Abstract
This study determined whether, and by how much, the cost-effectiveness of contingency management (CM) varied across the eight clinics in the National Institute on Drug Abuse Clinical Trials Network MIEDAR trial. Incremental costs, incremental outcomes, and incremental cost-effectiveness ratios (ICERs) of CM compared to usual care were calculated, compared and contrasted for each of the clinics. Results showed that the incremental cost of using CM compared to usual care varied by a factor of 1.9 across the clinics, ranging from an additional $306 to an additional $582 per patient. The effect of CM on the longest duration of continuous stimulant abstinence (LDA) varied by a factor of 8.0 across the clinics, ranging from an additional 0.5 to an additional 4.0 weeks. The ICERs for the LDA varied by a factor of 4.6 across the clinics, ranging from $145 to $666. These results show that the cost-effectiveness of CM varied widely among the clinics in the MIEDAR trial. Future research should focus on identifying the sources of this variation, perhaps by identifying clinic-level best practices and/or identifying those subgroups of patients that respond the most cost-effectively, with the ultimate goal of improving the cost-effectiveness of CM overall.
INTRODUCTION
Contingency management (CM) interventions are based on behavioral research demonstrating that when a behavior is reinforced, it increases in frequency. In the substance abuse treatment field, CM interventions typically provide substance abusers with tangible incentives (e.g., chances to win prizes exchangeable for retail goods and services), contingent upon submission of urine specimens that test negative for recent drug use. Many studies have shown that CM interventions are effective tools for improving treatment outcomes across a wide range of substance-abusing populations and treatment modalities.1–8
Despite the strong evidence base for CM interventions, these tools have not been adopted widely in the United States or elsewhere.9 One major hindrance to wider adoption of CM interventions is that not much is known about their cost-effectiveness.10,11 At issue is whether the additional expense associated with CM is cost-effective in terms of the additional value gained. To shed light on this issue, Olmstead, Sindelar, and Petry12 recently examined the cost-effectiveness of using CM to treat stimulant abusers. This multi-site cost-effectiveness study was based on a randomized clinical trial conducted within the National Institute on Drug Abuse Clinical Trials Network (CTN).1 The underlying trial, called MIEDAR (Motivational Incentives for Enhanced Drug Abuse Recovery), took place in eight community-based outpatient psychosocial (i.e., drug-free) clinics with a diverse set of clienteles, usual care practices, and geographic locations. The purpose of the Olmstead, Sindelar, and Petry study was to estimate the average cost-effectiveness of CM compared to usual care, where the average was taken across all of the trial participants. Therefore, data (i.e., costs and patient outcomes) from the eight clinics were pooled, and the study reported only the overall (or aggregate) cost-effectiveness of CM compared to usual care. Given the diversity of clinics included in MIEDAR, the aggregate incremental cost-effectiveness ratios (ICERs) presented in the Olmstead, Sindelar, and Petry study provide useful estimates of the population-average incremental cost-effectiveness of CM. Said differently, the aggregate ICERs in the Olmstead, Sindelar, and Petry study should be useful to policy makers interested in predicting the average cost-effectiveness of CM were it to be implemented widely across the United States.
Although overall (or aggregate) cost-effectiveness results are useful for high-level decision making (perhaps related to the future expansion of CM), they do not provide insight into the potential heterogeneity in cost-effectiveness among clinics in multi-site trials. Thus, important questions remaining from the Olmstead, Sindelar, and Petry study are whether, and by how much, the cost-effectiveness of CM varies across clinics? If clinics are heterogeneous with respect to the cost-effectiveness of CM, then further research is warranted to identify the sources of such variation with the goal of developing more cost-effective methods of implementing CM in practice. For example, case studies may be able to identify ‘‘best practices’’ at those clinics where CM is relatively more cost-effective, and these ‘‘best practices’’ could then be shared with those clinics where CM is relatively less cost-effective, thereby improving the cost-effectiveness of CM overall.
This study extends our previous work by examining whether, and by how much, the cost-effectiveness of CM varies across the clinics in the MIEDAR trial. Specifically, for each of the eight clinics in the MIEDAR trial, we calculate, compare and contrast the incremental costs, incremental outcomes, and incremental cost-effectiveness ratios (ICERs) of CM compared to usual care. We also present acceptability curves to further illustrate the heterogeneity in the cost-effectiveness of CM among the clinics. Our study adds to the small but growing literature on the cost-effectiveness of CM12,13 and to the literature on the cost-effectiveness of treatment for substance abuse in general.14–19
METHODS
Methods and results of the MIEDAR effectiveness trial and overall cost-effectiveness study are described in detail in earlier publications,1,12 and summarized briefly below. Then we describe our clinic-specific analytical methods.
MIEDAR Effectiveness Trial1
The MIEDAR trial evaluated the effectiveness of prize-based CM as compared to usual care in each of eight outpatient psychosocial community-based substance abuse treatment clinics that were members of the CTN. The study intervention lasted for 12 weeks, and the final study sample comprised 415 stimulant-abusing participants who were randomly assigned to either usual care (UC) or usual care plus prize-based incentives. UC consisted primarily of group and possibly some individual and family counseling, depending on the clinic. Participants assigned to the prize-based CM condition, in addition to receiving usual care, earned the chance to win prizes for submitting substance-negative urine samples. Those who submitted substance-negative samples were invited to draw between one and 12 square plastic chips from an opaque container containing 500 chips. Half of the chips were marked ‘‘Good Job’’ and had no monetary value; the other half of the chips were exchangeable for goods ranging in value from $1 to $100. The number of draws earned increased with duration of continuous abstinence, but was reset to one draw after an unexcused absence or a positive sample. The escalating reward system was designed to reinforce long durations of abstinence.
Overall (Pooled) Cost-Effectiveness Study12
The Olmstead, Sindelar, and Petry overall cost-effectiveness study conducted an incremental cost-effectiveness analysis20,21 to answer the question of value per dollar spent on prize-based CM over usual care. All analyses used data that were pooled across the eight clinics in the study. Cost data were collected via surveys administered to each of the clinics, while data on resources used and patient outcomes were obtained from the MIEDAR trial. Incremental cost-effectiveness ratios (ICERs) were then calculated from the pooled data for each of the three patient outcomes in the study: longest duration of abstinence (LDA) from primary target drugs, length of stay in the study, and number of stimulant-negative urine samples submitted. The ICERs measure the incremental cost of using CM, compared to usual care, to produce an additional unit of effect for each of the patient outcomes. In addition, an acceptability curve for the primary patient outcome LDA was presented to illustrate the statistical uncertainty in the ICERs due to the study sample and to provide policy relevant information.22,23
Clinic-Specific Cost-Effectiveness Analysis
Following the methods described in Olmstead, Sindelar, and Petry,12 we first calculate the incremental costs, incremental outcomes, and ICERs for each of the three patient outcomes at each of the eight clinics in the study. We then use the following two straightforward measures to assess the degree of heterogeneity in the incremental costs, incremental outcomes, and ICERs among the clinics: the difference between the highest and lowest observations (i.e., the range) and the ratio of the highest and lowest observations. We also present acceptability curves for the primary patient outcome LDA to further illustrate the heterogeneity in the cost-effectiveness of CM among the clinics.
RESULTS
Table 1 shows the incremental costs, incremental outcomes, and ICERs of CM compared to usual care (UC) at each of the clinics in the study, as well as the associated ranges and ratios of the highest and lowest observations in each category. Clearly, there is substantial variation across the clinics in every category. For example, the incremental cost of using CM compared to UC varies by a factor of 1.9 across the clinics, ranging from an additional $306 per patient at clinic #8 to an additional $582 per patient at clinic #2. The effect of CM on patient outcomes varies even more. The effect of CM on the LDA, for example, varies by a factor of 8.0 across the clinics, ranging from an additional 0.5 weeks at clinic # 5 to an additional 4.0 weeks at clinic #1.
TABLE 1.
Incremental costs, incremental outcomes, and ICERs by clinic*
| Incremental Costs (CM–UC) |
Incremental Outcomes (CM–UC) |
ICERs |
||||||
|---|---|---|---|---|---|---|---|---|
| N | $ | LDA (weeks) | Neg urines | LOS (weeks) | $/week of LDA | $/neg urine | $/week of LOS | |
| Overall | 412 | 438† | 1.7† | 3.0† | 1.1† | 258 | 146 | 398 |
| Clinic 1 | 67 | 581† | 4.0† | 6.0† | 2.3† | 145 | 97 | 253 |
| Clinic 2 | 48 | 582† | 2.4 | 3.8 | 0.9 | 243 | 153 | 647 |
| Clinic 3 | 24 | 451† | 2.5 | 6.7† | 4.2† | 180 | 67 | 107 |
| Clinic 4 | 32 | 535† | 2.1 | 5.7† | 2.3 | 255 | 94 | 233 |
| Clinic 5 | 55 | 333 | 0.5 | 1.6 | 1.6 | 666 | 208 | 208 |
| Clinic 6 | 88 | 411† | 1.1 | 2.7 | 0.5 | 374 | 152 | 822 |
| Clinic 7 | 59 | 368† | 0.8 | 1.3 | 0.5 | 460 | 283 | 736 |
| Clinic 8 | 39 | 306† | 2.0 | 1.2 | − 0.2 | 153 | 255 | − 1530 |
| Max/Min | 1.9 | 8.0 | 5.6 | n/a | 4.6 | 4.2 | n/a | |
| Max–Min | 276 | 3.5 | 5.5 | 4.4 | 521 | 216 | 2352 | |
Three of the 415 participants in the MIEDAR study were excluded from the present study due to missing data; all three occurred at clinic #3.
p value <.05.
Abbreviations: ICER = incremental cost-effectiveness ratio; CM = prize-based contingency management; UC = usual care; LDA = longest duration abstinent during study (weeks); Neg urines = number of stimulant-negative urines submitted during study; LOS = length of stay in study (weeks).
Given the substantial variation across clinics in both incremental costs and incremental outcomes, it is not surprising that the ICERs vary considerably as well. The ICERs for the patient outcome LDA, for example, vary by a factor of 4.6 across the clinics, ranging from $145 at clinic #1 to $666 at clinic #5. Said differently, compared to UC, the incremental cost of using prize-based CM to extend the LDA by one week is, on average, 4.6 times more expensive at clinic #5 than it is at clinic #1.
To further illustrate the heterogeneity in the cost-effectiveness of CM among the clinics, Figure 1 shows acceptability curves for the patient outcome LDA at each of the clinics. Each acceptability curve in Figure 1 shows the probability that CM would be considered cost-effective at a given clinic for a large set of alternative values that decision makers could place on extending the LDA by one week. As Figure 1 shows, for a wide range of possible threshold values, there is substantial variation among the clinics with respect to the probabilities that CM would be considered cost-effective. For example, if the threshold value (perhaps determined by society’s willingness to pay) to extend the LDA by one week were $250, then the probability that CM would be considered cost-effective ranges from 10% at clinic #5 to 50% at clinics #2 and #4 to 98% at clinic #1.
Figure 1.
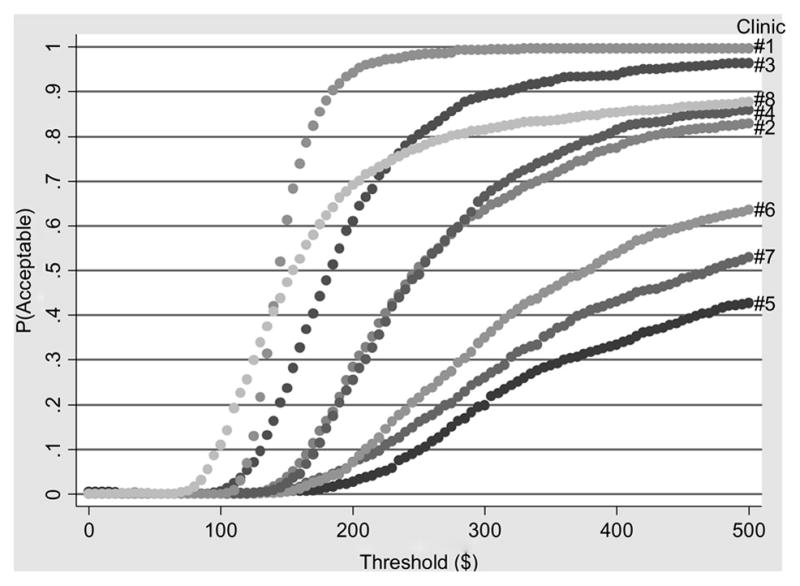
Acceptability Curves for Longest Duration Abstinent (LDA) by Clinic
DISCUSSION
This study found strong evidence of heterogeneity in the cost-effectiveness of CM among the eight clinics in the MIEDAR trial. Specifically, substantial variation among the clinics was found in the incremental costs, incremental outcomes, and incremental cost-effectiveness ratios of CM compared to usual care. Heterogeneity was also readily apparent among the acceptability curves associated with the clinics.
These findings suggest that it may be possible to improve the cost-effectiveness of CM overall. The next step is to understand the sources of the clinic variation in the cost-effectiveness of CM. Is the variation due to specific clinic practices, client characteristics, the interaction between clinic and clients, or to something else? The answers to these questions have important implications for both how CM should be implemented and who should receive it. For example, if the variation is due to clinic practices, then case studies may be able to identify both ‘‘best practices’’ at those clinics where CM is relatively more cost effective and barriers to implementing ‘‘best practices’’ at those clinics where CM is relatively less cost effective. In addition, if the variation is due to client characteristics, then it may be possible to target CM at those clients that are likely to be the most cost-effective to treat with this intervention.
In conclusion, we find that the cost-effectiveness of CM varied widely among the clinics in the MIEDAR trial. Unfortunately, because the MIEDAR trial did not collect detailed data on clinic practices and case studies are beyond the scope of this paper, we are unable to identify the sources of this clinic variation in the cost-effectiveness of CM. Nevertheless, our findings demonstrate the need for future research that focuses on understanding the reasons why CM is more cost-effective at some clinics than at others. By identifying clinic-level ‘‘best practices’’ and/or identifying those subgroups of patients that respond the most cost-effectively to CM, it may be possible to improve the cost-effectiveness of CM overall.
Acknowledgments
The authors gratefully acknowledge research support from grants RO1-DA14471 (Dr. Sindelar), U10DA13034 (Dr. Sindelar), R01-DA13444 (Dr. Petry), RO1-DA016855 (Dr. Petry), RO1-DA14618 (Dr. Petry), R01-DA018883 (Dr. Petry), P50-DA09241 (Dr. Petry), and P50-AA03510 (Dr. Petry) from the National Institute on Drug Abuse, Bethesda, Md.
The authors thank Maxine Stitzer, Ph.D., for use of the MIEDAR clinical trial data.
References
- 1.Petry NM, Pierce J, Stitzer ML, et al. Prize-based incentives improve outcomes of stimulant abusers in outpatient psychosocial treatment programs: a national drug abuse treatment clinical trials network study. Arch Gen Psychiatry. 2005;62:1148–1156. doi: 10.1001/archpsyc.62.10.1148. [DOI] [PubMed] [Google Scholar]
- 2.Peirce JM, Petry NM, Stitzer ML, et al. Effects of lower-cost incentives on stimulant abstinence in methadone maintenance treatment: a National Drug Abuse Treatment Clinical Trials Network Study. Arch Gen Psychiatry. 2006;63:201–208. doi: 10.1001/archpsyc.63.2.201. [DOI] [PubMed] [Google Scholar]
- 3.Higgins ST, Sigmon SC, Wong CJ, et al. Community reinforcement therapy for cocaine-dependent outpatients. Arch Gen Psychiatry. 2003;60:1043–1052. doi: 10.1001/archpsyc.60.9.1043. [DOI] [PubMed] [Google Scholar]
- 4.Rawson RA, Huber A, McCann M, et al. A comparison of contingency management and cognitive-behavioral approaches during methadone maintenance treatment for cocaine dependence. Arch Gen Psychiatry. 2002;59:817–824. doi: 10.1001/archpsyc.59.9.817. [DOI] [PubMed] [Google Scholar]
- 5.Budney AJ, Higgins ST, Radonovich KJ, Novy PL. Adding voucher-based incentives to coping skills and motivational enhancement improves outcomes during treatment for marijuana dependence. J Consult Clin Psychol. 2000;68:1051–1061. doi: 10.1037//0022-006x.68.6.1051. [DOI] [PubMed] [Google Scholar]
- 6.Petry NM, Martin B, Cooney JL, Kranzler HR. Give them prizes and they will come: Contingency management for the treatment of alcohol dependence. J Consult Clin Psychol. 2000;68:250–257. doi: 10.1037//0022-006x.68.2.250. [DOI] [PubMed] [Google Scholar]
- 7.Petry NM, Tedford J, Austin M, Nich C, Carroll K, Rounsaville B. Prize reinforcement contingency management for treating cocaine user: how low can we go, and with whom? Addiction. 2004;99:349–360. doi: 10.1111/j.1360-0443.2003.00642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petry NM, Martin B. Lower-cost contingency management for treating cocaine-abusing methadone patients. J Consult Clin Psychol. 2002;70:398–405. doi: 10.1037//0022-006x.70.2.398. [DOI] [PubMed] [Google Scholar]
- 9.Ritter A, Cameron J. Australian clinician attitudes towards contingency management: comparing down under with America. Drug Alcohol Depend. 2007;87:312–315. doi: 10.1016/j.drugalcdep.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Carroll KM, Rounsaville BJ. Bridging the gap between research and practice in substance abuse treatment: a hybrid model linking efficacy and effectiveness research. Psychiatr Serv. 2003;54:333–339. doi: 10.1176/appi.ps.54.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petry NM. A comprehensive guide for the application of contingency management procedures in standard clinic settings. Drug Alcohol Depend. 2000;58:9–25. doi: 10.1016/s0376-8716(99)00071-x. [DOI] [PubMed] [Google Scholar]
- 12.Olmstead TA, Sindelar JL, Petry NM. Cost-effectiveness of prize-based incentives for stimulant abusers in outpatient psychosocial treatment programs. Drug Alcohol Depend. 2007;87:175–182. doi: 10.1016/j.drugalcdep.2006.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sindelar JL, Elbel B, Petry NM. What do we get for our money? Cost-effectiveness of adding contingency management. Addiction. 2007;102:309–316. doi: 10.1111/j.1360-0443.2006.01689.x. [DOI] [PubMed] [Google Scholar]
- 14.French MT, Mausopf JA, Teague JL, Roland J. Estimating the dollar value of health outcomes from drug abuse interventions. Med Care. 1996;34:890–910. doi: 10.1097/00005650-199609000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Cartwright WS. Cost benefit and cost-effectiveness analysis of drug abuse treatment services. Eval Rev. 1998;22:609–636. doi: 10.1177/0193841X9802200503. [DOI] [PubMed] [Google Scholar]
- 16.Cartwright WS. Cost-benefit analysis of drug treatment services: Review of the literature. J Ment Health Policy Econ. 2000;3:11–26. doi: 10.1002/1099-176x(200003)3:1<11::aid-mhp66>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 17.Barnett PG, Zaric GS, Brandeau ML. The cost-effectiveness of buprenorphine maintenance therapy for opiate addiction in the United States. Addiction. 2001;96:1267–1278. doi: 10.1046/j.1360-0443.2001.96912676.x. [DOI] [PubMed] [Google Scholar]
- 18.Jofre-Bonet M, Sindelar JL. Creating an aggregate outcome index: cost-effectiveness analysis of substance abuse treatment. J Behav Health Serv Res. 2004;31:229–241. doi: 10.1007/BF02287287. [DOI] [PubMed] [Google Scholar]
- 19.Sindelar JL, Jofre-Bonet M, French MT, McLellan AT. Cost-effectiveness analysis of addition treatments for illicit drug dependence: paradoxes with multivariate outcomes. Drug Alcohol Depend. 2004;73:41–50. doi: 10.1016/j.drugalcdep.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Drummond MF, O’Brien B, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programs. 2. Oxford, UK: Oxford University Press; 1997. [Google Scholar]
- 21.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-Effectiveness in Health and Medicine. Oxford, UK: Oxford University Press; 1996. [Google Scholar]
- 22.Briggs A. Handling uncertainty in economic evaluation and presenting the results. In: Drummond M, McGuire A, editors. Economic Evaluation in Health Care: Merging Theory with Practice. Oxford, UK: Oxford University Press; 2001. pp. 172–214. [Google Scholar]
- 23.Fenwick E, Claxton K, Schulpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ. 2001;10:779–787. doi: 10.1002/hec.635. [DOI] [PubMed] [Google Scholar]


