Abstract
Somatic sex determination in Drosophila depends on the expression of Sex-lethal (Sxl), whose level is determined by the relative number of X chromosomes and sets of autosomes (X:A ratio). The first step in regulation of Sxl expression is transcriptional control from its early promoter and several genes encoding transcription factors of the helix-loop-helix (HLH) family such as daughterless (da), sisterless-b (sis-b), deadpan (dpn) and extramacrochaetae (emc) have been implicated. By the use of transfection assays and in vitro binding experiments, here we show that da/sis-b heterodimers bind several sites on the Sxl early promoter with different affinities and consequently tune the level of active transcription from this promoter. Interestingly, our data indicate that repression by the dpn product of da/sis-b dependent activation results from specific binding of dpn protein to a unique site within the promoter. This contrasts with the mode of emc repression, which inhibits the formation of the da/sis-b heterodimers. These results reveal the molecular mechanisms by which Sxl gene transcription is positively or negatively regulated to control somatic sex determination.
Full text
PDF
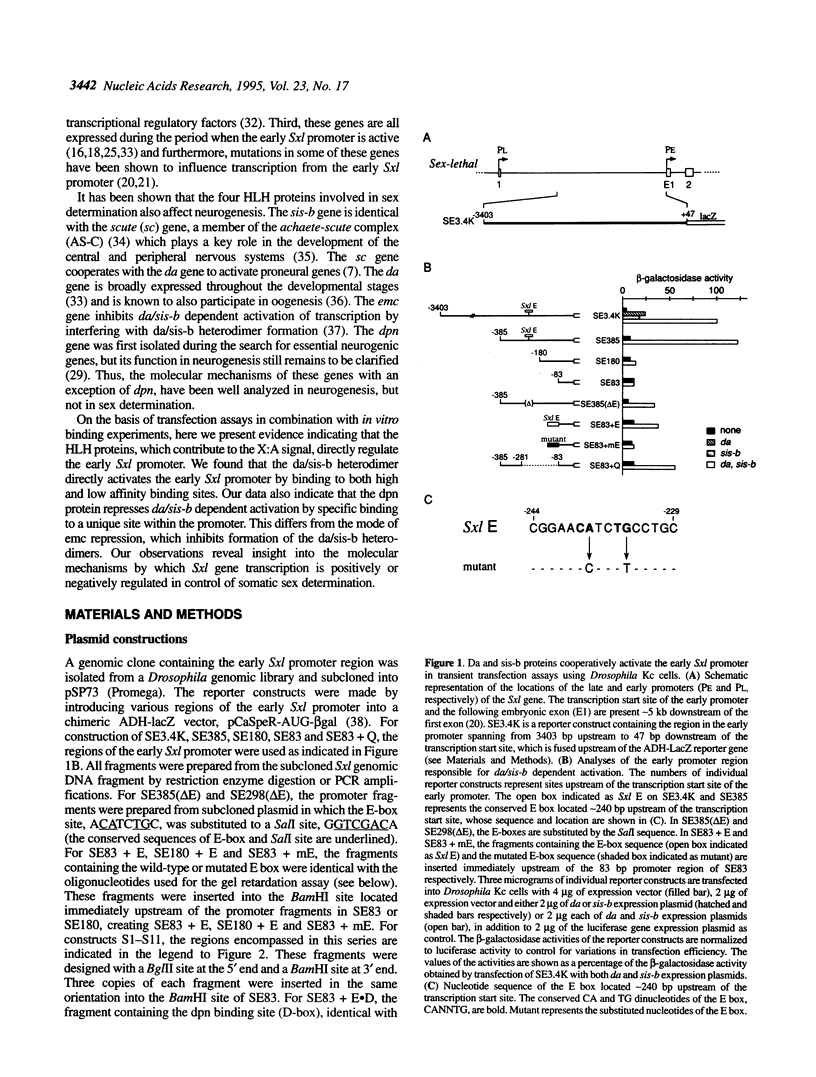



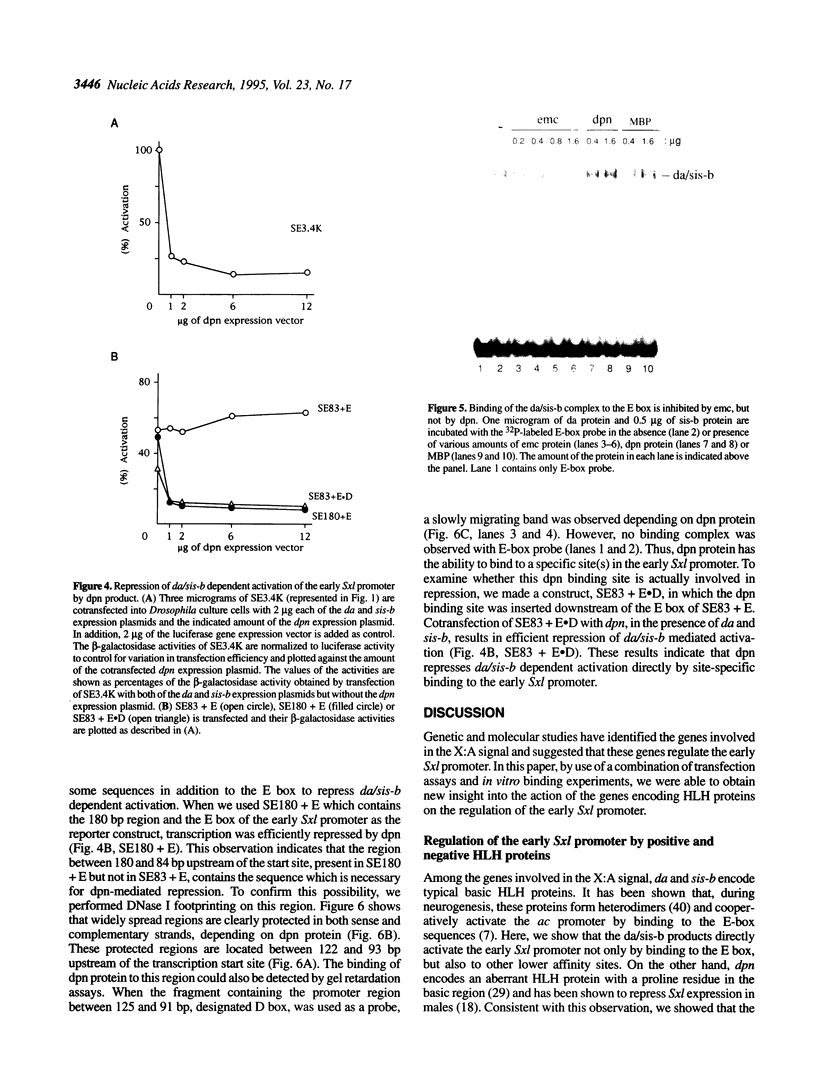
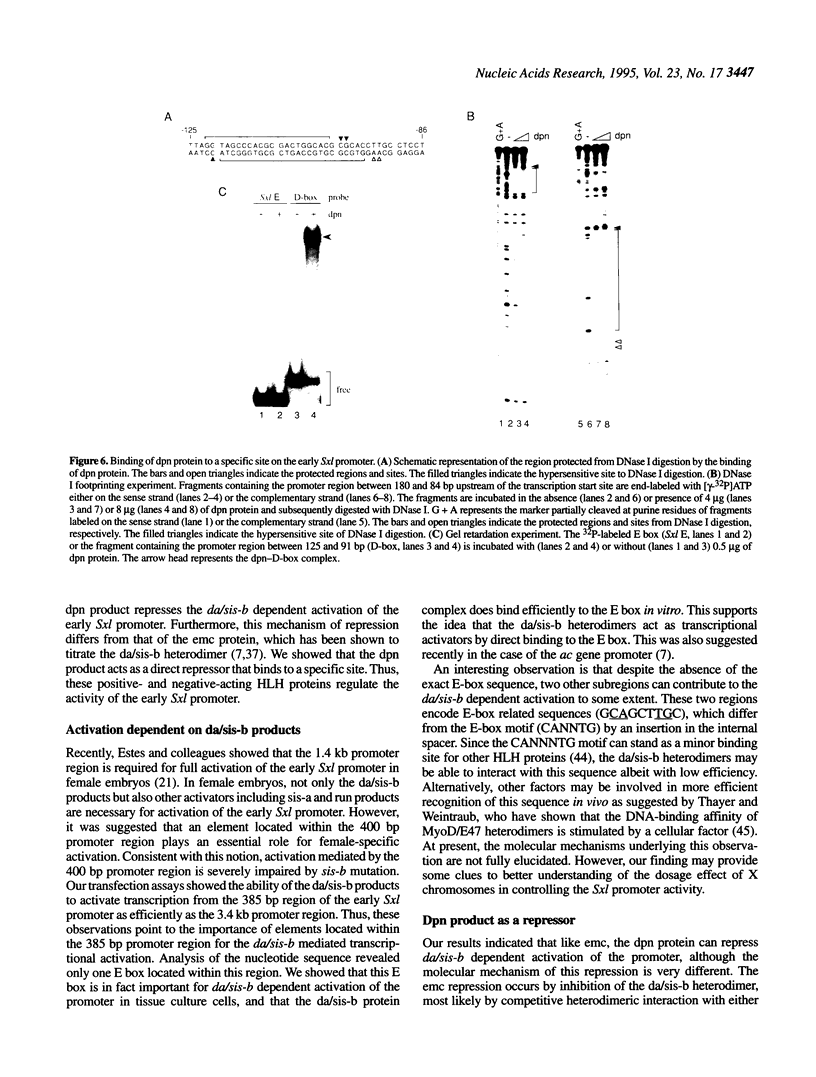

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell L. R., Horabin J. I., Schedl P., Cline T. W. Positive autoregulation of sex-lethal by alternative splicing maintains the female determined state in Drosophila. Cell. 1991 Apr 19;65(2):229–239. doi: 10.1016/0092-8674(91)90157-t. [DOI] [PubMed] [Google Scholar]
- Benezra R., Davis R. L., Lockshon D., Turner D. L., Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990 Apr 6;61(1):49–59. doi: 10.1016/0092-8674(90)90214-y. [DOI] [PubMed] [Google Scholar]
- Bier E., Vaessin H., Younger-Shepherd S., Jan L. Y., Jan Y. N. deadpan, an essential pan-neural gene in Drosophila, encodes a helix-loop-helix protein similar to the hairy gene product. Genes Dev. 1992 Nov;6(11):2137–2151. doi: 10.1101/gad.6.11.2137. [DOI] [PubMed] [Google Scholar]
- Bopp D., Bell L. R., Cline T. W., Schedl P. Developmental distribution of female-specific Sex-lethal proteins in Drosophila melanogaster. Genes Dev. 1991 Mar;5(3):403–415. doi: 10.1101/gad.5.3.403. [DOI] [PubMed] [Google Scholar]
- Cabrera C. V., Alonso M. C. Transcriptional activation by heterodimers of the achaete-scute and daughterless gene products of Drosophila. EMBO J. 1991 Oct;10(10):2965–2973. doi: 10.1002/j.1460-2075.1991.tb07847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera C. V., Martinez-Arias A., Bate M. The expression of three members of the achaete-scute gene complex correlates with neuroblast segregation in Drosophila. Cell. 1987 Jul 31;50(3):425–433. doi: 10.1016/0092-8674(87)90496-x. [DOI] [PubMed] [Google Scholar]
- Caudy M., Vässin H., Brand M., Tuma R., Jan L. Y., Jan Y. N. daughterless, a Drosophila gene essential for both neurogenesis and sex determination, has sequence similarities to myc and the achaete-scute complex. Cell. 1988 Dec 23;55(6):1061–1067. doi: 10.1016/0092-8674(88)90250-4. [DOI] [PubMed] [Google Scholar]
- Cline T. W. A female-specific lethal lesion in an X-linked positive regulator of the Drosophila sex determination gene, Sex-lethal. Genetics. 1986 Jul;113(3):641–663. doi: 10.1093/genetics/113.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline T. W. Evidence that sisterless-a and sisterless-b are two of several discrete "numerator elements" of the X/A sex determination signal in Drosophila that switch Sxl between two alternative stable expression states. Genetics. 1988 Aug;119(4):829–862. doi: 10.1093/genetics/119.4.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline T. W. The Drosophila sex determination signal: how do flies count to two? Trends Genet. 1993 Nov;9(11):385–390. doi: 10.1016/0168-9525(93)90138-8. [DOI] [PubMed] [Google Scholar]
- Cline T. W. The affairs of daughterless and the promiscuity of developmental regulators. Cell. 1989 Oct 20;59(2):231–234. doi: 10.1016/0092-8674(89)90280-8. [DOI] [PubMed] [Google Scholar]
- Cline T. W. The interaction between daughterless and sex-lethal in triploids: a lethal sex-transforming maternal effect linking sex determination and dosage compensation in Drosophila melanogaster. Dev Biol. 1983 Feb;95(2):260–274. doi: 10.1016/0012-1606(83)90027-1. [DOI] [PubMed] [Google Scholar]
- Cronmiller C., Cummings C. A. The daughterless gene product in Drosophila is a nuclear protein that is broadly expressed throughout the organism during development. Mech Dev. 1993 Aug;42(3):159–169. doi: 10.1016/0925-4773(93)90005-i. [DOI] [PubMed] [Google Scholar]
- Cronmiller C., Schedl P., Cline T. W. Molecular characterization of daughterless, a Drosophila sex determination gene with multiple roles in development. Genes Dev. 1988 Dec;2(12A):1666–1676. doi: 10.1101/gad.2.12a.1666. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Cheng P. F., Lassar A. B., Weintraub H. The MyoD DNA binding domain contains a recognition code for muscle-specific gene activation. Cell. 1990 Mar 9;60(5):733–746. doi: 10.1016/0092-8674(90)90088-v. [DOI] [PubMed] [Google Scholar]
- Duffy J. B., Gergen J. P. The Drosophila segmentation gene runt acts as a position-specific numerator element necessary for the uniform expression of the sex-determining gene Sex-lethal. Genes Dev. 1991 Dec;5(12A):2176–2187. doi: 10.1101/gad.5.12a.2176. [DOI] [PubMed] [Google Scholar]
- Edlund T., Walker M. D., Barr P. J., Rutter W. J. Cell-specific expression of the rat insulin gene: evidence for role of two distinct 5' flanking elements. Science. 1985 Nov 22;230(4728):912–916. doi: 10.1126/science.3904002. [DOI] [PubMed] [Google Scholar]
- Ellis H. M., Spann D. R., Posakony J. W. extramacrochaetae, a negative regulator of sensory organ development in Drosophila, defines a new class of helix-loop-helix proteins. Cell. 1990 Apr 6;61(1):27–38. doi: 10.1016/0092-8674(90)90212-w. [DOI] [PubMed] [Google Scholar]
- Erickson J. W., Cline T. W. A bZIP protein, sisterless-a, collaborates with bHLH transcription factors early in Drosophila development to determine sex. Genes Dev. 1993 Sep;7(9):1688–1702. doi: 10.1101/gad.7.9.1688. [DOI] [PubMed] [Google Scholar]
- Erickson J. W., Cline T. W. Molecular nature of the Drosophila sex determination signal and its link to neurogenesis. Science. 1991 Mar 1;251(4997):1071–1074. doi: 10.1126/science.1900130. [DOI] [PubMed] [Google Scholar]
- Estes P. A., Keyes L. N., Schedl P. Multiple response elements in the Sex-lethal early promoter ensure its female-specific expression pattern. Mol Cell Biol. 1995 Feb;15(2):904–917. doi: 10.1128/mcb.15.2.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrell J., Modolell J. The Drosophila extramacrochaetae locus, an antagonist of proneural genes that, like these genes, encodes a helix-loop-helix protein. Cell. 1990 Apr 6;61(1):39–48. doi: 10.1016/0092-8674(90)90213-x. [DOI] [PubMed] [Google Scholar]
- Horabin J. I., Schedl P. Regulated splicing of the Drosophila sex-lethal male exon involves a blockage mechanism. Mol Cell Biol. 1993 Mar;13(3):1408–1414. doi: 10.1128/mcb.13.3.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K., Hoshijima K., Sakamoto H., Shimura Y. Binding of the Drosophila sex-lethal gene product to the alternative splice site of transformer primary transcript. Nature. 1990 Mar 29;344(6265):461–463. doi: 10.1038/344461a0. [DOI] [PubMed] [Google Scholar]
- Jan Y. N., Jan L. Y. HLH proteins, fly neurogenesis, and vertebrate myogenesis. Cell. 1993 Dec 3;75(5):827–830. doi: 10.1016/0092-8674(93)90525-u. [DOI] [PubMed] [Google Scholar]
- Kagoshima H., Shigesada K., Satake M., Ito Y., Miyoshi H., Ohki M., Pepling M., Gergen P. The Runt domain identifies a new family of heteromeric transcriptional regulators. Trends Genet. 1993 Oct;9(10):338–341. doi: 10.1016/0168-9525(93)90026-e. [DOI] [PubMed] [Google Scholar]
- Keyes L. N., Cline T. W., Schedl P. The primary sex determination signal of Drosophila acts at the level of transcription. Cell. 1992 Mar 6;68(5):933–943. doi: 10.1016/0092-8674(92)90036-c. [DOI] [PubMed] [Google Scholar]
- Matsuo I., Kitamura M., Okazaki K., Yasuda K. Binding of a factor to an enhancer element responsible for the tissue-specific expression of the chicken alpha A-crystallin gene. Development. 1991 Oct;113(2):539–550. doi: 10.1242/dev.113.2.539. [DOI] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989 Mar 10;56(5):777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- Ohsako S., Hyer J., Panganiban G., Oliver I., Caudy M. Hairy function as a DNA-binding helix-loop-helix repressor of Drosophila sensory organ formation. Genes Dev. 1994 Nov 15;8(22):2743–2755. doi: 10.1101/gad.8.22.2743. [DOI] [PubMed] [Google Scholar]
- Sakamoto H., Inoue K., Higuchi I., Ono Y., Shimura Y. Control of Drosophila Sex-lethal pre-mRNA splicing by its own female-specific product. Nucleic Acids Res. 1992 Nov 11;20(21):5533–5540. doi: 10.1093/nar/20.21.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai Y., Kageyama R., Tagawa Y., Shigemoto R., Nakanishi S. Two mammalian helix-loop-helix factors structurally related to Drosophila hairy and Enhancer of split. Genes Dev. 1992 Dec;6(12B):2620–2634. doi: 10.1101/gad.6.12b.2620. [DOI] [PubMed] [Google Scholar]
- Sun X. H., Baltimore D. An inhibitory domain of E12 transcription factor prevents DNA binding in E12 homodimers but not in E12 heterodimers. Cell. 1991 Jan 25;64(2):459–470. doi: 10.1016/0092-8674(91)90653-g. [DOI] [PubMed] [Google Scholar]
- Sánchez L., Nöthiger R. Sex determination and dosage compensation in Drosophila melanogaster: production of male clones in XX females. EMBO J. 1983;2(4):485–491. doi: 10.1002/j.1460-2075.1983.tb01451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer M. J., Weintraub H. A cellular factor stimulates the DNA-binding activity of MyoD and E47. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6483–6487. doi: 10.1073/pnas.90.14.6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thummel C. S., Boulet A. M., Lipshitz H. D. Vectors for Drosophila P-element-mediated transformation and tissue culture transfection. Gene. 1988 Dec 30;74(2):445–456. doi: 10.1016/0378-1119(88)90177-1. [DOI] [PubMed] [Google Scholar]
- Torres M., Sánchez L. The scute (T4) gene acts as a numerator element of the X:a signal that determines the state of activity of sex-lethal in Drosophila. EMBO J. 1989 Oct;8(10):3079–3086. doi: 10.1002/j.1460-2075.1989.tb08459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres M., Sánchez L. The segmentation gene runt is needed to activate Sex-lethal, a gene that controls sex determination and dosage compensation in Drosophila. Genet Res. 1992 Jun;59(3):189–198. doi: 10.1017/s0016672300030470. [DOI] [PubMed] [Google Scholar]
- Van Doren M., Bailey A. M., Esnayra J., Ede K., Posakony J. W. Negative regulation of proneural gene activity: hairy is a direct transcriptional repressor of achaete. Genes Dev. 1994 Nov 15;8(22):2729–2742. doi: 10.1101/gad.8.22.2729. [DOI] [PubMed] [Google Scholar]
- Van Doren M., Ellis H. M., Posakony J. W. The Drosophila extramacrochaetae protein antagonizes sequence-specific DNA binding by daughterless/achaete-scute protein complexes. Development. 1991 Sep;113(1):245–255. doi: 10.1242/dev.113.1.245. [DOI] [PubMed] [Google Scholar]
- Van Doren M., Powell P. A., Pasternak D., Singson A., Posakony J. W. Spatial regulation of proneural gene activity: auto- and cross-activation of achaete is antagonized by extramacrochaetae. Genes Dev. 1992 Dec;6(12B):2592–2605. doi: 10.1101/gad.6.12b.2592. [DOI] [PubMed] [Google Scholar]
- Villares R., Cabrera C. V. The achaete-scute gene complex of D. melanogaster: conserved domains in a subset of genes required for neurogenesis and their homology to myc. Cell. 1987 Jul 31;50(3):415–424. doi: 10.1016/0092-8674(87)90495-8. [DOI] [PubMed] [Google Scholar]
- Younger-Shepherd S., Vaessin H., Bier E., Jan L. Y., Jan Y. N. deadpan, an essential pan-neural gene encoding an HLH protein, acts as a denominator in Drosophila sex determination. Cell. 1992 Sep 18;70(6):911–922. doi: 10.1016/0092-8674(92)90242-5. [DOI] [PubMed] [Google Scholar]