Abstract
Background:
Aristolochic Acids (AAs) are major components of plants in Aristolochia and have been found to be nephrotoxic, carcinogenic and mutagenic. Herein reported are the isolation, identification and quantity determination methods of Aristolochic Acid-I (AA-I) and Aristolochic Acid-II (AA-II) toxic compounds of Aristolochia bracteolata indigenous to Central Sudan and medicinally used in diverse biological functions including analgesic and diuretic effects, treatment of tumors, malaria and/or fevers.
Methods and results:
AAs mixture was extracted with methanol from the defatted material of Aristolochia bracteolata whole plant at room temperature and was isolated from the aqueous methanol extract by chloroform. Moreover, Silica-gel column chromatography and Preparative Thin Layer Chromatography (PTLC) using chloroform/methanol gradient mixtures were used to isolate AAs mixtures as a yellow crystalline solid. A preliminary detection of AAs was made by Thin Layer Chromatography (silica-gel, chloroform: methanol (6:1)). The Rf value of the acids mixture was 0.43–0.46. The presence of AAs in plant sample was confirmed by High Performance Liquid Chromatography/Ultraviolet (HPLC/UV) analysis using 1% acetic acid and methanol (40:60) as mobile phase and maximum absorption wave length of 250 nm. Quantitative determination of AA-II (49.03 g/kg) and AA-I (12.98 g/kg) was also achieved by HPLC/UV.
Recommendation:
It is recommended that the use of Aristolochia bracteolata as a medicinal plant should be extremely limited or strictly prohibited. The chromatograms obtained in this study can serve as fingerprints to identify AAs in plant samples.
Keywords: Aristolochic acid-I, Aristolochic acid-II, Aristolochia, Aristolochia bracteolata
Introduction
The genus Aristolochia grows over a wide area of different climatic zones and consists of about 500 species.1 Various Aristolochia species have been used in herbal medicines since antiquity in obstetrics and in treatment of snakebite,2 festering wounds, and tumors, and they remain in use particularly in Chinese herbal medicine.3,4 Aristolochia bracteolata is known as “worm killer” due to supposed antihelminthic activity and trypanocidal effect.5 Aristolochia bracteolata also possess a potent antiallergic activity6 and has pronounced antibacterial and antifungal activities.7
In Sudan, Aristolochia is traditionally used as an analgesic, antiscorpion, and antisnake. It is also used in the treatment of tumors, malaria and for fevers.8
Aristolochic acids (Fig. 1) are compounds of nitrophenanthrene carboxylic acids that occur naturally in plants of the family Aristolochiaceae, primarily in the genera Aristolochia and Asarum9,10 as well as in butterflies that feed on such plants.11 Botanical products from plants containing aristolochic acids are used in traditional folk medicines, particularly in Chinese herbal medicine, especially those used as a part of weight-loss regimens.4
Figure 1.
Chemical structure of aristolochic acid I and aristolochic acid II.
Aristolochic acids are known to be toxic and a rodent carcinogen, in addition to their carcinogenicity, aristolochic acids are also highly nephrotoxic agents.1,10–12 In the present work Aristolochia bracteolata grown in Sudan was investigated for the presence of Aristolochic acids.
Materials and Methods
Plant material
The plant Aristolochia bracteolata was collected during December 2009 from the Southern Gezira scheme, Sudan. The plant was taxonomically identified in the Department of Chemistry and Pharmacognosy, University of Gezira, and a voucher specimen was deposited.
Methods
Extraction of plant material
The shade dried whole plant sample (200 g) of Aristolochia bracteolata were milled in the form of a coarse powder. The powdered sample was defatted by maceration at room temperature using petroleum ether (40–60 °C) for 6 hours to yield dark green oily residue after evaporation of the solvent. As described in previous studies,13,14 and with some modifications (defatting before methanol extraction), the dried defatted residue was macerated in methanol at room temperature for 72 hours and filtered. Solvent was evaporated to dryness under reduced pressure using a rotary evaporator at 60 °C to give a dark green methanol extract.
Liquid-liquid extraction
Twenty grams of Aristolochia bracteolata methanol extract was partitioned between water (450 ml) and chloroform (800 ml). Chloroform layer was separated and dried by rotary evaporator at 40 °C to give chloroform extract.
Column chromatography
Chloroform extract of Aristolochia bracteolata (4.0 g) was chromatographed on a silica-gel column. For column fractionation the chloroform extract was dissolved in 20 ml chloroform and applied to the top of a column (8.0 cm ID) backed with 160 g silica-gel of a mesh size of 60–120.
Elution was commenced with gradient chloroform/methanol mixture (8:1). Polarity of the solvent was increased in a gradient of 8:1, 4:1, 1:1, 0:1 and finally the column was washed with 100 ml of water. 200 ml of eluents were used and fractions of 50 ml were collected and thin layer chromatography (TLC) monitored. Similar fractions were combined to give three fractions 1–7 (a), 8–14 (b) and 15–17 (c). Fraction (b) was further subjected to column chromatography (3.5 cm ID) backed with 40 g silica-gel of mesh size 60–120, and eluted with a chloroform/methanol mixture of increasing polarity as: 8:1, 4:1, 1:1, 0:1. Fractions of 25 ml were collected and TLC monitored using chloroform/methanol mixture (6:1) as mobile phase (Fig. 2).
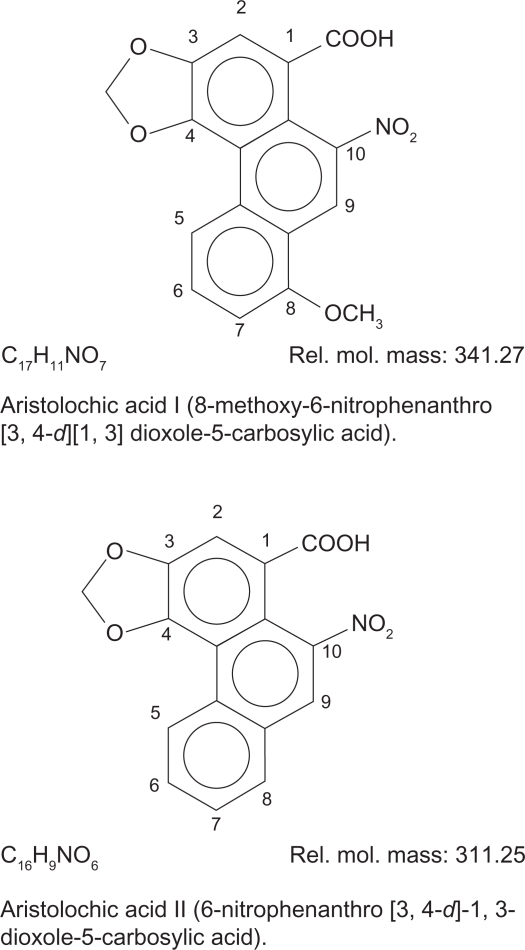
Figure 2.
Isolated Aristolochic acids (left), silica-gel fluorescence TLC aluminum sheet, chloroform/methanol (6:1) mobile phase (under UV at 365 nm), compare to standard Aristolochic acids (right).
Thin layer chromatography (TLC)
TLC glass backed plates were prepared using silica-gel G type 60 (s d Fine-Chem Limited, Mumbai, India). A slurry of silica-gel in water was applied by means of a spreader to glass plates, previously cleaned with methanol. A layer 0.25 mm thick was used routinely. Plates were activated by heating at 100–110 °C for an hour. TLC aluminum sheets 20 × 20 cm, 1 mm thick, silica-gel 60 F254 (Merk, Germany) and Cellulose plastic papers 20 × 20 cm, 1 mm thick (Macherey-Nagel, Germany) were also used. Different mobile phases chloroform/methanol (6:1);15 chloroform/methanol/acetic acid (65:20:5);16 benzene/acetone (14:1),17 chloroform/methanol/water (60:40:10)18 were checked for the separation of aristolochic acids.
Chromatographic responses (Table 1) were obtained and compared to those of Aristolochic acids reference sample Aristolochic acid mixture of AA-I and AA-II (Aldrich Chemical Company, USA). Day light, ultraviolet light (UV), 0.5% Diphenylamine in 60% H2SO4 (DPA/H2SO4) and Dragendurff’s reagents were used as locating agents to disclose the aristolochic acids on TLC and preparative TLC chromatograms (Fig. 2).
Table 1.
Observed chromatographic responses of Aristolochic acids on TLC chromatograms.
| Type of adsorbent | Solvent system | Rf value |
Appearance of spots/bands |
||
|---|---|---|---|---|---|
| Day light | UV at 365 nm | 0.5% DPA/60% H2SO4 | |||
| Silica-gel 60 F254, precoated aluminum sheets, 20 × 20 cm (Merk, Germany) | CHCL3/MeOH (6:1) | 0.43 | Yellow fluorescence | Black spot | Pale grey spot |
| Silica-gel G glass plates (s d Fine-Chem., Limited, Mumbai, India) | CHCL3/MeOH (6:1) | 0.46 | Yellow fluorescence | Faint brown spot | Pale grey spot |
| Cellulose, precoated plastic sheets, 20 × 20 cm (Macherey-Nagel, Germany) | CHCL3/MeOH/Water (6:4:1) | 0.83 | Yellow fluorescence | Faint brown spot | Pale grey spot |
Preparative thin layer chromatography (PTLC)
The dried material obtained from the combined column chromatography fractions which are rich in aristolochic acids (Fraction b) was dissolved in 10 ml chloroform/methanol mixture (6:1) and subjected to preparative thin layer chromatography (PTLC) using 20 × 20 cm cellulose papers for TLC with chloroform/methanol/water (6:4:1) as mobile phase and TLC aluminum sheets: (20 × 20 cm), silica-gel 60 F254 with chloroform/methanol (6:1) as mobile phase. The disclosed bands of aristolochic acids were then scraped off, dissolved in chloroform/methanol mixture (6:1) and filtered. The solvent was removed by evaporation and the obtained isolate containing aristolochic acids was subjected to further PTLC for purification using silica-gel fluorescent TLC aluminum sheets as described above (Figs. 3 and 4). Scraped Aristolochic acids were dissolved and filtered. Solvent was removed to yield a yellow crystalline aristolochic acids mixture.
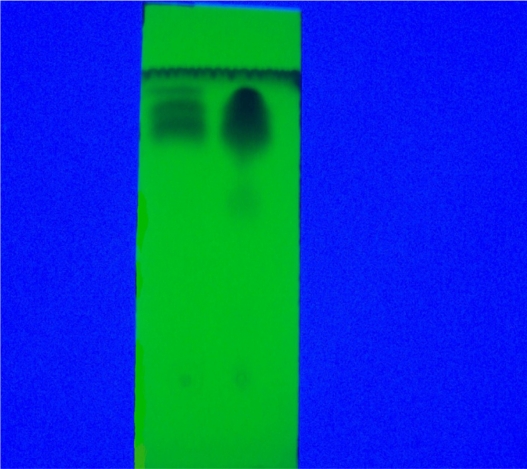
Figure 3.
Aristolochic acids PTLC chromatogram, silica-gel fluorescence TLC aluminum sheet, chloroform/methanol (6:1) mobile phase, (day light).
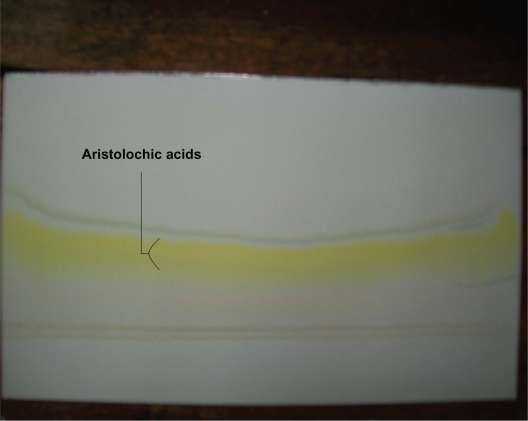
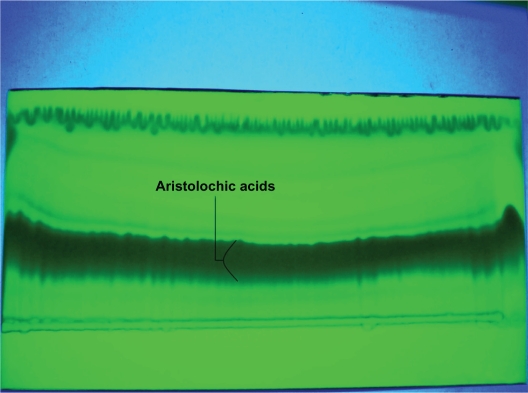
Figure 4.
Aristolochic acids PTLC chromatogram, silica-gel fluorescence TLC aluminum sheet, chloroform/methanol (6:1) mobile phase, (under UV at 365 nm).
C18-High-performance liquid chromatography (HPLC)
The separation and quantification of aristolochic acids was achieved on HPLC chromatograph, (Waters® 2996, USA); C18 250 × 4.6 mm column with UV detector. A mixture of 1% acetic acid and methanol (40:60) utilized as mobile phase, flow rate 1.0 ml/min, pressure 3015 psr, temperature 20–25 °C, UV detection 250 nm, Helium degassing, and injection volume 20 μL.
Preparation of standard Aristolochic acids, isolated Aristolochic acids and methanol extract of Aristolochia bracteolata
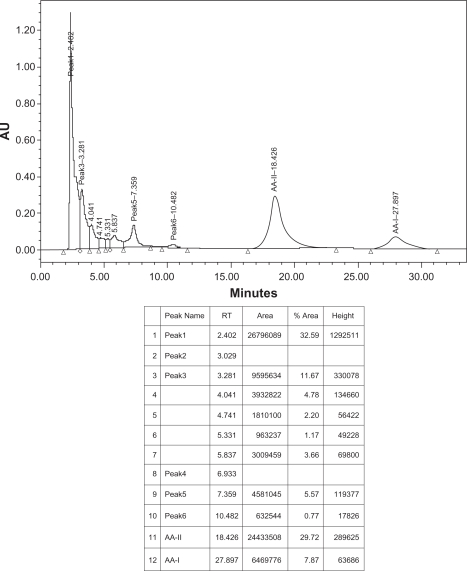
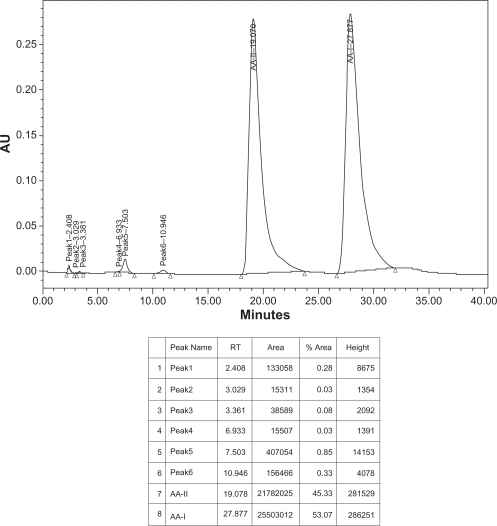
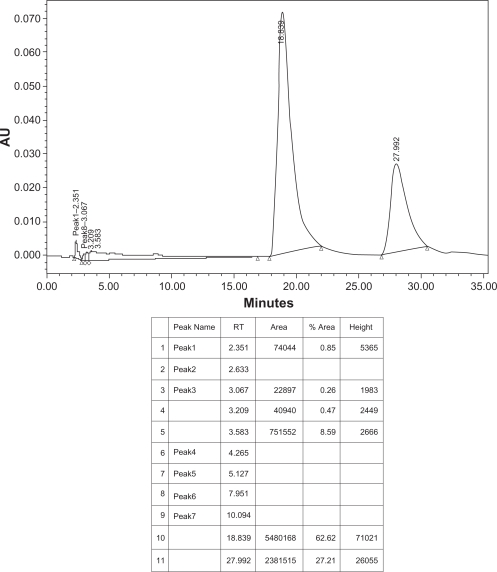
Fifteen milligrams of Aristolochic acids mixture of AA-I and AA-II (Aldrich Chemical Company, USA), fifteen mg of isolated aristolochic acids of Aristolochia bracteolata and one g of methanol extract of Aristolochia bracteolata were weighed separately using digital balance, each was placed in a small beaker, transferred into volumetric flask (50 ml), washed by methanol-HPLC grade and adjusted to 50 ml, filtered and sonicated for 10 minutes. 20 μL were separately injected in the HPLC system. Identification was made by comparing the samples retention times with those of the standard reference (Figs. 5–7).
Figure 5.
HPLC chromatogram of methanol extract sample of Aristolochia bracteolata.
Condition: column, C18 (Varian® microsorb-MV 300–8 C18 250 × 4.6 mm), mobile phase, 1% acetic acid/methanol (40:60), temperature 20–25 °C, flow rate 1.0 ml/min, UV detection 250 nm.
Figure 7.
HPLC chromatogram of AAs standard sample.
Condition: column, C18 (Varian® microsorb-MV 300–8 C18 250 × 4.6 mm), mobile phase, 1% acetic acid/methanol (40:60), temperature 20–25 °C, flow rate 1.0 ml/min, UV detection 250 nm.
Results and Discussion
To isolate and identify Aristolochic acids (AAs) in Aristolochia bracteolata L., the whole plant (200 g) was defatted to yield dark green oily residue (5.35%). As described in previous studies13,14 and with some modifications (defatting before methanol extraction, further n-butanol was not used in successive partitioning), the defatted plant material produced on drying 16.5% crude dark green extract on subsequent methanol maceration. On column chromatography of chloroform fraction obtained from the aqueous methanol extract, fraction (b) eluted by 4:1 and 1:1 solvent mixture was found to be rich in Aristolochic acids as revealed by TLC monitoring. Column chromatography fractionation of fraction (b) with the same gradient mobile phase and TLC monitoring showed that Aristolochic acids were obtained as major spots in the first ten fractions. Sixteen milligrams of a yellow powder of Aristolochic acids mixture (Fig. 2) was produced thereafter by preparative thin layer chromatography.
It was noted in previous studies that many attempts have been made to assay active components in Aristolochiaceae by various methods including Thin Layer Chromatography, High Performance Liquid Chromatography-Ultraviolet. However, most of these methods focus on analysis of AA-I and AA-II in samples.11,14,15,19,20
In the present study TLC and PTLC chromatograms clearly demonstrated the presence of Aristolochic acids. Results also indicated that the obtained AAs produced varying chromatographic responses with different chromatographic solvent systems and/or locating agents (Table 1). Aristolochic acids generally acquired black color on fluorescent silica-gel in UV-light at 365 nm while they fluoresce yellow in day light. Rf values ranged 0.43–0.46 (chloroform/methanol (6:1); silica-gel) and 0.83 (chloroform/methanol/water (6:4:1); cellulose).
For separation and quantification of Aristolochic acids by HPLC earlier reports mainly used a mobile phase of methanol and water21–24 or acetonitrile and water21,25,26 and the optimum chromatographic conditions were investigated by varying the content of methanol and water.
In this study, and for the first time in Sudan, the presence of Aristolochic acids in Aristolochia bracteolata methanol extract was confirmed and quantified by High Performance Liquid Chromatography as shown in Figures 5–7 using a mixture of 1% acetic acid and methanol (40:60) as mobile phase. Aristolochic acid II (AA-II) and Aristolochic acid I (AA-I) were detected as major peaks of components at a retention time of about 18.4 min and 27.9 min respectively and exactly corresponding to data profile exhibited by the Aristolochic acid reference sample. HPLC data also showed that AA-II was represented in a higher calculated amount of 49.03 g/kg compared to AA-I (12.98 g/kg) in Aristolochia bracteolata L. whole plant while in literature, other species of Aristolochia had recorded far more lower amounts of AAs such as Aristolochia debilis that contained 0.18 g/kg of AA-II and 1.01 g/kg of AA-I; Aristolochia manshuriensi contained 1.0 g/kg of AA-II and 8.82 g/kg of AA-I; Aristolochia fangchi also contained 0.22 g/kg of AA-II and 2.22 g/kg of AA-I.1,11,27–32
It is worth noting that, in the reported data AA-I obtained from different Aristolochia species was found to be in a higher quantity than AA-II, contrary to results obtained in this study. This may be attributed to climatic or biological variations which could affect metabolic pathways of interconvertion of Aristolochic acids and analogues.
In conclusion, the presence of Aristolochic acids toxic compounds in Aristolochia bracteolata L. methanol extract was evident through the multicomponent method including Column Chromatography (CC), Thin Layer Chromatography (TLC), Preparative Thin Layer Chromatography (PTLC) and had been confirmed and quantified by High Performance Liquid Chromatography (HPLC). Thus it is recommended that the use of Aristolochia bracteolata as a medicinal plant should be extremely limited or strictly prohibited. Moreover, the chromatograms obtained in this study can serve as fingerprints to identify plants suspected to contain or adulterated with Aristolochic acids.
Figure 6.
HPLC chromatogram of isolated AAs sample of Aristolochia bracteolata.
Condition: column, C18 (Varian® microsorb-MV 300–8 C18 250 × 4.6 mm), mobile phase, 1% acetic acid/methanol (40:60), temperature 20–25 °C, flow rate 1.0 ml/min, UV detection 250.
Footnotes
Disclosure
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.IARC (International Agency for Research on Caner) IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans. Vol. 82. Lyon; France: 2002. Some Traditional Herbal Medicines, Some Mycotoxins, Napthalene and Styrene; pp. 69–128. [PMC free article] [PubMed] [Google Scholar]
- 2.Meenatchisundaram S, Prajish Parameswari G, Subbraj T, Michael A. Studies on antivenom activity of Andrographis paniculata and Aristolochia indica plant extracts against Echis carinatus venom. The Internet Journal of Toxicology. 2009;6(1):1559–3916. [Google Scholar]
- 3.Balachandran P, Wei F, Lin R, Khan I, Pasco D. Structure activity relationships of aristolochic acid analogues: Toxicity in cultured renal epithelial cells. Journal of Kidney International. 2005;67:1797–805. doi: 10.1111/j.1523-1755.2005.00277.x. [DOI] [PubMed] [Google Scholar]
- 4.NTP (National toxicological program), United Sates department of health and human services . Research Triangle Park, NC 27709. 2008. Final report on carcinogenens background document for aristolochic acids; pp. 1–12. [Google Scholar]
- 5.Samia HAR, Elmalik KH, Khalid HS. Therapeutic Effect of Aristolochia bracteolate Extract Against Experimental Trypanosoma evansi Infection. International Journal of Tropical Medicine. 2006;1(4):170–2. [Google Scholar]
- 6.Chitme H, Malipatil M, Chandrashekhar V, Prashand P. Antiallergic activity of Aristolochia bracteolata Lank in animal model. Indian Journal of Experimental Biology. 2010;48:46–5. [PubMed] [Google Scholar]
- 7.Kavitha D, Nirmaladevi R. Assessment of Aristolochia bracteolata leaf extracts for its biotherapeutic potential. African Journal of Biotechnology. 2007;8(17):4242–4. [Google Scholar]
- 8.El-Tahir A, Satti G, Khalid S. Antiplasmodial Activity of Selected Sudanese Medicinal Plants with Emphasis on Acacia nilotica. Phytotherapy Research. 1999;13:474–8. doi: 10.1002/(sici)1099-1573(199909)13:6<474::aid-ptr482>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 9.Huang C, Tseng M, Lin J. Analyzing Aristolochic Acids in Chinese Herbal Preparations Using LC/MS/MS. Journal of Food and Drug Analysis. 2005;13:125–31. [Google Scholar]
- 10.Hsieh S, Huangc M, Lin B, Chang H. Determination of aristolochic acid in Chinese herbal medicine by capillary electrophoresis with laser-induced fluorescence detection. Journal of Chromatography. 2006;1105:127–34. doi: 10.1016/j.chroma.2005.07.056. [DOI] [PubMed] [Google Scholar]
- 11.Yuan J, Liu Q, Wei G, Tang F, Ding L, Yao S. Characterization and determination of six aristolochic acids and three aristololactams in medicinal plants and their preparations by high-performance liquid chromatography-photodiode array detection/electrospray ionization mass spectrometry. Rapid Communication in Mass Spectrometry. 2007;21:2332–42. doi: 10.1002/rcm.3097. [DOI] [PubMed] [Google Scholar]
- 12.Jou J, Li CP, Schelonka E, Lin C, Wu T. Analysis of the Analogues of Aristolochic Acid and Aristolactam in the Plant of Aristolochia Genus by HPLC. Journal of Food and Drug Analysis. 2004;12:40–5. [Google Scholar]
- 13.Wu T, Chan Y, Leu Y. The constituent of the Root and Stem of Aristolochia cucubitifolia Hayata and Their Biological Activity. Chemical and Pharmaceutical Bulletin. 2000;48(7):1006–9. doi: 10.1248/cpb.48.1006. [DOI] [PubMed] [Google Scholar]
- 14.Stacheno E, Andres O, Armando M, Rene M. Determination of Volatile and Semi-volatile Secondary Metabolites, and Aristolochic Acids in Aristolochia Ringens Vahl. Journal of Chromatographic Science. 2009;47(9):817–21. doi: 10.1093/chromsci/47.9.817. [DOI] [PubMed] [Google Scholar]
- 15.Watanable K, Miyakado M, Iwai T, Izumi K, Yanagi K. Isolation of Aristolochic Acid and Aristolic Acid from Cocculus triolobus DC as Potent Seed Germination Inhibitor. Agricultural and Biological Chemistry. 1988;52(4):1079–82. [Google Scholar]
- 16.Ioset JR, Raoelison GE, Hostettmann K. Detection of aristolochic acid in Chinese phytomedicines and dietary supplements used as slimming regimens. Food and Chemical Toxicology. 2003;41(1):29–36. doi: 10.1016/s0278-6915(02)00219-3. [DOI] [PubMed] [Google Scholar]
- 17.Almahy HA, Khalid HE. Chemical Examination of the Leaves Nerium oleander. International Journal of Tropical Medicine. 2006;1(2):58–62. [Google Scholar]
- 18.Seto T, Hamano T, Shioda H, Kamimura H. Analysis of Aristolochic acid and in Kampo Medicine Preparations. Journal of Health and Science. 2002;48(5):412–7. [Google Scholar]
- 19.Chinese Pharmacopoeia Committe . Chemical Industry Press. Beijing: 2005. The Chinese Pharmacopoeia; p. 25.p. 35.p. 37.p. 101. [Google Scholar]
- 20.Sathornviriyapong S, Picheansoonthon C, Tiasakul R, Tiyaworanant S, Reutrakul V. Botanical Origin Identification of Krai-Krue Herbal Plant. Kasetsart Journal (Natural Science) 2007;41:420–432. [Google Scholar]
- 21.Sun Q, Wu Y, Jia L. Quantitative Determination of Aristolochic Acid in Asarum heterotropoides Fr. Schmidt var. mandshuricum (Maxim.) Kitag. Asian Journal of Traditional Medicines. 2001:5–13. [Google Scholar]
- 22.Xie ZM, Li SX, Liao HC. Determination of aristolochic acid A in herba asari by HPLC. Central South Pharmacy. 2003;1(3):165–7. [Google Scholar]
- 23.Jiang X, Wang ZM, You LS, et al. Determination of aristolochic acid in Radix Aristolociae and Herba Asari by RP-HPLC. China Journal of Chinese Materia Medica. 2004;29(5):408–10. [PubMed] [Google Scholar]
- 24.Guan WQ, Li XM, Xiao JS. Quantitative determination of aristolochic acid in herba asari and its preparations by RPHPLC. Chinese Remedies and Clinics. 2005;5(4):283–5. [Google Scholar]
- 25.Lu J. Studies on the aristolochic acid related crude traditional Chinese medicine. Chinese Material Medica Standard. 2002;3(2):49–50. 61. [Google Scholar]
- 26.Geng LJ, Li BG. Determination of aristolochic acid A in herba asari and its preparation. Chemical Analysis and Measure. 2005;14(2):25–7. [Google Scholar]
- 27.Hashimoto K, Higuchi M, Makino B, et al. Quantitative analysis of aristolochic acids, toxic compounds, contained in some medicinal plants. Journal of Ethnopharmacology. 1999;64:185–9. doi: 10.1016/s0378-8741(98)00123-8. [DOI] [PubMed] [Google Scholar]
- 28.Ong ES, Woo SO. Determination of aristolochic acids in medicinal plants (Chinese) prepared medicine using capillary zone electrophoresis. Electrophoresis. 2001;22:2236–41. doi: 10.1002/1522-2683(20017)22:11<2236::AID-ELPS2236>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 29.Lee TY, Wu ML, Deng JF, Hwang DF. High-performance liquid chromatographic determination for aristolochic acid in medicinal plants and slimming products. Journal Chromatography B, Analytical Technologies in the Biomedical and Life Sciences. 2001;766(1):169–74. doi: 10.1016/s0378-4347(01)00416-9. [DOI] [PubMed] [Google Scholar]
- 30.Zhai ZD, Luo XP, Shi YP. Separation and determination of aristolochic acids in herbal medicines by microemulsion electrokinetic chromatography. Analytica Chimica Acta. 2006;561(1–2):119–25. [Google Scholar]
- 31.Zhang C, Wang X, Shang M, et al. Simultaneous determination of five aristolochic acids and two aristololactams in Aristolochia plants by high-performance liquid chromatography. Biomedical Chromatography. 2006b;20(4):309–18. doi: 10.1002/bmc.565. [DOI] [PubMed] [Google Scholar]
- 32.Wu KM, Farrelly JG, Upton R, Chen J. Complexities of the herbal nomenclature system in traditional Chinese medicine (TCM): lessons learned from the misuse of Aristolochia-related species and the importance of the pharmaceutical name during botanical drug product development. Phytomedicine. 2007a;14(4):273–9. doi: 10.1016/j.phymed.2006.05.009. [DOI] [PubMed] [Google Scholar]