The HMGB1 C-terminal tail is responsible for the in vitro and in vivo inhibitory effects of HMGB1 on phagocytosis of apoptotic neutrophils.
Keywords: inflammation, neutrophils, macrophages, phagocytosis, apoptosis
Abstract
HMGB1 was described originally as a nuclear protein involved in DNA binding and transcriptional regulation. However, HMGB1 also has an extracellular role as a potent mediator of inflammation and can diminish the uptake of apoptotic cells by phagocytes, a process called efferocytosis. To explore the mechanism responsible for the ability of HMGB1 to inhibit efferocytosis, we examined the role of the C-terminal acidic tail, a region of HMGB1 that has been shown to participate in specific intramolecular interactions. Deletion of the C-terminal tail abrogated the ability of HMGB1 to decrease murine macrophage ingestion of apoptotic neutrophils and to diminish phagocytosis-induced activation of Erk and Rac-1 in macrophages. We found that RAGE plays a major role in efferocytosis, and deletion of the C-terminal tail of HMGB1 prevented binding of HMGB1 to RAGE but not to other macrophage receptors involved in efferocytosis, such as the αVβ3 integrin. Whereas HMGB1 decreased ingestion of apoptotic neutrophils significantly by alveolar macrophages under in vivo conditions in the lungs of mice, this effect was lost when the C-terminal acidic tail was absent from HMGB1. These results demonstrate that the HMGB1 C-terminal tail is responsible for the inhibitory effects of HMGB1 on phagocytosis of apoptotic neutrophils under in vitro and in vivo conditions.
Introduction
HMGB1 was described originally as a nuclear protein involved in DNA binding and transcriptional regulation [1–3]. However, HMGB1 also can function as an extracellularly secreted participant in inflammatory reactions. Circulating levels of HMGB1 are increased in patients with severe infections, including pneumonia, and treatment of mice with antibodies to HMGB1 reduces the severity of and improves survival from acute inflammatory conditions, such as sepsis and acute lung injury, in which activated neutrophils play a major role [4–7]. HMGB1 can associate with LPS, DNA, and cytokines, such as IL-1β, and acquires enhanced proinflammatory activity through such interactions [8–12]. Although recent data indicate that HMGB1 itself has little or no proinflammatory activity, at least in terms of activating macrophages and other cellular populations to secrete cytokines and additional mediators of inflammation, there is evidence that HMGB1 can enhance inflammation directly, through acting as a potent chemotactin for neutrophils and also by diminishing clearance of apoptotic neutrophils by macrophages [8–10, 12–14].
The uptake of apoptotic cells by macrophages and other phagocytic cell populations is called efferocytosis and involves interaction between “eat me” signals expressed on the apoptotic cells and ligands or receptors for the eat me signal on macrophages and other phagocytes [15, 16]. Ingestion and clearance of apoptotic neutrophils play a central role in the resolution of inflammation through reducing the numbers of activated neutrophils in inflammatory foci. Efferocytosis also decreases the severity of inflammation by preventing apoptotic neutrophils from becoming necrotic and releasing proinflammatory intracellular contents into the extracellular milieu.
The ability of HMGB1 to diminish the ability of macrophages to ingest apoptotic neutrophils appears to be related to binding between HMGB1 and phosphatidyl serine, a well-described eat me signal, which is exposed on the membrane of neutrophils undergoing programmed cell death [14]. The receptor on the macrophage surface for phosphatidyl serine-associated HMGB1 has not been well characterized, although recent data from our laboratory suggest that binding between the αVβ3 integrin and HMGB1 participates in the ability of HMGB1 to inhibit efferocytosis of neutrophils and other apoptotic cells (unpublished results). Other receptors for HMGB1, including RAGE, have been identified [17, 18]. Although interactions between HMGB1 and RAGE have been shown to be involved in modulating HMGB1-induced cellular activation [13, 19, 20], including phosphorylation of the p38 MAPK, nuclear translocation of NF-κB, and chemotaxis of neutrophils, there is no evidence, at present, that binding between HMGB1 and RAGE is involved in affecting efferocytosis.
HMGB1 is comprised of 3 domains: A box, B box, and a highly acidic C-terminal tail [2, 3, 21–23]. Although previous studies demonstrated that HMGB1 diminishes efferocytosis, the domain responsible for this effect has not been delineated. In these experiments, we show that the HMGB1 C-terminal tail is responsible for the inhibitory effects of HMGB1 on phagocytosis of apoptotic neutrophils under in vitro and in vivo conditions.
MATERIALS AND METHODS
Mice
Male C57BL/6 mice, 8 weeks of age, were purchased from Jackson Laboratory (Bar Harbor, ME, USA). Transgenic RAGE−/− mice were a kind gift from Angelika Bierhaus (University of Heidelberg, Germany) [24, 25]. Mice were housed and studied under University of Alabama at Birmingham (Birmingham, AL, USA) Institutional Animal Care and Use Committee-approved protocols. Experiments were performed on 8- to 10-week-old mice.
Materials
Custom cocktail antibodies and negative selection columns for neutrophil isolation were from Stem Cell Technologies (Canada). RPMI media, penicillin-streptomycin, and Brewer thioglycollate were from Sigma-Aldrich (St. Louis, MO, USA). FBS (heat-inactivated) was from Gibco-BRL (Grand Island, NY, USA). Anti-Flag mouse mAb (M2) was from Sigma-Aldrich. Rabbit anti-HMGB1 pAb was from Abcam (Cambridge, MA, USA). Antibodies to phosphorylated and total Erk1/2 were obtained from Cell Signaling Technology Inc. (Beverly, MA, USA). Mouse soluble RAGE-Fc chimeric protein and human soluble αVβ3 protein were from R&D Systems (Minneapolis, MN, USA). Anti-RAGE rabbit pAb (H-300) was from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-β3 rabbit pAb was from Cell Signaling Technology Inc.
Construction of expression plasmids
Full-length human HMGB1 cDNA was purchased from Open Biosystems (Huntsville, AL, USA). To generate constructs that express full-length HMGB1 and ΔC-HMGB1, cDNAs with proper endonuclease sites that express HMGB1 or ΔC-HMGB1 were generated by PCR amplification and cloned into a mammalian expression vector, 3× FLAGCMV10 (Sigma-Aldrich), in-frame with 3× Flag. Human embryo kidney-293-human TLR4/MD2-CD14 cells (Catalog Number 293-htlr4-md2-cd14, Invivogen, San Diego, CA, USA), which stably express human TLR4, MD2, and CD14, were maintained in RPMI-1640 medium (Sigma-Aldrich) containing 10% FBS (Atlanta Biologicals, Lawrenceville, GA, USA) and penicillin/streptomycin solution (1:100; Sigma-Aldrich). To generate a stable cell line that overexpresses Flag-tagged HMGB1 or ΔC-HMGB1, 293-human TLR4/MD2-CD14 cells were transfected with the HMGB1-Flag or ΔC-HMGB1-Flag construct using Lipofectamine 2000TM (Invitrogen, Carlsbad, CA, USA), followed by culture in medium containing 500 μg/ml G418 (Sigma-Aldrich). A clone that stably expresses HMGB1-Flag or ΔC- HMGB1-Flag was selected and expanded for purification of HMGB1-Flag or ΔC- HMGB1-Flag (10×150 mm dish/cell line). Cells were lysed with MCLB [50 mM Tris, pH 8.0, 5 mM EDTA, 150 mM NaCl, 10 mM NaF, 2 mM Na3VO4, protease inhibitor mixture (1:100, v/v); Sigma-Aldrich] and 0.5% Nonidet P-40. Cell lysates were incubated with anti-Flag agarose beads (M2-agarose, Sigma-Aldrich) for 2 h with rotation at 4°C, after which, the beads were washed with MCLB buffer, followed by washing 3 times with 50 mM Tris buffer (pH 7.5) to remove any detergent. Beads binding Flag-tagged proteins were eluted using 3× Flag peptide (250 μg/ml) in 50 mM Tris buffer. Proteins were concentrated using Amicon Ultra 0.5 filters (Ultracell 10k, Millipore, Bedford, MA, USA) and protein concentration determined by SDS-PAGE Coomassie staining using BSA as a standard.
Neutrophil purification and induction of apoptosis
Mouse neutrophils were purified from bone marrow cell suspensions as described previously [14, 26] using antibody-specific, negative selection techniques. Purified neutrophils were suspended in RPMI media containing 1% FBS (6×106 cells/ml) and apoptosis induced by heating at 43°C for 1 h followed by incubation at 37°C for 2 h as described previously [14].
Culture of mouse peritoneal macrophages
Peritoneal macrophages were elicited by i.p. injection of 1.5 ml 4% Brewer thioglycollate. Cells were harvested 5 days later by peritoneal lavage and plated on coverslips in 24-well plates at a concentration of 3 × 105 cells/well. After 1 h incubation at 37°C, nonadherent cells were removed by washing with RPMI medium. Fresh RPMI medium containing 5% FBS and penicillin-streptomycin was added to the cells and changed every 3 days.
In vitro phagocytosis assay
Macrophages cultured on coverslips were washed with serum-free media and then incubated for 1 h in serum-free media. The macrophages were then incubated with 1 μg/ml HMGB1, ΔC-HMGB1, or BSA protein in serum-free media for 1 h, followed by washing with RPMI media prior to being included in phagocytosis assays. Phagocytosis was assessed by adding apoptotic neutrophils (1:10 macrophages:neutrophils) to pretreated macrophages in RPMI media containing 1% FBS, followed by incubation at 37°C for 90 min. Noninternalized neutrophils were removed by washing the cells 3 times with PBS. The cells were then fixed with 100% methanol and stained with HEMA3. Phagocytosis was evaluated by an observer blinded to experimental conditions by counting 200–300 macrophages/slide from triplicate experiments. The phagocytic index is represented as the percentage of macrophages containing at least 1 ingested neutrophil.
In vivo phagocytosis assay
In vivo phagocytosis was determined as described previously [14]. Briefly, 107 apoptotic neutrophils were suspended in 50 μl PBS with 2 μg HMGB1, ΔC-HMGB1, or mouse albumin and then were injected intratracheally into isofluorane-anesthetized C57BL/6 mice (n=3 mice/group). After 90 min, the mice were killed and BAL performed with a total volume of 1 ml PBS containing 5 mM EDTA/mouse. Cytospin slides were prepared using 200 μl of the BALF. The phagocytic index was determined as described for the in vitro assays.
Erk 1/2 and Rac-1 activation assays
Peritoneal macrophages were treated with 1 μg/ml BSA, HMGB1, or ΔC-HMGB1 for 1 h in serum-free media followed by washing with PBS. Apoptotic neutrophils were then added as described above for in vitro phagocytosis assays and the macrophages and apoptotic neutrophils incubated together for 15 min. The macrophages were then washed with PBS and lysed using MCLB buffer containing 0.1% Triton X-100. Erk1/2 activation was determined by Western blot analysis using antiphosphorylated Erk1/2 antibodies. The same blot was stripped and then reprobed with antibodies to total Erk1/2. To determine Rac-1 activation (level of GTP-bound Rac-1), a Rac-1 activation assay kit was used (Millipore). Briefly, cell lysates from macrophages were incubated with PBD-agarose beads for 1 h to pull down activated Rac-1 (PBD-bound Rac-1). PBD-bound Rac-1, precipitated by the beads, was then resolved by 15% SDS-PAGE and detected by anti-Rac-1 antibodies. To demonstrate an equal amount of inputs used in each sample, 1/20 of the cell lysates was analyzed by Western blotting using anti-Rac-1 antibodies.
In vitro binding assays
HMGB1-Flag or ΔC-HMGB1-Flag (50 ng) was incubated with 100 ng αvβ3 or soluble RAGE-Fc in buffer containing 50 mM Tris (pH 7.4), 150 mM NaCl, and 10% (v/v) glycerol for 1 h at 4°C, and then 20 μl M2-agarose beads was added to each sample and incubated for 2 h at 4°C. The beads were washed 3 times using buffer containing 50 mM Tris (pH 7.4), 300 mM NaCl, and 1 mM DTT. The immunocomplexes were resolved by 15% SDS-PAGE and analyzed by Western blotting using anti-β3 or anti-RAGE antibodies. The blots were reprobed with anti-Flag antibodies.
Statistical analysis
For each experimental condition, the entire group of animals was prepared and studied at the same time. Data are presented as mean ± sd (in vitro experiments) or mean ± sem (in vivo experiments) for each experimental group. One-way ANOVA followed by analysis with the Tukey-Kramer test was performed for comparisons among multiple groups. A value of P < 0.05 was considered significant.
RESULTS
The C-terminal acidic tail of HMGB1 is responsible for inhibition of efferocytosis
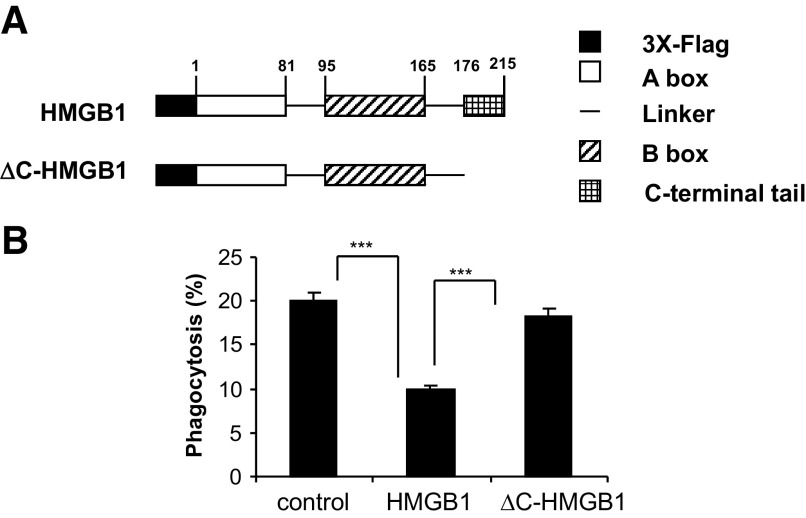
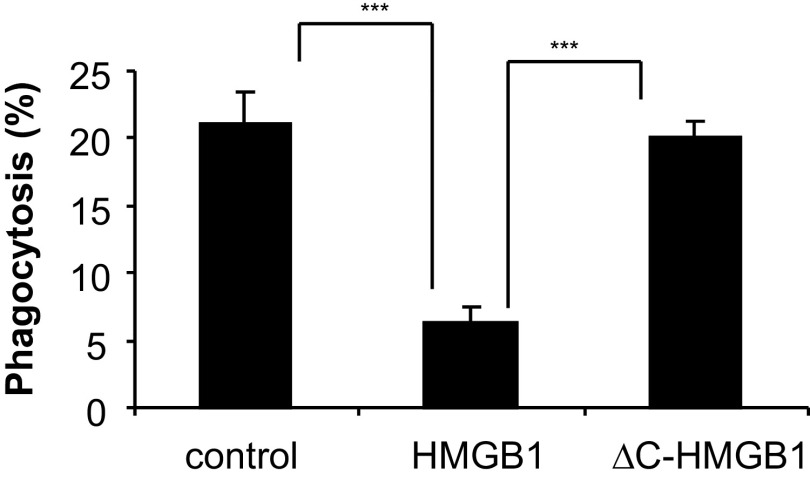
In previous studies, we found that HMGB1 inhibits the ingestion of apoptotic neutrophils by macrophages [14]. As the C-terminal tail of HMGB1 has been reported to play a major role in determining the binding of HMGB1 to DNA, NF-κB, and other molecules involved in cell signaling [22, 27–29], we hypothesized that this region of HMGB1 might also be involved in modulating the inhibitory effect of HMGB1 on efferocytosis. To address this issue, we purified Flag-tagged, full-length HMGB1 (1–215 aa; HMGB1) and a C-terminal acidic tail deletion mutant of HMGB1 (1–176 aa; ΔC-HMGB1) from 293-human TLR4/MD2-CD14 cells stably expressing these proteins (Fig. 1A).
Figure 1. The C-terminal acidic tail is required for HMGB1 to inhibit efferocytosis.
(A) Schematic diagram showing constructs that express 3× Flag-fused human HMGB1 and ΔC-HMGB1. The amino acid number at the end of each domain is shown. (B) Effects of full-length HMGB1 and ΔC-HMGB1 on phagocytosis of apoptotic neutrophils by peritoneal macrophages.. Phagocytosis assays were performed as described in Materials and Methods. Three additional independent experiments provided similar results; ***P<0.001.
To examine the role of the C-terminal tail of HMGB1 in efferocytosis, peritoneal macrophages were incubated with HMGB1 or ΔC-HMGB1 for 1 h, then washed, and incubated with apoptotic neutrophils. As shown in Fig. 1B, whereas full-length HMGB1 diminished ingestion of apoptotic neutrophils by ∼50% [P<0.001 compared with macrophages (control) that had been incubated with albumin], exposure of macrophages to ΔC-HMGB1 had no significant effect on their uptake of apoptotic neutrophils. Of note, the degree of suppression of efferocytosis induced by the Flag-tagged, full-length HMGB1 was similar to that found with HMGB1 purified from porcine thymus (data not shown).
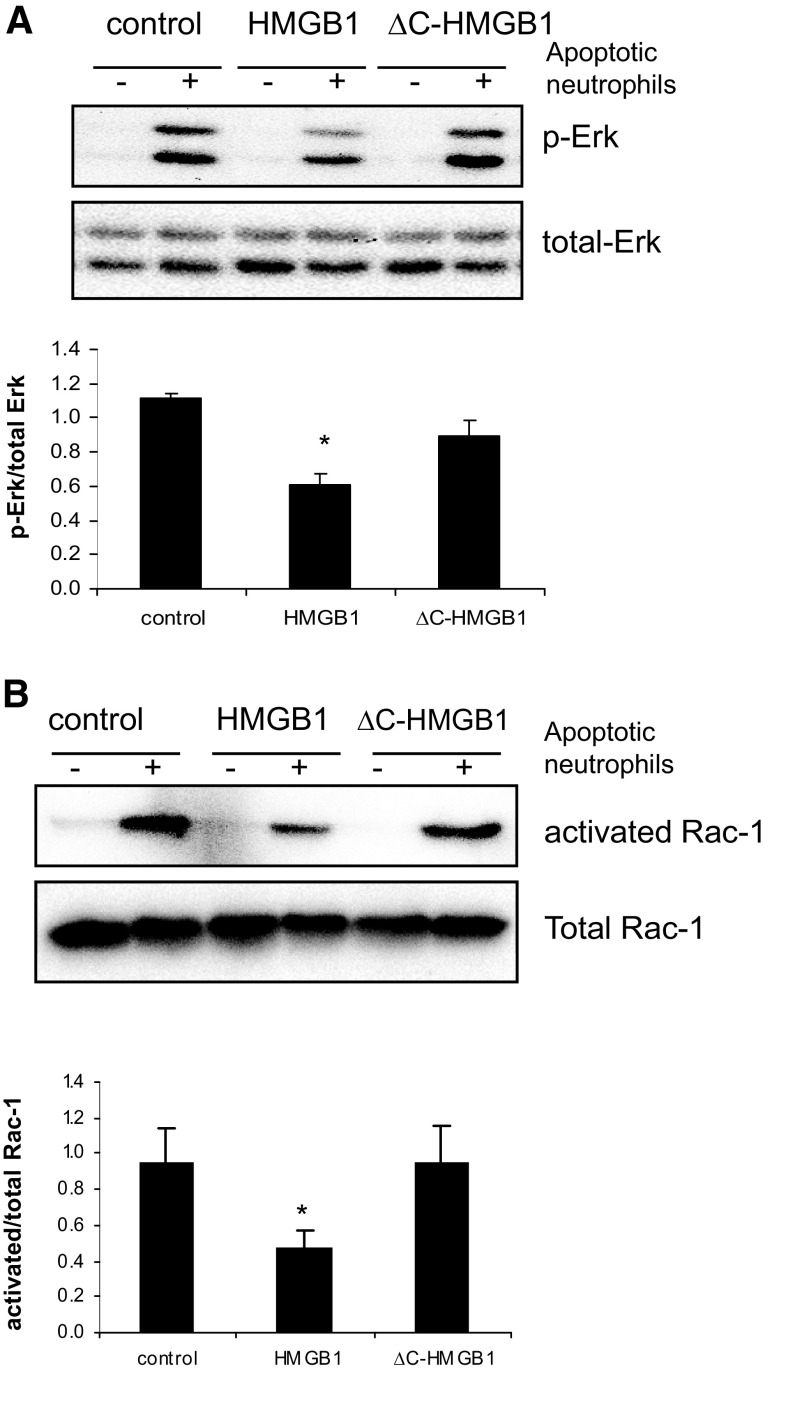
Deletion of the C terminus of HMGB1 abolishes its inhibitory effect on phagocytosis-induced activation of Erk and Rac-1
Exposure of macrophages to apoptotic cells enhances Erk phosphorylation and activates Rac-1 [30–33]. To determine if deletion of the C-terminal acidic tail of HMGB1 affects the ability of HMGB1 to modulate Erk and Rac-1 activation during efferocytosis, macrophages were incubated with HMGB1, ΔC-HMGB1, or HSA, followed by exposure to apoptotic neutrophils; phosphorylation of Erk1/2 and activation of Rac-1 were then determined 15 min later.
As shown in Fig. 2A, incubation of macrophages with apoptotic neutrophils resulted in enhanced phosphorylation of Erk1/2, an effect that was diminished significantly by pretreatment of macrophages with full-length HMGB1 protein but not by pretreatment with ΔC-HMGB1. Similarly, preincubation of macrophages with full-length HMGB1, but not with ΔC-HMGB1, attenuated efferocytosis-induced Rac-1 activation significantly (Fig. 2B).
Figure 2. Absence of the C-terminal acidic tail abolishes the inhibitory effect of HMGB1 on efferocytosis-induced Erk phosphorylation and Rac-1 activation.
(A) Peritoneal macrophages were washed with serum-free medium and incubated in serum-free media for 1 h followed by exposure for 1 h to 1 μg/ml BSA (control), full-length HMGB1, or ΔC-HMGB1 in serum-free media. The macrophages were washed and incubated with RPMI-1640 medium with 1% FBS, with or without apoptotic neutrophils. After 15 min, the cells were washed, and cell extracts from the macrophages were prepared, Western blotting was performed to determine the levels of phosphorylated (p-Erk) and total Erk. A representative gel as well as mean ± sd of phosphorylated Erk/total Erk ratios for each condition using optical band intensity measurements from 3 independent experiments are shown. (B) Phagocytosis assays were performed for 15 min as described above. The macrophages were then washed 3 times and cell lysates prepared to determine Rac-1 activation. A representative gel as well as mean ± sd of activated/total Rac-1 ratios for each condition using optical band intensity measurements from 3 independent experiments are shown; *P < 0.05 versus control.
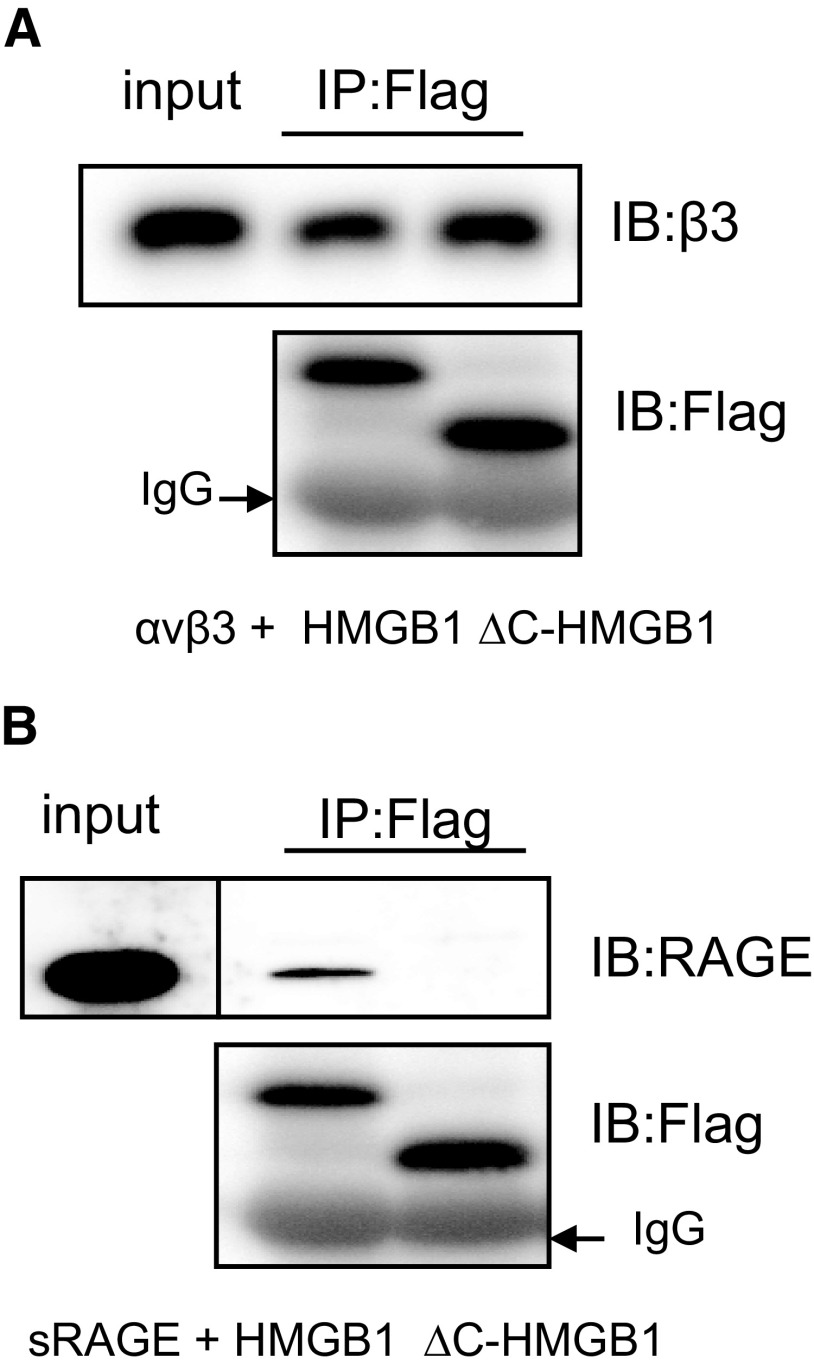
The C-terminal acidic tail of HMGB1 is required for binding to RAGE but not to the αVβ3 integrin
In previous studies (unpublished results), we found that interactions between HMGB1 and the αVβ3 integrin on the macrophage surface are involved in the inhibition of phagocytosis by HMGB1. To determine whether the importance of the C-terminal tail of HMGB1 in modulating efferocytosis is a result of interactions with αVβ3, we performed coimmunoprecipitation assays using Flag-tagged rHMGB1 or ΔC-HMGB1 and rαVβ3. As shown in Fig. 3A, there did not appear to be any difference in the binding affinity of full-length HMGB1 or ΔC-HMGB1 to αVβ3.
Figure 3. The C terminus is required for binding of HMGB1 to RAGE but not to the αVβ3 integrin.
HMGB1-Flag or ΔC-HMGB1-Flag (50 ng) was incubated with 100 ng soluble αVβ3 (A) or soluble RAGE (sRAGE)-Fc chimeric protein (B). αVβ3- or RAGE-bound HMGB1 or ΔC-HMGB1 was pulled down using agarose beads conjugated with anti-Flag antibody. The precipitated proteins were eluted and resolved by 12% SDS-PAGE, and Western blotting was performed using anti-β3 antibody (A) or anti-RAGE antibody. (B) Blots were stripped and reprobed with anti-Flag antibody to demonstrate the amount of Flag-tagged HMGB1 or ΔC-HMGB1 that was pulled down by the beads. A second independent experiment provided similar findings. IP, Immunoprecipitation; IB, immunoblotting.
A previous study showed that the C-terminal motif in HMGB1 (aa 150–183) is responsible for binding to RAGE [34]. As there is overlap in aa 177–183, between the C-terminal-binding region of RAGE and the C-terminal tail of HMGB1, we next examined the binding affinity of HMGB1 and ΔC-HMGB1 with RAGE. As shown in Fig. 3B, the binding of HMGB1 to RAGE is abrogated by deletion of the C-terminal acidic tail.
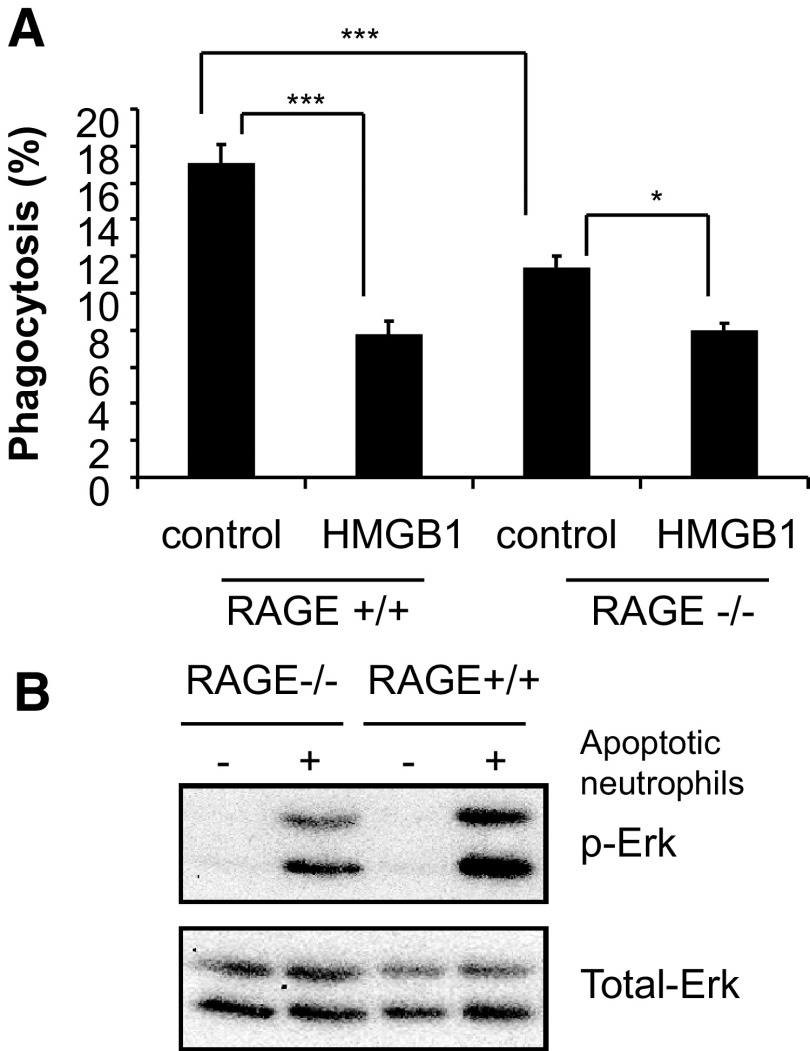
Given the ability of the C-terminal tail of HMGB1 to determine binding of HMGB1 to RAGE and the lack of inhibitory activity of HMGB1 lacking the C-terminal tail on efferocytosis, it seemed possible that RAGE itself might have an important role in modulating the uptake of apoptotic cells by macrophages. To examine this hypothesis, we incubated peritoneal macrophages from RAGE−/− and control RAGE+/+ mice with albumin or full-length HMGB1 and then examined their ability to ingest apoptotic neutrophils. As shown in Fig. 4A, RAGE−/− macrophages had decreased phagocytic activity significantly as compared with those from RAGE+/+ mice. The magnitude of the inhibitory effects of HMGB1 on efferocytosis, although still present with RAGE−/− macrophages, was substantially less than that found after incubation of RAGE+/+ macrophages with HMGB1 (17.0±1.3% albumin-treated vs. 7.8±0.8% after incubation with HMGB1 for RAGE+/+ macrophages as compared with 11.4±0.6% albumin-treated vs. 8.0±0.2% after incubation with HMGB1 for RAGE−/− macrophages; Fig. 4A). Phagocytosis-induced Erk activation in macrophages from RAGE−/− mice was also diminished as compared with that found in RAGE+/+ macrophages (Fig. 4B).
Figure 4. RAGE plays a major role in modulating phagocytosis of apoptotic neutrophils by macrophages.
(A) Peritoneal macrophages from RAGE+/+ and RAGE−/− mice were pretreated with BSA or HMGB1 protein (1 μg/ml) for 1 h followed by incubation with apoptotic neutrophils for 90 min and the phagocytic index then determined. Data shown are representative of 2 independent experiments; ***P < 0.001; *P < 0.05. (B) RAGE+/+ and RAGE−/− macrophages were incubated with apoptotic neutrophils for 15 min and then washed. Western blotting was performed to determine the levels of phosphorylated and total Erk. A representative experiment is shown. A second independent experiment provided similar results.
The C-terminal acidic tail of HMGB1 is responsible for the inhibitory effects of HMGB1 on efferocytosis under in vivo conditions in the lungs
The in vitro experiments shown in Fig. 1 indicated that the C-terminal acidic tail of HMGB1 was responsible for the inhibitory effects of HMGB1 on efferocytosis. To confirm whether the C-terminal tail had similar properties under in vivo conditions, we administered apoptotic neutrophils intratracheally with albumin, full-length HMGB1, or ΔC-HMGB1 and then performed BAL 90 min later to assess uptake of the alveolar neutrophils by alveolar macrophages.
As shown in Fig. 5, coadministration of full-length HMGB1 with apoptotic neutrophils resulted in a decrease of ∼70% in phagocytosis of apoptotic neutrophils as compared with control conditions, in which albumin was included with the apoptotic neutrophils (P<0.001). In contrast, there was no apparent difference in efferocytosis between apoptotic neutrophils administered with albumin or with ΔC-HMGB1.
Figure 5. Removal of the C-terminal acidic tail abrogates the inhibitory effect of HMGB1 on phagocytosis in vivo.
Apoptotic neutrophils (107) with HMGB1, ΔC-HMGB1, or mouse albumin (2 μg) were administered intratracheally in 50 μl PBS into anesthesized mice. BAL was performed with 1 ml cold PBS containing 5 mM EDTA 90 min later. Cytospin slides were prepared using 200 μl BALF, and phagocytosis of apoptotic neutrophils by alveolar macrophages was assessed. The phagocytosis index is represented as the percentage of alveolar macrophages containing at least 1 ingested neutrophil; n = 3 mice in each group; ***P < 0.001. A second independent experiment provided similar results.
DISCUSSION
HMGB1 is composed of 3 primary functional domains, comprising the A box, B box, and an acidic C terminus composed of 30 glutamic and aspartic residues [1, 3, 22, 35–37]. The A and B boxes are involved in DNA binding, and there is evidence that whereas the B box has intrinsic proinflammatory properties, the A box is able to inhibit the proinflammatory activity of extracellular HMGB1 [7, 38]. The C-terminal tail of HMGB1 can reduce the DNA-binding properties of HMGB1, presumably through interacting with the A box and B box, as well as with the N-terminal domain of HMGB1 [22, 35, 39, 40]. The acidic C terminus also participates in modulating acetylation of HMGB1, including reducing the level of acetylation of lysine 2 residues and inhibiting the acetylation of lysine 81 in HMGB1 by CREB-binding protein [41]. Interactions between HMGB1 and the p50 subunit of NF-κB are diminished in the presence of the C-terminal acidic tail [42]. There is recent evidence that the C-terminal acidic tail of HMGB1 and particularly, aa residues 201–205 are responsible for the antibacterial activity of HMGB1 [27]. However, there is only limited information concerning the role of the C-terminal tail in modulating the extracellular properties of HMGB1.
In the present experiments, we found that the C-terminal tail of HMGB1 is responsible for the ability of HMGB1 to inhibit the ingestion of apoptotic neutrophils by macrophages. In particular, whereas full-length HMGB1 diminished the uptake of apoptotic neutrophils under in vitro and in vivo conditions by more than 50%, this action was lost when the C-terminal tail was absent. Similarly, the ability of HMGB1 to decrease the activation of the Erk and Rac-1 kinases in macrophages during efferocytosis was no longer present when a deletion mutant of HMGB1 lacking the C terminus was used in phagocytosis assays in place of full-length HMGB1.
RAGE is a well-characterized receptor for HMGB1 and occupies a central role in modulating the ability of HMGB1 to affect multiple cellular functions, including p38 MAPK activation, chemotaxis, and NF-κB activation [13, 19, 43, 44]. In these studies, we found that RAGE also has an important role in efferocytosis, as macrophages lacking RAGE showed significantly diminished ability to ingest apoptotic neutrophils and also were less responsive to the suppressive effects of HMGB1 on efferocytosis. The C-terminal tail of HMGB1 appears to have a crucial role in facilitating binding between HMGB1 and RAGE, as elimination of the C terminus resulted in markedly decreased association of soluble RAGE with HMGB1, consistent with a previous report that a C-terminal motif in HMGB1, which overlaps partially with the C-terminal tail, is responsible for binding to RAGE [34]. The present results therefore suggest that the ability of HMGB1 to diminish efferocytosis is primarily a result of interaction between the C-terminal tail and RAGE, as the deletion mutant of HMGB1 lacking the C-terminal tail was unable to bind to RAGE and also did not diminish the uptake of apoptotic neutrophils by peritoneal macrophages in vitro and by alveolar macrophages under in vivo conditions in the lungs.
Although the present experiments show that the C-terminal acidic tail of HMGB1 is responsible for binding between RAGE and HMGB1, our results also indicate that receptors other than RAGE are involved in modulating the inhibitory effects of HMGB1 on efferocytosis. In particular, although the ability of HMGB1 to diminish uptake of apoptotic neutrophils was reduced in RAGE−/− macrophages, HMGB1 still diminished efferocytosis by the RAGE−/− macrophages significantly, indicating that interactions of HMGB1 with receptors other than RAGE participate in HMGB1-induced reduction in ingestion of apoptotic cells. In previous studies, we found that interactions between macrophage-based αVβ3 integrin and HMGB1 were involved in inhibiting ingestion of apoptotic neutrophils (unpublished results). However, there was no evidence in the present experiments that deletion of the C-terminal tail affected binding of HMGB1 to αVβ3, suggesting that interactions between HMGB1 and ligands or receptors in addition to RAGE and αVβ3 are likely to participate in diminishing efferocytosis.
The present experiments, by demonstrating that the C-terminal acidic tail of HMGB1 plays a central role in modulating the inhibitory effects of HMGB1 on efferocytosis, provide new insights into the functional importance of the different domains of HMGB1 in modulating the extracellular effects of this molecule on inflammatory processes. Although the A box of HMGB1 had been shown effectively to block interactions of HMGB1 with RAGE [13, 19], there had been no previous data to indicate that the C-terminal tail itself was responsible for the ability of HMGB1 to bind to RAGE. As receptors other than RAGE appear to be involved in modulating the inhibitory effects of HMGB1 on efferocytosis, it is likely that the C-terminal tail is also involved in interactions between HMGB1 and these receptors. In addition, although highly purified HMGB1 itself has minimal proinflammatory properties, its ability to activate macrophages and other cell populations is enhanced markedly by binding to relatively small amounts of proinflammatory mediators, including LPS, IL-1β, and DNA [9, 10, 12]. The domains of HMGB1 responsible for its association with proinflammatory mediators are presently unknown. We are actively investigating this issue.
Antibodies to HMGB1 are effective in diminishing organ injury and mortality in multiple models of sepsis, acute lung injury, ischemia-reperfusion, and other pathophysiologic situations, in which neutrophils play a major role in acute inflammation [5–7, 45, 46]. The ability of HMGB1 to diminish ingestion and clearance of apoptotic neutrophils is likely to contribute to organ dysfunction under in vivo conditions. In particular, HMGB1-associated reductions in efferocytosis would be expected to result in the persistence of activated neutrophils in inflammatory foci, as well as progression of apoptotic neutrophils to necrosis, with associated spillage of intracellular contents into the extracellular milieu, thereby enhancing inflammation-associated tissue injury. Of note, levels of HMGB1 are increased in airway secretions from patients with neutrophil-associated airway inflammation, such as cystic fibrosis, and the presence of HMGB1 in BALF in the setting of cystic fibrosis diminishes clearance of apoptotic neutrophils [14, 47]. Therapies able to block the association of HMGB1 with macrophages, including those directed at the C-terminal acidic tail, which these experiments show to be the primary domain involved in the effects of HMGB1 on efferocytosis, are likely to enhance clearance of apoptotic neutrophils and diminish inflammation-associated organ dysfunction.
ACKNOWLEDGMENT
This work was supported by National Institutes of Health grants GM087748 and HL076206 to E.A.
Footnotes
- ΔC-HMGB1
- high mobility group box protein 1 mutant lacking the C-terminal acidic tail
- BALF
- bronchoalveolar lavage fluid
- HMGB1
- high mobility group box protein 1
- MCLB
- mammalian cell lysis buffer
- MD2
- myeloid differentiation protein 2
- PBD
- p21-binding domain
- RAGE
- receptor for advanced glycation endproducts
AUTHORSHIP
S.B., G.L., and E.A. conceived of, designed, and supervised the experiments and wrote the manuscript. S.B., A.F., and G.L. conducted the experiments.
REFERENCES
- 1.Bianchi M. E., Agresti A. (2005) HMG proteins: dynamic players in gene regulation and differentiation. Curr. Opin. Genet. Dev. 15, 496–506. [DOI] [PubMed] [Google Scholar]
- 2.Dumitriu I. E., Baruah P., Manfredi A. A., Bianchi M. E., Rovere-Querini P. (2005) HMGB1: guiding immunity from within. Trends Immunol. 26, 381–387. [DOI] [PubMed] [Google Scholar]
- 3.Muller S., Scaffidi P., Degryse B., Bonaldi T., Ronfani L., Agresti A., Beltrame M., Bianchi M. E. (2001) New EMBO members′ review: the double life of HMGB1 chromatin protein: architectural factor and extracellular signal. EMBO J. 20, 4337–4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angus D. C., Yang L., Kong L., Kellum J. A., Delude R. L., Tracey K. J., Weissfeld L. (2007) Circulating high-mobility group box 1 (HMGB1) concentrations are elevated in both uncomplicated pneumonia and pneumonia with severe sepsis. Crit. Care Med. 35, 1061–1067. [DOI] [PubMed] [Google Scholar]
- 5.Abraham E., Arcaroli J., Carmody A., Wang H., Tracey K. J. (2000) HMG-1 as a mediator of acute lung inflammation. J. Immunol. 165, 2950–2954. [DOI] [PubMed] [Google Scholar]
- 6.Wang H., Bloom O., Zhang M., Vishnubhakat J. M., Ombrellino M., Che J., Frazier A., Yang H., Ivanova S., Borovikova L., Manogue K. R., Faist E., Abraham E., Andersson J., Andersson U., Molina P. E., Abumrad N. N., Sama A., Tracey K. J. (1999) HMG-1 as a late mediator of endotoxin lethality in mice. Science 285, 248–251. [DOI] [PubMed] [Google Scholar]
- 7.Yang H., Ochani M., Li J., Qiang X., Tanovic M., Harris H. E., Susarla S. M., Ulloa L., Wang H., DiRaimo R., Czura C. J., Roth J., Warren H. S., Fink M. P., Fenton M. J., Andersson U., Tracey K. J. (2004) Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc. Natl. Acad. Sci. USA 101, 296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bianchi M. E. (2009) HMGB1 loves company. J. Leukoc. Biol. 86, 573–576. [DOI] [PubMed] [Google Scholar]
- 9.Hreggvidsdottir H. S., Ostberg T., Wahamaa H., Schierbeck H., Aveberger A-C., Klevenvall L., Palmblad K., Ottosson L., Andersson U., Harris H. E. (2009) The alarmin HMGB1 acts in synergy with endogenous and exogenous danger signals to promote inflammation. J. Leukoc. Biol. 86, 655–662. [DOI] [PubMed] [Google Scholar]
- 10.Sha Y., Zmijewski J., Xu Z., Abraham E. (2008) HMGB1 develops enhanced proinflammatory activity by binding to cytokines. J. Immunol. 180, 2531–2537. [DOI] [PubMed] [Google Scholar]
- 11.Rouhiainen A., Tumova S., Valmu L., Kalkkinen N., Rauvala H. (2007) Pivotal advance: analysis of proinflammatory activity of highly purified eukaryotic recombinant HMGB1 (amphoterin). J. Leukoc. Biol. 81, 49–58. [DOI] [PubMed] [Google Scholar]
- 12.Tian J., Avalos A. M., Mao S. Y., Chen B., Senthil K., Wu H., Parroche P., Drabic S., Golenbock D., Sirois C., Hua J., An L. L., Audoly L., La Rosa G., Bierhaus A., Naworth P., Marshak-Rothstein A., Crow M. K., Fitzgerald K. A., Latz E., Kiener P. A., Coyle A. J. (2007) Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat. Immunol. 8, 487–496. [DOI] [PubMed] [Google Scholar]
- 13.Penzo M., Molteni R., Suda T., Samaniego S., Raucci A., Habiel D. M., Miller F., Jiang H-P., Li J., Pardi R., Palumbo R., Olivotto E., Kew R. R., Bianchi M. E., Marcu K. B. (2010) Inhibitor of NK-{κ}B kinases {α} and {β} are both essential for high mobility group box 1-mediated chemotaxis. J. Immunol. 184, 4497–4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu G., Wang J., Park Y. J., Tsuruta Y., Lorne E. F., Zhao X., Abraham E. (2008) High mobility group protein-1 inhibits phagocytosis of apoptotic neutrophils through binding to phosphatidylserine. J. Immunol. 181, 4240–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagata S., Hanayama R., Kawane K. (2010) Autoimmunity and the clearance of dead cells. Cell 140, 619–630. [DOI] [PubMed] [Google Scholar]
- 16.Erwig L-P., Henson P. M. (2007) Immunological consequences of apoptotic cell phagocytosis. Am. J. Pathol. 171, 2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park J. S., Gamboni-Robertson F., He Q., Svetkauskaite D., Kim J. Y., Strassheim D., Sohn J. W., Yamada S., Maruyama I., Banerjee A., Ishizaka A., Abraham E. (2006) High mobility group box 1 protein interacts with multiple Toll-like receptors. Am. J. Physiol. Cell Physiol. 290, C917–C924. [DOI] [PubMed] [Google Scholar]
- 18.Erlandsson Harris H., Andersson U. (2004) Mini-review: the nuclear protein HMGB1 as a proinflammatory mediator. Eur. J. Immunol. 34, 1503–1512. [DOI] [PubMed] [Google Scholar]
- 19.Qin Y-H., Dai S-M., Tang G-S., Zhang J., Ren D., Wang Z-W., Shen Q. (2009) HMGB1 enhances the proinflammatory activity of lipopolysaccharide by promoting the phosphorylation of MAPK p38 through receptor for advanced glycation end products. J. Immunol. 183, 6244–6250. [DOI] [PubMed] [Google Scholar]
- 20.Park J. S., Svetkauskaite D., He Q., Kim J. Y., Strassheim D., Ishizaka A., Abraham E. (2004) Involvement of Toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J. Biol. Chem. 279, 7370–7377. [DOI] [PubMed] [Google Scholar]
- 21.Wang H., Ward M. F., Sama A. E. (2009) Novel HMGB1-inhibiting therapeutic agents for experimental sepsis. Shock 32, 348–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knapp S., Muller S., Digilio G., Bonaldi T., Bianchi M. E., Musco G. (2004) The long acidic tail of high mobility group box 1 (HMGB1) protein forms an extended and flexible structure that interacts with specific residues within and between the HMG boxes. Biochemistry 43, 11992–11997. [DOI] [PubMed] [Google Scholar]
- 23.Guazzi S., Strangio A., Franzi A. T., Bianchi M. E. (2003) HMGB1, an architectural chromatin protein and extracellular signaling factor, has a spatially and temporally restricted expression pattern in mouse brain. Gene Expr. Patterns 3, 29–33. [DOI] [PubMed] [Google Scholar]
- 24.Schwenger V., Morath C., Salava A., Amann K., Seregin Y., Deppisch R., Ritz E., Bierhaus A., Nawroth P. P., Zeier M. (2006) Damage to the peritoneal membrane by glucose degradation products is mediated by the receptor for advanced glycation end-products. J. Am. Soc. Nephrol. 17, 199–207. [DOI] [PubMed] [Google Scholar]
- 25.Liliensiek B., Weigand M. A., Bierhaus A., Nicklas W., Kasper M., Hofer S., Plachky J., Grone H. J., Kurschus F. C., Schmidt A. M., Yan S. D., Martin E., Schleicher E., Stern D. M., Hammerling G. G., Nawroth P. P., Arnold B. (2004) Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. J. Clin. Invest. 113, 1641–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yum H. K., Arcaroli J., Kupfner J., Shenkar R., Penninger J. M., Sasaki T., Yang K. Y., Park J. S., Abraham E. (2001) Involvement of phosphoinositide 3-kinases in neutrophil activation and the development of acute lung injury. J. Immunol. 167, 6601–6608. [DOI] [PubMed] [Google Scholar]
- 27.Gong W., Li Y., Chao F., Huang G., He F. (2009) Amino acid residues 201–205 in C-terminal acidic tail region plays a crucial role in antibacterial activity of HMGB1. J. Biomed. Sci. 16, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watson M., Stott K., Thomas J. O. (2007) Mapping intramolecular interactions between domains in HMGB1 using a tail-truncation approach. J. Mol. Biol. 374, 1286–1297. [DOI] [PubMed] [Google Scholar]
- 29.Mitkova E., Ugrinova I., Pashev I. G., Pasheva E. A. (2005) The inhibitory effect of HMGB-1 protein on the repair of cisplatin-damaged DNA is accomplished through the acidic domain. Biochemistry 44, 5893–5898. [DOI] [PubMed] [Google Scholar]
- 30.Wu Y., Tibrewal N., Birge R. B. (2006) Phosphatidylserine recognition by phagocytes: a view to a kill. Trends Cell Biol. 16, 189–197. [DOI] [PubMed] [Google Scholar]
- 31.Henson P. M., Tuder R. M. (2008) Apoptosis in the lung: induction, clearance and detection. Am. J. Physiol. Lung Cell. Mol. Physiol. 294, L601–L611. [DOI] [PubMed] [Google Scholar]
- 32.Ravichandran K. S., Lorenz U. (2007) Engulfment of apoptotic cells: signals for a good meal. Nat. Rev. Immunol. 7, 964–974. [DOI] [PubMed] [Google Scholar]
- 33.Kinchen J. M., Ravichandran K. S. (2007) Journey to the grave: signaling events regulating removal of apoptotic cells. J. Cell Sci. 120, 2143–2149. [DOI] [PubMed] [Google Scholar]
- 34.Huttunen H. J., Fages C., Kuja-Panula J., Ridley A. J., Rauvala H. (2002) Receptor for advanced glycation end products-binding COOH-terminal motif of amphoterin inhibits invasive migration and metastasis. Cancer Res. 62, 4805–4811. [PubMed] [Google Scholar]
- 35.Gong W., Zheng Y., Chao F., Li Y., Xu Z., Huang G., Gao X., Li S., He F. (2010) The anti-inflammatory activity of HMGB1 A box is enhanced when fused with C-terminal acidic tail. J. Biomed. Biotechnol. 2010, 915234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watson M., Stott K., Thomas J. O. (2007) Mapping intramolecular interactions between domains in HMGB1 using a tail-truncation approach. J. Mol. Biol. 374, 1286–1297. [DOI] [PubMed] [Google Scholar]
- 37.Bergeron S., Madathiparambil T., Swanson P. C. (2005) Both high mobility group (HMG)-boxes and the acidic tail of HMGB1 regulate recombination-activating gene (RAG)-mediated recombination signal synapsis and cleavage in vitro. J. Biol. Chem. 280, 31314–31324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li J., Kokkola R., Tabibzadeh S., Yang R., Ochani M., Qiang X., Harris H. E., Czura C. J., Wang H., Ulloa L., Warren H. S., Moldawer L. L., Fink M. P., Andersson U., Tracey K. J., Yang H. (2003) Structural basis for the proinflammatory cytokine activity of high mobility group box 1. Mol. Med. 9, 37–45. [PMC free article] [PubMed] [Google Scholar]
- 39.Kawase T., Sato K., Ueda T., Yoshida M. (2008) Distinct domains in HMGB1 are involved in specific intramolecular and nucleosomal interactions. Biochemistry 47, 13991–13996. [DOI] [PubMed] [Google Scholar]
- 40.Wang Q., Zeng M., Wang W., Tang J. (2007) The HMGB1 acidic tail regulates HMGB1 DNA binding specificity by a unique mechanism. Biochem. Biophys. Res. Commun. 360, 14–19. [DOI] [PubMed] [Google Scholar]
- 41.Pasheva E., Sarov M., Bidjekov K., Ugrinova I., Sarg B., Lindner H., Pashev I. G. (2004) In vitro acetylation of HMGB-1 and -2 proteins by CBP: the role of the acidic tail. Biochemistry 43, 2935–2940. [DOI] [PubMed] [Google Scholar]
- 42.Agresti A., Lupo R., Bianchi M. E., Muller S. (2003) HMGB1 interacts differentially with members of the Rel family of transcription factors. Biochem. Biophys. Res. Commun. 302, 421–426. [DOI] [PubMed] [Google Scholar]
- 43.Dumitriu I. E., Bianchi M. E., Bacci M., Manfredi A. A., Rovere-Querini P. (2007) The secretion of HMGB1 is required for the migration of maturing dendritic cells. J. Leukoc. Biol. 81, 84–91. [DOI] [PubMed] [Google Scholar]
- 44.Rouhiainen A., Kuja-Panula J., Wilkman E., Pakkanen J., Stenfors J., Tuominen R. K., Lepantalo M., Carpen O., Parkkinen J., Rauvala H. (2004) Regulation of monocyte migration by amphoterin (HMGB1). Blood 104, 1174–1182. [DOI] [PubMed] [Google Scholar]
- 45.Suda K., Kitagawa Y., Ozawa S., Saikawa Y., Ueda M., Ebina M., Yamada S., Hashimoto S., Fukata S., Abraham E., Maruyama I., Kitajima M., Ishizaka A. (2006) Anti-high-mobility group box chromosomal protein 1 antibodies improve survival of rats with sepsis. World J. Surg. 30, 1755–1762. [DOI] [PubMed] [Google Scholar]
- 46.Kim J. Y., Park J. S., Strassheim D., Douglas I., Diaz Del Valle F., Asehnoune K., Mitra S., Kwak S. H., Yamada S., Maruyama I., Ishizaka A., Abraham E. (2005) HMGB1 contributes to the development of acute lung injury after hemorrhage. Am. J. Physiol. Lung Cell. Mol. Physiol. 288, L958–L965. [DOI] [PubMed] [Google Scholar]
- 47.Rowe S. M., Jackson P. L., Liu G., Hardison M., Livraghi A., Solomon G. M., McQuaid D. B., Noerager B. D., Gaggar A., Clancy J. P., O′Neal W., Sorscher E. J., Abraham E., Blalock J. E. (2008) Potential role of high-mobility group box 1 in cystic fibrosis airway disease. Am. J. Respir. Crit. Care Med. 178, 822–831. [DOI] [PMC free article] [PubMed] [Google Scholar]