Abstract
Although known for its acutely toxic action, palytoxin has also been identified as a type of carcinogenic agent called a tumor promoter. In general tumor promoters do not damage DNA, but instead contribute to carcinogenesis by disrupting the regulation of cellular signaling. The identification of palytoxin as a tumor promoter, together with the recognition that the Na+,K+-ATPase is its receptor, led to research on how palytoxin triggers the modulation of signal transduction pathways. This review focuses on mitogen activated protein (MAP) kinases as mediators of palytoxin-stimulated signaling. MAP kinases are a family of serine/threonine kinases that relay a variety of signals to the cellular machinery that regulates cell fate and function. The studies discussed in this review investigated how palytoxin stimulates MAP kinase activity and, in turn, how MAP kinases mediate the response of cells to palytoxin.
Keywords: mitogen activated protein kinase; dual specificity phosphatase; Na+,K+-ATPase; tumor promoter; palytoxin
1. Introduction
The carcinogenic action of palytoxin was revealed by the multi-stage mouse skin model of carcinogenesis (Fujiki et al., 1986). The development of cancer involves a series of genetic and epigenetic changes that occur at different stages of tumorigenesis, starting with early changes in cell behavior, proceeding to the development of benign tumors, and then progressing to the development of malignant tumors. The multi-stage mouse skin model has been instrumental in revealing important genetic and biochemical changes that occur during the process of carcinogenesis (Yuspa, 1998).
Traditionally, the multi-stage mouse skin model has been used to classify chemical carcinogens as either initiators, which typically damage DNA, or tumor promoters, which do not directly damage DNA, but instead alter signal transduction pathways. The first stage of carcinogenesis in this model is called initiation; in this stage the single application of an initiator results in the activation of the oncogene Ras (Balmain and Pragnell, 1983). Initiation is a rapid, irreversible stage. The second stage is called tumor promotion. This stage requires repeated application of the tumor promoter over the course of several weeks and results in the development of benign tumors called papillomas (Hennings et al., 1993; Yuspa, 1998). Tumor promotion is reversible if tumor promoter treatment is ceased.
The phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA) (also called phorbol-12-myristate-13-acetate or PMA) is the prototypical tumor promoter in the multistage mouse skin model. The identification of protein kinase C as the receptor for TPA represented a major breakthrough in the field of carcinogenesis because it suggested that tumor promoter action involves the aberrant modulation of protein kinases involved in signal transduction (Nishizuka, 1984). Once protein kinase C was identified as the phorbol ester receptor, however, studies were designed to determine if other types of compounds could also act as tumor promoters, and whether their action was necessarily mediated by protein kinase C.
Palytoxin was identified as a tumor promoter in a screen of naturally occurring compounds isolated from various sources, including plants and marine products (Fujiki et al., 1983). The screen included a series of short-term tests that detect typical cellular and physiological responses to tumor promoters. These tests included irritation of mouse skin, induction of ornithine decarboxylase in mouse skin, and induction of the adhesion of human promyelocytic leukemia cells (HL60). The compounds were also tested for tumor promoting activity in the multi-stage mouse skin model. Compounds that scored positive in the multi-stage mouse skin model were classified as non-TPA-type if they failed to bind to and activate protein kinase C in vitro. Palytoxin, like TPA, scored positive in the assays for skin irritation and tumor promotion. In contrast to TPA, however, palytoxin scored negative in the assays for induction of ornithine decarboxylase and HL60 cell adhesion. Importantly, palytoxin scored negative in the assays for protein kinase C binding and activation, and was therefore classified as a non-TPA-type tumor promoter. These novel properties suggested that studying palytoxin action might reveal new information about signal transduction and tumor promotion.
Cell culture studies provided further evidence that palytoxin stimulates signaling pathways that do not require phorbol ester-sensitive isoforms of protein kinase C (Wattenberg et al., 1987). A set of early studies compared the signaling pathways by which palytoxin and TPA-type tumor promoters modulate the epidermal growth factor (EGF) receptor in Swiss 3T3 fibroblasts (Wattenberg et al., 1987; Wattenberg et al., 1989a; Wattenberg et al., 1989b; Wattenberg et al., 1989c). Both palytoxin and phorbol esters stimulated a loss of EGF binding (Friedman et al., 1984; Wattenberg et al., 1987). Whereas down modulation of protein kinase C blocked the effect of phorbol esters on the EGF receptor, the loss of protein kinase C did not inhibit the effects of palytoxin (Wattenberg et al., 1987).
A clue to the nature of palytoxin-stimulated signaling was suggested by studies which identified the Na+,K+-ATPase as the palytoxin receptor and reports that palytoxin stimulated ion flux in a variety of excitable and non-excitable systems (Habermann, 1989). Although palytoxin-stimulated ion flux is often associated with acute toxicity, subsequent studies indicated that sodium influx plays an important role in the ability of palytoxin to stimulate the down modulation of the EGF receptor under noncytotoxic conditions (Wattenberg et al., 1989a; Wattenberg et al., 1989b). A search for biochemical mediators of palytoxin-stimulated ion flux led to the identification of mitogen activated protein (MAP) kinases as important targets of palytoxin action (Wattenberg, 2007).
2. Mitogen activated protein (MAP) kinases
The MAP kinase family of serine/threonine kinases mediates the action of a wide variety of stimuli and plays a key role in regulating cell fate and function in many systems (Roux and Blenis, 2004; Dhillon et al., 2007; Raman et al., 2007; Turjanski et al., 2007). Phosphorylation is a fundamental mechanism by which cells regulate the function of various types of proteins. For example, phosphorylation can affect protein stability, enzyme activity, and the activity of transcription factors (Roux and Blenis, 2004; Zeliadt et al., 2008). MAP kinases phosphorylate and thus modulate the function of various types of proteins that are located in the membrane, the cytosol and the nucleus; phosphatases that remove the phosphate groups reverse the action of MAP kinases.
There are at least six major types of MAP kinases (Raman et al., 2007). The three types that have been studied most extensively are extracellular signal regulated kinase 1 (ERK1) and ERK2 (referred to here as ERK1/2), c-Jun N-terminal kinase/stress-activated protein kinase (referred to here as JNK), and p38. MAP kinase family members are often initially characterized by the types of signals that stimulate enzyme activation. For example, agents that induce cell proliferation, such as growth factors, typically stimulate ERK1/2 activation. Agents that are stressful to the cell, such as UV light and proinflammatory cytokines, typically stimulate JNK and p38 activation. ERK5 (also called Big MAP kinase 1 or BMK1) is a MAP kinase family member that is not as well characterized as ERK1/2, JNK, or p38, but is receiving increasing attention as a mediator of signals stimulated by both mitogens, such as EGF, and by stress, including osmotic shock (Abe et al., 1996; Kato et al., 1998; Wang and Tournier, 2006).
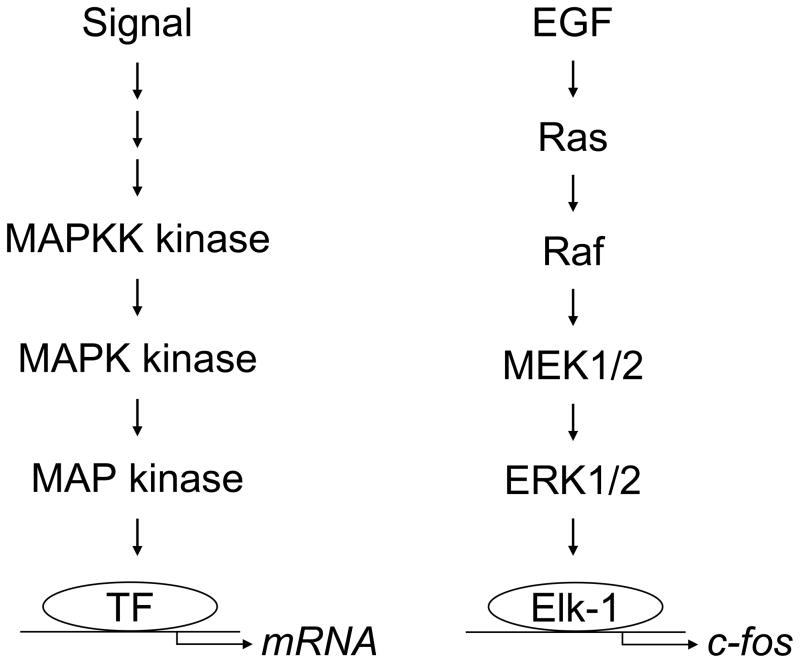
MAP kinases themselves are regulated by reversible phosphorylation (Turjanski et al., 2007). Full activation of MAP kinases requires dual phosphorylation of a conserved threonine residue and a conserved tyrosine residue, separated by one amino acid, which are located within the activation loop of the enzyme; restoring MAP kinases to the inactive state requires dephosphorylation of these residues. The signal to phosphorylate and activate MAP kinases is typically transmitted through the sequential phosphorylation and activation of three kinases; this is known as a protein kinase cascade (Fig. 1). In general, the cell receives a signal that stimulates the activation of a MAP kinase kinase kinase (MAPKK kinase). Once activated the MAPKK kinase phosphorylates and activates a MAP kinase kinase (MAPK kinase or MEK). The MAPK kinase can then phosphorylate and activate a MAP kinase. The phosphorylated, active MAP kinase can then phosphorylate various types of cellular substrates. For example, MAP kinases can translocate from the cytoplasm to the nucleus and then phosphorylate and activate transcription factors, resulting in changes in gene expression.
Fig. 1.
MAP kinases are regulated by protein kinase cascades. The cell receives a signal that stimulates the activation of a MAP kinase kinase kinase (MAPKK kinase). The MAPKK kinase then phosphorylates and activates a MAP kinase kinase (MAPK kinase or MEK). The MAPK kinase then phosphorylates and activates a MAP kinase. The phosphorylated, active MAP kinase can translocate from the cytoplasm to the nucleus and then phosphorylate and activate transcription factors (TF), resulting in changes in gene expression. For example, EGF stimulates the activation of the GTPase Ras. Ras activates Raf (a MAPKK kinase). Raf phosphorylates and activates MEK1/2 (the MAPK kinases MEK1 and MEK2). MEK1/2 phosphorylates and activates ERK1/2 (MAP kinases). ERK1/2 can then translocate from the cytoplasm to the nucleus to phosphorylate transcription factors such as Elk-1, which regulates the expression of c-Fos.
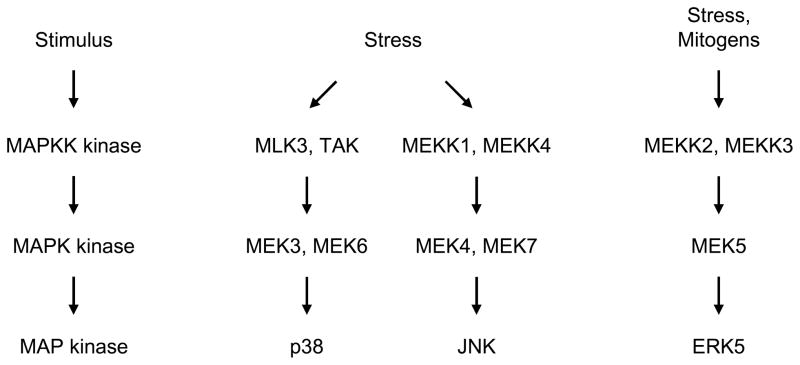
The major types of MAP kinases tend to be activated by different protein kinase cascades, although cross talk between the cascades can occur (Fig. 1 and Fig. 2) (Dhillon et al., 2007; Raman et al., 2007; Turjanski et al., 2007). The best-defined MAP kinase cascade is the one that regulates ERK1/2 (Fig. 1). For example, EGF binds to a transmembrane tyrosine kinase receptor, which stimulates the activation of the GTPase Ras. Ras activates a MAPKK kinase called Raf. The phosphorylated, active form of Raf can then phosphorylate and activate MEK1 and MEK2 (MAPK kinases referred to here as MEK1/2). The phosphorylated, active forms of MEK1/2 can then phosphorylate and activate ERK1/2 (a MAP kinase). ERK1/2 can then translocate from the cytoplasm to the nucleus and phosphorylate transcription factors such as Elk-1, which regulates the expression of c-Fos (Fig. 1) (Turjanski et al., 2007). JNK, p38, and ERK5 are regulated by similar protein kinase cascades (Fig. 2). Several MAPKK kinases can serve in the protein kinase cascades that regulate JNK and p38 (Cuevas et al., 2007; Raman et al., 2007). MEK4 (also called SEK1) and MEK7 are MAPK kinases that can phosphorylate and activate JNK; MEK3 and MEK6 are MAPK kinases that can phosphorylate and activate p38 (Raman et al., 2007). MEKK2 and MEKK3 are MAPKK kinases that can phosphorylate and activate MEK5, the MAPK kinase that phosphorylates and activates ERK5 (Chao et al., 1999; Sun et al., 2001).
Fig. 2.
p38, JNK, and ERK5 are regulated by different protein kinase cascades. Various MAPKK kinases, including MLK3, and TAK, can regulate p38. MEK3 and MEK6 are MAPK kinases that phosphorylate and activate p38 (a MAP kinase). MEKK1 and MEKK4 are among the MAPKK kinases that regulate JNK. MEK4 and MEK7 are MAPK kinases that phosphorylate and activate JNK (a MAP kinase). MEKK2 and MEKK3 are MAPKK kinases that can regulate ERK5. MEK5 is a MAPK kinase that phosphorylates and activates ERK5 (a MAP kinase).
The expression of specific MAP kinase modulators, such as upstream kinases, phosphatases, and downstream molecular targets, can vary between cell types. As a result, the ability of specific types of signals to selectively stimulate the activation of ERK1/2, JNK, p38, and ERK5, and the role of specific MAP kinase family members in regulating cell fate and function, can differ depending on the model system (Engelberg, 2004). For example, in some systems ERK5 can be involved in cell proliferation, while in others ERK5 is involved in apoptosis (Kato et al., 1998; Dinev et al., 2001; Sohn et al., 2008).
3. Palytoxin stimulates JNK activation through a pathway that involves ion flux
The first indication that MAP kinases can mediate signals triggered by palytoxin came from studies which showed that palytoxin stimulates JNK activation in Swiss 3T3 fibroblasts (Kuroki et al., 1996). JNK activity is stimulated by osmotic stress (Davis, 1994). Palytoxin causes a type of osmotic stress through its interaction with the Na+,K+-ATPase and the stimulation of ion flux. Together, these observations suggested that the osmotic stress often associated with palytoxin-induced toxicity might also trigger the activation of cellular signaling pathways.
The mechanisms by which palytoxin affects JNK activity were studied in Swiss 3T3, HeLa, and COS7 cells, which are commonly used to study signal transduction (Kuroki et al., 1996; Kuroki et al., 1997). Incubation of cells with picomolar concentrations of palytoxin stimulated sustained JNK activity (Kuroki et al., 1996). Interestingly, the dose-response curve was an inverted U-shape in Swiss 3T3 cells. Under nontoxic conditions (picomolar concentrations), JNK activity increased with increasing concentrations of palytoxin. Under toxic conditions (nanomolar concentrations), JNK activity decreased with increasing concentrations of palytoxin. Furthermore, there was a dose-dependent effect on the kinetics of JNK activation. Under conditions in which palytoxin was not toxic, the higher the concentration of palytoxin, the more rapidly JNK activation was detected.
Although initial studies indicated that palytoxin stimulates JNK activation through a mechanism that involves sodium influx, not calcium influx (Kuroki et al., 1996), a later report indicated that palytoxin stimulates JNK activation in rat fibroblasts through a mechanism that involves potassium efflux, as opposed to sodium influx (Iordanov and Magun, 1998). Finally, studies also demonstrated that palytoxin-stimulated signals are transmitted to JNK through the activation of a protein kinase cascade, such that the induction of ion flux by palytoxin results in the activation of MEK4, a MAPK kinase that phosphorylates and activates JNK (Sanchez et al., 1994; Derijard et al., 1995; Lin et al., 1995; Kuroki et al., 1997).
4. Palytoxin stimulates p38 activation through different protein kinases cascades, depending on the cell type
Palytoxin also stimulates the activation of p38, another major stress-activated MAP kinase that is activated in response to osmotic stress (Li and Wattenberg, 1999; Warmka et al., 2002; Raman et al., 2007). Studies on the mechanisms by which palytoxin stimulates p38 activity revealed an intriguing difference between HeLa and COS7 cells with regard to the protein kinase cascades that transmit palytoxin-induced signals to p38 (Li and Wattenberg, 1999). Although MEK3 and MEK6 are widely recognized as MAPK kinases that specifically phosphorylate and activate p38 (Han et al., 1996; Moriguchi et al., 1996; Stein et al., 1996), under some conditions MEK4, a MAPK kinase that phosphorylates and activates JNK, can also phosphorylate and activate p38 (Derijard et al., 1995; Lin et al., 1995). HeLa cells express MEK3, MEK6, and MEK4, and, as expected, palytoxin stimulated the activation of all three MAPK kinases cascades that required MEK3 and MEK6 in HeLa cells, MEK4 was not required. Like HeLa cells, COS 7 cells express MEK3, MEK6, and MEK4 (Li and Wattenberg, 1999). Surprisingly, and in contrast to HeLa cells, in COS7 cells palytoxin stimulated the activation of MEK6 and MEK4, but not MEK3; the protein kinase cascades that transmit palytoxin-stimulated signals to p38 in COS7 cells require MEK6 and MEK4. These studies illustrate how palytoxin-stimulated signaling cascades can differ between cell types.
A study conducted in MCF-7 human breast cancer cells suggests that heat shock protein 27 (Hsp27) may be one of the downstream targets of palytoxin-stimulated p38 activation (Sala et al., 2009). Proteomic analysis of MCF-7 cells treated with a cytotoxic concentration of palytoxin revealed the presence of Hsp27 phosphorylated on serine 82. p38 phosphorylates and activates MAP kinase activated protein kinase-2 (MAPKAP kinase-2), which phosphorylates Hsp27 (Stokoe et al., 1992; Roux and Blenis, 2004). It has thus been suggested that palytoxin stimulates the phosphorylation of Hsp27 through a MAPKAP kinase-2/p38 protein kinase cascade (Sala et al., 2009).
5. Palytoxin modulates ERK1/2 activity in cells that express oncogenic Ras
Early work supported the idea that the non-TPA-type tumor promoter palytoxin stimulates different signaling pathways than the prototypical tumor promoter TPA. This was supported by the observation that in COS7, HeLa, and Swiss 3T3 cells, palytoxin predominantly stimulates JNK and p38 activation, the stress-activated MAP kinases, whereas phorbol esters predominantly stimulate ERK1/2 activation, which is typically a mitogen-activated kinase (Kuroki et al., 1996; Kuroki et al., 1997; Li and Wattenberg, 1998). These results were consistent with the fact that palytoxin and TPA bind to very different types of receptors; palytoxin signaling is triggered by changes in ion flux that result from modulation of the Na+,K+-ATPase, whereas TPA directly modulates the activity of protein kinase C. Yet the discovery that ERK1/2, JNK, and p38 can modulate common substrates, despite their stimulation by different types of signals, suggested that MAP kinases might act as mediators through which the different signaling pathways stimulated by palytoxin and TPA could converge to regulate common targets involved in carcinogenesis. For example, all three major MAP kinases can phosphorylate and modulate the activity of the transcription factor Elk-1, which regulates the expression of c-Fos; ERK1/2, JNK, and p38 also all modulate the AP-1 family of transcription factors, which are dimers made up of various combinations of Jun and Fos family members (Angel and Karin, 1991; Gille et al., 1995; Whitmarsh et al., 1995; Raingeaud et al., 1996; Whitmarsh and Davis, 1996; Karin et al., 1997).
The concept that palytoxin and TPA modulate common transcription factors and stimulate the expression of common genes through the activation of MAP kinases was investigated in a keratinocyte cell line called 308 (Warmka et al., 2002; Zeliadt et al., 2003; Zeliadt et al., 2004). 308 cells, which were derived from initiated mouse skin and express oncogenic Ras, were chosen as a model for the likely target cells of tumor promoters in vivo (Strickland et al., 1988). The most surprising result from these studies was that in initiated mouse keratinocytes, as opposed to several other cell types, both palytoxin- and TPA-stimulated signaling pathways lead to the activation of ERK1/2 activation (Warmka et al., 2002; Zeliadt et al., 2004). These studies demonstrated again how the cellular context can dramatically affect how palytoxin-stimulated signals are transmitted through the cell.
The significance of ERK1/2 as a common target for non-TPA-type and TPA-type tumor promoters is supported by the observation that palytoxin and TPA modulate several common downstream effects, which are likely to be involved in tumor promotion, through ERK1/2-dependent pathways, including c-Fos gene expression, the modulation of AP-1, and the expression of matrix metalloproteinase 13 (MMP-13) (Warmka et al., 2002; Zeliadt et al., 2004). In addition, several studies indicate that aberrant regulation of ERK1/2 is involved in mouse and human carcinogenesis (Hoshino et al., 1999; Kim et al., 1999; Albanell et al., 2001; Cohen et al., 2002; Santen et al., 2002; Segrelles et al., 2002; Smalley, 2003; Bourcier et al., 2006). Interestingly, although palytoxin stimulates the activation of JNK and p38 in 308 cells, palytoxin does not require either JNK or p38 to increase c-Fos protein levels or MMP-13 gene expression (Zeliadt et al., 2003).
These studies also suggested that 308 mouse keratinocytes express modulators of ERK1/2 activity that are sensitive to palytoxin action. The identification of such ERK1/2 modulators could explain how the cellular context determines whether or not palytoxin can stimulate ERK1/2 activity. MAP kinase activity is determined by the balance between phosphorylation of specific tyrosine and threonine residues, which activates MAP kinases, and dephosphorylation, which inactivates the kinases (Camps et al., 2000; Keyse, 2000; Chen et al., 2001; Farooq and Zhou, 2004; Keyse, 2008). One possible explanation for the observation that palytoxin stimulates ERK1/2 activity in 308 cells is that these cells, but not others, express a novel MAPK kinase that is activated by palytoxin-stimulated signaling. Alternatively, 308 cells could express a different set of phosphatases than other cell types; in this case palytoxin could increase ERK1/2 activity by disrupting the activity of a phosphatase that is a negative regulator of ERK1/2.
5.1 Palytoxin modulates ERK1/2 activity by down modulating the phosphatase MKP-3
Palytoxin increases ERK1/2 activity through a mechanism that is quite distinct from the mechanisms by which it stimulates JNK and p38 activation. Whereas palytoxin stimulates JNK and p38 through the activation of upstream MAPK kinases, palytoxin increases ERK1/2 activity in 308 keratinocytes by stimulating a decrease in the cellular activity of an ERK1/2 phosphatase. ERK1/2 activity can be modulated by three different classes of protein phosphatases: tyrosine phosphatases, serine-threonine phosphatases, and a family of dual-specificity phosphatases called MAP kinase phosphatases (MKPs) that dephosphorylate both tyrosine and threonine residues (Camps et al., 2000; Keyse, 2000; Farooq and Zhou, 2004; Raman et al., 2007; Keyse, 2008).
The surprising result that palytoxin did not stimulate MEK1/2 activity in 308 mouse keratinocytes, together with the observation that incubation of 308 cells with palytoxin resulted in a delayed, yet prolonged increase in ERK1/2 activity, suggested that palytoxin increased ERK1/2 activity through the inactivation of a phosphatase (Warmka et al., 2004). Accordingly, it was found that the ability of palytoxin to increase ERK1/2 activity was linked to the down modulation of a phosphatase called MKP-3, a dual-specificity phosphatase that is highly selective for dephosphorylating and inactivating ERK1/2 (Muda et al., 1996). Palytoxin stimulates a dramatic loss of MKP-3 within one hour (Warmka et al., 2004). MKP-3 is a relatively unstable protein (Marchetti et al., 2004; Marchetti et al., 2005), and thus a particularly vulnerable target for agents that block the production of this phosphatase. In rat fibroblasts, palytoxin inhibits protein synthesis through a mechanism that requires potassium efflux (Iordanov and Magun, 1998). Such a block in translation could explain how palytoxin induces the loss of MKP-3.
Further studies demonstrated that the ability of palytoxin to increase ERK1/2 activity is not specific to mouse keratinocytes, but instead is related to the expression of oncogenic Ras. For example, palytoxin does not stimulate an increase in ERK1/2 activity in the human breast epithelial cell line called MCF10A (Warmka et al., 2004). Palytoxin can increase ERK1/2 activity when this cell line is engineered to express oncogenic Ras, however (Warmka et al., 2004). The expression of oncogenic Ras results in the increased expression of MKP-3 in many different systems, including MCF10A cells (Yip-Schneider et al., 2001; Croonquist et al., 2003; Furukawa et al., 2003; Warmka et al., 2004; Bloethner et al., 2005; Keyse, 2008). This suggests that cells adapt to the chronic activation of Ras by boosting negative feedback pathways that can dampen the activity of Ras-stimulated protein kinase cascades. Altogether, these studies suggest that in initiated cells that express oncogenic Ras, palytoxin can shift the balance toward the active, phosphorylated form of ERK1/2 by blocking the action of MKP-3, which directly dephosphorylates and inactivates ERK1/2. This is a potentially important mechanism by which palytoxin disrupts the regulation of ERK1/2 activity because the duration and magnitude of ERK1/2 activity affects cell behavior, such that in some systems the duration of ERK1/2 activity determines whether a cell proliferates or undergoes differentiation (Marshall, 1995; McCawley et al., 1999; Murphy et al., 2002).
5.2 Palytoxin stimulates ERK1/2 activity through autocrine stimulation
A study of the role of prostaglandins in the tumor promoting action of palytoxin in an in vitro Balb/c 3T3 cell transformation model indicates that palytoxin can also activate ERK1/2 through an autocrine mechanism (Miura et al., 2006). Balb/c 3T3 cells were initiated with 3-methylcholanthrene. The tumor promotion phase was modeled by a 2-week treatment with palytoxin. Nontoxic concentrations of palytoxin increased the production of prostaglandins, stimulated ERK1/2 phosphorylation, and increased the number of transformed foci. All of these palytoxin-stimulated effects were blocked by indomethacin, an inhibitor of prostaglandin synthesis. This study suggests that, in Balb/c 3T3 cells, palytoxin action triggers the production and release of prostaglandins, which then interact with neighboring cells to stimulate ERK1/2 activity.
5.3 The cytotoxic effects of palytoxin in cultured neurons involves ERK1/2
ERK1/2 has been implicated in the cytotoxic, as well as the carcinogenic, action of palytoxin (Vale et al., 2007). Pharmacological inhibitors were used to investigate the role of different protein kinases in palytoxin-induced increases in intracellular calcium, intracellular acidification, and cytotoxicity in cultured neurons. Genistein, which inhibits tyrosine kinases, blocked the ability of palytoxin to increase intracellular calcium (Vale et al., 2007). High concentrations (500 nM) of GF 109203X, which inhibits calcium-independent isoforms of protein kinase C, also inhibited the ability of palytoxin to increase intracellular calcium. The calcium-independent class of protein kinase C isoforms includes both novel isoforms that are activated by phorbol esters and also atypical isoforms that are not activated by phorbol esters (Griner and Kazanietz, 2007). Further studies are required to determine which isoforms mediate palytoxin action in neurons. Pretreatment of the cells with the MEK1/2 inhibitor PD98059 or a cell-permeable inhibitor of ERK2 blocked the ability of palytoxin to increase intracellular calcium levels and induce intracellular acidification, and blocked palytoxin-induced cytotoxicity. These studies suggest that ERK1/2 may play an important role in palytoxin-induced neurotoxicity (Vale et al., 2007).
6. ERK5 relays palytoxin-stimulated signals to the nucleus
Palytoxin-stimulated signals can also be mediated by ERK5 (Wang and Tournier, 2006). ERK5 is distinguished from other MAP kinase family members by its large C-terminal non-kinase domain (Lee et al., 1995; Zhou et al., 1995; Hayashi and Lee, 2004). This C-terminal domain, which contains a nuclear localization signal, makes ERK5 approximately twice the size of ERK1/2. Transcription factors, including MEF2C (myocyte enhancer factor), Sap1a, and c-Fos, are among the substrates of ERK5 (Kato et al., 1997; Kamakura et al., 1999; Terasawa et al., 2003).
Three lines of evidence prompted the investigation of the role of ERK5 in palytoxin-stimulated gene expression. First, osmotic stress stimulates ERK5 activation (Abe et al., 1996), suggesting that palytoxin-stimulated changes in ion flux, which causes a type of osmotic stress, might stimulate ERK5 activity. Second, early studies concluded that palytoxin modulates c-Fos through an ERK1/2-dependent mechanism based, in part, on the ability of the MEK1/2 inhibitor PD98059 to block palytoxin-stimulated ERK1/2 activity and increases in c-Fos levels (Warmka et al., 2002). Several types of MEK1/2 inhibitors can also block MEK5, the MAPK kinase that phosphorylates and activates ERK5 (Kamakura et al., 1999; Mody et al., 2003). Consequently, MEK1/2 inhibitors, such as PD98059, can block activation of ERK5, as well as ERK1/2, in some cell types. Finally, both ERK1/2 and ERK5 can regulate c-Fos gene expression (Sasaki et al., 2006).
The role of ERK5 in mediating palytoxin-stimulated c-Fos gene expression was investigated in HeLa and 308 cells (Charlson et al., 2009). Palytoxin stimulated transient ERK5 activation in these cell lines. By contrast, palytoxin stimulated prolonged ERK1/2, JNK, and, p38 activation. This indicated that the mechanism by which palytoxin modulates ERK5 differs from that by which it modulates the three major MAP kinases. These studies also demonstrated that palytoxin modulates ERK5 though a mechanism that requires its interaction with the Na+,K+-ATPase, but does not require inhibition of protein synthesis or inhibition of either serine/threonine phosphatases or tyrosine phosphatases.
Both ERK1/2 and ERK5 appear to be involved in palytoxin-stimulated c-Fos gene expression. Low concentrations of the MEK1/2 inhibitor U0126, which block palytoxin-stimulated ERK1/2 activation, but not ERK5 activation, partially inhibited palytoxin-stimulated c-Fos gene expression. By contrast, high concentrations of U0126, which block both palytoxin-stimulated ERK1/2 and ERK5 activation, caused an almost complete block in palytoxin-stimulated c-Fos gene expression. Complementary studies showed that in HeLa cells, knockdown of ERK5 by shRNA partially blocked palytoxin-stimulated c-Fos gene expression at 60 minutes. Interestingly, knockdown of ERK5 did not affect palytoxin-stimulated c-Fos gene expression at 180 minutes, a time point at which ERK1/2 activity is continuing to increase as ERK5 activity is decreasing. This suggests that at 180 minutes, the level of ERK1/2 activity may be high enough to maintain c-Fos expression even in the absence of ERK5 activity. Altogether, these studies support a role for ERK5 in mediating palytoxin-stimulated signals to the nucleus, although they also indicate that there is redundancy in the ability of MAP kinases to mediate palytoxin-stimulated gene expression.
7. Conclusions
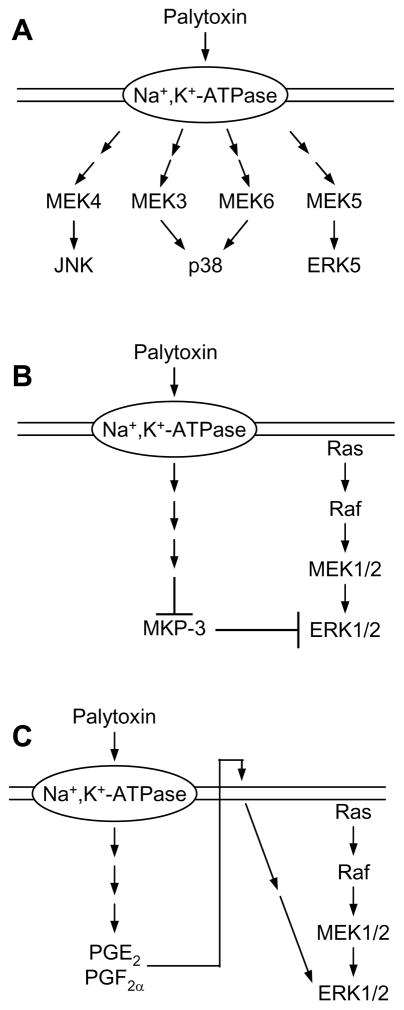
In summary, palytoxin can disrupt the regulation of MAP kinase signaling pathways by several different mechanisms (Fig. 3). First, the interaction of palytoxin with the Na+,K+-ATPase triggers a change in ion flux, which then stimulates the activation of protein kinase cascades that ultimately activate JNK, p38, and ERK5 (Fig. 3A) (Kuroki et al., 1997; Li and Wattenberg, 1999; Charlson et al., 2009). Second, palytoxin action results in the down modulation of MKP-3; the loss of this negative regulator of ERK1/2 in cells that express oncogenic Ras results in the accumulation of the phosphorylated, active form of ERK1/2 (Fig. 3B) (Warmka et al., 2004). Third, palytoxin can stimulate the production and release of prostaglandins in Balb/c 3T3 cells, which can then interact with cells to stimulate ERK1/2 activation (Fig. 3C) (Miura et al., 2006). Further research is needed to determine how the cellular context affects the mechanisms by which palytoxin modulates different MAP kinases, and how this affects the biological response of the cell to palytoxin exposure.
Fig. 3.
Palytoxin modulates MAP kinase activity by several mechanisms. (A) Palytoxin stimulates the activation of JNK, p38, and ERK5 through the stimulation of upstream protein kinase cascades. (B) In cells that express oncogenic Ras, palytoxin can increase ERK1/2 activity by stimulating the loss of MKP-3, a dual specificity protein phosphatase that specifically dephosphorylates and inactivates ERK1/2. (C) In Balb/c 3T3 cells, palytoxin stimulates ERK1/2 activation through an autocrine mechanism that involves the production of prostaglandins.
The complete biochemical pathways that directly link the interaction of palytoxin with the Na+,K+-ATPase to the modulation of MAP kinase cascades remain to be defined. One pathway may involve palytoxin-induced changes in ion flux, which can trigger a series of downstream effects that ultimately result in the stimulation of MAP kinase activity. Alternatively, palytoxin may modulate MAP kinase activity independent of its effects on ion flux by affecting how the Na+,K+-ATPase interacts with major signaling proteins located in the plasma membrane (Xie, 2003; Xie and Cai, 2003). For example, ouabain, a ligand for the Na+,K+-ATPase, alters the interaction of the Na+,K+-ATPase with the tyrosine kinase Src and the EGF receptor, which, in turn, stimulates the activation of ERK1/2, p38, phospholipase C, and protein kinase C, independent of changes in ion flux.
Palytoxin does not completely mimic ouabain action, however. For example, although ouabain and palytoxin both stimulated the expression of c-myc in immortalized human bronchial epithelial cells (BEAS-2B), the time courses differed significantly; ouabain-induced c-myc expression peaked within 4 hours, whereas palytoxin-induced c-myc expression peaked after 20 hours. This suggests that ouabain and palytoxin modulate c-myc gene expression through different mechanisms. Such a difference is further reflected by the observation that palytoxin stimulated an increase in DNA synthesis in BEAS-2B cells, whereas ouabain caused a decrease in DNA synthesis (Bonnard et al., 1988). In HeLa cells, both ouabain and palytoxin stimulate JNK activity through a signaling pathway that requires MEK4, but does not require Ras (Li and Wattenberg, 1998). It remains to be determined whether ouabain and palytoxin stimulate common pathways upstream of MEK4. Interestingly, in HeLa cells these ligands for the Na+,K+-ATPase predominantly stimulate different MAP kinase family members; palytoxin predominantly stimulates JNK and p38 activation, whereas ouabain stimulates ERK1/2 activity. These data support for the idea that palytoxin and ouabain do not stimulate identical signaling pathways. Further research is needed to determine whether palytoxin action is mediated through the modulation of the signaling function of the Na+,K+-ATPase.
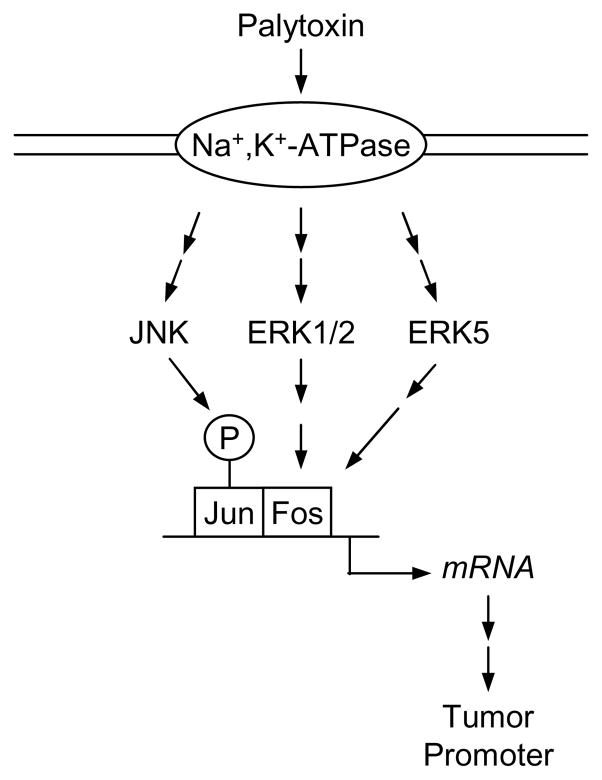
In conclusion, MAP kinases appear to be important mediators of palytoxin-stimulated signals. The central role of MAP kinases in regulating a variety of critical cellular functions, ranging from enzyme activity to gene expression and ultimately to proliferation and apoptosis, may help explain how palytoxin can stimulate the wide range of effects that are characteristic of tumor promoters. For example, the transcription factor AP-1 is a target of MAP kinase signaling that is widely recognized to play an important role in tumor promotion (Young et al., 1999). Palytoxin can modulate AP-1, a dimer made up of Jun and Fos family members, through different mechanisms that involve MAP kinases (Fig. 4). First, palytoxin stimulates ERK1/2 and ERK5 activation, which results in an increase in c-Fos gene expression, which in turn can alter the composition and function of AP-1 dimers (Warmka et al., 2002; Charlson et al., 2009). Second, palytoxin stimulates JNK activation; phosphorylation of c-Jun by JNK modulates AP-1 transcriptional activity (Kuroki et al., 1996; Karin et al., 1997). AP-1 may act as a type of master switch that regulates a constellation of downstream targets that are important for tumor promotion. The major challenge for future research is to fully establish the biochemical pathways by which MAP kinase cascades translate palytoxin-stimulated signaling into biological outcomes that contribute to carcinogenesis.
Fig. 4.
MAP kinases transmit palytoxin-stimulated signals to the nucleus. The transcription factor AP-1, a dimer made up of Jun and Fos family members, is an important target in tumor promotion. Palytoxin can modulate AP-1 through different mechanisms that involve MAP kinases. Palytoxin can stimulate JNK activation; JNK can directly phosphorylate c-Jun and modulate AP-1 transcriptional activity. Palytoxin can stimulate ERK1/2 and ERK5 activation, which results in an increase in c-Fos gene expression, which in turn can alter the composition and function of AP-1 dimers.
Acknowledgments
This work was supported by National Institutes of Health grant RO1-CA104609. The National Institutes of Health was not involved in study design, collection, analysis, or interpretation of data, writing the manuscript, or the decision to submit the manuscript for publication.
Footnotes
Conflicts of interest
There are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe J, Kusuhara M, Ulevitch RJ, Berk BC, Lee JD. Big mitogen-activated protein kinase 1 (BMK1) is a redox-sensitive kinase. J Biol Chem. 1996;271:16586–16590. doi: 10.1074/jbc.271.28.16586. [DOI] [PubMed] [Google Scholar]
- Albanell J, Codony-Servat J, Rojo F, Del Campo JM, Sauleda S, Anido J, Raspall G, Giralt J, Rosello J, Nicholson RI, Mendelsohn J, Baselga J. Activated extracellular signal-regulated kinases: association with epidermal growth factor receptor/transforming growth factor alpha expression in head and neck squamous carcinoma and inhibition by anti-epidermal growth factor receptor treatments. Cancer Res. 2001;61:6500–6510. [PubMed] [Google Scholar]
- Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991;1072:129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- Balmain A, Pragnell IB. Mouse skin carcinomas induced in vivo by chemical carcinogens have a transforming Harvey-ras oncogene. Nature. 1983;303:72–74. doi: 10.1038/303072a0. [DOI] [PubMed] [Google Scholar]
- Bloethner S, Chen B, Hemminki K, Muller-Berghaus J, Ugurel S, Schadendorf D, Kumar R. Effect of common B-RAF and N-RAS mutations on global gene expression in melanoma cell lines. Carcinogenesis. 2005;26:1224–1232. doi: 10.1093/carcin/bgi066. [DOI] [PubMed] [Google Scholar]
- Bonnard C, Lechner JF, Gerwin BI, Fujiki H, Harris CC. Effects of palytoxin or ouabain on growth and squamous differentiation of human bronchial epithelial cells in vitro. Carcinogenesis. 1988;9:2245–2249. doi: 10.1093/carcin/9.12.2245. [DOI] [PubMed] [Google Scholar]
- Bourcier C, Jacquel A, Hess J, Peyrottes I, Angel P, Hofman P, Auberger P, Pouyssegur J, Pages G. p44 mitogen-activated protein kinase (extracellular signal-regulated kinase 1)-dependent signaling contributes to epithelial skin carcinogenesis. Cancer Res. 2006;66:2700–2707. doi: 10.1158/0008-5472.CAN-05-3129. [DOI] [PubMed] [Google Scholar]
- Camps M, Nichols A, Arkinstall S. Dual specificity phosphatases: a gene family for control of MAP kinase function. Faseb J. 2000;14:6–16. [PubMed] [Google Scholar]
- Chao TH, Hayashi M, Tapping RI, Kato Y, Lee JD. MEKK3 directly regulates MEK5 activity as part of the big mitogen-activated protein kinase 1 (BMK1) signaling pathway. J Biol Chem. 1999;274:36035–36038. doi: 10.1074/jbc.274.51.36035. [DOI] [PubMed] [Google Scholar]
- Charlson AT, Zeliadt NA, Wattenberg EV. Extracellular signal regulated kinase 5 mediates signals triggered by the novel tumor promoter palytoxin. Toxicol Appl Pharmacol. 2009;241:143–153. doi: 10.1016/j.taap.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Gibson TB, Robinson F, Silvestro L, Pearson G, Xu B, Wright A, Vanderbilt C, Cobb MH. MAP kinases. Chem Rev. 2001;101:2449–2476. doi: 10.1021/cr000241p. [DOI] [PubMed] [Google Scholar]
- Cohen C, Zavala-Pompa A, Sequeira JH, Shoji M, Sexton DG, Cotsonis G, Cerimele F, Govindarajan B, Macaron N, Arbiser JL. Mitogen-actived protein kinase activation is an early event in melanoma progression. Clin Cancer Res. 2002;8:3728–3733. [PubMed] [Google Scholar]
- Croonquist PA, Linden MA, Zhao F, Van Ness BG. Gene profiling of a myeloma cell line reveals similarities and unique signatures among IL-6 response, N-ras-activating mutations, and coculture with bone marrow stromal cells. Blood. 2003;102:2581–2592. doi: 10.1182/blood-2003-04-1227. [DOI] [PubMed] [Google Scholar]
- Cuevas BD, Abell AN, Johnson GL. Role of mitogen-activated protein kinase kinase kinases in signal integration. Oncogene. 2007;26:3159–3171. doi: 10.1038/sj.onc.1210409. [DOI] [PubMed] [Google Scholar]
- Davis RJ. MAPKs: new JNK expands the group. Trends Biochem Sci. 1994;19:470–473. doi: 10.1016/0968-0004(94)90132-5. [DOI] [PubMed] [Google Scholar]
- Derijard B, Raingeaud J, Barrett T, Wu IH, Han J, Ulevitch RJ, Davis RJ. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267:682–685. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- Dinev D, Jordan BW, Neufeld B, Lee JD, Lindemann D, Rapp UR, Ludwig S. Extracellular signal regulated kinase 5 (ERK5) is required for the differentiation of muscle cells. EMBO Rep. 2001;2:829–834. doi: 10.1093/embo-reports/kve177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelberg D. Stress-activated protein kinases-tumor suppressors or tumor initiators? Semin Cancer Biol. 2004;14:271–282. doi: 10.1016/j.semcancer.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Farooq A, Zhou MM. Structure and regulation of MAPK phosphatases. Cell Signal. 2004;16:769–779. doi: 10.1016/j.cellsig.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Friedman B, Frackelton AR, Jr, Ross AH, Connors JM, Fujiki H, Sugimura T, Rosner MR. Tumor promoters block tyrosine-specific phosphorylation of the epidermal growth factor receptor. Proc Natl Acad Sci U S A. 1984;81:3034–3038. doi: 10.1073/pnas.81.10.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki H, Suganuma M, Nakayasu M, Hakii H, Horiuchi T, Takayama S, Sugimura T. Palytoxin is a non-12-O-tetradecanoylphorbol-13-acetate type tumor promoter in two-stage mouse skin carcinogenesis. Carcinogenesis. 1986;7:707–710. doi: 10.1093/carcin/7.5.707. [DOI] [PubMed] [Google Scholar]
- Fujiki H, Suganuma M, Tahira T, Yoshioka A, Nakayasu M, Endo Y, Shudo K, Takayama S, Moore RE, Sugimura T. Nakahara memorial lecture. New classes of tumor promoters: teleocidin, aplysiatoxin, and palytoxin. Princess Takamatsu Symp. 1983;14:37–45. [PubMed] [Google Scholar]
- Furukawa T, Sunamura M, Motoi F, Matsuno S, Horii A. Potential tumor suppressive pathway involving DUSP6/MKP-3 in pancreatic cancer. Am J Pathol. 2003;162:1807–1815. doi: 10.1016/S0002-9440(10)64315-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gille H, Strahl T, Shaw PE. Activation of ternary complex factor Elk-1 by stress-activated protein kinases. Curr Biol. 1995;5:1191–1200. doi: 10.1016/s0960-9822(95)00235-1. [DOI] [PubMed] [Google Scholar]
- Griner EM, Kazanietz MG. Protein kinase C and other diacylglycerol effectors in cancer. Nat Rev Cancer. 2007;7:281–294. doi: 10.1038/nrc2110. [DOI] [PubMed] [Google Scholar]
- Habermann E. Palytoxin acts through Na+,K+-ATPase. Toxicon. 1989;27:1171–1187. doi: 10.1016/0041-0101(89)90026-3. [DOI] [PubMed] [Google Scholar]
- Han J, Lee JD, Jiang Y, Li Z, Feng L, Ulevitch RJ. Characterization of the structure and function of a novel MAP kinase kinase (MKK6) J Biol Chem. 1996;271:2886–2891. doi: 10.1074/jbc.271.6.2886. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Lee JD. Role of the BMK1/ERK5 signaling pathway: lessons from knockout mice. J Mol Med. 2004;82:800–808. doi: 10.1007/s00109-004-0602-8. [DOI] [PubMed] [Google Scholar]
- Hennings H, Glick AB, Greenhalgh DA, Morgan DL, Strickland JE, Tennenbaum T, Yuspa SH. Critical aspects of initiation, promotion, and progression in multistage epidermal carcinogenesis. Proc Soc Exp Biol Med. 1993;202:1–8. doi: 10.3181/00379727-202-43511a. [DOI] [PubMed] [Google Scholar]
- Hoshino R, Chatani Y, Yamori T, Tsuruo T, Oka H, Yoshida O, Shimada Y, Ari-i S, Wada H, Fujimoto J, Kohno M. Constitutive activation of the 41-/43-kDa mitogen-activated protein kinase signaling pathway in human tumors. Oncogene. 1999;18:813–822. doi: 10.1038/sj.onc.1202367. [DOI] [PubMed] [Google Scholar]
- Iordanov MS, Magun BE. Loss of cellular K+ mimics ribotoxic stress. Inhibition of protein synthesis and activation of the stress kinases SEK1/MKK4, stress-activated protein kinase/c-Jun NH2-terminal kinase 1, and p38/HOG1 by palytoxin. J Biol Chem. 1998;273:3528–3534. doi: 10.1074/jbc.273.6.3528. [DOI] [PubMed] [Google Scholar]
- Kamakura S, Moriguchi T, Nishida E. Activation of the protein kinase ERK5/BMK1 by receptor tyrosine kinases. Identification and characterization of a signaling pathway to the nucleus. J Biol Chem. 1999;274:26563–26571. doi: 10.1074/jbc.274.37.26563. [DOI] [PubMed] [Google Scholar]
- Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- Kato Y, Kravchenko VV, Tapping R, Han J, Ulevitch RJ, Lee JD. BMK1/ERK5 regulates serum-induced early gene expression through transcription factor MEF2C. EMBO J. 1997;16:7054–7066. doi: 10.1093/emboj/16.23.7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y, Tapping RI, Huang S, Watson MH, Ulevitch RJ, Lee JD. Bmk1/Erk5 is required for cell proliferation induced by epidermal growth factor. Nature. 1998;395:713–716. doi: 10.1038/27234. [DOI] [PubMed] [Google Scholar]
- Keyse SM. Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Curr Opin Cell Biol. 2000;12:186–192. doi: 10.1016/s0955-0674(99)00075-7. [DOI] [PubMed] [Google Scholar]
- Keyse SM. Dual-specificity MAP kinase phosphatases (MKPs) and cancer. Cancer Metastasis Rev. 2008;27:253–261. doi: 10.1007/s10555-008-9123-1. [DOI] [PubMed] [Google Scholar]
- Kim SC, Hahn JS, Min YH, Yoo NC, Ko YW, Lee WJ. Constitutive activation of extracellular signal-regulated kinase in human acute leukemias: combined role of activation of MEK, hyperexpression of extracellular signal-regulated kinase, and downregulation of a phosphatase, PAC1. Blood. 1999;93:3893–3899. [PubMed] [Google Scholar]
- Kuroki DW, Bignami GS, Wattenberg EV. Activation of stress-activator protein kinase/c-Jun N-terminal kinase by the non-TPA-type tumor promoter palytoxin. Cancer Res. 1996;56:637–644. [PubMed] [Google Scholar]
- Kuroki DW, Minden A, Sanchez I, Wattenberg EV. Regulation of a c-Jun amino-terminal kinase/stress-activated protein kinase cascade by a sodium-dependent signal transduction pathway. J Biol Chem. 1997;272:23905–23911. doi: 10.1074/jbc.272.38.23905. [DOI] [PubMed] [Google Scholar]
- Lee JD, Ulevitch RJ, Han J. Primary structure of BMK1: a new mammalian map kinase. Biochem Biophys Res Commun. 1995;213:715–724. doi: 10.1006/bbrc.1995.2189. [DOI] [PubMed] [Google Scholar]
- Li S, Wattenberg EV. Differential activation of mitogen-activated protein kinases by palytoxin and ouabain, two ligands for the Na+,K+-ATPase. Toxicol Appl Pharmacol. 1998;151:377–384. doi: 10.1006/taap.1998.8471. [DOI] [PubMed] [Google Scholar]
- Li S, Wattenberg EV. Cell-type-specific activation of p38 protein kinase cascades by the novel tumor promoter palytoxin. Toxicol Appl Pharmacol. 1999;160:109–119. doi: 10.1006/taap.1999.8754. [DOI] [PubMed] [Google Scholar]
- Lin A, Minden A, Martinetto H, Claret FX, Lange-Carter C, Mercurio F, Johnson GL, Karin M. Identification of a dual specificity kinase that activates the Jun kinases and p38-Mpk2. Science. 1995;268:286–290. doi: 10.1126/science.7716521. [DOI] [PubMed] [Google Scholar]
- Marchetti S, Gimond C, Chambard JC, Touboul T, Roux D, Pouyssegur J, Pages G. Extracellular signal-regulated kinases phosphorylate mitogen-activated protein kinase phosphatase 3/DUSP6 at serines 159 and 197, two sites critical for its proteasomal degradation. Mol Cell Biol. 2005;25:854–864. doi: 10.1128/MCB.25.2.854-864.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti S, Gimond C, Roux D, Gothie E, Pouyssegur J, Pages G. Inducible expression of a MAP kinase phosphatase-3-GFP chimera specifically blunts fibroblast growth and ras-dependent tumor formation in nude mice. J Cell Physiol. 2004;199:441–450. doi: 10.1002/jcp.10465. [DOI] [PubMed] [Google Scholar]
- Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- McCawley LJ, Li S, Wattenberg EV, Hudson LG. Sustained activation of the mitogen-activated protein kinase pathway. A mechanism underlying receptor tyrosine kinase specificity for matrix metalloproteinase-9 induction and cell migration. J Biol Chem. 1999;274:4347–4353. doi: 10.1074/jbc.274.7.4347. [DOI] [PubMed] [Google Scholar]
- Miura D, Kobayashi M, Kakiuchi S, Kasahara Y, Kondo S. Enhancement of Transformed Foci and Induction of Prostaglandins in Balb/c 3T3 Cells by Palytoxin: In Vitro Model Reproduces Carcinogenic Responses in Animal Models Regarding the Inhibitory Effect of Indomethacin and Reversal of Indomethacin’s Effect by Exogenous Prostaglandins. Toxicol Sci. 2006;89:154–163. doi: 10.1093/toxsci/kfi342. [DOI] [PubMed] [Google Scholar]
- Mody N, Campbell DG, Morrice N, Peggie M, Cohen P. An analysis of the phosphorylation and activation of extracellular-signal-regulated protein kinase 5 (ERK5) by mitogen-activated protein kinase kinase 5 (MKK5) in vitro. Biochem J. 2003;372:567–575. doi: 10.1042/BJ20030193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T, Toyoshima F, Gotoh Y, Iwamatsu A, Irie K, Mori E, Kuroyanagi N, Hagiwara M, Matsumoto K, Nishida E. Purification and identification of a major activator for p38 from osmotically shocked cells. Activation of mitogen-activated protein kinase kinase 6 by osmotic shock, tumor necrosis factor-alpha, and H2O2. J Biol Chem. 1996;271:26981–26988. doi: 10.1074/jbc.271.43.26981. [DOI] [PubMed] [Google Scholar]
- Muda M, Boschert U, Dickinson R, Martinou JC, Martinou I, Camps M, Schlegel W, Arkinstall S. MKP-3, a novel cytosolic protein-tyrosine phosphatase that exemplifies a new class of mitogen-activated protein kinase phosphatase. J Biol Chem. 1996;271:4319–4326. doi: 10.1074/jbc.271.8.4319. [DOI] [PubMed] [Google Scholar]
- Murphy LO, Smith S, Chen R, Fingar DC, Blenis J. Molecular Interpretation of ERK Signal Duration by Immediate Early Gene Products. Nature Cell Biology. 2002;4:556–564. doi: 10.1038/ncb822. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984;308:693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Raingeaud J, Whitmarsh AJ, Barrett T, Derijard B, Davis RJ. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol. 1996;16:1247–1255. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene. 2007;26:3100–3112. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala GL, Bellocci M, Rossini GP. The cytotoxic pathway triggered by palytoxin involves a change in the cellular pool of stress response proteins. Chem Res Toxicol. 2009;22:2009–2016. doi: 10.1021/tx900297g. [DOI] [PubMed] [Google Scholar]
- Sanchez I, Hughes RT, Mayer BJ, Yee K, Woodgett JR, Avruch J, Kyriakis JM, Zon LI. Role of SAPK/ERK kinase-1 in the stress-activated pathway regulating transcription factor c-Jun. Nature. 1994;372:794–798. doi: 10.1038/372794a0. [DOI] [PubMed] [Google Scholar]
- Santen RJ, Song RX, McPherson R, Kumar R, Adam L, Jeng MH, Yue W. The role of mitogen-activated protein (MAP) kinase in breast cancer. J Steroid Biochem Mol Biol. 2002;80:239–256. doi: 10.1016/s0960-0760(01)00189-3. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Kojima H, Kishimoto R, Ikeda A, Kunimoto H, Nakajima K. Spatiotemporal regulation of c-Fos by ERK5 and the E3 ubiquitin ligase UBR1, and its biological role. Mol Cell. 2006;24:63–75. doi: 10.1016/j.molcel.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Segrelles C, Ruiz S, Perez P, Murga C, Santos M, Budunova IV, Martinez J, Larcher F, Slaga TJ, Gutkind JS, Jorcano JL, Paramio JM. Functional roles of Akt signaling in mouse skin tumorigenesis. Oncogene. 2002;21:53–64. doi: 10.1038/sj.onc.1205032. [DOI] [PubMed] [Google Scholar]
- Smalley KS. A pivotal role for ERK in the oncogenic behaviour of malignant melanoma? Int J Cancer. 2003;104:527–532. doi: 10.1002/ijc.10978. [DOI] [PubMed] [Google Scholar]
- Sohn SJ, Lewis GM, Winoto A. Non-redundant function of the MEK5-ERK5 pathway in thymocyte apoptosis. EMBO J. 2008;27:1896–1906. doi: 10.1038/emboj.2008.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein B, Brady H, Yang MX, Young DB, Barbosa MS. Cloning and characterization of MEK6, a novel member of the mitogen-activated protein kinase kinase cascade. J Biol Chem. 1996;271:11427–11433. doi: 10.1074/jbc.271.19.11427. [DOI] [PubMed] [Google Scholar]
- Stokoe D, Engel K, Campbell DG, Cohen P, Gaestel M. Identification of MAPKAP kinase 2 as a major enzyme responsible for the phosphorylation of the small mammalian heat shock proteins. FEBS Lett. 1992;313:307–313. doi: 10.1016/0014-5793(92)81216-9. [DOI] [PubMed] [Google Scholar]
- Strickland JE, Greenhalgh DA, Koceva-Chyla A, Hennings H, Restrepo C, Balaschak M, Yuspa SH. Development of murine epidermal cell lines which contain an activated rasHa oncogene and form papillomas in skin grafts on athymic nude mouse hosts. Cancer Res. 1988;48:165–169. [PubMed] [Google Scholar]
- Sun W, Kesavan K, Schaefer BC, Garrington TP, Ware M, Johnson NL, Gelfand EW, Johnson GL. MEKK2 associates with the adapter protein Lad/RIBP and regulates the MEK5-BMK1/ERK5 pathway. J Biol Chem. 2001;276:5093–5100. doi: 10.1074/jbc.M003719200. [DOI] [PubMed] [Google Scholar]
- Terasawa K, Okazaki K, Nishida E. Regulation of c-Fos and Fra-1 by the MEK5-ERK5 pathway. Genes Cells. 2003;8:263–273. doi: 10.1046/j.1365-2443.2003.00631.x. [DOI] [PubMed] [Google Scholar]
- Turjanski AG, Vaque JP, Gutkind JS. MAP kinases and the control of nuclear events. Oncogene. 2007;26:3240–3253. doi: 10.1038/sj.onc.1210415. [DOI] [PubMed] [Google Scholar]
- Vale C, Gomez-Limia B, Vieytes MR, Botana LM. Mitogen-activated protein kinases regulate palytoxin-induced calcium influx and cytotoxicity in cultured neurons. Br J Pharmacol. 2007;152:256–266. doi: 10.1038/sj.bjp.0707389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Tournier C. Regulation of cellular functions by the ERK5 signalling pathway. Cell Signal. 2006;18:753–760. doi: 10.1016/j.cellsig.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Warmka JK, Mauro LJ, Wattenberg EV. Mitogen-activated protein kinase phosphatase-3 is a tumor promoter target in initiated cells that express oncogenic Ras. J Biol Chem. 2004;279:33085–33092. doi: 10.1074/jbc.M403120200. [DOI] [PubMed] [Google Scholar]
- Warmka JK, Winston SE, Zeliadt NA, Wattenberg EV. Extracellular signal-regulated kinase transmits palytoxin-stimulated signals leading to altered gene expression in mouse keratinocytes. Toxicol Appl Pharmacol. 2002;185:8–17. doi: 10.1006/taap.2002.9519. [DOI] [PubMed] [Google Scholar]
- Wattenberg EV. Palytoxin: exploiting a novel skin tumor promoter to explore signal transduction and carcinogenesis. Am J Physiol Cell Physiol. 2007;292:C24–32. doi: 10.1152/ajpcell.00254.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattenberg EV, Byron KL, Villereal ML, Fujiki H, Rosner MR. Sodium as a mediator of non-phorbol tumor promoter action. Down-modulation of the epidermal growth factor receptor by palytoxin. J Biol Chem. 1989a;264:14668–14673. [PubMed] [Google Scholar]
- Wattenberg EV, Fujiki H, Rosner MR. Heterologous regulation of the epidermal growth factor receptor by palytoxin, a non-12-O-tetradecanoylphorbol-13-acetate-type tumor promoter. Cancer Res. 1987;47:4618–4622. [PubMed] [Google Scholar]
- Wattenberg EV, McNeil PL, Fujiki H, Rosner MR. Palytoxin down-modulates the epidermal growth factor receptor through a sodium-dependent pathway. J Biol Chem. 1989b;264:213–219. [PubMed] [Google Scholar]
- Wattenberg EV, Uemura D, Byron KL, Villereal ML, Fujiki H, Rosner MR. Structure-activity studies of the nonphorbol tumor promoter palytoxin in Swiss 3T3 cells. Cancer Res. 1989c;49:5837–5842. [PubMed] [Google Scholar]
- Whitmarsh AJ, Davis RJ. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med. 1996;74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- Whitmarsh AJ, Shore P, Sharrocks AD, Davis RJ. Integration of MAP kinase signal transduction pathways at the serum response element. Science. 1995;269:403–407. doi: 10.1126/science.7618106. [DOI] [PubMed] [Google Scholar]
- Xie Z. Molecular mechanisms of Na/K-ATPase-mediated signal transduction. Ann N Y Acad Sci. 2003;986:497–503. doi: 10.1111/j.1749-6632.2003.tb07234.x. [DOI] [PubMed] [Google Scholar]
- Xie Z, Cai T. Na+-K+--ATPase-mediated signal transduction: from protein interaction to cellular function. Mol Interv. 2003;3:157–168. doi: 10.1124/mi.3.3.157. [DOI] [PubMed] [Google Scholar]
- Yip-Schneider MT, Lin A, Marshall MS. Pancreatic tumor cells with mutant K-ras suppress ERK activity by MEK-dependent induction of MAP kinase phosphatase-2. Biochem Biophys Res Commun. 2001;280:992–997. doi: 10.1006/bbrc.2001.4243. [DOI] [PubMed] [Google Scholar]
- Young MR, Li JJ, Rincon M, Flavell RA, Sathyanarayana BK, Hunziker R, Colburn N. Transgenic mice demonstrate AP-1 (activator protein-1) transactivation is required for tumor promotion. Proc Natl Acad Sci U S A. 1999;96:9827–9832. doi: 10.1073/pnas.96.17.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuspa SH. The pathogenesis of squamous cell cancer: lessons learned from studies of skin carcinogenesis. J Dermatol Sci. 1998;17:1–7. doi: 10.1016/s0923-1811(97)00071-6. [DOI] [PubMed] [Google Scholar]
- Zeliadt NA, Mauro LJ, Wattenberg EV. Reciprocal regulation of extracellular signal regulated kinase 1/2 and mitogen activated protein kinase phosphatase-3. Toxicol Appl Pharmacol. 2008;232:408–417. doi: 10.1016/j.taap.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeliadt NA, Warmka JK, Wattenberg EV. Mitogen activated protein kinases selectively regulate palytoxin-stimulated gene expression in mouse keratinocytes. Toxicol Appl Pharmacol. 2003;192:212–221. doi: 10.1016/s0041-008x(03)00298-9. [DOI] [PubMed] [Google Scholar]
- Zeliadt NA, Warmka JK, Winston SE, Kahler R, Westendorf JJ, Mauro LJ, Wattenberg EV. Tumor promoter-induced MMP-13 gene expression in a model of initiated epidermis. Biochem Biophys Res Commun. 2004;317:570–577. doi: 10.1016/j.bbrc.2004.03.081. [DOI] [PubMed] [Google Scholar]
- Zhou G, Bao Z, Dixon JE. Components of a new human protein kinase signal transduction pathway. J Biol Chem. 1995;270:12665–12669. doi: 10.1074/jbc.270.21.12665. [DOI] [PubMed] [Google Scholar]