Abstract
Earlier studies in monkeys have reported mild impairment in recognition memory following nonselective neonatal hippocampal lesions (Bachevalier, Beauregard, & Alvarado, 1999; Rehbein, Killiany, & Mahut, 2005). To assess whether the memory impairment could have resulted from damage to cortical areas adjacent to the hippocampus, we tested adult monkeys with neonatal focal hippocampal lesions and sham-operated controls in three recognition tasks: delayed nonmatching-to-sample, object memory span, and spatial memory span. Further, to rule out that normal performance on these tasks may relate to functional sparing following neonatal hippocampal lesions, we tested adult monkeys that had received the same focal hippocampal lesions in adulthood and their controls in the same three memory tasks. Both early and late onset focal hippocampal damage did not alter performance on any of the three tasks, suggesting that damage to cortical areas adjacent to the hippocampus was likely responsible for the recognition impairment reported by the earlier studies. In addition, given that animals with early and late onset hippocampal lesions showed object and spatial recognition impairment when tested in a visual paired comparison task (Zeamer, Meunier, & Bachevalier, Submitted; Zeamer, Heuer & Bachevalier, 2010), the data suggest that not all object and spatial recognition tasks are solved by hippocampal-dependent memory processes. The current data may not only help explain the neural substrate for the partial recognition memory impairment reported in cases of developmental amnesia (Adlam, Malloy, Mishkin, & Vargha-Khadem, 2009), but they are also clinically relevant given that the object and spatial memory tasks used in monkeys are often translated to investigate memory functions in several populations of human infants and children in which dysfunction of the hippocampus is suspected.
Keywords: delayed nonmatching-to-sample, object memory span, spatial memory span, developmental amnesia
Introduction
Medial temporal lobe (MTL) structures are critically involved in item-specific recognition memory (Mishkin, 1978; Zola-Morgan & Squire, 1985). Although the contribution of the medial temporal cortical areas to these memory processes seems unequivocal, that of the hippocampus is still debated. Thus, lesion studies in adult monkeys, using a delayed nonmatching-to-sample (DNMS) task to measure object recognition abilities, have indicated profound recognition memory impairment after damage to the medial temporal cortical areas, such as the perirhinal, entorhinal and parahippocampal cortex (Malkova, Bachevalier, Mishkin, & Saunders, 2001; Meunier, Bachevalier, Mishkin, & Murray, 1993; Suzuki, Zola-Morgan, Squire, & Amaral, 1993). However, milder recognition impairment, mainly at longer delays (Beason-Held, Rosene, Killiany, & Moss, 1999; Zola et al., 2000), or even no recognition memory deficits (Murray & Mishkin, 1998) followed selective lesions of the hippocampus that did not encroach onto these adjacent cortical areas. Additionally, the lack of recognition memory decay with increasing delays following hippocampal lesions has been further substantiated by others (Baxter & Murray, 2001; Ringo, 1988, 1991).
Earlier developmental studies in monkeys seem to provide further support to the view that the hippocampus may be less critical for object recognition memory when the medial temporal cortical areas are mostly spared. Thus, Rehbein and colleagues (2005) reported severe recognition impairment after neonatal hippocampal lesions that was present at all delays and all list lengths of a delayed matching-to-sample task. By contrast, only milder recognition memory deficits were reported in another developmental study (Bachevalier, Beauregard, & Alvarado, 1999). In this later study, adult monkeys with neonatal hippocampal lesions performed as well as unoperated controls at all delays of the DNMS task, including the longest delay of 10 minutes, as well as for all list lengths, except the longest list of 10 objects for which the operated animals showed a slight but significant decrease in performance. In both studies the hippocampal lesions were made by aspiration procedures, which inevitably included damage to the adjacent cortical areas. This additional cortical damage appeared more extended in the Rehbein’s study, in which portions of the entorhinal and perirhinal cortex together with parahippocampal areas were also damaged, than in the Bachevalier’s study, in which the lesions extended to the parahippocampal areas but spared most of the entorhinal and perirhinal cortex. The mild impairment in recognition memory when the neonatal lesions spared most of the cortical areas is in fact in line with recent reports of patients with developmental amnesia indicating mild but significant impairment in recognition memory due to hypoxia/ischemic damage to the hippocampus early in life (Adlam, Malloy, Mishkin, & Vargha-Khadem, 2009). Because investigation of the integrity of the adjacent rhinal cortical areas in these human cases is difficult to demonstrate, it is possible that the mild recognition impairment in the humans may (Adlam et al., 2009) have resulted from residual damage to the adjacent parahippocampal cortex as was the case for the monkeys (Bachevalier et al., 1999). In the present study, we sought to directly explore this possibility and tested adult monkeys that had received neonatal neurotoxic lesions of the hippocampus, which spared the adjacent cortical areas, and sham-operated controls in the DNMS task. Additionally, given that the effects of hippocampal lesions on recognition memory may vary depending on task demands, such as longer delays (Zola et al., 2000) and increasing memory load (Beason-Held et al., 1999), the same monkeys were also tested on two versions of the delayed recognition span task, which measured the ability to recognize novel stimuli (Object Memory Span, OMS) or novel locations (Spatial Memory Span, SMS) when an increasing number of stimuli or locations need to be maintained in memory for good performance on the task. Finally, to directly evaluate whether the magnitude of the memory impairment after neonatal hippocampal lesions is similar to that following adult onset lesions, we also tested adult monkeys that had received similar neurotoxic hippocampal lesions in adulthood in the three recognition tasks (DNMS, OMS and SMS).
Methods
Subjects
Eighteen adult rhesus monkeys (Macaca mulatta) of 3–5 years of age and weighing between 5–8 kg participated in the current study. Twelve were obtained as newborns (1–4 days of age) from the breeding facility of the University of Texas, M.D. Anderson Cancer Center Science Park (Bastrop, TX) and were brought to the primate nursery of the M.D. Anderson Cancer Center (Houston, TX) to be raised by human caregivers and age-matched peers. They received either sham operations (Group Neo-C, 3 males, 3 females) or neurotoxic lesions of the hippocampus (Group Neo-Hibo, 4 males, 2 females) when they were 10–15 days of age. Extensive descriptions of the rearing conditions, neuroimaging and surgical procedures are provided elsewhere (Goursaud & Bachevalier, 2007) but a brief description is given here.
The infants were grouped into 6 cohorts of four age-matched animals each. Each cohort included one animal of Group Neo-C and one animal of Group Neo-H along with two other animals that participated in other ongoing studies. Upon arrival at the primate nursery, animals were kept in adjacent individual cages that allowed social contacts with neighboring animals. Each cage contained a soft stuffed animal (approximately 30cm long) that served as a surrogate and the infants received extensive contacts with a principal human caregiver who spent a minimum of 6 hours a day with the subjects (5 days/week), supplemented on the weekends with contacts from other familiar humans. By 3 months of age, each tetrad was given 3–4 hrs (5 days/week) of social interactions in a play cage, and between 1–2 years of age each tetrad lived in a large enclosure 24 hrs/day. Thereafter, animals were maintained in pairs.
The remaining six monkeys were obtained as young adults (2.4–3.2 years of age) from the California National Primate Research Center (Davis, CA). They were reared in semi-naturalistic outdoor enclosures in small matrilineal groups, but where individually housed once transferred to the MD Anderson Cancer Center primate facility (Houston, TX). They received either sham operations (Group C, 3 males) or neurotoxic lesions of the hippocampus (Group Hibo, 3 males) at approximately 3.5 years of age.
At the time of the current study, all subjects were housed individually, maintained in a 12 hour light/dark cycle (7AM:7PM) and fed Purina Old World Primate chow (Lab Diet #5037, PMI Nutrition International Inc., Brentwood, MO) supplemented with fruit (water ad libitum). During behavioral testing, food was minimally restricted to sufficiently motivate the animal to retrieve food rewards during the tasks, but their body weight was maintained at or above 85% of their full feed weight. Although all surgeries and the behavioral testing of the adult lesion groups as well as the surgeries of the infant lesion groups were conducted at the M.D. Anderson Cancer Center (Houston, TX), the behavioral testing of the infant lesion groups was conducted at the Yerkes National Primate Research Center- Emory University (Atlanta, GA). The behavioral procedures were kept constant across the two Institutions. All experimental procedures conformed to the NIH Guide for the care and use of Laboratory Animals (HHS publication 85–23, 1985) and were approved by the Institutional Animal Care and Use Committee at the University of Texas at Houston and at Emory University at Atlanta.
Surgical Procedures
The surgical procedures were performed using MRI-guided injections of neurotoxin and have been described in earlier reports for the infant monkeys (Goursaud & Bachevalier, 2007; Zeamer et al., 2010) and for the adult monkeys (Zeamer et al., Submitted). They are summarized below and included only minor changes between the infant and adult procedures.
Pre-surgical and post-surgical MRI procedures
All animals were initially sedated using either ketamine hydrochloride and xylazine (10mg/kg of 7:3 mixture of ketamine hydrochloride, 100mg/ml, and xylazine, 20 mg/ml, i.m.) for the adults or isoflurane inhalation (1–2% to effect) in an induction box for the infants. The sedated animals were then intubated and kept anesthetized with isoflurane for the entire imaging session. EMLA cream was applied in the ear canals as a topical anesthetic and ophthalmic ointment was applied to the eyes to prevent ocular dryness. The MR images were acquired with a GE Signa 1.5 Tesla Echo Speed scanner (GE Medical Systems, Milwaukee, WI) using a 3” surface coil (5” surface coil for adults). For both pre- and post-surgical scanning sessions, two types of MR images were obtained in the coronal plane: a series of high-resolution T1-weighted MR images (echo time (TE) = 11ms, repetition time (TR) = 450ms in contiguous 4mm sections, 12cm field of view (FOV), 256 × 256 matrix), and three series of Fluid Attenuated Inversion Recovery (FLAIR) images (Parameters: TE = 140ms, TR = 10,000ms, inversion time (TI) = 2200ms, contiguous 3mm sections, 14cm FOV, 256 × 256 matrix) offset 1mm in the posterior axis. The pre-surgical scans were taken one week prior to surgery for the adults or on the day prior to surgery for the infants. Post-surgical scans, utilized to estimate the extent of lesions, were taken 1–2 weeks after surgery using the same procedures and the same MR sequences. An additional high-resolution T1 scan was taken 1-year post-surgery in both the infant and the adult animals and was used to estimate the volume reduction of the hippocampus.
Surgical procedures
For all surgeries, the animal’s head was shaved and disinfected with Nolvasan solution. An intravenous drip of 5% dextrose and 0.5% sodium chloride was infused to maintain hydration, a heating pad was wrapped around the animal to prevent hypothermia and all vitalsigns (heart and respiration rates, blood pressure, body temperature and expired CO2) were monitored continuously throughout surgical procedures. To reduce pain, Marcaine (25%, 1.5 ml, s.c.) was injected along the skin incision, which extended from the occiput to a middle point between the two eyebrows. The connective tissue was then displaced laterally to expose the skull and small craniotomies were made in both hemispheres above the hippocampus. Small slits were made into the dura to expose the underlying brain tissue.
For the neurotoxic lesions of the hippocampus, injections of ibotenic acid (Biosearch Technologies, Novato, CA, 10mg/ml in PBS, pH 7.4, 0.4 to 0.6 μl per site at 0.2μl/min) were made simultaneously in the two hemispheres using 30 gauge needles attached to 10μl Hamilton syringes held in Kopf manipulators (David Kopf Instruments, Tujunga, CA). A total of 2.8–4.2μl of ibotenic acid was injected into 11 sites for the infants (3.0 to 3.8 μl into 11 sites for the adults) to target the uncus and the hippocampus along its entire length. After each injection, the needles were left in place for an additional 3 min to allow diffusion of the drug at the tip of the needle and minimize its spread in the needle track during retraction of the needles. For the sham operation, the surgical procedures were identical, except that no needle was lowered into the brain.
At the completion of the surgical procedure, the wound was closed in anatomical layers and the animals were then placed in an incubator until they fully recovered from the anesthesia. Pre- and post-surgical treatment included a seven-day regimen of dexamethasone sodium phosphate (0.3mg/kg, i.m.) to reduce swelling that began the day before surgery (3 times/day the day before and the day of surgery) and ended after 5–8 days post-surgery with progressive withdrawal of the drug (3times/day for 3 days, 2 times/day for 3 days, once/day for two days). Cefazolin (25mg/kg, i.m.) to minimize infection and acetaminophen (10mg/kg, p.o.) for pain management were only administered for several days after surgery.
Lesion Assessment
For estimation of hippocampal lesions in both infant and adult monkeys, pre- and post-surgical FLAIR images were matched to corresponding pre-surgical T1-weighted images. Hypersignals on FLAIR images were identified and plotted onto corresponding drawings of coronal sections of a normal infant or adult rhesus monkey atlas. These drawings were imported into a Java-based image analysis program (ImageJ®; http://rsb.info.nih.gov/ij/) to measure the surface area (in pixels2) of hippocampal damage targets, as well as for unintended damage to all surrounding areas (entorhinal, perirhinal, and parahippocampal cortex and amygdala). For any given region of interest (ROI), the measured surface area of damage on each section for each hemisphere was summed and then multiplied by image thickness to calculate a total volume of damage (Gundersen & Jensen, 1987). The volume of damage was then divided by the normal volume of the ROI and multiplied by 100 to indicate a percent of the total hippocampal volume damaged (X%). In addition, the weighted average (W% = (L% × R%)/100) was calculated for each subject and provided a measure of laterality of the damage as described by Hodos and Bobko (1984). A weighted average of less than 25% indicates a particularly unilateral lesion, whereas a weighted average of greater than 50% indicates an extensive and relatively symmetrical lesion.
Behavioral procedures
Both infants and adult animals had received extensive but similar cognitive testing before they participated in this experiment. Briefly, this cognitive testing included incidental recognition memory (visual paired comparison at 1, 6 and 18 months post surgery for the infants and 2 months post-surgery for the adults), oddity learning (3 and 15 months post-surgery for the infants and 8 months post-surgery for the adults), spatial working memory (48 months post-surgery for the infants and 12 months post-surgery for the adults), and concurrent discrimination learning with devaluation (60 months post-surgery for the infants and 14 months post-surgery in the adults). In this study, the testing apparatus, stimuli and behavioral procedures were identical for all animals.
Apparatus and stimuli
Monkeys were tested in a darkened room containing a white noise generator to mask extraneous noise. Two trays were used to display the stimuli. One had a single centered row of three recessed food wells (2 cm in diameter, 1cm deep and 13 cm apart from each other) for testing in the DNMS task. The other contained 19 wells (2 cm in diameter and spaced 7 cm apart from each other) arranged on two rows of 6 wells and a middle row of 7 wells (see Figure 1) for testing on the OMS and SMS tasks.
Figure 1.

Memory Span Tasks. Examples of one object memory span (A) and one spatial memory span (B). For object memory span (OMS) task, 3-D objects were used and added one at a time at 10-s intervals. Note that for each trial the locations of the objects on the testing board were shuffled so that animal’s performance had to rely on the last object added on the board. For spatial memory span (SMS) task, red checkers pieces were used and added one at a time at 10-s intervals. Animal’s performance relied on selecting the last well (or location on the board) covered. For both tasks, adding an object or a checker piece continued until the animal made an error. + indicates the item rewarded on each trial.
The stimuli used for DNMS consisted of a pool of 1000 objects varied in size, shape and color, which were novel to the animal prior to testing. A new set of 500 variegated objects served as stimuli for the OMS task and 19 identical round red checker pieces served as stimuli for the SMS task.
Delayed Nonmatching-to-Sample (DNMS)
The testing procedures for this task were identical to those used in a previous publication (Bachevalier et al., 1999). On each trial, the monkey was presented with a single object overlying the center well containing a food reward. After displacing the object and retrieving the reward, a 10-s delay was imposed and followed by a choice test in which the familiar object and a novel baited object each covering the lateral wells were presented for choice. New objects were used for each trial and 20 trials, separated by a 30-s inter-trial interval, were given per daily session until the monkey met the criterion of 90 correct responses in 100 consecutive trials. After achieving the learning criterion, their recognition memory was taxed further with performance tests including 100 trials at delays of 30, 60 and 120s and 50 trials at delay of 600s.
Memory span tasks
The testing procedures for the object and spatial memory span tasks followed those described in an earlier report (Beason-Held et al., 1999), except for a minor modification of the testing tray that was composed of three rows of wells that were staggered instead of being inline. This modification was implemented to ensure that objects positioned on the row closest to the animal will not obstruct the view of objects positioned on the row further away from the animal.
Object Memory Span (OMS)
On each day, animals received eight novel-spans using 19 novel stimuli for each span, and 2 repeat-spans using two sets of 19 objects that were re-used on every testing day, keeping their order constant but varying their location on each day. Within a daily session, the repeat-spans were randomly intermixed within the novel-spans and a 30-s interval was used between spans. To prevent the animals from using a spatial strategy to succeed on the task, the locations of the objects on the tray were changed for each trial of a given span. For each span (Figure 1A), Trial 1 began with an object covering a reward placed in one of the 19 wells. After the subject displaced the object and retrieved the food reward, a 10-s delay was imposed during which the familiar object was moved to a novel location on the tray and a novel object was placed in a different location with a reward hidden under it (Trial 2). After the animal made a choice, another 10-s delay was imposed during which the two familiar objects were moved to different locations and a third new object was covering a reward in a different location (Trial 3). Objects were added on the tray in this way until the animal made an error, i.e. selected a previously presented object. The number of novel objects the animal correctly selected before making an error served as a measure of object memory span. Animals received 10 daily sessions, thus totaling 80 novel spans and 10 presentations of each repeat-span.
Spatial Memory Span (SMS)
Procedures for the SMS task were similar to those described for the OMS task, except that identical plaques were used instead of objects, such that monkeys had to select the new well covered on each trial. Thus, each daily session consisted of 8 novel-spans in which the sequence of wells covered on each trial changed for each span and 2 repeat-spans in which the sequence of the wells covered on each trial remained constant for each span presentation. The repeat-spans were randomly intermixed within the novel-spans and 30-s interval was used between spans. For each span (Figure 1B), Trial 1 began by placing a single red plaque over one of the 19 wells that was baited. After a 10-s delay, the same well, this time un-baited, was re-covered by a red plaque and a new well was baited and covered with a red plaque (Trial 2). After the animal made a choice, another 10-s delay was imposed during which the two previous wells were un-baited and re-covered by the red plaques and another well in another location of the tray was baited and covered with an identical plaque (Trial 3). Identical plaques were added to the tray until the animals made an error, i.e. selected a previously rewarded well (or location). The number of wells visited prior to committing an error was used as a measure of spatial memory span. The 10 spatial spans were given daily for a total of 10 days, thus totaling 80 novel spatial memory spans and 10 repeat spatial memory spans.
Results
Lesion Assessment
Estimates of hippocampal lesions were already published for both animals with neonatal hippocampal damage (Goursaud & Bachevalier, 2007; Zeamer et al., 2010) and adult onset hippocampal damage (Alvarado, Kazama, Zeamer, & Bachevalier, In Press; Zeamer et al., Submitted). Table 1 shows the extent of the hippocampal lesions for adult animals with neonatal lesions (Group Neo-Hibo) and adult animals that had acquired the same lesions as adults (Group Hibo), as estimated from the FLAIR-MRI images taken one-week post-surgery.
Table 1.
Intended and Unintended Lesion Extent after Neonatal and Adult Hippocampal Lesions
| Group |
Intended Damage |
Unintended Damage |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subjects | Hippocampus | Amygdala | TH/TF | |||||||||
| L% | R% | X% | W% | L% | R% | X% | W% | L% | R% | X% | W% | |
| Neo-Hibo | ||||||||||||
| Neo-Hibo-1 | 63.8 | 2.9 | 33.2 | 1.8 | 14.0 | 0.0 | 7.0 | 0.0 | 3.1 | 0.5 | 1.8 | 0.0 |
| Neo-Hibo-2 | 54.4 | 80.9 | 67.6 | 44.0 | 0.0 | 0.0 | 0.0 | 0.0 | 21.4 | 2.7 | 12.1 | 0.6 |
| Neo-Hibo-3 | 78.5 | 96.3 | 87.4 | 75.6 | 1.7 | 0.0 | 0.8 | 0.0 | 6.1 | 5.5 | 5.8 | 0.3 |
| Neo-Hibo-4 | 20.3 | 67.3 | 43.8 | 13.6 | 0.0 | 4.7 | 2.4 | 0.0 | 15.3 | 0.0 | 7.6 | 0.0 |
| Neo-Hibo-5 | 20.7 | 84.0 | 52.6 | 17.5 | 0.0 | 4.9 | 2.4 | 0.0 | 6.1 | 4.0 | 5.1 | 0.2 |
| Neo-Hibo-6 | 7.9 | 0.0 | 3.9 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Mean | 40.9 | 55.2 | 48.0 | 25.4 | 2.6 | 1.6 | 2.1 | 0.0 | 8.6 | 2.1 | 5.4 | 0.1 |
| Hibo | ||||||||||||
| Hibo-7 | 12.6 | 58.5 | 35.6 | 7.4 | 0 | 10.9 | 5.4 | 0 | 0 | 0 | 0 | 0 |
| Hibo-8 | 79.7 | 98.1 | 88.9 | 78.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hibo-9 | 90.9 | 89.3 | 90.1 | 81.3 | 12.8 | 0.9 | 6.9 | 0.1 | 0.9 | 1.3 | 1.1 | 0.0 |
| Mean | 61.0 | 81.9 | 71.5 | 55.6 | 4.2 | 3.9 | 4.1 | 0.0 | 0.3 | 0.4 | 0.3 | 0 |
Note: L%: percent damage in the left hemisphere; R%: percent damage in the right hemisphere; X% average damage to both hemispheres; W%: weighted average damage to both hemispheres (W% = L% × R%)/100; weighted index as defined by Hodos and Bobko (1984). Mean: average damage per group; Neo-Hibo: animals that received hippocampal lesions in infancy and Hibo: animals that received hippocampal lesions in adulthood. TH/TF: ventral cortical areas of the temporal lobe as defined by Von Bonin and Bailey (1947). Estimates were from one-week postsurgical FLAIR images.
For the neonatal lesions, the extent of hippocampal damage across the two hemispheres ranged from 3.9% to 87.4% (average: 41.7%). Cases Neo-Hibo-2 and 3 had extensive (mean X: 77.5%) and symmetrical (mean W: 59.8%) lesions. The remaining four animals had significant (mean X: 43.2%) but asymmetrical (mean W: 11%) lesions of the hippocampus, except for Case-Neo-ibo-7 for which only small amount of hypersignals could be identified in the anterior portion of the hippocampus. For two of these four cases (Neo-Hibo-4 and Neo-Hibo-5), the mild extent of hypersignals on the left hippocampus (20%) were mainly located in the CA2 and CA1 fields of almost the entire length of the hippocampus, thus disrupting the normal functioning of the tri-synaptic hippocampal circuit as well as the direct entorhinal-CA1 pathway. For all cases, unintended damage was minor (5%) and limited to the parahippocampal areas TH/TF (mean X: 5.4%, see Table 1). In addition, Figure 2 displays volume reduction of the hippocampus in case Neo-Hibo-4, which was extensive on the right hemisphere (67.3%) but milder in the left hemisphere (20.3%) as estimated by the 1-year postsurgical T1 images, as compared to a sham-operated control (Neo-C-4).
Figure 2.

Coronal T1 images through the hippocampus taken one-year postsurgically in a sham-operated monkey (Neo-C-4, left column) and a monkey with neonatal hippocampal lesion (Neo-Hibo-4, right column). Note that, in case Neo-Hibo-4, hippocampal volume reduction is more extensive on the right than on the left, which confirms the percent damage (R: 67.3%; L: 20.3%) that was estimated for that case from the one-week postdurgical FLAIR scan (see Table 1). White arrows point to reduced hippocampal volume as compared to sham-operated control.
For the adult hippocampal lesions, the extent of damage as estimated from the FLAIR images is listed for each case in Table 1. Two of the three animals (Hibo-8 and Hibo-9) had nearly complete and bilateral lesions of the hippocampus (mean X: 89%; mean W: 79%). In Case Hibo-7, hippocampal damage was extensive on the right hemisphere (58.5%) but limited on the left (12.6%). However, this limited damage included most of the CA1 field and portions of the CA2 and CA3 fields through almost the entire length of the hippocampus (see Figs. 4–5) and thus disconnected information flowing through the tri-synaptic pathway. Unintended damage to adjacent structures was minor and included less than 7% of the posterior amygdala unilaterally in two cases (Hibo-7 and Hibo-9) and less than 2% of areas TH/TF bilaterally in case Hibo-9. Additionally, case Hibo-9 sustained moderate damage to the tail of the caudate nucleus (average 30% bilaterally). Figure 34 displays hippocampal volume reduction in case Hibo-8 as estimated from the 1-year post-surgical T1 images as compared to a sham-operated monkey (C-7). In addition, for the animals with adult onset hippocampal lesions, detailed histological estimation of the lesions is also available and is illustrated in Figures 4 and 5 for case H-ibo7 that had greater hippocampal damage on the right than on the left and for case H-ibo-9 that had extensive bilateral damage (details of histological procedures are provided in Zeamer et al., submitted).
Figure 4.

Photomicrographs through the anterior body of the hippocampus in a normal animal (top row) and two cases with adult onset hippocampal lesions (middle and bottom rows). As estimated from the one-week FLAIR scan (Table 1), the cell loss in case Hibo-7 was extensive on the right, sparing only portion of the uncus, whereas on the left the cell loss was mostly restricted to the CA1 and CA2 subfields. For case Hibo-9, the cell loss was extensive bilaterally in the body of the hippocampus, spanning all CA fields and dentate gyrus, but sparing medial most portions of the uncus. Asterisks indicate region of intense cell loss.
Figure 5.

Photomicrographs through the mid-body of the hippocampus in a normal animal (top row) and two cases with adult onset hippocampal lesions (middle and bottom rows). As estimated from the one-week FLAIR scan (Table 1), the cell loss in case Hibo-7 was restricted to the CA1 and CA2 subfields on the right and was moderate in the CA1-CA3 subfields on the left. For case Hibo-9, the cell loss was again extensive bilaterally, spanning all CA fields and dentate gyrus. Asterisks indicate region of moderate to intense cell loss.
Delayed NonMatching- to-Sample
Scores that each animal obtained in the acquisition of the DNMS rule and for the four delays of the performance test are given in Table 2.
Table 2.
DNMS Acquisition and Performance Test
| Groups |
Acquisition |
Delays |
||||||
|---|---|---|---|---|---|---|---|---|
| Subjects | Trials | Errors | % Correct | 30s | 60s | 120s | 600s | Mean |
| Neonatal Lesion | ||||||||
| Neo-C | ||||||||
| Neo-C-1 | 80 | 15 | 90 | 98 | 96 | 90 | 92 | 94 |
| Neo-C-2 | 80 | 21 | 81 | 93 | 91 | 93 | 84 | 90 |
| Neo-C-3 | 480 | 125 | 90 | 87 | 83 | 89 | 72 | 83 |
| Neo-C-4 | 200 | 57 | 92 | 93 | 98 | 86 | 95 | 93 |
| Neo-C-5 | 120 | 26 | 90 | 96 | 95 | 94 | 86 | 93 |
| Neo-C-6 | 40 | 8 | 91 | 97 | 95 | 97 | 98 | 98 |
| X | 166 | 42 | 90 | 94 | 93 | 91 | 87 | 92 |
| Neo-Hibo | ||||||||
| Neo-Hibo-1 | 260 | 32 | 94 | 92 | 95 | 88 | 88 | 91 |
| Neo-Hibo-2 | 60 | 15 | 93 | 94 | 92 | 96 | 90 | 93 |
| Neo-Hibo-3 | 40 | 10 | 92 | 84 | 88 | 81 | 86 | 85 |
| Neo-Hibo-4 | 60 | 13 | 90 | 96 | 97 | 93 | 78 | 91 |
| Neo-Hibo-5 | 40 | 8 | 91 | 75 | 73 | 81 | 72 | 76 |
| Neo-Hibo-6 | 60 | 11 | 91 | 89 | 93 | 81 | 84 | 87 |
| X | 86 | 14 | 91 | 88 | 89 | 86 | 83 | 87 |
| Adult Lesion | ||||||||
| C | ||||||||
| C-7 | 160 | 65 | 91 | 88 | 92 | 81 | 88 | 87 |
| C-8 | 40 | 8 | 90 | 83 | 85 | 84 | 66 | 80 |
| C-9 | 140 | 28 | 91 | 90 | 91 | 89 | 80 | 88 |
| X | 113 | 32 | 90 | 87 | 89 | 84 | 78 | 85 |
| Hibo | ||||||||
| Hibo-7 | 100 | 25 | 92 | 97 | 96 | 91 | 84 | 92 |
| Hibo-8 | 60 | 15 | 90 | 92 | 94 | 94 | 92 | 93 |
| Hibo-9 | 180 | 39 | 90 | 82 | 94 | 89 | 84 | 87 |
| X | 113 | 26 | 90 | 90 | 94 | 91 | 86 | 91 |
Note: Scores are number of trials (Trials) and total number of errors (Errors) before attainment of learning criterion, and percent correct during the five sessions to criterion (% Correct). For delays of 30, 60 and 120s, scores are percent correct over 100 trials, and at delay of 600s, scores are percent correct over 50 trials. Mean is average scores across the four delays. Neo-C: monkeys with neonatal sham lesions, Neo-Hibo: monkeys with neonatal neurotoxic acid lesions of the hippocampus, C: monkeys with adult sham operations; Hibo: monkeys with adult neurotoxic acid lesions of the hippocampus.
Effects of hippocampal lesions in infancy
Animals with neonatal hippocampal lesions required an average of 86 trials (14 errors) to reach the learning criterion as compared to an average of 166 trials (42 errors) for the sham-operated controls. Group Neo-C poorer performance was due to case Neo-C-3, which took three times more trials to reach the criterion that the other sham-operated animals. However, the group difference did not reach significance [t(10) = 1.066, p = 0.312 and t(10) = 1.482, p = 0.169, for trials and errors, respectively]. Similarly, animals with neonatal lesions of the hippocampus averaged 87% correct across all delays slightly below the average of 92% for the sham-operated controls. The slight overall group difference did not reach significance [ANOVA Group effect: F(1, 10) = 2.011, p = 0.186; and Group X Delay: F(1,10) = 0.1464, p = 0.931], although in both groups performance decreased with increasing delays (Delay effect: F(3,32) = 8.500, p < 0.05).
Effects of hippocampal lesions in adulthood
Animals with lesions of the hippocampus or sham operations acquired in adulthood reached criterion at the same rate, averaging 113 trials (26 errors) and 113 trials (32 errors) to criterion, respectively [t(4) = 0.00, p = 1.000 and t(4) = 0.405, p = 0.706, for trials and errors, respectively]. Similarly, animals with adult onset hippocampal lesions performed as well as their age-matched sham-operated controls, averaging 91% vs. 85% across all delays, respectively [Group: F(1,4) = 3.385, p = 0.140; and Group X Delay: F(1,4) = 0.893, p = 0.398], although in both groups performance decreased with increasing delays (Delay: F(1,4) = 4.128, p = 0.032).
Comparisons between the effects of hippocampal lesions acquired in infancy or adulthood and revealed comparable performance for all animals in learning the DNMS rule as revealed by no significant interaction between Group and Age [F(1,18) = 0.485, p = 0.49 for trials to criterion and F(1,18) = 0.457, p = 0.51 for errors to criterion]. Similarly, as shown in Figure 6, comparable performance between adult and infant onset lesion groups was found for all delays [Group X Age: F(1,18) = 1.943, p = 0.185 for 30s; F(1,18) = 1.889, p = 0.185 for 60 s; and F(1,18) = 2.654, p = 0.126 for 600s], except for the 120-s delay [Age x Group: F(1,18) = 5.318, p = 0.037]. The significant interaction at the 120s delay was due to Group Neo-C (X: 91%) performing slightly better than Group Neo-Hibo (X: 86%), whereas the reverse was true for the adult groups, i.e. Group Hibo (X: 91%) performed slightly better than Group C (X: 84%). Thus, the results indicate normal performance in learning the DNMS rule as well as memory performance at increasing delays following selective lesions of the hippocampus either in infancy or adulthood.
Figure 6.

DNMS Delay Performance. Mean percent correct on DNMS at delays of 30, 60 and 120-s (100 trials) and 600-s (50 trials) for animals with neonatal (Neo-Hibo) or adult (Hibo) lesions of the hippocampus and their sham-operated controls (Neo-C and C, respectively. Error bars are standard error of the mean (S.E.M.).
Object Memory Span
The mean number of both novel and repeat object spans across ten days of testing is shown for each animal of the neonatal and adult lesion groups in Table 3.
Table 3.
Object and Spatial Memory Span Performance
| Subjects |
Object Memory Span |
Spatial Memory Span |
||
|---|---|---|---|---|
| Novel Span | Repeat Span | Novel Span | Repeat Span | |
| Neonatal Lesion | ||||
| Neo-C | ||||
| Neo-C-1 | 6.1 | 4.9 | 2.9 | 2.4 |
| Neo-C-2 | 5.0 | 3.8 | 2.3 | 1.4 |
| Neo-C-3 | 3.5 | 3.3 | 2.5 | 1.4 |
| Neo-C-4 | 4.1 | 2.7 | 2.0 | 1.7 |
| Neo-C-5 | 3.0 | 3.2 | 2.0 | 1.4 |
| Neo-C-6 | 4.4 | 2.9 | 3.2 | 2.3 |
| Mean | 4.4 | 3.5 | 2.5 | 1.8 |
| Neo-Hibo | ||||
| Neo-Hibo-1 | 4.8 | 2.4 | 3.1 | 2.4 |
| Neo-Hibo-2 | 5.8 | 2.2 | 2.3 | 2.1 |
| Neo-Hibo-3 | 5.5 | 3.0 | 2.7 | 2.2 |
| Neo-Hibo-4 | 5.4 | 3.4 | 2.5 | 1.5 |
| Neo-Hibo-5 | 4.5 | 3.9 | 2.0 | 1.7 |
| Neo-Hibo-6 | 5.1 | 4.7 | 2.6 | 2.0 |
| Mean | 5.2 | 3.3 | 2.5 | 2.0 |
| Adult Lesion | ||||
| C | ||||
| C-7 | 3.7 | 3.2 | 1.8 | 1.2 |
| C-8 | 4.9 | 4.4 | 2.1 | 1.6 |
| C-9 | 5.9 | 5.4 | 2.2 | 1.4 |
| Mean | 4.8 | 4.3 | 2.0 | 1.4 |
| Hibo | ||||
| Hibo-7 | 5.3 | 4.3 | 3.0 | 2.5 |
| Hibo-8 | 7.6 | 4.4 | 2.7 | 3.3 |
| Hibo-9 | 5.9 | 5 | 1.9 | 2.1 |
| Mean | 6.3 | 4.5 | 2.5 | 2.6 |
Note: Scores are average span for 80 Novel spans and 20 Repeat-spans. Conventions as in Table 2.
Effects of hippocampal lesions in infancy
Animals with neonatal lesions of the hippocampus averaged spans of 5 and 3 for novel and repeat object spans, respectively similar to the averaged spans of 4 and 3 (novel and repeat, respectively) for sham-operated controls [Group: F(1,10) = 0.522, p = 0.487]. Both groups did significantly better on the novel versus repeat spans [Span: F(1,10) = 24.119, p < 0.01; Group X Span: F(1,10) = 3.224, p = 0.103].
Effects of hippocampal lesions in adulthood
Animals with adult lesions of the hippocampus obtained spans of 6 and 4 for novel and repeat object spans comparable to spans of 5 and 4 for novel and repeat object spans obtained by the sham-operated controls. This was reflected by a group effect that failed just short of significance [F(1,4) = 6.071, p = 0.06] and no significant interaction between the two factors [Group X Span: F(1,4) = 0.855, p = 0.408], although both groups performed better on the novel than on the repeat spans [F(1,4) = 23.320, p = 0.006].
As with the DNMS data, the results demonstrate that selective lesions of the hippocampus acquired in infancy or adulthood did not impair object memory span performance on the novel [Group X Age: F(1,18) = 0.360, p = 0.403] or repeat [Group x Age F(1,18) = 0.188, p = 0.255] spans. These comparable results following the early and late onset hippocampal lesions are depicted in Figure 7.
Figure 7.
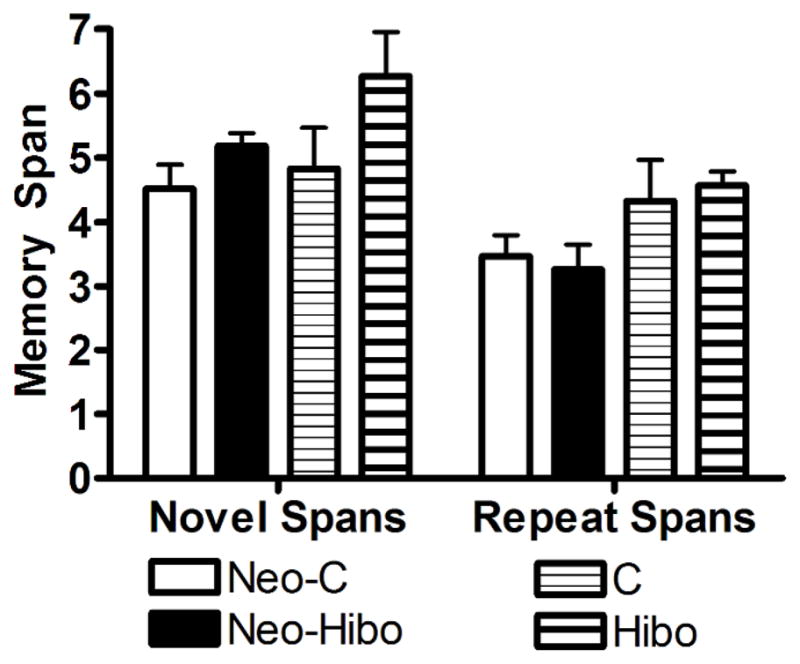
Object Memory Span. Mean memory spans for both novel and repeated spans across 10 days of testing for animals with neonatal (Neo-Hibo) or adult (Hibo) lesions of the hippocampus and their sham-operated controls (Neo-C and C, respectively. Error bars are standard error of the mean (S.E.M.).
Spatial Memory Span
The mean number of both novel and repeat spatial spans across ten days of testing is shown for each animal of the neonatal and adult lesion groups in Table 3.
Effects of hippocampal lesions in infancy
Animals with both neonatal lesions of the hippocampus or sham lesions obtained spans of 3 and 2 for novel and repeat spatial spans, respectively [Group effect: F(1,10) = 0.312, p = 0.589], and both groups performed better on the novel versus repeat spans as indicated by a significant effect of span type [Span type effect: F(1,10) = 57.443, p < 0.001].
Effects of hippocampal lesions in adulthood
Animals with adult lesions of the hippocampus obtained spans of 3 for both novel and repeat spatial spans and performed slightly better than sham-operated controls (average span: 2 and 1 for novel and repeat spans, respectively). Thus, although performance was comparable for novel and repeat spatial spans in both groups [Span type effect: F(1,4) = 2.560, p = 0.185], Group Hibo performed better overall than Group C [F(1,14) = 5.862, p = 0.03].
As with the DNMS and OMS, hippocampal lesions in infancy or in adulthood did not impair performance on SMS with novel spans [Age X Group: F(1,14) = 1.620, p = 0.224]. However, the Group and Age reached significance for the repeat spatial spans [F(1,14) = 5.862, p = 0.03] and was due to the animals with adult onset hippocampal lesions that slightly outperformed their sham-operated controls. These comparable results following the early and late onset hippocampal lesions are depicted in Figure 8.
Figure 8.

Spatial Memory Span. Mean memory spans for both novel and repeated spans across 10 days of testing for animals with neonatal (Neo-Hibo) or adult (Hibo) lesions of the hippocampus and their sham-operated controls (Neo-C and C, respectively. Error bars are standard error of the mean (S.E.M.).
There were no significant correlations between performance on the three tasks and extent of lesions in both the early and late onset hippocampal lesion groups and these correlations remained unreliable when the sample size included all 9 subjects with hippocampal lesions in the correlation analyses (Acquisition DNMS: r = − 0.10; Performance DNMS: r = 0.001; OMS: r = 0.61; SMS: r = − 0.38). However, to ascertain that the lack of effect of neonatal hippocampal lesions on the three tasks has not resulted from the inclusion in the statistical analyses of one case (Neo-ibo-6) that had only limited hippocampal damage (3.9%), we re-analyzed the data for all behavioral measures after removing Neo-Hibo-6 from the group. The data showed as before, that neonatal hippocampal lesions resulted in sparing of DNMS rule learning [t(10)= 0.902, p = 0.391 and t(10) = 1.304, p = 0.370 for trials and errors to criterion, respectively] and delay performance [Group: F(1,9) = 1.622, p = 0.235]. Similarly, these neonatal lesions did not impact performance on the memory span tasks [Group: F(1,8) = 0.184, p = 0.678 and F(1,8) = 0.248, p = 0.630 for the object and spatial span tasks, respectively].
Discussion
In keeping with previous work in adult monkeys (Murray & Mishkin, 1998; Nemanic, Alvarado, & Bachevalier, 2004), neonatal damage to the hippocampus that did not encroach onto adjacent cortical areas does not alter recognition memory performance when assessed with DNMS, at least at delays lasting as long as 10 minutes. This normal recognition memory performance following neonatal hippocampal lesions persisted even when memory load increased progressively as assessed by the object memory span task or when the task required to recognize locations that had been visited few seconds earlier (spatial memory span). These results demonstrate that (a) the recognition deficit reported in earlier studies investigating the effects of neonatal hippocampal lesions on DNMS performance in monkeys was likely due to additional damage to medial temporal cortical areas (Bachevalier et al., 1999; Rehbein et al., 2005), which have been shown to be critical to recognition memory processes (Malkova et al., 2001; Meunier et al., 1993; Suzuki et al., 1993); (b) neonatal hippocampal damage results in sparing of object and spatial memory similar to that reported after adult onset hippocampal lesions; (c) this memory sparing in animals with early onset hippocampal lesions contrasts with the impairment found in the same animals on other tasks of object and spatial memory (Blue, Kazama, & Bachevalier, 2009; Glavis-Bloom, Alvarado, & Bachevalier, 2006; A. Zeamer et al., 2010), suggesting that not all object and spatial memory tasks are sensitive to hippocampal damage; and finally (d) they indicate that the mild recognition memory deficits found in human cases suffering from perinatal hippocampal damage (Adlam et al., 2009; Baddeley, Vargha-Khadem, & Mishkin, 2001) could likewise be the result of additional damage to the cortical areas adjacent to the hippocampus.
Object Recognition memory
The results that focal neurotoxic hippocampal lesions in adulthood do not impair either the acquisition of DNMS rule or recognition memory performance at least at delays as long as 10 minutes confirmed our previous findings (Nemanic et al., 2004). In the two studies, the sparing of object recognition memory occurs despite nearly complete bilateral hippocampal lesions in most cases and when DNMS rule training was not given to the animals prior to the hippocampal removals. Also, in both studies, animals with hippocampal lesions in adulthood slightly outperformed control animals in the performance test (90% vs. 85% in the present study and 94% vs 91% for the re-test in Nemanic et al.,(2004). Consistent with the results obtained on the DNMS task, animals with adult onset hippocampal lesions performed as well as control animals when memory load was increased in the object memory span task. The sparing of recognition abilities on both the DNMS and OMS contrasts with the severe impairment in both tasks reported earlier by Beason-Held and colleagues (1999).
Because the behavioral procedures were identical in the two studies, the different outcomes may have resulted from variation in hippocampal damage extent. As shown in Table 1 and Figures 3–6, hippocampal damage in two of the cases of the present study included almost all subfields of the hippocampus in its entire length and, in the third case, the damage was extensive on one side but more restricted on the other side. By contrast, the hippocampal damage in all 5 cases of the Beason-Held’s study included only small percentages of damage in all subfields of the hippocampus bilaterally, though this mild damage most likely altered the normal functioning of the structure. The difference in lesion size between the two studies suggests that greater impairment in the two recognition tasks followed smaller hippocampal damage than more complete hippocampal lesions. Although counterintuitive, this negative correlation has already been reported and discussed by others (Bachevalier & Meunier, 1996; Baxter & Murray, 2001). Further, Mumby and colleagues (1996) directly demonstrated that partial ischemic hippocampal damage in rats yielded severe recognition memory deficits that were alleviated by completely removing the hippocampus thereafter. The authors have suggested that a dysfunctioning hippocampus may directly impact the functioning of the adjacent medial temporal cortex to which it projects. An alternative explanation at least for the DNMS task relates to the number of delays tested. As compared to the delays used in the Beason-Held’s study (10s, 120s, 10 min), two additional delays (30s and 60s) were used in the present study. This additional training at 30s and 60s (200 trials) may have enable the hippocampectomized animals to learn a strategy to old information of the sample stimuli to bridge the longer delays of 120s and 10 min. In addition, for the OMS task, all subjects (lesion and control) performed better on the novel than they did on the repeat spans in both object and spatial span types. This finding contrasts with that reported by Beason-Held and colleagues (1999) who noted that normal animals, but not those with hippocampal lesions, performed better on the repeat spans than on the novel spans, suggesting that they were essentially learning the span. Thus, it is possible that the animals in the current study solved the object memory span task using a different strategy. Although the reasons for the different outcomes between the two studies need to be further investigated, it is interesting to note that, despite their normal performance on the DNMS and OMS tasks, the three animals with adult hippocampal lesions showed impaired incidental recognition memory, as assessed by the visual paired comparison task (VPC: Zeamer et al., Submitted). This pattern of normal performance on problem-solving object recognition tasks together with impaired performance on incidental preferential viewing task replicates that reported in our earlier study (Nemanic et al., 2004) and suggests that normal performance on problem-solving recognition tasks (DNMS and OMS) after hippocampal lesions may be supported by alternate strategies that are independent of the processes mediated by the hippocampus (For further discussion see: Nemanic et al., 2004).
Figure 3.

Coronal T1 images through the hippocampus taken one-year postsurgically in a sham-operated monkey (C-7, left column) and a monkey with adult hippocampal lesion (Hibo-8, right column). Note that, in case Hibo-8, hippocampal volume reduction is extensive in both hemispheres, which confirms the percent damage (R: 79.7%; L: 98.1%) that was estimated for that case from the one-week postsurgical FLAIR scan (see Table 1). White arrows point to reduced hippocampal volume as compared to sham-operated control.
Interestingly, the same pattern of results followed focal neurotoxic lesions of the hippocampus in infancy. In the present study, neonatal hippocampal lesions spared performance on DNMS when delays up to 10 min were used as well as when memory load was increased in the OMS task. This object recognition memory sparing after early onset hippocampal lesions contrasts with the mild to moderate DNMS impairment reported in earlier studies using nonselective neonatal hippocampal lesions (Bachevalier et al., 1999; Rehbein et al., 2005). The present findings indicate that additional damage to perirhinal and/or parahippocampal cortex after the nonselective hippocampal lesions (Buffalo et al., 1999; Malkova et al., 2001; Meunier et al., 1993; Nemanic et al., 2004) is most likely responsible for the impairment observed. Nonetheless, one might argue that the sparing of recognition memory abilities after neonatal focal hippocampal lesions may have also resulted from either incomplete damage to the hippocampal tissue, brain reorganization after early insult or both. This proposal seems unlikely, however, given that further testing on the same animals revealed significant object recognition memory deficits when tested in the visual paired comparison task (Zeamer et al., 2010). Thus, as for the hippocampal lesions in adulthood, those performed in infancy yielded sparing of recognition memory as measured by problem-solving tasks but recognition memory impairment in incidental recognition task. The finding that recognition memory deficits after hippocampal lesions depends on the tasks used has also been reported in human amnesics with either early or late onset hippocampal damage (Baddeley et al., 2001; Mayes, Holdstock, Isaac, Hunkin, & Roberts, 2002; Munoz, Chadwick, Perez-Hernandez, Vargha-Khadem, & Mishkin, 2010; Pascalis, Hunkin, Holdstock, Isaac, & Mayes, 2004) and suggests that recognition memory tasks may be solved by cognitive processes that are not all dependent of the hippocampus (Holdstock et al., 2002). This conclusion provides support to recent proposals indicating that recognition memory entails two different processes, i.e. familiarity judgments supported by the medial temporal cortical areas and recollection supported by the hippocampus (see for reviews Brown & Aggleton, 2001; Eichenbaum, Fortin, Sauvage, Robitsek, & Farovik, 2010; but see Wixted, Mickes, & Squire, 2010 for an alternative view). Thus, performance on the DNMS and OMS tasks in the absence of a functioning hippocampus could be solved by familiarity judgments mediated by the cortical areas.
Recognition memory for spatial locations
Adult lesions of the hippocampus also spared recognition memory for spatial locations as measured by the SMS task; a finding strikingly opposite to the significant impairment reported by Beason-Held and colleagues (1999) using the same spatial location task. Although the reasons for these different outcomes may relate to differences in lesion size as discussed above, it is interesting to note that, as for object recognition memory, the presence of spatial memory impairment after selective hippocampal lesions depends on the tasks used. Thus, selective hippocampal lesions in adult monkeys impaired performance on a delayed nonmatching-to-location task (Alvarado & Bachevalier, 2005), open-field delayed matching-to-locations (Hampton, Hampstead, & Murray, 2004), open-field foraging task (P. B. Lavenex, Amaral, & Lavenex, 2006), and object-in-place visual paired comparison task (Bachevalier & Nemanic, 2008); but not on one-trial object-in-place task (Malkova & Mishkin, 2003) and on spatial location visual comparison task (Bachevalier & Nemanic, 2008). Thus, it is becoming clear that, as for the object recognition tasks, abilities to solve spatial memory tasks are not all dependent on the integrity of the hippocampus. Recent reviews of both the human and animal work indicate that the hippocampus may be crucial only in tasks of spatial navigation and spatial-memory that requires the formation of precise allocentric cognitive maps (Banta Lavenex & Lavenex, 2009; Kessels, de Haan, Kappelle, & Postma, 2001; King, Trinkler, Hartley, Vargha-Khadem, & Burgess, 2004; O’Keefe & Nadel, 1978; Spiers, Burgess, Hartley, Vargha-Khadem, & O’Keefe, 2001).
Finally, as for the adult hippocampal lesions, neonatal hippocampal lesions spared performance on the spatial memory span task. This normal spatial location memory performance after neonatal hippocampal lesions parallels similar findings recently reported in 9-month-old monkeys (Lavenex, Banta Lavenex, & Amaral, (2007) tested in an open-field foraging task known to be impaired by adult onset hippocampal lesions (Lavenex et al., 2006). Although the authors concluded that the sparing of spatial memory functions was likely due to functional brain reorganization following the neonatal hippocampal lesions, an alternative explanation for this sparing could be that the impairment will emerge when the animals will reach maturity or again could be related to the type of spatial memory processes measured by a given task. Thus, we found that the animals with neonatal hippocampal lesions that performed normally in the SMS task, showed significant spatial memory deficits on two other spatial memory tasks. As compared to control animals, in an object-in-place visual paired comparison task they showed no novelty preference towards a familiar array of 5 objects in which the locations of the objects had been re-arranged (Blue et al., 2009), and in a spatial foraging task they had difficulty matching a specific food item with a specific location in an open-field (Glavis-Bloom et al., 2006).
Relationship to developmental amnesia in humans
Studying the effects of neonatal hippocampal lesions in animals in which there is a better control over the extent and location of the lesions and the timing of the lesions can provide the best platform for understanding the pattern of sparing and impairment of memory functions described in human cases of developmental amnesia. Several reports have shown that damage to the hippocampus in human infants and children completely (Baddeley et al., 2001) or partially (Adlam et al., 2009; Baddeley et al., 2001) spared visual and verbal recognition memory. In this respect, the data in monkeys suggest that the partial recognition impairment found in some of the developmental amnesia cases could have resulted from additional damage to adjacent medial temporal cortical regions. For example, in the DNMS task, no impairment was reported after focal neonatal lesions (current results), whereas mild recognition memory deficits were reported when the hippocampal lesions encroached onto the parahippocampal cortex (Bachevalier et al., 1999). Interestingly, in both species, incidental recognition memory as measured by the visual paired comparison task was significantly altered (Munoz et al., 2010; Zeamer et al., 2010), suggesting that the incidental recognition memory deficits in human developmental amnesia could also occur with lesions that are restricted to the hippocampus.
The findings in monkeys indicate that the pattern of memory sparing and impairment following neonatal hippocampal lesions is identical to that after adult onset hippocampal damage. Yet, the incidental recognition memory impairment is generally milder after the neonatal hippocampal lesions than after the late lesions (Zeamer et al., 2010), suggesting the existence of some functional sparing. Possible candidates for this sparing are the remaining undamaged hippocampal tissue, neural reorganization within cortical areas interconnected with the hippocampus, or both. Thus, milder recognition memory deficits in developmental amnesia could also indicate similar functional sparing.
Further, as for object recognition memory, the present findings indicate that the effects of neonatal hippocampal lesions on spatial memory were directly related to the types of spatial memory tasks used. A similar pattern was also reported in one case of developmental amnesia in that Jon, who suffers from perinatal damage to the hippocampus, scored in the normal range in the Camden topographical memory test but his ability to navigate in a virtual maze was severely impaired (Spiers et al., 2001).
Finally, it is worth noting that, although the present findings report no effects of neonatal hippocampal lesions on object and spatial memory, together with additional testing on the same animals (Blue et al., 2009; Glavis-Bloom et al., 2006; A. E. Zeamer et al., 2010), they provide knowledge on which object and spatial memory tasks are critically dependent on hippocampal functioning. This knowledge is clinically relevant given that object and spatial memory tasks developed in monkeys have been translated to investigate memory functions in populations of human infants and children in which dysfunction of the hippocampus is suspected, such as infants with temporal lobe epilepsy or perinatal anoxic insults, babies born pre-termed or from diabetic mothers, infants receiving brain irradiation.
Acknowledgments
This work was supported by grants from the National Institute of Mental Health (MH-58846), the Yerkes Base Grant NIH RR00165 and the Center for Behavioral Neuroscience grant NSF IBN-9876754. We thank the University of Texas Health Science Center at Houston veterinary and animal husbandry staff for expert animal care, Belinda Rivera for the care and handling of animals during the MR imaging procedures, Edward F. Jackson for assistance in neuroimaging techniques, and Diana Lay, Martin J. O’Malley, Rebecca Grizzard and Bronwyn Welsh for help with the behavioral training of the animals.
References
- Adlam AL, Malloy M, Mishkin M, Vargha-Khadem F. Dissociation between recognition and recall in developmental amnesia. Neuropsychologia. 2009;47(11):2207–2210. doi: 10.1016/j.neuropsychologia.2009.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado MC, Bachevalier J. Comparison of the effects of damage to the perirhinal and parahippocampal cortex on transverse patterning and location memory in rhesus macaques. Journal of Neuroscience. 2005;25(6):1599–1609. doi: 10.1523/JNEUROSCI.4457-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado MC, Kazama AM, Zeamer A, Bachevalier J. The effects of selective hippocampal damage on tests of oddity in rhesus macaques. Hippocampus. doi: 10.1002/hipo.20827. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachevalier J, Beauregard M, Alvarado MC. Long-term effects of neonatal damage to the hippocampal formation and amygdaloid complex on object discrimination and object recognition in rhesus monkeys (Macaca mulatta) Behavioral Neuroscience. 1999;113(6):1127–1151. doi: 10.1037//0735-7044.113.6.1127. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Meunier M. Cerebral ischemia: are the memory deficits associated with hippocampal cell loss? Hippocampus. 1996;6(5):553–560. doi: 10.1002/(SICI)1098-1063(1996)6:5<553::AID-HIPO8>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Nemanic S. Memory for spatial location and object-place associations are differently processed by the hippocampal formation, parahippocampal areas TH/TF and perirhinal cortex. Hippocampus. 2008;18(1):64–80. doi: 10.1002/hipo.20369. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Vargha-Khadem F, Mishkin M. Preserved recognition in a case of developmental amnesia: implications for the acquisition of semantic memory? J Cogn Neurosci. 2001;13(3):357–369. doi: 10.1162/08989290151137403. [DOI] [PubMed] [Google Scholar]
- Banta Lavenex P, Lavenex P. Spatial memory and the monkey hippocampus: not all space is created equal. Hippocampus. 2009;19(1):8–19. doi: 10.1002/hipo.20485. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Murray EA. Opposite relationship of hippocampal and rhinal cortex damage to delayed nonmatching-to-sample deficits in monkeys. Hippocampus. 2001;11(1):61–71. doi: 10.1002/1098-1063(2001)11:1<61::AID-HIPO1021>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Beason-Held LL, Rosene DL, Killiany RJ, Moss MB. Hippocampal formation lesions produce memory impairment in the rhesus monkey. Hippocampus. 1999;9(5):562–574. doi: 10.1002/(SICI)1098-1063(1999)9:5<562::AID-HIPO10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Blue SN, Kazama AM, Bachevalier J. Neuroscience Meeting Planner. Washington DC: Society for Neuroscience; 2009. The normal development of object-place association memory is altered by neonatal hippocampal lesions in rhesus monkeys. Online, 98–7. [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2(1):51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Buffalo EA, Ramus SJ, Clark RE, Teng E, Squire LR, Zola SM. Dissociation between the effects of damage to perirhinal cortex and area TE. Learning & Memory. 1999;6(6):572–599. doi: 10.1101/lm.6.6.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Fortin N, Sauvage M, Robitsek RJ, Farovik A. An animal model of amnesia that uses Receiver Operating Characteristics (ROC) analysis to distinguish recollection from familiarity deficits in recognition memory. Neuropsychologia. 2010;48(8):2281–2289. doi: 10.1016/j.neuropsychologia.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glavis-Bloom C, Alvarado MC, Bachevalier J. Neuroscience Meeting Planner. Washington DC: Society for Neuroscience; 2006. Neonatal hippocampal damage impairs specific place/food associations in adult macaques. Online, 574.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goursaud AP, Bachevalier J. Social attachment in juvenile monkeys wth neonatal lesion of the hippocampus, amygdala and orbitofrontal cortex. Behavioural Brain Research. 2007;176:75–93. doi: 10.1016/j.bbr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. Journal of Microscopy. 1987;147(Pt 3):229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Hampton RR, Hampstead BM, Murray EA. Selective hippocampal damage in rhesus monkeys impairs spatial memory in an open-field test. Hippocampus. 2004;14(7):808–818. doi: 10.1002/hipo.10217. [DOI] [PubMed] [Google Scholar]
- Hodos W, Bobko P. A weighted index of bilateral brain lesions. Journal of Neuroscience Methods. 1984;12(1):43–47. doi: 10.1016/0165-0270(84)90046-3. [DOI] [PubMed] [Google Scholar]
- Holdstock JS, Mayes AR, Roberts N, Cezayirli E, Isaac CL, O’Reilly RC, Norman KA. Under what conditions is recognition spared relative to recall after selective hippocampal damage in humans? Hippocampus. 2002;12(3):341–351. doi: 10.1002/hipo.10011. [DOI] [PubMed] [Google Scholar]
- Kessels RP, de Haan EH, Kappelle LJ, Postma A. Varieties of human spatial memory: a meta-analysis on the effects of hippocampal lesions. Brain Res Brain Res Rev. 2001;35(3):295–303. doi: 10.1016/s0165-0173(01)00058-3. [DOI] [PubMed] [Google Scholar]
- King JA, Trinkler I, Hartley T, Vargha-Khadem F, Burgess N. The hippocampal role in spatial memory and the familiarity--recollection distinction: a case study. Neuropsychology. 2004;18(3):405–417. doi: 10.1037/0894-4105.18.3.405. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Lavenex PB, Amaral DG. Spatial relational learning persists following neonatal hippocampal lesions in macaque monkeys. Nature Neuroscience. 2007;10(2):234–239. doi: 10.1038/nn1820. [DOI] [PubMed] [Google Scholar]
- Lavenex PB, Amaral DG, Lavenex P. Hippocampal lesion prevents spatial relational learning in adult macaque monkeys. Journal of Neuroscience. 2006;26(17):4546–4558. doi: 10.1523/JNEUROSCI.5412-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkova L, Bachevalier J, Mishkin M, Saunders RC. Neurotoxic lesions of perirhinal cortex impair visual recognition memory in rhesus monkeys. Neuroreport. 2001;12(9):1913–1917. doi: 10.1097/00001756-200107030-00029. [DOI] [PubMed] [Google Scholar]
- Mayes AR, Holdstock JS, Isaac CL, Hunkin NM, Roberts N. Relative sparing of item recognition memory in a patient with adult-onset damage limited to the hippocampus. Hippocampus. 2002;12(3):325–340. doi: 10.1002/hipo.1111. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Mishkin M, Murray EA. Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. Journal of Neuroscience. 1993;13(12):5418–5432. doi: 10.1523/JNEUROSCI.13-12-05418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishkin M. Memory in monkeys severely impaired by combined but not by separate removal of amygdala and hippocampus. Nature. 1978;273(5660):297–298. doi: 10.1038/273297a0. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Wood ER, Duva CA, Kornecook TJ, Pinel JP, Phillips AG. Ischemia-induced object-recognition deficits in rats are attenuated by hippocampal ablation before or soon after ischemia. Behavioral Neuroscience. 1996;110(2):266–281. doi: 10.1037//0735-7044.110.2.266. [DOI] [PubMed] [Google Scholar]
- Munoz M, Chadwick M, Perez-Hernandez E, Vargha-Khadem F, Mishkin M. Novelty preference in patients with developmental amnesia. Hippocampus. 2010 doi: 10.1002/hipo.20836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Mishkin M. Object recognition and location memory in monkeys with excitotoxic lesions of the amygdala and hippocampus. Journal of Neuroscience. 1998;18(16):6568–6582. doi: 10.1523/JNEUROSCI.18-16-06568.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemanic S, Alvarado MC, Bachevalier J. The hippocampal/parahippocampal regions and recognition memory: insights from visual paired comparison versus object-delayed nonmatching in monkeys. Journal of Neuroscience. 2004;24(8):2013–2026. doi: 10.1523/JNEUROSCI.3763-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The hippocampus as a cognitive map. Oxford New York: Clarendon Press, Oxford University Press; 1978. [Google Scholar]
- Pascalis O, Hunkin NM, Holdstock JS, Isaac CL, Mayes AR. Visual paired comparison performance is impaired in a patient with selective hippocampal lesions and relatively intact item recognition. Neuropsychologia. 2004;42(10):1293–1300. doi: 10.1016/j.neuropsychologia.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Rehbein L, Killiany R, Mahut H. Developmental study of the hippocampal formation in rhesus monkeys (Macaca mulatta): I. Early ablations spare discrimination learning but not recognition memory. Behavioral Neuroscience. 2005;119(3):635–650. doi: 10.1037/0735-7044.119.3.635. [DOI] [PubMed] [Google Scholar]
- Ringo JL. Seemingly discrepant data from hippocampectomized macaques are reconciled by detectability analysis. Behavioral Neuroscience. 1988;102(1):173–177. doi: 10.1037//0735-7044.102.1.173. [DOI] [PubMed] [Google Scholar]
- Ringo JL. Memory decays at the same rate in macaques with and without brain lesions when expressed in d’ or arcsine terms. Behavioural Brain Research. 1991;42(2):123–134. doi: 10.1016/s0166-4328(05)80003-8. [DOI] [PubMed] [Google Scholar]
- Spiers HJ, Burgess N, Hartley T, Vargha-Khadem F, O’Keefe J. Bilateral hippocampal pathology impairs topographical and episodic memory but not visual pattern matching. Hippocampus. 2001;11(6):715–725. doi: 10.1002/hipo.1087. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Zola-Morgan S, Squire LR, Amaral DG. Lesions of the perirhinal and parahippocampal cortices in the monkey produce long-lasting memory impairment in the visual and tactual modalities. Journal of Neuroscience. 1993;13(6):2430–2451. doi: 10.1523/JNEUROSCI.13-06-02430.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wixted JT, Mickes L, Squire LR. Measuring recollection and familiarity in the medial temporal lobe. Hippocampus. 2010;20(11):1195–1205. doi: 10.1002/hipo.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeamer A, Heuer E, Bachevalier J. Developmental trajectory of object recognition memory in infant rhesus macaques with and without neonatal hippocampal lesions. Journal of Neuroscience. 2010;30(27):9157–9165. doi: 10.1523/JNEUROSCI.0022-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeamer A, Meunier M, Bachevalier J. Stimulus discriminabliity and encoding time influence incidental recognition memory in adult monkeys with selective hippocampal lesions. Learning & Memory. doi: 10.1101/lm.2076811. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeamer AE, Heuer E, Bachevalier J. Developmental trajectory of object recognition memory in infant rhesus macaques with and without neonatal hippocampal lesions. Journal of Neuroscience. 2010;30(27):9157–9165. doi: 10.1523/JNEUROSCI.0022-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola SM, Squire LR, Teng E, Stefanacci L, Buffalo EA, Clark RE. Impaired recognition memory in monkeys after damage limited to the hippocampal region. Journal of Neuroscience. 2000;20(1):451–463. doi: 10.1523/JNEUROSCI.20-01-00451.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR. Medial temporal lesions in monkeys impair memory on a variety of tasks sensitive to human amnesia. Behavioral Neuroscience. 1985;99(1):22–34. doi: 10.1037//0735-7044.99.1.22. [DOI] [PubMed] [Google Scholar]


