting (3). CD34+ stem cells are then genetically transduced with the anti-HIV factor (4), and the cells are then reinfused back into the individual (5). Following infusion, the anti-HIV gene containing stem cells should migrate to the bone marrow where they take up residence as long-term hematopoietic progenitors. Anti-HIV gene containing cells protected from infection should undergo selection by virus in the body and/or cells engineered to target HIV should respond to the virus and proliferate (6). The effects of this are protection of anti-HIV gene containing cells (7a) or directed targeting of HIV or HIV infected cells by anti-HIV gene expressing cells (7b), resulting in the regeneration of antiviral immune responses and targeted eradication of HIV.
Protecting Cells from Infection: Intracellular Immunization
Introduction
Over the past 20 years, researchers have developed numerous gene based reagents capable of inhibiting HIV infection by intracellular immunization. The intracellular immunization approach to HIV treatment intends to make HIV target cells resistant to HIV by introducing anti-HIV genes (Baltimore, 1988). Here, we discuss anti-HIV gene reagents by classifying them as late step inhibitors (post integration) and early step inhibitors (preintegration).
Late step anti-HIV gene development
Initially, anti-HIV genes were designed to inhibit HIV transcription and translation, which occur in the late steps of the viral life cycle. The transdominant negative HIV Rev mutant RevM10 inhibits HIV RNA nuclear cytoplasmic transport by binding to the rev responsive element (RRE) and interfering with Rev function (Bogerd et al., 1995). Intrabodies against HIV Tat and transactivating response region (TAR) decoys inhibit HIV transcription by sequestering the Tat protein (Mhashilkar et al., 1999; Sullenger et al., 1990). Ribozymes were designed to cleave HIV RNA transcripts by enzymatic activities (Amado et al., 2004; Sarver et al., 1990). Antisense RNAs were designed to hybridize with HIV RNA transcripts and inhibit HIV expression and/or replication (Goodchild et al., 1988; Humeau et al., 2004; Levine et al., 2006). Since the discovery of RNA interference (RNAi) (Fire et al., 1998), many small interfering RNAs (siRNA) directed to HIV RNA sequences have been developed. siRNAs directed against various HIV RNA sequences inhibited HIV in various experimental settings (Capodici, Kariko, and Weissman, 2002; Lee et al., 2002; Novina et al., 2002; Park et al., 2003). One weakness of the RNAi approach was recognized when HIV quickly escaped from siRNAs with minimum mutations (Boden et al., 2003; Senserrich et al., 2008). Efforts to make combinations of siRNAs targeting multiple HIV RNA sequences prevented emergence of escape mutations (ter Brake et al., 2006). All of these anti-HIV genes mediated efficient HIV inhibition in various experimental settings. Some of the anti-HIV genes (RevM10, env antisense, ribozymes, RRE decoy and tat/rev siRNA) were tested in clinical trials, as discussed in more detail below.
Early step anti-HIV gene development
Several labs have developed HIV inhibitors that are designed to target HIV at early life cycle steps before genome integration. Theoretically, early step HIV inhibitors have advantages over strategies that inhibit late steps in the viral life cycle. They can protect cells from the establishment of chronic HIV infection and these protected cells may have selective advantage against being killed by HIV-mediated cytotoxicity. Further, inhibitors that work on steps before reverse transcription have a better chance of preventing HIV mutations because they stop the virus prior to the mutagenic effects of the reverse transcription process.
HIV receptor inhibitors
There is ongoing investigation into gene-based reagents that are capable of inhibiting HIV cell surface receptors, HIV fusion, or incoming virions. Gene based reagents to reduce CD4, CCR5 or CXCR4 have been developed using ribozyme, antisense RNAs, intrabodies and siRNAs (Anderson, Banerjea, and Akkina, 2003; Bai, Rossi, and Akkina, 2001; Qureshi et al., 2006; Zhou et al., 2004). Among HIV receptor inhibitors, CCR5 inhibitors provide great promise to protect cells from HIV without adversely affecting human health, because individuals who lack CCR5 expression due to the homozygous Δ32/Δ32 deletion in the CCR5 gene are highly resistant to HIV infection and have an apparently normal health status (Alkhatib et al., 1996; Dragic et al., 1996; Liu et al., 1996; Mummidi et al., 1998; Smith et al., 1997). Heterozygous individuals with a 50% decrease in CCR5 surface expression have lower plasma viral load and a substantially prolonged course of HIV disease (Dean et al., 1996; Rappaport et al., 1997). Recently, the proof of concept for HIV stem cell therapy using CCR5 defective HSC treatment was demonstrated by the “Berlin patient”, who demonstrated long-term control of HIV infection following myeloablation and an allogeneic bone marrow transplant with CCR5 defective cells (Hutter et al., 2009). In this report, Hutter and colleagues intentionally selected a human leukocyte antigen (HLA) matched CCR5 Δ32/Δ32 homozygous bone marrow donor to treat a HIV+ patient with acute myelogenous leukemia. Intensive bone marrow ablation and stem cell transplants resulted in complete replacement of the patient’s peripheral blood system with the CCR5 Δ32/Δ32 homozygous donor cells. Interestingly CCR5 expressing host-derived macrophages remained in the Gut. Though the patient had discontinued HAART at the time of the bone marrow transplant, the patient’s HIV plasma viral load and HIV proviral DNA have remained negative for more than 3.5 years. This case provides the first well documented evidence of a functional cure for HIV infection. A recent follow up paper by the same group provided further evidence of a cure (Kristina Allers, Blood, prepublished online December 8, 2010; DOI 10.1182/blood-2010-09-309591). In this report, the same patient’s blood system remained completely replaced with cells derived from the CCR5 Δ32/Δ32 donor cells and the host-derived CCR5+ macrophages became undetectable after 24 months. HIV DNA and RNA remained undetectable in plasma and peripheral blood mononuclear cells. One of the major limitations of this strategy is the difficulty of identifying HLA matched donors with the CCR5 Δ32/Δ32 homozygous deletion. This is confounded by the fact that the CCR5 Δ32/Δ32 homozygous deletion exists naturally in only one percent of the Caucasian population, and is rarer in other ethnic populations (Lucotte, 1997). In all, this report of a functional cure for HIV, although not practicable for widespread use as a viable therapeutic strategy, is an exciting observation and forms the foundation for strategies that target the CCR5 molecule in treating HIV infection.
Stable CCR5 inhibition by gene reagents
Gene therapy strategies enable the use of genetically modified HSCs for autologous transplant in patients. Several groups have developed gene therapy strategies using ribozyme, intrabodies, shRNA or zinc finger nuclease aiming to make cells resistant to HIV by CCR5 inhibition.
Ribozyme directed to CCR5
Feng and colleagues developed a hairpin ribozyme against CCR5 (Feng et al., 2000). They transduced the CCR5 ribozyme by recombinant adeno associated viral vector into human PM1 cells and demonstrated reduced CCR5 expression and CCR5 tropic HIV replication inhibition in vitro. In independent studies, Cagnon and colleagues developed a CCR5 ribozyme (Cagnon and Rossi, 2000). This ribozyme was combined with a HIV tat/rev shRNA and a TAR decoy in a lentiviral vector and was recently tested in a phase I clinical trial as described further below (DiGiusto et al., 2010).
Intrabodies to inhibit cell surface CCR5 expression
Using a different approach, Steinberger and colleagues developed a CCR5-specific single-chain intrabody that efficiently reduced CCR5 cell surface expression by retaining CCR5 protein in the endoplasmic reticulum (Steinberger et al., 2000). Intrabody expressing PM-1 cell lines, primary CD4+T cells, and human thymocytes derived from thymus/liver implants generated in the NOD-SCID-hu thymus/liver (thy/liv) model were protected from CCR5 tropic HIV infection by CCR5 surface down-regulation (Swan et al., 2006). Further, CCR5 blocked cells were selected by HIV infection during dendritic cell mediated R5 tropic HIV challenge experiments in vitro.
RNA interference to knock down CCR5
Several groups have developed shRNAs to stably knock down CCR5 expression (Anderson and Akkina, 2005; Anderson et al., 2009; Butticaz et al., 2003; Kim et al., 2010; Shimizu et al., 2010). Lentiviral vector transduction of CCR5 directed shRNAs efficiently reduced CCR5 expression and confered resistance against CCR5 tropic HIVs in vitro (Anderson and Akkina, 2005; Anderson et al., 2009; Butticaz et al., 2003; Kim et al., 2010; Shimizu et al., 2010). However, we and others recognized that over expression of shRNA using an U6 RNA polymerase III promoter could induce cytotoxicity in human primary T lymphocytes (An et al., 2006; Kiem et al., 2010; Lo et al., 2007). This cytotoxicity could be caused by saturation of endogenous micro RNA biogenesis (Grimm et al., 2006; Grimm et al., 2010). To avoid the cytotoxicity, we lowered shRNA expression by utilizing a transcriptionally weaker H1 RNA polymerase III promoter. To achieve robust CCR5 knock down with the H1 promoter, we selected a potent CCR5 shRNA from an enzymatically generated shRNA library directed to CCR5 (An et al., 2006). This optimization resulted in a 25 fold CCR5 reduction in human primary T lymphocytes and CD34+ cell-derived macrophages without toxic effects in vitro (An et al., 2007; Liang et al., 2010). CCR5 shRNA transduced CD34+ cells transplanted into myeloablated rhesus macaques resulted in a stable 3–10 fold reduction in CCR5 expression in peripheral blood T lymphocytes in vivo. No apparent adverse effects due to the shRNA were evident in transplanted macaques for 3 years. Importantly, these cells were less susceptible to simian immunodeficiency virus infection ex vivo than were control cells (An et al., 2007). We further examined in vivo human CCR5 knock down in a recently developed humanized NOD/SCID/IL2rγnull (NSG) bone marrow/liver/thymus (BLT) transplanted mouse model (Shimizu et al., 2010). Lentiviral vector transduction of the CCR5 shRNA into human fetal liver CD34+ cells and subsequent transplant in the BLT humanized mouse resulted in stable CCR5 down regulation in the transplanted thy/liv implant, and in primary and secondary lymphoid organs including the gut-associated lymphoid tissue, which is the major site of HIV replication in humans (Brenchley et al., 2004). CCR5 expression was efficiently reduced more than 5 fold in human CD4+ T cells and CD14+/CD33+ monocytes/macrophage populations. The shRNA-mediated CCR5 knockdown had no apparent adverse effects on T cell development as assessed by polyclonal T cell receptor Vβ family development and naïve/memory T cell differentiation. CCR5 knockdown continued to be observed in mice receiving a secondary transplant from the bone marrow of the mice receiving the first set of CCR5 shRNA modified CD34+ HSCs. This suggests long-term engraftment and self-renewal potential by the shRNA transduced HSCs. Down regulation of CCR5 was sufficient to protect T-cells from HIV challenge ex vivo. These studies demonstrated the feasibility and potential of lentiviral vector-mediated delivery of CCR5 shRNA through HSC transplant as a means of intracellular immunization for the treatment of HIV.
Zinc finger nuclease to disrupt the CCR5 gene
A recent novel approach to disrupt the CCR5 gene was developed by using engineered zinc finger nuclease proteins (ZFNs) (Perez et al., 2008). ZFNs are comprised of custom-made zinc finger DNA binding domains fused to an endonuclease fok I domain to generate a double-strand break at a specific DNA target site. When these double-strand breaks are repaired, deletions and insertions can be introduced at the site of cleavage through a non-homologous end joining (NHEJ) cellular DNA repair mechanism. Holt and colleagues optimized the method to introduce CCR5 gene specific ZFNs into human cord blood and fetal liver derived CD34+ cells (Holt et al., 2010). The group achieved a mean disruption rate of approximately 17% of the total CCR5 alleles in the population of CD34+ cells. They estimated that 5–7% of ZFNs treated cells would be mutated at both alleles. ZFN treated CD34+ cells were transplanted into irradiated neonatal NSG mice. ZFN treated CD34+ cells reconstituted human lymphocytes in systemic lymphoid organs in the mice. Mice were subsequently challenged with highly pathogenic CCR5 tropic HIV-1BaL. Remarkably, CCR5 negative CD4+ T were rapidly selected in the HIV challenged mice. Genomic DNA PCR analysis of the CCR5 gene revealed accumulations of polyclonal insertion/deletion mutations at the ZFN target sites, suggesting that the enriched CCR5 gene disrupted cells were polyclonal. Viral load decreased over time in the ZFN treated mice. These results were highly remarkable in that the relatively small fraction of CCR5 gene disrupted cells can be selected in vivo in CCR5 tropic HIV-1 infected NSG humanized mice suggesting protection of these cells from infection.
HIV entry inhibitors
In another strategy that targets HIV entry, Egelhofer and colleagues developed a HIV fusion inhibitor, termed C46, which can be stably expressed using retroviral vectors (Egelhofer et al., 2004). C46 is derived from the C-terminal heptad repeat of HIV gp41. It blocks HIV fusion by binding to the N terminal coiled coil domain of HIV gp41 fusion intermediate and prevents the six-helix bundle formation, analogous to the FDA approved soluble peptide drug enfuvirtide (T20) (Lalezari et al., 2003). C46 expression on the cell surface inhibited HIV replication more than 2 logs in cell lines and more than 1 log in primary human T lymphocytes (Perez et al., 2005). A recent report confirmed the robustness of C46 over tat/rev specific shRNAs and a long antisense RNA targeted against HIV envelope (termed VRX496) (Kimpel et al., 2010). C46 expressing cells were effectively selected after HIV challenge in vitro. The safety of C46 has been tested in a phase I clinical trial where autologous T-cells transduced with a retroviral vector expressing C46 were infused into patients, and showed no gene therapy related adverse effects (van Lunzen et al., 2007).
Host restriction factors as anti-HIV reagents for gene therapy
Recent investigations of cross primate HIV permissiveness identified natural host HIV restriction factors (Strebel, Luban, and Jeang, 2009). In Rhesus Macaques, it was found that TRIM5α inhibits HIV infection (Stremlau et al., 2004). Although, the precise inhibitory mechanism is under investigation, TRIM5α inhibits HIV through binding the capsid of incoming virions (Strebel, Luban, and Jeang, 2009). Further, TRIM5α can inhibit HIV infection when it is expressed in human cells as a transgene (Stremlau et al., 2004). However, rhesus TRIM5α is not suitable for gene therapy because of the potential immunogenicity in humans. Human-rhesus chimeric TRIM5α and a single amino acid substituted human TRIM5α (R322P) were created through the investigation of active domains responsible for the HIV restriction (Stremlau et al., 2005; Yap, Nisole, and Stoye, 2005). These minimally modified human TRIM5αs inhibit HIV (Li et al., 2006). Anderson and colleagues demonstrated CD34+ cell transduction of human-rhesus chimeric TRIM5α using a lentiviral vector and thymocyte differentiation in thy/liv tissue in the SCID hu thy/liv mouse model (Anderson and Akkina, 2008). The resultant human thymocytes were protected from ex vivo HIV challenge. In another instance of host restriction, owl monkey cells block HIV infection by an endogenous TRIM5α-cyclophilinA fusion protein (TRIMcyp) (Sayah et al., 2004). The Owl monkey TRIMcyp gene was created by a LINE-1 mediated retrotransposion of cyclophilinA cDNA into TRIM5α gene locus. Interestingly, the TRIMcyp fusion genes were created by independent retrotransposition events in Old World and New World primates, suggesting critical roles of TRIMcyp proteins as host restriction factors in primates (Brennan, Kozyrev, and Hu, 2008; Newman et al., 2008; Virgen et al., 2008; Wilson et al., 2008). Human genome does not carry endogenous TRIMcyp to restrict HIV. Neagu and colleagues recently engineered a human TRIMcyp aiming to use it as a human gene therapy reagent (Neagu et al., 2009). Human TRIMcyp potently inhibited HIV in human CD4+ T cells and macrophages in vitro and in vivo in human TRIMcyp transduced PBMCs trasplanted into Rag2−/−γc−/− mice. Other host HIV restriction factors that could be potentially utilized as gene therapy reagents are APOBEC 3G (Sheehy et al., 2002), APOBEC 3F (Holmes et al., 2007) and Tetherin (Neil, Zang, and Bieniasz, 2008). However, HIV is naturally equipped with the vif and vpu proteins to counteract these host restriction factors. Several researchers developed vif resistant APOBEC 3G by a single amino acid substitution at D128K (Bogerd et al., 2004; Schrofelbauer, Chen, and Landau, 2004; Xu et al., 2004). Gupta and colleagues developed a vpu resistant Tetherin by a single amino acid substitution at T45I (Gupta et al., 2009). These HIV accessory gene resistant HIV restriction factors can be gene therapy candidates because of their resistance to the HIV counter attack. Other cellular genes that restrict HIV infection were identified by cDNA screenings. Mov10 over expression inhibits HIV at multiple steps (Burdick et al., 2010; Furtak et al., 2010). Truncated poly adenylation factor 6 (CPSF6) inhibits HIV nuclear entry by capsid binding (Lee et al., 2010). Recent intensive investigations aimed at better understanding of host factors and HIV interaction may identify novel host restriction factors near future. The ongoing efforts to increase the list of anti-HIV genes may provide new strategies to inhibit HIV by a gene therapy approach.
Combinational anti-HIV strategy to produce highly HIV resistant cells and prevent escape mutations
Effective gene therapy applications against HIV disease will likely require a combination of multiple reagents directed against HIV. Single anti-HIV therapy will likely fail due to the development of escape mutations in the virus that confer resistance to the therapy. Emergence of CXCR4 and dual tropic HIVs will be a concern in the case of CCR5 inhibitors. Utilizing the same rationale as combination antiretroviral therapy, it would be important to develop gene therapy strategies to protect target cells with combinations of anti-HIV genes to inhibit multiple steps in the viral life cycle. With this approach, several groups have incorporated multiple anti-HIV genes into a single lentiviral vector (Anderson and Akkina, 2008; DiGiusto et al., 2010; Kiem et al., 2009; Schopman, ter Brake, and Berkhout, 2010). Combinations of anti-HIV genes were designed to target distinct steps in the viral life cycle to increase the antiviral effect but were also aimed to block the emergence of resistant HIVs. Multiple shRNAs targeting different sites of HIV transcripts were combined within a lentiviral vector (Li et al., 2005; Schopman, ter Brake, and Berkhout, 2010; ter Brake et al., 2006). The combination of three RNA reagents (HIV tat/rev shRNA, TAR decoy and CCR5 ribozyme) was transduced into human CD34+ cells which were then differentiated into monocytes in vitro (Li et al., 2005) and thymocytes in the SCID hu thy/liv mouse model (Anderson et al., 2007). The resultant monocytes and human thymocytes were protected from HIV infection ex vivo (Li et al., 2003; ter Brake et al., 2006). This study provided the preclinical data for a recent phase I clinical trial using the triple anti-RNA combination vector in AIDS lymphoma patients (DiGiusto et al., 2010). Using a different approach, a triple combination of CCR5 shRNA, a human/rhesus chimeric TRIM5α, and a TAR decoy was expressed from a lentiviral vector. This anti-viral RNA combination efficiently inhibited HIV replication in CD34+ cell-derived macrophages in vitro (Anderson et al., 2009). In a separate study, a combination of CCR5 shRNA and a human/rhesus chimeric TRIM5α were induced into induced pluripotent stem cells (iPSc) cells by a lentiviral vector and successfully differentiated through CD133+ cells into HIV resistant macrophages in vitro (Kambal et al., 2010). A membrane anchored fusion inhibitor C46 and multiple tat/rev shRNAs were combined in a lentiviral vector (Kiem et al., 2009). While many of these studies are promising, it is expected that more effective combinations will be developed with newly identified anti-HIV genes in the future.
Clinical trials
Several anti-HIV HSC based gene therapy protocols have been tested in clinical trials. Most clinical trials were phase I studies aimed at evaluating the safety and feasibility of anti-HIV gene transduced autologus hematopoietic stem/progenitor cell transplantation in patients. In early trials, transdominant RevM10 (Kang et al., 2002; Podsakoff et al., 2005), RRE decoy (Kohn et al., 1999), or an anti HIV ribozymeribozyme (Amado et al., 2004) were introduced into patient’s CD34+ cells with Molony murine leukemia virus based gammaretroviral vectors. In summary, all of these phase I clinical studies demonstrated safety and feasibility of the procedures. Gene transfer and stem cell transplantation were well tolerated in all clinical trials and no significant adverse events have been observed. In all of these studies, there were detectable levels of anti-HIV gene expressing cells in patients. However, the gene marking levels were too low to achieve therapeutic benefits.
Mitsuyasu and colleagues recently reported the first phase II clinical trial of an anti-HIV gene therapy. This trial involved 74 subjects enrolled in randomized, double-blind and placebo-controlled groups in a multi-centre trial (Mitsuyasu et al., 2009). A murine gamma retroviral vector was used to transduce a tat/vpr specific ribozyme into granulocyte-colony stimulating factor (G-CSF) mobilized peripheral blood CD34+ cells. Cells were genetically modified ex vivo and re-infused back into the patients without bone marrow conditioning. This procedure did not result in apparent adverse events. Ribozyme DNA and RNA were detectable in 94% of patients but gradually declined to 7% of patients 100 weeks following treatment. Although the levels of ribozyme DNA and RNA were low, lower viral loads and higher CD4+ cell counts were observed in the ribozyme group. These results suggest a marginal effect in the anti-HIV ribozyme group. This study demonstrated a proof of concept that anti- tat/vpr ribozyme HIV transduced autologous HSC transplant in humans is safe and has a capability to produce gene modified cells in a large numbers of human subjects.
In the most recent clinical study, a triple combination of an anti tat/rev shRNA, a TAR decoy and an anti-CCR5 ribozyme was introduced in AIDS lymphoma patients through HSC transplant (DiGiusto et al., 2010). This is the first clinical trial applying a lentiviral vector for anti-HIV gene transduction into patient CD34+ cells. Four AIDS lymphoma patients underwent myeloablative conditioning and anti-HIV gene transduced CD34+ cell infusion. The team infused unmanipulated CD34+ cells into the patients to ensure safety. Gene marking in peripheral blood mononuclear cells was low at 0.02–0.32% of the cells during the follow up period up to 24 months and the RNA transgenes were detectable by PCR. The low levels of vivo gene marking might be caused by the safety transplant procedure with co-infusion of genetically unmodified cells and resultant dilution of the gene modified CD34+ cells. Another possibility is that high expression of shRNA from the transcriptionally strong U6 promoter might have caused cytotoxicity. Their in vitro gene tracking experiment resulted in a sharp decline of vector DNA copies in transduced CD34+ cells within 4 weeks. These results suggest a growth disadvantage of vector transduced CD34+ cells. We and other reported that over expression of shRNA from the U6 promoter can induce cytotoxicity in human T lymphocytes (An et al., 2006; Kiem et al., 2010; Lo et al., 2007). shRNA utilize the endogenous microRNA pathway to mature and therefore may interfere with miRNA biogenesis. Therefore, the level of shRNA expression must be carefully optimized. Switching to a transcriptionally weaker H1 promoter to minimize shRNA expression may provide stable maintenance of shRNA expressing cells. Further optimizations in the vector design and transplant protocol may improve the efficiency of gene modified cells in the future trials. In summary, this clinical trial provided the first time usage of a lentiviral vector with a triple combination of anti-HIV genes through stem cell transplant in AIDS related lymphoma patients.
Summary of intracellular HSC therapeutic protection strategies
Stem cell based gene therapy approaches hold great potential for controlling HIV infection through a single treatment. The challenge of achieving therapeutic levels of genetically modified cells for patient transplants cell in clinical trials remains a major obstacle. Current gene therapy technologies have reached the point where an HSC based gene therapy is able to provide therapeutic benefits to single gene deficiency diseases. Examples of diseases where investigators have observed effective single gene therapies include Adenosine deaminase deficiency severe combined immunodeficiency (ADA SCID) and X-linked SCID, where gene corrected cells have strong in vivo selection in gene- corrected- HSC transplanted patients (Aiuti et al., 2009; Cavazzana-Calvo et al., 2000; Gaspar et al., 2004). In HIV single gene therapyies, in cells expressing anti-HIV genes are predicted to be selected due to resistance to HIV mediated T-cell killing. The recent success of gene therapy clinical trials using lentiviral vectors for the treatment of adrenoleukodystrophy (ALD) patients provided great promise of this vector system to repopulate the human hematopoietic system with genetically modified cells (Cartier et al., 2009). In the clinical trial, 9–14% of the peripheral blood cells express the ALD transgene product and gene corrected microglial cells migrated into the brain and stopped progressive demyelination. However, a recent thalassaemia stem cell based gene therapy using a lentiviral vector provides us a cautionary tale (Cavazzana-Calvo et al., 2010). In one individual, the treatment achieved the criteria for clinical benefit but this was achieved by a single cell clonal out-growth in the erythroid population caused by the over expression of high mobility group AT-hook 2 (HMGA2) via vector insertion into control elements of the gene. The clonal growth was not a malignancy and it is not clear whether this event is a unique case in the erythroid lineage. This thalassaemia example illustrates the notion that we currently do not have enough experience with lentiviral vectors in clinical trials to be certain of their safety. Further investigation will show whether lentiviral vectors can be used as an efficient and safe vector delivery system for HIV gene therapy. A non-viral vector approach using a CCR5 directed zinc finger nuclease showed great promise in a humanized mouse model (Holt et al., 2010), though the fidelity of CCR5 disruption and off target gene disruptions need to be fully investigated with a genome wide analysis. Successful application of stem cell based gene therapy strategies for HIV infection requires further investigation and development of effective anti-HIV genes, efficient gene delivery vehicles, a greater understanding of stem cell biology, and a safe and effective bone marrow transplant procedure. Our hope is to provide a long-term control of HIV infection by a single treatment using stem cell based gene therapy in the near future.
Engineering HIV Immunity
Introduction
In addition to developing HSC gene therapies to protect cells from infection with HIV, there are efforts to “engineer” resultant immune cells to specifically target and kill HIV in the body. As a basis for these approaches, antiviral immune responses towards HIV are naturally generated in most affected individuals; however, in the large majority of cases this response is insufficient at preventing replication and clearing the virus from the body. In the natural course of HIV infection, the levels of virus in the body following the acute stage represent the ongoing struggle between natural immunity and the virus’s ability to replicate and evade these responses. Therapeutic intervention strategies, while largely successful in lowering the levels of virus and prolonging disease progression, are currently incapable of eradicating the virus and repairing the damage done to the immune system by the infection. Pertaining to this, there is a strong demand for new and novel strategies that augment or enhance natural antiviral immune responses and allow reconstitution of other virally perturbed aspects of immunity.
Immune Therapy for HIV Infection
While the potential benefits of immune therapy for HIV infection utilizing a stem cell based gene therapy approach is discussed below, it is important to first discuss the rationale behind therapeutic strategies that attempt to enhance natural immune responses against the virus. The ultimate goal of all of these strategies is to manipulate antiviral immune responses to eradicate the virus from the body. A fundamental problem in HIV infection is that the virus does not possess the immunogenicity to elicit adequate protective responses. This is evident in the natural course of infection and has been a confounding factor in attempts to develop an effective preventative or therapeutic vaccine (McElrath and Haynes, 2010). In addition, HIV attacks the immune system itself and actively subverts it through a variety of mechanisms that further contribute to viral persistence (reviewed by (Trono et al., 2010)).
A relatively new focus has been to develop strategies that are directed at manipulating virus-specific humoral an/or cellular immune responses in hopes of overcoming these barriers to allow immune suppression and clearance. Relatively simple strategies aimed at enhancing antiviral humoral immune responses have demonstrated that passive immunization of rhesus macaques with neutralizing antibodies is protective against simian immunodeficiency virus (SIV) or simian-human immunodeficiency virus (SHIV) challenge and is associated with lower viral loads in infected animals (Baba et al., 2000; Hessell et al., 2009; Kramer, Siddappa, and Ruprecht, 2007; Mascola et al., 2000; Ng et al., 2010; Shibata et al., 1999). In humans, passive administration of HIV-specific neutralizing monoclonal antibodies resulted in delay of viremia following cessation of antiretroviral therapy (Mehandru et al., 2007; Trkola et al., 2005). While recent data strongly suggest that humoral immunity and neutralizing antibodies play an important role in controlling the levels of virus in chronic HIV infection (Huang et al., 2010), enhancing these responses could be therapeutically beneficial.
Efforts have also been made to enhance existing virus antigen-specific cellular immunity in people infected with cytomegalovirus (CMV) and Epstein-Barr Virus (EBV) by expanding virus-antigen specific cytotoxic T lymphocytes (CTLs) ex vivo followed by subsequent infusion. This approach has been demonstrated to be relatively safe and effective at enhancing T cell immunity to these viruses and virally transformed cells in vivo (Bollard et al., 2004; Heslop et al., 2010; Riddell et al., 1992). Ex vivo expansion of autologous HIV-specific CTL from infected individuals has been demonstrated to produce cells that retain their HIV-specific lytic function and migrate to sites of HIV replication in the lymph nodes (Brodie et al., 1999; Brodie et al., 2000; Lieberman et al., 1997). These cells transiently lowered the levels of productively infected CD4+ T cells. However, they did not have a significant effect on lowering viral loads and appear to persist for a relatively short period of time following infusion (Brodie et al., 1999; Brodie et al., 2000). The inability of these cells to have a significant effect on viral loads is likely due to the functional perturbation of these cells prior to ex vivo expansion by an altered immune environment resultant from HIV infection. The lack of persistence of these adoptively transferred cells is likely due to immune exhaustion in the presence of ongoing viral antigenic exposure. These cells likely lack full functional competency and altered effector function and may not represent the numbers necessary to have any effects on enhancing the CTL response when infused into the individual. However, ex vivo expansion and adoptive transfer of antigen-specific cells appears to be more successful when targeting malignancy related antigens where perturbations in the immune environment are not as dramatic as they are in the context of HIV infection (Berry, Moeller, and Darcy, 2009; DiGiusto and Cooper, 2007; June, 2007; Rosenberg, 2004; Rosenberg et al., 2008; Zhou et al., 2005). A methodology to generate large numbers of functionally competent HIV-specific CTL would be a desirable strategy to enhance CTL responses against HIV. These adoptive strategies and the success in the targeting of other viral antigens provide impetus towards the development of strategies that involve genetic enhancement of immune responses towards HIV.
Engineering Antiviral Immunity—Genetic Vaccination for HIV Infection
Gene therapy based approaches that program the immune response to target HIV are undergoing a period of rapid growth and interest. Several approaches that augment or enhance humoral and/or cellular antiviral immune responses are in various phases of development and attempts have been made and are currently under investigation to genetically program cells to target HIV infection in clinical trails. Approaches that rely on “redirecting” peripheral immune cells to target HIV infection are the most developed to this point, with the technology having moved further along and with several clinical trials either completed or in progress (reviewed by (June, Blazar, and Riley, 2009; Rossi, June, and Kohn, 2007)). A primary reason for this is simply that peripheral cells are relatively easy to obtain and manipulate. Stem cell-based approaches that produce immune cells that target viral infection are a newer concept and are more difficult to perform, with most of the preliminary development occurring in mouse systems as proof-of-principle studies (Rossi, June, and Kohn, 2007). However, as will be discussed, there are potentially significant advantages in using stem cell based approaches to engineer antiviral immune responses and their development is rapidly advancing.
Peripheral “redirection” of Antiviral Immunity
Peripheral blood T cells, which are particularly suitable for genetic manipulation, have been the focus of most efforts to date to “redirect” cells to target HIV infection. Studies have involved the utilization of peripheral T cells that have been genetically altered to express a chimeric molecule or a molecularly cloned T cell receptor (TCR) that targets cells to HIV antigens (Clay et al., 1999; Cooper et al., 2000; Deeks et al., 2002; Hofmann et al., 2008; Joseph et al., 2008; Miles, Silins, and Burrows, 2006; Mitsuyasu et al., 2000; Roberts et al., 1994; Varela-Rohena et al., 2008; Walker et al., 2000; Yang et al., 1997). There were early studies and clinical trials based on redirecting T cells using a chimeric receptor that has the gp120 binding domain of the human CD4 molecule that is fused to the zeta chain signaling domain of T cell receptor, termed a universal T cell receptor (UTR) (Deeks et al., 2002; Mitsuyasu et al., 2000; Roberts et al., 1994; Walker et al., 2000; Yang et al., 1997). This chimeric receptor T cell redirection approach avoids problems associated with human leukocyte antigen (HLA) restriction by an antigen-specific TCR and would allow more broad recognition of virally expressing cells. The clinical studies, in sum, showed persistence of cells harboring the chimeric transgene for more that one year. One study demonstrated rises in CD4 T cell levels in people receiving ex vivo expanded autologous T cells and modest decreases in viral reservoirs in HIV infected individuals receiving the UTR versus control (Deeks et al., 2002). Several other approaches that utilize chimeric receptors to redirect T cells to target malignancy related antigens have been performed to varying degrees of success (reviewed in (June, Blazar, and Riley, 2009; Sadelain, Brentjens, and Riviere, 2009)), and thus remain a viable option to target antigen expressing cells.
In addition, studies have been performed to redirect peripheral T cells to express a molecularly cloned TCR specific to HIV (Cooper et al., 2000; Hofmann et al., 2008; Joseph et al., 2008; Miles, Silins, and Burrows, 2006; Varela-Rohena et al., 2008). This approach involves molecularly cloning an HLA-restricted, peptide specific TCR from reactive T cells from an infected individual and subsequent modification of peripheral cells by retroviral vectors (Cooper et al., 2000), lentiviral vectors (Joseph et al., 2008; Varela-Rohena et al., 2008), or RNA electroporation (Hofmann et al., 2008). All of these approaches resulted in functional expression of the transgenic HIV-specific TCR which redirected the transduced peripheral CD8+ T cells in vitro and (in one case) in vivo in humanized SCID mice (Joseph et al., 2008), to respond to HIV. This approach has been used to successfully redirect cells to target malignancy related antigens, in particular the MART-1 melanoma antigen (Clay et al., 1999; Johnson et al., 2006; June, Blazar, and Riley, 2009; Morgan, Dudley, and Rosenberg, 2010; Morgan et al., 2003) and has proven to be safe in patients and resulted in clinical regression of metastatic melanoma lesions (Morgan et al., 2006). In HIV infection, there are efforts to “enhance” this redirection approach by modifying peripheral cells using high-affinity TCRs molecularly cloned, modified, and selected for higher peptide-HLA binding affinity from individuals with robust, HIV-specific CTL responses (Varela-Rohena et al., 2008). These high-affinity TCRs (in this case specific to the relatively conserved, HLA-A*0201-restricted, p17 Gag SLYNTVATL (SL9) viral peptide) appear to have an enhanced functional ability in producing polyfunctional cells that are capable of suppressing viral replication of wild type and naturally occurring SL9 escape variants. A phase 1 clinical trial of this approach is currently underway (see http://clinicaltrials.gov/show/NCT00991224). This or similar approaches that attempt to redirect peripheral T cells to target cells expressing lower levels of antigen or to constrain viral evolution could be beneficial in lowering viral loads or at least provide further insight as to the constraints that TCR interaction with antigen-expressing cells confers on the generation of effective antiviral responses.
However, there are several limitations to the peripheral redirection approach that may hinder its therapeutic development in HIV disease. The ex vivo manipulation of mature T cells inherent in this strategy can functionally alter the cells and result in subsequent and important effects on cellular differentiation in vivo following re-infusion. As recent studies suggest, cellular differentiation phenotypes and methods of in vitro manipulation of peripheral T cells can have dramatic effects in functional outcomes following handling (Hinrichs et al., 2010; Kaneko et al., 2009; Zhang et al., 2007). Further, ex vivo manipulation has a significant impact on the lifespan of the cells once re-infused back in the body (Berry, Moeller, and Darcy, 2009). In addition, a particular problem with the introduction of antigen-specific TCRs involves the fact that the altered cells retain the expression of their endogenous T cell receptors. Modification of peripheral T cells with a cloned TCR could result in cross-receptor paring with the expressed endogenous TCR, producing mixed TCR α and β chain pairs. This could produce self-reactive T cells by overriding peripheral tolerance mechanisms since these cells expressing these cross-paired TCR chains are not subjected to normal thymic negative selection processes. There is evidence to suggest that this mechanism may contribute to graft-versus-host disease (GVHD) in a mouse model that involved the modification of mouse peripheral T cells with a cloned TCR, where mixed TCRs were produced and resulted in autoreactive cells (Bendle et al., 2010). In addition, a study that introduced human TCRs into human peripheral T cells demonstrated the formation of alloreactive cells expressing mixed TCRs in vitro (van Loenen et al., 2010). However, while these studies show that this is a potential consequence of introduced TCR in peripheral T cells expressing an endogenous TCR and that this approach must be performed in humans with careful safeguards, cross-pairing of TCRs may be limited to certain settings as the generation of GVHD does not appear to occur following autologous reinfusion of TCR modified T cells in human clinical trials (Rosenberg, 2010). In addition, methods to optimize transgenic α and β pairing may bypass or reduce the likelihood of this scenario (Govers et al., 2010). As will be discussed below, an approach utilizing human HSCs may overcome many of these problems by allowing natural developmental mechanisms to produce genetically engineered immune cells that target HIV infection that are tolerant of the host and possess prolonged self-renewal capability.
Stem Cell-based Immune Programming
The fact that the immune system is incapable of naturally eradicating HIV infection mandates that a successful immune-based therapeutic strategy would allow long-lived, renewable immune responses capable of generating the quantity of antiviral cells and quality needed to allow complete eradication of HIV from the body. A new focus on this approach is the utilization of human HSCs that would allow the development of human immune cells capable of targeting HIV. The use of a HSC based therapeutic approach would allow multilineage hematopoietic development and the potential of targeting one or more of these arms of the immune response towards HIV. The development of these genetically modified, HIV-targeted cells from HSCs in the body following normal cellular differentiation pathways would allow proper and normal “education” and selection of these cells to produce cells that would, theoretically, be physiologically like unmodified cells. Thus, the activation and expansion of antigen reactive cells in the periphery would allow the differentiation of these cells into long-term memory cells through natural mechanisms. Further, a HSC based approach would allow long-term engraftment of genetically modified cells capable of generating continual lymphopoietic development of antigen-naïve HIV-targeted cells, potentially repairing defects in exhausted HIV-specific cells. They would thus lack the issues of functional impairment, developmental biasing, or exhaustion that other peripheral cell ex vivo modification methods would have. A human stem cell based approach is one of the more recent to gain impetus and one of the more difficult strategies to develop due to the paucity of experimental systems that allow the close examination of human hematopoietic events and cellular function. However, recent advances have been made in the early development of HSC based immune programming as a viable therapeutic strategy.
One initial aim of stem cell based immune therapeutic strategies has focused on engineering the development of B cells that produce neutralizing antibodies or CTL that target and kill HIV infected cells. Recent studies have demonstrated the ability to program B cells resultant from human HSCs to express an anti-HIV neutralizing antibody following differentiation in vitro and differentiation in vivo in humanized mice (Joseph et al., 2010; Luo et al., 2009). Further, expression of the neutralizing antibody following development into B cells in vivo in humanized mice significantly lowered viral loads and levels of infected cells (Joseph et al., 2010). While this approach using a single monoclonal antibody would almost certainly drive viral mutation in vivo, these studies provide the proof-of-principle that can be further explored in the utilization of multiple antibodies directed against HIV. Engineered B cell responses may be useful in enhancing cellular immune responses, particularly in the mucosal and innate immune compartments, and a therapeutic benefit may be obtained alone or in combination with other strategies. Further development in this approach may provide insight in the development of other chimeric receptors or single-chain therapeutic antibodies with recognition domains that target cellular immunity towards HIV infected cells (Roberts et al., 1994; Rossi, June, and Kohn, 2007; Yang et al., 1997).
Due to their importance in controlling HIV infection, engineering HSCs to augment or enhance antiviral T cell immunity is another enticing strategy for investigation as a potential therapeutic approach. Studies performed in mice examining antigen specific T cell responses utilizing transgenic mice that express a mouse TCR specific to one of a variety of antigens provide a basis for exploring this type of genetic modification in human cells (Pircher et al., 1989; Tian et al., 2007). Further, mouse-based studies demonstrated that mouse HSCs modified with cloned mouse TCRs can develop into antigen-specific T cells in vivo (Yang and Baltimore, 2005; Yang et al., 2002). Early studies with human cells demonstrated the ability of human HSCs to develop into T cells following differentiation on murine Delta-like 1 molecule-expressing stromal cell lines (van Lent et al., 2007; Zhao et al., 2007). However, the resultant cells in these studies that expressed the transgenic TCR did not undergo normal positive and negative selection events that a developing T cell would encounter in the human thymic environment. In what we believe is a major step forward in this approach, we recently determined that a molecularly cloned TCR specific for the SL9 peptide allows the development of functional HIV-specific CTL in human thymus tissue in vivo in SCID-hu mice (Kitchen et al., 2009). This TCR recognizes the SL9 epitope in the context of HLA-A*0201, and we demonstrated the necessity for expression of this allele in the thymus tissue to allow proper development of CD8 single positive thymocytes. These studies are the first to demonstrate that a human TCR can allow the development of human stem cells into mature, functional CTLs in human tissue in vivo and further demonstrate the necessity of matching the cloned TCR to the proper HLA type.
A therapeutic strategy involving molecularly cloned, antigen specific TCRs, much like with the modification of peripheral blood T cells with a cloned TCR, would therefore have to be tailored to the HLA type of the individual receiving treatment in order to produce cells that survive T cell selection processes. However, this process of development and thymic positive and negative selection from HSC progenitors would theoretically reduce the possibility of producing cells that are autoreactive, bypassing a major drawback of peripheral T cell modification. A further advantage of using a stem cell based approach is longer engraftment of functionally capable gene marked cells than with peripheral cell based modification. As with the necessity of utilizing multiple molecularly cloned monoclonal antibodies for that approach to be a viable therapeutic strategy, multiple TCRs targeted to different, relatively conserved epitopes of HIV within defined HLA molecules would likely reduce the possibility of viral immune escape and increase the numbers of suppressive antiviral CTLs. Indentifying and further characterizing conserved HIV TCR epitopes is important in the expansion and utilization of an engineered T cell approach. Immune escape to avoid certain CTL epitopes may render a fitness cost to the virus, and therefore lower the capacity of the virus to replicate. There is evidence that viral evolution and immune escape to alter certain conserved epitopes occurs relatively slowly, and immune pressure caused by T cell responses engineered against them may be therapeutically beneficial in forcing the evolution of the virus into a less fit state (Althaus and De Boer, 2008; Troyer et al., 2009).
Due to the technological development of more rapid antigen-specific TCR identification strategies (Balamurugan, Ng, and Yang, 2010), it may become possible to develop multiple “off the shelf” molecularly cloned TCRs to a variety of viral antigens in the context of different HLA types that could be rapidly matched to the individual HLA type and viral genotypes they possess. In addition, chimeric receptors, such as the aforementioned UTR, may provide a way of allowing the generation of antigen-specific cells outside of HLA restriction. Due to the fact that natural antigen specific TCR precursor cell frequencies are relatively low and that a single precursor can produce thousands of antigen-specific progeny cells (Harty and Badovinac, 2008; Wiesel et al., 2009), it would not be necessary to achieve the high levels of genetic transduction efficiencies necessary to protect progeny cells from infection or to redirect peripheral blood cells in other gene therapy models. A recent study, using a highly sensitive, enrichment-based technique, identified the naïve CD8+ T cell precursor frequency for the SL9 epitope in uninfected HLA-A*0201 individuals as approximately one in 3.3 × 106 cells in the peripheral blood (Alanio et al., 2010). This precursor frequency is similar to that of cells with a specificity to a variety of other viral antigens. Increasing this naïve cell, HIV-specific precursor frequency through molecularly cloned HIV-specific TCRs to conserved epitopes of HIV could overcome limits in the magnitude of the CTL response, reconstitute defects that appear in the ability of antigen-specific cells to respond by supplying newly developed, naïve cells, and diversify the breadth of the responses by targeting new epitopes in treated individuals. While the current preclinical development of this approach is ongoing, engineering T cell immunity through the use of molecularly cloned, HIV-specific TCRs and HSCs represents a potential means to increase the quantity and quality of the CTL response in infected individuals. With the ultimate goal of immune control of viral replication, this approach could provide benefit towards delaying or preventing disease progression.
Conclusions
In sum, therapeutic strategies to treat HIV infection utilizing stem cellbased approaches have recently become a major focus of interest. Recently published studies have demonstrated the reproducibility and feasibility of performing genetic manipulation and transplantation of HSCs in an expanded clinical setting. There are currently several therapeutic strategies under investigation that are aimed at treating HIV infection by protecting the cell from infection or by specifically engineering and targeting immune responses towards the virus using adult-derived HSCs. Alternative approaches are also under investigation that use stem cells derived from different sources. For instance, there have been recent reports that human embryonic stem cells (hESC) can differentiate into the major HIV target cells, T cells, dendritic cells, and macrophages (Anderson et al., 2006; Bandi and Akkina, 2008; Galic et al., 2006; Galic et al., 2009; Subramanian et al., 2009; Timmermans et al., 2009). As further investigation into better differentiation protocols for these cells allows this to become more efficient, it is potentially feasible that stem cells sourced from hESC as well as induced pluripotent stem cells (iPSC) (Lowry et al., 2008; Park et al., 2008; Takahashi et al., 2007; Yu et al., 2007) could be used for gene therapeutic strategies to combat multiple diseases, including HIV infection. The coming years will likely see the development of greater numbers of safety and efficacy studies from the next generation of stem cell based therapeutic approaches that will serve as the basis towards the use of these strategies in therapeutic applications for many chronic diseases.
Acknowledgments
We thank Lauren Pokomo for her critical review. This work was supported by NIH grants RO1 AI078806 to SGK, NHLBI 1R01HL086409 to DSA, California HIV/AIDS Research Program (CHRP) grant 163893 to SGK, California Institute of Regenerative Medicine (CIRM) grant RC1-00149 to SGK and the UCLA Center for AIDS Research P30 AI28697.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Aiuti A, Cattaneo F, Galimberti S, Benninghoff U, Cassani B, Callegaro L, Scaramuzza S, Andolfi G, Mirolo M, Brigida I, Tabucchi A, Carlucci F, Eibl M, Aker M, Slavin S, Al-Mousa H, Al Ghonaium A, Ferster A, Duppenthaler A, Notarangelo L, Wintergerst U, Buckley RH, Bregni M, Marktel S, Valsecchi MG, Rossi P, Ciceri F, Miniero R, Bordignon C, Roncarolo MG. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med. 2009;360(5):447–458. doi: 10.1056/NEJMoa0805817. [DOI] [PubMed] [Google Scholar]
- Alanio C, Lemaitre F, Law HK, Hasan M, Albert ML. Enumeration of human antigen-specific naive CD8+ T cells reveals conserved precursor frequencies. Blood. 2010;115(18):3718–3725. doi: 10.1182/blood-2009-10-251124. [DOI] [PubMed] [Google Scholar]
- Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, Berger EA. CC CKR5: a RANTES, MIP-1alpha, MIP- 1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272(5270):1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- Althaus CL, De Boer RJ. Dynamics of Immune Escape during HIV/SIV Infection. PLoS Comput Biol. 2008;4(7):e1000103. doi: 10.1371/journal.pcbi.1000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amado RG, Mitsuyasu RT, Rosenblatt JD, Ngok FK, Bakker A, Cole S, Chorn N, Lin LS, Bristol G, Boyd MP, MacPherson JL, Fanning GC, Todd AV, Ely JA, Zack JA, Symonds GP. Anti-human immunodeficiency virus hematopoietic progenitor cell-delivered ribozyme in a phase I study: myeloid and lymphoid reconstitution in human immunodeficiency virus type-1-infected patients. Hum Gene Ther. 2004;15(3):251–262. doi: 10.1089/104303404322886101. [DOI] [PubMed] [Google Scholar]
- An DS, Donahue RE, Kamata M, Poon B, Metzger M, Mao SH, Bonifacino A, Krouse AE, Darlix JL, Baltimore D, Qin FX, De Boer RJ. Stable reduction of CCR5 by RNAi through hematopoietic stem cell transplant in non-human primates. Proc Natl Acad Sci U S A. 2007;104(32):13110–13115. doi: 10.1073/pnas.0705474104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An DS, Qin FX, Auyeung VC, Mao SH, Kung SK, Baltimore D, Chen IS. Optimization and functional effects of stable short hairpin RNA expression in primary human lymphocytes via lentiviral vectors. Mol Ther. 2006;14(4):494–504. doi: 10.1016/j.ymthe.2006.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J, Akkina R. HIV-1 resistance conferred by siRNA cosuppression of CXCR4 and CCR5 coreceptors by a bispecific lentiviral vector. AIDS Res Ther. 2005;2(1):1. doi: 10.1186/1742-6405-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J, Akkina R. Human immunodeficiency virus type 1 restriction by human-rhesus chimeric tripartite motif 5alpha (TRIM 5alpha) in CD34(+) cell-derived macrophages in vitro and in T cells in vivo in severe combined immunodeficient (SCID-hu) mice transplanted with human fetal tissue. Hum Gene Ther. 2008;19(3):217–228. doi: 10.1089/hum.2007.108. [DOI] [PubMed] [Google Scholar]
- Anderson J, Banerjea A, Akkina R. Bispecific short hairpin siRNA constructs targeted to CD4, CXCR4, and CCR5 confer HIV-1 resistance. Oligonucleotides. 2003;13(5):303–312. doi: 10.1089/154545703322616989. [DOI] [PubMed] [Google Scholar]
- Anderson J, Li MJ, Palmer B, Remling L, Li S, Yam P, Yee JK, Rossi J, Zaia J, Akkina R. Safety and efficacy of a lentiviral vector containing three anti-HIV genes--CCR5 ribozyme, tat-rev siRNA, and TAR decoy--in SCID-hu mouse-derived T cells. Mol Ther. 2007;15(6):1182–1188. doi: 10.1038/sj.mt.6300157. [DOI] [PubMed] [Google Scholar]
- Anderson JS, Bandi S, Kaufman DS, Akkina R. Derivation of normal macrophages from human embryonic stem (hES) cells for applications in HIV gene therapy. Retrovirology. 2006;3:24. doi: 10.1186/1742-4690-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JS, Javien J, Nolta JA, Bauer G. Preintegration HIV-1 inhibition by a combination lentiviral vector containing a chimeric TRIM5 alpha protein, a CCR5 shRNA, and a TAR decoy. Mol Ther. 2009;17(12):2103–2114. doi: 10.1038/mt.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba TW, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S, Cavacini LA, Posner MR, Katinger H, Stiegler G, Bernacky BJ, Rizvi TA, Schmidt R, Hill LR, Keeling ME, Lu Y, Wright JE, Chou TC, Ruprecht RM. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med. 2000;6(2):200–206. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- Bai J, Rossi J, Akkina R. Multivalent anti-CCR ribozymes for stem cell-based HIV type 1 gene therapy. AIDS Res Hum Retroviruses. 2001;17(5):385–399. doi: 10.1089/088922201750102427. [DOI] [PubMed] [Google Scholar]
- Balamurugan A, Ng HL, Yang OO. Rapid T cell receptor delineation reveals clonal expansion limitation of the magnitude of the HIV-1-specific CD8+ T cell response. J Immunol. 2010;185(10):5935–5942. doi: 10.4049/jimmunol.1002236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D. Gene therapy. Intracellular immunization. Nature. 1988;335(6189):395–396. doi: 10.1038/335395a0. [DOI] [PubMed] [Google Scholar]
- Bandi S, Akkina R. Human embryonic stem cell (hES) derived dendritic cells are functionally normal and are susceptible to HIV-1 infection. AIDS Res Ther. 2008;5:1. doi: 10.1186/1742-6405-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendle GM, Linnemann C, Hooijkaas AI, Bies L, de Witte MA, Jorritsma A, Kaiser AD, Pouw N, Debets R, Kieback E, Uckert W, Song JY, Haanen JB, Schumacher TN. Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat Med. 2010;16(5):565–570. doi: 10.1038/nm.2128. 1p following 570. [DOI] [PubMed] [Google Scholar]
- Berry LJ, Moeller M, Darcy PK. Adoptive immunotherapy for cancer: the next generation of gene-engineered immune cells. Tissue Antigens. 2009;74(4):277–289. doi: 10.1111/j.1399-0039.2009.01336.x. [DOI] [PubMed] [Google Scholar]
- Boden D, Pusch O, Lee F, Tucker L, Ramratnam B. Human immunodeficiency virus type 1 escape from RNA interference. J Virol. 2003;77(21):11531–11535. doi: 10.1128/JVI.77.21.11531-11535.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogerd HP, Doehle BP, Wiegand HL, Cullen BR. A single amino acid difference in the host APOBEC3G protein controls the primate species specificity of HIV type 1 virion infectivity factor. Proc Natl Acad Sci U S A. 2004;101(11):3770–3774. doi: 10.1073/pnas.0307713101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogerd HP, Fridell RA, Madore S, Cullen BR. Identification of a novel cellular cofactor for the Rev/Rex class of retroviral regulatory proteins. Cell. 1995;82(3):485–494. doi: 10.1016/0092-8674(95)90437-9. [DOI] [PubMed] [Google Scholar]
- Bollard CM, Kuehnle I, Leen A, Rooney CM, Heslop HE. Adoptive immunotherapy for posttransplantation viral infections. Biol Blood Marrow Transplant. 2004;10(3):143–155. doi: 10.1016/j.bbmt.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Nguyen PL, Khoruts A, Larson M, Haase AT, Douek DC. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200(6):749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan G, Kozyrev Y, Hu SL. TRIMCyp expression in Old World primates Macaca nemestrina and Macaca fascicularis. Proc Natl Acad Sci U S A. 2008;105(9):3569–3574. doi: 10.1073/pnas.0709511105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie SJ, Lewinsohn DA, Patterson BK, Jiyamapa D, Krieger J, Corey L, Greenberg PD, Riddell SR. In vivo migration and function of transferred HIV-1-specific cytotoxic T cells. Nat Med. 1999;5(1):34–41. doi: 10.1038/4716. [DOI] [PubMed] [Google Scholar]
- Brodie SJ, Patterson BK, Lewinsohn DA, Diem K, Spach D, Greenberg PD, Riddell SR, Corey L. HIV-specific cytotoxic T lymphocytes traffic to lymph nodes and localize at sites of HIV replication and cell death. J Clin Invest. 2000;105(10):1407–1417. doi: 10.1172/JCI8707. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Burdick R, Smith JL, Chaipan C, Friew Y, Chen J, Venkatachari NJ, Delviks-Frankenberry KA, Hu WS, Pathak VK. P body-associated protein Mov10 inhibits HIV-1 replication at multiple stages. J Virol. 2010;84(19):10241–10253. doi: 10.1128/JVI.00585-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butticaz C, Ciuffi A, Munoz M, Thomas J, Bridge A, Pebernard S, Iggo R, Meylan P, Telenti A. Protection from HIV-1 infection of primary CD4 T cells by CCR5 silencing is effective for the full spectrum of CCR5 expression. Antivir Ther. 2003;8(5):373–377. [PubMed] [Google Scholar]
- Cagnon L, Rossi JJ. Downregulation of the CCR5 beta-chemokine receptor and inhibition of HIV-1 infection by stable VA1-ribozyme chimeric transcripts. Antisense Nucleic Acid Drug Dev. 2000;10(4):251–261. doi: 10.1089/108729000421439. [DOI] [PubMed] [Google Scholar]
- Capodici J, Kariko K, Weissman D. Inhibition of HIV-1 infection by small interfering RNA-mediated RNA interference. J Immunol. 2002;169(9):5196–5201. doi: 10.4049/jimmunol.169.9.5196. [DOI] [PubMed] [Google Scholar]
- Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I, Vidaud M, Abel U, Dal-Cortivo L, Caccavelli L, Mahlaoui N, Kiermer V, Mittelstaedt D, Bellesme C, Lahlou N, Lefrere F, Blanche S, Audit M, Payen E, Leboulch P, l'Homme B, Bougneres P, Von Kalle C, Fischer A, Cavazzana-Calvo M, Aubourg P. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326(5954):818–823. doi: 10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]
- Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P, Selz F, Hue C, Certain S, Casanova JL, Bousso P, Deist FL, Fischer A. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288(5466):669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- Cavazzana-Calvo M, Payen E, Negre O, Wang G, Hehir K, Fusil F, Down J, Denaro M, Brady T, Westerman K, Cavallesco R, Gillet-Legrand B, Caccavelli L, Sgarra R, Maouche-Chretien L, Bernaudin F, Girot R, Dorazio R, Mulder GJ, Polack A, Bank A, Soulier J, Larghero J, Kabbara N, Dalle B, Gourmel B, Socie G, Chretien S, Cartier N, Aubourg P, Fischer A, Cornetta K, Galacteros F, Beuzard Y, Gluckman E, Bushman F, Hacein-Bey-Abina S, Leboulch P. Transfusion independence and HMGA2 activation after gene therapy of human beta-thalassaemia. Nature. 2010;467(7313):318–322. doi: 10.1038/nature09328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun TW, Davey RT, Jr, Engel D, Lane HC, Fauci AS. Re-emergence of HIV after stopping therapy. Nature. 1999;401(6756):874–875. doi: 10.1038/44755. [DOI] [PubMed] [Google Scholar]
- Clay TM, Custer MC, Sachs J, Hwu P, Rosenberg SA, Nishimura MI. Efficient transfer of a tumor antigen-reactive TCR to human peripheral blood lymphocytes confers anti-tumor reactivity. J Immunol. 1999;163(1):507–513. [PubMed] [Google Scholar]
- Cooper LJ, Kalos M, Lewinsohn DA, Riddell SR, Greenberg PD. Transfer of specificity for human immunodeficiency virus type 1 into primary human T lymphocytes by introduction of T-cell receptor genes. J Virol. 2000;74(17):8207–8212. doi: 10.1128/jvi.74.17.8207-8212.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean M, Carrington M, Winkler C, Huttley GA, Smith MW, Allikmets R, Goedert JJ, Buchbinder SP, Vittinghoff E, Gomperts E, Donfield S, Vlahov D, Kaslow R, Saah A, Rinaldo C, Detels R, O'Brien SJ. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene, Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science. 1996;273(5283):1856–1862. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- Deeks SG. HIV Infection, Inflammation, Immunosenescence, and Aging. Annu Rev Med. 2010 doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks SG, Wagner B, Anton PA, Mitsuyasu RT, Scadden DT, Huang C, Macken C, Richman DD, Christopherson C, June CH, Lazar R, Broad DF, Jalali S, Hege KM. A phase II randomized study of HIV-specific T-cell gene therapy in subjects with undetectable plasma viremia on combination antiretroviral therapy. Mol Ther. 2002;5(6):788–797. doi: 10.1006/mthe.2002.0611. [DOI] [PubMed] [Google Scholar]
- DiGiusto DL, Cooper LJ. Preparing clinical grade Ag-specific T cells for adoptive immunotherapy trials. Cytotherapy. 2007;9(7):613–629. doi: 10.1080/14653240701650320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGiusto DL, Krishnan A, Li L, Li H, Li S, Rao A, Mi S, Yam P, Stinson S, Kalos M, Alvarnas J, Lacey SF, Yee JK, Li M, Couture L, Hsu D, Forman SJ, Rossi JJ, Zaia JA. RNA-based gene therapy for HIV with lentiviral vector-modified CD34(+) cells in patients undergoing transplantation for AIDS-related lymphoma. Sci Transl Med. 2010;2(36) doi: 10.1126/scitranslmed.3000931. 36ra43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinoso JB, Kim SY, Wiegand AM, Palmer SE, Gange SJ, Cranmer L, O'Shea A, Callender M, Spivak A, Brennan T, Kearney MF, Proschan MA, Mican JM, Rehm CA, Coffin JM, Mellors JW, Siliciano RF, Maldarelli F. Treatment intensification does not reduce residual HIV-1 viremia in patients on highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 2009;106(23):9403–9408. doi: 10.1073/pnas.0903107106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA, Cayanan C, Maddon PJ, Koup RA, Moore JP, Paxton WA. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381(6584):667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- Egelhofer M, Brandenburg G, Martinius H, Schult-Dietrich P, Melikyan G, Kunert R, Baum C, Choi I, Alexandrov A, von Laer D. Inhibition of human immunodeficiency virus type 1 entry in cells expressing gp41-derived peptides. J Virol. 2004;78(2):568–575. doi: 10.1128/JVI.78.2.568-575.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Leavitt M, Tritz R, Duarte E, Kang D, Mamounas M, Gilles P, Wong-Staal F, Kennedy S, Merson J, Yu M, Barber JR. Inhibition of CCR5-dependent HIV-1 infection by hairpin ribozyme gene therapy against CC-chemokine receptor 5. Virology. 2000;276(2):271–278. doi: 10.1006/viro.2000.0536. [DOI] [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391(6669):806, 811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Furtak V, Mulky A, Rawlings SA, Kozhaya L, Lee K, Kewalramani VN, Unutmaz D. Perturbation of the P-body component Mov10 inhibits HIV-1 infectivity. PLoS One. 2010;5(2):e9081. doi: 10.1371/journal.pone.0009081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galic Z, Kitchen SG, Kacena A, Subramanian A, Burke B, Cortado R, Zack JA. T lineage differentiation from human embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103(31):11742–11747. doi: 10.1073/pnas.0604244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galic Z, Kitchen SG, Subramanian A, Bristol G, Marsden MD, Balamurugan A, Kacena A, Yang O, Zack JA. Generation of T lineage cells from human embryonic stem cells in a feeder free system. Stem Cells. 2009;27(1):100–107. doi: 10.1634/stemcells.2008-0813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar HB, Parsley KL, Howe S, King D, Gilmour KC, Sinclair J, Brouns G, Schmidt M, Von Kalle C, Barington T, Jakobsen MA, Christensen HO, Al Ghonaium A, White HN, Smith JL, Levinsky RJ, Ali RR, Kinnon C, Thrasher AJ. Gene therapy of X-linked severe combined immunodeficiency by use of a pseudotyped gammaretroviral vector. Lancet. 2004;364(9452):2181–2187. doi: 10.1016/S0140-6736(04)17590-9. [DOI] [PubMed] [Google Scholar]
- Goodchild J, Agrawal S, Civeira MP, Sarin PS, Sun D, Zamecnik PC. Inhibition of human immunodeficiency virus replication by antisense oligodeoxynucleotides. Proc Natl Acad Sci U S A. 1988;85(15):5507–5511. doi: 10.1073/pnas.85.15.5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govers C, SebestyÈn Z, Coccoris M, Willemsen RA, Debets R. T cell receptor gene therapy: strategies for optimizing transgenic TCR pairing. Trends in Molecular Medicine. 2010;16(2):77–87. doi: 10.1016/j.molmed.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Grimm D, Streetz KL, Jopling CL, Storm TA, Pandey K, Davis CR, Marion P, Salazar F, Kay MA. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature. 2006;441(7092):537–541. doi: 10.1038/nature04791. [DOI] [PubMed] [Google Scholar]
- Grimm D, Wang L, Lee JS, Schurmann N, Gu S, Borner K, Storm TA, Kay MA. Argonaute proteins are key determinants of RNAi efficacy, toxicity, and persistence in the adult mouse liver. J Clin Invest. 2010;120(9):3106–3119. doi: 10.1172/JCI43565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RK, Hue S, Schaller T, Verschoor E, Pillay D, Towers GJ. Mutation of a single residue renders human tetherin resistant to HIV-1 Vpu-mediated depletion. PLoS Pathog. 2009;5(5):e1000443. doi: 10.1371/journal.ppat.1000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8(2):107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- Heslop HE, Slobod KS, Pule MA, Hale GA, Rousseau A, Smith CA, Bollard CM, Liu H, Wu MF, Rochester RJ, Amrolia PJ, Hurwitz JL, Brenner MK, Rooney CM. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115(5):925–935. doi: 10.1182/blood-2009-08-239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessell AJ, Rakasz EG, Poignard P, Hangartner L, Landucci G, Forthal DN, Koff WC, Watkins DI, Burton DR. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog. 2009;5(5) doi: 10.1371/journal.ppat.1000433. e1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichs CS, Borman ZA, Gattinoni L, Yu Z, Burns WR, Huang J, Klebanoff CA, Johnson LA, Kerkar SP, Yang S, Muranski P, Palmer DC, Scott CD, Morgan RA, Robbins PF, Rosenberg SA, Restifo NP. Human effector CD8+ T cells derived from naive rather than memory subsets possess superior traits for adoptive immunotherapy. Blood. 2010 doi: 10.1182/blood-2010-05-286286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann C, Harrer T, Kubesch V, Maurer K, Metzner KJ, Eismann K, Bergmann S, Schmitt-Haendle M, Schuler G, Dorrie J, Schaft N. Generation of HIV-1-specific T cells by electroporation of T-cell receptor RNA. Aids. 2008;22(13):1577–1582. doi: 10.1097/QAD.0b013e3283063a17. [DOI] [PubMed] [Google Scholar]
- Holmes RK, Koning FA, Bishop KN, Malim MH. APOBEC3F can inhibit the accumulation of HIV-1 reverse transcription products in the absence of hypermutation. Comparisons with APOBEC3G. J Biol Chem. 2007;282(4):2587–2595. doi: 10.1074/jbc.M607298200. [DOI] [PubMed] [Google Scholar]
- Holt N, Wang J, Kim K, Friedman G, Wang X, Taupin V, Crooks GM, Kohn DB, Gregory PD, Holmes MC, Cannon PM. Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. Nat Biotechnol. 2010;28(8):839–847. doi: 10.1038/nbt.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang KH, Bonsall D, Katzourakis A, Thomson EC, Fidler SJ, Main J, Muir D, Weber JN, Frater AJ, Phillips RE, Pybus OG, Goulder PJ, McClure MO, Cooke GS, Klenerman P. B-cell depletion reveals a role for antibodies in the control of chronic HIV-1 infection. Nat Commun. 2010;1(7):102. doi: 10.1038/ncomms1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humeau LM, Binder GK, Lu X, Slepushkin V, Merling R, Echeagaray P, Pereira M, Slepushkina T, Barnett S, Dropulic LK, Carroll R, Levine BL, June CH, Dropulic B. Efficient lentiviral vector-mediated control of HIV-1 replication in CD4 lymphocytes from diverse HIV+ infected patients grouped according to CD4 count and viral load. Mol Ther. 2004;9(6):902–913. doi: 10.1016/j.ymthe.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Hutter G, Nowak D, Mossner M, Ganepola S, Mussig A, Allers K, Schneider T, Hofmann J, Kucherer C, Blau O, Blau IW, Hofmann WK, Thiel E. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360(7):692–698. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- Johnson LA, Heemskerk B, Powell DJ, Jr, Cohen CJ, Morgan RA, Dudley ME, Robbins PF, Rosenberg SA. Gene transfer of tumor-reactive TCR confers both high avidity and tumor reactivity to nonreactive peripheral blood mononuclear cells and tumor-infiltrating lymphocytes. J Immunol. 2006;177(9):6548–6559. doi: 10.4049/jimmunol.177.9.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph A, Zheng JH, Chen K, Dutta M, Chen C, Stiegler G, Kunert R, Follenzi A, Goldstein H. Inhibition of in vivo HIV infection in humanized mice by gene therapy of human hematopoietic stem cells with a lentiviral vector encoding a broadly neutralizing anti-HIV antibody. J Virol. 2010;84(13):6645–6653. doi: 10.1128/JVI.02339-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph A, Zheng JH, Follenzi A, Dilorenzo T, Sango K, Hyman J, Chen K, Piechocka-Trocha A, Brander C, Hooijberg E, Vignali DA, Walker BD, Goldstein H. Lentiviral vectors encoding human immunodeficiency virus type 1 (HIV-1)-specific T-cell receptor genes efficiently convert peripheral blood CD8 T lymphocytes into cytotoxic T lymphocytes with potent in vitro and in vivo HIV-1-specific inhibitory activity. J Virol. 2008;82(6):3078–3089. doi: 10.1128/JVI.01812-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Invest. 2007;117(6):1466–1476. doi: 10.1172/JCI32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June CH, Blazar BR, Riley JL. Engineering lymphocyte subsets: tools, trials and tribulations. Nat Rev Immunol. 2009;9(10):704–716. doi: 10.1038/nri2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambal A, Mitchell G, Cary W, Gruenloh W, Jung Y, Kalomoiris S, Nacey C, McGee J, Lindsey M, Fury B, Bauer G, Nolta JA, Anderson JS. Generation of HIV-1 Resistant and Functional Macrophages From Hematopoietic Stem Cell-derived Induced Pluripotent Stem Cells. Mol Ther. 2010 doi: 10.1038/mt.2010.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko S, Mastaglio S, Bondanza A, Ponzoni M, Sanvito F, Aldrighetti L, Radrizzani M, La Seta-Catamancio S, Provasi E, Mondino A, Nagasawa T, Fleischhauer K, Russo V, Traversari C, Ciceri F, Bordignon C, Bonini C. IL-7 and IL-15 allow the generation of suicide gene-modified alloreactive self-renewing central memory human T lymphocytes. Blood. 2009;113(5):1006–1015. doi: 10.1182/blood-2008-05-156059. [DOI] [PubMed] [Google Scholar]
- Kang EM, De Witte M, Malech H, Morgan RA, Carter C, Leitman SF, Childs R, Barrett AJ, Little R, Tisdale JF. Gene therapy-based treatment for HIV-positive patients with malignancies. J Hematother Stem Cell Res. 2002;11(5):809–816. doi: 10.1089/152581602760404612. [DOI] [PubMed] [Google Scholar]
- Kiem HP, Wu RA, Sun G, von Laer D, Rossi JJ, Trobridge GD. Foamy combinatorial anti-HIV vectors with MGMTP140K potently inhibit HIV-1 and SHIV replication and mediate selection in vivo. Gene Ther. 2009;17(1):37–49. doi: 10.1038/gt.2009.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiem HP, Wu RA, Sun G, von Laer D, Rossi JJ, Trobridge GD. Foamy combinatorial anti-HIV vectors with MGMTP140K potently inhibit HIV-1 and SHIV replication and mediate selection in vivo. Gene Ther. 2010;17(1):37–49. doi: 10.1038/gt.2009.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SS, Peer D, Kumar P, Subramanya S, Wu H, Asthana D, Habiro K, Yang YG, Manjunath N, Shimaoka M, Shankar P. RNAi-mediated CCR5 silencing by LFA-1-targeted nanoparticles prevents HIV infection in BLT mice. Mol Ther. 2010;18(2):370–376. doi: 10.1038/mt.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimpel J, Braun SE, Qiu G, Wong FE, Conolle M, Schmitz JE, Brendel C, Humeau LM, Dropulic B, Rossi JJ, Berger A, von Laer D, Johnson RP. Survival of the fittest: positive selection of CD4+ T cells expressing a membrane-bound fusion inhibitor following HIV-1 infection. PLoS One. 2010;5(8):e12357. doi: 10.1371/journal.pone.0012357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchen SG, Bennett M, Galic Z, Kim J, Xu Q, Young A, Lieberman A, Joseph A, Goldstein H, Ng H, Yang O, Zack JA. Engineering antigen-specific T cells from genetically modified human hematopoietic stem cells in immunodeficient mice. PLoS ONE. 2009;4(12):e8208. doi: 10.1371/journal.pone.0008208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn DB, Bauer G, Rice CR, Rothschild JC, Carbonaro DA, Valdez P, Hao Q, Zhou C, Bahner I, Kearns K, Brody K, Fox S, Haden E, Wilson K, Salata C, Dolan C, Wetter C, Aguilar-Cordova E, Church J. A clinical trial of retroviral-mediated transfer of a rev-responsive element decoy gene into CD34(+) cells from the bone marrow of human immunodeficiency virus-1-infected children. Blood. 1999;94(1):368–371. [PubMed] [Google Scholar]
- Kramer VG, Siddappa NB, Ruprecht RM. Passive immunization as tool to identify protective HIV-1 Env epitopes. Curr HIV Res. 2007;5(6):642–655. doi: 10.2174/157016207782418506. [DOI] [PubMed] [Google Scholar]
- Lalezari JP, DeJesus E, Northfelt DW, Richmond G, Wolfe P, Haubrich R, Henry D, Powderly W, Becker S, Thompson M, Valentine F, Wright D, Carlson M, Riddler S, Haas FF, DeMasi R, Sista PR, Salgo M, Delehanty J. A controlled Phase II trial assessing three doses of enfuvirtide (T-20) in combination with abacavir, amprenavir, ritonavir and efavirenz in non-nucleoside reverse transcriptase inhibitor-naive HIV-infected adults. Antivir Ther. 2003;8(4):279–287. [PubMed] [Google Scholar]
- Lee K, Ambrose Z, Martin TD, Oztop I, Mulky A, Julias JG, Vandegraaff N, Baumann JG, Wang R, Yuen W, Takemura T, Shelton K, Taniuchi I, Li Y, Sodroski J, Littman DR, Coffin JM, Hughes SH, Unutmaz DQ, Engelman A, KewalRamani VN. Flexible use of nuclear import pathways by HIV-1. Cell Host Microbe. 2010;7(3):221–233. doi: 10.1016/j.chom.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NS, Dohjima T, Bauer G, Li H, Li MJ, Ehsani A, Salvaterra P, Rossi J. Expression of small interfering RNAs targeted against HIV-1 rev transcripts in human cells. Nat Biotechnol. 2002;20(5):500–505. doi: 10.1038/nbt0502-500. [DOI] [PubMed] [Google Scholar]
- Levine BL, Humeau LM, Boyer J, MacGregor RR, Rebello T, Lu X, Binder GK, Slepushkin V, Lemiale F, Mascola JR, Bushman FD, Dropulic B, June CH. Gene transfer in humans using a conditionally replicating lentiviral vector. Proc Natl Acad Sci U S A. 2006;103(46):17372–17377. doi: 10.1073/pnas.0608138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MJ, Bauer G, Michienzi A, Yee JK, Lee NS, Kim J, Li S, Castanotto D, Zaia J, Rossi JJ. Inhibition of HIV-1 infection by lentiviral vectors expressing Pol III-promoted anti-HIV RNAs. Mol Ther. 2003;8(2):196–206. doi: 10.1016/s1525-0016(03)00165-5. [DOI] [PubMed] [Google Scholar]
- Li MJ, Kim J, Li S, Zaia J, Yee JK, Anderson J, Akkina R, Rossi JJ. Long-term inhibition of HIV-1 infection in primary hematopoietic cells by lentiviral vector delivery of a triple combination of anti-HIV shRNA, anti-CCR5 ribozyme, and a nucleolar-localizing TAR decoy. Mol Ther. 2005;12(5):900–909. doi: 10.1016/j.ymthe.2005.07.524. [DOI] [PubMed] [Google Scholar]
- Li Y, Li X, Stremlau M, Lee M, Sodroski J. Removal of arginine 332 allows human TRIM5alpha to bind human immunodeficiency virus capsids and to restrict infection. J Virol. 2006;80(14):6738–6744. doi: 10.1128/JVI.00270-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M, Kamata M, Chen KN, Pariente N, An DS, Chen IS. Inhibition of HIV-1 infection by a unique short hairpin RNA to chemokine receptor 5 delivered into macrophages through hematopoietic progenitor cell transduction. J Gene Med. 2010;12(3):255–265. doi: 10.1002/jgm.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman J, Skolnik PR, Parkerson GR, 3rd, Fabry JA, Landry B, Bethel J, Kagan J. Safety of autologous, ex vivo-expanded human immunodeficiency virus (HIV)-specific cytotoxic T-lymphocyte infusion in HIV-infected patients. Blood. 1997;90(6):2196–2206. [PubMed] [Google Scholar]
- Liu R, Paxton WA, Choe S, Ceradini D, Martin SR, Horuk R, MacDonald ME, Stuhlmann H, Koup RA, Landau NR. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86(3):367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- Lo HL, Chang T, Yam P, Marcovecchio PM, Li S, Zaia JA, Yee JK. Inhibition of HIV-1 replication with designed miRNAs expressed from RNA polymerase II promoters. Gene Ther. 2007;14(21):1503–1512. doi: 10.1038/sj.gt.3303011. [DOI] [PubMed] [Google Scholar]
- Lowry WE, Richter L, Yachechko R, Pyle AD, Tchieu J, Sridharan R, Clark AT, Plath K. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(8):2883–2888. doi: 10.1073/pnas.0711983105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucotte G. Frequencies of the CC chemokine receptor 5 delta 32 allele in various populations of defined racial background. Biomed Pharmacother. 1997;51(10):469–473. doi: 10.1016/s0753-3322(97)82328-1. [DOI] [PubMed] [Google Scholar]
- Luo XM, Maarschalk E, O'Connell RM, Wang P, Yang L, Baltimore D. Engineering human hematopoietic stem/progenitor cells to produce a broadly neutralizing anti-HIV antibody after in vitro maturation to human B lymphocytes. Blood. 2009;113(7):1422–1431. doi: 10.1182/blood-2008-09-177139. [DOI] [PubMed] [Google Scholar]
- Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, Beary H, Hayes D, Frankel SS, Birx DL, Lewis MG. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6(2):207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- McElrath MJ, Haynes BF. Induction of immunity to human immunodeficiency virus type-1 by vaccination. Immunity. 2010;33(4):542–554. doi: 10.1016/j.immuni.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehandru S, Vcelar B, Wrin T, Stiegler G, Joos B, Mohri H, Boden D, Galovich J, Tenner-Racz K, Racz P, Carrington M, Petropoulos C, Katinger H, Markowitz M. Adjunctive passive immunotherapy in human immunodeficiency virus type 1-infected individuals treated with antiviral therapy during acute and early infection. J Virol. 2007;81(20):11016–11031. doi: 10.1128/JVI.01340-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhashilkar AM, LaVecchio J, Eberhardt B, Porter-Brooks J, Boisot S, Dove JH, Pumphrey C, Li X, Weissmahr RN, Ring DB, Ramstedt U, Marasco WA. Inhibition of human immunodeficiency virus type 1 replication in vitro in acutely and persistently infected human CD4+ mononuclear cells expressing murine and humanized anti-human immunodeficiency virus type 1 Tat single-chain variable fragment intrabodies. Hum Gene Ther. 1999;10(9):1453–1467. doi: 10.1089/10430349950017798. [DOI] [PubMed] [Google Scholar]
- Miles JJ, Silins SL, Burrows SR. Engineered T cell receptors and their potential in molecular medicine. Curr Med Chem. 2006;13(23):2725–2736. doi: 10.2174/092986706778521959. [DOI] [PubMed] [Google Scholar]
- Mitsuyasu RT, Anton PA, Deeks SG, Scadden DT, Connick E, Downs MT, Bakker A, Roberts MR, June CH, Jalali S, Lin AA, Pennathur-Das R, Hege KM. Prolonged survival and tissue trafficking following adoptive transfer of CD4zeta gene-modified autologous CD4(+) and CD8(+) T cells in human immunodeficiency virus-infected subjects. Blood. 2000;96(3):785–793. [PubMed] [Google Scholar]
- Mitsuyasu RT, Merigan TC, Carr A, Zack JA, Winters MA, Workman C, Bloch M, Lalezari J, Becker S, Thornton L, Akil B, Khanlou H, Finlayson R, McFarlane R, Smith DE, Garsia R, Ma D, Law M, Murray JM, von Kalle C, Ely JA, Patino SM, Knop AE, Wong P, Todd AV, Haughton M, Fuery C, Macpherson JL, Symonds GP, Evans LA, Pond SM, Cooper DA. Phase 2 gene therapy trial of an anti-HIV ribozyme in autologous CD34+ cells. Nat Med. 2009;15(3):285–292. doi: 10.1038/nm.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RA, Dudley ME, Rosenberg SA. Adoptive cell therapy: genetic modification to redirect effector cell specificity. Cancer J. 2010;16(4):336–341. doi: 10.1097/PPO.0b013e3181eb3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314(5796):126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RA, Dudley ME, Yu YY, Zheng Z, Robbins PF, Theoret MR, Wunderlich JR, Hughes MS, Restifo NP, Rosenberg SA. High efficiency TCR gene transfer into primary human lymphocytes affords avid recognition of melanoma tumor antigen glycoprotein 100 and does not alter the recognition of autologous melanoma antigens. J Immunol. 2003;171(6):3287–3295. doi: 10.4049/jimmunol.171.6.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mummidi S, Ahuja SS, Gonzalez E, Anderson SA, Santiago EN, Stephan KT, Craig FE, O'Connell P, Tryon V, Clark RA, Dolan MJ, Ahuja SK. Genealogy of the CCR5 locus and chemokine system gene variants associated with altered rates of HIV-1 disease progression. Nat Med. 1998;4(7):786–793. doi: 10.1038/nm0798-786. [DOI] [PubMed] [Google Scholar]
- Neagu MR, Ziegler P, Pertel T, Strambio-De-Castillia C, Grutter C, Martinetti G, Mazzucchelli L, Grutter M, Manz MG, Luban J. Potent inhibition of HIV-1 by TRIM5-cyclophilin fusion proteins engineered from human components. J Clin Invest. 2009;119(10):3035–3047. doi: 10.1172/JCI39354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451(7177):425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- Newman RM, Hall L, Kirmaier A, Pozzi LA, Pery E, Farzan M, O'Neil SP, Johnson W. Evolution of a TRIM5-CypA splice isoform in old world monkeys. PLoS Pathog. 2008;4(2):e1000003. doi: 10.1371/journal.ppat.1000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng CT, Jaworski JP, Jayaraman P, Sutton WF, Delio P, Kuller L, Anderson D, Landucci G, Richardson BA, Burton DR, Forthal DN, Haigwood NL. Passive neutralizing antibody controls SHIV viremia and enhances B cell responses in infant macaques. Nat Med. 2010;16(10):1117–1119. doi: 10.1038/nm.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novina CD, Murray MF, Dykxhoorn DM, Beresford PJ, Riess J, Lee SK, Collman RG, Lieberman J, Shankar P, Sharp PA. siRNA-directed inhibition of HIV-1 infection. Nat Med. 2002;8(7):681–686. doi: 10.1038/nm725. [DOI] [PubMed] [Google Scholar]
- Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338(13):853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451(7175):141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- Park WS, Hayafune M, Miyano-Kurosaki N, Takaku H. Specific HIV-1 env gene silencing by small interfering RNAs in human peripheral blood mononuclear cells. Gene Ther. 2003;10(24):2046–2050. doi: 10.1038/sj.gt.3302099. [DOI] [PubMed] [Google Scholar]
- Perez EE, Riley JL, Carroll RG, von Laer D, June CH. Suppression of HIV-1 infection in primary CD4 T cells transduced with a self-inactivating lentiviral vector encoding a membrane expressed gp41-derived fusion inhibitor. Clin Immunol. 2005;115(1):26–32. doi: 10.1016/j.clim.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Perez EE, Wang J, Miller JC, Jouvenot Y, Kim KA, Liu O, Wang N, Lee G, Bartsevich VV, Lee YL, Guschin DY, Rupniewski I, Waite AJ, Carpenito C, Carroll RG, Orange JS, Urnov FD, Rebar EJ, Ando D, Gregory PD, Riley JL, Holmes MC, June CH. Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nat Biotechnol. 2008;26(7):808–816. doi: 10.1038/nbt1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pircher H, Burki K, Lang R, Hengartner H, Zinkernagel RM. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989;342(6249):559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- Podsakoff GM, Engel BC, Carbonaro DA, Choi C, Smogorzewska EM, Bauer G, Selander D, Csik S, Wilson K, Betts MR, Koup RA, Nabel GJ, Bishop K, King S, Schmidt M, von Kalle C, Church JA, Kohn DB. Selective survival of peripheral blood lymphocytes in children with HIV-1 following delivery of an anti-HIV gene to bone marrow CD34(+) cells. Mol Ther. 2005;12(1):77–86. doi: 10.1016/j.ymthe.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Qureshi A, Zheng R, Parlett T, Shi X, Balaraman P, Cheloufi S, Murphy B, Guntermann C, Eagles P. Gene silencing of HIV chemokine receptors using ribozymes and single-stranded antisense RNA. Biochem J. 2006;394(Pt 2):511–518. doi: 10.1042/BJ20051268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport J, Cho YY, Hendel H, Schwartz EJ, Schachter F, Zagury JF. 32 bp CCR-5 gene deletion and resistance to fast progression in HIV-1 infected heterozygotes. Lancet. 1997;349(9056):922–923. doi: 10.1016/S0140-6736(05)62697-9. [DOI] [PubMed] [Google Scholar]
- Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. The challenge of finding a cure for HIV infection. Science. 2009;323(5919):1304–1307. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]
- Riddell SR, Watanabe KS, Goodrich JM, Li CR, Agha ME, Greenberg PD. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257(5067):238–241. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- Roberts MR, Qin L, Zhang D, Smith DH, Tran AC, Dull TJ, Groopman JE, Capon DJ, Byrn RA, Finer MH. Targeting of human immunodeficiency virus-infected cells by CD8+ T lymphocytes armed with universal T-cell receptors. Blood. 1994;84(9):2878–2889. [PubMed] [Google Scholar]
- Rosenberg SA. Shedding light on immunotherapy for cancer. N Engl J Med. 2004;350(14):1461–1463. doi: 10.1056/NEJMcibr045001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA. Of mice, not men: no evidence for graft-versus-host disease in humans receiving T-cell receptor-transduced autologous T cells. Mol Ther. 2010;18(10):1744–1745. doi: 10.1038/mt.2010.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8(4):299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi JJ, June CH, Kohn DB. Genetic therapies against HIV. Nat Biotechnol. 2007;25(12):1444–1454. doi: 10.1038/nbt1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadelain M, Brentjens R, Riviere I. The promise and potential pitfalls of chimeric antigen receptors. Current Opinion in Immunology. 2009;21(2):215–223. doi: 10.1016/j.coi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarver N, Cantin EM, Chang PS, Zaia JA, Ladne PA, Stephens DA, Rossi JJ. Ribozymes as potential anti-HIV-1 therapeutic agents. Science. 1990;247(4947):1222–1225. doi: 10.1126/science.2107573. [DOI] [PubMed] [Google Scholar]
- Sayah DM, Sokolskaja E, Berthoux L, Luban J. Cyclophilin A retrotransposition into TRIM5 explains owl monkey resistance to HIV-1. Nature. 2004;430(6999):569–573. doi: 10.1038/nature02777. [DOI] [PubMed] [Google Scholar]
- Schopman NC, ter Brake O, Berkhout B. Anticipating and blocking HIV-1 escape by second generation antiviral shRNAs. Retrovirology. 2010;7:52. doi: 10.1186/1742-4690-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrofelbauer B, Chen D, Landau NR. A single amino acid of APOBEC3G controls its species-specific interaction with virion infectivity factor (Vif) Proc Natl Acad Sci U S A. 2004;101(11):3927–3932. doi: 10.1073/pnas.0307132101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senserrich J, Pauls E, Armand-Ugon M, Clotet-Codina I, Moncunill G, Clotet B, Este JA. HIV-1 resistance to the anti-HIV activity of a shRNA targeting a dual-coding region. Virology. 2008;372(2):421–429. doi: 10.1016/j.virol.2007.10.045. [DOI] [PubMed] [Google Scholar]
- Sheehy AM, Gaddis NC, Choi JD, Malim MH. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418(6898):646–650. doi: 10.1038/nature00939. [DOI] [PubMed] [Google Scholar]
- Shibata R, Igarashi T, Haigwood N, Buckler-White A, Ogert R, Ross W, Willey R, Cho MW, Martin MA. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat Med. 1999;5(2):204–210. doi: 10.1038/5568. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Hong P, Arumugam B, Pokomo L, Boyer J, Koizumi N, Kittipongdaja P, Chen A, Bristol G, Galic Z, Zack JA, Yang O, Chen IS, Lee B, An DS. A highly efficient short hairpin RNA potently down-regulates CCR5 expression in systemic lymphoid organs in the hu-BLT mouse model. Blood. 2010;115(8):1534–1544. doi: 10.1182/blood-2009-04-215855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MW, Dean M, Carrington M, Winkler C, Huttley GA, Lomb DA, Goedert JJ, O'Brien TR, Jacobson LP, Kaslow R, Buchbinder S, Vittinghoff E, Vlahov D, Hoots K, Hilgartner MW, O'Brien SJ. Contrasting genetic influence of CCR2 and CCR5 variants on HIV-1 infection and disease progression. Hemophilia Growth and Development Study (HGDS), Multicenter AIDS Cohort Study (MACS), Multicenter Hemophilia Cohort Study (MHCS), San Francisco City Cohort (SFCC), ALIVE Study. Science. 1997;277(5328):959–965. doi: 10.1126/science.277.5328.959. [DOI] [PubMed] [Google Scholar]
- Steinberger P, Andris-Widhopf J, Buhler B, Torbett BE, Barbas CF., 3rd Functional deletion of the CCR5 receptor by intracellular immunization produces cells that are refractory to CCR5-dependent HIV-1 infection and cell fusion. Proc Natl Acad Sci U S A. 2000;97(2):805–810. doi: 10.1073/pnas.97.2.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strebel K, Luban J, Jeang KT. Human cellular restriction factors that target HIV-1 replication. BMC Med. 2009;7:48. doi: 10.1186/1741-7015-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P, Sodroski J. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature. 2004;427(6977):848–853. doi: 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
- Stremlau M, Perron M, Welikala S, Sodroski J. Species-specific variation in the B30.2(SPRY) domain of TRIM5alpha determines the potency of human immunodeficiency virus restriction. J Virol. 2005;79(5):3139–3145. doi: 10.1128/JVI.79.5.3139-3145.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian N, Mani P, Roy S, Gnanasundram SV, Sarkar DP, Das S. Targeted delivery of hepatitis C virus-specific short hairpin RNA in mouse liver using Sendai virosomes. J Gen Virol. 2009;90(Pt 8):1812–1819. doi: 10.1099/vir.0.010579-0. [DOI] [PubMed] [Google Scholar]
- Sullenger BA, Gallardo HF, Ungers GE, Gilboa E. Overexpression of TAR sequences renders cells resistant to human immunodeficiency virus replication. Cell. 1990;63(3):601–608. doi: 10.1016/0092-8674(90)90455-n. [DOI] [PubMed] [Google Scholar]
- Swan CH, Buhler B, Steinberger P, Tschan MP, Barbas CF, 3rd, Torbett BE. T-cell protection and enrichment through lentiviral CCR5 intrabody gene delivery. Gene Ther. 2006;13(20):1480–1492. doi: 10.1038/sj.gt.3302801. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- ter Brake O, Konstantinova P, Ceylan M, Berkhout B. Silencing of HIV-1 with RNA interference: a multiple shRNA approach. Mol Ther. 2006;14(6):883–892. doi: 10.1016/j.ymthe.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Tian S, Maile R, Collins EJ, Frelinger JA. CD8+ T cell activation is governed by TCR-peptide/MHC affinity, not dissociation rate. J Immunol. 2007;179(5):2952–2960. doi: 10.4049/jimmunol.179.5.2952. [DOI] [PubMed] [Google Scholar]
- Timmermans F, Velghe I, Vanwalleghem L, De Smedt M, Van Coppernolle S, Taghon T, Moore HD, Leclercq G, Langerak AW, Kerre T, Plum J, Vandekerckhove B. Generation of T cells from human embryonic stem cell-derived hematopoietic zones. J Immunol. 2009;182(11):6879–6888. doi: 10.4049/jimmunol.0803670. [DOI] [PubMed] [Google Scholar]
- Trkola A, Kuster H, Rusert P, Joos B, Fischer M, Leemann C, Manrique A, Huber M, Rehr M, Oxenius A, Weber R, Stiegler G, Vcelar B, Katinger H, Aceto L, Gunthard HF. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat Med. 2005;11(6):615–622. doi: 10.1038/nm1244. [DOI] [PubMed] [Google Scholar]
- Trono D, Van Lint C, Rouzioux C, Verdin E, Barre-Sinoussi F, Chun TW, Chomont N. HIV persistence and the prospect of long-term drug-free remissions for HIV-infected individuals. Science. 2010;329(5988):174–180. doi: 10.1126/science.1191047. [DOI] [PubMed] [Google Scholar]
- Troyer RM, McNevin J, Liu Y, Zhang SC, Krizan RW, Abraha A, Tebit DM, Zhao H, Avila S, Lobritz MA, McElrath MJ, Le Gall S, Mullins JI, Arts EJ. Variable Fitness Impact of HIV-1 Escape Mutations to Cytotoxic T Lymphocyte (CTL) Response. PLoS Pathog. 2009;5(4):e1000365. doi: 10.1371/journal.ppat.1000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lent AU, Nagasawa M, van Loenen MM, Schotte R, Schumacher TN, Heemskerk MH, Spits H, Legrand N. Functional human antigen-specific T cells produced in vitro using retroviral T cell receptor transfer into hematopoietic progenitors. J Immunol. 2007;179(8):4959–4968. doi: 10.4049/jimmunol.179.8.4959. [DOI] [PubMed] [Google Scholar]
- van Loenen MM, de Boer R, Amir AL, Hagedoorn RS, Volbeda GL, Willemze R, van Rood JJ, Falkenburg JH, Heemskerk MH. Mixed T cell receptor dimers harbor potentially harmful neoreactivity. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(24):10972–10977. doi: 10.1073/pnas.1005802107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lunzen J, Glaunsinger T, Stahmer I, von Baehr V, Baum C, Schilz A, Kuehlcke K, Naundorf S, Martinius H, Hermann F, Giroglou T, Newrzela S, Muller I, Brauer F, Brandenburg G, Alexandrov A, von Laer D. Transfer of autologous gene-modified T cells in HIV-infected patients with advanced immunodeficiency and drug-resistant virus. Mol Ther. 2007;15(5):1024–1033. doi: 10.1038/mt.sj.6300124. [DOI] [PubMed] [Google Scholar]
- Varela-Rohena A, Molloy PE, Dunn SM, Li Y, Suhoski MM, Carroll RG, Milicic A, Mahon T, Sutton DH, Laugel B, Moysey R, Cameron BJ, Vuidepot A, Purbhoo MA, Cole DK, Phillips RE, June CH, Jakobsen BK, Sewell AK, Riley JL. Control of HIV-1 immune escape by CD8 T cells expressing enhanced T-cell receptor. Nat Med. 2008;14(12):1390–1395. doi: 10.1038/nm.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgen CA, Kratovac Z, Bieniasz PD, Hatziioannou T. Independent genesis of chimeric TRIM5-cyclophilin proteins in two primate species. Proc Natl Acad Sci U S A. 2008;105(9):3563–3568. doi: 10.1073/pnas.0709258105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volberding PA, Deeks SG. Antiretroviral therapy and management of HIV infection. The Lancet. 2010;376(9734):49–62. doi: 10.1016/S0140-6736(10)60676-9. [DOI] [PubMed] [Google Scholar]
- Walker RE, Bechtel CM, Natarajan V, Baseler M, Hege KM, Metcalf JA, Stevens R, Hazen A, Blaese RM, Chen CC, Leitman SF, Palensky J, Wittes J, Davey RT, Jr, Falloon J, Polis MA, Kovacs JA, Broad DF, Levine BL, Roberts MR, Masur H, Lane HC. Long-term in vivo survival of receptor-modified syngeneic T cells in patients with human immunodeficiency virus infection. Blood. 2000;96(2):467–474. [PubMed] [Google Scholar]
- Wiesel M, Walton S, Richter K, Oxenius A. Virus-specific CD8 T cells: activation, differentiation and memory formation. APMIS. 2009;117(5–6):356–381. doi: 10.1111/j.1600-0463.2009.02459.x. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Webb BL, Ylinen LM, Verschoor E, Heeney JL, Towers GJ. Independent evolution of an antiviral TRIMCyp in rhesus macaques. Proc Natl Acad Sci U S A. 2008;105(9):3557–3562. doi: 10.1073/pnas.0709003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Svarovskaia ES, Barr R, Zhang Y, Khan MA, Strebel K, Pathak VK. A single amino acid substitution in human APOBEC3G antiretroviral enzyme confers resistance to HIV-1 virion infectivity factor-induced depletion. Proc Natl Acad Sci U S A. 2004;101(15):5652–5657. doi: 10.1073/pnas.0400830101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Baltimore D. Long-term in vivo provision of antigen-specific T cell immunity by programming hematopoietic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(12):4518–4523. doi: 10.1073/pnas.0500600102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Qin XF, Baltimore D, Van Parijs L. Generation of functional antigen-specific T cells in defined genetic backgrounds by retrovirus-mediated expression of TCR cDNAs in hematopoietic precursor cells. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(9):6204–6209. doi: 10.1073/pnas.092154599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang OO, Tran AC, Kalams SA, Johnson RP, Roberts MR, Walker BD. Lysis of HIV-1-infected cells and inhibition of viral replication by universal receptor T cells. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(21):11478–11483. doi: 10.1073/pnas.94.21.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap MW, Nisole S, Stoye JP. A single amino acid change in the SPRY domain of human Trim5alpha leads to HIV-1 restriction. Curr Biol. 2005;15(1):73–78. doi: 10.1016/j.cub.2004.12.042. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zhang H, Snyder KM, Suhoski MM, Maus MV, Kapoor V, June CH, Mackall CL. 4-1BB is superior to CD28 costimulation for generating CD8+ cytotoxic lymphocytes for adoptive immunotherapy. J Immunol. 2007;179(7):4910–4918. doi: 10.4049/jimmunol.179.7.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Parkhurst MR, Zheng Z, Cohen CJ, Riley JP, Gattinoni L, Restifo NP, Rosenberg SA, Morgan RA. Extrathymic generation of tumor-specific T cells from genetically engineered human hematopoietic stem cells via Notch signaling. Cancer Res. 2007;67(6):2425–2429. doi: 10.1158/0008-5472.CAN-06-3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Dudley ME, Rosenberg SA, Robbins PF. Persistence of multiple tumor-specific T-cell clones is associated with complete tumor regression in a melanoma patient receiving adoptive cell transfer therapy. J Immunother. 2005;28(1):53–62. doi: 10.1097/00002371-200501000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N, Fang J, Mukhtar M, Acheampong E, Pomerantz RJ. Inhibition of HIV-1 fusion with small interfering RNAs targeting the chemokine coreceptor CXCR4. Gene Ther. 2004;11(23):1703–1712. doi: 10.1038/sj.gt.3302339. [DOI] [PubMed] [Google Scholar]