Abstract
Memories are initially stored in a labile state and are subject to modification by a variety of treatments, including disruption of hippocampal function. We infused a sodium channel blocker (or CNQX) to inactivate the rat dorsal hippocampus reversibly for 1 week following training on a task of spatial memory (the water maze). Previous work with conventional lesions has established that the dorsal hippocampus is essential for both the acquisition and expression of memory in this task. The question in the present study was whether chronic disruption of neuronal activity in the dorsal hippocampus after training would abolish memory or whether memory would survive extended disruption of hippocampal activity. As expected from earlier work, we found that performance was impaired during the infusion period. The critical test occurred 1 week after the lesion was reversed. We found that retention of the water maze recovered to control levels. Accordingly, sustained hippocampal activity following training is not obligatory for either the maintenance of long-term spatial memory or its subsequent retrieval.
Keywords: reversible lesion, minipump, rat, water maze, lidocaine
INTRODUCTION
After memory is initially acquired, it remains subject to modification by a variety of treatments, including disruption of hippocampal function (McGaugh, 2002; Martin and Clark, 2007). Gradually, memory becomes resistant to disruption, a process that in experimental animals typically occurs across a period of a few weeks (Squire et al., 2004; Frankland and Bontempi, 2005). Little is known about how this process occurs or what exactly happens to stored representations when memory is disrupted. Here we explored the effect of chronic disruption of hippocampal activity on the long-term maintenance of hippocampus-dependent memory.
The water maze has been the benchmark task for spatial learning and memory in the rodent. A substantial literature indicates that both acquisition and expression of memory in the water maze requires the hippocampus (Bolhuis et al. 1994, Mumby et al. 1999, Sutherland et al. 2001; Martin et al., 2005; Broadbent et al., 2006; Clark et al., 2005a,b, 2007). However, the importance of the hippocampus during the retention interval itself is poorly understood. In principle, this issue could be addressed by reversibly disrupting hippocampal function for a lengthy period during the retention interval. Only a few studies have taken this approach. In two studies, pharmacological blockade of AMPA receptors in the rat hippocampus for 7 days after water maze training completely impaired subsequent performance (Riedel et al., 1999; Micheau et al., 2004). Two other studies used a different manipulation, blocking NMDA-mediated synaptic plasticity in the hippocampus for 7 days after water maze training, and these studies obtained conflicting results (no impairment, Day and Morris, 2001; impairment, Shimizu et al., 2000).
We have used a sodium channel blocker to reversibly inactivate the rat hippocampus for 1 week following training on the water maze. Specifically, we infused lidocaine into dorsal hippocampus using osmotic minipumps. The question of interest was whether chronic disruption of neuronal activity in the hippocampus after training would abolish memory for the water maze task or whether memory storage would survive prolonged disruption of hippocampal activity.
MATERIALS AND METHODS
Subjects
The subjects were 142 naïve male, Long-Evans rats weighing 300–350 g at the beginning of the study. One hundred eighteen rats underwent cannula implantation surgery, behavioral training, minipump implantation, and intrahippocampal infusion of lidocaine (n = 58) or aCSF (n = 60). Six rats received behavioral training and sham surgeries and served as an untreated control group. Eighteen rats were infused with aCSF (n = 9) or lidocaine (n = 9) and then used for immunohistochemistry to determine the extent of lidocaine inactivation. All rats were housed individually and maintained on a 12:12 h light:dark cycle. Food and water were available ad libitum. Prior to training, rats were implanted bilaterally with guide cannulae in the dorsal hippocampus.
Stereotaxic Bilateral Hippocampal Cannula Implants
Anesthesia was maintained throughout surgery with isoflurane gas (0.8–2.0% isoflurane delivered in O2 at 1 l min−1). The rat was placed in a stereotaxic instrument (Kopf Instruments, Tujunga, CA), and the incisor bar was adjusted until Bregma was level with Lambda. Sterile, stainless steel guide cannulae (22 gauge; Plastics One, Roanoke, VA) were implanted bilaterally into the dorsal hippocampus (millimeters from Bregma, AP = −4.3, ML = ±3.5, DV = −2.0; Paxinos and Watson, 1998). Anchoring screws and dental acrylic secured the guide cannula to the skull. The skin was approximated around the implant and sutured in place. At completion of surgery, a dummy cannula (Plastics One) was inserted into each guide cannula to maintain patency. Each rat received Baytril (Bayer Corporation, Shawnee Mission, KS) antibiotic for prophylaxis against infection (0.2 ml s.c. for 2 days). All rats were given at least 4-day recovery before water maze training began. For the six rats receiving sham surgeries, the skin above the skull was opened and then sutured.
Minipump Implantation
On the day following completion of water maze training, rats were anesthetized with isoflurane gas and secured in the stereotaxic instrument. Sterile osmotic minipumps (Alzet; Durect Corporation, Cupertino, CA) were implanted under the skin of the back of each rat. Each minipump was attached via sterile polyethylene tubing (Durect Corporation) to sterile internal cannula (28 gauge; Plastics One).
Initially we used three different infusion rates (1, 2.5, and 5 μl/hr) to determine the rate needed to affect performance during the infusion period. For a 1 μl/hr infusion rate, we implanted two individual pumps (one per cannula), each of which infused at 1 μl/hr. For a 2.5 μl/hr infusion rate, a single 5 μl/hr minipump was attached to a cannula bifurcation connector (Plastics One), which was then routed to the two cannulae to achieve an infusion rate of 2.5 μl/hr. For the 5 μl/hr infusion rate, we used the same procedure, except that we replaced the 5 μl/hr pump with a 10 μl/hr pump. During pump implantation surgery, the dummy cannulae were removed from the guide cannulae, and internal cannulae inserted until each tip extended 1.5-mm beyond the end of the guide cannula at a depth of 3.5-mm below Bregma. The mini-pumps began infusing compounds as soon as they were implanted and provided continuous infusion for up to 7 days. Each rat received Baytril antibiotic and was monitored until awake and alert.
Drugs
To create a reversible lesion we used lidocaine, a fast voltage-gated sodium channel blocker (4% lidocaine hydrochloride solution in aCSF; Sigma-Aldrich, St. Louis, MO). The vehicle aCSF (Harvard Apparatus, Holliston, MA) served as the control infusion.
Minipump Removal
Seven days after minipump implantation, rats were anesthetized with isoflurane gas, the minipump removed, and the tubing attached to the internal cannula sealed. Each rat received Baytril antibiotic and was monitored until awake and alert.
At completion of testing, rats were administered an overdose of sodium pentobarbital and perfused transcardially with buffered 0.9% NaCl solution followed by 10% formaldehyde solution (in 0.1 M phosphate buffer). The brains were then removed and cryoprotected in 20% glycerol/10% formaldehyde. Coronal sections (50 μm) were cut with a freezing microtome beginning at the level of the anterior commissure and continuing caudally through the length of the hippocampus. Every fifth section was mounted and stained with thionin to verify the cannula placements within the dorsal hippocampus.
Apparatus
Testing was conducted in the Morris water maze (diameter 1.8 m) with an “Atlantis Platform” (diameter = 12.7 cm; Spooner et al., 1994), which could be raised or lowered remotely. The platform was located in the center of the northeast quadrant of the pool throughout the experiment. The water was rendered opaque by the addition of powdered milk, and four 30 w spotlights pointed at a white ceiling illuminated the room. The water was maintained at room temperature (~23°C). The testing room contained a number of constant, salient visual cues (posters, objects, and equipment), and an opaque curtain shielded the experimenter from the rat once a trial began. A video camera was mounted on the ceiling directly above the pool and was used, in conjunction with a video tracking system (San Diego Instruments), to record the swim path of each rat.
PROCEDURE
Spatial Training
Rats received four training trials each day for 9 days (except for rats that received 1 μl/hr infusion in which case only 4 days of training were used). Each daily session began with a single reinforced probe trial, followed by the four training trials. For the probe trials, the platform was lowered so that it was inaccessible, and the rat was placed in the water facing the pool wall at one of four start points (North, South, East, or West). The start points were counterbalanced across trials for all animals. Upon release into the water, the rat was allowed to swim for 60 s, at which point the platform was raised to within 1.5 cm of the water surface. An additional 60 s were then allowed for the rat to locate the platform and escape from the water. After escaping, the rat remained on the platform for 30 s before being removed. If the rat failed to escape, it was guided to the platform and remained there for 30 s.
The performance measure was the percentage of time that animals spent in a 30 cm-diameter circular zone surrounding the platform location during the first 60 s of swimming (4% of the total pool surface area, chance = 4%). Previous work has indicated that basing water maze performance on the time spent in a small circle around the platform location provides a more sensitive measure of memory than the standard measure of time spent in the training quadrant (Moser et al., 1993). Whereas the quadrant measure indicates whether animals remember the general location of the platform, the small circle measure assesses how well animals remember its exact location. Nonetheless, because the quadrant measure is commonly reported, we have also reported it.
After completion of the daily probe trial, four training trials were given with the platform in the raised position (1.5 cm below the water surface) so that it provided a means of escape from the water. The procedure was the same as for the probe trials, except that the rat was allowed 120 s to find the platform. On completion of training, rats were assigned to a drug condition (aCSF or lidocaine [LIDO]) and to a retention test group (Infusion test vs. Postinfusion test) such that the average percent time spent in the training quadrant on the final training session (Day 9) was equivalent across groups.
Retention Testing
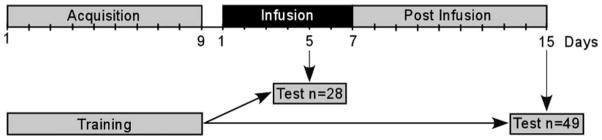
Rats in the infusion group were given a single 60 s probe trial during the 5th day of infusion. Rats in the postinfusion group were given a single 60 s probe trial 7 days after removal of the minipump (and 15 days after completion of spatial water maze training, Fig. 1).
FIGURE 1.
Animals were trained for 9 days and then tested either during the 5th day of a 7-day infusion period or tested on Day 15, 8 days after the completion of infusion. An additional group of unoperated controls were also trained for 9 days and tested 15 days after completion of training.
New Learning
After retention testing, a subset of animals that had received aCSF infusion (n = 8) or lidocaine infusion (n = 9) were trained with a new platform location (opposite quadrant) using the same training protocol used for initial acquisition.
CNQX Group
Previous work by Riedel et al., (1999) and Micheau et al., (2004) assessed the effects of inhibiting hippocampal neural activity during a retention period of a spatial task using an AMPA/Kainate receptor blocker, LY326325. In those studies, chronic hippocampal inactivation resulted in impaired spatial memory at the retention test. To test the effect of reversible lesions created by AMPA blockers using our experimental design, a separate group of animals (CNQX Group, n = 35) were tested during (n = 24) or following (n = 11) a 7-day infusion of 6-cyano-7-nitroquinoxaline-2,3dione disodium (CNQX). All of the testing and infusion protocols were identical to the aCSF and LIDO groups except the infused compound was a water-soluble form of CNQX (0.75 mM solution in aCSF; Tocris, Ellisville, MO) (LY326325 was not available). Previous studies that have infused CNQX (e.g., Bast et al., 2005) used concentrations of 3 mM. However, these studies were acute infusions that suspended the drug in DMSO (allowing higher concentrations). We were unable to use concentrations greater than 0.75 mM, as pilot work revealed that the drug did not remain stable and in solution for 7 days at these higher concentrations (using DMSO was not possible due to the chronic nature of the infusion protocol).
Characterizing the Extent of the Reversible Lesion
We stained for the activity-dependent immediate early gene protein c-Fos to determine the area of the hippocampus that was disrupted by the 5 μl/hr lidocaine infusion relative to 5 μl/hr aCSF infusion.
For the c-Fos study, each rat was transported to a new testing room on the 5th day of infusion and placed in a large novel environment (93 × 93 × 61 cm3 high black Plexiglas) for 10 min. To maximize c-Fos expression, the environment was constructed to be as stimulating as possible and contained moving objects, a pool of water, a variety of materials covering the floor (bedding, sand, plastic bubble wrap), scents hidden in objects, glow sticks, flashing lights, novel objects, tubes and loud music (~90 dB). Ninety minutes after exposure to the novel environment, rats were administered an overdose of sodium pentobarbital and perfused transcardially with buffered 0.9% NaCl solution followed by 4% paraformaldehyde solution (in 0.1 M phosphate buffer). Brains were cryoprotected in 30% sucrose (in phosphate buffer 0.1 M, 7.4pH). Coronal sections (50 μm) were cut through the entire length of the hippocampus.
Immunohistochemistry
Every third section was prepared for immunohistochemistry using anti Fos (1:10,000) rabbit polyclonal antibodies (Oncogene Research Products). A biotinated goat antirabbit antibody (1:2,000; Jackson Immunoresearch) was used as the secondary. Staining was visualized using the avidin-biotin peroxidase method (Vectorstain ABC kit, Vector) with diaminobenzidine as the chromagen. Quantitative analysis of reactive nuclei was performed using a Leica MZ6 microscope with Leica digital camera (DC300) and Kodak digital image capture software. Images were then imported into Adobe Photoshop, and immunoreactive neurons in each section were counted through all the cell fields of the dorsal and ventral hippocampus (CA 1–3) and the dentate gyrus (DG) by an experimenter blind to the experimental condition. Across animals, sections were compared that were an equivalent distance (anterior or posterior) from the cannula tract.
The hippocampus was further divided into dorsal and ventral regions by the following criteria. Sections within −1.8 to −3.8 mm from Bregma (Paxinos and Watson, 1998) were designated as dorsal hippocampus (there was no ventral tissue in these sections). To determine the dorsal/ventral boundary for the sections posterior to −3.8 mm from Bregma (that include both dorsal and ventral tissue), we divided the hippocampus using the following anatomical landmarks; the middle of the acoustic radiation/superior thalamic radiation, the middle of the marginal zone of the medial geniculate nucleus. These landmarks approximate a DV distance from Bregma of ~6 mm (Paxinos and Watson, 1998), with tissue above ~6 mm designated as dorsal and tissue below ~6 mm designated as ventral. A mean number of immunoreactive cells was then calculated for the sections at each anterior or posterior distance from the cannula tract for the animals that received lidocaine infusion (n = 9). Based on these values, the counts at each level for the animals that received lidocaine were expressed as percent reductions in c-Fos counts relative to the mean counts for animals that received aCSF (n = 9).
RESULTS
Infusion Rate
Figure 2 shows the performance of the three groups that were infused with lidocaine compared to the group that was infused with aCSF (data from all infusion rates were pooled). Only the 5 μl/hr group was impaired relative to the aCSF group (t[47] = 3.7, P < 0.001). The other LIDO groups did not differ from the aCSF group (P > 0.10). On the basis of these findings, we used a 5 μl/hr infusion rate for the behavioral studies and the c-Fos studies described below.
FIGURE 2.
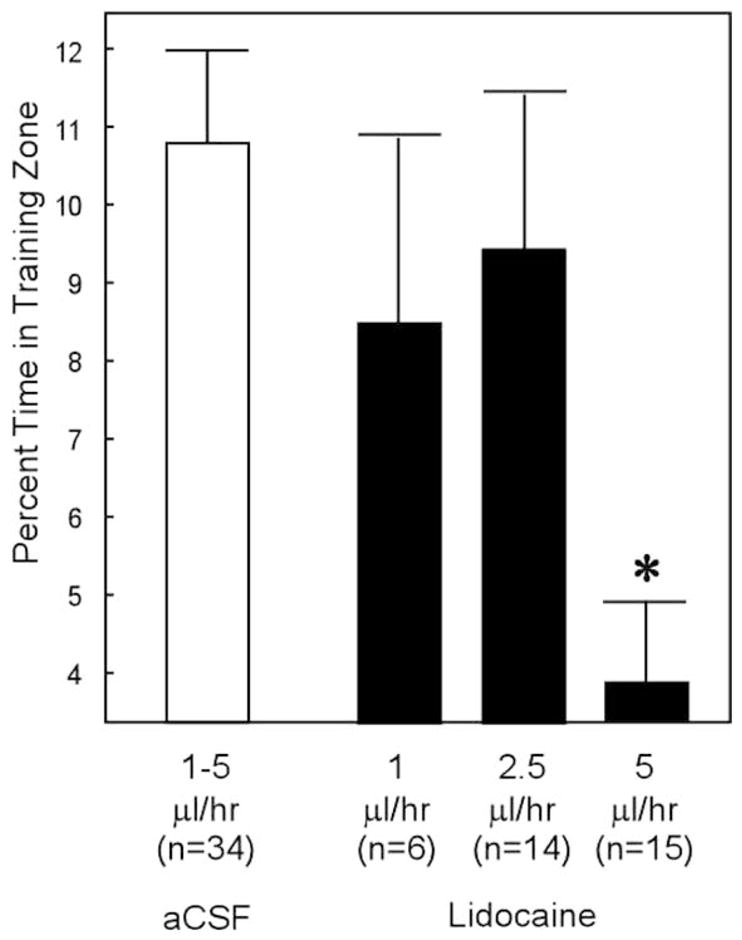
Probe trial performance following acquisition and during the 5th day of the infusion period. The aCSF group (white bar) consisted of animals that received infusions at 1 μl/hr (n = 5), 2.5 μl/hr (n = 16), and 5 μl/hr (n = 13). Animals infused with lidocaine at a rate of 1 μl/hr were given 4 days of acquisition training, and the other groups were given 9 days of acquisition training. Asterisk indicates performance different from the other groups (P < 0.05; chance = 4%).
Histology
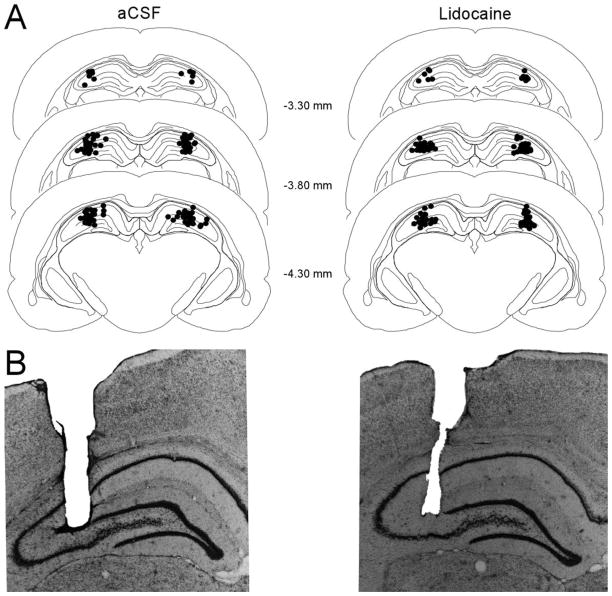
For all the animals included in the behavioral and c-Fos studies, the tip of the injection cannulae was located within the dorsal hippocampus. Figure 3 illustrates the tip location of the internal (injection) cannulae for the animals that received a 5 μl/hr infusion of either aCSF (n = 39) or lidocaine (n = 38).
FIGURE 3.
(A) Tip location of the internal (injection) cannulae for animals that received 5 μl/hr aCSF infusions (n = 39) and those that received 5 μl/hr lidocaine infusions (n = 38). (B) Representative illustrations of the cannula tracks for a rat that received aCSF infusion (left) or a rat that received lidocaine infusion (right).
Extent of the Reversible Lesion
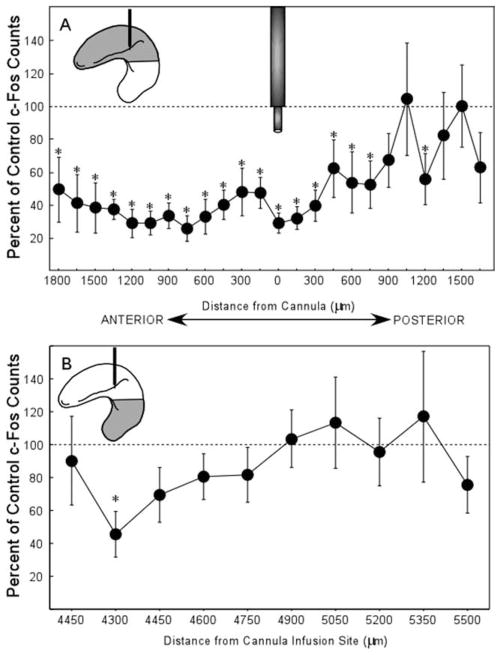
Figure 4A shows the average percent of c-Fos counts for the LIDO group relative to the aCSF group for each coronal section through the entire dorsal hippocampus at intervals of 150 μm. Significant reductions (one-sample t-test against the control mean of 100%) were observed for 700 μm in the posterior direction from the cannula and 1,800 μm in the anterior direction from the cannula (ts > 2.2, Ps < 0.05). We also examined 10 anterior-posterior levels at intervals of 150 μm through the ventral hippocampus. The distance from the cannula was estimated based on the anterior–posterior location of the cannula, histology sections, a rat brain atlas (Paxinos and Watson, 1998), and was further corrected for the approximate dorsal-ventral distance between the tip of the cannula to the middle of the ventral sections (~4,300 μm). Figure 4B shows the average percent of c-Fos counts for the LIDO group relative to the aCSF group for each coronal section through the ventral hippocampus. The data points are organized from anterior to posterior going from left to right. As such, position 4,300 corresponds to the same anterior–posterior location as the cannula, except these counts were collected from tissue 4,300 microns ventral to the infusion site. Significant reductions (one-sample t-test against the control mean of 100%) were observed only at 4,300 μm from the cannula tip. Interestingly, this position corresponds to the same anterior–posterior location as the cannula. Accordingly, we suggest that this significant reduction was due to the loss of the excitatory CA3 input from the dorsal hippocampus.
FIGURE 4.
(A) C-Fos counts for the lidocaine group (% of control) for each coronal section through the entire dorsal hippocampus at intervals of 150 μm. Significant reductions were observed at the level of the cannula (level 0, see cannula icon) and extended posteriorly for at least 700 μm and to the anterior border of the hippocampus (1,800 μm from the cannula). Asterisks indicate P < 0.05. (B) C-fos counts for the lidocaine group (% of control) for each coronal section through the ventral hippocampus at intervals of 150 μm. The distance from the cannula was estimated based on the anterior–posterior location of the cannula, histology sections, a rat brain atlas (Paxinos and Watson, 1998) and further corrected for the approximate dorsal ventral distance between the tip of the cannula to the middle of the ventral sections (~4,300 μm). The data points are organized from anterior to posterior going from left to right. As such, position 4,300 corresponds to the same anterior–posterior location as the cannula, except these counts were collected from tissue 4,300 μm ventral to the infusion site. Icons in the upper left portion of each panel illustrate the area of the hippocampus that is being represented by the c-Fos results and also show the target location of the infusion cannula (black icon). Asterisks indicate P < 0.05.
These findings indicate that the lidocaine infusion was sufficient to disrupt activity in the dorsal hippocampus for ~2,550 μm in the anterior–posterior plane. Within this area of lidocaine disruption, c-Fos expression was 40% of control values.
We also analyzed the c-Fos counts separately for the aCSF group to determine if aCSF infusion caused a systematic reduction of c-Fos expression, particularly around the infusion tip. However, there were no c-Fos count differences across the anterior-posterior levels for the group receiving aCSF infusion (all Ps > 0.1).
BEHAVIORAL RESULTS
Acquisition
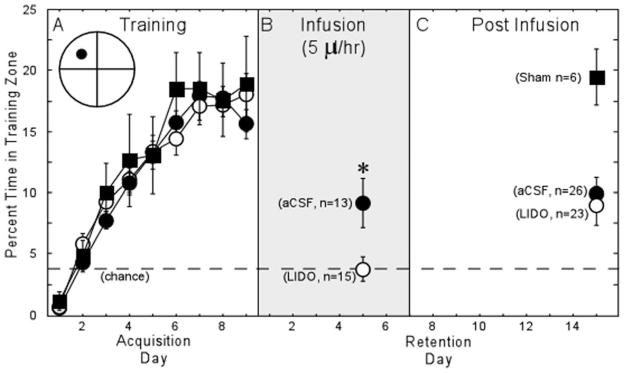
Figure 5A shows the probe trial performance at the beginning of each training day for the Sham group (n = 6), and for animals to be infused with aCSF (n = 39) or lidocaine (n = 38). A repeated measures analysis of variance (ANOVA) indicated a significant effect of training day (F[8] = 44.7, P < 0.0001) but no effect of group (F[2] = 0.5, P > 0.1). Performance during the probe trials increased from 0.6, 0.7, and 1.1% to 15.6, 18.0, and 18.9% across the 9 days of training for the aCSF, LIDO, and sham groups, respectively. These results indicate that all three groups learned the location of the hidden platform at a similar rate. Thus, the indwelling cannulae and the implantation surgery did not disrupt acquisition compared to the sham group.
FIGURE 5.
Probe trial performance across 9 days of acquisition training (A) and performance on a retention probe trial given either during the infusion period (B) or after the infusion period (C). An additional group of animals that did not receive infusions (sham, n = 6) was also tested in the “Post Infusion” period. When the probe trial was given during the infusion period, the LIDO group (n = 15) was impaired relative to the aCSF group (n = 13; P = 0.018). When the probe trial was given after the pumps were removed (and the lesion reversed), the LIDO (n = 23) and aCSF (n = 26) groups performed similarly (P > 0.1). Further, both groups performed above chance (chance = 4%; P < 0.01), and the LIDO group tested in the postinfusion period performed better than the LIDO group tested during the infusion period (P = 0.013). The icon in panel (A) illustrates the relative size of the small circular zone used to measure probe trial performance.
Retention Probe (During Infusion)
Five days after the final day of acquisition training and during the 5th day of aCSF or lidocaine infusion, subgroups of the trained animals (aCSF, n = 13; lidocaine, n = 15) were given a single 60-s retention probe trial. Figure 5B shows the percentage of time the two groups spent in the circular zone surrounding the trained platform location. The aCSF group spent more time in the training zone than did the LIDO group (9.2% ± 2.0% vs. 3.7% ± 1.0%; t[26] = 2.5, P < 0.05). The aCSF group performed well above chance (t[12] = 2.61, P < 0.05), but the LIDO group performed at chance level (3.7% ± 1.0%, P > 0.1). These results indicate that a 5 μl/hr infusion of lidocaine disrupted retention when the infusion occurred during the probe trial. For the quadrant measure, the group difference was not reliable (43.1% ± 3.3% vs. 36.2% ± 3.5%; t[26] = 1.4, P = 0.17).
Retention Probe (Post Infusion)
Fifteen days after the final day of acquisition training and 7 days after the completion of the infusion, the remaining animals (aCSF, n = 26; LIDO, n = 23; sham, n = 6) were given a single 60-s probe trial. Figure 5C shows the percentage of time the two groups spent in the circular zone surrounding the trained platform location. The two groups performed similarly (aCSF, 9.9% ± 1.4% of the time in the training zone vs. LIDO, 9.0% ± 1.6%). Both groups performed well above chance levels (Ps < 0.001). The sham group spent 19.5 % ± 2.3% of the time in the training zone, a score better than either of the infusion groups (Ps < 0.01). For the quadrant measure, the aCSF and LIDO groups performed similarly (40.3% ± 3.5% vs. 38.5% ± 3.5% respectively; t[26] = 0.4, P = 0.72).
Comparisons of the post-infusion and infusion groups indicated that the impairment exhibited by the LIDO group during the infusion period was reversible. Whereas the aCSF groups performed similarly during and after the infusion (9.2% ± 2.0% vs. 9.9% ± 1.4%, P > 0.10), the LIDO group performed better after the infusion than during the infusion (3.7% ± 1.0% vs. 9.0% ± 1.6%, t[36] = 2.5, P < 0.05).
These results indicate that chronic 5 μl/hr infusion of lidocaine and disruption of dorsal hippocampal activity for 7 days does not permanently impair spatial memory retention. Note that, although animals recovered after 7 days of infusion, neither the aCSF nor the LIDO group performed as well as the sham animals. Thus, the combination of the cannula implantation surgery, minipump implantation and 1-week drug infusion impaired behavioral performance to a noticeable extent for both the LIDO and aCSF groups.
New Learning
We next compared the ability to acquire a new platform location (following minipump removal) for subgroups of animals that had previously received retention probe trials during the infusion of either aCSF (n = 8) or lidocaine (n = 9). Figure 6 shows probe trial performance at the beginning of each training day. A repeated measures ANOVA indicated an effect of training day (F[8] = 15.8, P < 0.0001) but no effect of group (F[1] = 1.0, P > 0.1). Thus, the reversible lesion created by chronic lidocaine infusion had no persistent effect on new learning ability subsequent to the infusion.
FIGURE 6.
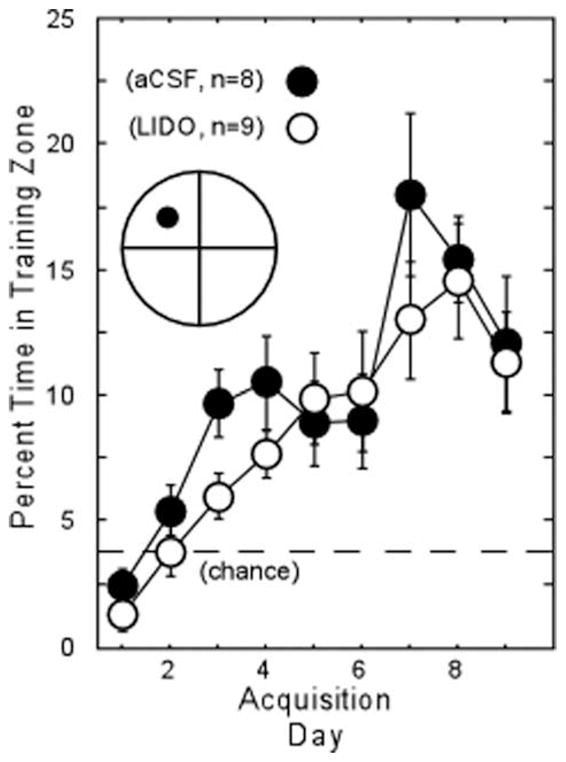
Probe trial performance across 9 days of acquisition training for a new platform location in subgroups of animals that had initially received a retention probe trial during the 7-day infusion period (aCSF, n = 8; LIDO, n = 9). Training was initiated after the minipumps had been removed. The icon illustrates the relative size of the small circular zone used to measure probe trial performance.
CNQX Group
The two CNQX groups acquired the water maze task similarly, and the probe trial performance on the final day of training was similar for both groups (infusion group, 13.5% ± 1.3%; post infusion group, 11.4% ± 0.94%; t[34] = 1.06, P = 0.3) and significantly above chance (chance = 4%, Ps < 0.0001). Comparison of performance for the infusion test group (6.7% ± 0.7%) and the post-infusion test group (11.2% ± 1.6%) revealed that the post-infusion group performed better than the infusion group (t[33] = 3.0, P < 0.01). However, during infusion, CNQX only moderately impaired performance. Specifically, performance was above chance (6.7% ± 0.7%, P < 0.01) and only marginally worse than the aCSF group (P = 0.08). These data are consistent with the idea that infusion of CNQX during the retention interval disrupts memory during the infusion, but performance improves once the infusion has stopped, suggesting that hippocampal inactivation with CNQX does not permanently impair spatial memory. But, this interpretation must remain tentative due to the relatively mild effect of CNQX infusion during the drug-on condition.
DISCUSSION
The dorsal hippocampus was inactivated with lidocaine (or CNQX) for 7 days beginning directly after training in the water maze. Retention was impaired when animals were tested during the infusion period (significantly during lidocaine, marginally during CNQX), suggesting that these agents effectively interfered with normal hippocampal function. Yet, 7 days after the completion of infusion, animals that had been infused with lidocaine (or CNQX) performed as well as control animals given aCSF and significantly better than animals that were tested during the drug infusion period. Furthermore, animals that had been infused with lidocaine learned a new platform location as well as rats that had been infused with aCSF.
The present results suggest that neural activity in the dorsal hippocampus can be chronically blocked, and memory expression correspondingly impaired, without permanently impairing either memory storage or the capacity for retrieval. Accordingly, sustained activity in the dorsal hippocampus appears not to be obligatory for either the maintenance of long-term spatial memory or its subsequent retrieval.
One way to understand these findings is that during learning critical modifications are established within the hippocampus that support water maze performance, but persisting neural activity during the days after training is not essential. The plasticity sufficient to support performance remains intact even after extended inactivation of neuronal activity.
Another way to view these results was that lidocaine (and CNQX) reversibly impaired performance. When lidocaine (or CNQX) infusion was stopped, memory expression was again possible. In this view, when the dorsal hippocampus was disrupted, expression of spatial memory was blocked because the dorsal hippocampus was necessary to meet performance demands of the probe trial retention test. For example, the contribution of the hippocampus might be to support a navigational requirement of the probe test, rather than representing recently acquired spatial information. When the disruption of the dorsal hippocampus was reversed, performance was restored and memory could again be expressed.
The c-fos results indicate that drug infusion disrupted the dorsal hippocampus and left the ventral hippocampus functional. Accordingly, it is possible that persistent activity in the ventral hippocampus might have sustained the capacity for memory that was observed in the postinfusion, drug-off condition. We note that activity in the ventral hippocampus did not allow the lidocaine group to perform above chance (by the small circle analysis) during infusion. Nevertheless, we cannot rule out the possibility that such activity helped maintain memory during the 7-day infusion period and contributed to the recovered performance 1 week later. A related issue is that the c-fos findings estimate the spatial extent of the disruption, not the magnitude of disruption. Accordingly, some residual dorsal hippocampal function might have survived the disruption and been able to sustain memory. Nonetheless, it is worth noting that whatever residual function persisted, it was not sufficient to support above-chance performance (by the small circle analysis) during the infusion period.
It is also worth noting that learning in the water maze proceeded across multiple days of training. Accordingly, several days elapsed between the time when the animals began acquiring spatial memory and the time when the reversible lesions were introduced. Perhaps this passage of time allowed sufficient consolidation to occur to protect memory from permanent disruption. Thus, permanent memory impairment might have been observed had we used a memory task that could be acquired in a single training session (like context fear conditioning or the novel object recognition task) or had we introduced the reversible lesion earlier in training (e.g., following the third day of training, when the group had just begun to perform above chance). In these cases the disruption could have been introduced soon after acquisition and before substantial consolidation had taken place (Squire et al., 2001; Frankland and Bontempi, 2005).
There are two other notable features of our findings. First, the vehicle control group performed quite poorly relative to the sham group (Fig. 5C). This result makes it clear that sustained infusion of fluid into the dorsal hippocampus using the osmotic minipump technique (even with sterile pyrogen-free aCSF) adversely impacts performance in the water maze task. We note, however, that despite this effect of infusion itself, a clear group difference during infusion did emerge between the aCSF and LIDO groups (and a marginal difference emerged between the aCSF and CNQX groups). Indeed, we were able to document both impairment in performance (during infusion) and recovery from impairment (1 week later). Nonetheless, it remains true that had there not been a substantial infusion effect, the infusion technique might have proven more useful, and differences between groups could have been detected more readily at each test interval. Second, during infusion, performance was impaired in the drug group as measured by how much time the animals spent in a small circular zone surrounding the platform, but this difference was not reliable when the more conventional quadrant measure was used. This finding indicates that, even during the infusion period, both LIDO and CNQX groups continued to express some general spatial memory of the platform location.
Though the preceding discussion identifies a number of factors that deserve consideration and that complicate the interpretation, the fact remains that our results differ from an earlier study, which used similar methods and reported a complete and permanent memory impairment (Riedel et al., 1999). In that study, rats were infused with an AMPA/ Kainate receptor antagonist (LY326325) into the dorsal hippocampus for 7 days beginning one day after the completion of 4 days of water maze training. Contrary to our own findings, spatial memory was subsequently absent (even as assessed by the quadrant measure) 8 days after the final day of infusion.
Despite the different findings in that study and the present study, both studies used similar methods and experimental designs. Specifically, both studies inactivated the dorsal hippocampus with osmotic minipumps for 7 days beginning one day after multiple days of water maze training, and both assessed spatial memory at similar times after the infusion was completed. In fact, in the earlier study (Riedel et al., 1999), memory was permanently impaired even when the disruption was delayed by 5 days (instead of 1 day; Fig. 4, Riedel et al., 1999).
In addition, both studies targeted a similar region of the dorsal hippocampus. In the present study we estimated our inactivation to extend across a diameter of 2.5 mm centered on the cannula. In the Riedel et al. (1999) study, the extent appears to be more limited (a diameter of ~1.5 mm). Because in our study we appear to have produced as least as much disruption as Riedel et al., (1999), based on the substantial reduction in c-fos expression and the spatial extent of inactivation, it appears unlikely that residual dorsal hippocampal function could account for our different findings. Furthermore, these same considerations make it unlikely that residual activity in the ventral hippocampus could explain why we found recovery following the completion of infusion. At least as much spared ventral hippocampal function was likely in the Riedel et al., (1999) study as in our study, but spatial memory was completely and permanently abolished in Riedel et al. (1999).
It is also noteworthy that both studies used aCSF as the control vehicle. The Riedel et al., (1999) study did not include a sham control group to evaluate possible infusion effects. However, in the condition where animals were trained and then infused for 7 days and then tested 8 days after the infusion (7 days in our study), the aCSF group in that study performed similarly to our aCSF group on the quadrant measure (estimated at 42.5% from Fig. 4B, Riedel et al., 1999) vs. 40.3% from our study). Our Sham group performed at 55.0% ± 5.9% under this condition. Thus, it appears likely that in both studies, the procedures produced substantial and relatively similar infusion effects. Accordingly, infusion effects are not likely to account for the different results.
The most salient difference between the studies that we can identify concerns the specific drugs used to inactivate the hippocampus and the rates of their infusion. We used the sodium channel blocker lidocaine (138.5 mM/ml) and an AMPA receptor blocker CNQX (0.75 mM/ml, which was the highest concentration that would maintain the drug in solution for 7 days). Riedel et al. (1999) used a water-soluble, selective AMPA/kainate receptor antagonist LY326325 (1.5 mM/ml) (a drug that is no longer available for experimental use). We infused at a rate of 5.0 μl/hr, which was the lowest infusion rate that would impair performance (Fig. 2). Riedel et al. (1999) infused at a rate of 0.5 μl/hr (10 times slower than our study). Thus there is a curious difference between the two studies. Despite the fact that we infused our drugs at 10 times the rate of Riedel et al. (1999), and despite the fact that we appear to have disrupted at least as much (if not more) hippocampal tissue, we found that performance recovered after completion of the infusion (and by the quadrant measure was only moderately impaired during the infusion). Yet in the earlier study (Riedel et al., 1999), all of the drug groups performed at chance on the quadrant measure after completion of the infusion. We cannot account for these earlier results. Note that the aCSF groups (drug-off condition, see above) performed similarly in the two studies, ruling out the possibility that the animals in the two studies differed in their initial learning of the water maze.
Spatial memory has been studied previously after extended, reversible disruption of hippocampal function. For example, reversible deletion of the NR1 subunit of the NMDA receptor from hippocampal area CA1 for 1 week following water maze training impaired retention when tested 1 week later (Shimizu et al., 2000). The authors suggested that normal memory consolidation depends on NMDA-mediated neural plasticity. Yet in other studies, retrieval of a previously acquired spatial memory was reported to be independent of the NMDA receptor (Steele and Morris, 1999; Day and Morris, 2001; Bast et al., 2005). Finally, using the zeta inhibitory peptide (ZIP) (an inhibitor of PKMζ), it was reported that following 5 days of water maze training, a single, 1-μl bilateral infusion of ZIP, 24 h after training and 2 h before the retention test, abolished performance. Specifically, performance was impaired as measured by platform crossings (an analog of the small circle analysis), but performance was spared on the quadrant measure (Serrano et al., 2008).
In summary, our results are consistent with the idea that dorsal hippocampal function and memory expression can be disrupted for 1 week without permanently disrupting memory retention or the ability to acquire new spatial memory once the blockade is reversed. Our findings identify a number of challenges associated with the chronic infusion technique (e.g., substantial vehicle infusion effects; the difficulty of producing a total disruption of hippocampal activity). It is also notable that our results run counter to the findings from the single earlier attempt to use chronic blockade of neural transmission to study memory (Riedel et al., 1999). Further work on this issue would be facilitated by using improved reversible lesion methods where both the dorsal and ventral hippocampus can be inactivated for extended intervals and without the substantial infusion effects encountered in our study.
Acknowledgments
The authors thank Laura Entwistle, Daniel Guadarrama, and Brittany Masatsugu for their assistance.
Grant sponsor: National Institute of Aging; Grant number: P50 AG05131; Grant sponsor: National Science Foundation; Grant numbers: SBE 0542013, 237053; Grant sponsors: Medical Research Service of the Department of Veterans Affairs; The National Institute of Mental Health; The Metropolitan Life Foundation; The James S. McDonnell Foundation; The Kavli Foundation; National Alliance for Research on Schizophrenia and Depression Effie Beeman Investigator Award.
References
- Bast T, da Silva BM, Morris RG. Distinct contributions of hippocampal NMDA and AMPA receptors to encoding and retrieval of one-trial place memory. J Neurosci. 2005;25:5845–5856. doi: 10.1523/JNEUROSCI.0698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolhuis J, Stewart CA, Forrest EM. Retrograde amnesia and memory reactivation in rats with ibotenate lesions to the hippocampus or subiculum. Q J Exp Psychol. 1994;47:129–150. [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE. Reversible hippocampal lesions disrupt water maze performance during both recent and remote memory tests. Learn Mem. 2006;13:187–191. doi: 10.1101/lm.134706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RE, Broadbent NJ, Squire LR. Hippocampus and remote spatial memory in rats. Hippocampus. 2005a;15:260–272. doi: 10.1002/hipo.20056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RE, Broadbent NJ, Squire LR. Impaired remote spatial memory after hippocampal lesions despite extensive training beginning early in life. Hippocampus. 2005b;15:340–346. doi: 10.1002/hipo.20076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RE, Broadbent NJ, Squire LR. The hippocampus and spatial memory: Findings with a novel modification of the water maze. J Neurosci. 2007;27:6647–6654. doi: 10.1523/JNEUROSCI.0913-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day M, Morris RG. Memory consolidation and NMDA receptors: Discrepancy between genetic and pharmacological approaches. Science. 2001;290:755. doi: 10.1126/science.293.5531.755a. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Clark RE. The rodent hippocampus and spatial memory: From synapses to systems. Cell Mol Life Sci. 2007;64:401–431. doi: 10.1007/s00018-007-6336-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SJ, de Hoz L, Morris RG. Retrograde amnesia: Neither partial nor complete hippocampal lesions in rats result in preferential sparing of remote spatial memory, even after reminding. Neuropsychologia. 2005;43:609–624. doi: 10.1016/j.neuropsychologia.2004.07.007. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory—A century of consolidation. Science. 2002;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- Micheau J, Riedel G, Roloff EL, Inglis J, Morris RG. Reversible hippocampal inactivation partially dissociates how and where to search in the water maze. Behav Neurosci. 2004;118:1022–1032. doi: 10.1037/0735-7044.118.5.1022. [DOI] [PubMed] [Google Scholar]
- Moser E, Moser M-B, Andersen P. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J Neurosci. 1993;13:3916–3925. doi: 10.1523/JNEUROSCI.13-09-03916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby DG, Astur RS, Weisend MP, Sutherland RJ. Retrograde amnesia and selective damage to the hippocampal formation: Memory for places and object discriminations. Behav Brain Res. 1999;106:97–107. doi: 10.1016/s0166-4328(99)00097-2. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Amsterdam: Elsevier Academic Press; 1998. [Google Scholar]
- Riedel G, Micheau J, Lam AG, Roloff EL, Martin SJ, Bridge H, de Hoz L, Poeschel B, McCulloch J, Morris RG. Reversible neural inactivation reveals hippocampal participation in several memory processes. Nat Neurosci. 1999;2:898–905. doi: 10.1038/13202. [DOI] [PubMed] [Google Scholar]
- Serrano P, Friedman EL, Kenney J, Taubenfeld SM, Zimmerman JM, Hanna J, Alberini C, Kelley AE, Maren S, Rudy JW, Yin JC, Sacktor TC, Fenton AA. PKMzeta maintains spatial, instrumental, and classically conditioned long-term memories. PLoS Biol. 2008;6:2698–2706. doi: 10.1371/journal.pbio.0060318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu E, Tang YP, Rampon C, Tsien JZ. NMDA receptor-dependent synaptic reinforcement as a crucial process for memory consolidation. Science. 2000;290:1170–1174. doi: 10.1126/science.290.5494.1170. [DOI] [PubMed] [Google Scholar]
- Spooner RIW, Thomson A, Hall J, Morris RGM, Salter SH. The atlantis platform: a new design and further developments of Buresova’s on-demand platform for the water made. Learn Mem. 1994;3:203–211. [PubMed] [Google Scholar]
- Squire LR, Clark RE, Knowlton BJ. Retrograde amnesia. Hippocampus. 2001;11:50–55. doi: 10.1002/1098-1063(2001)11:1<50::AID-HIPO1019>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Squire LR, Clark RE, Bayley PJ. Medial temporal lobe function and memory. In: Gazzinaga M, editor. The Cognitive Neurosciences. 3. Cambridge: The MIT Press; 2004. pp. 691–708. [Google Scholar]
- Steele RJ, Morris RG. Delay-dependent impairment of a matching-to-place task with chronic and intrahippocampal infusion of the NMDA-antagonist D-AP5. Hippocampus. 1999;9:118–136. doi: 10.1002/(SICI)1098-1063(1999)9:2<118::AID-HIPO4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Weisend MP, Mumby D, Astur RS, Hanlon FM, Koerner A, Thomas MJ, Wu Y, Moses SN, Cole C, Hamilton DA, Hoesing JM. Retrograde amnesia after hippocampal damage: Recent vs. remote memories in three tasks. Hippocampus. 2001;11:27–42. doi: 10.1002/1098-1063(2001)11:1<27::AID-HIPO1017>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]