Abstract
Leptin acts in the brain to regulate food intake and energy expenditure. Leptin also increases renal sympathetic nerve activity (SNA) and arterial pressure. The divergent signaling capacities of the leptin receptor (ObRb) mediate the stimulation of various intracellular pathways that are important for leptin control of physiological processes. Here, we evaluated the cardiovascular and sympathetic consequences of disrupting the signal emanating from tyrosine985 of ObRb. For this, we used LeprL985 (l/l) mice which carry a loss of function mutation replacing tyrosine985 of ObRb with leucine. Body weight of l/l mice was not significantly different from wild type controls. In contrast, radiotelemetry measurements revealed that the l/l mice had higher arterial pressure and heart rate as compared to controls. Ganglionic blockade caused a greater arterial pressure fall in the l/l mice relative to controls. In addition, leptin treatment induced a larger increase in arterial pressure and heart rate in the l/l vs. wild type mice. Finally, we compared the response of renal and BAT SNA to intracerebroventricular (ICV) injection of leptin (2 μg) between l/l and control mice. Leptin-induced increase in renal SNA was greater in l/l mice relative to controls. In contrast, the BAT SNA response to leptin was attenuated in the l/l mice relative to controls. These data indicate that selective loss of leptin receptor signaling emanating from tyrosine985 enhances the cardiovascular and renal sympathetic effects of leptin. These findings provide important insight into the molecular mechanisms underlying leptin’s effects on the sympathetic cardiovascular function and arterial pressure.
Keywords: Leptin, cardiovascular regulation, autonomic function
Introduction
Leptin is an adipocyte hormone that acts in the central nervous system to modulate food intake and enhance energy expenditure through activation of sympathetic nerve traffic to thermogenic brown adipose tissue.1;2 Leptin action in the central nervous system also increases blood pressure through activation of the sympathetic nervous system.3;4 Indeed, in animal studies, elevating circulating leptin levels increase arterial pressure which can be prevented by adrenergic blockade.5;6 In addition, leptin has emerged as an important culprit linking obesity, sympathetic overdrive and hypertension based on epidemiological and experimental animal studies.3;4;7 For instance, several animal models of obesity that have elevated arterial pressure were found to have a preserved renal sympathetic and arterial pressure responses to leptin in spite of the resistance to the anorectic and weight-reducing actions of leptin.8–11 Despite this, little is known about the molecular mechanisms underlying the sympathetic and cardiovascular responses triggered by leptin.
The long form of the leptin receptor (ObRb) is the major signaling isoform of the leptin receptor.12 Leptin binding triggers the activation of the receptor-associated Janus kinase (Jak) 2 tyrosine kinase. Once activated, Jak2 phosphorylates other tyrosine residues within the ObRb including Tyr1138 , Tyr1077 and Tyr985, each of which mediates the activation of distinct downstream signaling pathways.13;14 ObRb activation also promotes the activation of phosphatidylinositol (PI) 3 kinase, although this appears to be cell-type specific and the mechanisms underlying this regulation remain unclear.13 Whereas phosphorylated Tyr1138 of ObRb recruits and activates signal transducer and activator of transcription (STAT) 3, the phosphorylation of Tyr1077 promotes the tyrosine phosphorylation and activation of STAT5.15 Activated STAT3 and STAT5 translocate to the nucleus to modulate gene transcriptional with important implications for the regulation of metabolism and body energy balance.16 For instance, knock-in mice that have a mutation at Tyr1138 of ObRb (LeprS1138; s/s mice), which disrupts leptin-induced STAT3 signaling, are severely obese and hyperphagic, but in contrast to the mice lacking leptin or ObRb these mice remain fertile and are less diabetic.17
On the other hand, phosphorylation of Tyr985 creates a binding site for the COOH-terminal SH2 domain of the tyrosine phosphatase, PTPN11 (aka SHP2), leading to the activation of extracellular signal–related kinase (ERK) signaling pathway.13;14 While Tyr985 mediates most ERK stimulation during ObRb signaling, tyrosine phosphorylation sites on Jak2 appears to account for a fraction of ERK activation by leptin independently from ObRb phosphorylation.13 Tyr985 also binds suppressor of cytokine signaling-3 (SOCS3) which act as a negative regulator to inhibit STAT3 signaling.
A knock-in mouse model, LeprL985 (l/l mice), carries a point mutation resulting in the substitution of the Tyr985 with a Leu residue.18 This presumably blocks the recruitment of SHP2 and SOCS3. The l/l mice tend to be lean and exhibit a resistance to diet-induced obesity likely because of enhanced leptin sensitivity in the hypothalamus.18 In addition, the l/l mice exhibited a robust elevation in urinary excretion of norepinephrine, suggesting a higher sympathetic nerve discharge.19
In the present study, we evaluated the consequences on the cardiovascular and sympathetic functions of disrupting Tyr985 ObRb signaling in the l/l mice. We also examined the effects of leptin on arterial pressure, heart rate, and regional sympathetic nerve traffic in the l/l mice.
Methods
Animals
We used LeprL985 (l/l) and LeprS1138(s/s) mice on a C57BL/6 background generated previously. 17;18 Heterozygous mice were bred to generate experimental mice (l/l and s/s) and wild type (+/+) controls needed for our study. Mice were genotyped using a custom designed SNP assay which enabled accurate and reliable detection of the point mutation in these knock-in mouse models (Custom TaqManR SNP Genotype Assay, Applied Biosystems) as described previously.17;18
We used adult mice (12–17 weeks of age) which werehoused in a temperature-controlled room with a 12:12 hour light-darkcycle with free access to standard laboratory chow and tap water. All studieswere approved by the University of Iowa Animal Research Committee.
Arterial Pressure and Heart Rate Measurement
Arterial pressure and heart rate were recorded in conscious state using continuous radiotelemetric measurement (PA-C10, Data Science Instruments) as described.9;10 Mice were anesthetized with intraperitoneal (IP) administration of Ketamine (91 mg/kg) and Xylazine (9.1 mg/kg) cocktail. Under aseptic surgical conditions, the catheter of the telemeter was inserted in the left carotid artery and tied securely using 6-0 silk suture. The transmitter was tunneled sub-dermally from neck area until the unit has reached the mid-abdominal region. The neck incision was sutured closed with 4-0 absorbable cat gut and then further sealed with tissue adhesive (Vet-Bond) along the incision line.
Animals were allowed to recover for 7–10 days before arterial pressure, heart rate and locomotor activity were recorded continuously in the conscious unrestrained state for 7 days. The effect of ganglionic blockade on the hemodynamic parameters was tested using hexamethonium bromide (1 μg/g body weight, IP) during the daytime recording period (11 AM). Control period correspond to the 15 minutes before injection, and the effect of hexamethonium bromide was considered the first recording acquired after injection with data collected every 5 minutes. The effect of leptin (60 μg, IP, twice daily) on arterial pressure and heart rate was measured for 6 days. Hemodynamic parameters were recorded for 10 seconds every 5 minutes and stored on a personal computer using Data Science Dataquest software.
Sympathetic Nerve Recording
Mice were equipped with a lateral cerebroventricular (ICV) cannula as described elswehere.9;10 One week after recovery, mice were anesthetized with the Ketamine/Xylazine cocktail, IP, and intubated (PE-50) to allow for spontaneous respiration of oxygen-enriched room air. Body temperature was kept constant at 37.5 °C using a surgical heat lamp and a heat pad. The right jugular vein was cannulated to maintain anesthesia with α-chloralose (initial dose: 25 mg/kg; sustaining dose of 6 mg/kg/hr). Finally, the left carotid artery was cannulated for continuous measurement of arterial pressure and heart rate.
The nerves to the left kidney or BAT were identified using a dissecting scope and then mounted on a bipolar 36-gauge platinum-iridium electrode (Cooner Wire Co., Chadsworth, CA). Once optimal recording parameters were established, the nerve fibers were fixed to the electrode with silicone gel (Kwik-Sil, World Precision Instruments Inc., Sarasota, FL). After completing the surgery, animals were allowed to stabilize before a 10-minute control period was obtained, followed by ICV administration of leptin (2 μg) or vehicle (saline, 2 μl). In one cohort of l/l and control mice, we tested the role of PI3 kinase in mediating leptin-induced increase in renal SNA. For this, LY294002 (0.1 μg) was administered ICV, 10 min before ICV leptin (2 μg). SNA responses were followed for 4 hours.
Data on SNA were acquired and analyzed as previously described.9 The nerve electrodes were attached to a high-impedance probe (HIP-511, Grass Instruments Co., Quincy, Ma). The signal was amplified 105 times with a Grass P5 AC preamplifier, and filtered at both low (100 Hz) and high-frequency (1000 Hz) cut-off. This amplified, filtered signal was then sent to a speaker system and oscilloscope (model 54501A, Hewlett-Packard Co., Palo Alto, CA). The signal was also routed to a MacLab analogue-digital converter (Ad Instruments, Castle Hill, New South Wales, Australia) for recording and data analysis on a Macintosh computer. Background noise was excluded from the measurements of both renal and BAT SNA by correcting for post-mortem activities.
Data Analysis
Sympathetic nerve responses are expressed as the percent change from the 10 minute baseline recording period. Results are shown as mean±SEM. Data were analyzed using Student’s t-test, 1- or 2- way analysis of variance (ANOVA) with or without repeated measures. When ANOVA reached significance, a post-hoc comparison was made using Fisher test. A P<0.05 was considered to be statistically significant.
Results
Cardiovascular effects of disrupting Tyr985 of ObRb in l/l mice
Body weight of l/l mice (23.6±0.7 g) and wild type controls (23.1±0.7 g) did not differ significantly (P=0.3), but visceral fat pads in the l/l mice (0.36±0.06 g) were significantly (P<0.02) less than wild type controls (0.54±0.04 g) (please see http://hyper.ahajournals.org, Fig. S1).
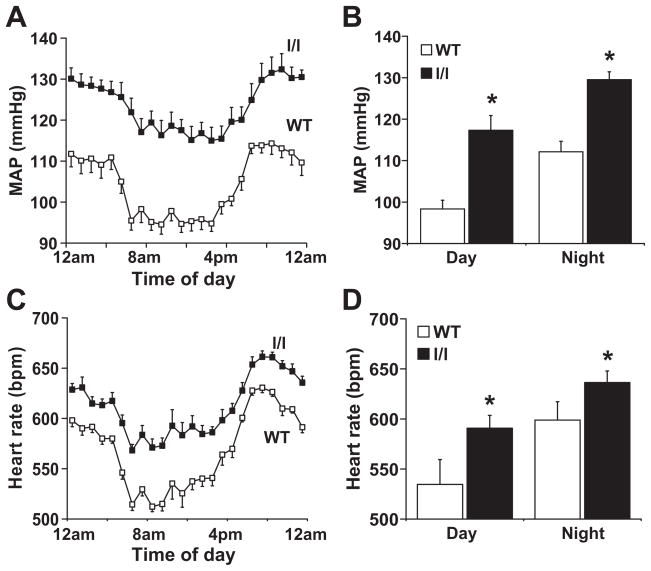
To evaluate the cardiovascular consequences of disrupting Tyr985 of ObRb, we used radiotelemetry to assess arterial pressure, heart rate and locomotor activity. Significant differences between l/l mice and wild type controls were observed (Fig. 1). Mean arterial pressure was significantly (P<0.05) elevated in the l/l mice during the 24-hour recoding period (Fig. 1A). The elevated mean arterial pressure in the l/l mice resulted from an increase in both systolic (+20.0±3 mmHg) and diastolic (+26±9 mmHg) arterial pressure.
Figure 1.
Comparison of radiotelemetric mean arterial pressure and heart rate between l/l mice wild type controls. (A and C) represent the 24-hour recordings and (B and D) the 12-hour day and night averages. *P<0.05 vs. WT; n=10 each group.
As shown in Fig. 1B, the elevation in mean arterial pressure in l/l mice occurred during both the light phase (+26 mmHg during the 12-hour day period, P=0.05 vs. controls) and the dark phase (+23 mmHg during the 12-hour night period, P=0.04 vs. controls).
Relative to wild type controls, the l/l mice had a significantly (P=0.04) higher diurnal heart rate measured over the 24-hour period (Fig. 1C). The elevation in heart rate in the l/l was present during both the light (+52 bpm, P=0.01) and dark phases (+44 bpm, P=0.02; Fig. 1D).
Locomotor activity tended to be higher in the l/l mice relative to controls during the active dark phase, but this was not statistically significant (P=0.14, Fig. S2).
Exaggerated arterial pressure response to ganglionic blockade in l/l mice
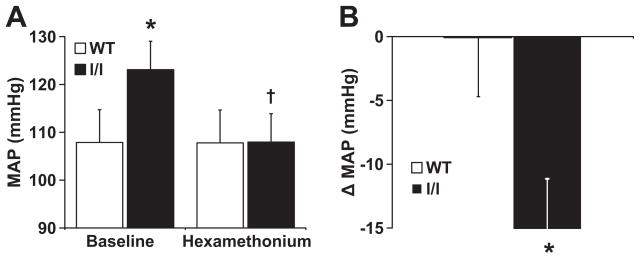
To investigate the contribution of neurogenic mechanisms to the elevated arterial pressure in the l/l mice, we compared the arterial pressure response to ganglionic blockade with IP hexamethonium bromide (1 μg/g body weight) between l/l and control mice. As shown in Fig. 2, in the l/l mice, the magnitude of the decrease in mean arterial pressure in response to ganglionic blockade was significantly greater than in wild type controls (−15.1±5.4 mmHg in l/l mice vs. −0.1±4.6 mmHg in controls, P<0.05) indicating the importance of neurogenic mechanisms, presumably sympathetic tone, in maintaining arterial pressure elevation in the l/l mice.
Figure 2.
Arterial pressure response to ganglionic blockade in l/l mice and wild type controls. (A) Mean arterial pressure in wild type and l/l mice, at baseline and after injection of hexamethonium bromide (1 μg/g body weight, IP). (B) Change in mean arterial pressure, from baseline, after treatment with hexamethonium bromide. *P<0.05 vs. WT, †P<0.05 vs. baseline; n=5 l/l and 6 WT.
Enhanced leptin-induced increases in arterial pressure in the l/l mice
To examine whether disruption of Tyr985 of ObRb affected the hemodynamic response to leptin, we evaluated the effect of 6 days of IP leptin treatment (60 μg, twice daily) in l/l mice and control littermates that were instrumented for radiotelemetric recording of arterial pressure. The leptin-induced increase in arterial pressure was significantly (P<0.05) greater in the l/l mice from days 2 through 6 (+7.3±2.1 mmHg at day 6) as compared to the wild type controls (+3.2±1.7 mmHg at day 6, Fig. 3A). Similarly, the leptin-induced increase in heart rate was augmented in the l/l mice relative to the controls, but statistical significance was reached only at day 5 of leptin treatment (Fig. 3B). Leptin treatment did not change significantly locomotor activity in the l/l and control mice (Fig. 3C).
Figure 3.
Changes in mean arterial pressure, heart rate and locomotor activity in response to leptin (60 μg, IP, twice daily for 6 days) in l/l mice and wild type controls. Data represent the changes from baseline control period for mean arterial pressure (A), heart rate (B) and locomotor activity (C). *P<0.05 vs. WT; n=5 l/l and 6 WT.
Greater renal sympathetic nerve response to leptin in l/l mice
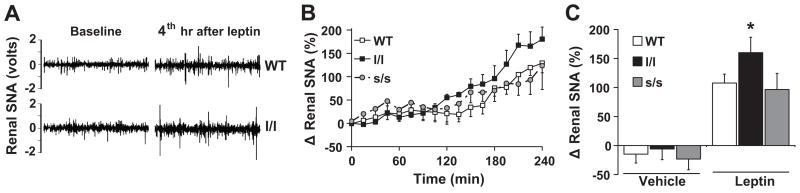
Given the importance of the renal sympathetic nerve tone for leptin-induced increase in arterial pressure,10;20;21 we asked if the sensitivity of renal sympathetic nerve activity (SNA) to leptin is augmented in l/l mice. We found that ICV leptin (2 μg) caused increases in renal SNA in both the wild type and l/l mice. However, the response was significantly (P<0.05) greater in the l/l mice (Fig. 4A-C). Indeed, ICV leptin increased renal SNA by 160±27% in the l/l mice versus 108±15% in the controls (P<0.02, Fig. 4B). In the anesthetized state, the l/l mice tend to have higher baseline mean arterial pressure (85±4 mmHg) and heart rate (335±17 bpm) as compared to the controls (80±4 mmHg and 325 ± 25 bpm, respectively). Consistent with our previous data in anesthetized mice,9;20;22 leptin caused no change in arterial pressure and heart rate relative to vehicle in either l/l or control mice (data not shown). In l/l and wild-type mice, ICV administration of vehicle caused no significant change in renal SNA during the 4 h recording period (The effects of vehicle on renal SNA at 4 hr are shown in Fig. 4B).
Figure 4.
Renal SNA response to leptin (2 μg, ICV) in wild type, l/l and s/s mice. (A) Segments of original records (1 sec each) of renal SNA from wild type and l/l mice at baseline and 4 h after leptin. (A and B) Data are presented as percent increase from baseline and data in (B) represent the average of last (4th) h of recording after leptin or vehicle injection in wild type, l/l and s/s mice. *P<0.05 vs. WT; n=5 veh-WT, 6 veh-l/l, 3 veh-s/s, 17 leptin-WT, 12 leptin-l/l and n=5 leptin-s/s.
We previously demonstrated that leptin-induced sympathetic activation to kidney is PI3 kinase-dependent.22;23 Therefore, we tested whether inhibition of PI3 kinase with LY294002 attenuate renal sympathetic activation to leptin in l/l mice. We found that ICV pre-treatment with LY294002 (0.1 μg) substantially attenuated the increase in renal SNA induced by ICV leptin (2 μg) in controls (18±33%, n=4, P=0.005 vs. leptin alone) as well as l/l mice (30±39%, n=3, P=0.02 vs. leptin alone).
Next, we tested whether the enhanced renal sympathetic activation to leptin is specific to disruption of Tyr985. For this, we examined the effect of leptin on renal SNA in the s/s mice. These mice carry a point mutation resulting in the replacement of the Tyr1138 in ObRb with a serine residue which selectively abolishes the activation of STAT3 by leptin.17 We found that despite severe obesity (Fig. S3), in the s/s mice, ICV administration of leptin (2 μg) increased renal SNA by 97±28% which was not different (P=0.3) from the response obtained in the wild type mice (Fig. 4A-B).
Attenuated BAT SNA response to leptin in l/l mice
Finally, we asked if the enhanced leptin-induced sympathetic nerve activation in the l/l mice is uniform or is specific to renal SNA. For this, we compared the effect of ICV administration of leptin (2 μg) on SNA to brown adipose tissue (BAT) between l/l and wild type mice (Fig. 5). As expected, in wild type mice, ICV leptin caused a gradual, but robust increase in BAT SNA (Fig 5A). ICV leptin also increased BAT SNA in the l/l mice, but this increase was significantly (P<0.05) less in the l/l mice (159±27%) compared to control mice (363±72%, Fig. 5B). Vehicle had no effect on BAT SNA in either control or l/l mice (Fig. 5B).
Figure 5.
BAT SNA response to leptin (2 μg, ICV) in wild type and l/l mice. (A and B) Data are presented as percent increase from baseline and data in (B) represent the average of last (4th) h of recordings after leptin or vehicle injection. *P<0.05 vs. WT; n=3 veh-WT, 6 veh-l/l, 9 leptin-WT and 9 leptin-l/l.
Discussion
This study demonstrates that loss of function of Tyr985 of the leptin receptor in l/l mice increases arterial pressure and sympathetic cardiovascular responses to leptin. First, despite normal body weight, the l/l mice have elevated arterial pressure and heart rate. Second, the elevation in arterial pressure is neurogenically and presumably sympathetically mediated as indicated by augmented depressor response to ganglionic blockade. These data are further supported by a previous report documenting higher urinary norepinephrine levels in l/l mice.19 Third, the l/l mice have enhanced sensitivity of renal sympathetic and arterial pressure responses to leptin. Fourth, and importantly, the enhanced sympathetic nerve responses to leptin in the l/l mice are selective and not uniform as indicated by the fact that leptin-induced increases in renal SNA are enhanced whereas increases in BAT SNA are attenuated. Such contrasting change in the sensitivity of regional SNA to leptin in the l/l mice is consistent with the differential regulation of SNA subserving various tissues.24;25 Together, these findings provide important insight into the molecular mechanisms underlying the influence of leptin on sympathetic regulation of the sympathetic tone regulating cardiovascular function and arterial pressure.
Divergent signaling capacities of the leptin receptor are important for leptin control of physiological processes.4;16 Several intracellular pathways can be triggered by leptin through various tyrosine residues of ObRb. Our data indicate that selective loss of ObRb signaling emanating from Tyr985 (l/l mice) enhances the cardiovascular and renal sympathetic effects of leptin. Similarly, leptin effects on bone mass were found to be enhanced in l/l mice.19 Such increase in leptin sensitivity following loss of function mutation of Tyr985 of ObRb could best be explained by the inability of the mutant receptor to recruit SOCS3 which antagonize the signaling pathways activated by leptin.18 SOCS3 binds to Tyr985 to mediate inhibition of Jak2 tyrosine kinase activity through the N-terminal kinase inhibitory region.13 On the other hand, an inability of ObRb with a mutant Tyr985 to recruit SHP-2 and, therefore, fail to activate the ERK pathway may explain the attenuated BAT SNA response to leptin in l/l mice.26 We previously demonstrated the critical role of ObRb-ERK axis in mediating the BAT sympathetic activation to leptin.23
Specific disruption of the ObRb-STAT3 pathway in s/s mice did not alter leptin-induced renal sympathetic activation which excludes a role for STAT3 signaling in the control of renal sympathetic traffic by leptin. This finding adds renal SNA to the list of physiological processes regulated by leptin independent of the STAT3 signaling. The ObRb-STAT3 pathway was previously shown to be critical in mediating leptin regulation of energy homeostasis as indicated by the severe obesity and hyperphagia in s/s mice with loss of function of the ObRb-STAT3 axis.17 In contrast, the ObRb-STAT3 pathway does not contribute importantly to leptin regulation of linear growth, reproductive function and glucose metabolism.17
The fact that STAT3 signaling is not involved in the renal SNA response to leptin is consistent with the notion that PI3 kinase plays a major role in the transduction of leptin-induced changesin renal sympathetic outflow and arterial pressure.4 In mice, we found that both inhibitors of PI3 kinase, namely LY294002 and wortmannin, markedly attenuated the leptin-induced increase in renal SNA.22 These inhibitors did not affect the renal sympathetic activation to stimulation of the melanocortin system suggesting that their inhibitory effect on the responses to leptin was a specific effect. In a subsequent study in rat, we found that the role of PI3 kinase in leptin-induced sympathetic activation was specific to the kidney, as pre-treatment with LY294002 prevented the effect of leptin on renal SNA without altering the SNA responses to other beds including BAT, hindlimb and adrenal gland.23 Our finding in the present study that inhibition of PI3 kinase blocks the renal SNA response to leptin in the l/l mice confirms the importance of the PI3 kinase pathway in the transduction of leptin-induced renal sympathetic activation. Although the mechanism by which ObRb activates PI3 kinase remains unclear, it is known that in the hypothalamus, leptin phosphorylates and stimulates the insulin receptor substrates, which in turn can activate PI3 kinase.14;27;28
We found that l/l mice have attenuated leptin-induced increase in thermogenic BAT SNA yet these mice remain lean. Activity of BAT is critical for thermogenesis and a reduction in leptin ability to regulate BAT activity may be expected to increase fat mass leading to an obese phenotype.29 Recently, You et al., reported the development of a knock-in mouse model resembling the l/l mice, in which Tyr985 was replaced with a phenylalanine residue (Y985F).30 Similar to the l/l mice, the Y985F mice were found to have a lean phenotype at younger age, but as these mice get older they tend to accumulate fat ultimately leading to the development of an obese phenotype. This observation prompted us to examine body weight in our l/l mice over time. In a preliminary study, we noticed a similar pattern of excess weight gain in l/l mice with aging. Although a larger study is needed to confirm this observation, this seems to indicate that the metabolic consequences of attenuated leptin-induced increases in BAT sympathetic nerve activity are manifest over time as an obese phenotype.
Perspectives
Despite the recent advances in understanding the pathophysiological role of leptin in obesity-induced hypertension and other cardiovascular disorders, little is known about the molecular mechanisms underlying the sympathetic and cardiovascular responses to leptin. Detailed characterization of the molecular mechanisms and individual ObRb signals in the control of arterial pressure, regional sympathetic activity and metabolism will greatly enhance our understanding of the obesity-associated cardiovascular disorders. Such advances might make it possible to therapeutically modulate thermogenic and metabolic functions without altering cardiovascular function. It might also offer the possibility to selectively interfere with potentially deleterious renal/cardiovascular sympathetic actions of leptin, while preserving the beneficial thermogenic, weight-reducing actions.
Supplementary Material
Acknowledgments
None.
Funding
This work was supported by US National Institutes of Health (HL084207). We also gratefully acknowledge the generous research support of the Roy J. and Lucille A. Carver Trust. SMH was supported by NIH NRSA research fellowship HL007121 and AHA postdoctoral fellowship 10POST2600278.
Footnotes
Disclosure. None.
References
- 1.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 2.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 3.Rahmouni K, Correia ML, Haynes WG, Mark AL. Obesity-associated hypertension: new insights into mechanisms. Hypertension. 2005;45:9–14. doi: 10.1161/01.HYP.0000151325.83008.b4. [DOI] [PubMed] [Google Scholar]
- 4.Hall JE, da Silva AA, do Carmo JM, Dubinion J, Hamza S, Munusamy S, Smith G, Stec DE. Obesity-induced hypertension: role of sympathetic nervous system, Leptin, and Melanocortins. J Biol Chem. 2010;285:17271–17276. doi: 10.1074/jbc.R110.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlyle M, Jones OB, Kuo JJ, Hall JE. Chronic cardiovascular and renal actions of leptin: role of adrenergic activity. Hypertension. 2002;39:496–501. doi: 10.1161/hy0202.104398. [DOI] [PubMed] [Google Scholar]
- 6.Aizawa-Abe M, Ogawa Y, Masuzaki H, Ebihara K, Satoh N, Iwai H, Matsuoka N, Hayashi T, Hosoda K, Inoue G, Yoshimasa Y, Nakao K. Pathophysiological role of leptin in obesity-related hypertension. J Clin Invest. 2000;105:1243–1252. doi: 10.1172/JCI8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeppesen J, Asferg C. Positive relationship between plasma leptin levels and hypertension: from an epidemiological perspective. Hypertension. 2010;56:573–574. doi: 10.1161/HYPERTENSIONAHA.110.158519. [DOI] [PubMed] [Google Scholar]
- 8.Correia ML, Haynes WG, Rahmouni K, Morgan DA, Sivitz WI, Mark AL. The concept of selective leptin resistance: evidence from agouti yellow obese mice. Diabetes. 2002;51:439–442. doi: 10.2337/diabetes.51.2.439. [DOI] [PubMed] [Google Scholar]
- 9.Rahmouni K, Morgan DA, Morgan GM, Mark AL, Haynes WG. Role of selective leptin resistance in diet-induced obesity hypertension. Diabetes. 2005;54:2012–2018. doi: 10.2337/diabetes.54.7.2012. [DOI] [PubMed] [Google Scholar]
- 10.Rahmouni K, Fath MA, Seo S, Thedens DR, Berry CJ, Weiss R, Nishimura DY, Sheffield VC. Leptin resistance contributes to obesity and hypertension in mouse models of Bardet-Biedl syndrome. J Clin Invest. 2008;118:1458–1467. doi: 10.1172/JCI32357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prior LJ, Eikelis N, Armitage JA, Davern PJ, Burke SL, Montani JP, Barzel B, Head GA. Exposure to a high-at diet alters leptin sensitivity and elevates renal sympathetic nerve activity and arterial pressure in rabbits. Hypertension. 2010;55:862–868. doi: 10.1161/HYPERTENSIONAHA.109.141119. [DOI] [PubMed] [Google Scholar]
- 12.Tartaglia LA. The leptin receptor. J Biol Chem. 1997;272:6093–6096. doi: 10.1074/jbc.272.10.6093. [DOI] [PubMed] [Google Scholar]
- 13.Myers MG, Jr, Cowley MA, Munzberg H. Mechanisms of leptin action and leptin resistance. Ann Rev Physiol. 2008;70:537–556. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- 14.Morris DL, Rui LY. Recent advances in understanding leptin signaling and leptin resistance. Am J Physiol Endocrinol Metab. 2009;297:E1247–E1259. doi: 10.1152/ajpendo.00274.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gong Y, Ishida-Takahashi R, Villanueva EC, Fingar DC, Muenzberg H, Myers MG., Jr The long form of the leptin receptor regulates STAT5 and ribosomal protein S6 via alternate mechanisms. J Biol Chem. 2007;282:31019–31027. doi: 10.1074/jbc.M702838200. [DOI] [PubMed] [Google Scholar]
- 16.Myers MG., Jr Deconstructing leptin: from signals to circuits. Diabetes. 2010;59:2708–2714. doi: 10.2337/db10-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers MG., Jr STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- 18.Bjornholm M, Munzberg H, Leshan RL, Villanueva EC, Bates SH, Louis GW, Jones JC, Ishida-Takahashi R, Bjorbaek C, Myers MG., Jr Mice lacking inhibitory leptin receptor signals are lean with normal endocrine function. J Clin Invest. 2007;117:1354–1360. doi: 10.1172/JCI30688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi Y, Yadav VK, Suda N, Liu XS, Guo XE, Myers MG, Jr, Karsenty G. Dissociation of the neuronal regulation of bone mass and energy metabolism by leptin in vivo. Proc Natl Acad Sci U S A. 2008;105:20529–20533. doi: 10.1073/pnas.0808701106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Role of melanocortin-4 receptors in mediating renal sympathoactivation to leptin and insulin. J Neurosci. 2003;23:5998–6004. doi: 10.1523/JNEUROSCI.23-14-05998.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tallam LS, da Silva AA, Hall JE. Melanocortin-4 receptor mediates chronic cardiovascular and metabolic actions of leptin. Hypertension. 2006;48:58–64. doi: 10.1161/01.HYP.0000227966.36744.d9. [DOI] [PubMed] [Google Scholar]
- 22.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Intracellular mechanisms involved in leptin regulation of sympathetic outflow. Hypertension. 2003;41:763–767. doi: 10.1161/01.HYP.0000048342.54392.40. [DOI] [PubMed] [Google Scholar]
- 23.Rahmouni K, Sigmund CD, Haynes WG, Mark AL. Hypothalamic ERK mediates the anorectic and thermogenic sympathetic effects of leptin. Diabetes. 2009;58:536–542. doi: 10.2337/db08-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hausberg M, Morgan DA, Chapleau MA, Sivitz WI, Mark AL, Haynes WG. Differential modulation of leptin-induced sympathoexcitation by baroreflex activation. J Hypertens. 2002;20:1633–1641. doi: 10.1097/00004872-200208000-00027. [DOI] [PubMed] [Google Scholar]
- 25.Hausberg M, Morgan DA, Mitchell JL, Sivitz WI, Mark AL, Haynes WG. Leptin potentiates thermogenic sympathetic responses to hypothermia: a receptor-mediated effect. Diabetes. 2002;51:2434–2440. doi: 10.2337/diabetes.51.8.2434. [DOI] [PubMed] [Google Scholar]
- 26.Bjorbaek C, Buchholz RM, Davis SM, Bates SH, Pierroz DD, Gu H, Neel BG, Myers MG, Jr, Flier JS. Divergent roles of SHP-2 in ERK activation by leptin receptors. J Biol Chem. 2001;276:4747–4755. doi: 10.1074/jbc.M007439200. [DOI] [PubMed] [Google Scholar]
- 27.Niswender KD, Schwartz MW. Insulin and leptin revisited: adiposity signals with overlapping physiological and intracellular signaling capabilities. Front Neuroendocrinol. 2003;24:1–10. doi: 10.1016/s0091-3022(02)00105-x. [DOI] [PubMed] [Google Scholar]
- 28.Carvalheira JB, Torsoni MA, Ueno M, Amaral ME, Araujo EP, Velloso LA, Gontijo JA, Saad MJ. Cross-talk between the insulin and leptin signaling systems in rat hypothalamus. Obes Res. 2005;13:48–57. doi: 10.1038/oby.2005.7. [DOI] [PubMed] [Google Scholar]
- 29.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 30.You J, Yu Y, Jiang L, Li WX, Yu XX, Gonzalez L, Yang GQ, Ke ZJ, Li WJ, Li C, Liu Y. Signaling through Tyr(985) of leptin receptor as an age/diet-dependent switch in the regulation of energy balance. Mol Cell Biol. 2010;30:1650–1659. doi: 10.1128/MCB.01307-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.