Abstract
Rationale
The action of serotonin (5-HT) at the 5-HT2A receptor subtype is thought to be involved in cocaine-seeking behavior that is motivated by exposure to drug-associated cues and drug priming. 5-HT2A receptors are densely clustered in the ventromedial prefrontal cortex (vmPFC), an area that plays a role in mediating cocaine-seeking behavior.
Objectives
This study examined the hypothesis that M100907, a 5-HT2A receptor antagonist, infused directly in the vmPFC attenuates cue- and cocaine-primed reinstatement of cocaine-seeking behavior.
Methods
Rats trained to self-administer cocaine (0.75 mg/kg, i.v.) paired with light and tone cues underwent extinction training during which operant responses produced no consequences. Once behavior extinguished, rats were tested for reinstatement of responding elicited by either response-contingent presentations of the cocaine-paired light/tone cues or by cocaine-priming injections (10 mg/kg, i.p.) within 1 min after pretreatment with microinfusions of M100907 (0.1, 0.3, 1.0, or 1.5 μg/0.2 μl/side) into the vmPFC.
Results
Intra-vmPFC M100907 decreased cue-elicited reinstatement at the two highest doses (1.0 and 1.5 μg) but produced only a slight decrease in cocaine-primed reinstatement that was not dose dependent. The decrease in cue reinstatement was not likely due to impaired ability to respond since intra-vmPFC M100907 infusions had minimal effect on cocaine self-administration and no effect on cue-elicited sucrose-seeking behavior, or spontaneous or cocaine-induced locomotion. M100907 infusions into the adjacent anterior cingulate cortex had no effect on cue reinstatement.
Conclusions
The results suggest that the blockade of 5-HT2A receptors in the vmPFC selectively attenuates the incentive motivational effects of cocaine-paired cues.
Keywords: Cocaine, Serotonin, Prefrontal cortex, Reinstatement, Self-administration
Introduction
Cocaine craving can be triggered in several ways, including exposure to cocaine-related cues, stress, or acute administration of cocaine (Childress et al. 1988; Jaffe et al. 1989; Sinha et al. 1999). These events also trigger cocaine-seeking behavior in rats as demonstrated using the extinction/reinstatement model. In this model, animals are trained to press a lever for cocaine reinforcement. Subsequently, they undergo extinction sessions in which cocaine is withheld and operant responding under this condition is referred to as cocaine-seeking behavior. Once the behavior is extinguished, it can be reinstated by cocaine priming or by presenting cues previously associated with cocaine. Reinstatement of extinguished cocaine-seeking behavior is thought to measure incentive motivational effects of the reinstating stimuli, as well as conditioned reinforcing effects of cocaine cues (de Wit and Stewart 1981; Markou et al. 1993).
Incentive motivation for cocaine, as well as cocaine reinforcement, is modulated by serotonin (5-HT). For instance, acute administration of the 5-HT transport inhibitor, fluoxetine, decreases cocaine self-administration (Carroll et al. 1990; Peltier and Schenk 1993; Richardson and Roberts 1991) and cocaine-seeking behavior during extinction and cue reinstatement, but not during cocaine-primed reinstatement (Burmeister et al. 2003). Furthermore, chronic administration of the 5-HT reuptake inhibitor, fluoxetine, decreases sensitivity to the rewarding effects of cocaine (Lee and Kornetsky 1998) and decreases cocaine-seeking behavior during extinction (Baker et al. 2001).
Among the seven families of 5-HT receptors, the 5-HT2 family is known to play a role in cocaine-seeking behavior. Previous work with 5-HT2A selective antagonists found that peripheral administration decreases cocaine-induced locomotor activity as well as cue-elicited and cocaine-primed reinstatement, but has no effect on cocaine self-administration (Fantegrossi et al. 2002; Filip et al. 2006; Fletcher et al. 2002; Nic Dhonnchadha et al. 2009; Orejarena et al. 2010). It is believed that 5-HT action at 5-HT2A receptors may oppose its action at 5-HT2C receptors (Bubar and Cunningham 2006; Higgins and Fletcher 2003). For example, in contrast to the inhibitory effects of the 5-HT2A receptor antagonist on cocaine-seeking behavior, the 5-HT2C receptor antagonist was found to enhance cocaine-primed reinstatement of extinguished cocaine-seeking behavior, as well as cocaine self-administration and cocaine-induced locomotor activity (Fletcher et al. 2002; Nic Dhonnchadha et al. 2009). Furthermore, 5-HT2C receptor agonists inhibit cue-elicited and cocaine-primed reinstatement of extinguished cocaine-seeking behavior when injected systemically (Fletcher et al. 2002; Neisewander and Acosta 2007) or directly into the ventral medial prefrontal cortex (vmPFC), which includes the prelimbic and infralimbic subregions (Gabbott et al. 2005; Pentkowski et al. 2010).
The vmPFC plays a critical role in cue-elicited and cocaine-primed reinstatement of extinguished cocaine-seeking behavior, as well as cocaine reinforcement (Di Pietro et al. 2008; Goeders and Smith 1983; McGregor et al. 1996; Olsen and Duvauchelle 2006). Exposure to drug-associated cues causes an increase in activity-related gene expression in the infralimbic, prelimbic, anterior cingulate, and orbitofrontal subregions of the prefrontal cortex (PFC) (Hearing et al. 2008; Kufahl et al. 2009; Neisewander et al. 2000; Zavala et al. 2008). Furthermore, excitoxic lesions or reversible pharmacological inactivation of these subregions of the PFC prevents cue-elicited reinstatement of extinguished cocaine-seeking behavior (Di Pietro et al. 2006; Fuchs et al. 2004; McLaughlin and See 2003; Weissenborn et al. 1997). Conversely, cocaine injections directly into the mPFC are reinforcing (Goeders and Smith 1983; Guzman et al. 2009) and reinstate cocaine self-administration (Goeders et al. 1986). Given our recent findings that stimulation of 5-HT2C receptors in the vmPFC attenuates cue-elicited and cocaine-primed reinstatement of extinguished cocaine-seeking behavior (Pentkowski et al. 2010) together with research demonstrating opposing roles of 5-HT2C and 5-HT2A receptors in modulating cocaine-seeking behavior (Fletcher et al. 2002; Nic Dhonnchadha et al. 2009), we hypothesized that blockade of 5-HT2A receptors in the vmPFC would attenuate cue- and cocaine-primed reinstatement of extinguished cocaine-seeking behavior.
Additional rationale for this hypothesis is that 5-HT2A receptors are densely distributed throughout the cortex including the vmPFC, as well as the ventral tegmental area (VTA), substantia nigra, and the striatum which have also been implicated in addiction (Doherty and Pickel 2000; Lopez-Gimenez et al. 1997; Pompeiano et al. 1994). The highly selective 5-HT2A receptor antagonist, M100907, has been shown to decrease extracellular dopamine levels in the vmPFC and striatum when infused directly into these regions (Pehek et al. 2001; Schmidt et al. 1992, 1994). Furthermore, elevated glutamate release in the PFC is thought to excite outputs to the nucleus accumbens (NAc) resulting in potentiation of cue- and cocaine-primed reinstatement of extinguished cocaine-seeking behavior (Di Ciano and Everitt 2001; McFarland et al. 2003), and increases in glutamate in the vmPFC are attenuated by systemic injections of M100907 (Ceglia et al. 2004). These findings are consistent with the idea that 5-HT2A receptors in the vmPFC may mediate the inhibitory effects of M100907 on cue- and cocaine-primed reinstatement of extinguished cocaine-seeking behavior.
This study investigated the hypothesis that 5-HT2A receptor stimulation in the vmPFC contributes to the incentive motivational effects of cocaine-conditioned cues and cocaine itself. To test this hypothesis, we examined the effects of localized microinjections of M100907 on reinstatement of extinguished cocaine-seeking behavior elicited by cocaine-paired cues or cocaine-priming injections. The effects of M100907 on cocaine self-administration, cue-elicited reinstatement of sucrose-seeking behavior, and spontaneous and cocaine-induced locomotor activity were also examined in order to assess the specificity of the effects for cocaine-seeking behavior.
Materials and methods
Animals and housing
Adult male Sprague–Dawley rats weighing 300–325 g at the start of the experiments were used in this study. Animals were housed in a climate-controlled colony room with a 12-h reversed light/dark cycle (lights off at 7:00 a.m.) and were cared for in accordance with the “Guide for the Care and Use of Laboratory Animals” (Institute of Laboratory Animal Resources on Life Sciences, National Research Council 1996).
Surgery
Animals were handled for at least 6 days before implanting catheters into the right jugular vein. Catheters were connected to a bent 22-gauge metal cannula within a plastic screw connector (Plastics One, Roanoke, VA, USA) attached to a 10-cm silastic tube (inner diameter 0.012×outer diameter 0.025 in., Dow Corning, Midland, MI, USA) with a small ball of aquarium sealant ~4 cm from the other end. Animals were anesthetized with approximately 3% isoflurane throughout the surgery. Incisions were made in clean, shaven areas on the head to expose the skull and on the neck to expose the right jugular vein. A small incision was made in the jugular vein, the catheter was then inserted until flush with the ball of the aquarium sealant, and the catheter was secured to the vein with sutures on either side of the ball. The catheter was then pulled through a burrow made subcutaneously between the two incisions and the rat was then placed into a stereotaxic instrument. Connective tissue was removed from the skull surface and four small screws were drilled into the skull to serve as an anchor. Small holes were then drilled into the skull and stainless steel guide cannulae were lowered to a point 2 mm above the targeted site of the vmPFC (n=59) and 1 mm above the targeted site of the Cg2 region of the anterior cingulate cortex (n=9). The coordinates for the medial prefrontal cortex were selected based on previous research (Filip and Cunningham 2003; Pentkowski et al. 2010) and were as follows: AP=+2.7 and ML=+/−0.75 mm relative to bregma; DV=−3 mm from the skull surface (Paxinos and Watson 2007). The coordinates for the Cg2 were the following: AP=+2.0 and ML=+/−0.75 mm relative to bregma; DV=−3 mm from the skull surface (Paxinos and Watson 2007). The guide cannulae were secured to the skull along with the metal end of the catheter and the anchor screws using dental acrylic cement. Metal stylets were inserted with the cannulae during surgery. All incisions were sutured and treated with a topical antibiotic. Catheters were flushed with a solution of 0.1 ml saline containing heparin sodium (70 U/ml; APP Pharmaceuticals, Schaumburg, IL, USA), Abbokinase (20 mg/ml; ImaRx Therapeutics, Tucson, AZ, USA), and Timentin (66.7 mg/ml; GlaxoSmithKline, Research Triangle Park, NC, USA) for 5 days after surgery. Throughout the rest of self-administration training and testing, catheters were flushed daily with a solution containing only the Timentin and heparin sodium in order to maintain catheter patency. Animals were given at least 7 days of recovery from surgery before beginning self-administration training. Catheter patency was tested periodically by administering 0.05 ml Brevital (16.6 mg/ml, Jones Pharma Inc., St. Louis, MO, USA), which briefly anesthetized the animal only if delivered i.v.
Cocaine self-administration training
Cocaine self-administration training took place daily for 2-h sessions, 6 days per week. Animals were trained in operant conditioning chambers (28×10×20 cm; Med Associates, St Albans, VT, USA), each containing an active lever, a cue light 4 cm above the active lever, an inactive lever, a tone generator (500 Hz, 10 dB above ground noise), and a house light on the wall opposite the levers. Upon pressing the active lever to complete a schedule of reinforcement, the light and tone cues were simultaneously activated and followed 1 s later by a 0.1-ml cocaine infusion delivered over 6 s. The house light was then activated for a 20-s timeout period, during which active lever presses were recorded but had no effects. Responses on the inactive lever were recorded but had no effects.
For the first 5 days of training, all animals began on a fixed ratio (FR) 1 schedule of reinforcement with the capability to progress to a variable ratio (VR) 2, VR3, and finally VR5 schedule. After ending the session on a VR5 schedule for five consecutive days, animals then began the remaining sessions on a VR5 schedule. In this experiment, all animals were starting on a VR5 schedule by day 14 and were on a VR5 schedule exclusively for at least the last 5 days of self-administration. All animals were restricted to 16 g of food to facilitate acquisition of self-administration (Carroll et al. 1981) and remained food-restricted until they ended on a VR5 schedule for three consecutive sessions. Animals were then given food ad libitum for the rest of the experiment.
Intracranial drug infusions
M100907 (RTI International, Research Triangle Park, NC, USA) was dissolved in phosphate-buffered saline containing hydrochloric acid, titrated to pH 6.9. Microinjections were delivered over a 1-min period using a 30-gauge injector (Plastics One) connected via polyethylene 50 tubing (Becton Dickinson, Sparks, MD, USA) to a 25-μl syringe (Hamilton Co., Reno, NV, USA) housed in an infusion pump (CMA Microdialysis, North Chelmsford, MA, USA). Injection cannulae extended 2 mm below the guide cannulae for the vmPFC and 1 mm below for the Cg2. Successful infusion of the drug was confirmed by movement of an air bubble through the drug infusion line. After the infusion was complete, the injectors remained for 1 min to ensure thorough diffusion. After removing the injectors, metal stylets and caps were replaced before the animal was placed into the conditioning chamber for the test sessions.
Cocaine self-administration testing
A subset of animals (n=23) with vmPFC cannulae was tested for the effects of M100907 on cocaine self-administration once they reached a self-administration stability criterion of less than 15% variability of infusions per session for three consecutive days without any upward or downward trends. Rats were assigned to one of four dose groups (0.1, 0.3, 1.0, or 1.5 μg/0.2 μl/side). All animals were tested twice for self-administration, once with a vehicle microinjection into the vmPFC and once with their assigned dose of M100907, with order counterbalanced. At least three additional self-administration sessions were given in between each test in order to re-establish stable self-administration baseline rates. The bilateral microinjections of M100907 or vehicle into the vmPFC were administered 1 min before testing. Three additional sessions of self-administration were conducted after these tests were completed. Test sessions lasted for 2 h; however, there were no differences between results from the first hour and full two hours, so the data are reported for the first hour for consistency with reinstatement test data, which was collected for 1 h only.
Extinction phase
Upon completing self-administration training, and testing if applicable, all animals began receiving daily 1-h extinction sessions. Rats were placed into the self-administration chambers as before and lever presses were recorded, but produced no consequences (i.e., no infusions or cues were presented). Catheters were connected to the infusion lines during extinction, as well as during all reinstatement tests, even though no cocaine was infused. Extinction sessions continued for 10–14 days and until there was an 80% reduction in active lever pressing from the animals’ highest response rate during extinction or to less than 20 active lever presses.
Cue reinstatement of cocaine-seeking behavior
Following extinction training, a subset of animals with vmPFC (n=59) cannulae was assigned (or re-assigned if they had undergone self-administration testing) to one of four M100907 dose groups (0.1, 0.3, 1.0, or 1.5 μg/0.2 μl/side), counterbalanced based on the amount of cocaine intake during self-administration, as this has been shown to affect reinstatement response rates (Deroche et al. 1999; Baker et al. 2001). Another subset of animals with anterior cingulate cannulae (n=9) was assigned to receive 1.5 μg/0.2 μl/side M100907 or vehicle. Animals underwent two tests for the effects of M100907 on cue reinstatement of extinguished cocaine-seeking behavior, receiving a vehicle microinjection prior to one test and their assigned dose of M100907 prior to the other test, with the order of these pretreatments counterbalanced. Animals were given a minimum of three extinction days between tests to allow extinction baseline rates to stabilize. If animals failed to meet a reinstatement criteria of doubling extinction baseline response rates and at least ten responses on the active lever on both of the two test days, they were considered “nonreinstaters” and excluded from the analysis.
Five minutes after receiving their assigned microinjection, animals were tested for 1 h with the same stimulus complex as that paired with cocaine during training available response-contingently on an FR1 schedule; however, no cocaine was delivered during cue tests. The FR1 schedule was used in place of the VR5 training schedule because we have previously shown that under tests for cue reinstatement the FR1 schedule yields higher response rates, and thus greater sensitivity for detecting the predicted decrease, than the training schedule (Acosta et al. 2008). A noncontingent cue presentation was delivered if the animal did not receive a response-contingent cue within the first 5 min of the session to minimize the possibility that animals would fail to press the lever leaving them unaware that cues were available.
Cocaine-primed reinstatement of cocaine-seeking behavior
After the two cue reinstatement tests, a subset of animals with vmPFC cannulae (n=55) received at least five extinction sessions to re-establish a stable baseline extinction rate of responding. They were then given two tests for cocaine-primed reinstatement of extinguished cocaine-seeking behavior. Prior to one test, they received the same dose of M100907 as they had received during cue reinstatement testing (0.1, 0.3, 1.0, or 1.5 μg/0.2 μl/side). For the other test, they received a vehicle microinjection. The order of the two pretreatments was counterbalanced within a group. Five minutes after the microinjection, animals received a priming injection of cocaine (10 mg/kg, i.p.) and were then immediately placed into the conditioning chamber. Lever presses were recorded, but produced no consequences (i.e., no cues or cocaine were delivered). To control for injection stress, animals were given saline i.p. injections on the day preceding their cocaine reinstatement tests, and the average response rates during these sessions were used as the extinction baseline. Animals were given a minimum of three extinction sessions between tests to allow extinction baseline rates to stabilize. If animals failed to meet the reinstatement criteria of doubling baseline and at least ten responses on the active lever during at least one of the reinstatement tests, they were considered “nonreinstaters” and were excluded from the analysis. All animals were tested for cue reinstatement before cocaine reinstatement.
M100907-primed reinstatement of cocaine-seeking behavior
After cocaine reinstatement testing, a subset of animals with vmPFC cannulae (n=44) was given a minimum of five extinction sessions to allow extinction baseline response rates to stabilize. Animals were then given two reinstatement tests with a microinjection of M100907 (0.1, 0.3, 1.0, or 1.5 μg/0.2 μl/side) prior to one test and vehicle prior to the other test, counterbalanced for order of pretreatment. Animals received the same assigned dose of M100907 that they had received for cue- and cocaine-primed reinstatement testing. Animals were placed into the self-administration chambers 1 min after the microinjection for a 1-h test. Responding on neither the active nor the inactive lever had any scheduled consequences during these test sessions.
Cue reinstatement of sucrose-seeking behavior
After cocaine-primed reinstatement tests, a subset of animals with vmPFC cannulae (n=10) was food-restricted to approximately 18 g of food/day for 2 days prior to beginning sucrose reinforcement training. The animals were also given approximately 30 sucrose pellets (45 mg, Bio-Serv, French-town, NJ, USA) in their home cage to familiarize them with the pellets. Animals were trained in a different room with a different set of operant conditioning chambers than those used for cocaine self-administration training. These chambers were each equipped with a food pellet dispenser and a food well located between two levers. The location of the active and inactive levers from cocaine self-administration was reversed for sucrose reinforcement training. In all other respects, the training was similar to that used for cocaine self-administration. Upon completion of a schedule of reinforcement, a cue light was presented above the active lever that oscillated on for 1 s and off for 1 s for a total of 7 s, and a 45-mg sucrose pellet was delivered 1 s after the onset of the light. The house light remained on during the session aside from when the cue light was on as well as a 20-s timeout period after completion of a schedule during which active lever presses had no effects. Rats were given 30-min sessions daily beginning on an FR1 schedule of reinforcement with the capability to progress to a VR3 and then a VR5 schedule. A 30-min session was used here to avoid satiation and a reduction in responding that can occur when rats are given 1-h access to sucrose (Bizo et al. 1998). Once animals ended the session on a VR5 schedule, they began the next session on a VR3 schedule. If they ended on a VR5 schedule again, they began the next session on a VR5 schedule and stayed on this schedule for the remainder of the training. Animals remained food-restricted until they ended three sessions on a VR5 schedule, at which point they were given food ad libitum for the rest of the experiment. All animals began on a VR5 schedule during the last seven sessions of training and all were given a total of 14 sucrose training sessions.
Next, the animals underwent a total of 14 days of 1-h extinction training sessions, during which there was at least an 80% reduction in lever pressing from the animals’ highest response rate during extinction. Subsequently, animals were tested twice for cue reinstatement. They received a 1.5-μg/0.2-μl/side M100907 microinjection prior to one test and a vehicle microinjection prior to the other test, counterbalanced for order of pretreatment. Five minutes after receiving a microinjection, animals were tested for 1-h with the same stimulus complex as was paired with sucrose during training on an FR1 schedule; however, no sucrose was available. A noncontingent cue was delivered if a rat did not receive a response-contingent cue within the first 5 min of the test session. Animals were given a minimum of three extinction sessions between tests to allow extinction baseline rates to stabilize.
Locomotor activity
A subset of animals with vmPFC cannulae (n=24) that had a history of cocaine intake from the previous experiments was assigned to receive a microinjection of M100907 at an effective dose from cue-primed reinstatement testing (1.0 μg/0.2 μl/side) or vehicle. For 2 days before testing, animals received 1-h habituation sessions in the locomotor activity chambers. They were then tested twice, receiving either an injection of cocaine (10 mg/kg, i.p.) or saline, counterbalanced for order, immediately after receiving their assigned microinjection. Rats were then placed into Plexiglas locomotor chambers (44×24×20 cm high) and were tested for 90 min. A computer-automated video tracking system (Clever Systems, Reston, VA, USA) was used to measure the distance traveled by each animal. Animals were given five rest days between the two tests.
Statistical analyses
Data were analyzed using mixed-factor analyses of variance (ANOVAs) with session (e.g., extinction baseline, vehicle test, and M100907 test) as a within-subjects factor and dosage group (0.1, 0.3, 1.0, or 1.5 μg/0.2 μl/side) as a between-subject factor. A Greenhouse–Geisser correction was used to correct for heterogeneity of variance in the data. Subsequent post hoc comparisons were made using tests of simple main effects. In addition, planned t tests were used to test the prediction that cocaine-seeking behavior is attenuated after M100907 relative to vehicle pretreatment. Baseline values were calculated as the average of the two sessions that occurred before each test day (e.g., the day before cue testing with M100907 and the day before cue testing with vehicle). All statistics were run using SPSS, version 16.
Histology
Animals were deeply anesthetized with 3% isoflurane and given intracranial infusions (0.2 μl/side) of 1% methylene blue to verify cannulae placements. Animals were then decapitated and the brains were removed, cryoprotected, frozen, and stored at −20°C. Brains were sliced in coronal sections (40 μm), stained with thionin, and examined under a microscope by observers unaware of group assignment who determined the point of drug infusion.
Timeline of testing and summary of attrition
All 68 animals were trained to self-administer cocaine and underwent extinction training followed by cue reinstatement testing. Although this study was conducted using four different cohorts of rats, each cohort included rats tested at each of the M100907 doses, except for the highest dose which was included only in the last cohort. The cue reinstatement tests were the only tests that animals with Cg2 cannulae (n=9) underwent. During cue reinstatement tests, two animals with vmPFC cannulae and one animal with a Cg2 cannula failed to meet reinstatement criteria of double baseline or at least ten lever presses and were omitted from the analysis. Almost all animals with vmPFC cannulae (n=55) underwent cocaine-primed reinstatement testing following the cue reinstatement tests, and of these, two failed to meet the reinstatement criteria and were omitted from the analysis. One animal with vmPFC cannulae given the 1.5 μg dose of M100907 prior to cocaine-primed reinstatement was considered an outlier (3+ standard deviations above the mean) and was also excluded from the analysis. A subset of 23 animals with vmPFC cannulae underwent self-administration testing prior to extinction training, and a different subset of ten animals with vmPFC cannulae underwent testing for cue reinstatement of sucrose-seeking behavior. Finally, 24 animals with vmPFC cannulae were also tested for the effects of M100907 on locomotor activity after reinstatement testing had been completed. In summary, animals with vmPFC cannulae received a total of six to eight microinfusions whereas animals with anterior cingulate cannulae received a total of two microinfusions. The order of specific test types is summarized in Table 1.
Table 1.
Order of testing
| Test 1 | Test 2 | Test 3 | Test 4 |
|---|---|---|---|
| Self-administration testing (n=23)a | Cue reinstatement (n=42) | Cocaine-primed reinstatement (n=39) | M100907 reinstatement (n=22) |
| Cue reinstatement (n=22) | Cocaine-primed reinstatement (n=14) | M100907 reinstatement (n=14) | Cue reinstatement—sucrose (n=10) |
| M100907 reinstatement (n=17) | Locomotor activity (n=24) |
The number of animals shown for each test excludes those that did not reinstate or were outliers. Each animal received no more than four types of tests, and for each test type, they received a vehicle infusion prior to one test and their assigned dose of M100907 prior to the other test, with order counterbalanced, resulting in a maximum of a total of eight microinfusions
Results
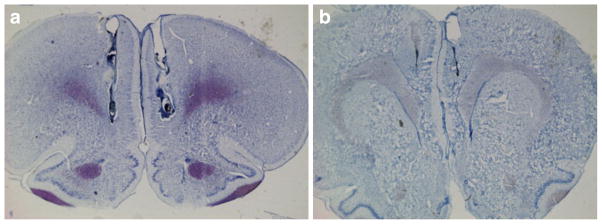
Panels a and b of Fig. 1 show the representative cannulae tip placements for each region. None of the animals had misplaced cannulae. All descriptive statistics given below are presented as the mean ± SEM.
Fig. 1.
Thionin-stained sections taken in the coronal plane demonstrating representative cannula placements in the vmPFC (a) and Cg2 (b)
Effects of M100907 on cocaine self-administration
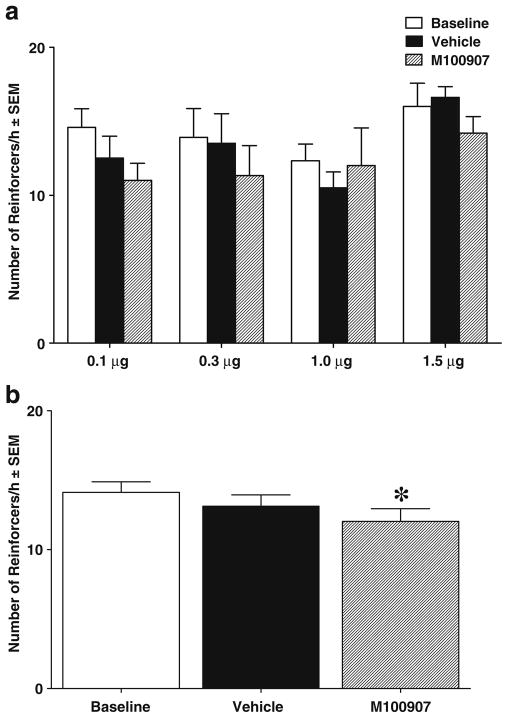
There was no significant difference between groups for total cocaine intake before testing. The average number of infusions ± SEM across the last 5 days of self-administration training for the groups ranged from 24.7±0.37 to 29.9±0.72. Figure 2 illustrates the effects of vmPFC M100907 infusions on the number of reinforcers obtained during self-administration testing. The ANOVA of the number of reinforcers/hour showed that there was a main effect of test day [F(2, 38)=5.23, P<0.05] but no main effect of group or interaction with test day on self-administration behavior. When collapsed across doses (see Fig. 2b), there was a significant decrease in reinforcers obtained on the M100907 test day versus baseline [t(22)=3.29, P<0.05].
Fig. 2.
Effects of M100907 on cocaine self-administration, expressed as the mean ± SEM number of reinforcers (infusions of cocaine with cues) received over a 1-h test session in each dosage group (a) and collapsed across dosage groups (b). Animals assigned to receive 0.1 (n=6), 0.3 (n= 6), 1.0 (n=6), or 1.5 (n=5) μg/0.2 μl/side M100907 into the vmPFC were tested on 1 day with their assigned dose (striped bar) and on another day with the vehicle (black bar), with order counterbalanced. Baselines (white bar) were calculated as the average number of reinforcers obtained during the first hour of the self-administration sessions immediately preceding each test. There was a small, but significant decrease in responding on the M100907 test day relative to baseline when collapsed across dose (i.e., main effect of test day). The asterisk (*) represents a significant difference from extinction baseline, test of simple main effects, P<0.05
Extinction
Active and inactive lever presses during the first session of extinction training are shown in Table 2. All animals had at least 13 extinction sessions before reinstatement testing began. For animals with vmPFC cannulae, ANOVAs of the number of active and inactive lever presses/hour on the first day of extinction versus the last day of extinction before testing showed main effects of day [F(1, 54)=144.70 and 18.79, respectively, P<0.01] but no dose effect or interaction with dose. Similarly, for animals with Cg2 cannulae, the ANOVA of the number of active lever presses/hour during the first extinction session versus the last extinction session before testing showed only a main effect of day [F (1, 7)=29.45, P<0.01]. In each case, the main effects indicated a significant drop in responding across training sessions. There were no significant effects for inactive lever presses/hour in animals with Cg2 cannulae, likely because initial response rates during the first sessions were low.
Table 2.
Lever presses/hour (mean ± SEM) during the first day of extinction and during the M100907 reinstatement tests
| Brain region and dose assignments | Active lever presses | Inactive lever presses |
|||
|---|---|---|---|---|---|
| First day extinction | First day extinction | Cue reinstatement | Cocaine reinstatement | M100907 reinstatement | |
| PFC | |||||
| 0.1 μg/side | 109.2±16.5 | 24.4±6.9 | 8.8±4.1 | 10.2±5.1 | 2.7±0.8 |
| 0.3 μg/side | 106.2±11.5 | 23.8±4.7 | 8.4±1.9 | 3.7±1.5 | 5.7±1.6 |
| 1.0 μg/side | 104.9±18.3 | 27.5±10.0 | 4.9±1.0 | 10.0±7.1 | 6.2±2.6 |
| 1.5 μg/side | 83.8±12.9 | 20.9±4.0 | 4.7±1.0 | 12.6±8.1 | 2.8±1.4 |
| Cg2 | |||||
| 1.5 μg/side | 70.8±13.3 | 24.8±6.3 | 12.7±3.0 | ||
Effects of M100907 on cue reinstatement of cocaine-seeking behavior
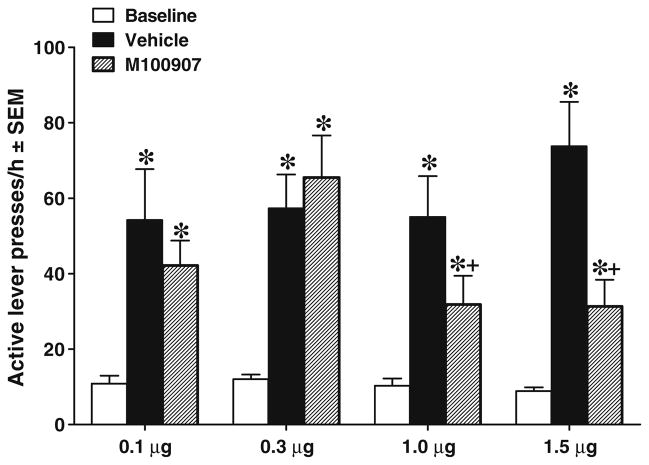
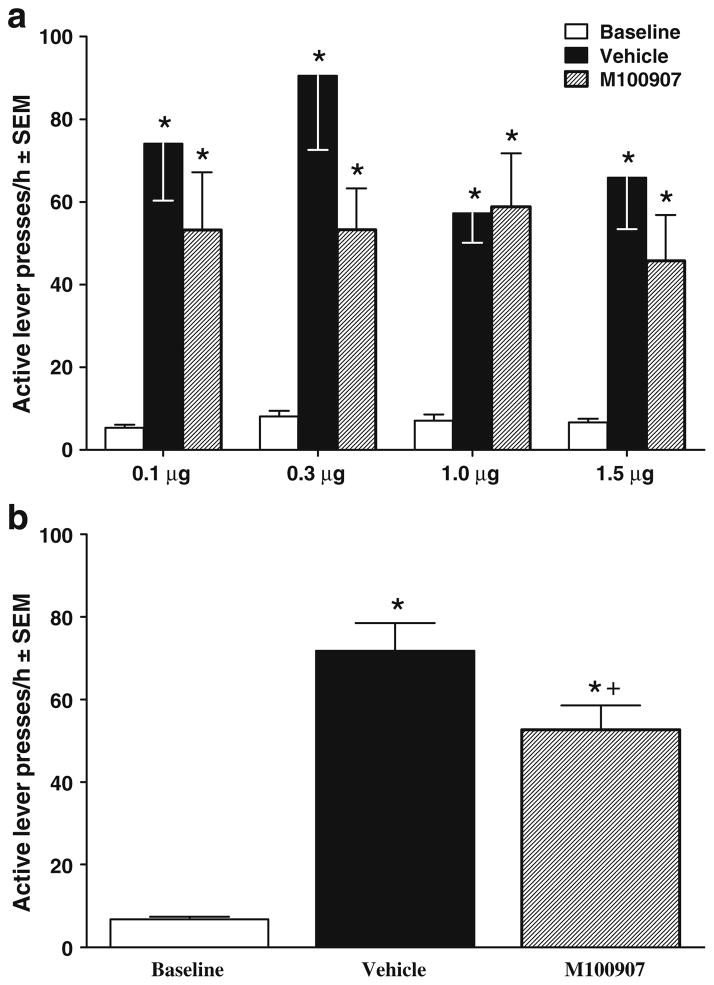
Figure 3 shows the effects of M100907 infusions into vmPFC on cue-elicited reinstatement of extinguished cocaine-seeking behavior. The ANOVA of responses/hour on the active lever for animals with vmPFC cannulae indicated a significant interaction between test session and M100907 dosage group [F(6, 104)=2.20, P<0.05]. Post hoc t tests with Bonferonni correction indicated that all groups exhibited cue reinstatement evident as an increase in responding on the vehicle pretreatment test day relative to the extinction baseline (P<0.05). In addition, M100907 pretreatment significantly decreased responding in animals receiving the 1.0- and 1.5-μg doses relative to their vehicle pretreatment [t(13)=2.00, P<0.05 and t(16)=3.56, P<0.05, respectively], demonstrating a decrease in cue reinstatement at these doses.
Fig. 3.
Effects of M100907 pretreatment on cue-elicited reinstatement of extinguished cocaine-seeking behavior when injected directly into the vmPFC, expressed as mean responses/hour ± SEM on the active lever. Animals assigned to receive 0.1 (n=13), 0.3 (n=12), 1.0 (n= 14), or 1.5 (n=17) μg/0.2 μl/side M100907 into the vmPFC were tested on 1 day with their assigned dose (striped bar) and on another day with the vehicle (black bar), with order counterbalanced. These pretreatments were infused within 1 min before placing the animals into the self-administration chambers, where light and tone cues were available response-contingently on an FR1 schedule. Baselines (white bar) were calculated as the average number of active lever presses during the extinction sessions immediately preceding each test. The asterisk (*) represents a significant difference from extinction baseline, test of simple main effects, P<0.05. The plus sign (+) represents a significant difference from vehicle pretreatment session, planned t tests, P<0.05
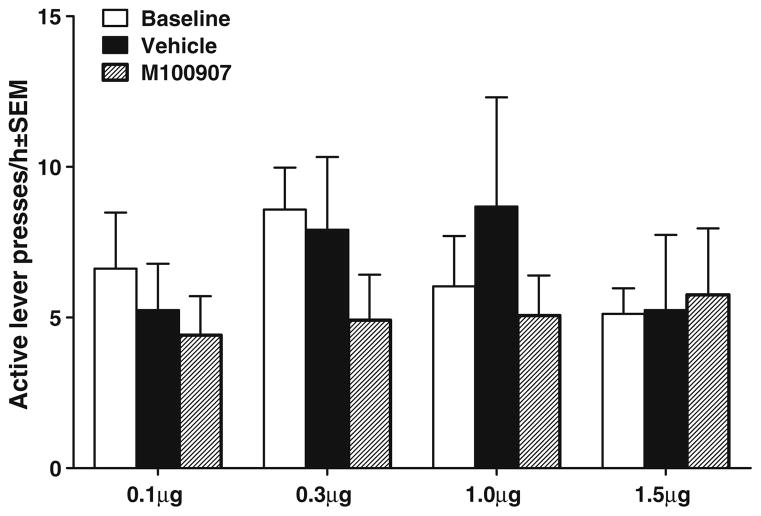
Figure 4 illustrates the effects of M100907 infusions on cue reinstatement of extinguished cocaine-seeking behavior in animals with Cg2 cannulae. The ANOVA of responses/hour on the active lever indicated a significant main effect of test day [F(2, 14)=9.242, P<0.05]. Tests of simple main effects indicated that animals exhibited cue reinstatement evident as an increase in responding on the vehicle pretreatment test day relative to the extinction baseline (P<0.05); however, there were no significant differences between vehicle and M100907 test days. Table 2 shows inactive lever presses on M100907 test days for all groups. There were no significant differences for inactive lever presses.
Fig. 4.
The effects of 1.5μg/0.2μl/side M100907 on cue-elicited reinstatement of extinguished cocaine-seeking behavior when injected directly into the Cg2 region of the anterior cingulate cortex (n=8), which served as an anatomical control site. Animals received 1.5 μg/0.2 μl/side M100907 and were tested on 1 day with their assigned dose (striped bar) and on another day with the vehicle (black bar), with order counterbalanced. These pretreatments were infused within 1 min before placing the animals into the self-administration chambers, where light and tone cues were available response-contingently on an FR1 schedule. Baselines (white bar) were calculated as the average number of active lever presses during the extinction sessions immediately preceding each test. The asterisk (*) represents a significant difference from extinction baseline, test of simple main effects, P<0.05
Effects of M100907 on cocaine-primed reinstatement of cocaine-seeking behavior
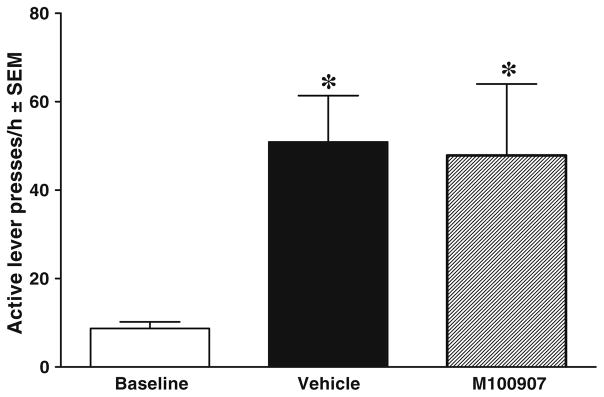
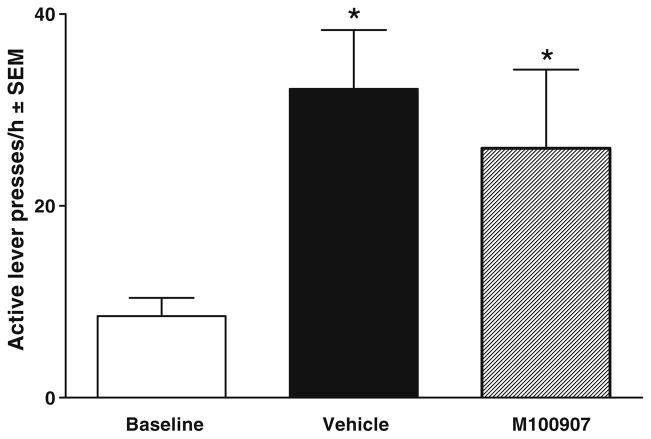
Figure 5 illustrates the effects of intra-vmPFC infusions of M100907 on cocaine-primed reinstatement of extinguished cocaine-seeking behavior. There were 2 animals out of 55 that failed to meet the reinstatement criteria and were excluded from the analyses. The ANOVA of responses/hour on the active lever indicated a significant main effect of test day [F(1.6, 76.6)=42.2, P<0.001] but no interaction with dose or main effect of dose. The planned comparisons also failed to show any differences between vehicle and M100907 test days for any of the groups. Also shown is the main effect of M100907 on cocaine-primed reinstatement collapsed across all four doses. Tests of simple main effects indicated an increase in responding relative to the extinction baseline on both the vehicle and M100907 test days (P<0.001). There was also a significant decrease in responding on M100907 test day compared to vehicle test day (P<0.05). There were no differences in inactive lever presses (see Table 2). Because these results were contrary to our hypothesis, we examined whether the effects occurred during the first 30 min when the drug effects should be maximal. Responses during the first 30 min showed a similar pattern across groups in the 1-h analysis (data not shown) with a main effect of test day [F(1.6, 78.6)=35.2, P<0.001) but no dose or interaction effects.
Fig. 5.
Effects of M100907 pretreatment on cocaine-primed reinstatement of extinguished cocaine-seeking behavior, expressed as mean responses/hour ± SEM on the active lever in each dosage group (a) and collapsed across dosage groups (b). Animals assigned to receive 0.1 (n=13), 0.3 (n=13), 1.0 (n=13), or 1.5 (n=14) μg/0.2 μl/side M100907 into the vmPFC were tested on 1 day with their assigned dose (striped bar) and on another day with the vehicle (black bar), with order counterbalanced. These pretreatments were infused immediately before the animals received the cocaine prime (10 mg/kg, i.p.) and were then immediately placed into the self-administration chambers. No cues were presented during the test sessions. Baselines (white bar) were calculated as the average number of active lever presses during the extinction sessions immediately preceding each test. When collapsed across doses (b), there was a significant increase in responding on both the vehicle and M100907 test days relative to extinction baseline and a significant decrease in responding on the M100907 test day relative to the vehicle test day. The star (★) represents a significant difference from extinction baseline, test of simple main effects, P<0.05. The plus sign (+) represents a significant difference from vehicle test day, test of simple main effects, P<0.05
Effects of M100907 on reinstatement of cocaine-seeking behavior
Figure 6 illustrates that M100907 priming injections infused into vmPFC prior to testing failed to alter responding relative to extinction baseline. The ANOVA of responses/hour indicated that there were no significant effects on response rates on either the active or inactive levers (see Table 2).
Fig. 6.
Effects of M100907 priming injections on reinstatement of extinguished cocaine-seeking behavior, expressed as mean responses/hour ± SEM on the active lever. Animals received 0.1 (n=13), 0.3 (n= 13), 1.0 (n=14), or 1.5 (n=13) μg/0.2 μl/side M100907 infused into the vmPFC on one test day (striped bar) and vehicle on another day (black bar), with order counterbalanced. They were placed into the self-administration chambers immediately after these pretreatments. Baselines (white bar) were calculated as the average number of active lever presses obtained during the extinction sessions immediately preceding each test. No cues were presented during the test sessions
Effects of M100907 on cue reinstatement of sucrose-seeking behavior
Figure 7 shows the effects of intra-vmPFC infusions of 1.5 μg M100907 on reinstatement of sucrose-seeking behavior. The ANOVA of responses/hour on the active lever indicated a significant main effect of test day [F(2, 18)=6.87, P<0.05] but no interaction with dose or main effect of dose. Tests of simple main effects indicated that animals exhibited cue reinstatement evident as an increase in responding on the vehicle pretreatment test day relative to the extinction baseline (P<0.05); however, reinstatement was also evident on the M100907 test day and there was no significant difference between vehicle and M100907 test days. There were no differences in inactive lever presses (see Table 2).
Fig. 7.
Effects of M100907 pretreatment on cue reinstatement of sucrose-seeking behavior (n=10), expressed as mean responses/hour ± SEM on the active lever. Animals were tested on 1 day with 1.5 μg/side M100907 (striped bar) and on another day with the vehicle (black bar), with order counterbalanced. These pretreatments were infused within 1 min before placing the animals into the self-administration chambers, where light and tone cues were available response-contingently on an FR1 schedule. Baselines (white bar) were calculated as the average number of active lever presses obtained during the extinction sessions immediately preceding each test. There was a significant increase in responding on both the vehicle and M100907 test days relative to extinction baseline. The star (★) represents a significant difference from extinction baseline, test of simple main effects, P<0.05
Effects of M100907 on locomotor activity
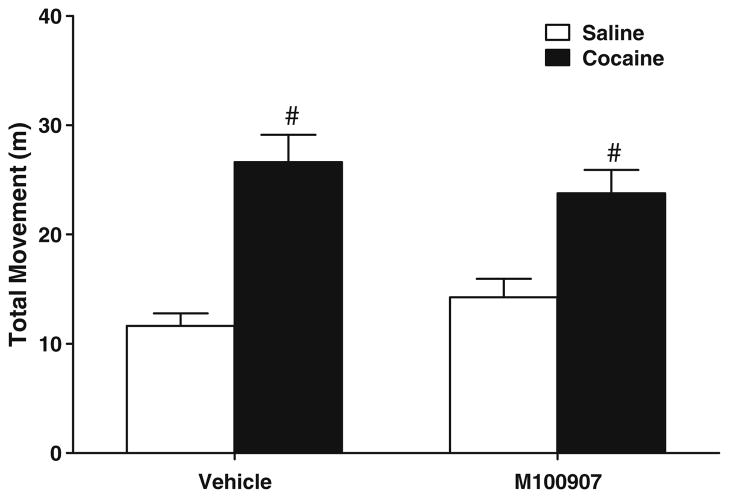
Figure 8 shows the effects of 1.0 μg/0.2 μl/side M100907 infused into vmPFC on spontaneous and cocaine-induced locomotor activity. The ANOVA indicated a significant effect of test session [F(1, 22)=76.292, P<0.05] but no M100907 dose effect or test by dose interaction. Tests of simple main effects indicated that animals exhibited significantly more distance traveled when given cocaine as compared to saline, but there was no difference in distance traveled in animals pretreated with vehicle versus M100907.
Fig. 8.
Effects of M100907 pretreatment on locomotor activity, expressed as total movement (m) during a 90-min test session. Animals received either vehicle or 1.0 μg/side M100907 into the vmPFC (n=12/group) and were given an injection 1 min later of cocaine (10 mg/kg, i.p.) for one test and saline (10 mg/kg, i.p.) for the other test, with order counterbalanced. They were then immediately placed into the test chambers. A pound sign (#) indicates a difference from saline test day, ANOVA main effect, P<0.05
Discussion
The present findings are the first to demonstrate that a 5-HT2A receptor antagonist infused into the vmPFC dose-dependently decreases cue-elicited reinstatement of extinguished cocaine-seeking behavior. These results are consistent with the findings that peripheral injections of M100907 attenuate cue-elicited reinstatement (Nic Dhonnchadha et al. 2009). Furthermore, the M100907-induced attenuation of cue reinstatement supports our hypothesis that stimulation of 5-HT2A receptors in the vmPFC modulates incentive motivational effects of cocaine-paired cues. In contrast to the effects on cue reinstatement, intra-vmPFC infusions of M100907 failed to dose-dependently alter cocaine self-administration or cocaine-primed reinstatement. There was a main effect of test day in both cases reflecting a mild attenuation of these behaviors when collapsed across dose; however, there was no significant difference between the numbers of reinforcers obtained or the number of active lever presses following vehicle pretreatment relative to M100907 pretreatment at any given dose of M100907. The lack of a dose-dependent effect suggests that the attenuation of both cocaine self-administration and cocaine-primed reinstatement when the data were collapsed across dose (i.e., main effect of test day) is not likely due to antagonism of 5-HT2A receptors.
It is unlikely that the decrease in cue reinstatement was due to M100907 interfering with motor function. For instance, intra-vmPFC infusions of M100907 did not alter spontaneous or cocaine-induced locomotor activity at the 1.0-μg dose, which was a dose that effectively reduced cue reinstatement. Furthermore, the higher effective dose of 1.5 μg/side did not affect cue reinstatement of sucrose-seeking behavior. This finding further mitigates a possible motor confound and also suggests that memory for cues was intact; however, this finding does not rule out the possibility that the vmPFC is involved in the incentive motivational effects of other reinforcers given the difficulties of equating the motivational value of the different reinforcers.
The effect of M100907 on cue reinstatement appeared to be region-specific given that the 1.5-μg/side dose was only effective when injected into the vmPFC and not when injected into the neighboring Cg2 subregion of the anterior cingulate cortex. It is somewhat surprising that no effect was observed in the Cg2 since this subregion of the PFC is thought to be involved in cocaine-seeking behavior (Neisewander et al. 2000; Weissenborn et al. 1997). Again, the finding does not rule out the possibility that Cg2 5-HT2A receptors are involved in cocaine-seeking behavior, but rather suggests that the effect observed in vmPFC was not likely due to spread to a neighboring region. Interestingly, the pattern of results is consistent with previous research from our laboratory demonstrating that a 5-HT2C receptor agonist effectively attenuates cue reinstatement when infused into the vmPFC, but not when injected into Cg2 (Pentkowski et al. 2010). In the present study, we targeted the infralimbic subregion of the PFC which is directly below the prelimbic subregion, and together they comprise the vmPFC (Gabbott et al. 2005). It seems likely that M100907 diffused upward from the site of infusion such that 5-HT2A receptors in both subregions may have been affected. In our previous study of 5-HT2C receptors, we found the effects in animals with cannulae in either the prelimbic or infralimbic subregion, suggesting that 5-HT innervation of both areas likely modulates cocaine-seeking behavior.
The M100907 attenuation of cue reinstatement was likely due to the antagonism of 5-HT2A receptors in the vmPFC. M100907 has >100-fold selectivity for 5-HT2A versus 5-HT2C receptors (Kehne et al. 1996), and several studies have demonstrated that doses of 0.005–0.4 mg/kg reverse the behavioral effects of 5-HT2A, but not 5-HT2C, receptor agonists (Dekeyne et al. 1999; Gresch et al. 2007; Hitchcock et al. 1997; McCreary et al. 2003; Vickers et al. 2001; Wettstein et al. 1999). We did not investigate the effects of a 5-HT2A agonist in the present study because when administered into the vmPFC, these drugs cause head shakes that may reflect hallucinogenic effects and would likely interfere with reinstatement. If M100907 was acting on another receptor subtype, the most likely candidate is the closely related 5-HT2C receptor. This is unlikely, however, because peripheral injections of the selective 5-HT2C receptor antagonist SB242084 do not affect cue reinstatement (Burbassi and Cervo 2008; Burmeister et al. 2004), and in fact, reverse the attenuation of cue reinstatement observed with 5-HT2C receptor agonists and enhance cocaine-primed reinstatement (Burmeister et al. 2004; Fletcher et al. 2002; Neisewander and Acosta 2007). Furthermore, SB242084 infused into the vmPFC has no effect on cue- or cocaine-primed reinstatement whereas the selective 5-HT2C agonist MK212 attenuates both behaviors (Pentkowski et al. 2010). Finally, if M100907 infusions had antagonized 5-HT2C receptors in this study, that may explain why only attenuation, and not complete reversal, of cue reinstatement was observed. Specifically, as the dose of M100907 is increased, antagonism of additional 5-HT2A receptor may be accompanied by antagonism of 5-HT2C receptors with the latter functionally opposing any additional reduction of cue reinstatement by 5-HT2A receptor antagonism.
In contrast to the predicted lack of effect of intra-vmPFC M100907 on cocaine self-administration, which was based on previous research demonstrating that systemic administration does not affect this behavior, the lack of effect of intra-vmPFC infusions on cocaine-primed reinstatement was unexpected given that this behavior is attenuated by systemic administration (Nic Dhonnchadha et al. 2009; Fletcher et al. 2002). As negative findings must always be interpreted with caution, we cannot rule out the possibility that 5-HT2A receptors in the vmPFC are involved in cocaine-primed reinstatement. Such an effect may require a higher dose of M100907 than that needed for attenuating cue reinstatement because endogenous 5-HT levels are likely higher following cocaine priming relative to cues. Note, however, that doses even lower than those used in the present study (0.1–0.3μg/0.2μl/side) attenuate cocaine-induced (10 mg/kg) locomotor activity when infused into the VTA (McMahon et al. 2001). Furthermore, infusions of 0.1–0.5μg M100907 into the NAc decrease impulsive responding on a five-choice serial reaction time task with a possible nonspecific impairment at the highest dose (Robinson et al. 2008), suggesting that the effects of higher doses are difficult to interpret. We also argue that it is unlikely that brain damage or tolerance with repeated M100907 administration occurred based on our unpublished observation that the 1.0-μg dose significantly attenuated headshakes induced by the 5-HT2A receptor agonist DOI after animals had undergone M100907-, cue-, and cocaine-primed reinstatement testing. We also found no effect of M100907 on cocaine-primed reinstatement during the first 30 min of testing, so it is unlikely that M100907 effects were obscured by testing beyond a period of maximal drug levels. Thus, presently it appears that at the very least, 5-HT2A receptors in vmPFC are more sensitive to modulating motivational effects of cocaine-associated cues relative to other cocaine-related behaviors.
The attenuation of cue reinstatement is consistent with literature suggesting that the mPFC plays a critical role in drug abuse-related behavior, including the incentive motivational effects of drug cues (Childress et al. 1999; Jentsch and Taylor 1999; Volkow et al. 2002). Although baseline activity of the mPFC may be reduced in drug-dependent rodents and humans relative to controls, they exhibit increased activity in the mPFC when exposed to drug-associated cues (Childress et al. 1999; Ciccocioppo et al. 2001; Grant et al. 1996; Maas et al. 1998; Neisewander et al. 2000). Furthermore, other pharmacological manipulations in the mPFC suggest that it is involved in the incentive motivational effects of drug-paired cues (Bossert et al. 2005; Kalivas and McFarland 2003; McLaughlin and See 2003). Indeed, it has been hypothesized that there is a “final common pathway” for the neurocircuitry involved in stress-, cocaine-, and cue-primed reinstatement (Capriles et al. 2003; Kalivas 2008; Neisewander et al. 2000) that likely involves glutamatergic projections from the mPFC to the NAc (Feltenstein and See 2008; Kalivas and McFarland 2003; Kalivas et al. 2006; Shaham et al. 2003). To the extent that differential effects of 5-HT2A receptor manipulations in the vmPFC on cue- and cocaine-primed reinstatement are confirmed, the findings would suggest that there may be independent circuitries involving the vmPFC that mediate the effects of cue- and cocaine-primed reinstatement.
Future research will be needed to determine the specific neuroanatomical pathways involved in the attenuation of cue-elicited reinstatement of extinguished cocaine-seeking behavior that we observed in this study. In the mPFC, 5-HT2A receptors are located postsynaptically, primarily on apical dendrites of pyramidal neurons with a minority found on GABA interneurons (Cornea-Hebert et al. 1999; Hamada et al. 1998; Jakab and Goldman-Rakic 1998; Santana et al. 2004; Willins et al. 1997). 5-HT has an excitatory effect on glutamate release from pyramidal cells originating in the mPFC (Aghajanian and Marek 1997). Studies showing activation of the mPFC by cocaine-paired cues (Childress et al. 1999; Grant et al. 1996; Kilts et al. 2001) and attenuation of cue-primed reinstatement of extinguished cocaine-seeking behavior by an AMPA antagonist in the NAc core (Di Ciano and Everitt 2001) have led to the hypothesis that glutamate transmission from the PFC to NAc core is involved in reinstatement (McFarland et al. 2003). Therefore, one potential effect of blocking 5-HT2A receptors in the vmPFC is a decrease in the activity of glutamatergic projection neurons to the VTA and NAc (Pierce and Vanderschuren 2010; Sesack and Carr 2002).
Another potential mechanism for M100907 effects is via modulation of dopamine, as 5-HT2A receptor antagonism decreases mesocortical dopamine release (Alex and Pehek 2007) and is thought to inhibit excitatory inputs from the mPFC to the VTA (Vazquez-Borsetti et al. 2009). Furthermore, systemic injection studies have shown that M100907 attenuates DOI-elicited increases in dopamine in the mPFC (Gobert and Millan 1999; Pehek et al. 2001) and dorsal raphe stimulated release of dopamine in the NAc (De Deurwaerdere and Spampinato 1999). These findings lead to the conclusion that 5-HT2A receptors in the mPFC modulate phasic, but not tonic, dopamine release in the mPFC and striatum (De Deurwaerdere and Spampinato 1999; Gobert and Millan 1999; Lucas and Spampinato 2000; Zhang et al. 2000). Blocking 5-HT2A receptors in the vmPFC may decrease activation of excitatory projections to the VTA, thereby attenuating glutamate receptor stimulation in the VTA and decreasing dopamine release in the PFC, NAc, and amygdala (Alex and Pehek 2007; Kalivas et al. 2006; See 2005). While we can only speculate on the brain regions more involved in mediating the effects of cocaine priming, it seems likely that the VTA is involved, as this region has been implicated with repeated cocaine injections (Kalivas and Duffy 1998; Parsons and Justice 1993).
Overall, our results indicate that selectively blocking 5-HT2A receptors in the vmPFC dose-dependently reduced cue-elicited reinstatement of drug-seeking behavior. The lack of a dose-dependent effect of M100907 on cocaine self-administration and cocaine-primed reinstatement of extinguished cocaine-seeking behavior suggests that the attenuation of these behaviors regardless of M100907 dose is not likely receptor-mediated. Further, M100907 in the vmPFC did not affect locomotor activity or cue reinstatement of sucrose-seeking behavior, suggesting that the effects on cue reinstatement were not due to general performance or a memory deficit. Therefore, we conclude that 5-HT2A receptors in the vmPFC play a role in mediating the incentive motivational effects of cocaine-paired cues. The findings contribute novel information about the neural circuitry underlying the incentive motivational effects of cocaine cues and further suggest that 5-HT2A receptor blockade may be a potential therapeutic mechanism to treat cocaine craving and relapse.
Acknowledgments
The authors thank Suzanne Weber, Lindsey Robertson, Kenneth Thiel, Timothy Cheung, Natalie Peartree, Ryan Bastle, Marisa Ostos, and Anthony Shepard for their expert technical and surgical assistance. This research was supported by NIDA grant R01DA11064. The experiments performed comply with the current laws of the United States of America.
Footnotes
Conflict of interest None of the authors have any financial conflicts to disclose.
Contributor Information
Lara A. Pockros, Department of Psychology, Arizona State University, 950 S. McAllister, Tempe, AZ 85287, USA
Nathan S. Pentkowski, School of Life Sciences, Arizona State University, 427 East Tyler Mall, Tempe, AZ 85287, USA
Sarah E. Swinford, Department of Psychology, Arizona State University, 950 S. McAllister, Tempe, AZ 85287, USA
Janet L. Neisewander, Email: Janet.Neisewander@asu.edu, School of Life Sciences, Arizona State University, 427 East Tyler Mall, Tempe, AZ 85287, USA
References
- Acosta JI, Thiel KJ, Sanabria F, Browning JR, Neisewander JL. Effect of schedule of reinforcement on cue-elicited reinstatement of cocaine-seeking behavior. Behav Pharmacol. 2008;19:129–136. doi: 10.1097/FBP.0b013e3282f62c89. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Marek GJ. Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology. 1997;36:589–599. doi: 10.1016/s0028-3908(97)00051-8. [DOI] [PubMed] [Google Scholar]
- Alex KD, Pehek EA. Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol Ther. 2007;113:296–320. doi: 10.1016/j.pharmthera.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, Tran-Nguyen TL, Fuchs RA, Neisewander JL. Influence of individual differences and chronic fluoxetine treatment on cocaine-seeking behavior in rats. Psychopharmacol Berl. 2001;155:18–26. doi: 10.1007/s002130000676. [DOI] [PubMed] [Google Scholar]
- Bizo LA, Bogdanov SV, Killeen PR. Satiation causes within-session decreases in instrumental responding. J Exp Psychol Anim Behav Process. 1998;24:439–452. [PubMed] [Google Scholar]
- Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. Eur J Pharmacol. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA. Serotonin 5-HT2A and 5-HT2C receptors as potential targets for modulation of psychostimulant use and dependence. Curr Top Med Chem. 2006;6:1971–1985. doi: 10.2174/156802606778522131. [DOI] [PubMed] [Google Scholar]
- Burbassi S, Cervo L. Stimulation of serotonin2C receptors influences cocaine-seeking behavior in response to drug-associated stimuli in rats. Psychopharmacol Berl. 2008;196:15–27. doi: 10.1007/s00213-007-0916-7. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Lungren EM, Neisewander JL. Effects of fluoxetine and d-fenfluramine on cocaine-seeking behavior in rats. Psychopharmacol Berl. 2003;168:146–154. doi: 10.1007/s00213-002-1307-8. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Lungren EM, Kirschner KF, Neisewander JL. Differential roles of 5-HT receptor subtypes in cue and cocaine reinstatement of cocaine-seeking behavior in rats. Neuropsychopharmacology. 2004;29:660–668. doi: 10.1038/sj.npp.1300346. [DOI] [PubMed] [Google Scholar]
- Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacol Berl. 2003;168:66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- Carroll ME, France CP, Meisch RA. Intravenous self-administration of etonitazene, cocaine and phencyclidine in rats during food deprivation and satiation. J Pharmacol Exp Ther. 1981;217:241–247. [PubMed] [Google Scholar]
- Carroll ME, Lac ST, Asencio M, Kragh R. Fluoxetine reduces intravenous cocaine self-administration in rats. Pharmacol Biochem Behav. 1990;35:237–244. doi: 10.1016/0091-3057(90)90232-7. [DOI] [PubMed] [Google Scholar]
- Ceglia I, Carli M, Baviera M, Renoldi G, Calcagno E, Invernizzi RW. The 5-HT receptor antagonist M100,907 prevents extracellular glutamate rising in response to NMDA receptor blockade in the mPFC. J Neurochem. 2004;91:189–199. doi: 10.1111/j.1471-4159.2004.02704.x. [DOI] [PubMed] [Google Scholar]
- Childress AR, McLellan AT, Ehrman R, O’Brien CP. Classically conditioned responses in opioid and cocaine dependence: a role in relapse? NIDA Res Monogr. 1988;84:25–43. [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D (1) antagonists. Proc Natl Acad Sci USA. 2001;98:1976–1981. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornea-Hebert V, Riad M, Wu C, Singh SK, Descarries L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol. 1999;409:187–209. doi: 10.1002/(sici)1096-9861(19990628)409:2<187::aid-cne2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- De Deurwaerdere P, Spampinato U. Role of serotonin(2A) and serotonin(2B/2C) receptor subtypes in the control of accumbal and striatal dopamine release elicited in vivo by dorsal raphe nucleus electrical stimulation. J Neurochem. 1999;73:1033–1042. doi: 10.1046/j.1471-4159.1999.0731033.x. [DOI] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacol Berl. 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- Dekeyne A, Girardon S, Millan MJ. Discriminative stimulus properties of the novel serotonin (5-HT)2C receptor agonist, RO 60-0175: a pharmacological analysis. Neuropharmacology. 1999;38:415–423. doi: 10.1016/s0028-3908(98)00203-2. [DOI] [PubMed] [Google Scholar]
- Deroche V, Le Moal M, Piazza PV. Cocaine self-administration increases the incentive motivational properties of the drug in rats. Eur J Neurosci. 1999;11:2731–2736. doi: 10.1046/j.1460-9568.1999.00696.x. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Dissociable effects of antagonism of NMDA and AMPA/KA receptors in the nucleus accumbens core and shell on cocaine-seeking behavior. Neuropsychopharmacology. 2001;25:341–360. doi: 10.1016/S0893-133X(01)00235-4. [DOI] [PubMed] [Google Scholar]
- Di Pietro NC, Black YD, Kantak KM. Context-dependent prefrontal cortex regulation of cocaine self-administration and reinstatement behaviors in rats. Eur J Neurosci. 2006;24:3285–3298. doi: 10.1111/j.1460-9568.2006.05193.x. [DOI] [PubMed] [Google Scholar]
- Di Pietro NC, Mashhoon Y, Heaney C, Yager LM, Kantak KM. Role of dopamine D1 receptors in the prefrontal dorsal agranular insular cortex in mediating cocaine self-administration in rats. Psychopharmacol Berl. 2008;200:81–91. doi: 10.1007/s00213-008-1149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty MD, Pickel VM. Ultrastructural localization of the serotonin 2A receptor in dopaminergic neurons in the ventral tegmental area. Brain Res. 2000;864:176–185. doi: 10.1016/s0006-8993(00)02062-x. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Ullrich T, Rice KC, Woods JH, Winger G. 3,4-Methylenedioxymethamphetamine (MDMA, “ecstasy”) and its stereoisomers as reinforcers in rhesus monkeys: serotonergic involvement. Psychopharmacol Berl. 2002;161:356–364. doi: 10.1007/s00213-002-1021-6. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. The neurocircuitry of addiction: an overview. Br J Pharmacol. 2008;154:261–274. doi: 10.1038/bjp.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip M, Cunningham KA. Hyperlocomotive and discriminative stimulus effects of cocaine are under the control of serotonin(2C) (5-HT(2C)) receptors in rat prefrontal cortex. J Pharmacol Exp Ther. 2003;306:734–743. doi: 10.1124/jpet.102.045716. [DOI] [PubMed] [Google Scholar]
- Filip M, Bubar MJ, Cunningham KA. Contribution of serotonin (5-HT) 5-HT2 receptor subtypes to the discriminative stimulus effects of cocaine in rats. Psychopharmacol Berl. 2006;183:482–489. doi: 10.1007/s00213-005-0197-y. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Grottick AJ, Higgins GA. Differential effects of the 5-HT(2A) receptor antagonist M100907 and the 5-HT(2C) receptor antagonist SB242084 on cocaine-induced locomotor activity, cocaine self-administration and cocaine-induced reinstatement of responding. Neuropsychopharmacology. 2002;27:576–586. doi: 10.1016/S0893-133X(02)00342-1. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MP, See RE. Differential involvement of orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. J Neurosci. 2004;24:6600–6610. doi: 10.1523/JNEUROSCI.1924-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Gobert A, Millan MJ. Serotonin (5-HT)2A receptor activation enhances dialysate levels of dopamine and noradrenaline, but not 5-HT, in the frontal cortex of freely-moving rats. Neuropharmacology. 1999;38:315–317. doi: 10.1016/s0028-3908(98)00188-9. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Smith JE. Cortical dopaminergic involvement in cocaine reinforcement. Science. 1983;221:773–775. doi: 10.1126/science.6879176. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Dworkin SI, Smith JE. Neuropharmacological assessment of cocaine self-administration into the medial prefrontal cortex. Pharmacol Biochem Behav. 1986;24:1429–1440. doi: 10.1016/0091-3057(86)90206-6. [DOI] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci USA. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresch PJ, Barrett RJ, Sanders-Bush E, Smith RL. 5-Hydroxytryptamine (serotonin)2A receptors in rat anterior cingulate cortex mediate the discriminative stimulus properties of d-lysergic acid diethylamide. J Pharmacol Exp Ther. 2007;320:662–669. doi: 10.1124/jpet.106.112946. [DOI] [PubMed] [Google Scholar]
- Guzman D, Moscarello JM, Ettenberg A. The effects of medial prefrontal cortex infusions of cocaine in a runway model of drug self-administration: evidence of reinforcing but not anxiogenic actions. Eur J Pharmacol. 2009;605:117–122. doi: 10.1016/j.ejphar.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S, Senzaki K, Hamaguchi-Hamada K, Tabuchi K, Yamamoto H, Yamamoto T, Yoshikawa S, Okano H, Okado N. Localization of 5-HT2A receptor in rat cerebral cortex and olfactory system revealed by immunohistochemistry using two antibodies raised in rabbit and chicken. Brain Res Mol Brain Res. 1998;54:199–211. doi: 10.1016/s0169-328x(97)00322-7. [DOI] [PubMed] [Google Scholar]
- Hearing MC, Miller SW, See RE, McGinty JF. Relapse to cocaine seeking increases activity-regulated gene expression differentially in the prefrontal cortex of abstinent rats. Psychopharmacol Berl. 2008;198:77–91. doi: 10.1007/s00213-008-1090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins GA, Fletcher PJ. Serotonin and drug reward: focus on 5-HT2C receptors. Eur J Pharmacol. 2003;480:151–162. doi: 10.1016/j.ejphar.2003.08.102. [DOI] [PubMed] [Google Scholar]
- Hitchcock JM, Lister S, Fischer TR, Wettstein JG. Disruption of latent inhibition in the rat by the 5-HT2 agonist DOI: effects of MDL 100,907, clozapine, risperidone and haloperidol. Behav Brain Res. 1997;88:43–49. doi: 10.1016/s0166-4328(97)02315-2. [DOI] [PubMed] [Google Scholar]
- Jaffe JH, Cascella NG, Kumor KM, Sherer MA. Cocaine-induced cocaine craving. Psychopharmacol Berl. 1989;97:59–64. doi: 10.1007/BF00443414. [DOI] [PubMed] [Google Scholar]
- Jakab RL, Goldman-Rakic PS. 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proc Natl Acad Sci USA. 1998;95:735–740. doi: 10.1073/pnas.95.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacol Berl. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Addiction as a pathology in prefrontal cortical regulation of corticostriatal habit circuitry. Neurotox Res. 2008;14:185–189. doi: 10.1007/BF03033809. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Repeated cocaine administration alters extracellular glutamate in the ventral tegmental area. J Neurochem. 1998;70:1497–1502. doi: 10.1046/j.1471-4159.1998.70041497.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacol Berl. 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Peters J, Knackstedt L. Animal models and brain circuits in drug addiction. Mol Interv. 2006;6:339–344. doi: 10.1124/mi.6.6.7. [DOI] [PubMed] [Google Scholar]
- Kehne JH, Baron BM, Carr AA, Chaney SF, Elands J, Feldman DJ, Frank RA, van Giersbergen PL, McCloskey TC, Johnson MP, McCarty DR, Poirot M, Senyah Y, Siegel BW, Widmaier C. Preclinical characterization of the potential of the putative atypical antipsychotic MDL 100,907 as a potent 5-HT2A antagonist with a favorable CNS safety profile. J Pharmacol Exp Ther. 1996;277:968–981. [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Kufahl PR, Zavala AR, Singh A, Thiel KJ, Dickey ED, Joyce JN, Neisewander JL. c-Fos expression associated with reinstatement of cocaine-seeking behavior by response-contingent conditioned cues. Synapse. 2009;63:823–835. doi: 10.1002/syn.20666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Kornetsky C. Acute and chronic fluoxetine treatment decreases the sensitivity of rats to rewarding brain stimulation. Pharmacol Biochem Behav. 1998;60:539–544. doi: 10.1016/s0091-3057(98)00020-3. [DOI] [PubMed] [Google Scholar]
- Lopez-Gimenez JF, Mengod G, Palacios JM, Vilaro MT. Selective visualization of rat brain 5-HT2A receptors by autoradiography with [3H]MDL 100,907. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:446–454. doi: 10.1007/pl00005075. [DOI] [PubMed] [Google Scholar]
- Lucas G, Spampinato U. Role of striatal serotonin2A and serotonin2C receptor subtypes in the control of in vivo dopamine outflow in the rat striatum. J Neurochem. 2000;74:693–701. doi: 10.1046/j.1471-4159.2000.740693.x. [DOI] [PubMed] [Google Scholar]
- Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW, Kukes TJ, Renshaw PF. Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Am J Psychiatry. 1998;155:124–126. doi: 10.1176/ajp.155.1.124. [DOI] [PubMed] [Google Scholar]
- Markou A, Weiss F, Gold LH, Caine SB, Schulteis G, Koob GF. Animal models of drug craving. Psychopharmacol Berl. 1993;112:163–182. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- McCreary AC, Filip M, Cunningham KA. Discriminative stimulus properties of (+/−)-fenfluramine: the role of 5-HT2 receptor subtypes. Behav Neurosci. 2003;117:212–221. doi: 10.1037/0735-7044.117.2.212. [DOI] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor A, Baker G, Roberts DC. Effect of 6-hydroxydopamine lesions of the medial prefrontal cortex on intravenous cocaine self-administration under a progressive ratio schedule of reinforcement. Pharmacol Biochem Behav. 1996;53:5–9. doi: 10.1016/0091-3057(95)00192-1. [DOI] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacol Berl. 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- McMahon LR, Filip M, Cunningham KA. Differential regulation of the mesoaccumbens circuit by serotonin 5-hydroxytryptamine (5-HT)2A and 5-HT2C receptors. J Neurosci. 2001;21:7781–7787. doi: 10.1523/JNEUROSCI.21-19-07781.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Coucil. Guide for the care and use of laboratory animals. National Academy Press; Washington, D.C: 1996. [Google Scholar]
- Neisewander JL, Acosta JI. Stimulation of 5-HT2C receptors attenuates cue and cocaine-primed reinstatement of cocaine-seeking behavior in rats. Behav Pharmacol. 2007;18:791–800. doi: 10.1097/FBP.0b013e3282f1c94b. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nic Dhonnchadha BA, Fox RG, Stutz SJ, Rice KC, Cunningham KA. Blockade of the serotonin 5-HT2a receptor suppresses cue-evoked reinstatement of cocaine-seeking behavior in a rat self-administration model. Behav Neurosci. 2009;123:382–396. doi: 10.1037/a0014592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen CM, Duvauchelle CL. Prefrontal cortex D1 modulation of the reinforcing properties of cocaine. Brain Res. 2006;1075:229–235. doi: 10.1016/j.brainres.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Orejarena MJ, Lanfumey L, Maldonado R, Robledo P. Involvement of 5-HT2A receptors in MDMA reinforcement and cue-induced reinstatement of MDMA-seeking behaviour. Int J Neuropsychopharmacol. 2010:1–14. doi: 10.1017/S1461145710001215. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Justice JB., Jr Serotonin and dopamine sensitization in the nucleus accumbens, ventral tegmental area, and dorsal raphe nucleus following repeated cocaine administration. J Neurochem. 1993;61:1611–1619. doi: 10.1111/j.1471-4159.1993.tb09794.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6. Academic; San Diego: 2007. [DOI] [PubMed] [Google Scholar]
- Pehek EA, McFarlane HG, Maguschak K, Price B, Pluto CP. M100,907, a selective 5-HT(2A) antagonist, attenuates dopamine release in the rat medial prefrontal cortex. Brain Res. 2001;888:51–59. doi: 10.1016/s0006-8993(00)03004-3. [DOI] [PubMed] [Google Scholar]
- Peltier R, Schenk S. Effects of serotonergic manipulations on cocaine self-administration in rats. Psychopharmacol Berl. 1993;110:390–394. doi: 10.1007/BF02244643. [DOI] [PubMed] [Google Scholar]
- Pentkowski NS, Duke FD, Weber SM, Pockros LA, Teer AP, Hamilton EC, Thiel KJ, Neisewander JL. Stimulation of medial prefrontal cortex serotonin 2C (5-HT(2C)) receptors attenuates cocaine-seeking behavior. Neuropsychopharmacology. 2010;35:2037–2048. doi: 10.1038/npp.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Vanderschuren LJ. Kicking the habit: the neural basis of ingrained behaviors in cocaine addiction. Neurosci Biobehav Rev. 2010;35(2):212–219. doi: 10.1016/j.neubiorev.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G. Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Brain Res Mol Brain Res. 1994;23:163–178. doi: 10.1016/0169-328x(94)90223-2. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Fluoxetine pretreatment reduces breaking points on a progressive ratio schedule reinforced by intravenous cocaine self-administration in the rat. Life Sci. 1991;49:833–840. doi: 10.1016/0024-3205(91)90248-a. [DOI] [PubMed] [Google Scholar]
- Robinson ES, Dalley JW, Theobald DE, Glennon JC, Pezze MA, Murphy ER, Robbins TW. Opposing roles for 5-HT2A and 5-HT2C receptors in the nucleus accumbens on inhibitory response control in the 5-choice serial reaction time task. Neuropsychopharmacology. 2008;33:2398–2406. doi: 10.1038/sj.npp.1301636. [DOI] [PubMed] [Google Scholar]
- Santana N, Bortolozzi A, Serrats J, Mengod G, Artigas F. Expression of serotonin1A and serotonin2A receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb Cortex. 2004;14:1100–1109. doi: 10.1093/cercor/bhh070. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Fadayel GM, Sullivan CK, Taylor VL. 5-HT2 receptors exert a state-dependent regulation of dopaminergic function: studies with MDL 100,907 and the amphetamine analogue, 3,4-methylene-dioxymethamphetamine. Eur J Pharmacol. 1992;223:65–74. doi: 10.1016/0014-2999(92)90819-p. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Sullivan CK, Fadayel GM. Blockade of striatal 5-hydroxytryptamine2 receptors reduces the increase in extracellular concentrations of dopamine produced by the amphetamine analogue 3,4-methylenedioxymethamphetamine. J Neurochem. 1994;62:1382–1389. doi: 10.1046/j.1471-4159.1994.62041382.x. [DOI] [PubMed] [Google Scholar]
- See RE. Neural substrates of cocaine-cue associations that trigger relapse. Eur J Pharmacol. 2005;526:140–146. doi: 10.1016/j.ejphar.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Carr DB. Selective prefrontal cortex inputs to dopamine cells: implications for schizophrenia. Physiol Behav. 2002;77:513–517. doi: 10.1016/s0031-9384(02)00931-9. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacol Berl. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Sinha R, Catapano D, O’Malley S. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacol Berl. 1999;142:343–351. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- Vazquez-Borsetti P, Cortes R, Artigas F. Pyramidal neurons in rat prefrontal cortex projecting to ventral tegmental area and dorsal raphe nucleus express 5-HT2A receptors. Cereb Cortex. 2009;19:1678–1686. doi: 10.1093/cercor/bhn204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers SP, Easton N, Malcolm CS, Allen NH, Porter RH, Bickerdike MJ, Kennett GA. Modulation of 5-HT(2A) receptor-mediated head-twitch behaviour in the rat by 5-HT(2C) receptor agonists. Pharmacol Biochem Behav. 2001;69:643–652. doi: 10.1016/s0091-3057(01)00552-4. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Goldstein RZ. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiol Learn Mem. 2002;78:610–624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- Weissenborn R, Robbins TW, Everitt BJ. Effects of medial prefrontal or anterior cingulate cortex lesions on responding for cocaine under fixed-ratio and second-order schedules of reinforcement in rats. Psychopharmacol Berl. 1997;134:242–257. doi: 10.1007/s002130050447. [DOI] [PubMed] [Google Scholar]
- Wettstein JG, Host M, Hitchcock JM. Selectivity of action of typical and atypical anti-psychotic drugs as antagonists of the behavioral effects of 1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane (DOI) Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:533–544. doi: 10.1016/s0278-5846(99)00014-7. [DOI] [PubMed] [Google Scholar]
- Willins DL, Deutch AY, Roth BL. Serotonin 5-HT2A receptors are expressed on pyramidal cells and interneurons in the rat cortex. Synapse. 1997;27:79–82. doi: 10.1002/(SICI)1098-2396(199709)27:1<79::AID-SYN8>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Zavala AR, Osredkar T, Joyce JN, Neisewander JL. Upregulation of Arc mRNA expression in the prefrontal cortex following cue-induced reinstatement of extinguished cocaine-seeking behavior. Synapse. 2008;62:421–431. doi: 10.1002/syn.20502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Perry KW, Wong DT, Potts BD, Bao J, Tollefson GD, Bymaster FP. Synergistic effects of olanzapine and other antipsychotic agents in combination with fluoxetine on norepinephrine and dopamine release in rat prefrontal cortex. Neuropsychopharmacology. 2000;23:250–262. doi: 10.1016/S0893-133X(00)00119-6. [DOI] [PubMed] [Google Scholar]