Abstract
Objective:
Role of reactive oxygen species (ROS) modified human Immunoglobulin G (IgG) in systemic lupus erythematosus (SLE) has been investigated.
Methods:
Human IgG was modified by hydroxyl-radicals. Immunogenicity of native and modified human IgG was probed by inducing polyclonal antibodies in rabbits. Cross-reactions of induced antibodies with nucleic acid, chromatin, different blood proteins and their ROS modified conformers were determined by competitive inhibition ELISA. The binding characteristics of circulating autoantibodies in SLE patients (n = 72) against native and modified IgG were screened by direct binding and competition ELISA and the results were compared with healthy age-matched controls (n = 39).
Results:
Induced antibodies against ROS-modified human IgG exhibited diverse antigen binding characteristics. Native DNA, native chromatin and their ROS-modified conformers were found to be effective inhibitors of induced antibody-immunogen interaction. Induced antibodies against native human IgG showed negligible binding to the above mentioned nucleic acid antigens. SLE sera (48.6%) showed strong binding to ROS-human IgG in comparison with its native analogue (P < 0.01). Normal human sera (NHS) showed negligible binding with either antigen (P > 0.05).
Conclusion:
ROS-induced modifications in human IgG present neo-epitopes, and make it a potential immunogen. The induced antibodies against ROS-modified human IgG resembled the diverse antigen-binding characteristics of naturally occurring SLE anti-DNA autoantibodies. ROS-modified IgG may be one of the factors for the induction of circulating SLE autoantibodies.
Keywords: human IgG, ROS-IgG, reactive oxygen species, cross-reactive-antibodies, SLE
Introduction
Systemic lupus erythematosus (SLE) is a multifactorial autoimmune disease characterized by several clinical manifestations and the appearance of multiple autoantibodies.1–4 Analysis of sera obtained from the same SLE patient over a long period of time has demonstrated that different autoantibody specificities appear at different time intervals with a general trend towards increasing the number of antigens recognized by the sera.3 These observations of increasing complexity of this autoimmune response over a period of time are very similar to those reported by Lehmann et al.4 in their mode of epitope spreading in experimental autoimmune encephalomyelitis. Thus, it was generally accepted that intermolecular epitope spreading was one of the mechanisms for the amplification and diversification of autoantibody responses in SLE.5,6 In short, SLE is a multisystem autoimmune disorder of unknown etiology6 or the agent (or agents) triggering this autoimmune response remains to be identified, but it is thought that a combination of genetic and environmental factors are required.6–8
Reactive oxygen species (ROS) has the potential to initiate damage to proteins, DNA and other cell biomolecules under pathological conditions.9 Protein oxidation, which results in functional disruption, is not random but appears to be associated with increased oxidation in specific proteins.10–14 We previously reported that many serum proteins were found to be oxidatively modified leading to the formation of neoantigens which could in turn initiate autoimmunity in various diseases15–20 including SLE.21 Oxidative stress and formation of oxidatively-modified protein are associated with SLE,8,21,22 however, the potential role of oxidative stress, especially the consequences of oxidative modification of proteins, in the pathogenesis and progression of SLE remains unresolved.
Immunoglobulin G (IgG) is the most abundant immunoglobulin and is approximately equally distributed in blood and in tissue liquids, constituting 75% of serum immunoglobulins in human. Function of IgG lies in the specific interactions with and clearance of antigen. It is well known that IgG is quite vulnerable to ROS.23–29 Many studies showed the presence of elevated levels of oxidized IgG in patients with rheumatic diseases.26–28 In patients with SLE, IgG dysfunction has been reported.26 Now, it is well documented that IgG behaves not only as an antibody, but also as a putative antigen for rheumatoid factor.20,26 Therefore, IgG is continuously exposed to oxidative stress, as the alterations in its conformation and function could occur, resulting in modification of its biological properties.
In the present study, we demonstrated that after modification with ROS, human IgG became highly immunogenic in experimental animals and the induced antibodies against ROS-modified IgG showed cross-reactions with native and ROS-modified nucleic acid conformers. Therefore, we hypothesized that oxidative by-products, such as hydroxyl radicals-damaged human IgG, help to initiate autoimmunity in SLE. To test this hypothesis, we investigated the binding characteristics of naturally occurring SLE autoantibodies to native and hydroxyl radical-modified IgG.
Methods
Purification of human immunoglobulin G, human DNA and chromatin
IgG from normal human sera was purified using Protein A-Sepharose CL-4B affinity column (Sigma-Aldrich St. Louis, MO, USA) as previously described.20 Briefly, serum diluted with equal volume of PBS, pH 7.4 and applied to the column equilibrated with the same buffer. The flow through was reloaded onto the column 2–3 times. Unbound proteins were removed by extensive washing with PBS, pH 7.4. The bound IgG was eluted with 0.58% acetic acid in 0.85% sodium chloride and neutralized with 1.0 ml of 1.0 mol/l Tris HCl, pH 8.5. The purified IgG was dialyzed against PBS, pH 7.4 and stored at −20 °C. Concentration of IgG was determined using 1.38 OD278 = 1.0 mg IgG/ml. The homogeneity of isolated IgG was checked by polyacrylamide gel electrophoresis.
Human DNA was purified from the blood of healthy human subjects, which was free of protein, RNA and single-stranded regions as previously described.30 Chromatin was purified from goat liver as described previously.31
ROS-modification of human IgG, HSA, human hemoglobin, human transferring, human DNA and chromatin
Human IgG, HSA (Sigma-Aldrich Co.), human hemoglobin (Sigma-Aldrich Co.), human transferring (Sigma-Aldrich Co.), human DNA and chromatin was modified in PBS (10 mM sodium phosphate buffer containing 150 mM NaCl, pH 7.4) by our published procedure.18 Proteins or nucleic acid conformers were modified by hydroxyl radicals, generated with UV irradiation (30 min) of hydrogen peroxide (15.1 mM) at 254 nm. Unbound hydrogen peroxide was removed by extensive dialysis against PBS, pH 7.4.
Preparation of advanced gylcation end products (AGEs)
AGEs was prepared by independent reactions of different plasma proteins (HSA, human IgG, human transferrin, or hemoglobin) with glycoaldehyde (Sigma-Aldrich, Co.) according to our published procedure.32–34 The reaction was terminated by removing non-reacted glycoaldehyde using dialyzing extensively against PBS, pH 7.2.
Immunization schedule
The immunization of random bred, female New Zealand white rabbits was performed as described previously18,20 Briefly, rabbits (n = 4; two each for native and ROS-human IgG antigens) were immunized intramuscularly at multiple sites with 25 μg of antigen, emulsified with an equal volume of Freund’s complete adjuvant (Sigma-Aldrich, Co.). The animals were boosted in Freund’s incomplete adjuvant (Sigma-Aldrich, Co.) at weekly intervals for 6 weeks with the same amount of antigen. Test bleeds were performed 7 days post boost, which gave appropriate titer of the antibody. The animals were bled and the serum separated from the blood (pre-immune and immune) was heated at 56 °C for 30 min to inactivate complement proteins and stored at −80 °C.
Human subjects
The study group included 72 patients (63 females and 9 males; aged (±SD) 43.6 ± 13.3 years) with SLE, as defined by the American College of Rheumatology 1997 revised criteria34 and the age range was 29–69 years (43.6 ± 13.3 years). The control group comprised 39 healthy volunteers (36 females and 3 males; aged (±SD) 45.7 ± 13.8 years). The study was approved by Qassim University Medical Review Board. Venous blood samples from the control subjects and from SLE patients were collected and serum was separated. Full informed consent was obtained prior to the blood extraction from SLE patients and healthy subjects. All serum samples were decomplemented at 56 °C for 30 min and stored in small aliquots at −80 °C.
Assay of carbonyl formation
Protein carbonyl contents in SLE patients sera and in healthy human sera were analyzed as described previously35 with slight modifications. The reaction mixture containing 0.5 ml of 10 mM 2,4-dinitrophenylhydrazine (DNPH)/2.5 MHCl and 1:100 diluted sera from study subjects and was thoroughly mixed. After addition of 250 mM TCA (20%) and centrifugation, the pellet was collected and washed three times with 1 ml ethanol:ethylacetate (1:1) mixture. The pellet was then dissolved in 1 ml of 6 M guanidine solution and incubated at 30 °C for 15 min. After centrifugation, the supernatant was collected and carbonyl contents were estimated using molar absorption coefficient of 22,000 M−1 cm1. Samples were spectrophotometrically analyzed against a blank of 1 ml of guanidine solution (6M). Protein concentration was determined in the samples by the method of Lowry et al.36
Enzyme linked immunosorbent assay (ELISA)
Antibodies against native and ROS-modified human IgG were detected and quantified using ELISA assays performed on flat bottom 96 wells, polystyrene immunoplates as previously described.15,16 Polystyrene polysorp/maxisorp immunoplates (Thermo Fisher Scientific, Fremont, CA, USA) were coated with 100 ml of native or modified IgG (5 μg/ml) or DNA (2.5 μg/ml) in carbonate–bicarbonate buffer (0.05 M, pH 9.6). The plates were coated for 2 h at 37 °C and overnight at 4 °C. Each sample was coated in duplicate and half of the plates served as control devoid of only antigen coating. Unbound antigen was washed thrice with TBS-T (20 mM Tris, 150 mM, NaCl, pH 7.4 containing 0.05% Tween-20) and unoccupied sites were blocked with 2% fat free milk in TBS (10 mM Tris, 150 mM NaCl, pH 7.4) for 4–6 h at 37 °C. After incubation the plates were washed four times with TBS-T. The test serum serially diluted in TBS-T in TBS (100 ml/well) was adsorbed for 2 h at 37 °C and overnight at 4 °C. Bound antibodies were assayed with human alkaline phosphatase conjugate using p-nitrophenyl phosphate as substrate. The absorbance (A) of each well was monitored at 410 nm on an automatic microplate reader. Each sample was run in duplicate. The control wells were treated similarly but were devoid of antigen. Results were expressed as a mean of Atest—Acontrol.
Competition ELISA
The antigenic specificity of the antibodies was determined by competition ELISA.17,18 Varying amounts of inhibitors (0–20 mg/ml) were mixed with a constant amount of serum samples. The mixture was incubated at room temperature for 2 h and overnight at 4 °C. The immune complex thus formed was coated in the wells instead of the serum. The remaining steps were the same as in direct binding ELISA. Percent inhibition was calculated using the formula:
Statistical analysis
All measurements were performed in duplicates and repeated at least 3 times using age- and sex-matched SLE or control samples. Comparisons were performed using Origin 6.1 software package (Northampton, MA, USA) (one paired two tailed t-test with one way ANOVA analysis). P values less than 0.05 were considered significant, and P values less than 0.001 were considered highly significant. Values shown are mean ± SEM unless stated otherwise.
Results
Human IgG was purified from normal human sera by affinity chromatography using Protein-A Sepharose CL-4B affinity column. The purified IgG was found to elute as a single symmetrical peak and gave a single band on SDS-PAGE (data not shown). Our earlier report20 showed alterations in human IgG following exposure to the hydroxyl radicals, generated by the UV-irradiation of hydrogen peroxide. Loss of secondary structures, hypochromicity at 280 nm, loss of tryptophan fluorescence intensity, increase in protein carbonyl contents were observed in hydroxyl treated human IgG. We also showed previously that immunization of ROS-modified human IgG in rabbits induced high titre antibodies (>1:12,800), whereas with native human IgG the titre was low (∼1:6400).20 In the present study, we studied the antigenic specificity of the experimentally induced antibodies against native and ROS-modified human IgG by competitive inhibition assay. A maximum of 97% inhibition of the affinity purified anti-ROS-human IgG antibodies with the immunogen as inhibitor, was observed (Table 1). Competition experiments with native human IgG used as inhibitor showed 51.2% inhibition at 20 μg/ml. The affinity purified anti-ROS-human IgG antibodies exhibited a variable recognition of chromatin, DNA, ROS-modified-chromatin and ROS-modified DNA (Table 1). Native chromatin and native DNA showed maximum inhibition of 48.2% and 56.3%, respectively, and 18.2 μg/ml of native DNA was required for 50% inhibition, whereas the ROS-modified conformers of chromatin and DNA showed maximum inhibition of 63.2% and 65.4%, respectively at 20 μg/ml of inhibitor concentration. 50% inhibition of anti-ROS-IgG antibodies was achieved at 7.4 and 10.2 μg/ml of ROS-modified DNA and ROS-modified chromatin, respectively. Percentage of relative affinity of anti-ROS-IgG antibodies in respect with inhibitors was also estimated which further confirmed that anti-ROS-human IgG antibodies exhibited diverse antigen binding characteristic with native and ROS-modified nucleic acid conformers (Table 1). Glycated IgG showed negligible inhibitions of 16.5%, whereas native HSA and glycated HSA showed inhibition of 18.0% and 14.1%, respectively. Native human hemoglobin and glycated hemoglobin showed inhibition of 11.0% and 17.0%, respectively, whereas ROS-modified conformers of HSA and hemoglobin showed moderate inhibition of 28.0% and 27.0%, respectively. Transferrin, ROS-modified transferrin was non-inhibitory. The complete antigen binding specificity of affinity purified anti-ROS-human IgG antibodies has been summarized in Table 1. Competitive inhibition ELISA results of antigen binding characteristics of affinity purified antihuman IgG antibodies were shown in Table 2. Our data with anti-native human IgG antibodies showed a maximum inhibition of 89.0% with the immunogen as inhibitor. Only 9.7 μg/ml of native IgG was required to inhibit 50% its activity. The induced antibodies partially recognized ROS-modified human IgG as it showed a maximum inhibition of 56.1%. Inhibitor 17.2 μg/ml was required to inhibit 50% antibody binding activity to native IgG. Native chromatin and native DNA showed maximum inhibition of 32.2% and 22.2%, respectively, whereas ROS-chromatin and ROS-DNA showed maximum inhibition of 41.1% and 34.1%, respectively (Table 2).
Table 1.
Antigen binding specificity of anti-ROS-modified human IgG antibodies.
| Inhibitors | Maximun % inhibition at 20 μg/ml | Concentration for 50% inhibition (μg/ml) | % relative affinity |
|---|---|---|---|
| ROS-Igg | 96.8 ± 3.2 | 0.83 ± 1.3 | 100 |
| Native Igg | 51.2 ± 4.3 | 19.1 ± 2.6 | 4.3 |
| Chromatin | 48.2 ± 2.2 | – | – |
| ROS-chromatin | 63.2 ± 2.7 | 10.2 ± 3.1 | 8.1 |
| Human DNA | 56.3 ± 4.2 | 18.2 ± 2.1 | 4.5 |
| ROS-human DNA | 65.3 ± 3.3 | 7.2 ± 2.3 | 11.5 |
| AGE-IgG | 26.5 ± 2.4 | – | – |
| Native HSA | 18.0 ± 4.4 | – | – |
| AGE-HSA | 14.1 ± 5.3 | – | – |
| ROS-HSA | 28.0 ± 3.2 | – | – |
| Hemoglobin | 11.0 ± 3.2 | – | – |
| AGE-hemoglobin | 19.0 ± 4.2 | – | – |
| ROS-hemoglobin | 27.0 ± 2.1 | – | – |
| Transferrin | 09.6 ± 3.2 | – | – |
| ROS-transferrin | 10.2 ± 2.5 | – | – |
Notes: The results represent mean ± SD of four independent assays. The ELISA plates were coated with ROS-IgG (10 mg/ml).
Table 2.
Antigen binding specificity of anti-human IgG antibodies.
| Inhibitors | Maximun % inhibition at 20 μg/ml | Concentration for 50% inhibition (μg/ml) | % relative affinity |
|---|---|---|---|
| Native-IgG | 89.0 ± 2.1 | 9.71 ± 2.3 | 100 |
| ROS-IgG | 56.1 ± 3.5 | 17.2 ± 1.4 | 56.4 |
| Chromatin | 32.2 ± 4.1 | – | – |
| ROS-chromatin | 41.1 ± 1.1 | – | – |
| Human DNA | 22.2 ± 3.5 | – | – |
| ROS-human DNA | 34.1 ± 2.1 | – | – |
| AGE-IgG | 13.2 ± 1.5 | – | – |
| Native HSA | 10.7 ± 4.2 | – | – |
| AGE-HSA | 11.2 ± 2.1 | – | – |
| ROS-HSA | 19.6 ± 2.8 | – | – |
| Hemoglobin | 13.0 ± 1.1 | – | – |
| ROS-hemoglobin | 13.3 ± 2.5 | – | – |
| AGE-hemoglobin | 11.0 ± 1.4 | – | – |
| Transferrin | 10.2 ± 2.7 | – | – |
| ROS-transferrin | 12.5 ± 3.1 | – | – |
Notes: The results represent mean ± SD of four independent assays. The ELISA plates were coated with native-IgG (10 mg/ml).
To probe the possible role of the ROS in the pathogenesis of SLE, 72 SLE sera were selected for binding to native and ROS-modified human IgG. Majority of SLE sera (35/72) showed strong binding to ROS-modified IgG over native IgG (P < 0.01) (Fig. 1). Normal human sera (NHS) showed negligible binding with native or modified IgG (P > 0.05). Native DNA was used as an immunochemical marker for SLE, showed strong binding to SLE serum antibodies; whereas antibodies from NHS showed negligible binding to native DNA (Fig. 1). The average absorbance at 410 nm (±SD) of 35 SLE sera binding to native and ROS-damaged IgG was 0.51 ± 0.10 and 1.3 ± 0.11, respectively. Whereas, 39 NHS binding to native and ROS-human IgG antigens was 0.19 ± 0.12 and 0.22 ± 0.10, respectively. Table 3 summaries the complete immunological studies of study subjects.
Figure 1.
Direct binding ELISA of 1:100 diluted SLE and normal human serum samples. The microtitre plates were individually coated with native DNA (2.5 μg/ml), native and ROS-modified IgG (5 μg/ml). The number of SLE serum samples was 35 and NH serum samples were 39. Data presented as mean ± SEM of five independent assays; data without a common letter differ, P < 0.05.
Table 3.
Immunological details of study subjects.
| Parameters | SLE-serum | NH-serum | |
|---|---|---|---|
| Age | 47.4 ± 15.3 (n = 35) | 45.7 ± 13.8 (n = 39) | |
| Sex | 33F/2M | 36F/3M | |
| Detection of anti-ROS-human | A410 (ROS-IgG) | 1.3 ± 0.11* | 0.22 ± 0.10 |
| IgG antibodies | MPI (ROS-IgG) | 54.4 ± 8.2** | 13.3 ± 5.7 |
| Detection of anti-human | A410 (n-IgG) | 0.51 ± 0.10 | 0.19 ± 0.12 |
| IgG antibodies | MPI (n-IgG) | 27.8 ± 7.9 | 12.9 ± 8.2 |
| Carbonyl contents (nmol/mg protein) | 3.9 ± 0.42# (n = 12) | 2.4 ± 0.21 (n = 12) |
Note:
P < 0.01 vs. A410 (n-IgG);
P < 0.01 vs. MPI (n-IgG);
P < 0.05 vs. carbonyl content (NH-serum).
Abbreviations: SLE-serum, serum from systemic lupus erythematosus patients; NH-serum, serum from normal human; n, number of samples tested; F, females; M, males; n-IgG, native IgG; A410, absorbance at 410 nm calculated by direct binding ELISA; MPI, maximum percent inhibition at 20 μg/ml of inhibitor concentration calculated by competitive inhibition ELISA.
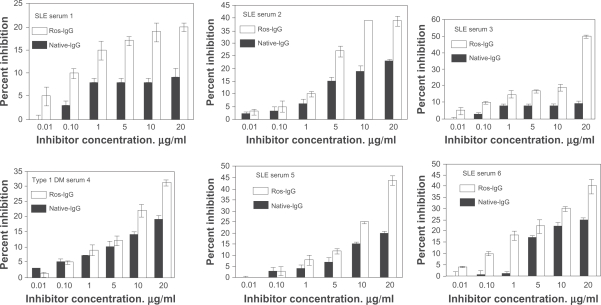
The binding specificity of antibodies from 35 selected SLE sera was evaluated by competition ELISA using native and ROS-modified IgG as inhibitors. Mircrotitre plates were coated with human DNA. Figure 2 illustrates inhibition of SLE autoantibodies (in sera 1 to 6) binding to native DNA by native or ROS-modified IgG. Microtiter plates were coated with native DNA. Our data showed strong reactivity of autoantibodies in patient’s sera towards ROS-IgG over native IgG (P < 0.01). Similarly, the rest of the sera showed high percent inhibitions with modified IgG over native IgG (data summarized in Table 3). The average percent inhibition (±SD) in the binding of 35 SLE sera to ROS-modified and unmodified IgG was 54.4 ± 8.2 and 27.8 ± 7.9, respectively. The data reveals striking differences in the recognition of native and oxidized IgG by SLE autoantibodies (P < 0.001) (Table 3).
Figure 2.
Competitive inhibition ELISA of SLE serum antibody from six patients (1 to 6). The inhibitors used were native human IgG and ROS-modified human IgG. The microtitre plates were coated with the native DNA (2.5 μg/ml). Varying amounts of inhibitors (0.01–20 μg/ml) were allowed to interact with a constant amount of antiserum for 2 h and overnight at 4 °C, the mixture was added to antigen-coated plates and the residual antibody level was detected by ELISA. Each histogram represents the mean ± SEM of three independent assays.
Elevated level of protein carbonyl contents in the serum is considered to be the most reliable biomarker of oxidative stress. The data showed significantly increase in serum protein carbonyl contents (P < 0.05) in SLE patients, compared with normal subjects of the same age group. The average carbonyl contents (±SD) of 12 independent assays of SLE serum proteins and normal human serum (NHS) proteins were 3.9 ± 0.42 and 2.4 ± 0.21 nmol/mg protein, respectively (Table 3). A P-value of <0.01 indicates significant difference in the carbonyl contents of SLE-serum and NH-serum.
Discussion
Reactive oxygen species (ROS) play a key role in both normal biological functions and in the pathogenesis of certain human diseases. These species are continuously generated in cells by cellular metabolism and by exogenous agents but increase in their steady states are thought to be responsible for a variety of pathological conditions, including SLE, cancer and aging.7,22,37 ROS in excessive amount have the ability, either directly or indirectly, to damage proteins, DNA and other cell biomolecules.7–22 Among the ROS, hydroxyl radicals are the most potent damaging ROS which can react with almost all biological macromolecules.15–21 Proteins are the major targets for free radical attack mainly by hydroxyl radicals.17 Protein oxidation, results in cellular dysfunctions, functional disruption and structural changes and contributes to the etiology of many human diseases.9,11–14 These oxidative modifications on protein may lead to the formation of neoantigens which could in turn initiate autoimmunity.
It is well established that IgG is an abundant protein in the circulatory system, whose redox modifications modulate its physiologic functions,23 as well as may serve as a biomarker of oxidative stress.23–25,27–29 IgG is continuously exposed to oxidative stress23–25 bringing about alterations in conformation and functions of IgG, resulting in modification of its biological properties. We previously demonstrated that ROS caused extensive damaged to human IgG and damaged IgG was found to be a potent antigenic stimulus inducing high titre antibodies in rabbits, whereas with native human IgG, antibodies titre was low.20 The antigenic specificity of affinity purified anti-ROS-human IgG and anti-native IgG antibodies reiterated that induced antibodies were immunogen specific. The substantially enhanced immunogenicity of ROS-human IgG in comparison to native analogue could possibly be due to the generation of potential neo-epitopes against which antibodies are raised.
In the present study, we demonstrated for the very first time that experimentally induced antibodies against ROS-damaged human IgG exhibited diverse antigen binding characteristics. Native DNA, native chromatin, and their ROS-modified conformers were found to be effective inhibitors of induced antibody-immunogen interaction. Whereas, induced antibodies against native IgG showed negligible binding to the above mentioned nucleic acid conformers. This notable feature of induced antibodies against ROS-modified IgG resembled the diverse antigen-binding characteristics of naturally occurring SLE anti-DNA autoantibodies.
SLE is a chronic autoimmune disease that is characterized by increased production of autoantibodies, but the initial immunizing antigens that drive the development of SLE are largely unknown. Autoantibody production in SLE has been attributed to either selective stimulation of autoreactive B cells by self antigens or antigens cross-reactive with self.1,2,7 Numerous modified forms of DNA have been found to be immunogenic and are recognized by SLE anti-DNA antibodies.7,31,38 Despite the power of modern molecular approaches and persistent investigative efforts, lupus remains an enigmatic disorder6 and the agent (or agents) triggering this autoimmune response remains to be identified. Patients with SLE have a diverse array of anti-nuclear autoantibodies, but the cellular and molecular mechanisms that are responsible for the production of anti-nuclear antibodies in SLE and the way by which these antibodies participate in tissue destruction remain highly controversial.39 It was thought worthwhile to investigate the binding characteristics of natural SLE autoantibodies to ROS-damaged human IgG as that the possible involvement of ROS-modified IgG in SLE could be ascertained. Sera from 72 SLE patients having high titre anti-DNA antibodies and 39 normal human subjects were collected for the present study. Of these, 48.6% SLE sera showed preferentially high binding to ROS-IgG as compared to its native analogue (P < 0.01) as determined by direct binding ELISA assays. Native or modified IgG showed no appreciable binding with normal subjects (P > 0.05). We also noticed that unmodified IgG showed some binding to SLE serum antibodies as compared with the antibodies from NHS (P < 0.05), this may be due to the partial structural similarities of native and modified IgG, which may shared common paratope of SLE autoantibodies. Competition ELISA assays further confirmed the direct binding results. Our data clearly indicated that SLE autoantibodies showed substantial difference in the recognition of modified IgG over native IgG (P < 0.01).
The oxidation of a protein typically results in an increase in carbonyl contents. This increase is due to the oxidation of lys, arg, pro or other amino acid residues. In short, protein carbonyl groups are the biomarker of oxidative stress.35 In human plasma, all amino acids in the protein are susceptible to oxidative modification by oxidants such as hydroxyl radicals and hypochlorous acid.35 In view of these, carbonyl contents present in the total serum protein of SLE were investigated. Our results showed that total serum protein carbonyl contents were significantly increased in SLE patients, when compared with the carbonyl contents present in total serum protein of healthy human subjects (P < 0.05). These results indicated that in SLE patients with increased oxidative stress, the oxidative modification of plasma proteins has been greatly enhanced. Since the abundant protein of plasma is IgG, it is likely to be extensively damaged and might be responsible for the pathological conditions associated with SLE. These results suggest that IgG is continuously exposed to oxidative stress, so much so that alterations in its biological properties could result in the conformational changes of IgG. Our results demonstrate the presence of ROS-induced human IgG damage in SLE patients, which might play an active part in the progression of disease. The present study further proposed that, in addition to IgG in serum concentration, the quality of IgG molecules may be not only a crucial factor affecting its protective effects, but also a risk factor as a pro-oxidant in SLE patients.
Conclusions
Ours is the first report to show the role of hydroxyl radical damaged immunoglobulin G in SLE. Our results provide new suggestions that hydroxyl radical modification of IgG causes perturbations, resulting in the generation of neo-epitopes, and making it a potential immunogen. The induced antibodies against hydroxyl radical-modified IgG resembled the diverse antigen-binding characteristics of naturally occurring SLE anti-DNA autoantibodies. The IgG modified with the hydroxyl radicals may be one of the factors for the induction of circulating SLE autoantibodies.
Acknowledgments
We would like to thank Dr. Lokender Kumar for providing SLE samples. Financial support from Qassim University is gratefully acknowledged.
Footnotes
Disclosure
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Gualtierotti R, Biggioggero M, Penatti AE, Meroni PL. Updating on the pathogenesis of systemic lupus erythematosus. Autoimmun Rev. 2010;10:3–7. doi: 10.1016/j.autrev.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Arbuckle MR, McClain MT, Rubertone MV, Scofied RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemin lupus erythematosus. N Engl J Med. 2003;349:1526–33. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 3.Greidinger EL, Hoffman RW. The appearance of U1 RNP antibody specificities in sequential autoimmune human antisera follows a characteristic order that implicates the U1–70 kd and B0/B proteins as predominant U1 RNP immunogens. Arthritis Rheum. 2001;44:368–75. doi: 10.1002/1529-0131(200102)44:2<368::AID-ANR55>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 4.Lehmann PV, Sercarz EE, Forsthuker T, Doyan CM, Gammon G. Determinant spreading and the dynamics of the autoimmune T-cell repertoire. Immunol Today. 1993;14:203–8. doi: 10.1016/0167-5699(93)90163-F. [DOI] [PubMed] [Google Scholar]
- 5.James JA, Harley JB. B-cell epitope spreading in autoimmunity. Immunol Rev. 1998;164:85–200. doi: 10.1111/j.1600-065x.1998.tb01220.x. [DOI] [PubMed] [Google Scholar]
- 6.Perl A. Pathogenesis and spectrum of autoimmunity. In: Perl A, editor. Autoimmunity, methods and protocols. Totowa, NJ: Humana Press; 2004. pp. 1–8. [DOI] [PubMed] [Google Scholar]
- 7.Ahsan H, Ali A, Ali R. Oxygen free radicals and systemic autoimmunity. Clin Exp Immunol. 2003;131:398–404. doi: 10.1046/j.1365-2249.2003.02104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scofield RH, Kurien BT, Granick S, McClain MJ, Pye Q, James JA, et al. Modification of lupus-associated 60-kDa Ro protein with the lipid oxidation product 4-hydroxy-2-nonenal increases antigenicity and facilitates epitopes spreading. Free Radic Biol Med. 2005;38:719–28. doi: 10.1016/j.freeradbiomed.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Griffiths HR. Is the generation of neo-antigenic determinants by free radicals central to the development of autoimmune rheumatoid disease? Autoimmun Rev. 2008;7:544–9. doi: 10.1016/j.autrev.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 10.Zerovnik E. Protein conformational pathology in Alzheimer’s and other neurodegenerative diseases; new targets for therapy. Curr Alzheimer Res. 2010;7:74–83. doi: 10.2174/156720510790274437. [DOI] [PubMed] [Google Scholar]
- 11.Shacter E. Quantification and significance of protein oxidation in biological samples. Drug Met Rev. 2000;32:307–26. doi: 10.1081/dmr-100102336. [DOI] [PubMed] [Google Scholar]
- 12.Poon HF, Vaishnav RA, Gelchell TV, Getchell ML, Butterfield DA. Quantitative proteomics analysis of differential protein expression and oxidative modification of specific proteins in the brains of old mice. Neurobiol Aging. 2006;27:1010–9. doi: 10.1016/j.neurobiolaging.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Arutyunova EI, Danshina PV, Domnina LV, Pleten AP, Muronetz VI. Oxidation of glyceraldehydes-3-phosphate dehydrogenase enhances its binding to nucleic acids. Biochem Biophys Res Commun. 2003;307:547–52. doi: 10.1016/s0006-291x(03)01222-1. [DOI] [PubMed] [Google Scholar]
- 14.Shacter E, Williams JA, Levine RL. Oxidative modification of fibrinogen inhibits thrombin-catalyzed clot formation. Free Radic Biol Med. 1995;18:815–21. doi: 10.1016/0891-5849(95)93872-4. [DOI] [PubMed] [Google Scholar]
- 15.Rasheed Z, Ahmad R, Ali R. Structure and immunological function of oxidised albumin in lung cancer: its potential role as a biomarker of elevated oxidative stress. Br J Biomed Sci. 2009;66:67–73. doi: 10.1080/09674845.2009.11730247. [DOI] [PubMed] [Google Scholar]
- 16.Rasheed Z, Ahmad R, Rasheed N, Ali R. Reactive oxygen species damaged human serum albumin in patients with hepatocellular carcinoma. J Exp Clin Cancer Res. 2007;26:395–404. [PubMed] [Google Scholar]
- 17.Rasheed Z, Khan MW, Ali R. Hydroxyl radical modification of human serum albumin generated cross reactive antibodies. Autoimmunity. 2006;39:479–88. doi: 10.1080/08916930600918472. [DOI] [PubMed] [Google Scholar]
- 18.Rasheed Z, Ali R. Reactive oxygen species damaged human serum albumin in patients with type 1 diabetes mellitus: biochemical and immunological studies. Life Sci. 2006;79:2320–8. doi: 10.1016/j.lfs.2006.07.041. [DOI] [PubMed] [Google Scholar]
- 19.Rasheed Z, Ahmad R, Rasheed N. Reactive oxygen species damaged hemoglobin presents unique epitopes for type 1 diabetes autoantibodies. Int J Bio Chem. 2008;2:1–13. [Google Scholar]
- 20.Rasheed Z. Hydroxyl radical damaged Immunoglobulin G in patients with rheumatoid arthritis: biochemical and immunological studies. Clin Biochem. 2008;2:1–13. doi: 10.1016/j.clinbiochem.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 21.Rasheed Z, Ahmad R, Rasheed N, Ali R. Enhanced recognition of reactive oxygen species damaged human serum albumin by circulating systemic lupus erythematosus autoantibodies. Autoimmunity. 2007;40:512–20. doi: 10.1080/08916930701574331. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Q, Ye DQ, Chen GP, Zheng Y. Oxidative protein damage and antioxidant status in systemic lupus erythematosus. Clin Exp Dermatol. 2010;35:287–94. doi: 10.1111/j.1365-2230.2009.03437.x. [DOI] [PubMed] [Google Scholar]
- 23.Uesugi M, Yoshida K, Jasin HE. Inflammatory properties of IgG modified by oxygen radicals and peroxynitrite. J Immunol. 2000;165:6532–7. doi: 10.4049/jimmunol.165.11.6532. [DOI] [PubMed] [Google Scholar]
- 24.Maninger K, Weblacher M, Zatloukal K, Estelberger W, Schauenstein K, Schauenstein E. IgG1-As the only subclass of human serum IgG spontaneously undergoes O2-induced, noncolavalent self aggregation upon storage at room temperature. Free Radical Biol Med. 1996;20:263–70. doi: 10.1016/0891-5849(95)02030-6. [DOI] [PubMed] [Google Scholar]
- 25.Kleinveld HA, Slulter W, Boonman AM, Swaak AJ, Hack CE, Koster JF. Differential stimulation by oxygen-free-radical-altered immunoglobulin G of the production of superoxide and hydrogen peroxide by human polymorphonuclear leucocytes. Clin Sci. 1991;80:385–91. doi: 10.1042/cs0800385. [DOI] [PubMed] [Google Scholar]
- 26.Chou C. Binding of rheumatoid and lupus synovial fluids and sera-derived human IgG rheumatoid factor to degalactosylated IgG. Arch Med Res. 2002;33:541–4. doi: 10.1016/s0188-4409(02)00406-x. [DOI] [PubMed] [Google Scholar]
- 27.Swaak AJ, Kleinveld HA, Kloster JF, Hack CE. Possible role of free radical altered IgG in the etiopathogenesis of rheumatoid arthritis. Rheumatol Int. 1989;9:1–6. doi: 10.1007/BF00270282. [DOI] [PubMed] [Google Scholar]
- 28.Griffiths HR, Lunec J. The C1q binding activity of IgG is modified in vitro by reactive oxygen species: implications for rheumatoid arthritis. Fed Eur Biochem Soc Lett. 1996;388:161–4. doi: 10.1016/0014-5793(96)00542-x. [DOI] [PubMed] [Google Scholar]
- 29.Griffiths HR, Lunec J. The effect of oxygen free radicals on the carbohydrate moiety of IgG. Fed Eur Biochem Soc. 1989;245:95–9. doi: 10.1016/0014-5793(89)80199-1. [DOI] [PubMed] [Google Scholar]
- 30.Habib S, Moinuddin Ali A, Ali R. Preferential recognition of peroxynitrite modified human DNA by circulating autoantibodies in cancer patients. Cell Immunol. 2009;254:117–23. doi: 10.1016/j.cellimm.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Mansoor F, Ali R. Characterization of chromatin modified with reactive oxygen species: recognition by autoantibodies in cancer. Clin Biochem. 2007;40:928–35. doi: 10.1016/j.clinbiochem.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 32.Rasheed Z, Anbazhagan AN, Akhtar N, Ramamurthy S, Voss FR, Haqqi TM. Green tea polyphenol epigallocatechin-3-gallate inhibits advanced glycation end product-induced expression of tumor necrosis factor-alpha and matrix metalloproteinase-13 in human chondrocytes. Arthritis Res Ther. 2009;11:R71. doi: 10.1186/ar2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rasheed Z, Akhtar N, Haqqi TM. Rheumatology (Oxford) 2010. Advanced Glycation End Products (AGEs) induce the expression of interleukin (IL)-6 and IL-8 by RAGE mediated activation of MAPKs and NF-κB in human osteoarthritis chondrocytes. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 35.Levine RL, Williams J, Stadtman ER, Shacter E. Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol. 1994;233:346–57. doi: 10.1016/s0076-6879(94)33040-9. [DOI] [PubMed] [Google Scholar]
- 36.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 37.Ahmad R, Rasheed Z, Ahsan H. Biochemical and cellular toxicology of peroxynitrite: implications in cell death and autoimmune phenomenon. Immunopharmacol Immunotoxicol. 2009;31:388–96. doi: 10.1080/08923970802709197. [DOI] [PubMed] [Google Scholar]
- 38.Touma Z, Gladman DD, Tulloch-Reid D, Toloza SM, Ibañez D, Fortin PR, et al. Burden of autoantibodies and association with disease activity and damage in systemic lupus erythematosus. Clin Exp Rheumatol. 2010;28:525–31. [PubMed] [Google Scholar]
- 39.Ravirajan CT, Rowse L, MacGowan JR, Isenberg DA. An analysis of clinical disease activity and nephritis-associated serum autoantibody profiles in patients with systemic lupus erythematosus: a cross-sectional study. Rheumatology (Oxford) 2001;40:1405–12. doi: 10.1093/rheumatology/40.12.1405. [DOI] [PubMed] [Google Scholar]