Abstract
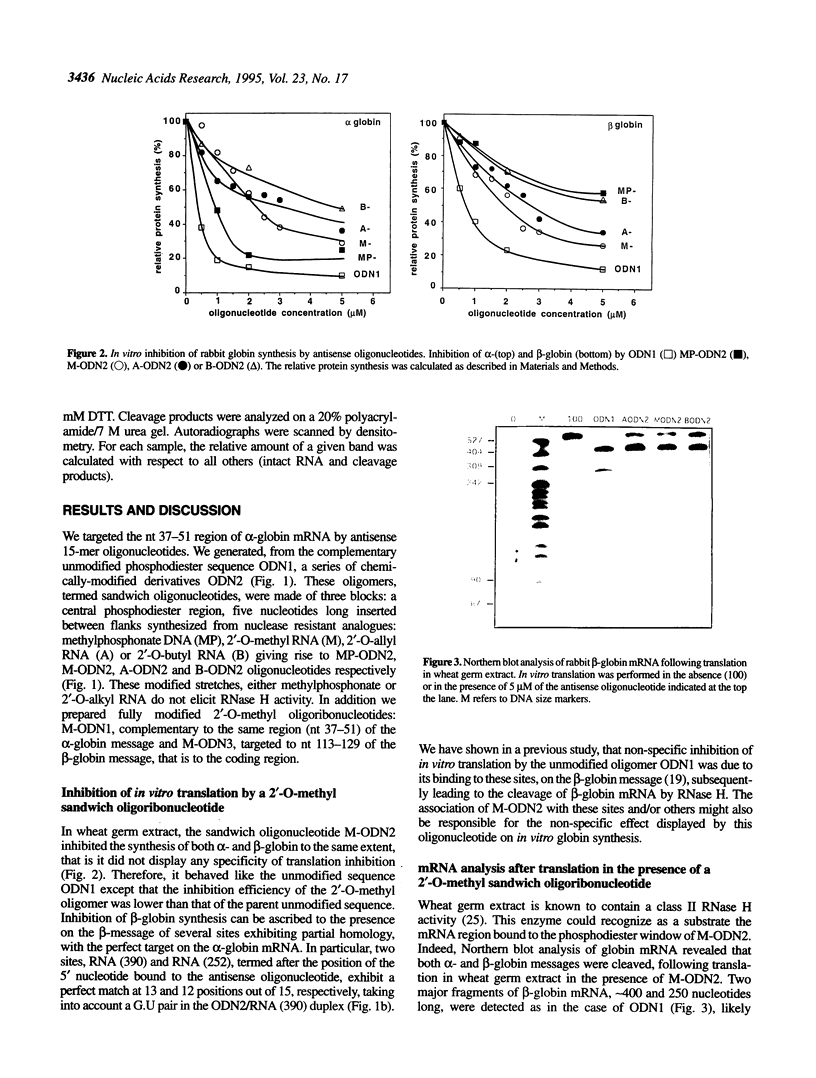
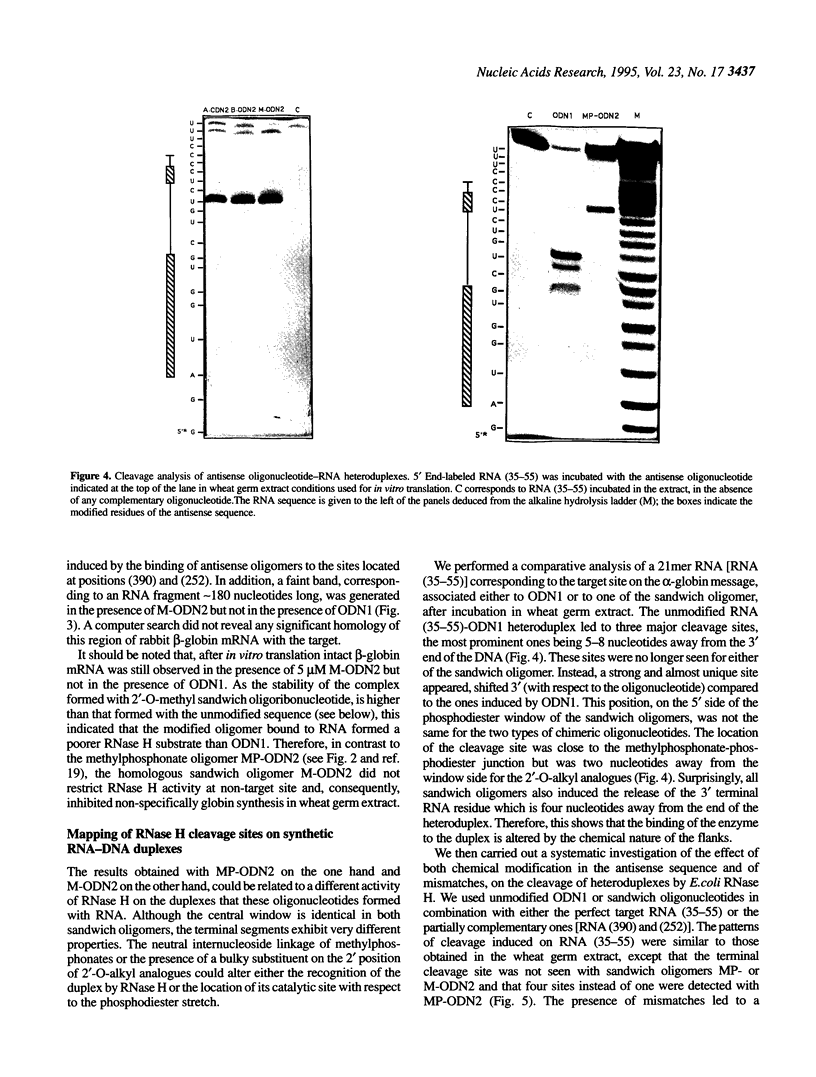
Ribonuclease H (RNase H) which recognizes and cleaves the RNA strand of mismatched RNA-DNA heteroduplexes can induce non-specific effects of antisense oligonucleotides. In a previous paper [Larrouy et al. (1992), Gene, 121, 189-194], we demonstrated that ODN1, a phosphodiester 15mer targeted to the AUG initiation region of alpha-globin mRNA, inhibited non-specifically beta-globin synthesis in wheat germ extract due to RNase H-mediated cleavage of beta-globin mRNA. Specificity was restored by using MP-ODN2, a methylphosphonate-phosphodiester sandwich analogue of ODN1, which limited RNase H activity on non-perfect hybrids. We report here that 2'-O-alkyl RNA-phosphodiester DNA sandwich analogues of ODN1, with the same phosphodiester window as MP-ODN2, are non-specific inhibitors of globin synthesis in wheat germ extract, whatever the substituent (methyl, allyl or butyl) on the 2'-OH. These sandwich oligomers induced the cleavage of non-target beta-globin RNA sites, similarly to the unmodified parent oligomer ODN1. This is likely due to the increased affinity of 2'-O-alkyl-ODN2 chimeric oligomers for both fully and partly complementary RNA, compared to MP-ODN2. In contrast, the fully modified 2'-O-methyl analogue of ODN1 was a very effective and highly specific antisense sequence. This was ascribed to its inability (i) to induce RNA cleavage by RNase H and (ii) to physically prevent the elongation of the polypeptide chain.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boiziau C., Kurfurst R., Cazenave C., Roig V., Thuong N. T., Toulmé J. J. Inhibition of translation initiation by antisense oligonucleotides via an RNase-H independent mechanism. Nucleic Acids Res. 1991 Mar 11;19(5):1113–1119. doi: 10.1093/nar/19.5.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazenave C., Frank P., Büsen W. Characterization of ribonuclease H activities present in two cell-free protein synthesizing systems, the wheat germ extract and the rabbit reticulocyte lysate. Biochimie. 1993;75(1-2):113–122. doi: 10.1016/0300-9084(93)90032-n. [DOI] [PubMed] [Google Scholar]
- Cazenave C., Loreau N., Thuong N. T., Toulmé J. J., Hélène C. Enzymatic amplification of translation inhibition of rabbit beta-globin mRNA mediated by anti-messenger oligodeoxynucleotides covalently linked to intercalating agents. Nucleic Acids Res. 1987 Jun 25;15(12):4717–4736. doi: 10.1093/nar/15.12.4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazenave C., Stein C. A., Loreau N., Thuong N. T., Neckers L. M., Subasinghe C., Hélène C., Cohen J. S., Toulmé J. J. Comparative inhibition of rabbit globin mRNA translation by modified antisense oligodeoxynucleotides. Nucleic Acids Res. 1989 Jun 12;17(11):4255–4273. doi: 10.1093/nar/17.11.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagle J. M., Walder J. A., Weeks D. L. Targeted degradation of mRNA in Xenopus oocytes and embryos directed by modified oligonucleotides: studies of An2 and cyclin in embryogenesis. Nucleic Acids Res. 1990 Aug 25;18(16):4751–4757. doi: 10.1093/nar/18.16.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furdon P. J., Dominski Z., Kole R. RNase H cleavage of RNA hybridized to oligonucleotides containing methylphosphonate, phosphorothioate and phosphodiester bonds. Nucleic Acids Res. 1989 Nov 25;17(22):9193–9204. doi: 10.1093/nar/17.22.9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles R. V., Tidd D. M. Enhanced RNase H activity with methylphosphonodiester/phosphodiester chimeric antisense oligodeoxynucleotides. Anticancer Drug Des. 1992 Feb;7(1):37–48. [PubMed] [Google Scholar]
- Giles R. V., Tidd D. M. Increased specificity for antisense oligodeoxynucleotide targeting of RNA cleavage by RNase H using chimeric methylphosphonodiester/phosphodiester structures. Nucleic Acids Res. 1992 Feb 25;20(4):763–770. doi: 10.1093/nar/20.4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hélène C., Toulmé J. J. Specific regulation of gene expression by antisense, sense and antigene nucleic acids. Biochim Biophys Acta. 1990 Jun 21;1049(2):99–125. doi: 10.1016/0167-4781(90)90031-v. [DOI] [PubMed] [Google Scholar]
- Johansson H. E., Belsham G. J., Sproat B. S., Hentze M. W. Target-specific arrest of mRNA translation by antisense 2'-O-alkyloligoribonucleotides. Nucleic Acids Res. 1994 Nov 11;22(22):4591–4598. doi: 10.1093/nar/22.22.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamond A. I., Sproat B. S. Antisense oligonucleotides made of 2'-O-alkylRNA: their properties and applications in RNA biochemistry. FEBS Lett. 1993 Jun 28;325(1-2):123–127. doi: 10.1016/0014-5793(93)81427-2. [DOI] [PubMed] [Google Scholar]
- Larrouy B., Blonski C., Boiziau C., Stuer M., Moreau S., Shire D., Toulmé J. J. RNase H-mediated inhibition of translation by antisense oligodeoxyribonucleotides: use of backbone modification to improve specificity. Gene. 1992 Nov 16;121(2):189–194. doi: 10.1016/0378-1119(92)90121-5. [DOI] [PubMed] [Google Scholar]
- Maher L. J., 3rd, Dolnick B. J. Comparative hybrid arrest by tandem antisense oligodeoxyribonucleotides or oligodeoxyribonucleoside methylphosphonates in a cell-free system. Nucleic Acids Res. 1988 Apr 25;16(8):3341–3358. doi: 10.1093/nar/16.8.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metelev V. G., Zayakina G. V., Ryabushenko I. L., Krynetskaya N. F., Romanova E. A., Oretskaya T. S., Shabarova Z. A. Influence of probe structure on unique (regiospecific) cleavage of RNA by RNase H. FEBS Lett. 1988 Jan 4;226(2):232–234. doi: 10.1016/0014-5793(88)81429-7. [DOI] [PubMed] [Google Scholar]
- Milligan J. F., Groebe D. R., Witherell G. W., Uhlenbeck O. C. Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res. 1987 Nov 11;15(21):8783–8798. doi: 10.1093/nar/15.21.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morvan F., Porumb H., Degols G., Lefebvre I., Pompon A., Sproat B. S., Rayner B., Malvy C., Lebleu B., Imbach J. L. Comparative evaluation of seven oligonucleotide analogues as potential antisense agents. J Med Chem. 1993 Jan 22;36(2):280–287. doi: 10.1021/jm00054a013. [DOI] [PubMed] [Google Scholar]
- Quartin R. S., Brakel C. L., Wetmur J. G. Number and distribution of methylphosphonate linkages in oligodeoxynucleotides affect exo- and endonuclease sensitivity and ability to form RNase H substrates. Nucleic Acids Res. 1989 Sep 25;17(18):7253–7262. doi: 10.1093/nar/17.18.7253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartin R. S., Wetmur J. G. Effect of ionic strength on the hybridization of oligodeoxynucleotides with reduced charge due to methylphosphonate linkages to unmodified oligodeoxynucleotides containing the complementary sequence. Biochemistry. 1989 Feb 7;28(3):1040–1047. doi: 10.1021/bi00429a018. [DOI] [PubMed] [Google Scholar]
- Shaw J. P., Kent K., Bird J., Fishback J., Froehler B. Modified deoxyoligonucleotides stable to exonuclease degradation in serum. Nucleic Acids Res. 1991 Feb 25;19(4):747–750. doi: 10.1093/nar/19.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth J., Colman A. Antisense oligonucleotide-directed cleavage of mRNA in Xenopus oocytes and eggs. EMBO J. 1988 Feb;7(2):427–434. doi: 10.1002/j.1460-2075.1988.tb02830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. C., Bement W. M., Dersch M. A., Dworkin-Rastl E., Dworkin M. B., Capco D. G. Nonspecific effects of oligodeoxynucleotide injection in Xenopus oocytes: a reevaluation of previous D7 mRNA ablation experiments. Development. 1990 Nov;110(3):769–779. doi: 10.1242/dev.110.3.769. [DOI] [PubMed] [Google Scholar]
- Tidd D. M., Warenius H. M. Partial protection of oncogene, anti-sense oligodeoxynucleotides against serum nuclease degradation using terminal methylphosphonate groups. Br J Cancer. 1989 Sep;60(3):343–350. doi: 10.1038/bjc.1989.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulmé J. J., Krisch H. M., Loreau N., Thuong N. T., Hélène C. Specific inhibition of mRNA translation by complementary oligonucleotides covalently linked to intercalating agents. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1227–1231. doi: 10.1073/pnas.83.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf T. M., Melton D. A., Jennings C. G. Specificity of antisense oligonucleotides in vivo. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7305–7309. doi: 10.1073/pnas.89.16.7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt J. R., Walker G. T. Deoxynucleotide-containing oligoribonucleotide duplexes: stability and susceptibility to RNase V1 and RNase H. Nucleic Acids Res. 1989 Oct 11;17(19):7833–7842. doi: 10.1093/nar/17.19.7833. [DOI] [PMC free article] [PubMed] [Google Scholar]