Abstract
Serotonergic systems in the dorsal raphe nucleus are thought to play an important role in the regulation of anxiety states. To investigate responses of neurons in the dorsal raphe nucleus to a mild anxiety-related stimulus, we exposed rats to an open-field, under low-light or high-light conditions. Treatment effects on c-Fos expression in serotonergic and non-serotonergic cells in the midbrain raphe nuclei were determined 2h following open-field exposure or home cage control (CO) conditions. Rats tested under both light conditions responded with increases in c-Fos expression in serotonergic neurons within subdivisions of the midbrain raphe nuclei compared to CO rats. However, the total numbers of serotonergic neurons involved were small suggesting that exposure to the open-field may affect a subpopulation of serotonergic neurons. To determine if exposure to the open-field activates a subset of neurons in the midbrain raphe complex that projects to forebrain circuits regulating anxiety states, we used Cholera Toxin B subunit (CTb) as a retrograde tracer to identify neurons projecting to the basolateral amygdaloid complex (BL) in combination with c-Fos immunostaining to identify cells that responded to open-field exposure. Rats received a unilateral injection of CTb into the BL. Seven to eleven days following CTb injection rats were either, 1) exposed to an open-field in low-light conditions, 2) briefly handled or 3) left undisturbed in home cages. Dual immunostaining for c-Fos and CTb revealed an increase in the percentage of c-Fos-immunoreactive BL-projecting neurons in open-field-exposed rats compared with handled and control rats. Dual immunostaining for tryptophan hydroxylase and CTb revealed that a majority (65%) of BL-projecting neurons were serotonergic, leaving open the possibility that activated neurons were serotonergic, non-serotonergic, or both. These data are consistent with the hypothesis that exposure to anxiogenic stimuli activates a subset of neurons in the midbrain raphe complex projecting to amygdala anxiety circuits.
Keywords: anxiety, serotonin, amygdala, retrograde tracing, basolateral nucleus
Anxiety is a complex emotional state associated with heightened physiological and behavioral arousal (Lowry et al., 2005). Although the mechanisms underlying the regulation of anxiety-states are not well understood, the physiological and behavioral arousal appears to be regulated by a distributed and interconnected system of forebrain and hindbrain structures (Singewald et al., 2003; Singewald and Sharp, 2000). Consistent with this hypothesis, administration of multiple anxiogenic drugs acting through diverse pharmacological mechanisms increases c-Fos expression in these anxiety-related brain regions (Singewald and Sharp, 2000) including topographically organized subpopulation of serotonergic and non-serotonergic neurons within the mid-rostrocaudal and caudal dorsal raphe nucleus (Abrams et al., 2005).
Several lines of evidence suggest that the serotonergic system is an important modulator of anxiety-related circuits and anxiety-related behavior. Direct microinjections into the dorsal raphe nucleus of drugs and peptides that increase serotonergic activity such as FG-7142, a partial inverse agonist at the benzodiazepine allosteric binding site of the GABAA receptor, and the anxiety-related neuropeptide urocortin 2 (Ucn 2) increase anxiety-related behavioral responses (Hammack et al., 2003; Sena et al., 2003). In contrast, direct microinjections into the dorsal raphe nucleus of drugs and other chemicals that inhibit the serotonergic system, such as the inhibitory neurotransmitter γ-aminobutyric acid (GABA), benzodiazepines, 5-hydroxytryptamine (5-HT, serotonin) and 5-HT1A receptor agonists such 8-hydroxy-2-(di-n-propylamino) tetralin (8-OH-DPAT), reduce anxiety-related behavioral responses (Higgins et al., 1988; Higgins et al., 1992; Maier and Watkins, 1998; Stein et al., 1975).
The ascending serotonergic system arises from the dorsal raphé nucleus (DR) and the median raphé nucleus (MnR). These nuclei contain anatomically distinct subpopulations of serotonergic neurons, each with unique afferent and efferent connections with forebrain structures. Serotonergic systems in the mid-rostrocaudal and caudal DR may play a particularly important role in the regulation of anxiety states and anxiety-related behavior (Abrams et al., 2005; Lowry et al., 2005). This region sends projections to the basolateral amygdaloid complex (Abrams et al., 2005; Ottersen, 1981) which is an important component of the distributed neuronal system regulating anxiety states. Activation of the basolateral amygdala with the GABAA receptor antagonist bicuculline methiodide, or the corticotropin-releasing factor (CRF) receptor ligands CRF or urocortin 1 (Ucn 1) increases anxiety-like behavior in the social interaction test (Sajdyk et al., 1999; Sajdyk et al., 2002; Spiga et al., 2006), while inhibition of the basolateral amygdala following blockade of N-methyl-D-aspartic acid (NMDA) and non-NMDA receptors or administration of the benzodiazepine receptor agonist, midazolam, decreases anxiety-like behavior in the social interaction test (Gonzalez et al., 1996; Sajdyk and Shekhar, 1997).
We have recently reported that exposure of rats to either low or high-light conditions in an open-field arena is associated with an increase in c-Fos expression in serotonergic and non-serotonergic neurons in the dorsal raphe nucleus (Bouwknecht et al., 2007) as well as in specific subdivisions of the basolateral amygdaloid nucleus, notably in the anterior part of the basolateral amygdala (BLA) (Hale et al., 2006). Although the basolateral amygdala receives projections from the dorsal raphe nucleus (Abrams et al., 2005; Ottersen, 1981) and contains serotonergic terminals (Muller et al., 2007) and several serotonin receptor subtypes (Campbell and Merchant, 2003; Mascagni and McDonald, 2007; McDonald and Mascagni, 2007), the interaction between the dorsal raphe nucleus and the basolateral nucleus in the regulation of anxiety-states is not clear.
The open-field test is a widely used behavioral paradigm for the measurement of exploratory and anxiety-related behaviors in rodents (Belzung and Griebel, 2001; Prut and Belzung, 2003). The test is based on a conflict between the internal drive to explore a novel environment (based on the potential rewarding outcomes) versus the internal drive to avoid a novel environment (based on the potential aversive outcomes). Behavior in the open-field is sensitive to anxiolytic drugs such as the classical benzodiazepines (Bruhwyler, 1990; Hard et al., 1985; Prut and Belzung, 2003) and 5-HT1A receptor agonists (Rex et al., 1998; Siemiatkowski et al., 2000), but is insensitive to compounds such as triazolobenzodiazepines (O'Connor et al., 1985) and selective serotonin reuptake inhibitors (Durand et al., 1999), which are effective in the treatment of anxiety disorders. The different behavioral responses in the open-field to anxiolytic drugs with diverse pharmacological profiles has led to the suggestion that the open-field test represents a rodent model of normal conflict anxiety but not a rodent model of anxiety disorders (Prut and Belzung, 2003).
In the present study we hypothesized that anxiety-states and anxiety-related behavior are regulated by a distributed network of forebrain and hindbrain structures, including projections from the midbrain raphe complex to the basolateral amygdaloid complex. To test this hypothesis we used c-Fos expression in serotonergic neurons as a marker of neuronal activation, in control rats and rats exposed to an open-field arena in low- or high-light conditions. In a second experiment, we investigated the effects of exposure to an open-field arena in low-light conditions on c-Fos expression in neurons in the dorsal raphe nucleus and median raphe nucleus that project to the basolateral amygdaloid complex. We chose to study the low-light condition instead of the high-light condition because we were interested in identifying a threshold for activation of neurons projecting to the basolateral amygdaloid complex by mild anxiety-related stimuli. Finally, we used double immunofluorescence techniques to determine the proportion of neurons projecting to the basolateral amygdaloid complex that are serotonergic.
Experimental Procedures
Experiment 1
Animals
Adult male Wistar rats arrived from the vendor (B & K Universal Ltd, Hull, UK) weighing 213 ± 2 g and were allowed to acclimatize for at least a week in group housing (4 rats per cage) at 23 °C with ad libitum tap water and standard rat chow (CRM, B&K Universal Ltd., Hull, UK). Rats were then housed singly for 4 days in RB3 cages (45 × 28 × 20 cm, North Kent Plastic Cages Ltd., Rochester, UK) under the same environmental conditions. The average body weight on the test day had increased to 270 ± 2.6 g. Rats were maintained on a 12L:12D light cycle with lights on at 6:00 A.M. All animal procedures in Experiment 1 were approved by the University of Bristol Ethical Review Group and were conducted in accordance with Home Office guidelines and the UK Animals (Scientific Procedures) Act, 1986. In addition, all studies were consistent with the NIH Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23) and were covered by Animal Welfare Assurance #A5057-01.
Experimental design
Rats were weighed and handled daily in a holding room for 2 min on 4 consecutive days to familiarize them with general procedures involved and to increase the stability of behavioral responses (Hirsjarvi et al., 1990). Rats were randomly assigned to one of 3 treatment groups (n = 9); control (CO) groups were left undisturbed in home cages, low-light open-field (LL) groups were exposed to the open-field arena in low-light conditions (8-13 lux throughout the box) and high-light open-field (HL) groups were exposed to the open-field test in high-light conditions (400-500 lux throughout the box). On the test day, LL and HL rats were individually moved to an adjacent room and put in the open-field box for 15 min in either low- or high-light conditions. Following the open-field test each rat was placed back in its home cage and then returned to the holding room. Two hours from the start of the open-field test, LL, HL and time-matched CO rats were injected with an overdose of sodium pentobarbital (0.5-1.0 ml of Lethobarb (200 mg/ml), Fort Dodge, Southampton, UK), rats were perfused with fixative, and brains were collected for immunohistochemistry. The selection of the 2 h time point was based on previous studies in which injections of anxiogenic drugs increased c-Fos expression 2 h later within anxiety-related neural circuits, including serotonergic and non-serotonergic neurons within the mid-rostrocaudal and caudal DR (Abrams et al., 2005; Bouwknecht et al., 2007; Singewald et al., 2003; Singewald and Sharp, 2000), and in studies documenting c-Fos expression within the midbrain raphe nuclei 2 h following exposure of rats to the elevated plus-maze or elevated T-maze (Silveira et al., 1993; Silveira et al., 2001). Rats were tested and perfused between 08:00 A.M. and 3:00 P.M. It was considered important to limit the experimental time window because of diurnal variation of c-Fos expression in the DR (Janusonis and Fite, 2001).
Behavior
The square open-field arena (90 × 90 cm and 40 cm height) was divided into a 6 × 6 grid of equally-sized squares using black tape. The outer section of the box was defined as the sum of all squares adjacent to a wall including the 4 corner squares (i.e. 20 out of 36 squares). The remaining region of the open-field arena (16 square) was defined as the center. The test started by placing the rat in the same side of the outer section (halfway along one of the four walls of the box, facing the center) such that the rat could visit the center area first or move to one of the corners. The behavior of each rat in the open-field arena was recorded on video and scored afterwards using The Observer® 5.0 software (Noldus Information Technologies BV, Wageningen, The Netherlands, supplied by Tracksys Ltd. Nottingham, UK). For behavior, the data were collapsed for the 4 identical quarters of the box (with each quarter of the box containing 3 × 3 squares, consisting of 5 outer section and 4 center squares). Time spent in the outer section and the center was recorded. Total locomotor activity was scored as the number of line crossings (all four paws crossing a line) during each of 3 blocks of 5 min of the 15-min open-field test. In addition, the frequency of the following behaviors was recorded: rearing (standing on hind legs, with or without contact with the walls of the arena), grooming (using paws or tongue to clean/scratch body), stretched-attend posture (stretching forward with the forelimbs extended) and corner-facing (i.e. standing or sitting with the face directed toward the corner of the box).
Tissue collection and preparation
Rats were transcardially perfused with 0.05 M phosphate buffered saline (PBS: pH 7.4) followed by 4% paraformaldehyde in 0.1 M sodium phosphate buffer (PB: pH 7.4), both at 4 °C. Brains were removed from the skull and stored in the same fixative at 4 °C. The next day, brains were put in PB for 2 × 12 h, after which they were stored in 30% sucrose in PB until they had sunk. At that point, the brain was cut in a rat brain matrix (RBM-4000C, ASI Instruments, Warren, MI USA) into forebrain and hindbrain sections that were stored at −80 °C until further processing. The hindbrain, including the midbrain raphe complex, was then cut using a cryostat (Leica CM1900, Leica Microsystems Ltd, Buckinghamshire, UK) into 30 μm sections. Sections were collected as six alternate sets of slices (with each containing a representative set of sections, at 180 μm intervals, throughout the midbrain raphe complex) and stored at −20 °C in a cryoprotectant storage buffer (30% ethylene glycol, 20% glycerol in 0.05 M PB; pH 7.4).
Immunohistochemistry for c-Fos and TrpOH and cell counting
One set of slices, including the midbrain raphe complex, was used for double immunostaining using primary antibodies directed against the protein product of the immediate-early gene c-fos (rabbit anti-c-Fos polyclonal antibody, Cat No. PC38 (Ab-5), 1:10 000; Oncogene Research Products, San Diego, CA, USA), and against tryptophan hydroxylase (TrpOH: sheep anti-tryptophan hydroxylase polyclonal antibody, Cat No. 9260-2505, 1:10 000; Biogenesis Ltd, Poole, UK). Tryptophan hydroxylase was chosen as a marker of serotonergic neurons as previous research in our laboratory has suggested that an anti-serotonin antibody tended to stain dendritic and axonal processes, making it more difficult to discern double-labeling in the perikarya. The anti-TrpOH antibody stains mainly the perikarya. Nevertheless, our previous studies have found comparable results using the anti-TrpOH and anti-serotonin antibodies on alternate sets of brain sections (Staub et al., 2005).
Free-floating tissue was incubated at room temperature (RT) in 12-well tissue culture plates, washed in plastic tubs using mesh wells (Corning Costar, Corning, NY, USA), and gently shaken on an orbital shaker throughout double immunostaining. The length of all washes, rinses and pre-incubations was 15 min. Tissue was first washed in 0.05 M PBS, then rinsed in 1% hydrogen peroxide in PBS, followed by washing in 0.05 M PBS and pre-incubation in PBS containing 0.3% Triton X-100 (PBST); sections were then incubated overnight at RT with rabbit anti-c-Fos antibody in 0.1% PBST. After 15 h, tissue was washed twice in 0.3% PBST followed by incubation with a biotinylated swine anti-rabbit polyclonal antibody (Cat. No. E0353, 1:200; DakoCytomation Ltd, Cambridgeshire, UK) in 0.1% PBST for 90 min. Tissue was washed twice in 0.3% PBST followed by incubation with an avidin-biotin-peroxidase complex (Elite ABC reagent, Cat. No. PK-6100, 1:200; Vector Laboratories, Peterborough, UK) in 0.1% PBST for 90 min. Last, tissue was washed in 0.3% PBST, then in PBS, and incubated in peroxidase chromogen substrate (Vector SG; Vector Laboratories; Cat. No. SK4700; diluted as recommended by the vendor) in PBS for 23 min. After the chromogen reaction, tissue was immediately washed in PBS, 1% hydrogen peroxide in PBS, PBS, and 0.3% PBST respectively. Then, slices were incubated with sheep anti-TrpOH antibody in 0.1% PBST for 18 h. All subsequent steps were identical to those described above used for the immuno-peroxidase localization of c-Fos-immunoreactivity, except for the secondary antibody and chromogen reaction steps; these used a rabbit anti-sheep secondary antibody (Cat. No. PK-6106, 1:200, Vector Laboratories Ltd.), and a peroxidase chromogen substrate solution consisting of 0.01% 3,3′-diaminobenzidine tetrahydrochloride (DAB) and 0.0015% hydrogen peroxide in PBS (20 min). Finally, sections were washed twice in PBS to stop the reaction. Brain sections were rinsed briefly in distilled water then mounted on SuperFrost microscope slides (Fisher Scientific UK, Leicestershire, UK), dehydrated through an alcohol series and cleared with xylene. Slides were then coverslipped using DPX mounting medium (RA Lamb, London, UK). The color reaction of the c-Fos immunostaining was blue-black and localized to the nucleus while TrpOH immunostaining was orange-brown and localized to the cytoplasm. The numbers of c-Fos-immunoreactive (c-Fos-ir) serotonergic neurons (i.e., c-Fos-ir/TrpOH-ir neurons), the numbers of c-Fos-ir, non-serotonergic cells (i.e., c-Fos ir/TrpOH-immunonegative cells), and the total numbers of TrpOH-ir neurons sampled (i.e., both c-Fos-ir/TrpOH-ir and c-Fos-immunonegative/TrpOH-ir neurons) were counted in different regions of the DR and MnR at 4 rostrocaudal levels (−7.46, −8.00, −8.18, and −8.54 mm Bregma, Fig. 1) (Paxinos and Watson, 1998). The subdivisions of the DR studied included the dorsal raphe nucleus, dorsal part (DRD) and dorsal raphe nucleus, ventral part (DRV) at −7.46 mm Bregma, the DRD, DRV and dorsal raphe nucleus, ventrolateral part (DRVL) at −8.00 mm Bregma, the DRD, DRV, DRVL and dorsal raphe nucleus, interfascicular part (DRI) and the median raphe nucleus (MnR) at −8.18 mm Bregma and the dorsal raphe nucleus, caudal part (DRC), DRI and MnR at −8.54 mm Bregma. The DRVL at −8.00 and −8.18 mm Bregma is a bilateral structure. Cells were counted in both the left and the right DRVL and the cell counts were summed to give a total number of cells in the DRVL. For the remaining subdivisions, which are all located on the midline, cells were counted on both the left and right sides of the midline and the cell counts were summed to give a total number of cells in each subdivision. One section from each rat at each anatomical level was sampled. Cell counts were conducted using brightfield microscopy using a 40× objective lens (400× total magnification) by an investigator blind to the assignment of treatment groups.
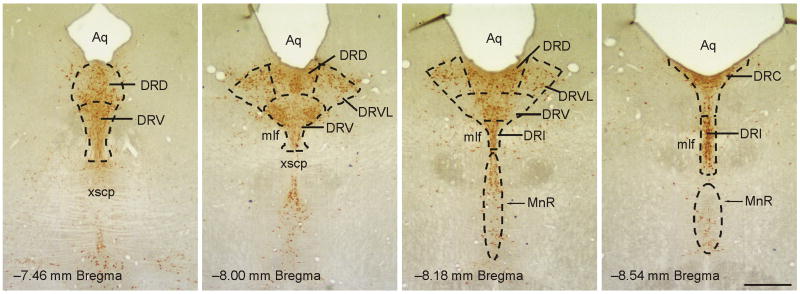
Figure 1.
Low magnification photomicrographs illustrating TrpOH/c-Fos-immunostained sections from different rostrocaudal levels of the midbrain raphe nuclei from a control rat. TrpOH-ir neurons and dendrites can be identified by the brown/orange precipitate within subdivisions of the DR and MnR. The subdivisions of the DR and MnR analyzed are illustrated by dashed lines (adapted from a standard stereotaxic atlas of the rat brain; Paxinos and Watson, 1998). Abbreviations: Aq, cerebral aqueduct; DRC, dorsal raphe nucleus, caudal part; DRD, dorsal raphe nucleus, dorsal part; DRI, dorsal raphe nucleus, interfascicular part; DRV, dorsal raphe nucleus, ventral part; DRVL, dorsal raphe nucleus, ventrolateral part; mlf, medial longitudinal fasciculus; MnR, median raphe nucleus; xscp, decussation of the superior cerebellar peduncle. Scale bar, 500 μm.
Experiment 2
In order to test the hypothesis that exposure to a mild anxiogenic stimulus selectively activates neurons within the DR that project to forebrain structures mediating anxiety-related behavioral responses, we used Cholera Toxin B subunit (CTb) as a retrograde tracer to identify neurons with direct afferent projections to the basolateral amygdaloid complex, in combination with c-Fos immunostaining, to identify cells responding to exposure to an open-field arena in low-light (8-13 lux) conditions. In addition, immunofluorescence for CTb and TrpOH was conducted to determine the proportion of the BL-projecting neurons within each subdivision of the midbrain raphe complex that were serotonergic.
Animals
Adult male Wistar rats arrived from the vendor (Møllengaarden, Denmark; N = 32) weighing approximately 200 g and were kept in standard laboratory conditions with ad libitum tap water and standard rat chow (Altromin, Lage, Germany) and maintained under a 12L:12D light cylce lights on at 6:00 AM. Experiment 2 was conducted under the authority of the Animal Core Facility of the Panum Institute, Department of Neuroscience and Pharmacology, The Panum Institute, University of Copenhagen, in accordance with and approved by The Animal Experiments Inspectorate, Ministry of Justice, Denmark. In all cases in Experiments 1 and 2 care was taken to minimize the number of animals used and their suffering.
Retrograde Tracing
Prior to CTb injection rats were anesthetized between 4 and 6 h after light onset with Hypnorm-Dormicum mixture (3 ml/kg s.c.) (one part fentanyl citrate (0.315 mg/ml) and fluanisone (10 mg/ml): one part midazolam (5 mg/ml): and two parts sterile water) and placed in a stereotaxic frame (Kopf Instruments, Model 1404, Turjunga, CA, USA). A glass microelectrode was broken to a final tip diameter of 15-20 μm and a solution of dialysed 4% CTb in PBS (Cat No. 104, LIST Biological Laboratories, Campbell, CA) was applied iontophoretically using positive current pulses of 10 mA (7 s on; 7 s off) for 10 min. The coordinates used for the basolateral amygdaloid complex were: posterior -2.8 mm; lateral 4.8 mm; ventral -8.5 mm with reference to Bregma according to a standard rat brain stereotaxic atlas (Paxinos and Watson, 1998). Rats were housed individually (cage size: 45 × 25 × 20 cm) for 7-11 days after CTb injections and handled for 2 min each day. On the test day rats were either 1) left undisturbed (control), 2) briefly handled in the testing room, or 3) exposed to open-field in low-light conditions (8-13 lux) for 15 min (as described above). Two hours after the start of the open-field test or handling, rats were re-anesthetized with Hypnorm and midazolam and perfused transcardially with PBS for 3 min followed by a solution of 10% formalin (Merck, Darmstadt, Germany) in 0.1 M phosphate buffer (pH 7.4) for 15 min. The brains were post-fixed in 10% formalin and 0.1 M phosphate buffer for 24 h, and stored in PBS + 0.1% sodium azide. Brains were cryoprotected for 3 days in 30% sucrose in PBS, frozen in dry ice and 40 μm sections were cut on a cryostat. Sections were divided into six alternate sets of slices, and stored in PBS +/0.1% sodium azide until immunohistochemical procedures were performed.
Immunohistochemistry for Cholera Toxin B-subunit and c-Fos
One set of slices was used for double immunostaining using primary antibodies directed against Cholera Toxin B-subunit (goat anti-CTb, Cat No. 703, 1:3000, List Biological Laboratories) and c-Fos (rabbit anti-c-Fos polyclonal antibody, Cat No. PC38 (Ab-5), 1:3000, Oncogene Research Products, San Diego, CA, USA).
Immunohistochemistry for CTb and c-Fos was conducted using free-floating brain sections in 30 ml tubes, gently shaken on an orbital shaker throughout double immunostaining. Tissue was rinsed in 3% hydrogen peroxide (H2O2) in PBS for 30 min, followed by washing in PBS containing 0.1% Triton X-100 (PBST) for 3 × 10 min; tissue was then incubated in a blocking solution of 1% human serum albumin (HSA) in 0.1% PBST for 30 min. Sections were incubated overnight at 4 °C with 1:3000 goat anti-CTb in 1% HSA/PBST. After 15 h, tissue was washed three times (for 10 min each) in 0.1% PBST followed by incubation with a biotinylated donkey anti-goat polyclonal antibody (Cat#705-096-147, Jackson ImmunoResearch Laboratories, West Grove, PA, USA) in 1% HSA/PBST for 60 min. Tissue was washed in 0.1% PBST for 3 × 10 min followed by incubation with Elite ABC reagent (Cat. No. PK-6100, 1:200; Vector Laboratories, Peterborough, UK) in 0.1% PBST for 60 min. Tissue was washed in 0.1% PBST for 3 × 10 min then incubated in 0.05% diaminobenzidine tetrahydrochloride (DAB) in PBS and 0.0066% H2O2 for 15 min. After the chromogen reaction, tissue was immediately washed in PBS (2 × 10 min), then 3% H2O2 in PBS (30 min), then 0.1% PBST (3 × 10 min) and preincubated in 1% HSA in 0.1% PBST for 30 min. Slices were then incubated with rabbit anti-c-Fos (Cat No. PC38 (Ab-5), 1:3000; Oncogene Research Products, San Diego, CA, USA) in 0.1% PBST overnight at room temperature. Tissue was then washed twice in 0.3% PBST for 15 min followed by incubation in biotinylated swine anti-rabbit secondary antibody (Cat. No. E0353, 1:200; DakoCytomation Ltd, Cambridgeshire, UK) in 0.1% PBST for 90 min. Following incubation with the secondary antibody, tissue was washed twice in 0.3% PBST for 15 min and incubated with an avidin-biotin-peroxidase complex (Elite ABC reagent, as above). Tissue was washed in 0.3% PBST for 15 min and rinsed in 0.05 M PBS. Tissue was then placed in a peroxidase chromogen substrate (Vector SG, Vector Laboratories; Cat. No. SK4700; diluted as recommended by the vendor) in PBS for 15 min. Finally, sections were washed twice in PBS to stop the reaction. Brain sections were rinsed briefly in distilled water then mounted on SuperFrost Plus microscope slides (Fisher Scientific UK, Leicestershire, UK), dehydrated through an alcohol series and cleared with xylene. Slides were then coverslipped using DPX mounting medium (RA Lamb, London, UK). The color reaction of the c-Fos immunostaining was blue-black and localized to the nucleus while CTb immunostaining was orange-brown and localized to the cytoplasm.
The numbers of c-Fos-ir/CTb-ir neurons, the numbers of c-Fos-ir/CTb-immunonegative cells), and the total numbers of CTb-ir neurons were counted in the same regions of the DR and MnR sampled in Experiment 1. Cell counts were conducted using brightfield microscopy using a 10× objective lens (100× total magnification; c-Fos-ir/CTb-ir cells were confirmed using a 40× objective lens; 400× total magnification) by an investigator blind to the assignment of treatment groups.
Immunofluorescence for CTb and TrpOH
In order to determine the proportion of BL-projecting neurons in the dorsal raphe nucleus that are serotonergic, an adjacent set of sections was used for double immunofluorescence using primary antibodies directed against CTb (goat anti-CTb, Cat No. 703, 1:2000, List Biological Laboratories) and TrpOH (mouse anti-TrpOH, Cat No. T0678, 1:2000, Sigma-Aldrich, Saint Louis, MO, USA).
Immunofluorescence for CTb and TrpOH was conducted on free-floating brain sections in 12-well plates (Corning Life Sciences, Lowell, MA, USA) and gently shaken on an orbital shaker throughout the procedure. Tissue was rinsed twice in 0.05 M PBS (15 min each time) followed by washing in PBS containing 0.1% Triton X-100 (PBST) for 60 min. Sections were incubated overnight at room temperature with 1:2000 goat anti-CTb and 1:2000 mouse anti-TrpOH in 0.1% PBST with 0.01% sodium azide. After 16 h, tissue was washed twice in 0.05 M PBS (15 min each time) and then incubated with donkey anti-mouse Cy5 (1:200, Cat No. 715-176-150, Jackson ImmunoResearch) in 0.1% PBST for 60 min. Tissue was then rinsed twice in 0.05 M PBS (15 min each time) and then incubated with donkey anti-goat FITC (1:200, Cat no. 705-096-147, Jackson ImmunoResearch) in 0.1% PBST for 60 min. Sections were then washed twice in PBS (15 min each time) and then transferred to 0.1M phosphate buffer (PB) prior to mounting. Tissue was mounted on SuperFrost Plus slides and coverslipped using Vectorshield mounting medium for fluorescence with DAPI (Cat No. H-1200, Vector Laboratories). Nail polish was used to seal the outer edges of the coverslips.
For cell counts, photomicrographs (100× total magnification) were generated for regions of interest using a Nikon 90i microscope and a Photometrics CoolSNAP ES digital camera linked to a computer with NIS Elements 3.00 imaging software (A.G. Heinze Inc., Lake Forest, CA, USA). For each rat, one section at each of four rostrocaudal levels (-7.46 mm, -8.00 mm, -8.18 mm and -8.54 mm Bregma) was photographed using FITC and Cy5 filters. Separate layers for CTb-ir and TrpOH-ir photomicrographs were created using Adobe Photoshop 6.0 and the numbers of CTb-ir and TrpOH-ir neurons were quantified by placing dots over each CTb-ir or TrpOH-ir profile in additional cell counting layers. The cell counting layers were superimposed and the numbers of single-labeled CTb, single-labeled TrpOH, and double labeled neurons were counted. Only full cell body profiles were counted. Double labeled (CTb-ir/TrpOH-ir) neurons were confirmed with the slides themselves using a 40× objective lens (400× total magnification).
Data analysis
Data were analyzed using analysis of variance (ANOVA) with repeated measures or independent samples t-tests. ANOVA with repeated measures was followed, when appropriate, by post hoc analysis using Bonferroni pairwise comparisons using SPSS (Version 14 for Windows, SPSS Inc., Chicago, IL, USA). A Huynh-Feldt correction epsilon (ε) was used for repeated measures analysis to correct for potential violation of the sphericity assumption.
Locomotor activity was analyzed using light condition (2 levels: LL and HL) as a between-subjects factor and time (3 levels: 0-5 min, 5-10 min, 10-15 min) as a within-subjects factor. The time spent in each square type (outer section, center) and the numbers of specific behavioral events were analyzed using independent samples t-tests.
In Experiment 1, cell counts for the numbers of c-Fos-ir/TrpOH-ir (serotonergic) neurons, the numbers of c-Fos-ir/TrpOH-immunonegative (non-serotonergic) neurons and the total numbers of TrpOH-ir neurons sampled were analyzed separately using treatment group (3 levels: CO, LL and HL) as a between-subjects factor and brain region (13 levels) as a within-subjects factor. In Experiment 2, cell counts for the numbers of c-Fos-ir/CTb-ir (basolateral amygdaloid complex-projecting) neurons, the number of c-Fos-ir/CTb-immunonegative (non-basolateral amygdaloid complex-projecting) neurons, total CTb-ir neurons and the percentage of c-Fos-ir/CTb-ir neurons (i.e. c-Fos-ir/CTb-ir / total CTb-ir × 100) were analyzed separately using treatment group (3 levels: CO, HA and LL) as a between-subjects factor and brain region (13 levels) as a within-subjects factor. For the immunofluorescence histochemistry, the total numbers of TrpOH-ir, CTb-ir, and TrpOH-ir/CTb-ir neurons were also analyzed separately using treatment group as a between-subjects factor and brain region as a within-subjects factor. Significance was accepted for the ANOVAs and post hoc comparisons when p < 0.05.
Outliers (Exp 1: 2.2%; Exp 2 (brightfield) 2.7%; Exp 2 (fluorescence) 2.7%) of the total cell count data) were identified by Grubbs test (Grubbs, 1969) and excluded. Replacement data for the repeated measures ANOVAs were calculated using the Petersen method (Petersen, 1985). Replacement data were not included in post hoc analyses and are not represented in graphical representation of the data. Significance was accepted for the ANOVAs and post hoc pairwise Bonferroni comparisons when p < 0.05.
Brightfield photographs were obtained using a Leica brightfield microscope (DMLB, Leica Mikroskopie and Systeme GmbH, Wetzler, Germany), an Insight digital camera (Diagnostics Instruments Inc., Sterling Heights, Michigan, USA) and SPOT 3.5.5 for Windows digital imaging software (Silicon Graphics, Mountain View, California, USA). Contrast and brightness of the photographs were adjusted using Adobe Photoshop 6.0.1 (Adobe Systems Incorporated, California, USA). Photographic plates were prepared in CorelDraw for Windows (Viglen Ltd., Wembley, UK).
Results
Experiment 1: Behavior in LL and HL groups
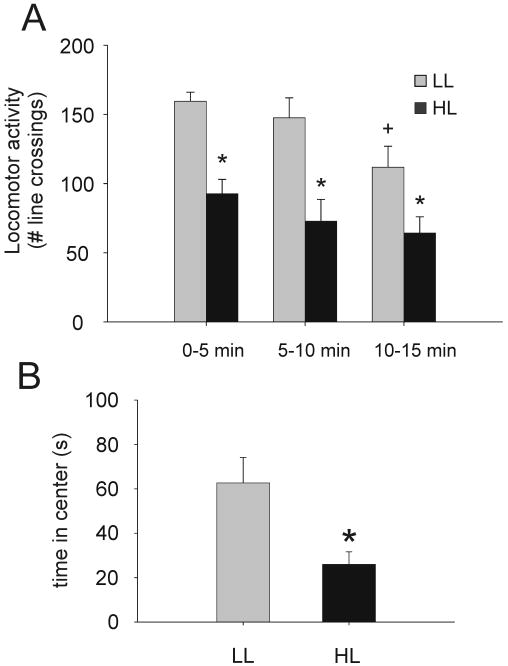
Consistent with a previous study conducted under similar conditions (Bouwknecht et al., 2007), exposure to the open-field in low- and high-light conditions differentially affected behavior (Fig. 2). Analysis of locomotor activity (Figure 2A), quantified as the number of line crossings in three 5-min blocks of the open-field test revealed main effects for time (F(2,32) = 8.16, p < 0.001, ε = 1.00) and treatment (LL and HL-open-field; F(1,16) = 19.35, p = 0.001). Rats exposed to the HL condition showed decreased locomotor activity compared to rats exposed to the LL condition in each of the 5 min time blocks of the 15 min open-field test. Post hoc Bonferroni pairwise comparisons showed a reduction in locomotor activity during the 10-15 min time block compared with the 0-5 min time blocks among rats exposed to the open-field in the LL condition.
Figure 2.
Graphs illustrating the effects of open-field exposure in low-light (LL: 8-13 lux) or high-light (HL: 400-500 lux) conditions on behavior in Experiment 1 (CO, n = 9; LL, n = 9; HL, n = 9). Graphs illustrate A) locomotor activity, scored as the number of square entries during each five min block of the 15 min open-field test (mean + SEM); *p < 0.05 versus LL within each time block; post hoc Bonferroni comparisons, +p < 0.05, versus 0-5 min time block within each light condition; post hoc Bonferroni pairwise comparisons, B) time spent in the outer section of the open-field, and C) time spent in the center of the open-field, *P < 0.05 versus LL group; independent samples t-tests.
The level of illumination also altered behavioral responses in the open-field test based on analysis of the time spent in each square type (Figure 2B). Rats exposed to the open-field in the HL condition spent less time in the center (t(16) = 2.86, p = 0.011) compared with rats exposed to the open-field in the LL condition.
The number of specific behavioral events was also examined. Rats exposed to the open-field in the LL condition displayed more rearing behavior than HL rats (t(16) = 2.45, p = 0.026; data not shown). There were no differences among treatment groups for grooming, stretched-attend posture or corner-facing behavior.
Experiment 1: Immunohistochemistry for c-Fos and TrpOH
Analysis of the numbers of c-Fos-ir serotonergic neurons, c-Fos-ir non-serotonergic cells, and total serotonergic neurons throughout the midbrain raphe nuclei
The total numbers of c-Fos-ir/TrpOH-ir (serotonergic) neurons (F(2,24) = 11.81, p < 0.001) and c-Fos ir/TrpOH-immunonegative (non-serotonergic) neurons (F(2,24) = 26.43, p < 0.001) across all subdivisions of the midbrain raphe nuclei studied were higher in LL and HL groups compared to CO (Table 1). The number of c-Fos ir serotonergic neurons was also higher in the HL group compared with the LL group, however there was no difference between the LL and HL groups in the total numbers of c-Fos-ir non-serotonergic cells.
Table 1.
Mean total numbers of cells counted within all subdivisions of the dorsal raphe nucleus and median raphe nucleus (mean ± SEM) in Experiment 1
| CO | LL | HL | |
|---|---|---|---|
| c-Fos+/TrpOH+ | 0.67 ± 0.37 | 6.67 ± 1.83* | 15.00 ± 3.10***+ |
| c-Fos+/TrpOH- | 11.2 ± 0.8 | 82.8 ± 11.5*** | 107.8 ± 12.1*** |
| Total TrpOH+ | 1084 ± 37 | 1106 ± 25 | 1091 ± 24 |
Abbreviations: c-Fos+/TrpOH+, c-Fos-ir/TrpOH-ir neurons; c-Fos+/TrpOH-, c-Fos-ir/TrpOH-immunonegative cells (CO, n = 9; LL, n = 9; HL, n = 9).
P < 0.05
P < 0.001 versus CO
P < 0.05 versus LL
post hoc Bonferroni comparisons.
c-Fos-ir serotonergic neurons in subdivisions of the midbrain raphe nuclei
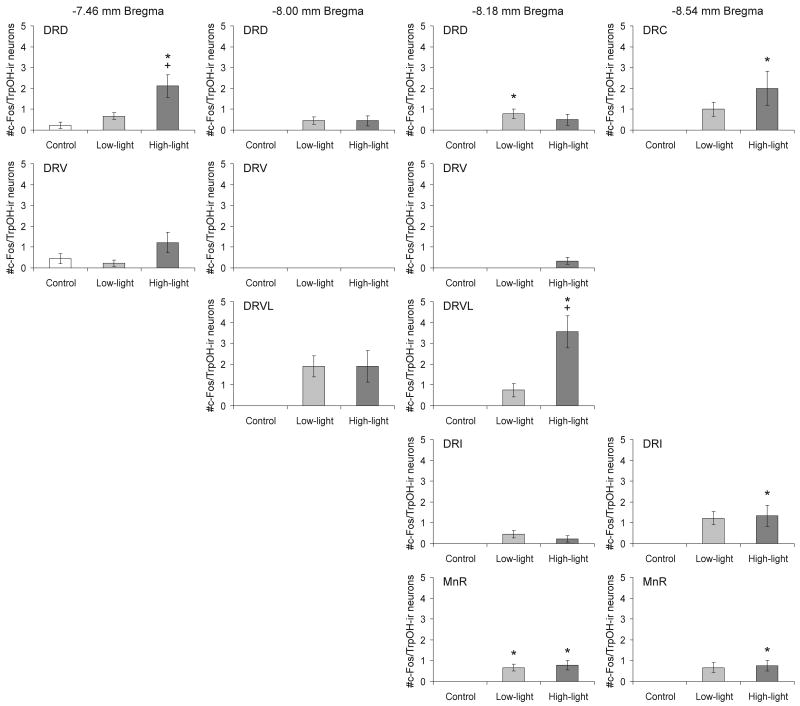
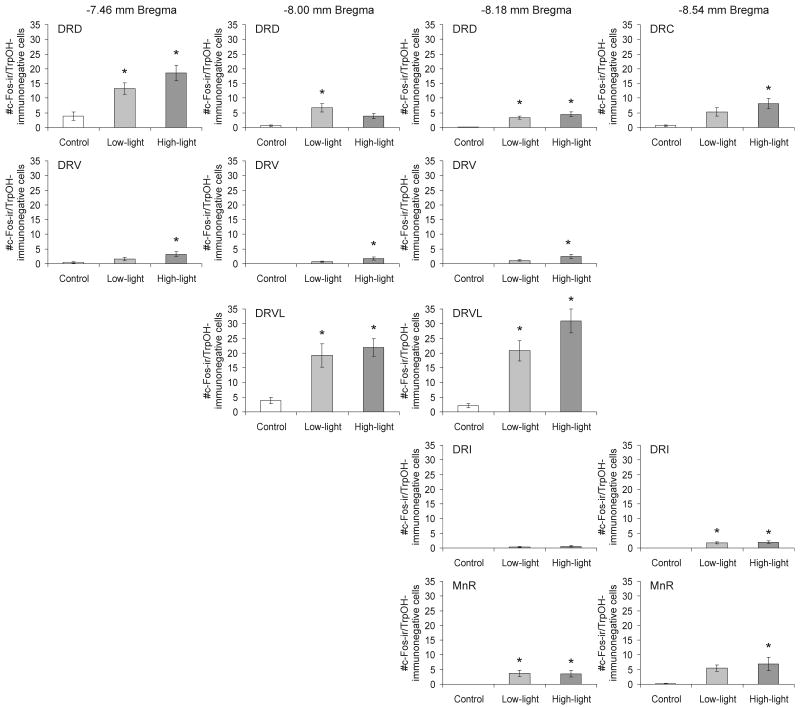
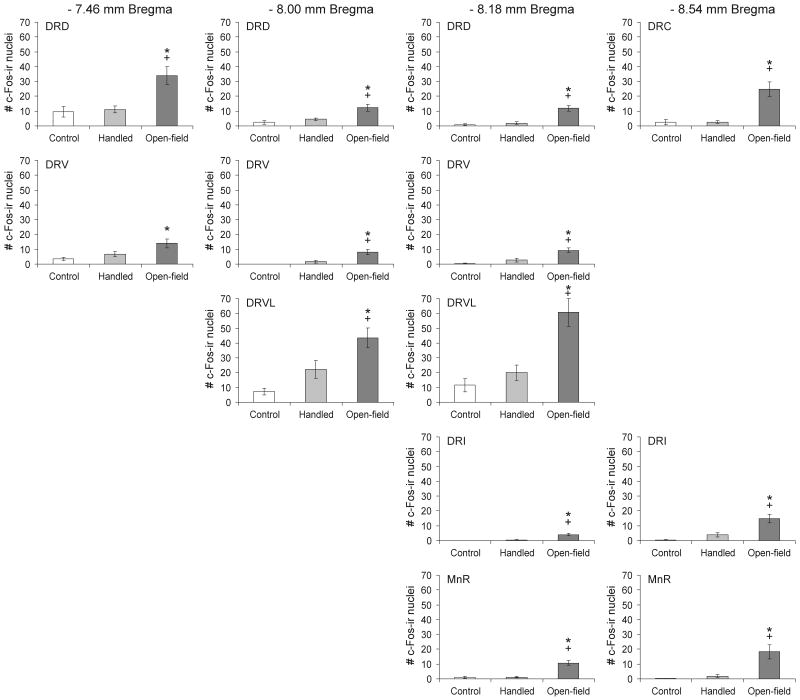
Open-field exposure in LL and HL conditions increased c-Fos expression in serotonergic neurons in subdivisions of the midbrain raphe nuclei (treatment × region interaction: F(24,288) = 4.22, p < 0.001, ε = 0.532; treatment: F(2,24) = 11.47, p < 0.001; region: F(12,288) = 8.15, p < 0.001, ε = 0.532). Post hoc analysis revealed effects of LL or HL open-field exposure on c-Fos expression in serotonergic neurons within all sampled subregions of the midbrain raphe nuclei studied except the DRV (Figure 3). Photographs in Figure 4 illustrate increases in c-Fos expression in serotonergic and non-serotonergic neurons within the DRD of control rats and rats exposed to either LL or HL open-field conditions.
Figure 3.
Graphs illustrating the effects of open-field exposure in low-light (LL: 8-13 lux) or high-light (HL: 400-500 lux) conditions on the number of c-Fos-ir/TrpOH-ir neurons (mean ± SEM) compared with home cage controls (CO) within different subdivisions of the midbrain raphe nuclei at the four rostrocaudal levels analyzed in Experiment 1. On the test day, rats were left undisturbed in their home cages (CO) or exposed to low-light (LL: 8-13 lux) or high-light (HL: 400-500 lux) conditions in an open-field arena for 15 min (CO, n = 9; LL, n = 9; HL, n = 9). *P < 0.05 versus CO group; +P < 0.05 versus LL group; post hoc Bonferroni pairwise comparisons. For abbreviations, see Fig. 1 legend.
Figure 4.
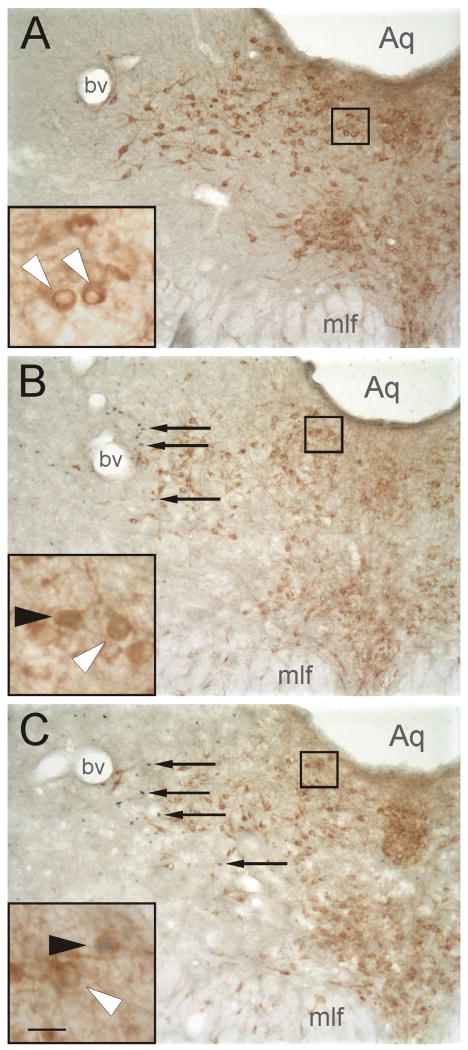
Photomicrographs illustrating the effects of exposure to the open-field in low-light, or high-light conditions compared to home cage controls, on c-Fos expression in serotonergic and non-serotonergic neurons in the mid-rostrocaudal dorsal raphe nucleus (−8.18 mm Bregma). Photomicrographs illustrate c-Fos-ir nuclei and TrpOH-ir neurons in sections from rats exposed to A) CO, B) LL, C) HL conditions in Experiment 1. Black boxes indicate regions shown at higher magnification in insets in the lower left hand corner of each panel. Arrows indicate examples of c-Fos-ir cells (blue/black nuclear staining); white arrowheads indicate c-Fos-immunonegative/TrpOH-ir (serotonergic) neurons (brown/orange cytoplasmic staining), filled arrowheads indicate c-Fos-ir/TrpOH-ir (double-immunostained) neurons. Abbreviation: Aq, cerebral aqueduct; bv, blood vessel, characteristic of the DRVL region at this rostrocaudal level; mlf, medial longitudinal fasciculus. Scale bar, 100 μm, inset 25 μm.
Post hoc Bonferroni pairwise comparisons detected increases in double-immunostained neurons in the LL and HL groups compared to the CO group in the MnR (−8.18 mm Bregma) and additional increases in the HL relative to the CO group in the DRD (−7.46 mm Bregma), the DRVL (−8.18 mm Bregma) and the DRC, DRI and MnR (−8.54 mm Bregma). There was also a small but statistically significant increase in the LL versus CO groups in the DRD (−8.18 mm Bregma). There were also increases in double-immunostained neurons in HL compared with LL rats in the DRD (−7.46 mm Bregma) and the DRVL (−8.18 mm Bregma).
c-Fos-ir non-serotonergic cells in subdivisions of the midbrain raphe nuclei
Open-field exposure in HL and LL conditions increased c-Fos expression in non-serotonergic cells in the dorsal raphe and median raphe nuclei (treatment × region: F(24,288) = 9.03, p < 0.001, ε = 0.309; treatment: F(2,24) = 22.77, p < 0.001; region: F(12,288) = 58.16, p < 0.001, ε = 0.309). Post hoc analysis revealed effects of LL or HL open-field exposure on c-Fos expression in non-serotonergic neurons within all subdivisions of the midbrain raphe nuclei studied except the DRI at -8.18 mm Bregma (Figure 5).
Figure 5.
Graphs illustrating the effects of open-field exposure in low-light (LL: 8-13 lux) or high-light (HL: 400-500 lux) conditions on the number of c-Fos-ir/TrpOH-immunonegative (non-serotonergic) cells (mean ± SEM) compared with home cage controls (CO) within different subdivisions of the midbrain raphe nuclei at the four rostrocaudal levels analyzed in Experiment 1. On the test day, rats were left undisturbed in their home cages (CO) or exposed to low-light (LL: 8-13 lux) or high-light (HL: 400-500 lux) conditions in an open-field arena for 15 min (CO, n = 9; LL, n = 9; HL, n = 9). *P < 0.05 versus CO group; post hoc Bonferroni pairwise comparisons. For abbreviations, see Fig. 1 legend.
Post hoc Bonferroni pairwise comparisons detected increases in c-Fos-ir/non-serotonergic cells in HL and LL groups compared to CO in the DRD (−7.46 mm Bregma), the DRVL (−8.00 mm Bregma), the DRD, DRVL and MnR (−8.18 mm Bregma) and the DRI (−8.54 mm Bregma). There were additional increases in HL compared to CO rats in the DRV (−7.46, −8.00 and −8.18 mm Bregma), DRC and MnR (−8.54 mm Bregma) and between the LL and CO groups in the DRD (−8.00 mm Bregma). There were no differences between LL and HL rats in any of the subdivisions of the midbrain raphe nuclei sampled.
Total TrpOH-ir neurons in subdivisions of the midbrain raphe nuclei
The numbers of TrpOH-ir cells sampled varied across the sampled subdivision of the midbrain raphe nuclei (F(12,288) = 117.11, p < 0.001, ε = 0.660). However, there were no differences in the total numbers of TrpOH-ir cells across the treatment groups and there was no interaction between region and treatment group (data not shown).
Experiment 2
The results from Experiment 1 suggest that exposure to an open-field increased c-Fos expression in serotonergic neurons within the DRD and MnR under low-light conditions, while the effects were more widespread under high-light conditions. The total numbers of c-Fos-ir serotonergic neurons were small (CO, 0.67 ± 0.37; LL, 6.67 ± 1.83; HL, 15.00 ± 3.1; mean ± SEM), suggesting that these effects may be restricted to a subset of serotonergic neurons. To determine if exposure to the open-field increases c-Fos expression in a subpopulation of neurons projecting to forebrain anxiety circuits, we examined the effects of exposure to the open-field environment on c-Fos expression in a subset of neurons in the dorsal raphe nucleus and median raphe nucleus that project to the basolateral amygdaloid complex.
Retrograde Tracing
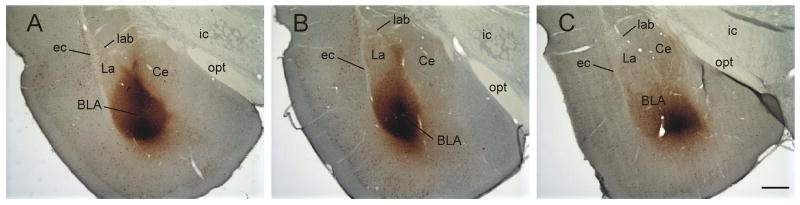
The distribution of CTb injection sites in the control rats, handled rats, and rats exposed to the open-field are summarized in Table 2 while figures illustrating the injection sites have been published (Hale et al., 2008). Representative injection sites for control, handled and open-field exposed groups are illustrated in Figure 6. The target of the injection was the basolateral amygdaloid complex. Although injection sites were often centered in two or more subdivisions of the basolateral amygdaloid complex, the major deposits of CTb were in the BLA (n = 13), BMP (n = 9), BLP (n = 3), BLV (n = 1) and BMA (n = 1). In 5 rats (16%) the injections sites were located outside the basolateral amygdaloid complex and therefore these rats were excluded from the study and are not included in Table 1. Of the 27 animals with appropriate injection sites, sections from an additional 5 cases (18.5%) were excluded that showed CTb immunostaining at -8.18 and -8.54 mm Bregma characteristic of CTb injections into the ventricle (Mikkelsen et al., 1997). In these cells, immunostaining of both the perikarya and dendritic structure appeared very dark brown/orange and frequently dendritic processes were observed extending several hundred micrometers from the perikarya toward the cerebral aqueduct. The sections from -8.18 and -8.54 mm Bregma from these rats were excluded from the analysis.
Table 2.
Table describing the center of each CTb injection site for control, handled and open-field-exposed rats in Experiment 2.
| Case Number | Treatment | Nuclei |
|---|---|---|
| T2822 | Control | BMP/BLA |
| T2823 | Control | BMA/BMP |
| T2825 | Control | BLA |
| T2824 | Control | BLA/BMP |
| T2826 | Control | BLA/BLP |
| T2820 | Control | BLA/CeA |
| T2816 | Handled | BLP/BLA |
| T2794 | Handled | BLA/BLV |
| T2796 | Handled | BLA |
| T2810 | Handled | BMP |
| T2808 | Handled | BMP/BLA |
| T2818 | Handled | BLA/BLP |
| T2812 | Handled | BMP |
| T2814 | Handled | BMP |
| T2804 | Handled | BMP |
| T2800 | Handled | BLV |
| T2815 | Open-field | BLA/BLP |
| T2799 | Open-field | BLA |
| T2811 | Open-field | BLA |
| T2801 | Open-field | BLA |
| T2819 | Open-field | BLP/BMP |
| T2817 | Open-field | BLA |
| T2803 | Open-field | BLA |
| T2795 | Open-field | BLP/BMP |
| T2807 | Open-field | BMP |
| T2805 | Open-field | BMP |
| T2809 | Open-field | BMP |
Abbreviations; BLA, basolateral amygdaloid nucleus, anterior part; BLP, basolateral amygdaloid nucleus, posterior part; BLV, basolateral amygdaloid nucleus, ventral part; BMP, basomedial amygdaloid nucleus, posterior part
Figure 6.
Photomicrographs of the basolateral amygdaloid complex (approximately -2.80 –- -3.14 mm Bregma (Paxinos and Watson, 1998)) showing CTb-injections from representative A) control (T2826), B) handled (T2818) and C) open-field exposed (T2811) rats from Experiment 2. Abbreviations: BLA, basolateral amygdaloid nucleus, anterior part; Ce, central amygdaloid nucleus; ec, external capsule; ic, internal capsule; La, lateral amygdaloid nucleus; lab, longitudinal association bundle. Scale bar 500 μm.
Retrogradely labeled cells were found in all subdivisions of the midbrain and pontine raphe complex studied, although the absolute number of retrogradely labeled cells varied among the different subnuclei. The number of retrogradely labeled neurons was highest in the mid-rostrocaudal DRD and DRV and lowest in the MnR, consistent with previous studies (Abrams et al., 2005).
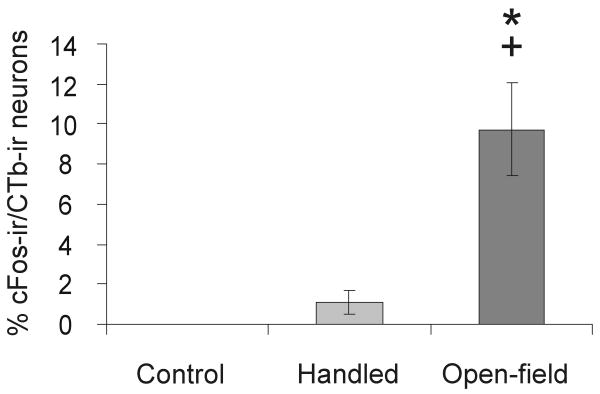
Exposure to the open-field arena increased the percentage of CTb-ir neurons expressing c-Fos in the midbrain raphe nuclei, irrespective of subdivision (F(2,24) = 9.72, p = 0.001; Figure 7, 8). Post hoc Bonferroni pairwise comparisons detected an increase in the percentage of c-Fos-positive, CTb-labeled neurons within the dorsal and median raphe nuclei of open-field exposed rats compared with either handled or home cage controls. There were no differences in c-Fos expression in BL-projecting raphe neurons among home-cage control and handled rats. Analysis of the total number of c-Fos-ir/CTb-ir neurons was also higher in rats exposed to the open-field compared with handled and control rats (F(2,24) = 7.82, p = 0.002; data not shown).
Figure 7.
Graph illustrating the effects of handling and open-field exposure, compared to home cage controls, on the percentage of CTb-ir neurons that were c-Fos-ir (i.e. c-Fos-ir/CTb-ir neurons / total CTb-ir neurons × 100; mean ± SEM) across 4 rostrocaudal levels of the dorsal raphe nucleus and median raphe nucleus (-7.46, -8.00, -8.18 and -8.54 mm Bregma) in Experiment 2. Rats were left undisturbed in their home cage, briefly handled or exposed to an open-field arena in low-light conditions (8-13 lux) for 15 min (Control, n = 6; Handled, n = 10; Open-field, n = 11). *p < 0.01 vs. control; +p < 0.01 versus handled group; post-hoc Bonferroni comparisons.
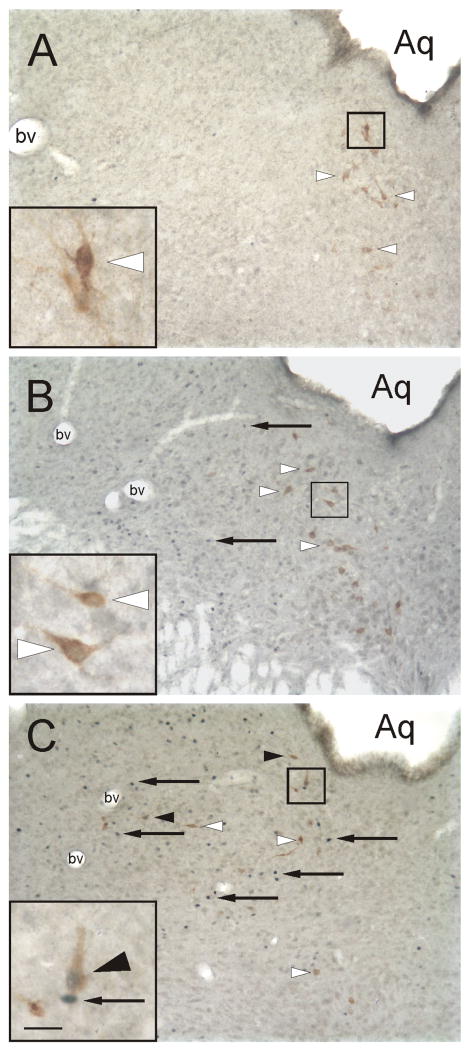
Figure 8.
Photomicrographs illustrating c-Fos-ir nuclei and CTb-ir neurons in the dorsal raphe nucleus (-8.18 mm Bregma) of rats exposed to A) Control (T2826), B) Handling (T2818), and C) Open-field conditions (T2801) in Experiment 2. Black boxes indicate regions shown at higher magnification in insets in the lower left-hand corner of each panel. Arrows indicate examples of c-Fos-ir nuclei (blue/black nuclear staining); white arrowheads indicate c-Fos-immunonegative/CTb-ir (basolateral amygdaloid complex-projecting) neurons (brown/orange cytoplasmic staining). Black arrowheads indicate c-Fos-ir/CTb-ir neurons. Abbreviation: Aq, cerebral aqueduct. Scale bar, 100 μm; inset, 25 μm.
Consistent with Experiment 1 and our previous findings (Bouwknecht et al., 2007) exposure to the open-field increased total numbers of c-Fos-ir nuclei (i.e. both CTb-ir and CTb-immunonegative cells) in subdivisions of the dorsal raphe and median raphe nuclei (Figure 9). Repeated measures ANOVA detected a treatment and subdivision interaction (F(24,288) = 5.67, p < 0.001, ε = 0.30) and main effects for treatment (F(2,24) = 3.55, p < 0.001) and subdivision (F(12,288) = 28.04, p < 0.001, ε = 0.30). Post hoc analysis revealed increases in c-Fos expression in both CTb-ir and CTb-immunonegative nuclei in open-field exposed rats compared to both control and handled rats in all subdivisions of the midbrain raphe nuclei sampled except in the DRV at -7.46 mm Bregma, where analysis detected an increase in rats exposed to the open-field compared with control but not with handled rats. There was no difference in total c-Fos expression among control and handled rats in any of the dorsal raphe and median raphe nuclei subdivisions studied.
Figure 9.
Graphs illustrating the effects of handling and open-field exposure, compared to home cage controls, on the total number of c-Fos-ir cells (mean ± SEM) within different subdivisions of the dorsal raphe and median raphe nuclei at the four rostrocaudal levels analyzed in Experiment 2. Rats were left undisturbed in their home cages (control), briefly handled (handled), or exposed to low-light conditions in an open-field arena for 15 min (Control, n = 6; Handled, n = 10; Open-field, n = 11). *P < 0.05 versus control group; +P < 0.05 versus HA group; post hoc Bonferroni pairwise comparisons. For abbreviations, see Fig. 1 legend.
The total number of CTb-ir neurons varied across midbrain raphe nuclei subdivisions (F(12,288) = 10.43, p < 0.001, ε = 0.38; Table 3). There was a small, but statistically significant difference in total CTb-ir neurons among treatment groups (F(2,24) = 3.55, p = 0.045). Post hoc analysis, however, revealed no differences among treatment groups in the subdivisions of the midbrain raphe complex except in the DRI at -8.18 mm Bregma (control versus open-field-exposed groups) and DRC at -8.54 (control versus handled groups and control versus open-field-exposed groups). There was no difference in the total numbers of CTb-ir neurons between the open-field exposed and handled rats in any subdivision of the dorsal and median raphe nuclei studied.
Table 3.
Total numbers of CTb-ir cells sampled in subdivisions of the dorsal raphe nucleus and median raphe nucleus (mean ± SEM) in Experiment 2 (Control, n = 6; Handled, n = 10; Open-field, n = 11).
| Rostrocaudal Level | Subdivision | Control | Handled | Open-field |
|---|---|---|---|---|
| -7.46 mm | DRD | 3.0 ± 0.9 | 2.2 ± 0.7 | 2.0 ± 0.6 |
| -8.00 mm | DRD | 2.5 ± 0.5 | 2.1 ± 0.7 | 1.9 ± 0.5 |
| -8.18 mm | DRD | 5.7 ± 1.8 | 5.5 ± 1.5 | 3.3 ± 0.7 |
| -7.46 mm | DRV | 2.7 ± 1.1 | 3.1 ± 1.0 | 1.3 ± 0.4 |
| -8.00 mm | DRV | 3.3 ± 0.9 | 7.0 ± 2.9 | 3.0 ± 0.8 |
| -8.18 mm | DRV | 5.3 ± 1.8 | 5.9 ± 1.8 | 2.3 ± 0.7 |
| -8.00 mm | DRVL | 4.7 ± 1.0 | 3.4 ± 0.8 | 1.8 ± 0.6 |
| -8.18 mm | DRVL | 2.7 ± 1.0 | 1.7 ± 0.5 | 0.8 ± 0.2 |
| -8.18 mm | DRI | 2.0 ± 0.4 | 1.6 ± 0.5 | 0.3 ± 0.2A |
| -8.54 mm | DRI | 5.5 ± 1.3 | 1.8 ± 0.5A | 2.1 ± 0.8A |
| -8.54 mm | DRC | 1.8 ± 0.7 | 1.3 ± 0.7 | 0.4 ± 0.3 |
| -8.18 mm | MRN | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.3 ± 0.2 |
| -8.54 mm | MRN | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.3 ± 0.2 |
P < 0.05 versus Control; post hoc Bonferroni comparisons
Immunofluorescence for CTb and TrpOH
Consistent with Experiment 1, the numbers of TrpOH-ir neurons varied across the subdivisions of the midbrain raphe nuclei sampled (F(12,264) = 72.94, p < 0.001, ε = 0.71; Figure 10) and there were no differences in the total numbers of TrpOH-ir cells across treatment and no interaction between region and treatment group. Consistent with the brightfield immunohistochemistry in Experiment 2, the numbers of CTb-ir neurons also varied across the subdivisions of the midbrain raphe sampled (F(12,264) = 12.65, p < 0.001, ε = 0.44; Figure 10), However unlike the brightfield immunohistochemistry, there was no effect of treatment on the numbers of CTb-ir neurons. An independent samples t-test indicated that there was no difference between the numbers of CTb-ir neurons detected in the brightfield and fluorescence techniques (t(571) = 0.390, p = 0.697).
Figure 10.
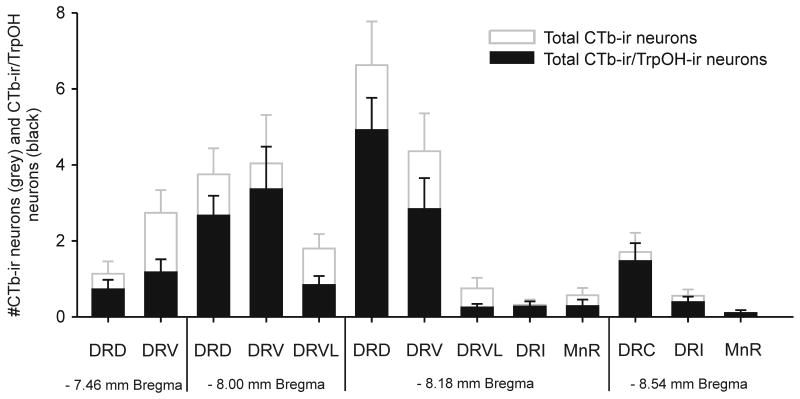
Histogram illustrating the distribution of BL-projecting neurons (grey open columns) and the distribution of BL-projecting serotonergic neurons (black filled columns) in subdivisions of the midbrain raphe complex across 4 rostrocaudal levels in combined control, handled and open-field conditions. See Table 1 for the distribution of CTb-ir neurons among treatment groups. For abbreviations see Fig 1 legend.
The total numbers of CTb-ir/TrpOH-ir (double-labeled) neurons varied among the subdivisions of the midbrain raphe nuclei sampled (F12,264) = 12.46, p < 0.001, ε = 0.30; Figure 10). Of the total numbers of BL-projecting neurons in the midbrain raphe nucleus, 65.2 ± 3.0% were serotonergic, however the percentage of BL-projecting serotonergic neurons varied among the sampled subdivisions (F(12,161) = 2.98, p = 0.001). Among the dorsal raphe nucleus subdivisions, the lowest percentages of serotonergic BL-projecting neurons were in the DRVL at both -8.00 and -8.18 mm Bregma (42.5 ± 9.5% and 46.7 ± 15.3% respectively) and the DRV at -7.46 mm Bregma (43.0 ± 9.0%). The highest percentage of serotonergic BL-projecting neurons were in the DRD at -8.00 and -8.18 mm Bregma (73.1 ± 5.8% and 85.5 ± 7.2%), DRV at -8.00 mm Bregma (77.7 ± 8.0%) and DRC (89.4 ± 5.6). The DRI and MnR contained very few CTb-ir neurons.
Discussion
Exposure of rats to an open-field arena under either LL or HL conditions was sufficient to increase c-Fos expression in both serotonergic and non-serotonergic cells in the midbrain raphe complex compared with home cage controls, including a subpopulation of neurons projecting to the basolateral amygdaloid complex. A high level of illumination in the open-field arena, compared to a low level of illumination, increased anxiety-like behavioral responses. In a few cases (i.e. the rostral DRD region and the DRVL region) the increased anxiety-related behavior in the HL condition compared to the LL condition was associated with an increase in c-Fos expression in serotonergic neurons. Although the effects of exposure to the open-field arena on c-Fos expression were statistically significant, they involved only small numbers of neurons, suggesting that exposure to the open-field arena may selectively affect subgroups of serotonergic and non-serotonergic cells. Combining immunostaining for c-Fos and retrograde tracing methods revealed that exposure to the open-field under LL conditions increased c-Fos expression in the population of neurons within the midbrain raphe complex that projects to the basolateral amygdaloid complex. Combined immunofluorescence for CTb and TrpOH indicated that most, but not all, BL-projecting neurons in the midbrain raphe complex are serotonergic and the proportion of BL-projecting neurons that are serotonergic varies depending on the anatomical subdivision of the raphe complex. These data are consistent with the hypothesis that mild anxiogenic stimuli engage serotonergic neurons that are part of a distributed system regulating anxiety-related behaviors.
A high level of illumination in the open-field arena, compared to a low level of illumination, increased anxiety-like behavioral responses. Exposure to HL conditions, relative to LL conditions, decreased overall locomotor activity, and decreased the time spent traversing center of the open-field. In the LL condition, rats showed a progressive decrease in locomotor activity during the 15 min open-field test, i.e. rats made fewer line crossings during the 10 -15 min time period compared with the 0 – 5 min time period. Consequently, c-Fos expression in serotonergic and non-serotonergic cells in the midbrain raphe complex may be associated with the initial anxiety-related behaviors observed in the open-field, or with the habituation to the test environment. These findings are consistent with our previous work (Bouwknecht et al., 2007; Hale et al., 2006) showing that the level of illumination in the open-field test is an important determinate of anxiety-related behaviors.
Open-field exposure in either low- or high-levels of illumination increased c-Fos expression in serotonergic neurons within all subdivisions of the dorsal raphe nucleus and median raphe nucleus studied, excluding the DRV, compared with home cage controls. Rats exposed to the open-field arena responded with small but statistically significant increases in c-Fos expression within the rostral DRD, DRVL, DRC, DRI, and MnR in the HL rats and mid-rostrocaudal (-8.18 mm Bregma) DRD and MnR in LL rats. Consistent with our previous research (Bouwknecht et al., 2007) there were few differences between LL and HL conditions in c-Fos expression in serotonergic neurons; however in the present study, exposure of rats to the HL condition, relative to the LL condition, showed increased c-Fos expression in serotonergic neurons in the rostral DRD and mid-rostrocaudal DRVL. This is the first time we have reported increases in c-Fos expression in serotonergic neurons in the MnR following open-field exposure. Exposure to the open-field, under either LL or HL conditions, increased c-Fos expression in small numbers of serotonergic neurons within both levels of the MnR studied.
Exposure to the open-field increased c-Fos expression in non-serotonergic cells in most subdivisions of the midbrain raphe nuclei studied. Consistent with our previous report (Bouwknecht et al., 2007) the majority of c-Fos-ir nuclei were non-serotonergic and the greatest effects of exposure to the open-field test under either LL or HL conditions were observed in the DRVL region and the rostral part of the DRD, a pattern that mirrored the regional effects on c-Fos expression in serotonergic neurons. Small but statistically significant increases in c-Fos expression in non-serotonergic cells were observed in HL, but not LL rats in the DRV at each rostrocaudal level. Exposure to the open-field, under either LL or HL conditions, increased c-Fos expression in non-serotonergic neurons within both levels of the MnR. There were no differences in c-Fos expression in non-serotonergic cells between the LL and HL rats in any of the regions studied.
Exposure to the open-field arena in low-light conditions increased c-Fos expression in an anatomically defined subpopulation of neurons within the dorsal raphe nucleus and median raphe nucleus projecting to the basolateral amygdaloid complex. CTb-ir neurons were found in all subdivisions of the midbrain raphe complex and although they were distributed across the entire rostrocaudal extent of the DR, the greatest numbers of basolateral amygdaloid complex-projecting neurons were found in the mid-rostrocaudal DRD and DRV, consistent with previous studies (Abrams et al., 2005; Lowry et al., 2005). Exposure to the open-field increased both the percentage of c-Fos-ir/CTb-ir neurons and the total numbers of c-Fos-ir/CTb-ir neurons in the midbrain raphe complex regardless of subdivision. Dual labeling immunofluorescence indicated that the majority of BL-projecting neurons in the midbrain raphe complex are serotonergic (65.2 ± 3.0%). However the numbers of BL-projecting neurons that are serotonergic vary depending on the anatomical subdivision of the midbrain raphe complex. Fewer than half of the BL-projecting neurons in the DRVL regions (both at -8.00 and -8.18 mm Bregma) were found to be serotonergic whereas BL-projecting neurons in the mid-rostrocaudal DRD were mostly serotonergic (73.1 ± 5.8% at -8.00 mm Bregma and 85.5 ± 7.2% at -8.18 mm Bregma).
Although we cannot be certain that c-Fos-ir basolateral amygdaloid-projecting neurons in the dorsal raphe nucleus were serotonergic, converging lines of evidence support a role for mid-rostrocaudal serotonergic systems in the regulation of anxiety states. We have previously reported that multiple anxiogenic drugs increase behavioral arousal and vigilance and have convergent effects on c-Fos expression in a topographically organized subpopulation of serotonergic neurons within the mid-rostrocaudal DRD (Abrams et al., 2005). Similarly, microinjections of an anxiogenic dose of the CRF receptor agonist urocortin 1 directly into the basolateral amygdala also increased c-Fos expression in the same subset of serotonergic neurons of the dorsal raphe nucleus (Spiga et al., 2006). In both of the above studies, much higher threshold anxiogenic stimuli were employed compared to the present study, and both systemic (Abrams et al., 2005) or localized basolateral amygdala injections (Spiga et al., 2006) of anxiogenic drugs elicited c-Fos expression in correspondingly greater numbers of serotonergic neurons in the dorsal raphe nucleus (about 20% and 5%, respectively, compared to about 1% in the present study). Future studies should determine if the increases in c-Fos expression in neurons projecting to the basolateral amygdaloid complex are localized within serotonergic or non-serotonergic neurons. Nevertheless, the current study demonstrates that exposure to a mild anxiogenic stimulus affects significant numbers of neurons in the midbrain raphe complex projecting to the basolateral amygdala (about 10%), and stimuli that are more anxiogenic are likely to engage higher percentages of the serotonergic neurons projecting to the amygdala circuits regulating anxiety states and anxiety-related behaviors.
The effects of exposure to the open-field on c-Fos expression in neurons projecting to the basolateral amygdaloid complex were evident even though there was some variability in the locations of injections of CTb in the basolateral amygdaloid complex. This may be because the innervation of the amygdala by the raphe complex is derived almost exclusively from the dorsal raphe forebrain tract traveling within the ventrolateral part of the medial forebrain bundle (Azmitia, 1981). Consequently, the innervation of different parts of the basolateral amygdaloid complex may be derived from the same group of raphe neurons.
Retrogradely labeled cells were found in all subdivisions of the midbrain and pontine raphe complex, however the numbers of retrogradely labeled neurons varied among subdivisions with the greatest numbers of basolateral amygdala-projecting cells located in the mid-rostrocaudal DRD and DRV. The DRD receives afferents from and sends efferents to a distributed system associated with stress- and anxiety-related behavioral responses (Abrams et al., 2005; Commons et al., 2003; Lowry et al., 2005; Peyron et al., 1998). The DRD receives projections from several forebrain and hypothalamic structures involved in the regulation of emotional behavior, including the lateral and ventral orbitofrontal and infralimbic cortices, the central nucleus of the amygdala, the bed nucleus of the stria terminalis, and the dorsal, dorsomedial, lateral and posterior hypothalamic nuclei (Peyron et al., 1998), and sends efferent projections to the dorsolateral periaqueductal gray (DLPAG; (Stezhka and Lovick, 1997)), basolateral and central nuclei of the amygdala (Abrams et al., 2005; Ottersen, 1981; Rizvi et al., 1991; Russchen, 1982), BNST ((Petit et al., 1995)) nucleus accumbens and medial prefrontal cortex (mPFC; (Van Bockstaele et al., 1993)). Basolateral amygdaloid complex-projecting cells are concentrated in the DRD but are not exclusively located in the DRD. Therefore without retrograde tracing, effects of anxiogenic stimuli on c-Fos expression may be more apparent in the DRD (Abrams et al., 2005) where cells projecting to the basolateral amygdaloid complex are numerous.
Conclusions
These studies demonstrate that exposure of rats to an open-field arena in low- and high-light conditions increases c-Fos expression in both serotonergic and non-serotonergic cells in the midbrain raphe complex compared with home cage controls, including a subpopulation of neurons projecting to the basolateral amygdaloid complex. These results are consistent with the hypothesis that a dorsal raphe nucleus-basolateral amygdala circuit participates in the regulation of anxiety states and anxiety-related behavior. Further studies should characterize the neurochemical phenotype of the basolateral amygdala-projecting neurons activated by exposure to mild anxiety-related stimuli and their role in anxiety-related physiological and behavioral responses.
Figure 11.
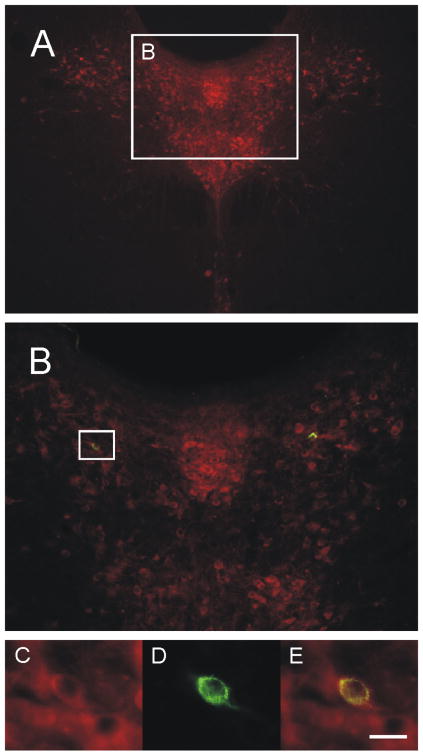
Double immunofluorescence photomicrograph illustrating a retrogradely labeled serotonergic neuron in the DRD region of the mid-rostrocaudal dorsal raphe nucleus (-8.18 mm Bregma) following unilateral intra-BL injection of CTb. A) Photomicrograph showing the mid-rostrocaudal dorsal raphe nucleus. White box in A indicates region shown at higher magnification in B. White box in B indicates region shown at higher magnification in C, D and E. TrpOH-ir (serotonergic) neurons appear red (C) while CTb-ir (retrogradely labeled) neuron appears green (D). Double TrpOH/CTb immunofluorescent neuron appears yellow (E). Scale bar (A) 200μm, (B) 80μm, (C), (D) & (E) 20μm.
Acknowledgments
This work was supported by NIMH R01 MH065702 and R01 MH52619 to AS and a Wellcome Trust Research Career Development Fellowship to CAL (RCDF 068558/Z/02/Z). CAL is a recipient of a NARSAD 2007 Young Investigator Award.
Reference List
- Abrams JK, Johnson PL, Hay-Schmidt A, Mikkelsen JD, Shekhar A, Lowry CA. Serotonergic systems associated with arousal and vigilance behaviors following administration of anxiogenic drugs. Neuroscience. 2005;133:983–997. doi: 10.1016/j.neuroscience.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Azmitia EC. The serotonin-producing neurons of the midbrain median and dorsal raphe nuclei. In: Iversen LL, Iversen SH, Snyder SH, editors. Chemical Pathways in the Brain. Plenum; New York: 1981. pp. 233–314. [Google Scholar]
- Belzung C, Griebel G. Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav Brain Res. 2001;125:141–149. doi: 10.1016/s0166-4328(01)00291-1. [DOI] [PubMed] [Google Scholar]
- Bouwknecht JA, Spiga F, Staub DR, Hale MW, Shekhar A, Lowry CA. Differential effects of exposure to low-light or high-light open-field on anxiety-related behaviors: Relationship to c-Fos expression in serotonergic and non-serotonergic neurons in the dorsal raphe nucleus. Brain Research Bulletin. 2007;72:32–43. doi: 10.1016/j.brainresbull.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhwyler J. Anxiolytic potential of a microgram dose of chlordiazepoxide in the open-field test. Eur J Pharmacol. 1990;187:547–549. doi: 10.1016/0014-2999(90)90385-j. [DOI] [PubMed] [Google Scholar]
- Campbell BM, Merchant KM. Serotonin 2C receptors within the basolateral amygdala induce acute fear-like responses in an open-field environment. Brain Res. 2003;993:1–9. doi: 10.1016/s0006-8993(03)03384-5. [DOI] [PubMed] [Google Scholar]
- Commons KG, Connolley KR, Valentino RJ. A neurochemically distinct dorsal raphe-limbic circuit with a potential role in affective disorders. Neuropsychopharmacology. 2003;28:206–215. doi: 10.1038/sj.npp.1300045. [DOI] [PubMed] [Google Scholar]
- Durand M, Berton O, Aguerre S, Edno L, Combourieu I, Mormede P, Chaouloff F. Effects of repeated fluoxetine on anxiety-related behaviours, central serotonergic systems, and the corticotropic axis axis in SHR and WKY rats. Neuropharmacology. 1999;38:893–907. doi: 10.1016/s0028-3908(99)00009-x. [DOI] [PubMed] [Google Scholar]
- Gonzalez LE, Andrews N, File SE. 5-HT1A and benzodiazepine receptors in the basolateral amygdala modulate anxiety in the social interaction test, but not in the elevated plus-maze. Brain Res. 1996;732:145–153. doi: 10.1016/0006-8993(96)00517-3. [DOI] [PubMed] [Google Scholar]
- Grubbs FE. Procedures for Detecting Outlying Observations in Samples. Technometrics. 1969;11:1–21. [Google Scholar]
- Hale MW, Bouwknecht JA, Spiga F, Shekhar A, Lowry CA. Exposure to high- and low-light conditions in an open-field test of anxiety increases c-Fos expression in specific subdivisions of the rat basolateral amygdaloid complex. Brain Res Bull. 2006;71:174–182. doi: 10.1016/j.brainresbull.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Hale MW, Hay-Schmidt A, Mikkelsen JD, Poulsen B, Shekhar A, Lowry CA. Exposure to an open-field arena increases c-Fos expression in a distributed anxiety-related system projecting to the basolateral amygdaloid complex. Neuroscience. 2008;155:659–672. doi: 10.1016/j.neuroscience.2008.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Schmid MJ, LoPresti ML, Der-Avakian A, Pellymounter MA, Foster AC, Watkins LR, Maier SF. Corticotropin releasing hormone type 2 receptors in the dorsal raphe nucleus mediate the behavioral consequences of uncontrollable stress. J Neurosci. 2003;23:1019–1025. doi: 10.1523/JNEUROSCI.23-03-01019.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hard E, Engel J, Larsson K, Musi B. Effect of diazepam, apomorphine and haloperidol on the audiogenic immobility reaction and on the open field behavior. Psychopharmacology (Berl) 1985;85:106–110. doi: 10.1007/BF00427332. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Bradbury AJ, Jones BJ, Oakley NR. Behavioural and biochemical consequences following activation of 5HT1-like and GABA receptors in the dorsal raphe nucleus of the rat. Neuropharmacology. 1988;27:993–1001. doi: 10.1016/0028-3908(88)90058-5. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Jones BJ, Oakley NR. Effect of 5-Ht1A Receptor Agonists in 2 Models of Anxiety After Dorsal Raphe Injection. Psychopharmacology. 1992;106:261–267. doi: 10.1007/BF02801982. [DOI] [PubMed] [Google Scholar]
- Hirsjarvi PA, Junnila MA, Valiaho TU. Gentled and non-handled rats in a stressful open-field situation; differences in performance. Scand J Psychol. 1990;31:259–265. doi: 10.1111/j.1467-9450.1990.tb00838.x. [DOI] [PubMed] [Google Scholar]
- Janusonis S, Fite KV. Diurnal variation of c-Fos expression in subdivisions of the dorsal raphe nucleus of the Mongolian gerbil (Meriones unguiculatus) J Comp Neurol. 2001;440:31–42. doi: 10.1002/cne.1368. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Johnson PL, Hay-Schmidt A, Mikkelsen JD, Shekhar A. Modulation of anxiety circuits by serotonergic systems. Stress. 2005;8:233–246. doi: 10.1080/10253890500492787. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability, anxiety, and serotonin. Cognitive Therapy and Research. 1998;22:595–613. [Google Scholar]
- Mascagni F, McDonald AJ. A novel subpopulation of 5-HT type 3A receptor subunit immunoreactive interneurons in the rat basolateral amygdala. Neuroscience. 2007;144:1015–1024. doi: 10.1016/j.neuroscience.2006.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Neuronal localization of 5-HT type 2A receptor immunoreactivity in the rat basolateral amygdala. Neuroscience. 2007;146:306–320. doi: 10.1016/j.neuroscience.2007.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen JD, Hay-Schmidt A, Larsen PJ. Central innervation of the rat ependyma and subcommissural organ with special reference to ascending serotoninergic projections from the raphe nuclei. J Comp Neurol. 1997;384:556–568. [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Serotonin-immunoreactive axon terminals innervate pyramidal cells and interneurons in the rat basolateral amygdala. J Comp Neurol. 2007;505:314–335. doi: 10.1002/cne.21486. [DOI] [PubMed] [Google Scholar]
- O'Connor WT, Earley B, Leonard BE. Antidepressant properties of the triazolobenzodiazepines alprazolam and adinazolam: studies on the olfactory bulbectomized rat model of depression. Br J Clin Pharmacol. 1985;19 1:49S–56S. doi: 10.1111/j.1365-2125.1985.tb02742.x. [DOI] [PubMed] [Google Scholar]
- Ottersen OP. Afferent connections to the amygdaloid complex of the rat with some observations in the cat. III. Afferents from the lower brain stem. J Comp Neurol. 1981;202:335–356. doi: 10.1002/cne.902020304. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press, Inc.; San Diego, CA: 1998. [Google Scholar]
- Petersen RG. Design and analysis of experiments. M. Dekker; New York: 1985. [Google Scholar]
- Petit JM, Luppi PH, Peyron C, Rampon C, Jouvet M. VIP-like immunoreactive projections from the dorsal raphe and caudal linear raphe nuclei to the bed nucleus of the stria terminalis demonstrated by a double immunohistochemical method in the rat. Neurosci Lett. 1995;193:77–80. doi: 10.1016/0304-3940(95)11669-n. [DOI] [PubMed] [Google Scholar]
- Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998;82:443–468. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Rex A, Voigt JP, Voits M, Fink H. Pharmacological evaluation of a modified open-field test sensitive to anxiolytic drugs. Pharmacol Biochem Behav. 1998;59:677–683. doi: 10.1016/s0091-3057(97)00461-9. [DOI] [PubMed] [Google Scholar]
- Rizvi TA, Ennis M, Behbehani MM, Shipley MT. Connections between the central nucleus of the amygdala and the midbrain periaqueductal gray: topography and reciprocity. J Comp Neurol. 1991;303:121–131. doi: 10.1002/cne.903030111. [DOI] [PubMed] [Google Scholar]
- Russchen FT. Amygdalopetal projections in the cat. II. Subcortical afferent connections. A study with retrograde tracing techniques. J Comp Neurol. 1982;207:157–176. doi: 10.1002/cne.902070205. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Schober DA, Gehlert DR. Neuropeptide Y receptor subtypes in the basolateral nucleus of the amygdala modulate anxiogenic responses in rats. Neuropharmacology. 2002;43:1165–1172. doi: 10.1016/s0028-3908(02)00234-4. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Schober DA, Gehlert DR, Shekhar A. Role of corticotropin-releasing factor and urocortin within the basolateral amygdala of rats in anxiety and panic responses. Behav Brain Res. 1999;100:207–215. doi: 10.1016/s0166-4328(98)00132-6. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Shekhar A. Excitatory amino acid receptors in the basolateral amygdala regulate anxiety responses in the social interaction test. Brain Res. 1997;764:262–264. doi: 10.1016/s0006-8993(97)00594-5. [DOI] [PubMed] [Google Scholar]
- Sena LM, Bueno C, Pobbe RL, Andrade TG, Zangrossi H, Jr, Viana MB. The dorsal raphe nucleus exerts opposed control on generalized anxiety and panic-related defensive responses in rats. Behav Brain Res. 2003;142:125–133. doi: 10.1016/s0166-4328(02)00399-6. [DOI] [PubMed] [Google Scholar]
- Siemiatkowski M, Sienkiewicz-Jarosz H, Czlonkowska AI, Bidzinski A, Plaznik A. Effects of buspirone, diazepam, and zolpidem on open field behavior, and brain [3H]muscimol binding after buspirone pretreatment. Pharmacol Biochem Behav. 2000;66:645–651. doi: 10.1016/s0091-3057(00)00200-8. [DOI] [PubMed] [Google Scholar]
- Silveira MC, Sandner G, Graeff FG. Induction of Fos immunoreactivity in the brain by exposure to the elevated plus-maze. Behav Brain Res. 1993;56:115–118. doi: 10.1016/0166-4328(93)90028-o. [DOI] [PubMed] [Google Scholar]
- Silveira MC, Zangrossi H, de B V, Silveira R, Graeff FG. Differential expression of Fos protein in the rat brain induced by performance of avoidance or escape in the elevated T-maze. Behav Brain Res. 2001;126:13–21. doi: 10.1016/s0166-4328(01)00233-9. [DOI] [PubMed] [Google Scholar]
- Singewald N, Salchner P, Sharp T. Induction of c-Fos expression in specific areas of the fear circuitry in rat forebrain by anxiogenic drugs. Biol Psychiatry. 2003;53:275–283. doi: 10.1016/s0006-3223(02)01574-3. [DOI] [PubMed] [Google Scholar]
- Singewald N, Sharp T. Neuroanatomical targets of anxiogenic drugs in the hindbrain as revealed by Fos immunocytochemistry. Neuroscience. 2000;98:759–770. doi: 10.1016/s0306-4522(00)00177-9. [DOI] [PubMed] [Google Scholar]
- Spiga F, Lightman SL, Shekhar A, Lowry CA. Injections of urocortin 1 into the basolateral amygdala induce anxiety-like behavior and c-Fos expression in brainstem serotonergic neurons. Neuroscience. 2006;138:1265–1276. doi: 10.1016/j.neuroscience.2005.12.051. [DOI] [PubMed] [Google Scholar]
- Staub DR, Spiga F, Lowry CA. Urocortin 2 increases c-Fos expression in topographically organized subpopulations of serotonergic neurons in the rat dorsal raphe nucleus. Brain Res. 2005;1044:176–189. doi: 10.1016/j.brainres.2005.02.080. [DOI] [PubMed] [Google Scholar]
- Stein L, Wise CD, Belluzzi JD. Effects of benzodiazepines on central serotonergic mechanisms. Adv Biochem Psychopharmacol. 1975:29–44. [PubMed] [Google Scholar]
- Stezhka VV, Lovick TA. Projections from dorsal raphe nucleus to the periaqueductal grey matter: studies in slices of rat midbrain maintained in vitro. Neurosci Lett. 1997;230:57–60. doi: 10.1016/s0304-3940(97)00464-3. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Biswas A, Pickel VM. Topography of serotonin neurons in the dorsal raphe nucleus that send axon collaterals to the rat prefrontal cortex and nucleus accumbens. Brain Res. 1993;624:188–198. doi: 10.1016/0006-8993(93)90077-z. [DOI] [PubMed] [Google Scholar]