Methods to target/isolate aorta resident immunocytes and study their angiogenic behavior.
Keywords: M-CSF, GM-CSF, IL-4, MHC II, dendritic cells, aortic ring, collagen
Abstract
Angiogenesis in the aortic ring model is preceded by activation of the immune system and impaired by ablation of adventitial macrophages. Treatment of aortic cultures with M-CSF induced extensive periaortic outgrowth of CD45+ CD68+ mononuclear cells with ultrastructural features of macrophages and DCs. Periaortic lysis of collagen caused many CD45+ CD68+ cells to attach to the bottom of the culture dish. Lifting the collagen gels left behind patches of CD45+ CD68+ cells, which focally organized into branching cords. These cells also expressed CD14, CD169, F4/80, and α-SMA but not CD31, vWF, desmin, or CD163. DNA synthesis studies showed that M-CSF-stimulated cells were actively proliferating. Aortic patch cells showed phagocytic properties and responded to IL-4 and GM-CSF by expressing MHC II, differentiating into DCs, and forming multinucleated giant cells. They also stimulated angiogenesis and VEGF production in aortic ring cultures. This study demonstrates that the rat aorta contains a distinct subset of immature immunocytes capable of proliferating, differentiating into macrophages and DCs, and stimulating angiogenesis. Isolation of these cells in patches from M-CSF-stimulated aortic rings provides a reproducible system to study the biology and angiogenic role of the resident immune system of the aortic wall.
Introduction
Angiogenesis, the formation of new blood vessels, plays an important role in many physiologic and pathologic processes. Neovessels are essential for embryogenesis, fetal development, and wound healing but also contribute to the progression of cancer, atherosclerosis, and other life-threatening diseases [1]. Neovessels develop from the endothelium of pre-existing vessels in response to soluble angiogenic factors produced by a variety of cell types including inflammatory cells [2]. Among these, monocytes/macrophages have emerged as important regulators of the angiogenic response [3, 4].
Macrophages are typically associated with neovessels as part of the inflammatory reaction that invariably accompanies the angiogenic response [5]. The macrophage populations present in the inflammatory infiltrate originate mostly from circulating bone marrow-derived monocytes but also comprise a component of tissue resident macrophages [6, 7], which appear first during embryonal development in the blood islands of the yolk sac from which they migrate into the bloodstream to colonize the liver and other organs prior to the onset of bone marrow hemopoiesis [8]. These cells proliferate and survive after birth into adulthood as resident macrophages capable of self-renewal [7].
In response to molecular cues generated by hypoxic or injured tissues, macrophages produce a broad array of proangiogenic growth factors and cytokines [4, 9]. Through their ability to modulate angiogenesis, macrophages regulate the healing of wounds [10, 11], the expansion of adipose tissue [12, 13], the morphogenesis of islet cells [14], the remodeling of arterial vessels [15, 16], and the progression of cancer [17]. These cells also regulate the angiogenic response of aortic rings in collagen gel culture [18].
The majority of the studies about macrophage-induced angiogenesis is based on bone marrow-derived macrophages. Limited data are available about the angiogenic role of tissue resident macrophages. We observed recently that pharmacological ablation of resident adventitial macrophages markedly impaired the angiogenic response of injured aortic rings in ex vivo collagen gel cultures [18]. These observations indicate that the resident immune system is a requisite component of the mechanisms that sense the injury of the dissection procedure and trigger the angiogenic response of the aortic rings.
A major limitation to the study of the aortic immune system has been the lack of suitable methods for the isolation and expansion of aorta resident immune cells. We report here that aortic ring cultures treated with M-CSF produce extensive outgrowths of CD45+ CD68+ immature mononuclear cells. M-CSF-stimulated cells form patches on the bottom of aortic ring cultures, which can be analyzed with immunocytochemical, immunochemical, molecular, and functional methods. These cells have the capacity to proliferate, take up fluorescent beads through phagocytosis, differentiate into DCs, form multinucleated giant cells, express α-SMA, organize into branching cords, and stimulate angiogenesis. M-CSF-stimulated aortic cultures provide a reproducible system to study the biology and angiogenic role of resident aortic immunocytes.
MATERIALS AND METHODS
Antibodies
Mouse mAb against rat CD45 (leukocyte common antigen, MCA43R, clone OX-1), CD68 (MCA341GA, clone ED1), CD163 (MCA342GA, clone ED2), CD169 (MCA343GA, clone ED3) [19, 20], MHC II molecules (MCA2687GA, clone OX-6), OX-62 (MCA1029G, clone MRC OX-62), F4/80 (MCA497GA, clone CI:A3-1), and desmin (MCA849, clone D33) were purchased from AbD Serotec (Raleigh, NC, USA). Mouse mAb against CD31 (platelet endothelial cell adhesion molecule 1, ab64543, clone 2Q854) and rabbit polyclonal to α-SMA (ab5694) were obtained from Abcam (Cambridge, MA, USA). Rabbit polyclonal antibody anti-human vWF (F3520) and mouse monoclonal anti-α-SMA (A5228, clone 1A4) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Mouse IgG isotype control was from R&D Systems (Minneapolis, MN, USA). Alexa Fluor 488- or 568-conjugated donkey anti-mouse, Alexa Fluor 488- or 568-conjugated goat anti-rabbit secondaries, and Alexa Fluor 568-conjugated Griffonia simplicifolia isolectin B4 were obtained from Invitrogen (Carlsbad, CA, USA).
Reagents
EBM was obtained from Lonza (Walkersville, MD, USA). Rat rM-CSF and rat rGM-CSF were from PeproTech Inc. (Rocky Hill, NJ, USA). Rat rIL-4 and Quantikine rat VEGF ELISA were obtained from R&D Systems. Neutral-buffered formalin (10%) was purchased from Biochemical Sciences Inc. (Swedesboro, NJ, USA). Collagen was isolated from rat tails as described [21]. The Click-iT EdU assay kit (Invitrogen) was used as a proliferation assay. Fluorescent latex beads (diameter 1 μm) for phagocytosis experiments were provided by Sigma-Aldrich.
Collagen gel cultures of rat aorta
All animal procedures were performed in accordance with Veterans Administration Puget Sound Health Care System Institutional Animal Care and Use Committee and National Institutes of Health guidelines. Thoracic aortas were dissected from killed 1- to 2-month-old Fischer 344 male rats (Harlan, Indianapolis, IN, USA), cleaned of fibroadipose tissue and blood, rinsed in several washes of EBM, and serially cross-sectioned into 1–2 mm rings, as described [22]. The aortic rings were embedded in 30 μl collagen gels and cultured in 16 mm wells (4-well Nunc dishes), each containing 500 μl serum-free EBM [23]. The aortic ring cultures were kept in a humidified CO2 incubator at 37°C. The medium was changed 3 times/week starting from Day 3.
Measurement of angiogenesis
The angiogenic response of aortic cultures was measured by counting the number of neovessels over time using a CK40 Olympus inverted microscope (Olympus American, Melville, NY, USA) [22]. Images of live or formalin-fixed cultures were captured with an Olympus MagnaFire S99800 digital camera (Olympus American) mounted on an IX71 Olympus inverted microscope.
Preparation of rat aortic patches
Patches of CD68+ cells were obtained by treating aortic ring cultures with 500 ng/ml M-CSF (PeproTech Inc.) for 14 days. Medium was changed twice/week. At Day 14 of treatment, collagen gels with embedded aortic rings were removed, leaving behind cellular patches on the bottom of the culture dish.
Cell isolation
Endothelial and mural cells, used as positive controls for immunohistochemical and RT-PCR studies, were isolated from the rat aorta as described [24, 25].
Immunoperoxidase histochemistry
Expression of proteins of interest in formalin-fixed whole-mounts of aortic ring cultures and in formalin-fixed aortic patches was evaluated using immunoperoxidase staining [23]. Collagen gel cultures and aortic patches were fixed in 10% neutral-buffered formalin for 10 min, washed twice with PBS, and stored in deionized water at 4°C for at least 12 h. Endogenous peroxidase was quenched with 3% hydrogen peroxide for 10 min. Samples were blocked in PBS with 0.1% BSA and 0.1% Tween 20 (Sigma-Aldrich) O.N. at 4°C, stained O.N. at 4°C with primary antibody diluted 1:100, washed in PBS (3×10 min), incubated for 2 h with biotin-conjugated secondary antibody diluted 1:100, and rinsed in PBS (3×10 min). Reactions were visualized with the standard Vestastain ABC kit and DAB, according to the manufacturer's recommendations. After washing in PBS (2×10 min), collagen gel cultures were mounted in an Aqua Polymount (Polysciences, Warrington, PA, USA) medium on glass slides and examined with an Olympus BX40 microscope. Immunostained aortic patches in culture dishes were visualized on an IX71 Olympus inverted microscope. Images were captured with Olympus MicroFire digital cameras.
Double immunofluorescence staining and confocal microscopy
For double immunofluorescence staining, patch cells were reacted with anti-CD68 mouse mAb and anti-α-SMA rabbit polyclonal antibodies followed by Alexa Fluor 488-conjugated goat anti-mouse and Alexa Fluor 568-conjugated goat anti-rabbit secondaries. Immunostained samples were mounted in Aqua Polymount (Polysciences). Images were taken with a Leica TCS-SP laser-scanning confocal microscope. Confocal images were obtained by z-plane analysis, followed by projection and overlay using Leica software.
Phagocytic assay
Phagocytic capacity of rat aorta patch cells was analyzed by studying the incorporation of fluorescent latex beads by these cells. Aortic patches were incubated O.N. in a humidified CO2 incubator at 37°C with latex beads (diluted 1:10,000 in EBM). Patches were then rinsed in PBS, fixed in 10% buffered formalin for 10 min, blocked O.N. in PBS with 0.1% BSA (Sigma-Aldrich), and stained for 1 h with mouse monoclonal anti-rat CD68. Patches were washed in PBS (10 min×3) and reacted (1 h) with Alexa Fluor 488-conjugated donkey anti-mouse antibody. Patches were then washed in PBS (10 min×2) and examined under an IX71 Olympus inverted microscope equipped with an Olympus MicroFire digital camera.
Proliferation assay
Cell proliferation in aortic ring cultures and patches was measured with the Click-iT EdU flow cytometry assay kit (Invitrogen). EdU, a thymidine analog incorporated during DNA synthesis, was added (15 μM) to day 13 collagen gel cultures and day 0 aortic cell patches. After O.N. incubation in a humidified CO2 incubator at 37°C, samples were rinsed in PBS with 1% BSA and incubated 1 h at room temperature in the dark with mouse anti-rat CD68 or anti-CD45 mAb (1:100). Samples were rinsed in PBS with 1% BSA, fixed with Click-iT fixative for 15 min in the dark at room temperature, and rinsed in PBS with 1% BSA. Samples were permeabilized with Triton X-100 for 30 min at room temperature in the dark, rinsed in PBS with 1% BSA, and incubated for 30 min at room temperature in the dark with a Click-iT reaction cocktail (containing Alexa Fluor 488 azide to visualize EdU+nuclei), followed by a 30-min incubation with Alexa Fluor 568-conjugated donkey anti-mouse antibody. Samples were washed twice in PBS with 1% BSA and visualized under an IX71 Olympus inverted microscope equipped with an Olympus MicroFire digital camera.
Detection of VEGF production by ELISA
VEGF production in control and M-CSF-treated aortic cultures and in cultures of aortic patches was measured with the Quantikine rat ELISA kit (R&D Systems).
RT-PCR
Total RNA from aortic cell patches, rat aortic endothelial cells [24], rat aortic mural cells [26], and bone marrow-derived macrophages [18] was extracted and purified with the RNeasy Micro kit (Qiagen, Valencia, CA, USA) and quantified with a Bioanalyzer 2100 spectrophotometer (Agilent, Palo Alto, CA, USA). cDNA templates for standard PCR were synthesized by RT, according to standard protocol. Briefly, 100 μg/ml total RNA was incubated for 5 min at 65°C with random hexamer primers (Promega, Madison, WI, USA). Each sample was then chilled on ice, incubated for 1 h at 50°C with 50 units Superscript III (Invitrogen), 5 mM dNTP (Promega), 50 mM DTT, 20 units RNase inhibitor (Promega), and 1× first-strand buffer (Invitrogen) in a final volume of 50 μl. Samples were then incubated for 15 min at 70°C, held at 4°C for 10 min, and stored at –20°C. Duplicate reactions were carried out without the Superscript III enzyme as negative controls. Standard PCR was carried out using 2 μl RT reaction templates with 1.5 mM MgCl2, 50 mM KCl, 10 pM each primer, and 1 unit Taq polymerase (Promega) in a final reaction volume of 20 μl. The following primers were used: GAPDH: 5′-CCTCTGGAAAGCTGTGGCGT-3′ and 5′-TTGGAGGCCATGTAGGCCAT-3′; CD68: 5′-CTGTTGCGGAAATACAAGCA-3′ and 5′-GGCAGCAAGAGAGATTGGTC-3′; CD163: 5′-GTGACGTTCCCTGCATAGGT-3′ and 5′-TCCTTGTGCCTGCAGTTATG-3′; CD14: 5′-CAGGAACTTTGGCTTTGCTC-3′ and 5′-CCCATTGAGCCATCTTGATT-3′; CD31: 5′-GCCCTGTCACGTTTCAGTTT-3′ and 5′-CCACGGAGCAAGAAAGACTC-3′; α-SMA: 5′-ACTGGGACGACATGGAAAAG-3′ and 5′-CATCTCCAGAGTCCAGCACA-3′. Thirty cycles of PCR were carried out, each cycle consisting of 30 s at 94°C, 30 s at 54°C, and 30 s at 72°C. PCR products were visualized on 1% agarose gels.
Rat aortic ring aortic patch cocultures
Patches of M-CSF-stimulated cells were washed in EBM and coembedded in collagen gel with freshly cut aortic rings. Controls included cultures of isolated aortic rings or of patches without aortic rings. Vessels were counted every 2 days starting on day 3. Growth medium was changed 3 times/week. Angiogenesis was scored by counting microvessels over time as described [21].
Electron microscopy
For ultrastructural studies, collagen gel cultures of rat aorta were fixed in 2.5% glutaraldehyde, 0.1 M Na cacodylate, pH 7.4, and processed for embedding in EPON-812 (Ted Pella Inc., Redding, CA, USA). Thin sections were stained with uranyl acetate and lead citrate and examined with a transmission electron microscope (Jeol USA, Peabody, MA, USA).
Statistical methods
All studies were repeated a minimum of 3 times, and each experimental group included 4 samples. This approach ensured a minimum of 12 data points per experimental group in all experiments. Data were analyzed with GraphPad statistical software. Unpaired Student's t test was used to evaluate differences between experimental groups. Probability values <0.05 were considered statistically significant.
RESULTS AND DISCUSSION
M-CSF promotes angiogenesis and immune cell outgrowth in aortic ring cultures
Angiogenesis in collagen gel cultures of rat aorta is a self-limited process triggered by the injury of the dissection procedure. Angiogenesis in this model is preceded by activation of the resident immune system and is critically dependent on the proangiogenic signals of adventitial macrophages [18, 27]. In a previous study, we were able to suppress angiogenesis by pharmacologically ablating the resident phagocytic cells of rat aortic rings with liposomal clodronate. We obtained a similar effect by depleting with DT aortic ring macrophages from genetically modified mice carrying the DT receptor under the control of the CD11b promoter [18]. In addition, priming the rat aorta with M-CSF prior to the preparation of the aortic rings enhanced angiogenesis significantly, whereas treatment with an anti-M-CSF antibody suppressed the angiogenic response [18].
To evaluate further the effect of M-CSF on the behavior of aorta resident immune cells, collagen gel cultures were treated continuously with increasing doses of this cytokine (10–500 ng/ml). M-CSF significantly enhanced the angiogenic response of the aortic rings but only if aortic cultures were maintained in the same conditioned medium from the beginning of the experiment without additional feedings. This effect was associated with a 3-fold increase in VEGF levels in cultures treated with high-dose M-CSF (60 pg/ml vs. 20 pg/ml in unstimulated controls, n=4; P<0.01). The proangiogenic effect of M-CSF was reduced significantly by depleting the system of endogenous VEGF through replacement of aorta-conditioned medium with fresh medium. Regardless of the frequency with which growth medium was changed, the roots of M-CSF-treated angiogenic outgrowths were invaded extensively by dense infiltrates of small cells with vacuolated and granular cytoplasm and refractile features under phase-contrast microscopy. These cells were also present, although in relatively small numbers, in control cultures. The M-CSF effect was dose-dependent, and M-CSF-stimulated cells were most numerous in cultures treated with 500 ng/ml M-CSF, where they formed thick and opaque mats around the aortic explants (Fig. 1).
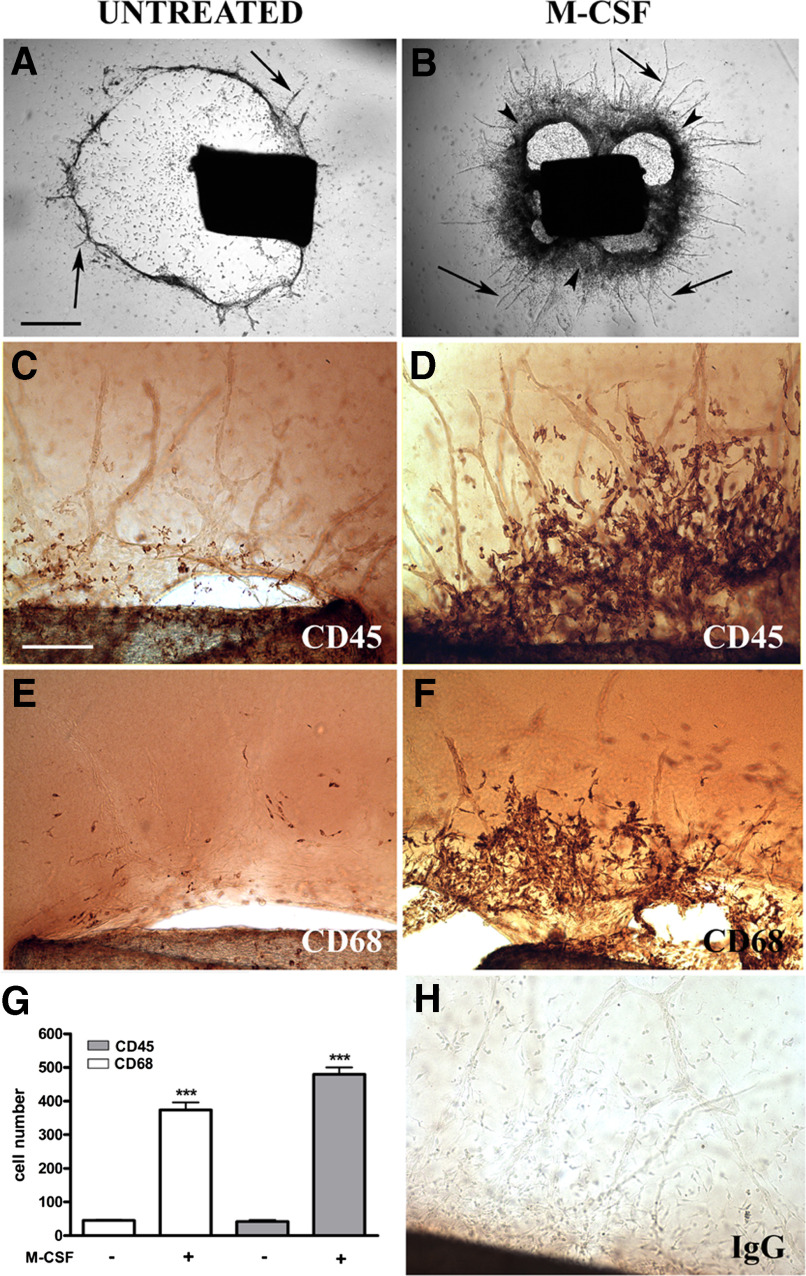
Figure 1. M-CSF promotes the outgrowth of CD45+ CD68+ cells in angiogenic cultures of rat aorta.
Unstimulated, 9-day-old control collagen gel culture of rat aorta undergoing vascular regression shows several residual microvessels (arrows) around a periaortic halo of collagen lysis (A). Parallel culture treated with M-CSF has a dense cellular rim at the roots of the angiogenic outgrowth (arrowheads, B). Immunoperoxidase staining demonstrates that M-CSF-stimulated cells at the root of neovessels are reactive for CD45 (D) and CD68 (F). Small numbers of CD45+ and C68+ cells are identified in unstimulated controls (C and E). Quantitative analysis of immunostained aortic cultures demonstrates a marked increase in the number of CD45+ and C68+ cells following M-CSF treatment (G). Negative control reacted with nonimmune IgG (H). Magnification bars: 1000 μm (A and B, bar shown in A); 200 μm (C–F and H, bar shown in C). ****P < 0.001.
Immunocytochemical and ultrastructural characterization of immune cells in collagen gel cultures of rat aorta
Immunoperoxidase staining of aortic cultures for CD45 confirmed the leukocyte nature of the M-CSF-stimulated cells that had aggregated around the aorta. These cells also stained for CD68, a monocyte/macrophage marker (Fig. 1). Quantitative evaluation of immunostained cultures demonstrated an 8- to 10-fold increase in CD45+ CD68+ cells following M-CSF treatment (n=16 20× fields from 4 cultures; P<0.001). Cells immunoreactive for these markers were identified next to the angiogenic outgrowths, where they attached frequently to the roots and stems of the neovessels (Fig. 2). This angiotropic behavior is likely to facilitate the proangiogenic activity of these cells, as reported previously for circulating mononuclear myeloid cells recruited at sites of angiogenesis in response to VEGF and stromal cell-derived factor 1 production [28]. The capacity of aortic immune cells to closely adhere to the vessel wall is comparable with that of endothelial progenitor cells [29, 30], but we found no evidence that the M-CSF-stimulated cells were incorporated into the endothelial layer of the vessel wall. By electron microscopy, M-CSF-stimulated cells exhibited cytoplasmic vacuoles, lysosomes, pseudopods, and numerous microvillous projections of the cell cytoplasm. Some of these cells also showed elaborate nets of tubular and vesicular structures. Adjacent cells were connected by junctional complexes with gap junction-like features (Fig. 2).
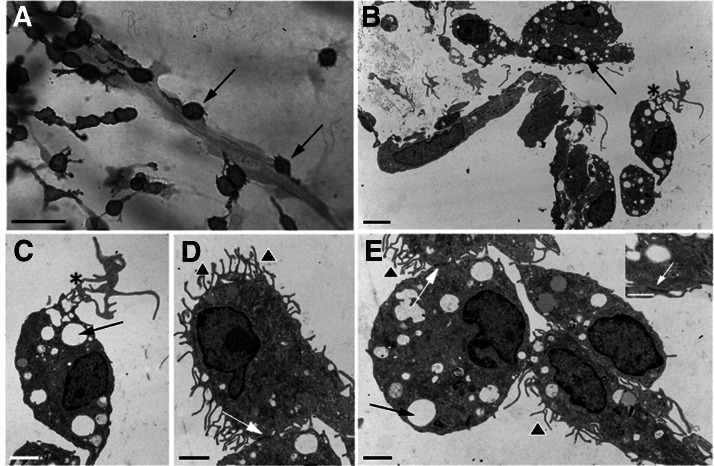
Figure 2. Outgrowth pattern and ultrastructural features of M-CSF-stimulated cells in aortic ring cultures.
(A) Immunoperoxidase staining of a microvessel surrounded by CD45+ cells, many of which have attached to the vessel wall (arrows). By electron microscopy, these cells show prominent cytoplasmic vacuoles (B, C, and E, arrows), elongated cytoplasmic processes (B and C, asterisks), and extensive surface microvillous projections (D and E, arrowheads). Adjacent cells are connected by cell junctions (D and E, white arrows). (E, inset) High magnification view of junctional complex with gap junction-like features (white arrow). Magnification bars = 50 μm (A), 5 μm (B), and 2 μm (C–E).
M-CSF-stimulated aortic ring cultures generate uniform patches of immune cells
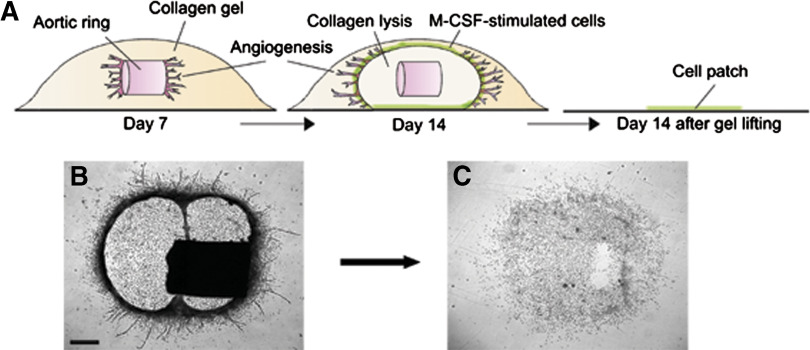
The periaortic collagen in M-CSF-treated aortic rings lysed over time. M-CSF-stimulated cells beneath the aortic explants attached to the bottom of the dish following collagen lysis. Lifting the collagen gel with the aortic ring and its outgrowth left behind a patch of 6000 cells on average (n=24; Fig. 3).
Figure 3. Formation of cellular patches in M-CSF-stimulated aortic ring cultures.
M-CSF-treated aortic ring cultures undergo collagen lysis over time. M-CSF-stimulated cells accumulate in the wall of the collagenolytic chamber, forming a dense circular band (A, schematic drawing; B, image of living culture). Lifting the collagen gel together with the explant leaves behind a patch of M-CSF-stimulated cells that have attached to the bottom of the culture dish (C). (B, C) Magnification bar = 400 μm.
Immunocytochemical characterization of M-CSF-stimulated aortic cells
Rat aortic cell patches formed in M-CSF-treated cultures were immunostained for CD45, CD68, F4/80, and CD163 and CD169, which recognize distinct subsets of mature macrophages [19, 20]. Patch cells stained strongly for CD45, CD68, CD169, and F4/80 but did not express CD163 (Fig. 4A). These marker studies indicate that the M-CSF-stimulated aortic cells have features of monocyte/macrophages. Macrophages with a CD163– CD169+ phenotype have been observed in distinct compartments of lymphoid organs [20] but have not been described in the aortic wall. Although the isolated patch cells from M-CSF-treated aortic cultures are not reactive for CD163, cells expressing this marker can be demonstrated in the adventitia of the native aorta [27] or of the cultured explants. This finding suggests that M-CSF-induced proliferation of adventitial immune cells results in the down-regulation or shedding of CD163, or M-CSF selects a CD68+ CD163– CD169+ subpopulation of immature cells capable of cell division. This second hypothesis implies that the aortic adventitia contains a heterogeneous population of immune cells, including macrophage progenitor cells.
Figure 4. Characterization of M-CSF-stimulated aortic patch cells.
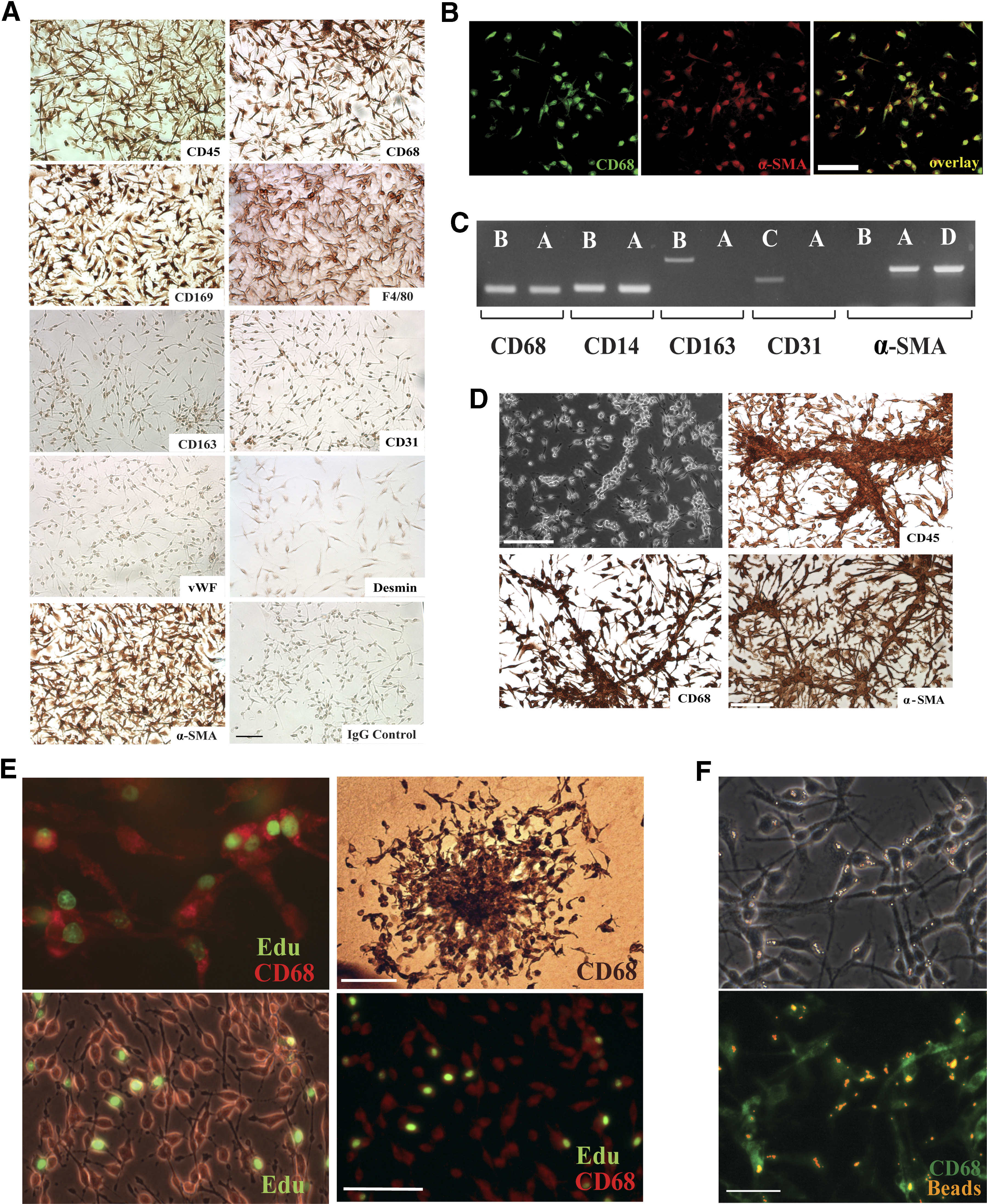
(A) Patch cells stained by the ABC immunoperoxidase method show strong immunoreactivity for CD45, CD68, CD169, F4/80, and α-SMA and are negative for CD163, CD31, vWF, desmin, and nonimmune IgG control; magnification bar = 100 μm. (B) Confocal images demonstrate coexpression in aortic patch cells of CD68 and α-SMA; Magnification bar = 20 μm. (C) RT-PCR shows that aortic patch cells (A) express CD68, CD14, and α-SMA and are negative for CD163 and CD31. Positive controls include rat bone marrow-derived macrophages (B), rat aortic endothelial cells (C), and rat aortic smooth muscle cells (D). (D) Living (upper left) and formalin-fixed cultures of aortic patch cells stained for CD45, CD68, and α-SMA demonstrate the capacity of these cells to organize into linear arrays and branching cord-like structures; magnification bars: 100 μm. (E) M-CSF-treated collagen gel culture of rat aorta (upper left) and an isolated aortic cell patch, photographed with (lower left) or without (lower right) phase contrast, demonstrate several CD68+ (red)-proliferating cells following incubation with the thymidine analog EdU (green). Proliferating CD68+ cells form colonies in aortic cultures (upper right, immunoperoxidase staining). Magnification bars = 50 μm (upper left and lower images, bar shown in lower right image) and 100 μm (upper right). (F) Aortic patch cells immunoreacted for CD68 show phagocytic activity following O.N. incubation with fluorescent latex beads (upper, phase contrast micrograph; lower, immunofluorescent image). Magnification bar = 50 μm.
To exclude the possibility of endothelial or smooth muscle cell differentiation, rat aortic patches were also stained for CD31, vWF, α-SMA, and desmin. Patch cells were negative for CD31, vWF, or desmin but surprisingly, stained intensely for α-SMA (Fig. 4A and B). The capacity of the aortic immunocytes to express α-SMA may be a constitutive feature of these cells, but it may also represent a phenotypic change in their differentiation status. Expression of α-SMA has been reported in prelabeled CD68+ PBMCs undergoing myofibroblastic differentiation in an animal model of granulation tissue formation [31]. Coexpression of CD68 and α-SMA has also been reported in the normal and atherosclerotic aorta, where CD68+ α-SMA+ cells were interpreted as a subset of intimal smooth muscle cells [32]. In our immunocytochemical study, CD68+ α-SMA+ cells also expressed the leukocyte/monocytic markers CD45 and F4/80 and did not express the mural cell marker desmin. These findings, taken together with the capacity of these cells to respond to M-CSF, favor a hematopoietic/leukocyte rather than smooth muscle/pericyte origin. The additional observation that α-SMA can be expressed in rat aortic endothelial cells indicates that this cell marker is not unique to the smooth muscle cell lineage and can be expressed by other cells of the vessel wall [33].
To further confirm the monocyte/macrophage nature of the M-CSF-stimulated aortic cells, aortic cell patches were analyzed by RT-PCR for CD14, for which there are no rat-specific antibodies. This study showed that aortic cell patches expressed CD14 and confirmed that they were positive for CD68 and α-SMA and negative for CD31 and CD163. Control bone marrow-derived macrophages were positive for CD14, CD68, and CD163 but negative for α-SMA (Fig. 4C).
M-CSF-stimulated aortic cells showed a tendency to align and reorganize into branching cords, which stained for CD45, CD68, and α-SMA (Fig. 4D) but not CD31 or vWF. These cords resembled the monocyte cell columns reported previously in Matrigel cultures and an in vivo Matrigel plug assay [34]. This pattern of collective cell migration has been interpreted as a mechanism by which monocytes/macrophage facilitate angiogenesis [34, 35]. The junctional complexes demonstrated between outgrowing macrophages in aortic ring cultures (Fig. 2) probably facilitated the assembly of these cells into cord-like structures, while promoting cell-to-cell communication, as reported previously in macrophage cell lines [36].
To evaluate the immunophenotype of resident adventitial immune cells, which are the source of macrophages in aortic cultures [27], whole-mount preparations of rat aorta were immunostained for the same macrophage markers used to characterize the cultures. The adventitia of the aorta contained numerous CD45+, CD68+, CD163+, and CD169+ cells (data not shown).
Rat aortic immune cells proliferate in response to M-CSF
The hypercellularity of M-CSF-stimulated aortic cultures suggested that this hematopoietic factor was promoting not only the migration but also the proliferation of aorta-resident immune cells. DNA synthesis studies of M-CSF-treated aortic ring cultures confirmed that CD45+ CD68+ cells were actively proliferating. Further proliferation study of aortic cell patches incubated for 24 h with EdU in the presence of aortic rings showed DNA synthesis in 28% of CD68+ cells (Fig. 4E). DNA synthesis decreased significantly to 20% in parallel cultures following removal of the aortic rings (P<0.05; n=16). This observation suggests that aortic rings promote macrophage proliferation. The finding that CD45+ CD68+ cells proliferate in response to M-CSF was substantiated further by the formation of colonies of these cells in M-CSF-treated aortic cultures (Fig. 4E). These findings demonstrate that the aorta contains a population of resident mononuclear immune cells capable of proliferating in response to M-CSF and paracrine stimuli originating from the vessel wall.
Rat aortic immune cells have phagocytic capacity
To characterize further the macrophage nature of the M-CSF-stimulated aortic cells, the phagocytic activity of these cells was tested by adding fluorescent latex beads to the cultures. All cells showed evidence of phagocytosis following O.N. incubation with the beads (Fig. 4F).
Rat aortic immune cells differentiate into DCs and form giant cells in response to GM-CSF and IL-4
To evaluate the capacity of aorta-derived CD45+ CD68+ cells to respond to cytokines known to modulate myeloid cell differentiation, aortic cell patches were treated with M-CSF, IL-4, GM-CSF, or the IL-4/GM-CSF combination or left untreated. Unstimulated patch cells appeared as short and stubby spindles or round cells. When treated with M-CSF, these cells maintained a spindly morphology but became highly elongated with tapering cytoplasmic processes. Patch cells treated with GM-CSF exhibited a pleomorphic morphology ranging from dendritic to star-like or fusiform. IL-4 induced DC differentiation in many but not all cells. IL-4 and GM-CSF induced expression of MHC II in 72% and 95% of cells, respectively. Combined treatment with the 2 cytokines induced expression of MHC II comparable with that of GM-CSF alone (Fig. 5). MHC II expression was not increased significantly by M-CSF compared with the untreated control (20%). Induction of DC differentiation by GM-CSF and/or IL-4 was confirmed by immunostaining the cultures with the OX-62 DC marker (data not shown). Thus, aorta-derived immune cells can differentiate into DCs in response to GM-CSF and IL-4. This finding suggests that immune cells isolated from M-CSF-stimulated aortic cultures are not fully committed to the macrophage cell lineage and have the capacity to differentiate into specialized APCs when exposed to GM-CSF or IL-4. Evidence of limited DC differentiation is observed in untreated collagen gel cultures, which contain scattered MHC II-positive cells. It is also possible that cells isolated from M-CSF-treated cultures are committed to the macrophage cell lineage but also capable of DC differentiation [37]. The demonstration that all M-CSF-stimulated cells are capable of phagocytosis would support this interpretation.
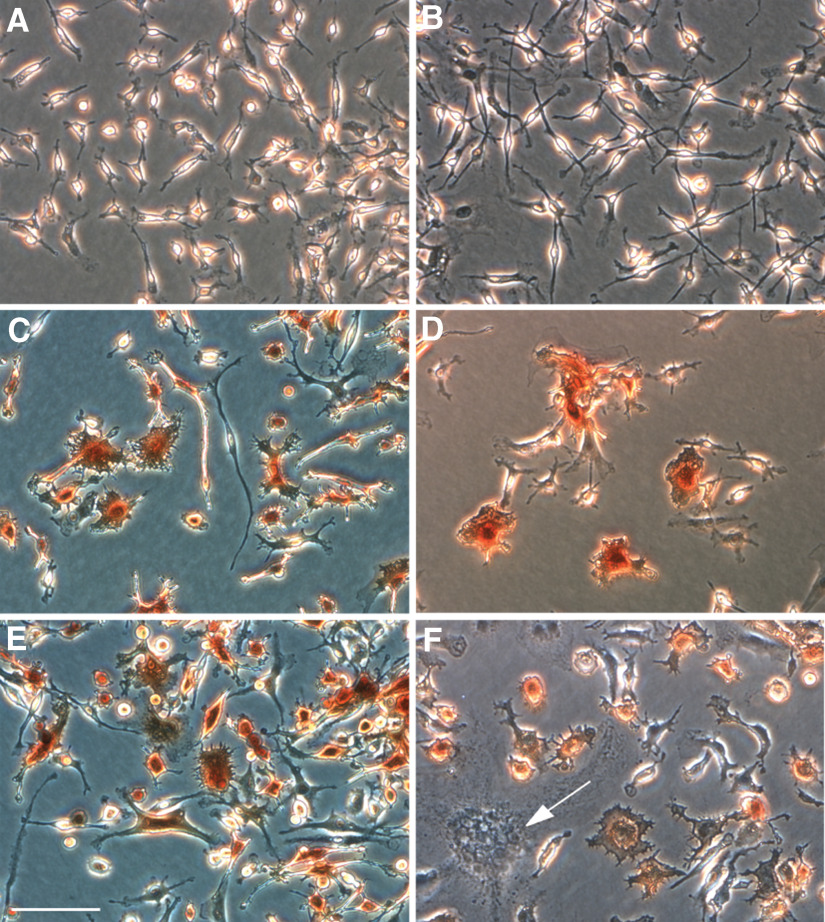
Figure 5. Aortic patch cells have the capacity to express MHC II, differentiate into DCs, and form multinucleated giant cells.
Micrographs show immunoperoxidase staining for MHC II of aortic patch cells left untreated (A) or treated with M-CSF (B), GM-CSF (C), IL-4 (D), or the GM-CSF/IL-4 combination (E and F). GM-CSF and IL-4, but not M-CSF, induce DC differentiation, including enlargement of cell cytoplasm, formation of peripheral spikes, and expression of MHC II. The GM-CSF/IL-4 combination also promotes formation of multinucleated giant cells (F, arrow). (E) Magnification bar = 100 μm.
Treatment of CD45+ CD68+ cells with GM-CSF and IL-4 resulted in the formation of many multinucleated giant cells within 48 h (Fig. 5), as reported previously for bone marrow-derived macrophages [38]. Induction of giant cell formation was also obtained by treating cultures with IL-4 or GM-CSF alone but at a slower rate than with the IL-4/GM-CSF combination. Giant cells were also obtained with the individual cytokines when treatment was prolonged for several days [39]. Giant cells characteristically form in inflammatory diseases of the arterial wall, and resident DCs have been implicated in the pathogenesis of these vasculitides [40]. The observation that the process of giant cell formation can be reproduced in primary cultures of aorta resident immune cells provides a new opportunity to study the inflammatory mechanisms of the vasculitic process under chemically defined culture conditions.
Aorta-derived immune cells stimulate the angiogenic sprouting of aortic rings
To evaluate the role of aortic immune cells on vessels sprouting, cell patches derived from M-CSF-stimulated aortic cultures were cocultured with freshly cut aortic rings in collagen gels. Aortic rings produced significantly more vessels when cocultured with aortic patch cells. This stimulatory effect was associated with increased production of VEGF (Fig. 6), which plays an important role in the endogenous regulation of angiogenesis in this system [41].
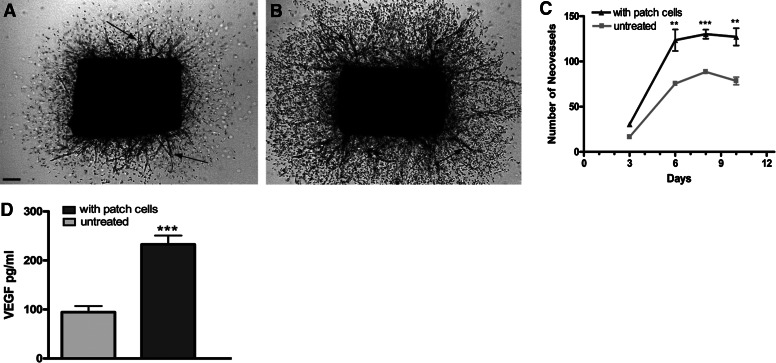
Figure 6. Aortic patch cells stimulate angiogenesis.
Photomicrographs of rat aortic rings cultured in collagen gel without (A) or with (B) aortic patch cells (day 7). Angiogenic sprouting from aortic rings (arrows) is stimulated significantly by aortic patch cells. Quantitative analysis of angiogenesis demonstrates a 50% increase in neovessel sprouting in the cocultures (C, n=4; **P<0.01; ***P<0.001). Stimulation of angiogenesis by aortic patch cells is associated with a 150% increase in VEGF concentration at day 7 (n=4;***P<0.001). (A) Magnification bar = 300 μm.
Summary and conclusion
In summary, our studies demonstrate that the aorta contains a population of immature immune cells that have the capacity to proliferate in response to M-CSF, phagocytose fluorescent beads, differentiate into DCs, and form multinucleated giant cells. The observation that these cells have angiotropic and angiogenic properties and are required for the formation of neovessels suggests that the aorta resident immune system plays a pivotal role in the angiogenic response of the vessel wall to injury. More studies are, however, needed to define the origin of these cells, characterize their functional properties, and identify the mechanisms that activate their angiogenic program in reactive and pathologic conditions.
ACKNOWLEDGMENTS
This study was supported by the National Heart Lung and Blood Institute grant HL-52585 and a Merit Review grant from the Department of Veterans Affairs Medical Research Services. We gratefully acknowledge Ms. Debbie Jones for her excellent technical assistance with the electron microscopy studies.
Footnotes
- α-SMA
- α smooth muscle actin
- DT
- diphtheria toxin
- EBM
- endothelial basal medium
- EdU
- 5-ethynyl-2′-deoxyuridine
- O.N.
- overnight
- vWF
- von Willebrand factor
AUTHORSHIP
P.Z. performed, analyzed, and interpreted experiments and prepared the manuscript. A.C.A. contributed to the characterization of aortic immunocytes and helped with the preparation of the manuscript. K.D.S. contributed to the design of the experiments. R.F.N. contributed to the experiment design, interpretation of data, and preparation of manuscript.
REFERENCES
- 1.Carmeliet P. (2005) Angiogenesis in life, disease and medicine. Nature 438, 932–936. [DOI] [PubMed] [Google Scholar]
- 2.Noonan D. M., De Lerma B. A., Vannini N., Mortara L., Albini A. (2008) Inflammation, inflammatory cells and angiogenesis: decisions and indecisions. Cancer Metastasis Rev. 27, 31–40. [DOI] [PubMed] [Google Scholar]
- 3.Sunderkotter C., Steinbrink K., Goebeler M., Bhardwaj R., Sorg C. (1994) Macrophages and angiogenesis. J. Leukoc. Biol. 55, 410–422. [DOI] [PubMed] [Google Scholar]
- 4.David Dong Z. M., Aplin A. C., Nicosia R. F. (2009) Regulation of angiogenesis by macrophages, dendritic cells, and circulating myelomonocytic cells. Curr. Pharm. Des. 15, 365–379. [DOI] [PubMed] [Google Scholar]
- 5.Polverini P. J. (1997) Role of the macrophage in angiogenesis-dependent diseases. EXS 79, 11–28. [DOI] [PubMed] [Google Scholar]
- 6.Leibovich S. J., Ross R. (1975) The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am. J. Pathol. 78, 71–100. [PMC free article] [PubMed] [Google Scholar]
- 7.Naito M., Umeda S., Yamamoto T., Moriyama H., Umezu H., Hasegawa G., Usuda H., Shultz L. D., Takahashi K. (1996) Development, differentiation, and phenotypic heterogeneity of murine tissue macrophages. J. Leukoc. Biol. 59, 133–138. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi K., Yamamura F., Naito M. (1989) Differentiation, maturation, and proliferation of macrophages in the mouse yolk sac: a light-microscopic, enzyme-cytochemical, immunohistochemical, and ultrastructural study. J. Leukoc. Biol. 45, 87–96. [DOI] [PubMed] [Google Scholar]
- 9.Naito M. (2008) Macrophage differentiation and function in health and disease. Pathol. Int. 58, 143–155. [DOI] [PubMed] [Google Scholar]
- 10.Van Amerongen M. J., Harmsen M. C., van Rooijen N., Petersen A. H., van Luyn M. J. (2007) Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice. Am. J. Pathol. 170, 818–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maruyama K., Asai J., Ii M., Thorne T., Losordo D. W., D′Amore P. A. (2007) Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am. J. Pathol. 170, 1178–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weisberg S. P., McCann D., Desai M., Rosenbaum M., Leibel R. L., Ferrante A. W., Jr. (2003) Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pang C., Gao Z., Yin J., Zhang J., Jia W., Ye J. (2008) Macrophage infiltration into adipose tissue may promote angiogenesis for adipose tissue remodeling in obesity. Am. J. Physiol. Endocrinol. Metab. 295, E313–E322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tessem J. S., Jensen J. N., Pelli H., Dai X. M., Zong X. H., Stanley E. R., Jensen J., DeGregori J. (2008) Critical roles for macrophages in islet angiogenesis and maintenance during pancreatic degeneration. Diabetes 57, 1605–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergmann C. E., Hoefer I. E., Meder B., Roth H., van Royen N., Breit S. M., Jost M. M., Aharinejad S., Hartmann S., Buschmann I. R. (2006) Arteriogenesis depends on circulating monocytes and macrophage accumulation and is severely depressed in op/op mice. J. Leukoc. Biol. 80, 59–65. [DOI] [PubMed] [Google Scholar]
- 16.Heil M., Ziegelhoeffer T., Pipp F., Kostin S., Martin S., Clauss M., Schaper W. (2002) Blood monocyte concentration is critical for enhancement of collateral artery growth. Am. J. Physiol. Heart Circ. Physiol. 283, H2411–H2419. [DOI] [PubMed] [Google Scholar]
- 17.Lewis C. E., Pollard J. W. (2006) Distinct role of macrophages in different tumor microenvironments. Cancer Res. 66, 605–612. [DOI] [PubMed] [Google Scholar]
- 18.Gelati M., Aplin A. C., Fogel E., Smith K. D., Nicosia R. F. (2008) The angiogenic response of the aorta to injury and inflammatory cytokines requires macrophages. J. Immunol. 181, 5711–5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Honda H., Kimura H., Rostami A. (1992) Isolation and characterization of macrophages from rat embryonic muscle culture. J. Leukoc. Biol. 52, 537–544. [DOI] [PubMed] [Google Scholar]
- 20.Dijkstra C. D., Dopp E. A., Joling P., Kraal G. (1985) The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in rat recognized by monoclonal antibodies ED1, ED2 and ED3. Adv. Exp. Med. Biol. 186, 409–419. [DOI] [PubMed] [Google Scholar]
- 21.Aplin A. C., Fogel E., Zorzi P., Nicosia R. F. (2008) The aortic ring model of angiogenesis. Methods Enzymol. 443, 119–136. [DOI] [PubMed] [Google Scholar]
- 22.Nicosia R. F., Ottinetti A. (1990) Growth of microvessels in serum-free matrix culture of rat aorta. A quantitative assay of angiogenesis in vitro. Lab. Invest. 63, 115–122. [PubMed] [Google Scholar]
- 23.Zhu W. H., Nicosia R. F. (2002) The thin prep rat aortic ring assay: a modified method for the characterization of angiogenesis in whole mounts. Angiogenesis 5, 81–86. [DOI] [PubMed] [Google Scholar]
- 24.Nicosia R. F., Villaschi S., Smith M. (1994) Isolation and characterization of vasoformative endothelial cells from the rat aorta. In Vitro Cell. Dev. Biol. Anim. 30A, 394–399. [DOI] [PubMed] [Google Scholar]
- 25.Nicosia R. F., Villaschi S. (1995) Rat aortic smooth muscle cells become pericytes during angiogenesis in vitro. Lab. Invest. 73, 658–666. [PubMed] [Google Scholar]
- 26.Villaschi S., Nicosia R. F., Smith M. R. (1994) Isolation of a morphologically and functionally distinct smooth muscle cell type from the intimal aspect of the normal rat aorta. Evidence for smooth muscle cell heterogeneity. In Vitro Cell. Dev. Biol. Anim. 30A, 589–595. [DOI] [PubMed] [Google Scholar]
- 27.Aplin A. C., Gelati M., Fogel E., Carnevale E., Nicosia R. F. (2006) Angiopoietin-1 and vascular endothelial growth factor induce expression of inflammatory cytokines before angiogenesis. Physiol. Genomics 27, 20–28. [DOI] [PubMed] [Google Scholar]
- 28.Grunewald M., Avraham I., Dor Y., Bachar-Lustig E., Itin A., Jung S., Chimenti S., Landsman L., Abramovitch R., Keshet E. (2006) VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell 124, 175–189. [DOI] [PubMed] [Google Scholar]
- 29.Asahara T., Murohara T., Sullivan A., Silver M., van der Z. R., Li T., Witzenbichler B., Schatteman G., Isner J. M. (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275, 964–967. [DOI] [PubMed] [Google Scholar]
- 30.Shi Q., Rafii S., Wu M. H., Wijelath E. S., Yu C., Ishida A., Fujita Y., Kothari S., Mohle R., Sauvage L. R., Moore M. A., Hammond W. P. (1998) Evidence for circulating bone marrow-derived endothelial cells. Blood 92, 362–367. [PubMed] [Google Scholar]
- 31.Jabs A., Moncada G. A., Nichols C. E., Waller E. K., Wilcox J. N. (2005) Peripheral blood mononuclear cells acquire myofibroblast characteristics in granulation tissue. J. Vasc. Res. 42, 174–180. [DOI] [PubMed] [Google Scholar]
- 32.Andreeva E. R., Pugach I. M., Orekhov A. N. (1997) Subendothelial smooth muscle cells of human aorta express macrophage antigen in situ and in vitro. Atherosclerosis 135, 19–27. [DOI] [PubMed] [Google Scholar]
- 33.Azuma K., Ichimura K., Mita T., Nakayama S., Jin W. L., Hirose T., Fujitani Y., Sumiyoshi K., Shimada K., Daida H., Sakai T., Mitsumata M., Kawamori R., Watada H. (2009) Presence of α-smooth muscle actin-positive endothelial cells in the luminal surface of adult aorta. Biochem. Biophys. Res. Commun. 380, 620–626. [DOI] [PubMed] [Google Scholar]
- 34.Anghelina M., Krishnan P., Moldovan L., Moldovan N. I. (2004) Monocytes and macrophages form branched cell columns in matrigel: implications for a role in neovascularization. Stem Cells Dev. 13, 665–676. [DOI] [PubMed] [Google Scholar]
- 35.De Palma M., Venneri M. A., Galli R., Sergi S. L., Politi L. S., Sampaolesi M., Naldini L. (2005) Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 8, 211–226. [DOI] [PubMed] [Google Scholar]
- 36.Fortes F. S., Pecora I. L., Persechini P. M., Hurtado S., Costa V., Coutinho-Silva R., Braga M. B., Silva-Filho F. C., Bisaggio R. C., De Farias F. P., Scemes E., De Carvalho A. C., Goldenberg R. C. (2004) Modulation of intercellular communication in macrophages: possible interactions between GAP junctions and P2 receptors. J. Cell Sci. 117, 4717–4726. [DOI] [PubMed] [Google Scholar]
- 37.Ferenbach D., Hughes J. (2008) Macrophages and dendritic cells: what is the difference? Kidney Int. 74, 5–7. [DOI] [PubMed] [Google Scholar]
- 38.Dugast C., Gaudin A., Toujas L. (1997) Generation of multinucleated giant cells by culture of monocyte-derived macrophages with IL-4. J. Leukoc. Biol. 61, 517–521. [DOI] [PubMed] [Google Scholar]
- 39.McNally A. K., Anderson J. M. (1995) Interleukin-4 induces foreign body giant cells from human monocytes/macrophages. Differential lymphokine regulation of macrophage fusion leads to morphological variants of multinucleated giant cells. Am. J. Pathol. 147, 1487–1499. [PMC free article] [PubMed] [Google Scholar]
- 40.Piggott K., Biousse V., Newman N. J., Goronzy J. J., Weyand C. M. (2009) Vascular damage in giant cell arteritis. Autoimmunity 42, 596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nicosia R. F., Lin Y. J., Hazelton D., Qian X. (1997) Endogenous regulation of angiogenesis in the rat aorta model. Role of vascular endothelial growth factor. Am. J. Pathol. 151, 1379–1386. [PMC free article] [PubMed] [Google Scholar]