Abstract
Although a great deal of research addresses the neural basis of deliberate and intentional emotion-regulation strategies, less attention has been paid to the neural mechanisms involved in implicit forms of emotion regulation. Behavioural research suggests that romantically involved participants implicitly derogate the attractiveness of alternative partners, and the present study sought to examine the neural basis of this effect. Romantically committed participants in the present study were scanned with functional magnetic resonance imaging (fMRI) while indicating whether they would consider each of a series of attractive (or unattractive) opposite-sex others as a hypothetical dating partner both while under cognitive load and no cognitive load. Successful derogation of attractive others during the no cognitive load compared to the cognitive load trials corresponded with increased activation in the ventrolateral prefrontal cortex (VLPFC) and posterior dorsomedial prefrontal cortex (pDMPFC), and decreased activation in the ventral striatum, a pattern similar to those reported in deliberate emotion-regulation studies. Activation in the VLPFC and pDMPFC was not significant in the cognitive load condition, indicating that while the derogation effect may be implicit, it nonetheless requires cognitive resources. Additionally, activation in the right VLPFC correlated with participants’ level of relationship investment. These findings suggest that the RVLPFC may play a particularly important role in implicitly regulating the emotions that threaten the stability of a romantic relationship.
Keywords: Implicit emotion regulation, Derogation of alternatives, Positive emotion, Relationship, Ventral striatum, Ventrolateral prefrontal cortex
Nearly all research on the psychological mechanisms involved in emotion regulation focuses on deliberate forms of emotion regulation, such as reappraisal and suppression (Gross & Thompson, 2007; Ochsner & Gross, 2005). These studies require that participants either be trained in explicit emotion-regulation strategies prior to study participation and/or be instructed to deliberately use specific emotion-regulation strategies at particular moments (e.g., Ochsner et al., 2009b). Although such research has been useful in determining the neural basis of emotion-regulation capacity, it does not assess whether the same mechanisms guide emotion regulation when it occurs outside of the individual’s awareness. Furthermore, it does not examine whether and how people might spontaneously regulate their emotions in everyday life.
Recent studies have begun to fill these gaps, finding that emotion regulation can operate at implicit levels (Eder & Rothermund, 2010; Koole, 2009; Koole & Jostmann, 2004; Mauss, Bunge, & Gross, 2007; Mauss, Evers, Wilhelm, & Gross, 2006; Rothermund, Voss, & Wentura, 2008; Schweiger-Gallo, Keil, McCulloch, Rockstroh, & Gollwitzer, 2009; Williams, Bargh, Nocera, & Gray, 2009), occurring outside of awareness and without the intention to regulate. However, research on the mechanisms guiding implicit emotion regulation is relatively sparse, most likely due to the difficulty in inducing implicit emotion regulation in participants and in developing methods to measure the phenomenon.
Some progress has been made by research using brain imaging techniques that measure neural activity during tasks that produce patterns of neural activation that resemble emotion regulation despite the fact that the tasks do not require emotion regulation explicitly (Enger, Etkins, Gale, & Hirsch, 2008; Etkins, Enger, Pereza, Kandel, & Hirsch, 2006; Hare, Tottenham, Davidson, Glover, & Casey, 2005; Hariri, Bookheimer, & Mazziotta, 2000; Kim et al., 2004; Lieberman et al., 2007). For example, using language to label an affective stimulus, even in the absence of a deliberate intention to regulate one’s response (Hariri et al., 2000; Lieberman et al., 2007), results in a pattern of increased right ventrolateral prefrontal cortical regions (RVLPFC) coupled with a decreased amygdala response. This pattern of neural activity is similar to the pattern observed in studies of intentional emotion regulation (Berkman & Lieberman, 2009). Similarly, a recent study examining inhibitory motor responses in the presence of emotional stimuli (in which the participant must regulate their emotional response in order to perform the inhibitory motor task) found that the degree of activation in the RVLPFC corresponded with reductions in amygdala activation during negative but not neutral stimuli (Berkman, Burklund, & Lieberman, 2009).
The RVLPFC is part of a network of brain regions known to engage in deliberate emotion regulation (Gross, 2007; Ochsner & Gross, 2005; Lieberman et al., 2007). Other regions in this network include the left ventrolateral prefrontal cortex (LVLPFC) as well as dorsal medial and lateral prefrontal cortex (DMPFC & LPFC; Gross, 2007; Ochsner & Gross, 2005; Ochsner et al., 2009b). Studies to date on implicit emotion regulation therefore suggest that emotional experiences, at the neurophysiological level, can be dampened through recruitment of at least part of the network (i.e., RVLPFC) known to engage in deliberate emotion regulation. Other regions in the network (e.g., LVLPFC, DMPFC, LPFC) may only be necessary for deliberate forms of emotion regulation. However, both affect labelling and motor inhibition require relatively sparse cognitive resources. It remains an open question whether other implicit regulation strategies that may use more cognitive resources will activate not only RVLPFC, but also more of the network associated with deliberate emotion regulation.
Another open question concerns the extent to which the brain network engaged in emotion-regulation studies is fully engaged in our everyday emotion-regulation experiences. To date, studies on implicit and explicit emotion regulation have examined externally induced emotion regulation. How does emotion regulation work when the task is not inherently regulatory across individuals, but rather when emotion regulation is recruited spontaneously by some people due to their social context and broader motivations? This question is related to an important limitation in emotion-regulation research, as the majority of studies examine what people can do (capacity, or one’s ability to regulate one’s emotions when explicitly instructed to do so) as opposed to what they actually do (tendency, or recruitment of emotion regulation processes when there is no explicit demand of emotion regulation) spontaneously in everyday life (Berkman & Lieberman, 2009).
In the present study, we capitalise on a well-documented, naturally occurring and implicit regulatory tendency to study the neural systems involved in implicit emotion regulation. In this phenomenon, the “derogation of attractive alternatives”, romantically involved individuals devalue the attractiveness of an objectively attractive potential partner, presumably as a result of motivation to maintain their current relationship (Johnson & Rusbult, 1989; Lydon, Fitzsimons, & Naidoo, 2003; Lydon, Maena, Sepinwalls, Richards, & Mayman, 1999; Simpson, Gangestad, & Lerma, 1990). For example, Simpson and colleagues (1990) found that participants involved in relationships rated the level of attractiveness of models from magazine ads systematically lower than single participants.
Importantly, the derogation of alternatives does not seem to reflect something about general attractiveness perceptions of people who choose to be in relationships. Instead, derogation of attractive alternatives is specific to age-matched, opposite-sex attractive others, and this effect persists when controlling for individual differences including participants’ level of attractiveness, self-esteem, self-monitoring, and empathy (Simpson et al., 1990). Moreover, the derogation effect is not restricted to ratings of physical attractiveness, as it extends to situations in which participants rate whether they would consider an attractive alternative as a potential romantic partner if they were hypothetically not in their current relationship (Ritter, Karremans, & van Schie, 2010).
Relationship scientists suggest that the derogation of alternatives is a relationship-maintenance strategy used by romantically involved individuals to regulate their attraction toward potential alternative partners (Johnson & Rusbult, 1989; Overall & Sibley, 2007; Simpson et al., 1990). Though several factors likely lead to the instability of a relationship, the lure of an attractive alternative partner is particularly influential (Kelly & Thibaut, 1978; Simpson et al., 1990). Past research has shown that people respond to attractive individuals with approach and affiliative tendencies (Van Leeuwen & Macrae, 2004) and that the gaze of an attractive person activates reward regions in the brain (Kampe, Frith, Dolan, & Frith, 2001). According to relationship theorists, the pleasing response elicited by attractive alternatives creates a dilemma for the romantically involved person such that the immediate pleasure and lure of the attractive alternative is inconsistent with the broader motivation to feel satisfied with their current partner (Kelly & Thibaut, 1978; Rusbult, Olsen, Davis & Hannon, 2004). As investment and interdependence in a relationship increases, individuals are motivated to protect the stability of their relationship, and the derogation of alternatives is one of many strategies romantically involved individuals draw from to preserve their relationships. In fact, the derogation of attractive alternatives corresponds with increasing levels of commitment, suggesting that as motivation to protect a relationship increases, the tendency to minimise the threat of an alternative increases (Johnson & Rusbult, 1989; Simpson et al., 1990).
Although the function of the derogation of alternatives is to maintain the stability of the current relationship, how people derogate has only recently been explored (Ritter et al., 2010). Findings suggest that the derogation effect is likely a regulatory response that occurs implicitly but nonetheless relies on cognitive resources. First, the derogation of alternatives seems to be consistent with “antecedent-focused” emotion-regulation strategies in which individuals change the external stimuli on some dimension(s) in order to prevent a full emotional response (Gross, 1998a, 1998b, 1999). Specifically, the derogation of alternatives helps romantically involved individuals reduce evoked feelings of attraction toward appealing opposite-sex others (Ritter et al., 2010). Second, the derogation of alternatives requires cognitive resources, and when the cognitive resources are unavailable, the derogation effect disappears. For example, romantically involved heterosexuals are more likely to judge attractive opposite-sex persons as potential romantic partners after emotion suppression (ego depletion condition) compared to emotion expression (no depletion), and while under time pressure (cognitive load) compared to no time pressure (no cognitive load; Ritter et al., 2010). Moreover, following depletion or during cognitive load, romantically involved participants considered attractive opposite sex persons as potential romantic partners at similar rates to those of single participants. Together, these results suggest that romantically involved participants are attracted to potential partners but engage in regulation of that attraction when cognitive resources are available during the derogation effect.
While the derogation of alternatives is similar to deliberate emotion regulation in that it reduces the emotional intensity of a stimulus and requires cognitive resources, it appears to operate implicitly. Simpson and colleagues (1990) found that romantically involved participants derogated the physical attractiveness of alternatives without engaging in extended reasoning. Additionally, participants from these studies reported being unaware of their different response tendencies after a funnelled debriefing procedure, suggesting that romantically involved participants are unaware of their bias (Karremans & Verwijmeren, 2008). Because derogation of attractive alternatives is similar to explicit forms of emotion regulation in some ways (i.e., down-regulation of attraction, resource dependent) but occurs spontaneously in real life and appears to operate outside of awareness, a paradigm capturing the derogation effect makes an ideal candidate for studying ecologically valid implicit emotion regulation.
In the present study we used functional magnetic resonance imaging (fMRI) to measure the neural responses of romantically involved participants while they evaluated attractive opposite-sex individuals as hypothetical romantic partners. Because the derogation effect shares some features with deliberate forms of emotion regulation—antecedent alteration of stimuli and necessity of cognitive resources—we hypothesised that derogation when cognitive resources were available, compared to when cognitive resources were unavailable, would show increased activation in areas of the emotion regulation network also engaged in deliberate forms of emotion regulation: namely the VLPFC and DMPFC. We did not predict activation in LPFC because although the derogation effect shares some features with deliberate forms of emotion regulation, the LPFC seems to play a role in allocating conscious attention to stimuli (McCrae et al., 2010), which is not characteristic of the implicitly occurring derogation effect.
Past research has also shown that recruiting increasing levels of RVLPFC during implicit regulation corresponds with decreased activation in limbic regions (Hariri et al., 2000; Lieberman et al., 2007). Accordingly, we hypothesised that the extent of activity in the RVLPFC when participants derogate attractive alternatives during the no cognitive load condition (when cognitive resources are available) should negatively correlate with degree of activation in the ventral striatum (VS), a region associated with reward processing (e.g., Haber & Knutson, 2009). Moreover, in extension of past work highlighting that the derogation effect increases with relationship investment (Johnson & Rusbult, 1989; Simpson et al., 1990), we hypothesised that extent of neural activation in RVLPFC during derogation should correspond with participants’ level of relationship investment.
METHODS
Participants
Fourteen heterosexual, right-handed, native English-speaking participants (9 females/5 males) in exclusive, romantic relationships participated in the study. Participants’ ages ranged from 21–41 years (mean = 27.2 years, SD = 5.55) and ethnicity breakdowns were as follows: 11 Caucasian/non-Hispanic origin participants; 2 Hispanic participants, and 2 Asian/Pacific Islander. Participants completed written consent in accordance with UCLA’s Institutional Review Board.
Procedure
Participants completed a computerised task based on Ritter et al. (2010), in which they made a yes/no decision regarding whether they would consider a depicted person as a hypothetical potential romantic partner. Participants rated 80 colour photographs (40 attractive and 40 unattractive) of the opposite sex. These pictures were taken from a pilot study, in which pictures of females and males (130 each) were evaluated by opposite-sex raters using a 7-point scale. For each sex, the 40 pictures with the lowest, and the 40 pictures with the highest mean were chosen for the current study (Munattractive males = 2.30; Mattractive males = 5.96), F(1, 129) = 60.99, p < .01; (Munattractive females = 2.05; Mattractive females = 5.10), F(1, 129) = 27.40, p < .01. Participants were instructed to indicate, by pressing the “yes” or “no” button, whether they would consider the pictured person to be a hypothetical potential partner.
During the scan, participants rated with or without time pressure and about either an attractive or unattractive target in a 2 × 2 within-subjects, repeated-measures design (Time Pressure: with/without × Attractiveness high/low). Time Pressure alternated by block, and each block contained half high- and half low-attractiveness trials. The eighty photos were randomly shown in four time-pressure and four no-time-pressure blocks that included 10 photos each. In addition to photo randomisation, the order of blocks was also randomised. Before each block of 10 photos, participants viewed an instruction screen for 12 seconds, indicating which type of block was about to follow. There were 2 to 2.5 seconds of fixation (randomised jitter interval between 0.00 and 0.5 seconds was added to each 2-second fixation cross to control for autocorrelation in haemodynamic responses) between blocks. For the no-time-pressure trials, participants had all of the 5 seconds to answer whether they would consider the individual pictured as a romantic partner (indicated by a green square presented above the photograph for the entire period), whereas for the time-pressure trials, participants only had 0.5 seconds to answer the question (indicated by the green square turning red after the first 0.5 seconds). Each trial was 5 seconds long, and the photograph was displayed for the entire duration in both conditions. After the scan, participants were probed for suspicion and asked what strategies they used to answer trials. Subjects did not report deliberately regulating their emotions and were unaware of the hypotheses.
fMRI data acquisition and analysis
Functional MRI data were collected using a Siemens Trio 3-Tesla head-only MRI scanner at the UCLA Ahmanson-Lovelace Brainmapping Center. The task was presented to participants on scanner-compatible goggles. Whole-brain blood oxygenation-level dependent functional scans were acquired during the 9-minute task (echo-planar T2-weighted gradient-echo, TR = 2000 ms, TE = 25 ms, flip angle = 90°, matrix size = 64 × 64, 34 axial slices, FOV = 20 cm; 4 mm thick; voxel size = 3 × 3 × 3 mm). In addition to the functional images acquired during the task, a set of high-resolution structural T2-weighted echo-planar images were acquired coplanar with the functional scans (spin-echo; TR = 5000 ms; TE = 34 ms; matrix size 128 × 128; 34 sagittal slices; FOV = 192 mm; 4 mm thick).
Neuroimaging data were pre-processed and analysed with Statistical Parametric Mapping (SPM5; Welcome Department of Cognitive Neurology, Institute of Neurology, London, UK). Pre-processing for each participant’s images included slice-timing to adjust for temporal differences in slice acquisition within each volume, spatial realignment to correct for head motion, normalisation into a standard stereotactic space as defined by the Montreal Neurological Institute, and spatial smoothing using an 8 mm Gaussian kernel, full width at half maximum, to increase the signal-to-noise ratio. The task was modelled at the first (subject) level as a 5-second event-related design with conditions for each response type (accept/reject) for each trial type (attractive time pressure/unattractive time pressure/attractive no time pressure/unattractive no time pressure) yielding a total of 8 within-participant conditions. The instructions before each block were grouped with the fixation periods between blocks to form the implicit baseline. Linear contrasts among these conditions were computed for each participant as a measure of differential blood oxyentation level-dependent (BOLD) activation, then entered into a random effects analyses at the group level for statistical inference. All analyses used a voxel-wise threshold of p < .005 with a 10-voxel extent threshold. We used this joint voxelwise and cluster-size threshold because this is the first study to investigate the neural basis of the derogation of alternatives, and these parameters provide a good balance between Type I and Type II errors and parallel false-detection rates in social psychological behavioural studies (Lieberman & Cunningham, 2009).
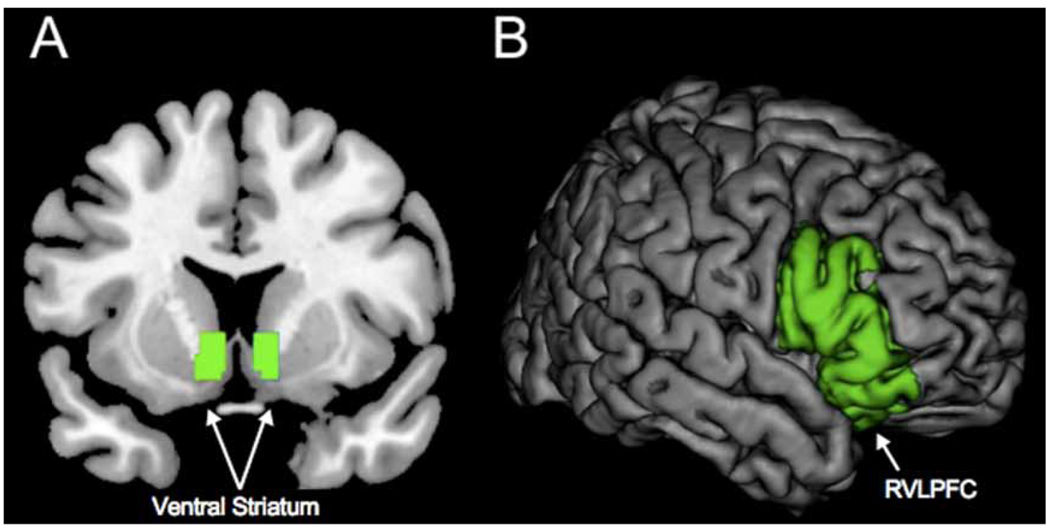
In addition to the whole-brain searches, we also constructed two anatomical regions-of-interest (ROIs) to be used in the subsequent analyses (Figure 1). First, we expected reward-system activation in the ventral striatum (VS) during unregulated viewing of attractive compared to unattractive others (Haber & Knutson, 2009). Second, we expected the RVLPFC to be involved in down-regulation of attraction when cognitive resources were available, indicated by a negative correlation between RVLPFC and VS when participants derogated attractive alternatives during no time pressure compared to time pressure conditions. Third, we examined whether the extent of RVLPFC activation during this contrast corresponded with participants’ scores on Rusbult, Martz, and Agnew’s (1998) commitment scale. We constructed ROIs in the bilateral VS bounded at x = −12 to +12, y = 4 to 8, and z = 0 to = −12 and RVLPFC(combining pars orbitalis, triangularis, and orbitalis) based on the Automated Anatomical Labelling (AAL; Tzourio-Mazoyer et al., 2002) atlas implemented in the Wake Forest University Pickatlas (Maldjian, Laurienti, Kraft, & Burdette, 2003). ROI analyses were computed by averaging across all voxels within these a priori defined ROIs. All coordinates are reported in MNI space.
Figure 1.
Clusters included in the construction of region of interests (ROIs) for (A) ventral striatum and (B) right ventrolateral prefrontal cortex (RVLPFC). [To view this figure in colour, please visit the online version of this Journal.]
Questionnaire data
After the brain scan, the Investment Model Scale (Rusbult et al., 1998) was used to measure participants’ level of relationship investment. The scale includes relationship-specific questions to measure: relationship commitment (e.g., “I want our relationship to last for a very long time”, α = .91); relationship satisfaction (e.g., “Our relationship makes me very happy”, α = .92); quality of alternatives (e.g., “My alternatives to our relationship are close to ideal”, α = .82); and investment size (e.g., “I feel very involved in our relationship—like I have put a great deal into it”, α = .82). Participants answered on a scale ranging from 1 (totally disagree) to 7 (totally agree). For the present study, we measured the composite score from these subscales to measure global relationship investment.
RESULTS
Behavioural results
Participants’ response times were faster for the time pressure (M = 1.47, SD = 0.34) compared to no time pressure conditions (M = 4.35, SD = 0.84); F(1, 13) = 9.34, p < .001. Participants accepted more attractive targets while under cognitive load (time pressure; mean acceptance rate = 74%, SD = 0.26) compared to no cognitive load (no time pressure; mean acceptance rate = 70%, SD = 0.29), though this difference was not significant, F(1, 13) = 0.53, ns. There was no effect of cognitive load on acceptance rate of unattractive targets (time pressure mean = 15%, SD = 0.23; no time pressure mean = 14%, SD = 0.22, ns).
Imaging results
Neural manipulation check: Attractiveness
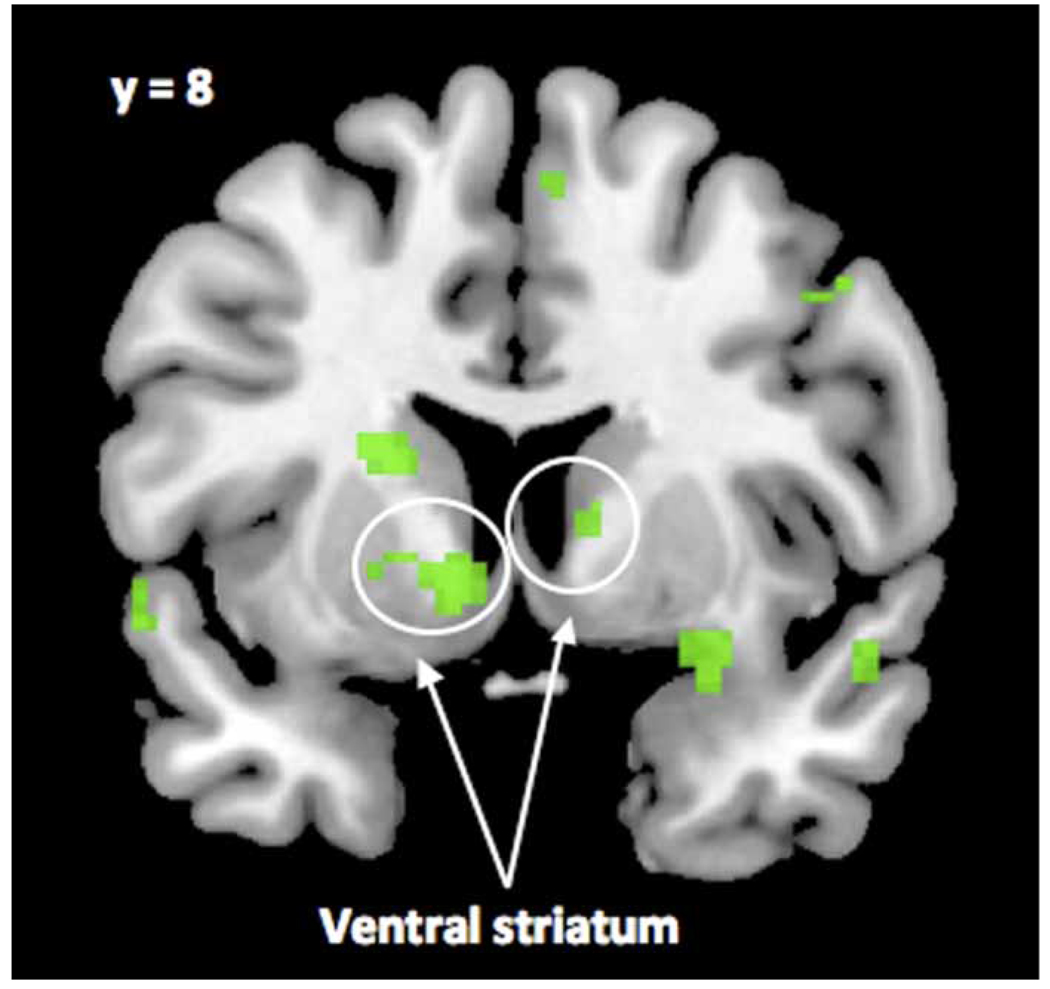
Although all photos were pre-tested for level of attractiveness, we wanted to check that our attractive photos did in fact elicit a neural response consistent with subjective attraction to these photos. To test this, we contrasted neural activity during viewing of attractive compared to unattractive photographs in the no time pressure condition in a whole-brain analysis. The logic of using this contrast is that a robust neural response to attractive photos is more likely when participants are not burdened with the task of making a rapid response. The ventral striatum (VS; centred at: x = −10, y = 8, z = −4; x = −22, y = 8, z = 16; x = 6, y = 12, z = 2) was significantly more active when participants viewed attractive compared to unattractive photos (Figure 2, Table 1). Importantly, bilateral activation in the ventral striatum remained significant in a region of interest (ROI) analysis (p < .05), which in this case is a more conservative test, as it limits the number of statistical tests performed on voxels by specifying a priori within which regions to compare voxel activation.
Figure 2.
Activation in the ventral striatum (VS) when participants rated attractive compared to unattractive alternative partners while under no time pressure, t(13) = 5.67 and 4.35 for clusters in right VS; t(13) = 3.38 for left VS, p < .005. [To view this figure in colour, please visit the online version of this Journal.]
Table 1.
Brain regions showing increased activity while rating attractive compared to unattractive alternatives under no time pressure
| Region | Laterality | x | y | z | t | Voxels |
|---|---|---|---|---|---|---|
| Ventral striatum (accumbens) | L | −10 | 8 | −4 | 4.35 | 29 |
| Ventral striatum (caudate) | L | −22 | 8 | 16 | 5.67 | 42 |
| R | 6 | 12 | 2 | 3.38 | 11 | |
| Frontal pole | L | −18 | 58 | −6 | 3.65 | 37 |
| L | −28 | 58 | −10 | 5.65 | 53 | |
| R | 10 | 56 | 40 | 4.22 | 29 | |
| R | 12 | 56 | 22 | 3.99 | 55 | |
| Medial frontal cortex | L | −6 | 50 | −18 | 4.59 | 58 |
| R | 2 | 58 | −14 | 4.26 | 40 | |
| Frontal orbital cortex | R | 40 | 30 | −14 | 3.26 | 21 |
| Anterior cingulate gyrus | R | 10 | 44 | 20 | 4.01 | 36 |
| L | −10 | 28 | 32 | 4.58 | 72 | |
| Superior frontal gyrus | R | 24 | 34 | 58 | 4.27 | 58 |
| Middle frontal gyrus | R | 36 | 28 | 52 | 4.12 | 66 |
| Putamen | L | −24 | 20 | −2 | 5.39 | 62 |
| Insular cortex | L | −28 | 18 | −8 | 4.3 | 71 |
| Temporal pole | R | 29 | 8 | −16 | 4.77 | 43 |
| Middle temporal gyrus | L | −50 | −36 | −10 | 3.95 | 67 |
| Supramarginal gyrus | R | 56 | −38 | 14 | 5.16 | 59 |
| Lingual gyrus | L | −14 | −56 | 0 | 4.36 | 67 |
| L | −2 | −70 | 4 | 4.56 | 78 | |
| R | 16 | −62 | 0 | 3.67 | 24 | |
| R | 4 | −64 | 0 | 3.76 | 39 | |
| Precuneus | L | −24 | −62 | 2 | 6.51 | 61 |
| Lateral occipital cortex | L | −20 | −92 | 0 | 5.87 | 71 |
Derogation of attractive alternatives
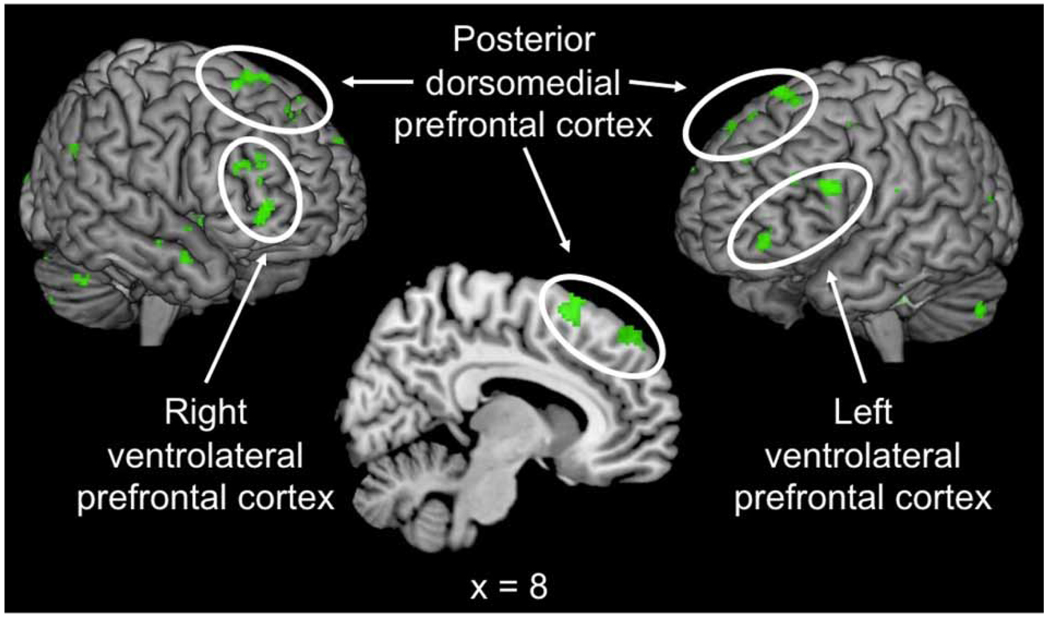
The main hypothesis in our study is that the derogation of attractive alternatives, a form of implicit emotion regulation, may be associated with activation in brain regions known to be associated with intentional emotion regulation. To test this hypothesis, we contrasted activation in the reject > accept comparison within no time pressure compared to time pressure conditions. Specifically, we searched for activations showing an interaction between decision (accept/reject) and time pressure for attractive targets in the contrast: [reject attractive > accept attractive during no time pressure] > [reject attractive > accept attractive during time pressure]. This is a highly specific analysis because it compares rejecting attractive alternatives when cognitive resources are available to rejecting those same targets when resources are not available, thereby controlling for general rejection effects such as response bias or random responding. Whole-brain analysis of this contrast showed significant activation in the bilateral VLPFC and pDMPFC (centred for RVLPFC at: x = 34, y = 46, z = 0 and x = 32, y = 38, z = 14; LVLPFC at: x = −48, y = 24, z = 20; pDMPFC at: x = 4, y = 22, z = 68; Figure 3, Table 1), and the RVLPFC activation remained significant in an ROI analysis (p < .001). Figure 3 shows activation and Figure 4 parameter estimates from the RVLPFC for each of the four conditions of the interaction minus their baseline activity, indicating that RVLPFC is most significant when participants reject attractive alternatives under no time pressure. Similar patterns of results for this interaction were also found for the LVLPFC and pDMPFC (Table 2).
Figure 3.
Activation in the bilateral ventrolateral prefrontal cortex (VLPFC) and posterior dorsomedial prefrontal cortex (pDMPFC) showed significantly increased activation when participants rejected compared to accepted attractive alternatives in the no time pressure compared to time pressure conditions, t(13) = 3.87 for right VLPFC (RVLPFC), t(13) = 3.75 for left VLPFC (LVLPFC) and t(13) = 4.06 for pDMPFC, p < .005. [To view this figure in colour, please visit the online version of this Journal.]
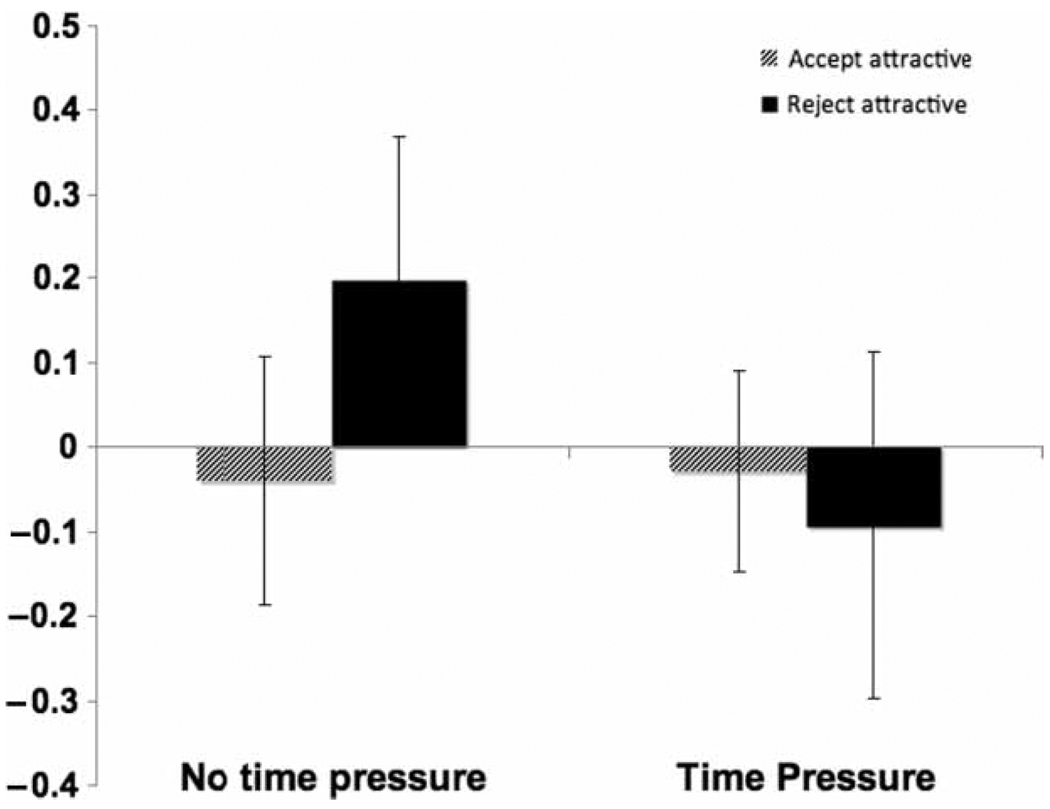
Figure 4.
Activation in the RVLPFC for each of the four conditions of the interaction minus their baseline activity (NTP accept attractive – base; NTP reject attractive – base; TP accept attractive – base; TP reject attractive – base), indicating that RVLPFC is most significant when participants reject attractive alternatives under no time pressure. Similar pattern of results for this interaction were also found for the LVLPFC and pDMPFC.
Table 2.
Brain regions showing increased activity while rejecting compared to accepting attractive alternatives in no time pressure compared to time pressure conditions
| Region | Laterality | x | y | z | t | Voxels |
|---|---|---|---|---|---|---|
| Ventrolateral PFC | R | 34 | 46 | 0 | 3.87 | 21 |
| R | 32 | 38 | 14 | 3.87 | 26 | |
| L | −48 | 24 | 20 | 3.75 | 27 | |
| Dorsomedial PFC | R | 4 | 22 | 68 | 4.06 | 32 |
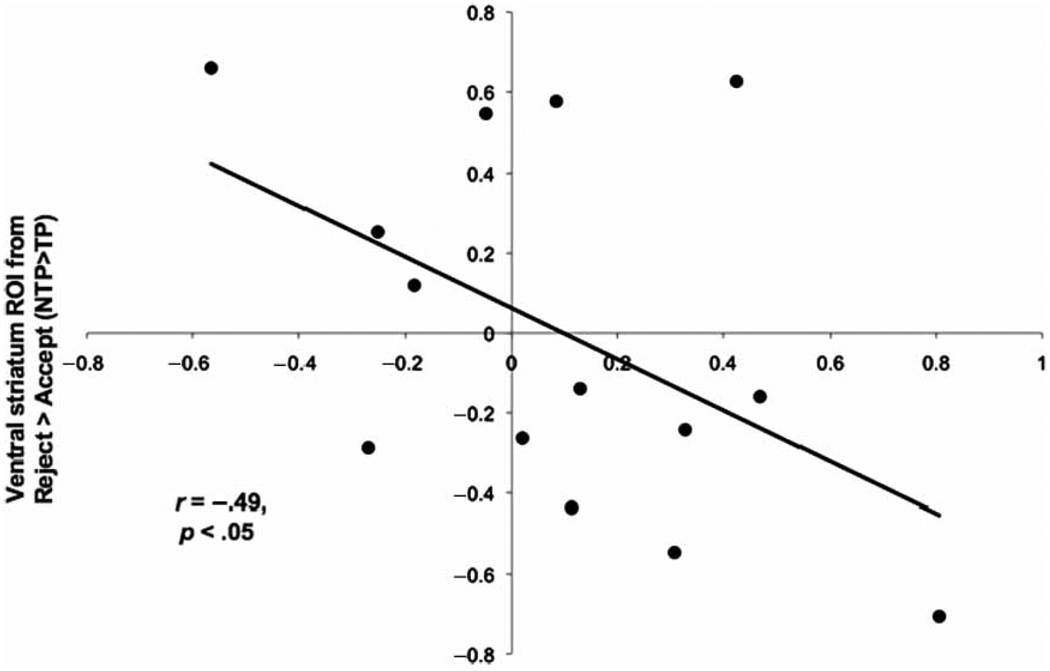
Based on an emotion regulation model of the derogation of alternatives effect whereby individuals are motivated to reduce their attraction to alternatives, the magnitude of reward-related neural activation in response to attractive alternatives should be associated with the magnitude of regulation-related activation. To test this possibility, we correlated activation associated with emotion regulation with activation associated with the reward of viewing an attractive photo. Activation in the RVLPFC ROI during the derogation of attractive targets while under no time pressure compared to time pressure conditions: [reject attractive > accept attractive during no time pressure] > [reject attractive > accept attractive during time pressure] significantly negatively correlated with activation in the VS ROI from the same contrast (r = −.49, p < .05; Figure 5). Activation from LVLPFC and pDMPFC during this contrast, however, did not significantly correlate with VS activation.
Figure 5.
Activation in the ventral striatum ROI and RVLPFC ROI from the contrast comparing rejected compared to accepted attractive alternatives during no time pressure compared to time pressure conditions negatively correlated, r = −.49, p < .05.
Association with relationship investment
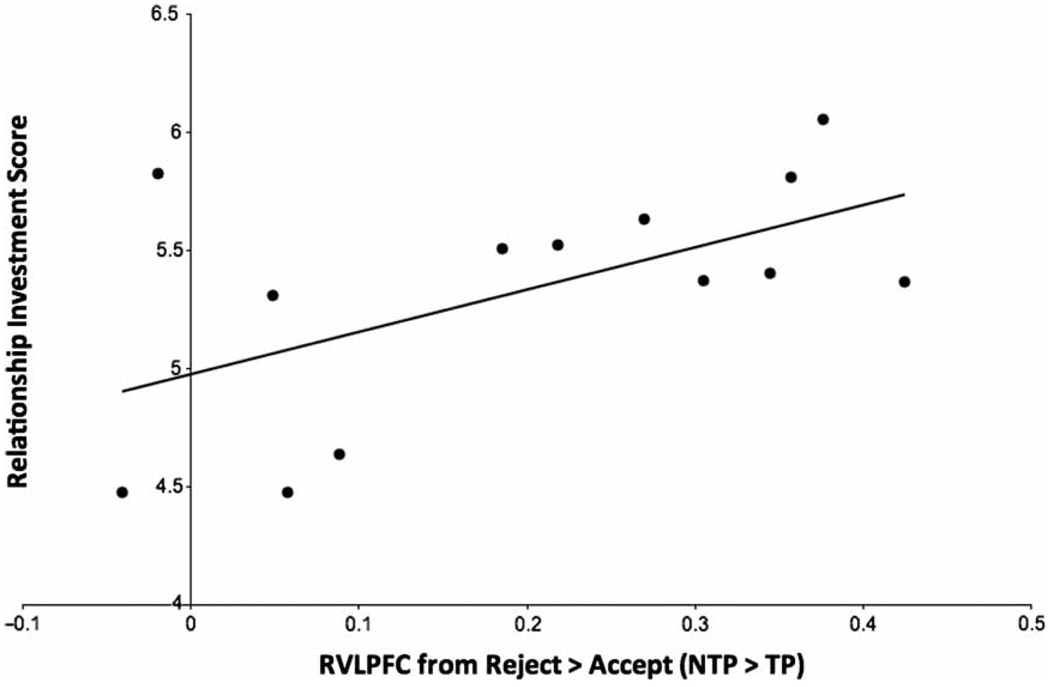
Based on findings reviewed above showing that derogation is moderated by investment in the relationship, we predicted that relationship investment should correspond with the extent of activation in emotion regulation regions during derogation. We searched within a functionally defined RVLPFC cluster using a 0.05 false discovery rate (FDR) correction based on that volume. The RVLPFC cluster was itself identified based on a 0.05 FDR corrected whole-brain search of the reject > accept (NTP > TP) contrast. Consistent with our prediction, a subset of voxels from the RLVPFC cluster (centred at: x = 34, y = 42, z = 10; x = 39, y = 34, z = 14) that were significant in the FDR corrected whole-brain search in the reject > accept (NTP > TP) contrast (p < .05), positively correlated with relationship investment scores (r = .56, p < .05; Figure 6). The relationship scores reported in this correlation exclude one outlier, whose score was more than two standard deviations below the mean. It is worth noting that when the outlier is included, activation from the LVLPFC and pDMPFC did not significantly correlate with investment, r = .42, p = .13.
Figure 6.
Activation in the RVLPFC when participants rejected compared to accepted attractive alternatives while under no time pressure compared to time pressure correlated with participants’ self-reported investment in their current relationship, r = .56, p < .05. One outlier participant, whose relationship investment score was more than 2 standard deviations below the mean, was removed from this figure.
DISCUSSION
The current study adds to the growing body of research on the mechanisms guiding implicit forms of emotion regulation by examining neural activation associated with a real-world, implicit emotion-regulation strategy. We found that romantically involved individuals’ regulation of attraction to an alternative partner corresponded with increases in bilateral VLPFC and pDMPFC. Moreover, for derogation when cognitive resources were available, the extent of RVLPFC activation negatively correlated with activation in the ventral striatum, suggesting that recruiting the RVLPFC may help minimise the felt attraction to an alternative partner. Consistent with relationship research showing that the derogation effect increases with relationship commitment, we found that the degree of RVLPFC activation also corresponded with participants’ self-reported level of investment in their relationship as measured by global investment scores (Rusbult et al., 1998).
In the present paradigm, participants decided whether they would consider attractive and unattractive alternatives as hypothetical romantic partners. As expected, participants were significantly faster to respond under cognitive load (“time pressure” trials) than when not under cognitive load (“no time pressure” trials), suggesting that in the former they relied on more time-and resource-intensive response strategies whereas in the latter they relied on more automatic, unregulated response strategies. Importantly, post-scan interviews indicated that participants did not knowingly (i.e., consciously) attempt to regulate their attraction in any of the conditions, suggesting that the response strategy engaged during the no load trials might be characterised as occurring outside of awareness (i.e., implicit) yet resource intensive.
It is worth mentioning that we did not replicate previous behavioural findings (Ritter et al., 2010) that, at the group level, participants accept significantly fewer attractive alternatives in the no time pressure compared to time pressure condition. This likely reflects our small sample size (N = 14). Nonetheless, we were able to narrow in on neural activation associated with derogation under no load compared to load conditions. We specifically examined neural activation for trials in which participants rejected attractive alternatives during the no time pressure and time pressure conditions. In this comparison there is significantly increased activation in LVLPFC, RVLPFC and pDMPFC, suggesting that although the derogation effect could not be captured behaviourally in our study due to small sample size, within-person level comparison in a trial-by-trial analysis suggests that the derogation effect relies on regions associated with both implicit and deliberate emotion regulation.
The present findings point to the value of brain imaging techniques in detecting psychological differences in the face of limited behavioural differences. In our comparison, participants derogated attractive alternatives on trials with and without cognitive load. Apart from the reaction time differences, the load versus no load behavioural responses appear similar. However, comparison of the blood oxygenated signal between these trials suggests that different psychological mechanisms guide these similar behaviours, with the no cognitive load condition relying on brain structures known to engage in emotion regulation.
To date, most studies examining the neural basis of emotion regulation focus on brain areas engaged during deliberate emotion regulation. However, there is growing interest in the mechanisms guiding implicit forms of emotion regulation in which individuals automatically and/or spontaneously regulate their emotional response. Consistent with this new body of research, we found that activation in the RVLPFC significantly increased when romantically involved individuals rejected attractive targets when cognitive resources were available, even though they were not instructed to regulate their attraction and did not report being aware of doing so. Additionally, the extent of activation of the RVLPFC corresponded with reduced ventral striatum activation during the observation of attractive targets, consistent with the notion that this regulation was successful in minimising the positive emotional reaction in response to viewing an attractive alternative partner. Previous brain imaging studies of implicit or unintended emotion regulation show RVLPFC increases and amygdala decreases during emotion-labelling (Hariri et al., 2000; Lieberman et al., 2007) and inhibition to emotional stimuli (Berkman et al., 2009). These findings converge to suggest that the RVLPFC plays a role in adjusting an emotional response in the limbic system—albeit regulation of a negative response in the amygdala or positive response in the ventral striatum.
To interpret the role of the RVLPFC in implicit regulation, it is worth mentioning that the cognitive mechanisms involved in the derogation of attractive alternatives may be similar to those engaged in rationalisation of choices post cognitive dissonance. In decision making, when individuals are faced with an attractive alternative option after they have already committed to a previous option, they report that the option they originally chose is superior to the alternative choice, even when options were initially rated as equally desirable (Brehm, 1956; Harmon-Jones & Harmon-Jones, 2002). In these situations, people adjust their initial attitudes (in which they report that options are equally desirable) to be in line with their choice. Importantly, research on cognitive dissonance suggests that the motivation to rationalise a decision is unconscious, yet effortful cognitive processes engage in service of the unconscious goal. Similarly, although participants in our study did not have a conscious intent to regulate their affective response to the pictures, they may have had a non-conscious goal to be satisfied with their relationship partner. This may promote effortful processes that never rise to the level of consciousness but still ensure that the goal is met. In this sense, derogation of alternatives may be considered “conditionally automatic” because the learned process of relationship maintenance is triggered and occurs outside of awareness, though still requires effort (Bargh, 1989).
Consistent with this suggestion, a recent neuroimaging study found that the extent of activation in the right inferior frontal gyrus, which shares anatomical overlap with the RVLPFC, positively corresponded with degree of attitude change post cognitive dissonance, and activation in RVLPFC correlated with reduced activation in the insula (Jarcho, Berkman, & Lieberman, in press), an area implicated in affective distress (Sanfey, Rilling, Aronson, Nystrom, & Cohen, 2003; reviewed by Ochsner & Gross, 2005). To the extent that attractive alternatives induce cognitive dissonance in romantically involved individuals, the RVLPFC may aid a shift toward decision-consistent attitudes in favour of their partner.
In addition to the RVLPFC activation, the LVLPFC and pDMPFC, regions typically associated with deliberate emotion regulation (McRae et al., 2010; Ochsner, Hughes, Robertson, Cooper,&Gabrieli, 2009a), were also significantly active when participants derogated attractive alternatives during the no time pressure condition. Recruitment of these regions may reflect the fact that the derogation effect, unlike other “pure” implicit emotion regulation processes like affect labelling, incorporates aspects of deliberate regulation. That is, the derogation of alternatives is similar to antecedent-focused strategies (Gross, 1998a, 1998b, 1999), because individuals seem to alter the attractiveness of the external stimuli to minimise attraction. Also, the derogation effect occurs only when cognitive resources are available.
The fact that the LVLPFC and pDMPFC, but not the dorsolateral PFC (DLPFC), significantly engaged during this contrast is consistent with this suggestion. The DLPFC likely plays a key role in allocating attentional resources during deliberate emotion regulation, whereas VLPFC and pDMPFC seem to be more integral to reappraising a stimulus over and above the attentional cost of doing so (McRae et al., 2010). Thus, it may be that the LVLPFC and pDMPFC contribute to the deliberate components of the derogation effect, whereas the RVLPFC plays a particularly important role in regulating an emotional response implicitly.
Future research should be able to test the relative roles of the VLPFC and pDMPFC in deliberate and implicit regulation by directly comparing deliberate and implicit emotion-regulation strategies within the same study. Surprisingly, no such study has been published to date. It is likely that this reflects the difficulty in inducing implicit emotion regulation in the laboratory. However, findings from the current study indicate that studying emotion regulation in the context of romantic relationships in general, and the derogation of attractive alternatives effect in particular, may be a promising paradigm to study the similarities and differences between implicit and deliberate forms of emotion regulation. For example, it would be interesting to compare whether asking romantically involved participants to deliberately try to change their attractiveness rating of an alternative (deliberate emotion regulation) to trials in which participants are not instructed to derogate but spontaneously do devalue a target’s attractiveness (implicit emotion regulation) is more or less effective a strategy in regulating attraction.
In addition to broadening the scientific understanding of implicit emotion regulation, the present study offers some insight into the physiological mechanisms that may help individuals maintain romantic relationships. Findings from behavioural studies seem to converge on the idea that partners’ self-regulatory ability may be a crucial predictor of relationship satisfaction and longevity (Finkel & Campbell, 2001; Karremans & Verwijmeren, 2008; Pronk, Karremans, Overbeek, Vermult, & Wigboidus, 2010; Ritter et al., 2010). For example, regulatory capacity has been shown to predict whether participants respond constructively to the romantic partner during conflict (e.g., Finkel & Campbell, 2001; Tangney, Baumeister, & Boone, 2004) particularly with forgiving of partner’s offences (Pronk et al., 2010). In extension of Ritter et al.’s (2010) finding that romantic partners derogate the appeal of an attractive alternative partner more when cognitive resources are high rather than low, we found that when cognitive resources are available (no time pressure condition), romantic partners recruit more RVLPFC activation when judging attractive alternatives compared to when their cognitive resources are depleted (time pressure condition).
One potential confound in our design is that the cognitive load condition induced stress either instead of or in addition to cognitive load. However, this is unlikely as previous research using a similar manipulation of cognitive load found no difference in stress rating between the high- and low-stress conditions (Ward & Mann, 2000, Study 2). Either way, the implication of these results may be that when cognitive resources are temporarily low, and/or in the presence of stress, individuals are unable to recruit relationship maintenance strategies.
On the other hand, available cognitive resources are likely not the sole process underlying implicit emotion-regulation strategies in romantic relationships. Previous behavioural studies find that the extent to which one devalues an attractive alternative correlates with their investment in their relationship (Johnson & Rusbult, 1989; Karremans & Verwijmeren, 2008; Ritter et al., 2010). In extension of these findings, the present study found that the extent to which individuals recruited the RVLPFC during the derogation of alternatives corresponds with their level of relationship investment. Thus, in the case of romantic relationships, implicit forms of emotion regulation may be inextricably linked with motivation to protect the relationship. An interesting question for future research would be to disentangle whether the extent to which one is good at spontaneously derogating an attractive alternative leads one to be more invested in one’s relationships, or whether increasing motivation to be in the relationship lends implicit derogation.
In conclusion, the current study demonstrates that the derogation of attractive alternatives by individuals in relationships corresponds with neural activity known to engage in both implicit and deliberate forms of emotion regulation. That RVLPFC activity during derogation correlated with relationship investment highlights the importance of considering motivation to regulate emotions as an important factor in implicit, everyday forms of emotion regulation. Studying implicit emotion regulation in the context of romantic relationships should be a promising direction to study real-world forms of implicit emotion regulation in both psychological and brain-imaging laboratories.
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
REFERENCES
- Bargh JA. Conditional automaticity: Varieties of automatic influence on social perception and cognition. In: Uleman J, Bargh J, editors. Unintended thought. New York, NY: Guilford Press; 1989. pp. 3–51. [Google Scholar]
- Berkman ET, Burklund L, Lieberman MD. Inhibitory spillover: Intentional motor inhibition produces incidental limbic inhibition via right inferior frontal cortex. NeuroImage. 2009;47:705–712. doi: 10.1016/j.neuroimage.2009.04.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman ET, Lieberman MD. Using neuroscience to broaden emotion regulation: Theoretical and methodological considerations. Social and Personality Psychology Compass. 2009;3:475–493. doi: 10.1111/j.1751-9004.2009.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm JW. Postdecision changes in the desirability of alternatives. The Journal of Abnormal and Social Psychology. 1956;52:384–389. doi: 10.1037/h0041006. [DOI] [PubMed] [Google Scholar]
- Eder AB, Rothermund K. Automatic influences of arousal information on evaluative processing: Valence-arousal interactions in an affective Simon task. Cognition and Emotion. 2010;24:1053–1061. [Google Scholar]
- Enger T, Etkins A, Gale S, Hirsch J. Dissociable neural systems resolve conflict from emotional versus non-emotional distracters. Cerebral Cortex. 2008;18:1475–1484. doi: 10.1093/cercor/bhm179. [DOI] [PubMed] [Google Scholar]
- Etkins A, Enger T, Pereza DM, Kandel ER, Hirsch J. Resolving emotional conflict: A role for the rostral anterior cingulated cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Finkel EJ, Campbell WK. Self-control and accommodation in close relationships: An interdependence analysis. Journal of Personality and Social Psychology. 2001;2:263–277. doi: 10.1037//0022-3514.81.2.263. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Antecedent- and response-focused emotion regulation: Divergent consequences for experience, expression, and physiology. Journal of Personality & Social Psychology. 1998a;74:224–237. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Gross JJ. The emerging field of emotion regulation: An integrative review. Review of General Psychology. 1998b;2:271–299. [Google Scholar]
- Gross JJ. Emotion regulation: Past, present, future. Cognition and Emotion. 1999;13:551–573. [Google Scholar]
- Gross JJ, editor. Handbook of emotion regulation. New York, NY: Guilford Press; 2007. [Google Scholar]
- Gross JJ, Thompson RA. Emotion regulation: Conceptual foundations. In: Gross JJ, editor. Handbook of emotion regulation. New York, NY: Guilford Press; 2007. pp. 3–24. [Google Scholar]
- Haber SN, Knutson B. The reward circuit: Linking primate anatomy and human neuroimaging. Neuropsychopharmacology Reviews. 2009;35:1–23. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Davidson MC, Glover GH, Casey BJ. Contributions of amygdala and striatal activity in emotion regulation. Biological Psychiatry. 2005;57(6):624–632. doi: 10.1016/j.biopsych.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: Effects of a neocortical network on the limbic system. NeuroReport. 2000;11:43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Harmon-Joes C. Testing the action-based model of cognitive dissonance: The effect of action orientation on postdecisional attitudes. Personality and Social Psychology Bulletin. 2002;28:711–723. [Google Scholar]
- Jarcho JM, Berkman ET, Lieberman MD. The neural basis of rationalization: Cognitive dissonance reduction during decision-making. Social Cognitive and Affective Neuroscience. doi: 10.1093/scan/nsq054. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DJ, Rusbult CE. Resisting temptation: Devaluation of alternative partners as a means of maintaining commitment in close relationships. Journal of Personality and Social Psychology. 1989;57:967–980. [Google Scholar]
- Kampe KKW, Frith CD, Dolan RJ, Frith U. Reward value of attractiveness and gaze. Nature. 2001;413:589–590. doi: 10.1038/35098149. [DOI] [PubMed] [Google Scholar]
- Karremans JC, Verwijmeren T. Mimicking attractive opposite-sex others: The role of romantic relationship status. Personality and Social Psychology Bulletin. 2008;34(7):939–950. doi: 10.1177/0146167208316693. [DOI] [PubMed] [Google Scholar]
- Kelly HH, Thibaut JW. Interpersonal relations: A theory of interdependence. New York, NY: Wiley; 1978. [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Polis S, Alexander AL, Shin LM, et al. Contextual modulation of amygdala responsivity to surprised faces. Journal of Cognitive Neuroscience. 2004;16:1730–1745. doi: 10.1162/0898929042947865. [DOI] [PubMed] [Google Scholar]
- Koole SL. The psychology of emotion regulation: An integrative review. Cognition and Emotion. 2009;23:4–41. [Google Scholar]
- Koole SL, Jostmann NB. Getting a grip on your feelings: Effects of action orientation and external demands on intuitive affect regulation. Journal of Personality and Social Psychology. 2004;87:974–990. doi: 10.1037/0022-3514.87.6.974. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: Re-balancing the scale. Social Cognitive Neuroscience. 2009;4:423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words: Affect labeling disrupts amygdala activity to affective stimuli. Psychological Science. 2007;18:421–428. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- Lydon JE, Fitzsimons GM, Naidoo L. Devaluation versus enhancement of attractive alternatives: A critical test using the calibration paradigm. Personality and Social Psychology Bulletin. 2003;29:349–359. doi: 10.1177/0146167202250202. [DOI] [PubMed] [Google Scholar]
- Lydon JE, Maena M, Sepinwalls D, Richards N, Mayman S. The commitment calibration hypothesis: When do people devaluate attractive alternatives? Personality and Social Psychology Bulletin. 1999;25:152–161. [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mauss IB, Bunge SA, Gross JJ. Automatic emotion regulation. Social and Personality Psychology Compass. 2007;1:146–167. [Google Scholar]
- Mauss IB, Evers C, Wilhelm FH, Gross JJ. How to bite your tongue without blowing your top: Implicit evaluation of emotion regulation predicts affective responding to anger provocation. Personality and Social Psychology Bulletin. 2006;32:589–602. doi: 10.1177/0146167205283841. [DOI] [PubMed] [Google Scholar]
- McRae K, Hughes B, Chopra S, Gabrieli JDE, Gross JJ, Ochsner KN. The neural bases of distraction and reappraisal. Journal of Cognitive Neuroscience. 2010;22:248–262. doi: 10.1162/jocn.2009.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Hughes BL, Robertson E, Cooper JC, Gabrieli J. Neural systems supporting the control of cognitive and affective conflict. Journal of Cognitive Neuroscience. 2009a;21:1841–1854. doi: 10.1162/jocn.2009.21129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Ray RR, Hughes B, McRae K, Cooper JC, Weber J, et al. Bottom-up and top-down processes in emotion generation: Common and distinct neural mechanisms. Psychological Science. 2009b;20:1322–1331. doi: 10.1111/j.1467-9280.2009.02459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronk TM, Karremans JC, Overbeek G, Vermult AA, Wigboidus D. What it takes to forgive: When and why executive functioning facilitates forgiveness. Journal of Personality and Social Psychology. 2010;98:119–131. doi: 10.1037/a0017875. [DOI] [PubMed] [Google Scholar]
- Overall CN, Sibley GC. Attachment and attraction toward romantic partners versus relevant alternatives within daily interactions. Personality and Individual Differences. 2007;44:1126–1137. [Google Scholar]
- Ritter SM, Karremans JC, van Schie HT. The role of self-regulation in derogating attractive alternatives. Journal of Experimental Social Psychology. 2010;46:631–637. [Google Scholar]
- Rothermund K, Voss A, Wentura D. Counter-regulation in affective attentional biases: A basic mechanism that warrants flexibility in emotion and motivation. Emotion. 2008;8:34–46. doi: 10.1037/1528-3542.8.1.34. [DOI] [PubMed] [Google Scholar]
- Rusbult CE, Martz JM, Agnew CR. The investment model scale: Measuring commitment level, satisfaction level, quality of alternatives, and investment size. Personal Relationships. 1998;5:357–391. [Google Scholar]
- Rusbult CE, Olsen N, Davis JL, Hannon PA. Commitment and relationship maintenance mechanisms. In: Reiss HT, Rusbult CE, editors. Close relationships. New York, NY: Psychology Press; 2004. pp. 287–303. [Google Scholar]
- Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD. The neural basis of economic decision-making in the Ultimatum Game. Science. 2003;300:1755–1758. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- Schweiger-Gallo I, Keil A, McCulloch KC, Rockstroh B, Gollwitzer PM. Strategic automation of emotion regulation. Journal of Personality and Social Psychology. 2009;96:11–31. doi: 10.1037/a0013460. [DOI] [PubMed] [Google Scholar]
- Simpson JA, Gangestad SW, Lerma M. Perception of physical attractiveness: Mechanisms involved in the maintenance of romantic relationships. Journal of Personality and Social Psychology. 1990;59:1192–1201. [Google Scholar]
- Tangney JP, Baumeister RF, Boone AL. High self-control predicts good adjustment, less pathology, better grades, and interpersonal success. Journal of Personality. 2004;72:271–322. doi: 10.1111/j.0022-3506.2004.00263.x. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Van Leeuwen ML, Macrae CN. Is beautiful always good? Implicit benefits of facial attractiveness. Social Cognition. 2004;22:637–649. [Google Scholar]
- Ward A, Mann T. Donșt mind if I do: Disinhibited eating under cognitive load. Journal of Personality and Social Psychology. 2000;78:753–763. doi: 10.1037//0022-3514.78.4.753. [DOI] [PubMed] [Google Scholar]
- Williams LE, Bargh JA, Nocera CC, Gray JR. The unconscious regulation of emotion: Nonconscious reappraisal goals modulate emotional reactivity. Emotion. 2009;9:847–854. doi: 10.1037/a0017745. [DOI] [PMC free article] [PubMed] [Google Scholar]