Abstract
A consensus about the functions of human wild-type or mutated α-synuclein (αSYN) is lacking. Both forms of αSYN are implicated in Parkinson’s disease, whereas the wild-type form is implicated in substance abuse. Interactions with other cellular proteins and organelles may meditate its functions. We developed a series of congenic mouse lines containing various allele doses or combinations of the human wild type αSYN (hwαSYN) or a doubly mutated (A30P*A53T) αSYN (hm2αSYN) in a C57Bl/6J line spontaneously deleted in mouse αSYN (C57BL/6JOla). Both transgenes had a functional role in the nigrostriatal system, demonstrated by significant elevations in striatal catecholamines, metabolites, and the enzyme tyrosine hydroxylase compared to null-mice without a transgene. Consequences occurred when the transgenes were expressed at a fraction of the endogenous level. Hemizygous congenic mice did not exhibit any change in the number or size of dopaminergic neurons in the ventral midbrain at nine months of age. Human αSYN was predominantly located in neuronal cell bodies, neurites, synapses, and in intraneuronal/intraneuritic aggregates. The hm2αSYN transgene resulted in more aggregates and dystrophic neurites than did the hw5 transgene. The hwαSYN transgene resulted in higher expression of two striatal proteins, synaptogamin 7 and UCHL1, compared to the levels of the hm2αSYN transgene.
These observations suggest that mutations in αSYN may impair specific functional domains, leaving others intact. These lines may also be useful for exploring interactions between hαSYN and environmental or genetic risk factors in dopamine-related disorders using a mouse model.
Keywords: Parkinson’s disease, substance abuse, substantia nigra pars compacta, ventral tegmental area, dopamine system
Introduction
The gene Snca encoding for the protein α-synuclein (αSYN) has been identified in diverse species, implicated in neurotransmitter function, and associated with different diseases (McKusick, 2009). A role for both wild-type (hwαSYN) and mutated αSYN (hmαSYN) has been recognized in multiple rare familial forms of the Parkinson’s disease phenotype (PDP) (McKusick, 2009). The risk associated with hwαSYN in sporadic forms of the PDP is less well understood, although polymorphisms in the noncoding portion of the gene have been variably associated with the PDP suggesting abnormal expression may be a risk factor (Holzmann et al., 2003; Tan et al., 2003; McKusick, 2009). A role for hwαSYN has recently been suggested in a variety of human substance abuse disorders including cocaine abuse (Mash et al., 2003; Qin et al., 2005; Boyer & Dreyer, 2007), cocaine abstinence (Mash et al., 2008), alcohol craving (Foroud et al., 2007), alcohol dependence (Bonsch et al., 2005b; Bonsch et al., 2005c; Clarimon et al., 2007), and methamphetamine psychosis/dependence (Kobayashi et al., 2004; Mauceli et al., 2006). The substantia nigra (SN) has also been implicated in reward-based learning and behavior(Zaghloul et al., 2009). Animal models of substance abuse also suggest a role for αSYN and might provide tools for identifying a mechanism (Liang et al., 2003; Ziolkowska et al., 2005; Liang & Carr, 2006; Oksman et al., 2006; Walker & Grant, 2006; Boyer & Dreyer, 2007; Ziolkowska et al., 2008). Additionally, the inverse association between the PDP and the use of tobacco (nicotine) and/or coffee and tea (caffeine) has been suggested to relate to a premorbid personality with a lower potential for substance use (Paulson & and Dadmehr, 1991; Kaasinen et al., 2001). How hwαSYN might participate in both substance abuse at a young age and neurodegeneration at a later age is conjecture, but might relate to the regional or cellular level of expression of hwαSYN or its interactions with other genetic or environmental risk factors over time.
Extensive in vitro and in vivo data suggest interactions of soluble and/or insoluble forms of the native and/or post-translationally modified forms of αSYN with other specific proteins and organelles (Yavich et al., 2004; Fortin et al., 2005; Mosharov et al., 2006; Vartianinen et al., 2006). Rodent models providing different levels of hαSYN expression in the presence or absence of mαSYN would benefit the understanding of these complex protein interactions. Although many mouse and rat models of hαSYN expression exist, they often lack certain features, such as: the demonstration of the physiological actions of hαSYN, the ability to contrast different levels, the ability to account for different forms of the protein, or expression at relevant concentrations in vivo. We have previously demonstrated that hαSYN transgenes expressed in multiple mouse lines had functional actions demonstrated by behavioral, pharmacological, biochemical, and pathological findings (Richfield et al., 2002a; Thiruchelvam et al., 2004; Chen et al., 2006). Now, for the first time, we have generated and characterized a series of congenic lines containing hwαSYN and/or hm2αSYN in a mouse line spontaneously deleted in the mouse homolog of αSYN (mαSYN). These congenic lines enable us to dissociate between endogenous mαSYN and transgenic hαSYN using a series of lines lacking mαSYN and replaced with varying amounts or combinations of either wild-type (hw5) or doubly mutated (hm239) hαSYN. We characterized these lines on both genomic and protein levels for αSYN and for measures related to dopamine (DA) and several striatal proteins. We demonstrate common yet dissociable alterations between the wild-type and mutated hαSYN suggesting that the specific point mutations may explicitly affect distinct functional domains. We suggest these lines may be useful for exploring and understanding the role of hαSYN in both neurodegeneration and substance abuse.
Materials and Methods
Congenic Line Generation and Identification
The original lines of mice containing the human wild type α-synuclein (hwαSYN, JAX Stock No. 008245) or a doubly mutated α-synuclein (hm2αSYN, JAX Stock No. 008239) were created and maintained in the C57BL/6J (B6J) background and are available from the Jackson lab (http://jaxmice.jax.org/query) (Richfield et al., 2002b; Thiruchelvam et al., 2004; Chen et al., 2006). In these original lines of mice, the hαSYN was under the control of the full length rat tyrosine hydroxylase (TH) promoter which resulted in expression in catecholaminergic neurons and other regions. C57BL/6JOlaHsd (B6Jola) mice were purchased from Harlan (Harlan, Indianapolis, IN). The B6JOla line is derived from the B6J line, but is spontaneously deleted in mouse α-synuclein (mαSYN) (Specht & Schoepfer, 2001; Chen et al., 2002). All mice were housed in a climate and light controlled (12/12 hr light/dark, light on at 6 A.M.) room at the University of Medicine and Dentistry of New Jersey (UMDNJ). Food and water were provided ad libitum. Animal use procedures were approved by the UMDNJ Institutional Animal Care and Use Committee (IACUC) and were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Congenic lines were generated by breeding and identified by genomic testing. Nontransgenic littermate controls (nTg) were always used in experiments. Genetic drift was avoided by periodically purchasing mice for breeding from the original supplier.
Genomic DNA was prepared from tail snips using DNAzol Direct (Molecular Research Center, Inc., Cincinnati, OH) according the manufacturers guidelines. Fluorescence based PCR using standard Taqman methodology, SDS 2.3 software and an Applied Biosystems 7900HT were used for all genotyping with the indicated probes and primers (Supplementary Table 1).
Lines C57Bl/6J-tg(hwαSYN-5) (B6J(hw5)) and C57Bl/6J-tg(hm2αSYN-39) (B6Jhm239) are hemizygous (containing one hαSYN transgenic allele) and were made congenic in the B6JOla strain by mating and genotyping over two generations (Supplementary Table 2). B6JOla mice were obtained from Harlan and used during initial generation and subsequently for mating. Once congenic, hemizygous mice were mated only with line B6JOla to maintain their background. All mice used in the experiments for this paper were ≥ generation F10. The current generation of the lines is ~F18. To make homozygous mice (containing two of the same hαSYN transgenic alleles), two congenic hemizygous mice containing the same transgene were mated with each other (Supplementary Table 2). To make compound hemizygous mice (containing two different hαSYN transgenic alleles), congenic hemizygous or homozygous mice were mated with mice containing a different transgene. The mouse N-methyl-D-aspartate (NMDA) NR1 receptor gene (mNMDA NR1) was used as a control and confirmed using a mNMDA NR1 probe and primer set (Supplementary Table 1) in all mice before the interpretation of other genotyping and was followed in selected genotyping by quantifying the presence of one or two alleles of either hαSYN transgene as appropriate by qRT-PCR (Supplementary Fig. A). The appropriate B6J(nTg), B6JOla(nTg), B6JOla hemizygote (B6JOla(hw5) or B6JOla(hm239)) or homozygote (B6JOla(hw5)×2 or B6JOla(hm239)×2) control DNA from prior testing was used for verification of assay conditions and quantifying. DNA containing the appropriate hαSYN transgene was always used as a positive control and for quantifying of zygosity because the two different hαSYN transgenes gave slightly different mean cycle time (CT) values. The absence of mαSYN was confirmed in all B6JOla mice (Supplementary Fig 1F). The presence of either hαSYN transgene was determined using the hαSYN probe and primer set (Supplementary Table 1 and Fig. B). The presence of two alleles of either hαSYN transgene was determined using the CT values of both the hαSYN transgene and the NMDA NR1 gene determined from the same two wells containing both probe and primer sets labeled with FAM or VIC (Supplementary Table 1 and Figs. C and D). The zygosity was determined using the ΔΔCT method (hαSYN ΔCT- NMDA NR1 ΔCT) using a known hemizygote control for each line in the assay. Values determined from a single experiment are reported as follows. One copy of the hwαSYN-5 transgene (n = 9) resulted in a mean ratio (± s.d.) of 1.02 ± 0.12 and two copies of the hwαSYN-5 transgene (n = 6) resulted in a mean ratio of 2.30 ± 0.19. For one copy of the hm2αSYN-39 transgene (n = 8) the mean ratio was 1.04 ± 0.35, and for two copies of the hm2αSYN-39 transgene (n = 12) the mean ratio was 2.13 ± 0.33. These ratios varied slightly from experiment to experiment depending on the CT value for a given control mouse used as the normalizer gene. However, homozygotes were always significantly different from hemizygotes. Zygosity determination by PCR was confirmed by breeding such that homozygotes with two alleles always generated hemizygote pups when mated with a nontransgenic mate. Hemizygotes mated with a nontransgenic mate always generated both hemizygotes and nTg pups. To specifically indentify the hwαSYN from the hm2αSYN transgene, we developed a SNP assay (Supplementary Table 1 and Fig E). Mating of a hemizygote B6JOla(hw5) with a hemizygote B6JOla(hm239) produced the expected ratio of different congenic mice including hemizygotes of either transgene (~50%) as well as compound hemizygotes (~25%) as determined by the SNP assay. Thus, all the lines containing one or two copies of either hαSYN transgene or their combination have been produced and confirmed by two PCR assays and breeding.
One phenotypic feature was noted in the generation and breeding of these congenic lines. The homozygote line B6JOla(hm239)×2 has an unusual furry phenotype manifested by increased and irregular fur that was presumed due to the homozygous disruption of a gene(s) related to the insertion site of the hm2αSYN-39 transgene. This trait was used to confirm genotyping data. All homozygous and hemizygous mice were fertile. All lines had normal B6JOla litter sizes and gained weight similarly. Hemizygous mice had a normal life span examined up to ~20 months. The life spans of homozygous and compound hemizygous congenic mice have not yet been examined.
Tissue Acquisition
Mice were either euthanized by cervical dislocation or anesthesia followed by perfusion depending on purpose. Mice that were perfused were anesthetized with either Nembutal (75 µg/gm, ~1.9 mg per 25 gm mouse) or Avertin (240 µg/gm, ~6.0 mg per 25 gm mouse). Nembutal was diluted to 5 mg/ml in saline and a volume of 0.375 ml used and Avertin was diluted to 17 mg/ml in saline and a volume of 0.35 ml used. After anesthesia, the mouse was slowly transcardially flush perfused by hand with heparin/saline (15 ml) and then fixation perfused with 50 ml of 4% paraformaldehyde (PFA) in saline. The brain was removed and postfixed in 4% PFA for 24 hrs and then transferred to 30% sucrose. Some brains were harvested fresh, dissected on ice to obtain regions of interest which were immediately frozen on dry ice. Some brains were divided in the midsaggital plane with one side immediately frozen and the other side postfixed in 4% PFA for 24 hrs and then transferred to 30% sucrose prior to sectioning. Tissues from dissected regions were stored at −80° C until used in assays described below.
HPLC-EC determination of catecholamines
High-performance liquid chromatography (HPLC) for catecholamine detection by electrochemical detection (HPLC-EC) was performed in two independent laboratories as described previously (Richardson et al., 2006). At EOHSI, dissected striata were sonicated in 0.1 M perchloric acid (PCA) containing 347 µM sodium bisulfite and 134 µM EDTA. Homogenates were centrifuged at 15,000 g for 20 min at 4°C, the supernatant removed, and filtered through a 0.22 micron filter by centrifugation at 15,000 g for 20 min. The supernatants were then analyzed for levels of DA, DOPAC, HVA, 5-HT and 5-HIAA. Catecholamine concentrations were determined using a Waters Alliance HPLC system equipped with an Antec Leyden electrochemical detector. Quantification was made by reference to calibration curves obtained from individual monoamine standards. Values are reported as ng/mg wet weight of tissue. At Eli Lilly, dissected striata were sonicated in 0.5ml buffer (0.1M PCA containing 0.1 mM EDTA and 2.5 mg/l ascorbic acid). The samples were centrifuged at 20,000g for 15 minutes at 4°C; the supernatants were then removed and filtered (Uniprep, Chromacol UK) and stored at −80°C pending analysis by HPLC-EC. The HPLC system consisted of a pump (Jasco, Great Dunmow, UK), an on-line degasser, a Hypersil BDS analytical column (150 × 3.0 mm, 3 m C18, Thermoquest, Runcorn, UK) maintained at 35°C using a Mistral column heater (Spark; Presearch Ltd., Hitchin, UK), and a Triathlon autosampler (Presearch Ltd., Hitchin, UK). The mobile phase consisted of a 100 mM phosphate buffer containing octane sulphonic acid (2mM), EDTA (1mM) and methanol (13%) with a final pH of 2.7. Detection of DA, DOPAC, HVA, 5-HT and 5-HIAA was accomplished with two LC4C electrochemical detectors (BAS, Kenilworth, UK) under oxidation (+775 mV) and reduction (+50 mV), respectively, versus an Ag/AgCl reference electrode. DA was measured under reduction; the remainder under oxidation. The flow-rate was 400 l/min. Chromatograms were displayed, integrated and stored using Empower (Waters, Milton Keynes, UK). Quantification was achieved by reference to calibration curves for individual monoamine standards and reported as ng/g wet weight of tissue.
Analysis was done independently using separate samples in two laboratories (EOHSI and Eli Lilly). Each laboratory received samples from groups of B6J(nTg), B6J transgenic, B6JOla(nTg), and B6JOla congenic mice (UMDNJ n = 41, Eli Lilly n = 41). The baseline level of neurotransmitters (mean ± sem, ng/mg wet weight tissue) differed slightly between labs for the B6J(nTg) line as follows: DA (Lilly 15.4 ± 1.1 and UMDNJ 17.9 ± 1.1), DOPAC (Lilly 1.8 ± 0.2 and UMDNJ 2.1 ± 0.1), HVA (Lilly 1.6 ± 0.1 and UMDNJ 2.9 ± 0.1), 5HT (Lilly 0.48 ± 0.04 and UMDNJ 0.84 ± 0.05), and 5HIAA (Lilly 0.43 ± 0.05 and UMDNJ 0.48 ± 0.03). Statistical analysis demonstrated that mean values for different measures (DA, DOPAC, HVA, 5HT and 5HIAA) differed by lab. Therefore, values were standardized by dividing each labs specific neurotransmitter value by the labs’ mean for that measure. Standard deviations were not significantly different between labs (Levene’s test of homogeneity of variances yielded p-values that were all ≥ 0.18). Scaled measures were used in all statistical analyses. Means and standard deviations for each measure were calculated and histograms examined for normality. All measures followed approximately a normal distribution.
Western blot analysis
Western blot analysis was performed similar to that described (Chen et al., 2006) when using chemiluminescence or modified when using fluorescence detection. Briefly, frozen striatal samples (~10 mg wet weight) were homogenized in buffer (5 mM HEPES, 320 mM sucrose with 1 µg/ml of protease inhibitors (aprotinin, leupeptin, and pepstatin)). Homogenized samples were centrifuged at 3,500×g for 5 min to remove nuclei and the supernatant was then centrifuged at 14,000×g for 45 min to pellet synaptosomes and proteins. The final pellet was resuspended in homogenization buffer, protein level determined, and then proteins were subjected to polyacrylamide gel (9%) electrophoresis. Between 15 and 40 µg of protein were loaded per lane depending on the primary antibody (1° Ab). Samples used for detection of α-SYN, tyrosine hydroxylase (TH), synaptotagmin 7 (syt7), and ubiquitin C-terminal hydrolase 1 (UCHL1) were boiled briefly (5 min) before loading whereas samples for the detection of the dopamine transporter (DAT), were not. Proteins were then transferred to nitrocellulose membranes (0.2 or 0.4 µm pore size) for chemiluminescence or Immobilon®-FL (Millipore, Billerica, MA). Membranes used for detection of αSYN were boiled for 5 min prior to blocking. Some membranes were stained with Ponceau S for confirmation of transfer.
Membranes were probed for the following proteins: human αSYN (SYN 211 mouse monoclonal, 1:500, 2 µg/ml, Sigma, St. Louis, MO) or total αSYN (human and/or mouse) (mouse monoclonal, 1:500, BD Transduction Labs, San Jose, CA), TH (rabbit polyclonal, 1:2,000, Millipore, Billerica, MA), α-tubulin (mouse monoclonal, 1:10,000, Sigma-Aldrich, St. Louis, MO), the DAT (rat polyclonal, 1:5,000, Millipore, Billerica, MA), synaptotagmin 7 (rabbit polyclonal, 1:1,000, Synaptic Systems. Göttingen, Germany), or UCHL1-Cterminal (rabbit polyclonal, 1:2,000, Invitrogen, Camarillo, CA) Abs were incubated overnight at RT. Appropriate species specific 2° Abs (1:10,000, GE Lifescience, Piscataway, NJ) were incubated for 45 min at RT for chemiluminescence detection or with Biotin-Goat (Gt) anti-Rabbit (Rbt) or BiotinGt anti-Mouse (Ms, 1:2,000, Invitrogen, Camarillo, CA). Bands were detected using enhanced chemiluminescence (PerkinElmer, Waltham, Massachusetts) on a Kodak Gel Logic 100 Imaging System (Kodak, Rochester, NY), and Kodak Molecular Imaging Software MI (v. 4.5.1, Kodak, Rochester, NY) or fluorescence detection (WesternDot™ 625 Western Blot Kit, Invitrogen, Catalog no. W10132) using the Gel Doc EQ (BioRad, Hercules, CA) and MCID Elite software (InterFocusImaging Ltd, Cambridge, England). Bands were quantified using their O.D. and area and normalized to their respective value for α-tubulin used as a loading control to yield a ratio. For syt7 a loading control on the same membrane was not possible due to multiple bands at a size comparable to all housekeeping proteins, so samples were normalized to the same control group (B6JOla(nTg) on each membrane. Sample sizes of 3–8 mice were used for each line and values are from 2 independent gels using separate samples with all reported lines used on each gel.
αSynuclein ELISA Assays
ELISA assays were performed at Elan Pharmaceuticals using fresh frozen samples from different transgenic and congenic lines. They were performed as described previously with the following details and modifications (Anderson et al., 2006). Total and human specific α-synuclein levels were quantified by sandwich ELISAs utilizing three Elan designed and generated monoclonal Abs: 1H7 as the capture Ab and either 5C12 (total α-SYN) or 9E4 (human preferring α-SYN) as reporter Abs. All steps were carried out at room temperature. Both ELISAs were performed using I.A./R.I.A 96-well, flat bottom, half-area (100 µl) high binding plates (Corning Cat. # 3690) coated overnight at 4° C with 10 µg/ml of 1H7. Plates were blocked for 1 hour with human serum albumin (0.25%) in a phosphate buffer containing sucrose (2.5%) and sodium azide (0.05%). Samples to be assayed were extracted with 5 M guanidine 50mM Tris-HCl (pH 8.0) plus a protease inhibitor mixture. Samples and standards (human αsynuclein peptide, non-phosphorylated, (R-Peptide, Cat. # S-1001-2) were diluted to 0.5 M guanidine in casein blocking solution and applied to the coated, blocked plates. Standards were run at 0, 0.15, 0.31, 0.62, 1.25, 2.5, 5.0, and 10 ng/ml. A test series was done to choose optimal dilutions and 2–3 dilutions were used for the final determination. Following an overnight incubation at 4 °C, plates were brought to room temperature, washed three times with 100 µl of TBST (150 mM sodium chloride, 50 mM Tris, pH 7.5, 0.1% Tween 20) and then incubated for 1 h at room temperature with the reporter Abs biotinylated 5C12 (1.5 µg/mL) or 9E4 (2.0 µg/mL). After washing, plates were incubated for 1 h with streptavidin alkaline phosphatase (GE Cat. #RPN4402) in specimen diluent (0.6% globulin-free bovine serum albumin, Na2HPO4-7H2O 2.16 g/L, NaH2PO4-H2O 0.2 g/L, NaCl 8.5 g/L, Na Azide 0.5 g./L, NaCl 8.5 g./L, and Triton X-405 0.5 ml/L). Wells were then washed with 0.05% TBST. Fluorescent substrate A (2 amino-2-methyl-1-propanol 31.2 g/L and 4-methyl belliferyl phosphate 0.03 gm/L, pH 9.5) was added to each well for exactly 20 minutes at room temperature. The reaction was stopped with 25 µl 3M K2PO4. Fluorescence values were read using a Molecular Devices Gemini Spectramax plate reader at an excitation wavelength of 360 nm and an emission wavelength of 460 nm. A curve fit for the standards used 4 parameters and the equation y = (A–D)/(1+(x/C^B)+D(Findlay & Dillard, 2007). For hemibrain samples, group sizes were 2–6. For striatal samples, group sizes were 2–9.
Fluorescence immunohistochemistry
For fluorescence detection in tissue sections, the methods were similar to those published in detail except two 1° and two different fluorescent labeled 2° Abs were used (Prasad & Richfield, 2008). For sections used for stereology, the fixed midbrains containing the entire SNpc and VTA were sectioned coronally from rostral to caudal into 30 µm sections using a freezing/sliding microtome and collected in cryoprotectant (30% sucrose, 30% ethylene glycol in 0.1 M phosphate buffer). Every 4th section was collected in a series having a random offset for each brain. Each series consists of 14–20 sections and was stored at −20° C until staining. For tissue staining, sections were rinsed in phosphate buffered saline (PBS) at room temperature before immunohistochemistry (IHC). Sections were first blocked with 10% normal goat serum and then incubated with a mouse monoclonal anti-tyrosine hydroxylase (TH) Ab (1:500 dilution, Millipore, Billerica, MA) and a rabbit polyclonal anti-hαSYN (1:5,000, BioMol International L.P., Plymouth Meeting, PA). Incubation with the 1° Abs was for 48 hrs and 2° Ab for 24 hrs at 4° C. The 2° Ab dilutions were 1:500 each with a goat anti-rabbit ALEXA fluor 594 and a goat anti-mouse ALEXA fluor 488 (Molecular Probes, Inc. Eugene, OR). Sections were counterstained without drying by incubation in 1 ug/ml 4’,6-diamidino-2-phenylindole (DAPI) for 5 minutes then washed in 0.1 M phosphate buffer for 15 minutes, mounted with a commercial aqueous mounting media containing an anti-fade agent, coverslipped, and dried overnight before nail polishing the edges to seal.
Similar staining methods were used for luminance measurements in striatal sections except only a limited number of sections were used. The 1° Abs detected synaptotagmin 7 (rabbit polyclonal, 1:4,000, Synaptic Systems. Göttingen, Germany) or ubiquitin C-terminal hydrolase 1 (rabbit polyclonal, 1:1,000, Invitrogen, Camarillo, CA).
Luminance measurements
Fluorescence luminance was measured blindly using Stereo Investigator (v. 9.0, MBF Bioscience, Williston, VT) and the luminance feature. All sections sampled for a comparison between groups were immunstained at the same time using the same master mix of antibodies and buffers. The sampling parameters for the camera and software were not altered during sampling. The same size area was used. The appropriate wavelength filter was selected based on secondary Ab used.
Confocal Image acquisition and Processing
Images from fluorescence stained sections were collected using an Olympus Fluoview FV10i confocal microscope. Images were obtained at 180x final magnification, final resolution of 1024.1024, and an aperture of 1.0. Confocal images were viewed and exported for final figures using Imaris (version 6.4.2, Bitplane Zurich, Switzerland). The image section feature was used to view planes in three dimensions.
Chromogenic immunohistochemistry for αSYN
Sections from PFA-fixed mouse hemibrains and human brain regions provided by the Institute for Brain Aging and Dementia Tissue Repository at the University of California, Irvine (CA) were prepared by NeuroScience Associates (Knoxville, TN). Briefly, brains were treated overnight with 20% glycerol and 2% dimethylsulfoxide to prevent freeze-artifacts and multiply embedded (16 brains per block) in a gelatin matrix using MultiBrain® Technology (NeuroScience Associates, Knoxville, TN). After curing, each block was rapidly frozen by immersion in isopentane chilled to −70°C with crushed dry ice and mounted on a freezing stage of an AO 860 sliding microtome (American Optical). A series of 40mm sagittal sections were taken through the MultiBrain® block. All sections were collected sequentially in containers which were filled with Antigen Preserve solution (50% PBS pH 7.0, 50% Ethylene glycol, 1% Polyvinyl Pyrrolidone).
For IHC, sagittal brain sections were immunostained following a standard protocol for immunoperoxidase. 1° Abs were a polyclonal anti-αSYN recognizing aa 115–122 (Ab1, Elan, EladW-47) in both human and mouse αSYN or a monoclonal Ab specific for hαSYN recognizing aa 121–125 (Ab2, Sigma, St, Louis, Missouri, clone SYN 211) with both used at 0.5µg/ml. Biotinylated 2° Abs (Vector, Burlingame, CA) were used at a concentration of 0.5 µg/ml and positive signals were visualized with the ABC Vector system (Vector) using diaminobenzidine and H2O2 as peroxidase substrates, based on manufacturer’s instructions. Sections were viewed with an Olympus BX-51 microscope (Tokyo, Japan) and digital images were taken using a Retiga Exi camera (Surrey, BC, Canada).
Stereology
The total number of tyrosine hydroxylase positive (TH+), hαSYN positive (hαSYN+), and TH−/hαSYN- neurons in the substantia nigra pars compacta (SNpc) and ventral tegmental area (VTA) were counted using the optical fractionator and our previously detailed counting criteria {Prasad, 2008 #8849;Prasad, 2010 #10309}. Briefly, neurons were counted as TH+ or hαSYN+ if: (a) they showed TH or hαSYN immunoreactivity within the cell body, (b) part of the nucleus of that cell resided inside the counting frame without touching the avoidance lines, and (c) the nucleolus within that nucleus was in focus between the top and bottom boundaries of the counting frame (i.e. not in the guard zones). TH−/ hαSYN- neurons were counted if (a) a neuronal nucleus was visualized without cytoplasmic TH or hαSYN staining, (b) the nucleus had a cross-sectional area similar to the average cross-sectional area of TH+/hαSYN+ nuclei, (c) it had a macronucleolus and more than one small nucleoli, (d) the shape of the nucleus was round to oval, (e) the nuclear membrane was smooth, (f) the nucleus was partially or entirely inside the counting frame without touching the avoidance lines, and (g) a portion of the nucleolus within that nucleus was in focus within the top and bottom boundaries of the sampling frame.
The mounted sections were ordered from rostral to caudal by visual inspection at low power and recorded on a data collection sheet. The regions of interest (SNpc and VTA) were outlined at low magnification (4× objective) and sampled at high magnification (100×/1.4 NA oil immersion objective) using StereoInvestigator (MicroBrightField, VT). IHC was performed using every 4th section from the midbrain using a random well and stereology was performed on every 8th section (total of 7–8 sections containing the SNpc and VTA) using a random first section to start. This provided two sets of sections should one set be uncountable due to a damaged or folded section. We counted one side of the SNpc and VTA.
The size of a subset of neurons was measured during stereology using the optical dissector. A five ray star was used to measure soma size of both TH+ and hαSYN+ neurons.
Statistical analysis
MANOVA was used for the analysis of HPLC data from the two different laboratories (2004). When p < 0.05 was found based on the MANOVA Wilk’s lambda (interpreted as the proportion of variance in the responses that is unexplained by mouse line) and its corresponding F test (for all lines combined), then select pairwise group comparisons were made using only the following a priori selected contrasts (lines B6J(nTg) vs. B6JOla(nTg), B6JOla(nTg) vs. B6JoOa(hw5), B6JOla(nTg) vs. B6JOla(hm239) and B6JOla (hw5) vs. B6JOla (hm239). If these comparisons yielded significant differences in the responses, then individual ANOVA results were used to determine which specific responses differed between lines. Significance levels for the ANOVA tests were set at the 0.05 level.
Levels of proteins quantified by Western blots were analyzed using one factor ANOVA implemented in SigmaStat 3.1 (San Jose, CA). All post-hoc assessments were carried out based on main effects or interactions as appropriate when the overall effect was significant. For all analyses, initial p value of ≤ 0.05 was considered statistically significant and posthoc comparisons (Holm-Sidak method) were performed that controlled for the number of comparisons made. The level of mouse or human αSYN was compared between groups using a two-sided Students t-test since a limited number of specific comparisons were made. A significant difference between groups was identified if p < 0.05.
Results
Striatal dopamine and metabolites
We measured the striatal content of catecholamines from the different lines at two laboratories. Both laboratories analyzed the same number of mice in all lines. Similar consequences of each line were seen in both laboratories before combining data as described in detail in the methods. MANOVA analysis of DA, DOPAC, HVA, 5HT and 5HIAA indicated a significant difference between the lines analyzed (B6J(nTg), B6JOla(nTg), B6JOla(hw5), B6JOla(hm239), B6JOla(hw5)×2, B6JOla(hm239)×2, and B6JOla(hw5× hm239): Wilk’s Lambda=0.25, F(25, 231.8)=4.12, p<0.0001). Pairwise comparisons indicated that responses were significantly different for mouse lines B6J(nTg) versus B6JOla(nTg) (Wilks’ Lambda=0.71, F(5,62)=5.14 p=0.0005), B6JOla(nTg) versus B6JOla(hw5) (Wilks’ Lambda=0.71, F(5, 62)=5.13 p=0.0005) and B6JOla(nTg) versus B6JOla(hm239) (Wilks’ Lambda=0.78, F(5,62)=3.46, p=0.0080), but not between lines B6JOla(hw5) versus B6JOla(hm239) (Wilks’ Lambda=0.89, F(5,62)=1.54, p=0.19).
For the line B6J(nTg) versus B6JOla(nTg) contrast, using ANOVA for comparison of univariate measures, DOPAC, HVA, 5HT and 5HIAA were shown to differ significantly between groups (Fig. 1, F(1,62)=15.50, 6.65, 6.40 and 9.99 with p=0.0002, 0.012, 0.014 and 0.0024, respectively), but not DA (F(1,62)=1.52 with p=0.22). For line B6JOla(nTg) versus B6JOla(hw5) comparison, DA, HVA, 5HT and 5HIAA were significantly different between the two lines (F(1,62)=11.07, 9.07, 14.0 and 8.20 with p=0.0014, 0.0037, 0.0004 and 0.0056, respectively), but not DOPAC (F(1,62)=3.90 with p=0.052). For line B6JOla(nTg) versus B6JOla(hm239) comparison, significant differences were found for HVA, 5HT and 5HIAA (F(1,62)=6.31, 11.64 and 6.12 with p= 0.015, 0.0011 and 0.016, respectively), but not DA or DOPAC (F(1,62)=2.42 and 3.51 with p=0.12 and 0.065).
Figure 1.
HPLC measurement of catecholamines in different lines of hαsyn transgenic/congenic mice at 9 months. HPLC was performed in two independent labs with each measurement normalized to the lab mean for the B6J group and later combined for statistical analysis and presentation. All neurotransmitters or metabolites were normalized to the B6J line and have a value of 1.0 with an error bar of associated variance. Each other line has a value normalized to the B6J line which might be less than 1.0 indicating a decreased value or greater than 1.0 indicating an elevated value compared to the B6J line. Values are shown for DA, DOPAC, HVA, 5HT, and 5HIAA. Samples sizes for each group (n=4–22) shown in the legend. Values indicated by 1 1* were significantly different from the B6J(nTg) line at the p < 0.05 and p < 0.01 level respectively and 2, 2* significantly different from the B6JOla(nTg) line at the p < 0.05 and p < 0.01 level respectively using appropriate post hoc contrasts.
Western blot analysis
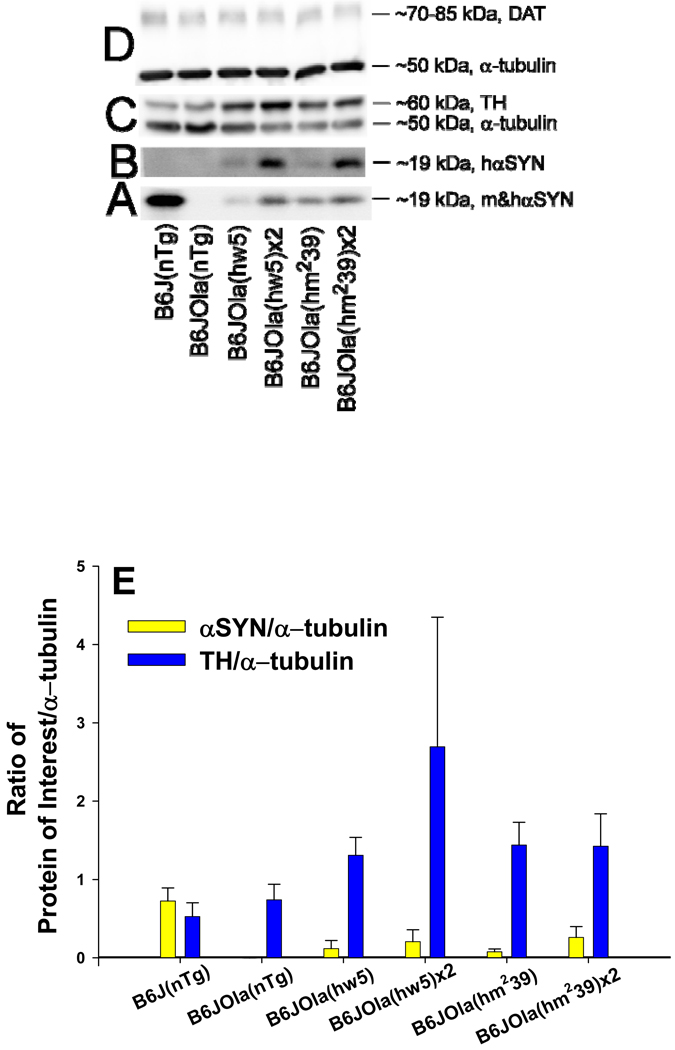
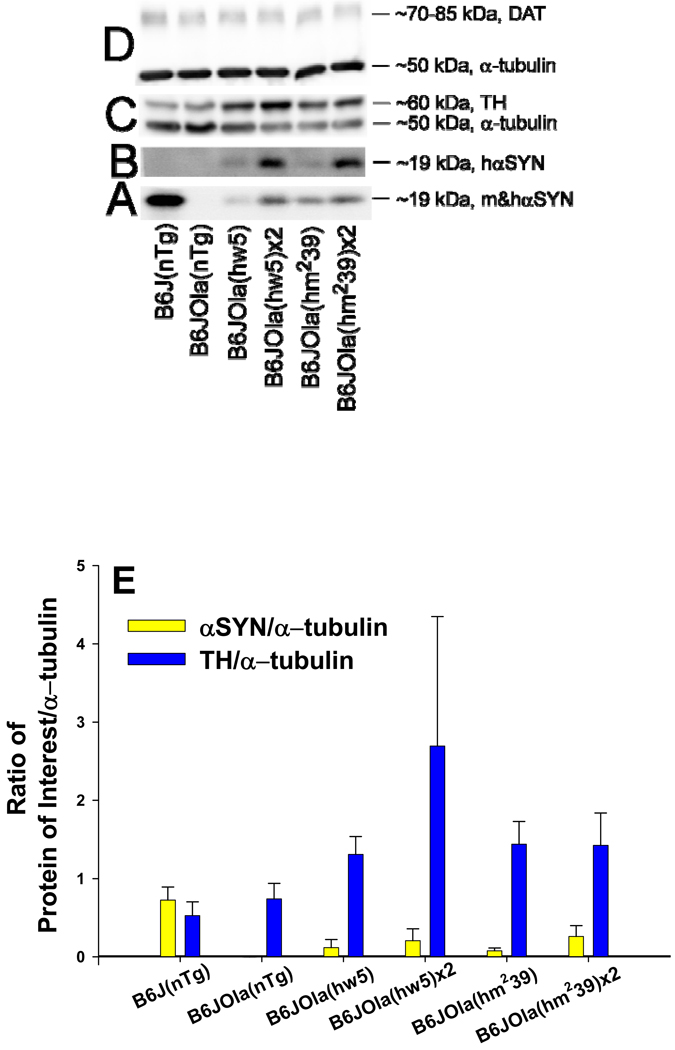
The expression of hαSYN and mαSYN protein in striatum agreed with genotyping data. Line B6J(nTg) expressed mαSYN protein detected using an Ab that recognized both hαSYN and mαSYN (BD Transduction Labs), but was not detected using a human specific αSYN Ab (Clone 211, Sigma, Fig. 2A, B). The B6JOla(nTg) mice, spontaneously deleted in the mαSYN gene, did not express detectable protein (Fig. 2A, B, E). Congenic lines containing either hαSYN transgene expressed hαSYN protein detected using either Ab (Fig. 2A, B, E) at varying levels depending on transgene copy number. Congenic lines containing the wild-type human transgene (hw5) expressed slightly less hαSYN protein than lines containing the doubly mutated transgene (hm239) and homozygous congenic lines expressed more hαSYN protein than their respective hemizygous line. The amount of striatal tyrosine hydroxylase (TH) was elevated in congenic hemi- or homozygous lines compared to lines without a transgene (Kruskal-Wallis One Way ANOVA H = 9.252 with 1 degree of freedom, p = 0.002, Fig. 2C, E). The level of the DAT did not differ significantly between congenic lines (Kruskal-Wallis One Way ANOVA 1 degree of freedom, p > 0.05, Fig. 2D).
Figure 2.
Western blot analysis. The amount of protein in six different lines was measured in the striatum. The specificity of two αSYN Abs (A, B) can be recognized based on the species of αSYN detected in the different lines and confirms prior genotyping and IHC. Representative blots for the following proteins: A. mouse and human αSYN detected using a species independent αSYN Ab (BD Transduction Labs), B. Human specific detection of αSYN (Ab2, Clone 211, Sigma), C. Tyrosine hydroxylase (TH, top) and α-tubulin (bottom), and D. Dopamine transporter (DAT, top) with loading controls for α-tubulin (bottom). E. Densitometric measurements of the ratio of protein of interest normalized to α-tubulin (to control for loading) in the six different lines. The amount of striatal αSYN in line B6J(nTg) was higher than in the congenic lines. The amount of hαSYN was higher in both homozygote compared to the respective hemizygote congenic line. The ratio of TH/α-tubulin was higher in all congenic lines than either nontransgenic line.
ELISA analysis
Total and human αSYN were measured first using a hemibrain without the cerebellum (Fig. 3 A, B) followed by separate striatal samples. Mouse specific αSYN (mαSYN) was determined in each B6J (expressing mαSYN) mouse sample using the total αSYN value (5C12) minus the mean value obtained from B6JOla nontransgenic group (nTg, not expressing mαSYN). Human specific αSYN (hαSYN) was determined in each B6J and B6JOla mouse expressing a human transgene (Tg+) using the hαSYN value (9E4) minus the corresponding mean value obtained from the B6J or B6JOla nontransgenic group (nTg). The curve fits for these assays were excellent with calibrators showing good accuracy and precision for the entire assay range. “Nonspecific” values obtained for each assay were quite low demonstrating the specificity of these assays. Values for “nonspecific” level using striatal samples were total αSYN (5C12) in line B6JOla 0.4 ± 0.2 µg/g, human αSYN (9E4) in line B6JOla (nTg) 0.6 ± 0.2 µg/g, and human αSYN (9E4) in line B6J(nTg) 2.1 ± 0.3 µg/g. In agreement with previous Western data, the B6J(nTg) line expressed an abundance of mαSYN in the striatum. The human αSYN transgenes were expressed at lower levels compared to the endogenous level in the B6J line. Although sample sizes were small (n = 3), a significant gender effect was not seen so gender data were combined. The sample size was also small (n = 3) for comparing the levels of the two human transgenes, but no significant difference was seen and that data was also combined. Expression of a human transgene significantly reduced expression of the mαSYN protein in the striatum, but not in hemibrain (Fig. 3 A, B). The level of the hαSYN protein was significantly lower in line B6JOla background compared to B6J background in the hemibrain (Fig. 3 B), but not in the striatum; although it was lower (Fig. 3). Mice containing one copy of each hαSYN transgene (B6JOla(hw5x hm239)) expressed significantly more protein than mice expressing one copy (Fig. 3). The absolute level of hαSYN was higher in the striatum than in the rest of the hemibrain (Fig. 3 B) reflecting the use of the tyrosine hydroxylase promoter to produce expression the nigrostriatal system.
Figure 3.
ELISA Measurements. Mouse (A) and human (B) αSYN were measured in hemibrain samples, then in striatal samples. The average protein levels of endogenous mouse αSYN were similar from hemibrain for the two B6J lines with (B6J(Tg+/−) or without (B6J(Tg−/−) the human transgene hm239 (A). However, mαSYN levels were significantly reduced in the striatum from the B6J(Tg+/) line (1, P < 0.001) compared to the B6J line. Statistical comparisons from entire hemibrains revealed a significantly reduced level of the human αSYN transgene (hm239) in the absence of mαSYN (B, 2 p < 0.001). Expressing two human transgenes (hw5 and hm239, Tg+/+) resulted in greater expression than expressing only one allele (3 p = 0.005). Bars represent group mean ± sem. 1Compares line B6J(nTg) to B6J(hm239), 2compares line B6J(hm239) to B6JOla(hm239), 3compares line B6JOla(hm239 or hw5) to B6JOla(hw5x hm239).
Stereology
The number of TH+, hαSYN+, and TH−/hαSYN- neurons in the SNpc and VTA did not differ between male mice from lines B6JOla(nTg), B6JOla(hw5), and B6JOla(hm239) at the age of 9 months (Fig. 4). The size of TH+ neurons did not differ between the three lines and was similar to the size of hαSYN+ neurons in the two congenic lines. The number of TH+ neurons was similar to the number of hαSYN+ neurons in both regions demonstrating expression of the transgene in almost every TH+ neuron in both congenic lines. TH+/hαSYN+ neurons were larger in the SNpc than in the VTA.
Figure 4.
Stereologic data from various brain regions of male mice from lines B6JOla(nTg), B6JOla(hw5), and B6JOla(hm239) at 9 months of age. The number of TH+ (green bars), hαSYN+ (red bars), and TH−/hαSYN− (blue bars) neurons did not differ between lines in the SNpc (A, (solid bars) or the VTA (B, (hatched bars). The size of TH+ neurons did not differ between the three lines and was similar to the size of hαSYN+ neurons in the two congenic lines in the SNpc and the VTA (C). TH+/ hαSYN+ neuronal size was smaller in the VTA compared to the SNpc.
Immunohistochemistry for αSYN
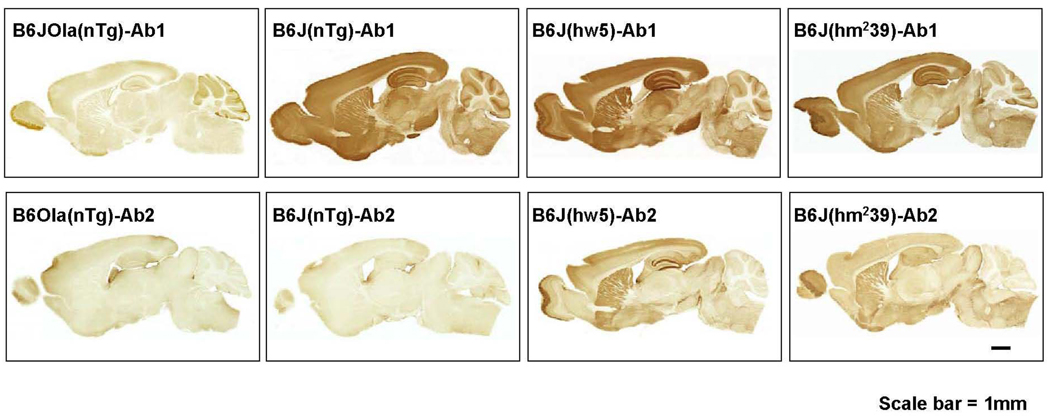
To demonstrate specificity of the two αSYN Abs used (Ab1, EladW-47 and Ab2, SYN 211) in this analysis, sagittal brain sections were immunostained and whole sections were photographed (Fig. 5). No specific staining in regions of interest was detected with either of the two Abs in line B6JOla(nTg) mice deficient in mαSYN. Ab 1 stained throughout the brain of B6J(nTg) and was regionally increased in lines B6J(hw5) and B6J(hm239) (Fig. 5, upper row) with a pattern suggesting mainly synaptic staining. This suggests that Ab1 recognized both mαSYN and hαSYN consistent with Western blot data. Ab2 stained only sections from lines containing a transgene, indicating that it is specific for hαSYN (Fig. 5, lower row).
Figure 5.
Specificity of mαSYN and hαSYN in various lines by IHC. Sagittal brain sections from B6JOla(nTg), B6J(nTg), B6J(hw5) and B6J(hm239) were immunostained with Ab1 (EladW-47, aa 115–122) or Ab2 (SYN 211, aa 121–125). Neither Ab1 nor Ab2 significantly labeled neuronal compartments in regions of interest from B6Ola(nTg) mice. Ab1 labeled neuronal compartments from B6J(nTg), B6J(hw5), and B6J(hm239) mice, whereas Ab2 labeled only neuronal compartments from B6J(hw5) and B6J(hm239) mice. This indicates that Ab1 recognized both hαSYN and mαSYN, whereas Ab2 only recognized hαSYN consistent with Western blotting data (Fig. 3). Scale bar = 1 mm.
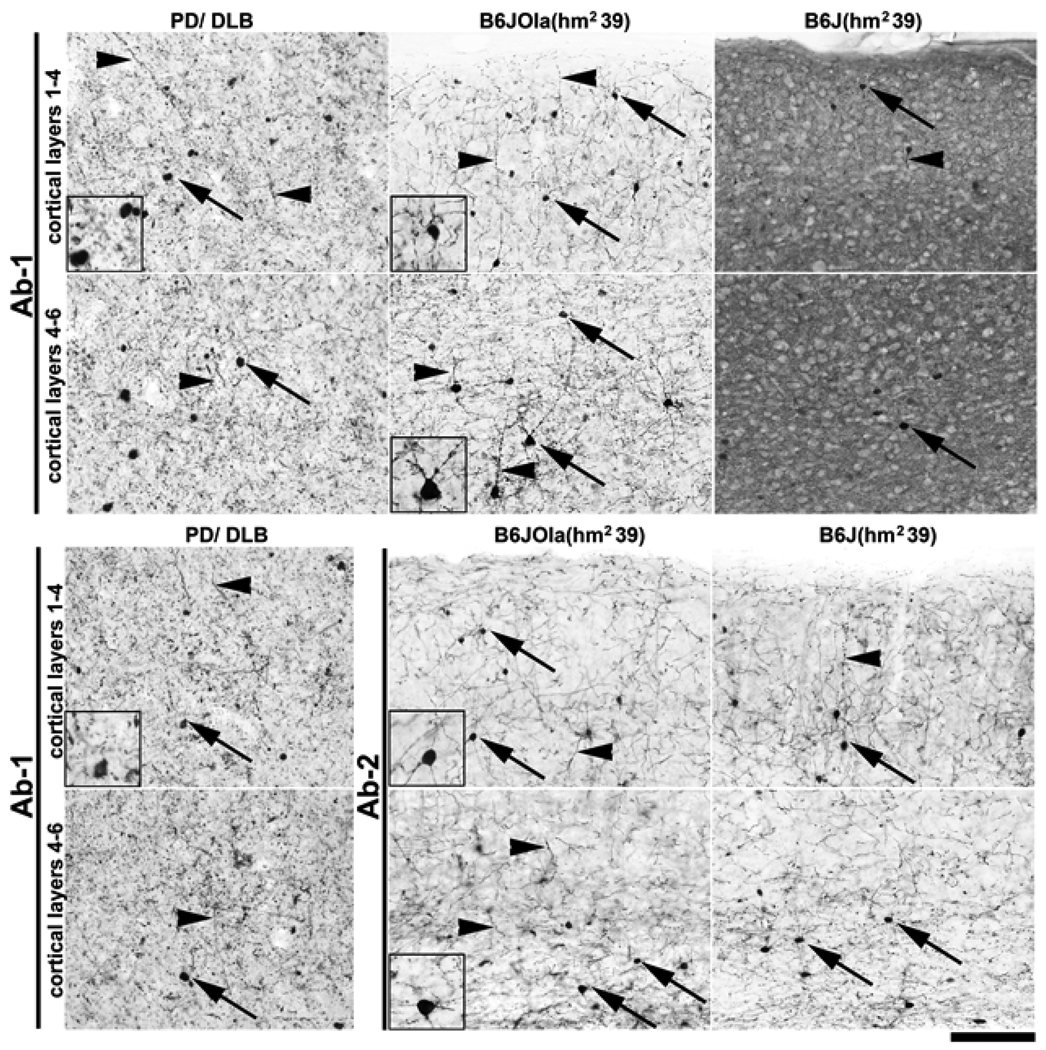
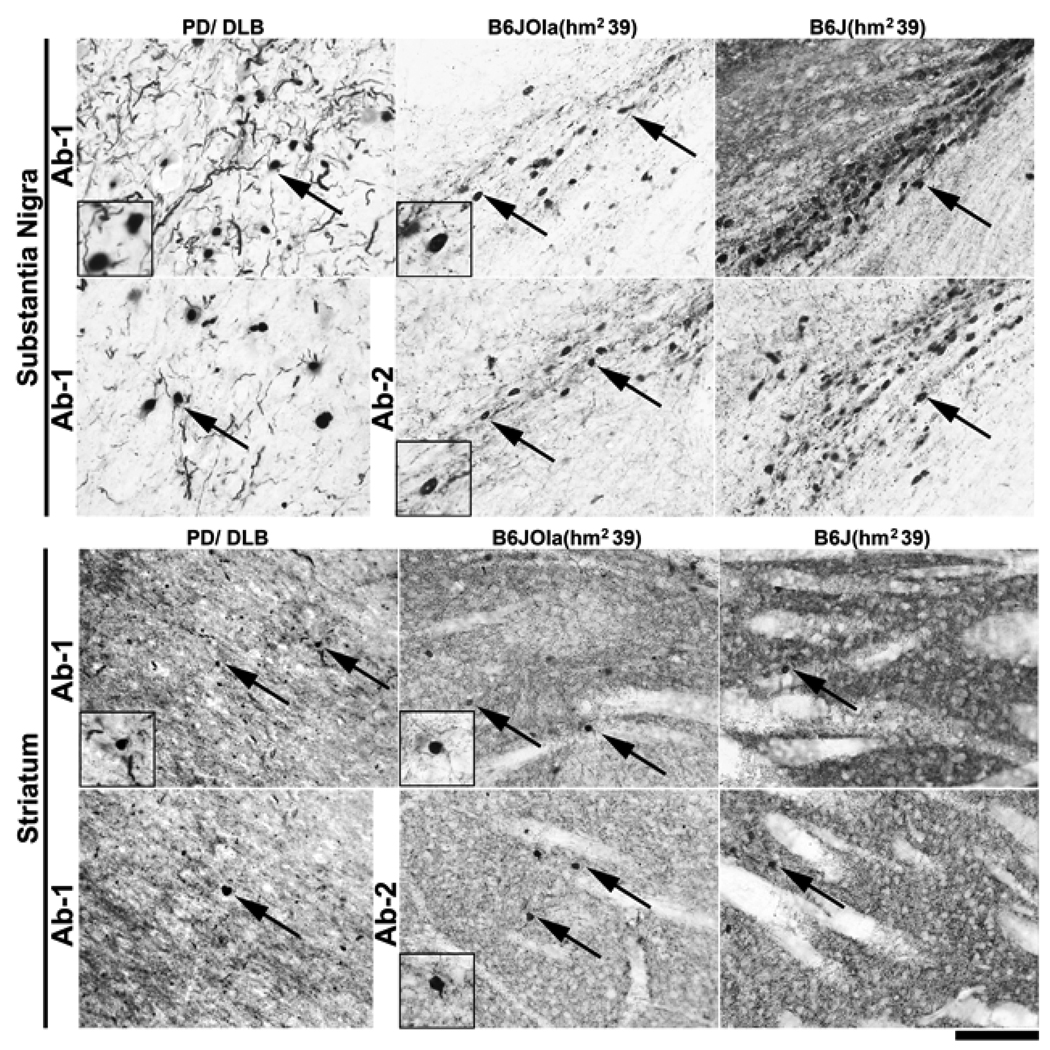
To compare mαSYN versus hαSYN staining patterns, the hippocampal dentate gyrus area was imaged using lines B6J(nTg), B6J(hw5), B6J(hm239), B6JOla(nTg), B6JOla(hw5), and B6JOla(hm239). Line B6JOla(nTg) served as a negative control expressing no αSYN. αSYN positive intraneuronal aggregates and neurites were detected with both Abs in all the transgenic lines (Fig. 6). Strikingly, in the B6JOla(hm239) line, the congenic hαSYN was localized almost exclusively in a non-synaptic compartment, i.e., in neuronal cell bodies and neurites, a phenomenon that was also observed in other brain regions such as the cerebral cortex and the substantia nigra, but not the striatum (Fig. 7). The hαSYN+ neurites in this line were similar to Lewy neurites observed under the same staining conditions in cortical tissues from 15 different PD and DLB (Dementia with Lewy Bodies) patients (Fig. 8). ). These results indicate that mutated human α-syn has a high propensity to be misplaced into non-synaptic compartments, and that the B6J(hm2-39) and the B6JOla(hm239) lines may be relevant models to study biological effects associated with abnormal αSYN localization consistent with that observed in PD.
Figure 6.
αSYN IHC in the hippocampus of transgenic and congenic mice. The typical synaptic staining of layers indicative of mαSYN synaptic staining appear in the B6J(nTg) mouse using Ab1. Intraneuronal aggregates can be seen in neurons of the dentate gyrus with either of the transgenes, however, are more prominent with the doubly mutated hαSYN (hm239) and more visible in line B6JOla(hm239) lacking the endogenous mαSYN. Scale bar = 100 µm.
Figure 7.
Comparison of αSYN expression in the neocortical layers of B6JOla(hm239) and B6J(hm239) mice with a human case of combined PD/DLB. Pictures show immunoperoxidase staining with Ab1 (α-syn aa 115–122) for the upper two rows, and with Ab1 and Ab2 (aa 121–125, human specific) for the lower two rows. α-syn was primarily in neuronal cell bodies and neurites in B6JOla(hm2-39) mice, but, with Ab1 (recognizing both human and mouse α-syn), in a pattern suggesting prominent synaptic localization in B6(hm2-39) mice. Staining with Ab2 revealed that transgenic (human) α-syn in B6J(hm2-39) was, just as in B6JOla(hm2-39) mice, primarily in neuronal cell bodies and neurites. Note the close similarity of staining between the transgenic mice and human PD/DLB in densely α-syn immunopositive neuronal cell bodies (arrows) and neurites (arrowheads) (see inset panels for higher magnification views). Scale bar = 100 µm.
Figure 8.
Comparison of αSYN staining pattern in the substantia Nigra pars compacta (SNpc) and striatum of B6JOla(hm239) and B6J(hm239) mice with a human case of combined PD/DLB. Pictures show immunoperoxidase staining for α-syn in the SNpc (upper two rows) and the Striatum (lower two rows) with either Ab1 (α-syn aa 115–122) or Ab2 (aa 121–125, human specific). In the SNpc, transgenic human α-syn was primarily located in neuronal cell bodies and neurites in both B6Ola(hm2-39 and B6J(hm2-39) mice. In contrast, in the striatum, α-syn staining was seen in a pattern primarily indicative of synaptic localization albeit a few neuronal aggregates can be detected (arrows). Note the similarities of staining between transgenic mice and human PD/DLB for both SNpc and striatum in αSYN immunopositive neuronal cell bodies (arrows) (see inset panels for higher magnification views). Scale bar = 100 µm.
Proteins altered by hαSYN expression
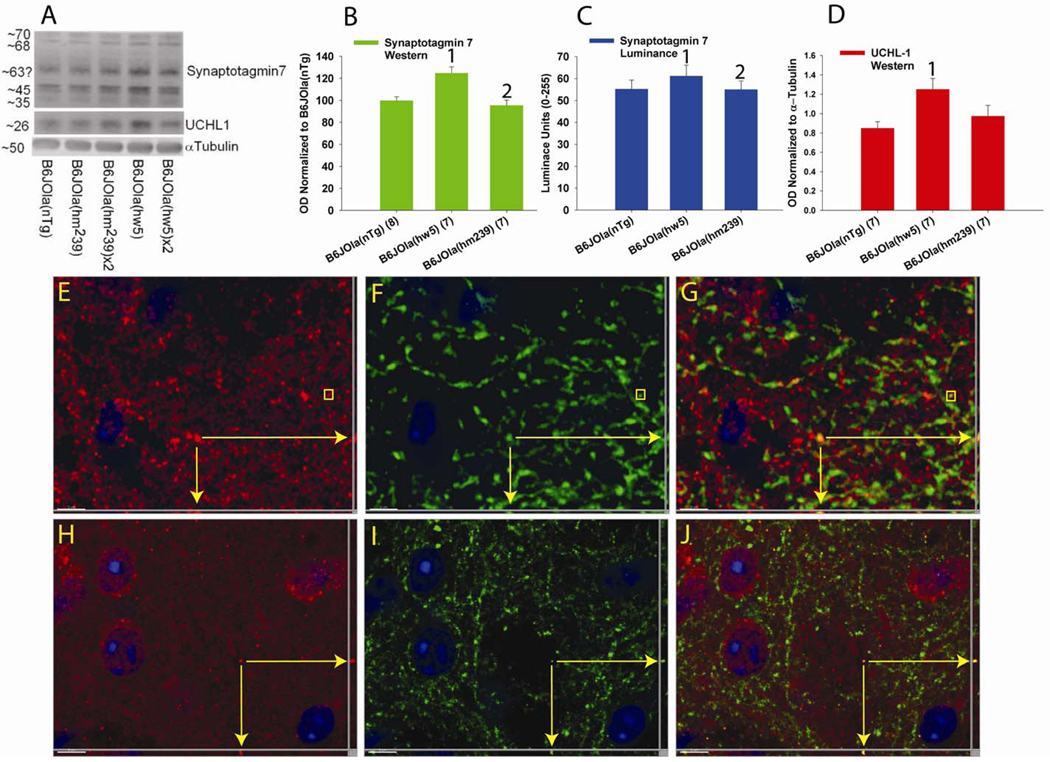
Based on findings provided by gene expression studies (not shown), two candidate proteins syt7 and UCHL1 were examined for expression using a variety of methods including Western blotting and IHC for luminance measurements and confocal co-localization examination (Fig. 9). Western blotting revealed the level of both syt7 (F2,21 = 11.683, p < 0.001) and UCHL1 (F2,20 = 4.30, p = 0.03) differed between the 3 lines examined. Syt7 was significantly elevated in the striatum of line B6JOla(hw5) compared to both line B6JOla(nTg) (p < 0.001) and B6JOla(hm239) (p = 0.003). UCHL1 was significantly elevated in the striatum of line B6JOla(hw5) compared to line B6JOla(nTg) (p = 0.031). Luminance measurements were made for syt7 and a similar difference between lines was found (F2,43 = 10.182, p < 0.001). The luminance was higher in line B6JOla(hw5) compared to both lines B6JOla(nTg) (p = 0.0002) and B6JOla(hm239) (p = 0.0007). IHC in the striatum revealed the expression of syt 7 to be present in distal axons and large puncta some of which also contained hαSYN using mice from line B6JOla(hw5) (Fig. 10 E–F). However, only a minority of syt 7 labeled processes and terminals also expressed hαSYN, suggesting expression of syt 7 occurs in additional projection systems. In contrast, UCHL1 was expressed in both cell bodies, smaller ‘micropuncta’ on dendritic spines as previously reported (Cartier et al., 2009) and rare distal axons (Fig. 10 H–J). Co-localization with hαSYN or synaptophysin (not shown) and UCHL1was extremely rare occurring only in distal axons, suggesting that UCHL1 was not highly expressed in the pre-synaptic compartment.
Figure 9.
Upregulation of synaptotagmin 7 (syt7) and ubiquitin C-terminal hydrolase 1 (UCHL1) expression. Western blot analysis demonstrated a significant increase in the striatal level of syt7 (A,B) in line B6JOla(hw5) compared to both B6JOla(nTg) and B6JOla(hm239). A similar pattern was also seen for luminance measurements performed in the striatum using syt7 and fluorescence detection (C). Western blot analysis demonstrated a significant increase in the striatal level of UCHL1 (D) in line B6JOla(hw5) compared to B6JOla(nTg), but not B6JOla(hm239). Double immunofluorescence revealed syt7 (E, Red) to be rarely co-localized with hαSYN (F, green) in axonal processes and puncta (E–G). Double label IHC revealed UCHL1 (H, red) also to be rarely co-localized with hαSYN (I, green) in axonal processes, but not puncta (E–G). UCHL1 was located in axonal processes, neuronal cytoplasm, and micropuncta. Vertical bar values are the mean ± sem. 1 indicates significant difference between B6JOla(hw5) and B6JOla(nTg). 2 indicates significant difference between B6JOla(hw5) and B6JOla(hm239). Yellow arrows (E–G and H–J) indicate distal axonal processes in 3 orientations that co-localize for indicated proteins. Yellow box (E–G) encompasses a puncta. Scale bar indicates 3 µm (E–G) or 5 µm (H–J).
Discussion
There are several major findings related to these new congenic lines that can be gleaned from this report. (1) The hαSYN was functional in the nigrostriatal system in vivo when replacing the mαSYN. Other transgenic lines expressing hαSYN have been reported, but none have reported these biochemical consequences for the transgene. (2) Post hoc testing revealed that in the absence of mαSYN, line B6JOla(nTg) showed significant decreases in DOPAC, HVA, 5HT and 5HIAA, with a trend for a decrease in DA levels compared to the B6J(nTg) line. (3) When mαSYN was replaced by hαSYN the levels of different catecholamines and metabolites were significantly elevated. (4) The striatal neurochemical consequences suggest that the effect of hαSYN exceeded that of mαSYN based on their protein levels in striatum. (5) The level of the protein TH was significantly elevated by the presence of hαSYN whereas the level of the DAT was not, consistent with the change in the level of DA. (6) These changes differed from findings in vitro, where a decrease in the level of those proteins were found, and demonstrate the insights gained from examining consequences in vivo. (7) The level of a presynaptic protein (syt 7) and a pre- and postsynaptic protein (UCHL1) were both increased by the presence of wild type hαSYN, but not the mutated form in the absence of mαSYN. (8) hwαSYN has functional domains that may be differentially altered point mutations.
Mouse models related to αSYN
There are several mouse lines absent in endogenous mαSYN created by either knocking out the gene (Abelovich et al., 2000; Cabin et al., 2002; Dauer et al., 2002; Schlüter et al., 2003) or a spontaneous deletion as used here (Specht & Schoepfer, 2001; Chen et al., 2002) and by others(Schlüter et al., 2003). Others have also examined the role of αSYN and other proteins in null mice(Robertson et al., 2004; Al-Wandi et al., 2008). Despite these, and other transgenic lines, and a large body of in vitro experiments, a consensus for the functions of αSYN remains elusive although roles in synaptic function are evident. The mouse lines absent in mαSYN may have disparate features. For example, sensitivity to the neurotoxicant MPTP varied in three of the lines from “striking resistance” (Dauer et al., 2002)) to “partly protected” in a dose dependent manner (Schlüter et al., 2003) to “no protection” (Schlüter et al., 2003). Two of the three KO lines demonstrated a decrease in striatal DA (Abelovich et al., 2000; Dauer et al., 2002). Unfortunately, the papers reporting these lines often described differing outcomes, used different methods, used different background strains, or reported results with hybrid rather than inbred strains. One model has replaced the endogenous mαSYN with a human mutated transgene (Cabin et al., 2005). This line was also hybrid, homozygous for the A53T human transgene, and was under control of the mouse prion promoter. No nigrostriatal or other measures were reported so no comparison could be made with the other lines absent in mαSYN.
An additional line of transgenic mice containing the same double mutation reported by us was recently published (Ikeda et al., 2009). This line was reported to have many similarities to our descriptions including alterations in DA, abnormal neurites, and locomotor abnormalities. This line was reported to have novel features of eosinophilic intracellular inclusions, the presence of phosphorylated and nitrated αSYN, and ubiquitin labeled structures. Abnormalities were identified in the substantia nigra and dentate gyrus.
Catecholamines and DA-ergic proteins
Both congenic lines (B6JOla(hw5) and B6JOla(hm239)) expressed hαSYN which was functional within the nigrostriatal pathway, as demonstrated by alterations in the levels of striatal catecholamines and the protein TH compared to the control line (B6JOla(nTg) absent in mαSYN. The decrease in catecholamines in the line B6JOla(nTg) compared to B6J(nTg) is consistent with a mild decrease described in other reports (Abelovich et al., 2000; Dauer et al., 2002; Al-Wandi et al., 2008), as well as their restoration by the human transgenes. The nonsignificant decline in DA found in the absence of mouse αSYN might be at the expense of lower levels of the DA metabolites DOPAC and HVA. The low level of hαSYN protein (compared to the endogenous mαSYN) may suggest a threshold effect. The dramatic increase in all catecholamines in congenic lines containing the transgene suggests both human forms are functional in this role and the human protein may be more potent than the mouse form (see below). The level of striatal catecholamines was not elevated in line B6JOla(hm239) to the extent it was in line B6JOla(hw5) suggesting that the mutated protein may not be as effective as the wild type protein in this effect. This consequence of the hαSYN transgenes was supported by the elevation in the level of striatal TH (but not the DAT). αSYN has been shown to interact with both of these proteins directly or indirectly (Lee et al., 2001; Yu et al., 2004), but in general resulted in the down regulation of these dopaminergic markers in vitro (for reviews see (Perez et al., 2002; Sidhu et al., 2004b). The difference in the direction of change between results reported here and those reported in the literature are most probably due to differences between in vivo versus in vitro methods and/or the presence of different proteins in cells in vitro versus striatal terminals in vivo (Mosharov et al., 2006). The most important interpretation is that human α-synuclein (at relevant levels) is functional in these terminals.
HαSYN protein level, distribution, and form
Measurement of the level of both human and mouse αSYN is valuable in the interpretation of findings. . The striatal protein level of human αSYN was much lower than the level of endogenous mouse αSYN, yet was capable of having functional effects. The origin of human or mouse αSYN in the striatum cannot be determined as several inputs contribute. Transgenic mRNA was detected in substantia nigra, hippocampus, and cerebral cortex as reported previously. Comparisons between the mRNA expression levels for hαSYN and mαSYN may be necessary for definitive determination. In the absence of mαSYN, the expression of hαSYN was reduced significantly in hemibrain, but less in the striatum. These effects may relate to the use of the TH promoter and regional levels of expression.
The level of expression of the transgenic hαSYN in the B6JOla background was lower (10–36% of mouse) than the level of endogenous mαSYN (B6J). The lower level may be due to the promoter used, its integration site, or may relate to the background strain (B6JOla). The results shown here indicate that hαSYN, particularly when mutated, has the tendency to be mislocalized to a non-synaptic compartment (neuritic) and that this phenomenon was accentuated by the absence of mαSYN as originally reported by others (Cabin et al., 2005). This feature of the human protein expressed in mice has been observed before (Kahle, 2000; Kahle et al., 2000; Kahle & Haass, 2001). Interestingly, hαSYN – positive neurons and neurites observed in B6JOla(hm239) mice lacking endogenous mαSYN appeared very similar to those found in PD/DLB tissues. Despite this potential adverse effect of hm2αSYN, neuronal loss was not identified in the SNpc or VTA of male mice at 9 months of age. Mice of these lines will need to be aged for clarity. Alternatively, the background absence of mαSYN may be protective of neuronal loss related to hm2αSYN yet still be associated with cellular displacement. The consequences of the morphological alterations in other regions of the brain over time are not yet known, but may be addressed by a variety of methods in other laboratories. The distribution of hαSYN agrees with that reported by others in the SNpc and VTA. We have previously described staining in those neurons in the cytoplasm, axons, neurites, synapse, and nucleus, avoiding the nucleolus, which agrees with recent findings in the rat (Yu et al., 2007). Expression of αSYN has also been reported in various layers of the hippocampus (Yu et al., 2007).
Striatal synaptic function
A variety of studies have implicated a role for αSYN in synaptic function (Clayton & George, 1998; 1999; Murphy et al., 2000; Cabin et al., 2002; Chandra et al., 2004; Sidhu et al., 2004b; Yavich et al., 2004; Al-Wandi et al., 2008). Data from these lines support both a direct role for αSYN acting in a presynaptic role via regulation of DA, TH, and syt7, but perhaps also an indirect postsynaptic role via UCHL1. Of note, mutations in αSYN may differentially affect these roles. Subtle biochemical changes may result, but their implications in neurodegeneration or substance abuse remain unclear and important for investigation.
Functional domains
These findings suggest that hαSYN has diverse synaptic functions and those functions may be related to different domains on the protein that can be altered by mutations{Payton, 2004 #10272} {Taymans, #10271} {Kim, 2002 #10270} {Perrin, 2000 #10269}. Despite their similar levels, the hm2SYN protein did not increase DA levels in the striatum to the level that the hwαSYN protein did. Also, the hm2SYN protein had a greater tendency to mislocalize to the neuritic compartment and form aggregates compared to the hwαSYN protein. Finally, hm2SYN was not able to elevate the level of two striatal proteins as was the wild type form. Thus, hαSYN, having different domains of function (Cookson, 2005; Sen & West, 2009), might have certain functions altered by mutations involved in the PDP while other functions may not be altered.
Implications for neurodegenerative diseases
Historically, αSYN gained great prominence as the first gene identified in familial Parkinson’s disease (fPD), although the gene had been previously identified (Polymeropoulos et al., 1997), for review see (McKusick, 2009). HαSYN has been implicated in the PDP, but also in other neurodegenerative diseases with aggregation or abnormal distribution/structure of hαSYN (including diffuse LB disease, multiple system atrophy, and others) (Spillantini & Goedert, 2000; Galvin et al., 2001). The mechanism by which wild-type and/or mutated hαSYN contribute to neurodegenerative disease remains poorly understood, although the connection has been frequently demonstrated. A role for aggregation leading to axonal and neuritic pathology has been identified and resembles the pathology seen in the line containing hm2αSYN (Wakabayashi et al., 1998; Galvin et al., 1999). Mutated forms of hαSYN may act by influencing the threshold for protein fibrillation or haploinsufficiency (Eriksen et al., 2003; Kobayashi et al., 2003). However, the number of patients with any form of inherited abnormality in hαSYN is small and the implications for the wild-type form as a risk factor are still unknown. These risks are based on the level of expression in rare families with duplication or triplication of the gene and in sporadic PD (sPD) due to hαSYN location in Lewy bodies (LBs) and Lewy neurites (LNs), and polymorphisms in the noncoding portions of the gene (Polymeropoulos et al., 1997; Spillantini et al., 1997; Eriksen et al., 2003; Miller et al., 2004; Maraganore et al., 2006; Halliday & McCann, 2007; Li et al., 2007; Miller et al., 2007). It is possible that wild-type hαSYN participates in the pathogenesis or consequences of these complex trait diseases due to interactions with other risk factors. Understanding the detailed mechanism of hαSYN function in contributing to the pathogenesis, risk assessment, and possible interventions of these disorders will be critical and benefit from these congenic lines since many environmental factors can be assessed over the life span of the mouse.
Implications for substance abuse disorders
An additional role for hαSYN has come into play recently as a risk factor for a variety of substance abuse disorders, including those associated with alcohol, cocaine, morphine, and methamphetamine (Heath et al., 1997; Liang et al., 2003; Mash et al., 2003; Bonsch et al., 2004; Kobayashi et al., 2004; Bonsch et al., 2005a; Bonsch et al., 2005b; Bonsch et al., 2005c; Qin et al., 2005; Ziolkowska et al., 2005; Halladay et al., 2006; Liang & Carr, 2006; Mauceli et al., 2006; Walker & Grant, 2006; Boyer & Dreyer, 2007; Clarimon et al., 2007; Foroud et al., 2007; Mash et al., 2008). The evidence for a link with αSYN is probably strongest for alcohol based on the number of publications and the strength of both human and animal data (Liang et al., 2003; Bonsch et al., 2004; Bonsch et al., 2005a; Bonsch et al., 2005b; Bonsch et al., 2005c; Liang & Carr, 2006; Walker & Grant, 2006; Clarimon et al., 2007; Foroud et al., 2007; Ziolkowska et al., 2008). Alcohol and αSYN have been linked to alcohol craving (Bonsch et al., 2004; Bonsch et al., 2005a; Foroud et al., 2007), dependence (Bonsch et al., 2005b; Clarimon et al., 2007), preference (Liang et al., 2003), and consumption/alcoholism (Bonsch et al., 2004; Bonsch et al., 2005c) in general. Alcohol studies include human (Bonsch et al., 2004; Bonsch et al., 2005a; Bonsch et al., 2005b; Bonsch et al., 2005c; Clarimon et al., 2007; Foroud et al., 2007) or rodent (Liang et al., 2003; Liang & Carr, 2006; Walker & Grant, 2006; Ziolkowska et al., 2008) subjects using genetic (Liang et al., 2003; Bonsch et al., 2005b; Bonsch et al., 2005c; Clarimon et al., 2007) or tissue-based outcomes (Bonsch et al., 2004; Bonsch et al., 2005a; Liang & Carr, 2006; Walker & Grant, 2006; Ziolkowska et al., 2008). Associations between αSYN and other substances of abuse including cocaine (Mash et al., 2003; Qin et al., 2005; Boyer & Dreyer, 2007; Mash et al., 2008), methamphetamine (Kobayashi et al., 2004; Mauceli et al., 2006), and morphine (Ziolkowska et al., 2005) exist. It seems likely that αSYN acts as a risk factor for the predisposition to or in concert with other risks, perhaps dictating the particular substance used. A compelling link between αSYN and the dopaminergic system exists based on the association between αSYN and substances directly tied to DA, including cocaine (Mash et al., 2003; Qin et al., 2005; Boyer & Dreyer, 2007; Mash et al., 2008) and methamphetamine (Kobayashi et al., 2004; Mauceli et al., 2006). Thus, the link between αSYN and substance abuse may be as strong as its link with the sporadic PDP and its associated disorders. The relationship between hαSYN and catecholamine levels in the striatum of mice may be a mechanism whereby hαSYN may act as risk factor for these disorders. Elevation of hαSYN in the ventral tegmental area may be risk factor for these disorders acting via DA in the ventral striatum. Thus, these mouse lines may serve as a useful model for understanding and intervening in a variety of substance abuse paradigms.
The interaction between αSYN and synaptic function seems most parsimonious with current data and is a potential mechanism for these diseases. Extensive evidence suggests that major functions of αSYN are related to interactions with neurotransmitter vesicles ((Murphy et al., 2000; Cabin et al., 2002), neurotransmitter transporters including DA (Sidhu et al., 2004a) Qin, 2005 #8756}, norepinephrine (Wersinger et al., 2006a), serotonin (Wersinger et al., 2006b), DA neurotransmitter synthesis (Perez et al., 2002), presynaptic DA recruitment (Yavich et al., 2004), and catecholamine concentration (Mosharov et al., 2006) among others. Many reviews on the biochemistry and function of αSYN exist (El-Agnaf & Irvine, 2000; Rajagopalan & Andersen, 2001; Kahle et al., 2002). Most data relates to in vitro methods and validation of these functions and interactions would greatly benefit from in vivo data using the congenic lines described here.
These new congenic mouse lines demonstrate a variety of features suggesting they will be useful for understanding the role of hαSYN (wild type and mutated) in vivo, particularly as it relates to its function within the DAergic nigrostriatal pathway. The absence of endogenous mαSYN allows for a more specific evaluation of the consequences of different forms or amounts of hαSYN. The ability to perform these evaluations over the duration of the lifespan of a mouse will be particularly useful. Finally, the ability to obtain consistent, defined regional and cellular expression at physiological levels using mice will assist in experimental design and sample size limitations.
Supplementary Material
Supplemental Figure. Fluorescence-based qRT-PCR amplification plots used for genotyping. All mice were tested in duplicate using adjacent wells. A. Mouse genomic NMDA NR1 receptor amplification plot for ten mice (5 hαSYN Tg+, 5 nTg) using the VIC detector, demonstrating all the mice express the NMDA NR1 receptor, each well contains a similar amount of DNA, and crosses the CT at about the same cycle. B. Transgenic hαSYN amplification plot using the same ten mice as A and the FAM detector, demonstrating the difference between hαSYN Tg+ and nTg mice in the same screen. Five mice express hαSYN and cross CT, whereas 5 did not. C. Mouse genomic NMDA NR1 receptor amplification plot for two mice demonstrating similar amounts of genomic DNA used per well and corresponding to the same mice used in D. D. The mouse with the lower CT pair was a homozygote with two alleles of hαSYN compared to the hemizygote mouse with one allele and the higher CT. CT values are normalized to the CT values for the NMDA receptor gene using the ΔΔCT method described in the text. E. Transgenic hαSYN SNP amplification plot for a single compound hemizygote mouse with one hm2αSYN allele and one hwαSYN allele. The hwαSYN transgene was detected in the VIC channel (higher CT) whereas the hm2αSYN was detected in the FAM channel (lower CT). F. Mouse αSYN amplification plot for multiple B6JOla mice using the FAM detector. Only the B6J(nTg) positive control mouse demonstrated amplification and curve crosses a threshold, whereas all B6JOla mice failed to cross a threshold and demonstrated the spontaneous deletion.
Acknowledgements
Support for P. O.-S. was received from an NIEHS center grant (P30ES005022). We thank Jason R. Richardson for performing HPLC on samples at EOHSI. We thank Ruth N. Motter and Pearl Tanaka (Élan Pharmaceuticals, Inc.) for the ELISA measurements, assistance with the interpretation of data, and technical training. We thank Wagner Zago (Élan Pharmaceuticals Inc.) for guidance on confocal microscopy. We’d also like to thank Dr. Mladen-Roko Rasin for the use of his confocal microscope and Victoria DiBona for training.
Abbreviations
- αSYN
α-synuclein
- DA
dopamine
- DAT
dopamine transporter
- FCtx
frontal cortex
- hαSYN
human α-synuclein
- hm2αSYN
human doubly mutated αSYN
- hwαSYN
human wild type αSYN
- IHC
immunohistochemistry
- mαSYN
mouse α-synuclein
- NMDA
N-methyl-D-aspartate
- PD
Parkinson’s disease
- PDP
Parkinson’s disease phenotype
- PFA
paraformaldehyde
- SNpc
substantia nigra pars compacta
- STR
striatum
- TH
tyrosine hydroxylase
- VM
ventral midbrain
- VTA
ventral tegmental area
References
- SAS System for Windows, version 9.1.3. North Carolina: SAS Institute Inc, Cary; 2004. [Google Scholar]
- Abelovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo P, Shiksky N, Verdugo JMC, Armanin M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking a-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- Al-Wandi A, Ninkina N, Millership S, Williamson SJ, Jones PA, Buchman VL. Absence of alpha-synuclein affects dopamine metabolism and synaptic markers in the striatum of aging mice. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JP, Walker DE, Goldstein JM, de Laat R, Banducci K, Caccavello RJ, Barbour R, Huang J, Kling K, Lee M, Diep L, Keim PS, Shen X, Chataway T, Schlossmacher MG, Seubert P, Schenk D, Sinha S, Gai WP, Chilcote TJ. Phosphorylation of Ser-129 Is the Dominant Pathological Modification of {alpha}-Synuclein in Familial and Sporadic Lewy Body Disease. J. Biol. Chem. 2006;281:29739–29752. doi: 10.1074/jbc.M600933200. [DOI] [PubMed] [Google Scholar]
- Bonsch D, Greifenberg V, Bayerlein K, Biermann T, Reulbach U, Hillermacher T, Kornhuber J, Bleich S. alpha-Synuclein protein levels are increased in alcoholic patients are are linked to craving. Alcoholism: Clinical and Experimental Research. 2005a;29:763–765. doi: 10.1097/01.alc.0000164360.43907.24. [DOI] [PubMed] [Google Scholar]
- Bonsch D, Lederer T, Reulbach U, Hothorn T, Kornhuber J, Bleich S. Joint analysis of the NACP-REP1 marker within the alpha-Synuclein gene concludes association with alcohol dependence. Human Molecular Genetics. 2005b;14:967–971. doi: 10.1093/hmg/ddi090. [DOI] [PubMed] [Google Scholar]
- Bonsch D, Lenz B, Kornhuber J, Bleich S. DNA hypermethylation of the alpha synuclein promoter in patients with alcoholism. Molecular Neuroscience. 2005c;16:167–170. doi: 10.1097/00001756-200502080-00020. [DOI] [PubMed] [Google Scholar]
- Bonsch D, Reulbach U, Bayerlein K, Hillemacher T, Kornhuber J, Bleich S. Elevated alpha-Synuclein mRNA levels are associated with craving in patients with alcoholism. Biol Psychiatry. 2004;56:984–986. doi: 10.1016/j.biopsych.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Boyer F, Dreyer J. alpha-Synuclein in the nucleus accumbens induces changes in cocaine behaviour in rats. European Journal of Neuroscience. 2007;26:2764–2776. doi: 10.1111/j.1460-9568.2007.05878.x. [DOI] [PubMed] [Google Scholar]
- Cabin DE, Gispert-Sanchex G, Murphy D, Auburger G, Myers RR, Nussbaum RL. Exacerbated synucleinopathy in mice expressing A53T SNCA on a SNCA null background. Neurobiology of Aging. 2005;26:25–35. doi: 10.1016/j.neurobiolaging.2004.02.026. [DOI] [PubMed] [Google Scholar]
- Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, McLlwain K, Orrison B, Chen A, Ellis CE, Paylor R, Lu B, Nussbaum RL. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking a-synuclein. Journal of Neuroscience Methods. 2002;22:9797–8807. doi: 10.1523/JNEUROSCI.22-20-08797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier AE, Djakovic SN, Salehi A, Wilson SM, Masliah E, Patrick GN. Regulation of synaptic structure by ubiquitin C-terminal hydrolase L1. J Neurosci. 2009;29:7857–7868. doi: 10.1523/JNEUROSCI.1817-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Fornai F, Kwon HB, Yazdani U, Atasoy D, Liu X, Hammer RE, et al. Double-knockout mice for µ- and b-synucleins: effect on synaptic functions. 2004:14966–14971. doi: 10.1073/pnas.0406283101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Thiruchelvam MJ, Madura K, Richfield EK. Proteasome dysfunction in aged human α-synuclein transgenic mice. Neurobiology of Disease. 2006 doi: 10.1016/j.nbd.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Chen SE, Specht CG, Morris RG, Schoepfer R. Spatial leaning is unaltered in mice containing a deletion of the apha-synuclein locus. Eur. J. Neuroscience. 2002;16:154–158. doi: 10.1046/j.1460-9568.2002.02062.x. [DOI] [PubMed] [Google Scholar]
- Clarimon J, Gray RR, Williams LN, Enoch M, Robin RW, Albaugh B, Singleton A. Linkage disequilibrium and association analysis of alpha-synuclein and alcohol and drug dependence in two American Indian populations. Alcoholism: Clinical and Experimental Research. 2007;31:546–554. doi: 10.1111/j.1530-0277.2007.00338.x. [DOI] [PubMed] [Google Scholar]
- Clayton DF, George JM. The synucleins: a family of proteins involved in synaptic function, plasticity, neurodegeneration and disease. Trends in Neurosciences. 1998;21:249–254. doi: 10.1016/s0166-2236(97)01213-7. [DOI] [PubMed] [Google Scholar]
- Clayton DF, George JM. Synucleins in synaptic plasticity and neurodegenerative disorders. Journal of Neuroscience Research. 1999;58:120–129. [PubMed] [Google Scholar]
- Cookson MR. The biochemistry of Parkinson's disease. Annu Rev Biochem. 2005;74:29–52. doi: 10.1146/annurev.biochem.74.082803.133400. [DOI] [PubMed] [Google Scholar]
- Dauer W, Kholodilov N, et al. Resistance of alpha-synuclein null mice to the parkinsonian neurotoxin mptp. 2002:14524–14529. doi: 10.1073/pnas.172514599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Agnaf OMA, Irvine GB. Review: formation and properties of amyloid-like fibrils derived from a-synuclein and related proteins. 2000:300–309. doi: 10.1006/jsbi.2000.4262. [DOI] [PubMed] [Google Scholar]
- Eriksen JL, Dawson TM, Dickson DW, Petrucelli L. Caught in the Act: alpha-synuclein is the culprit in Parkinson's disease. 2003:453–456. doi: 10.1016/s0896-6273(03)00684-6. [DOI] [PubMed] [Google Scholar]
- Findlay JW, Dillard RF. Appropriate calibration curve fitting in ligand binding assays. AAPS J. 2007;9:E260–E267. doi: 10.1208/aapsj0902029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foroud T, Wetherill LF, Liang T, Dick DM, Hesselbrock V, Kramer J, Nurnberger J, et al. Association of alcohol craving with alpha-synuclein (SNCA) Alcholism: Clinical and Experimental Research. 2007;31:537–545. doi: 10.1111/j.1530-0277.2007.00337.x. [DOI] [PubMed] [Google Scholar]
- Fortin DL, Nemani VM, Voglmaier SM, Anthony MD, Ryan TA, Edwards RH. Neural activity controls the synaptic accumulation of a-synuclein. 2005:10913–10921. doi: 10.1523/JNEUROSCI.2922-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin JE, Lee VMY, Tronjanowski JQ. Synucleinopathies. 2001:186–190. doi: 10.1001/archneur.58.2.186. [DOI] [PubMed] [Google Scholar]
- Galvin JE, Uryu K, Lee VML, Trojanowski JQ. Axon pathology in Parkinson’s disease and Lewy body dementia hippocampus contains a-, b-, and g-synuclein. PNAS. 1999;96:13450–13455. doi: 10.1073/pnas.96.23.13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halladay AK, Wagner GC, Sekowski A, Rothman RB, Baumann MH, Fisher H. Alterations in alcohol consumption, withdrawal seizures, and monoamine transmission in rats treated with phentermine and 5-hydroxy-L-tryptophan. Synapse. 2006;59:277–289. doi: 10.1002/syn.20239. [DOI] [PubMed] [Google Scholar]
- Halliday GM, McCann H. Human-based studies on α-synuclein deposition and relationship to Parkinson's disease symptoms. Exp. Neurol. 2007 doi: 10.1016/j.expneurol.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Heath AC, Bucholz KK, Madden PAF, Dinwiddie SH, Slutske WS, Bierut LJ, Statham DJ. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychological Medicine. 1997;27:1381–1396. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- Holzmann C, Krüger R, Vieira Saecker AMM, Schmitt I, Schöls L, et al. Polymorphisms of the alpha-synuclein promoter: expression analyses and association studies in Parkinson's disease. 2003:67–76. doi: 10.1007/s00702-002-0769-5. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Kawarabayashi T, Harigaya Y, Sasaki A, Yamada S, Matsubara E, Murakami T, Tanaka Y, Kurata T, Wuhua X, Ueda K, Kuribara H, Ikarashi Y, Nakazato Y, Okamoto K, Abe K, Shoji M. Motor impairment and aberrant production of neurochemicals in human alpha-synuclein A30P+A53T transgenic mice with alpha-synuclein pathology. Brain Res. 2009;1250:232–241. doi: 10.1016/j.brainres.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Nurmi E, Bergman J, Eskola O, Solin O, et al. Personality traits and brain dopaminergic function in Parkinson's disease. PNAS. 2001;98:13272–13277. doi: 10.1073/pnas.231313198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle PJ, Haass C. The emerging utility of animal models of chronic neurodegenerative diseases. Expert Opin Ther Targets. 2001;5:125–132. doi: 10.1517/14728222.5.1.125. [DOI] [PubMed] [Google Scholar]
- Kahle PJ, Haass C, Kretzschmar HA, Neumann M. Structure/function of alpha-synuclein in health and disease: rational development of animal models for Parkinson's and related diseases. J Neurochem. 2002;82:449–457. doi: 10.1046/j.1471-4159.2002.01020.x. [DOI] [PubMed] [Google Scholar]
- Kahle PJ, Neumann M, Ozmen L, Muller V, Jacobsen H, Schindzielorz A, Okochi M, Leimer U, van der Putten H, Probst A, Kremmer E, Kretzschmar HA, Haass C. Subcellular Localization of Wild-Type and Parkinson's Disease-Associated Mutant alpha -Synuclein in Human and Transgenic Mouse Brain. J. Neurosci. 2000;20:6365–6373. doi: 10.1523/JNEUROSCI.20-17-06365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle PJ, Neumann M, Ozman L, Hass C. Physiology and Pathophysiology of α-Synuclein: Cell Culture and Transgenic Animal Models Based on a Parkinson's Disease-associated Protein. Annals of the New York Academy of Sciences. 2000;920:33–41. doi: 10.1111/j.1749-6632.2000.tb06902.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Ide S, Hasegawa J, Ujike H, Sekine Y, Ozaki N, Inada T, et al. Study of association between alpha-synuclein gene polymorphism and methamphetamine psychosis/dependence. Ann. N.Y. Acad. Sci. 2004;1025:325–334. doi: 10.1196/annals.1316.040. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Kruger R, Markopoulou K, Wszolek Z, Chase B, Taka H, Mineki R. Haploinsufficiency at the alpha-synuclein gene underlies phenotypic severity in familial Parkinson's disease. Brain. 2003;126:32–42. doi: 10.1093/brain/awg010. [DOI] [PubMed] [Google Scholar]
- Lee FJS, Liu F, Pristupa ZB, Niznik HB. Direct binding and functional coupling of a-synuclein to the dopamine transporters accelerate dopamine-induced apoptosis. The FASEB Journal. 2001;15:916–926. doi: 10.1096/fj.00-0334com. [DOI] [PubMed] [Google Scholar]
- Li Q, Mok SS, Laughton KM, McLean CA, Cappai R, Masters CL, Culvenor JG. Plasma alpha-synuclein is decreased in subjects with Parkinson's disease. Experimental Neurology. 2007;204:583–588. doi: 10.1016/j.expneurol.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Liang T, Carr LG. Regulation of alpha-synuclein expression in alcohol-preferring and non-preferring rats. Journal of Neurochemistry. 2006;99:470–482. doi: 10.1111/j.1471-4159.2006.04111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang T, Spence J, Liu L, Strother WN, Chang HW, Ellison JA, Lumeng L. alpha-Synuclein maps to a quantitative trait locus for alcohol preference and is differentially expressed in alcohol-preferring and non-preferring rats. PNAS. 2003;100:4690–4695. doi: 10.1073/pnas.0737182100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraganore DM, Andrade M, Elbaz A, Farrer MJ, Ioannidis JP, Kruger R, Rocca WA. Collaborative analysis of alpha-synuclein gene promoter variability and Parkinson disease. JAMA. 2006;296:661–670. doi: 10.1001/jama.296.6.661. [DOI] [PubMed] [Google Scholar]
- Mash DC, Adi N, Duque L, Pablo J, Kumar M, Ervin FR. Alpha-synuclein protein levels are increased in serum from recently abstinent cocaine abusers. Drug and Alcohol Dependence. 2008;94:246–250. doi: 10.1016/j.drugalcdep.2007.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mash DC, Ouyang Q, Pablo J, Basile M, Izenwasser S, Lieberman A, Perrin RJ. Cocaine abusers have an overexpression of alpha-Synuclein in dopamine neurons. Journal of Neuroscience. 2003;23:2564–2571. doi: 10.1523/JNEUROSCI.23-07-02564.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauceli G, Busceti CI, Pellegrini A, Soldani P, Lenzi P, Paparelli A, Fornai F. Overexpression of alpha-synuclein following methamphetamine: Is it good or bad? Ann. N.Y. Acad. Sci. 2006;1074:191–197. doi: 10.1196/annals.1369.019. [DOI] [PubMed] [Google Scholar]
- McKusick VA. OMIM, Online Mendelian Inheritance in Man. 2009 doi: 10.1002/(SICI)1098-1004(200001)15:1<57::AID-HUMU12>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Miller DW, Hague SM, Clarimon J, Baptista M, Gwinn-Hardy K, Cookson MR, Singleton AB. alpha-synuclein in blood and brain from familial Parkinson disease with SNCA locus triplication. Neurology. 2004;62:1835–1838. doi: 10.1212/01.wnl.0000127517.33208.f4. [DOI] [PubMed] [Google Scholar]
- Miller RM, Kiser GL, Kaysser-Kranich T, Casaceli C, Colla E, Lee MK, Palaniappan C, Federoff HJ. Wild-type and mutant alpha-synuclein induce a multi-component gene expression profile consistent with shared pathophysiology in different transgenic mouse models of PD. Exp Neurol. 2007;204:421–432. doi: 10.1016/j.expneurol.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Mosharov EV, Staal RGW, Bove J, Prou D, Hananiya A, Markov D, Poulsen N, Larsen K, Moore CMH, Troyer MD, Edwards RH, et al. alpha-Synuclein overexpression increases cytosolic catecholamine concentration. Journal of Neuroscience. 2006;26:9304–9311. doi: 10.1523/JNEUROSCI.0519-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DD, Rueter SM, Trojanowski JQ, Lee VM. Synucleins are developmentally expressed, and alpha-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J Neurosci. 2000;20:3214–3220. doi: 10.1523/JNEUROSCI.20-09-03214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksman M, Tanila H, Yavich L. Brain reward in the absence of alpha-synuclein. Molecular Neuroscience. 2006;17:1191–1194. doi: 10.1097/01.wnr.0000230507.70843.51. [DOI] [PubMed] [Google Scholar]
- Paulson GW, Dadmehr N. Is there a premorbid personality typical for Parkinson's disease? Neurology. 1991;41:73–76. doi: 10.1212/wnl.41.5_suppl_2.73. [DOI] [PubMed] [Google Scholar]
- Perez RG, Waymire JC, Lin E, Liu JJ, Guo F, Zigmond MJ. A role for alpha-synuclein in the regulation of dopamine biosynthesis. J Neurosci. 2002;22:3090–3099. doi: 10.1523/JNEUROSCI.22-08-03090.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou APT, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha -Synuclein Gene Identified in Families with Parkinson's Disease. Science. 1997;5321:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Prasad K, Richfield EK. Sporadic midbrain dopamine neuron abnormalities in laboratory mice. Neurobiol Dis. 2008;32:262–272. doi: 10.1016/j.nbd.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Qin Y, Ouyang Q, Pablo J, Mash DC. Cocaine abuse elevates alpha-synuclein and dopamine transporter levels in the human striatum. Molecular Neuroscience. 2005;16:1489–1493. doi: 10.1097/01.wnr.0000175617.39054.ba. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Andersen JK. Alpha synuclein aggregation: is it the toxic gain of function responsible for neurodegeneration in Parkinson's disease? Mech Ageing Dev. 2001;122:1499–1510. doi: 10.1016/s0047-6374(01)00283-4. [DOI] [PubMed] [Google Scholar]
- Richardson JR, Caudle WM, Wang M, Dean ED, Pennell KD, Miller GW. Developmental exposure to the pesticide dieldrin alters the dopamine system and increases neurotoxicity in an animal model of Parkinson’s disease. FASEB Journal. 2006;20:1695–1697. doi: 10.1096/fj.06-5864fje. [DOI] [PubMed] [Google Scholar]
- Richfield EK, Cory-Slechta DA, Thiruchelvam MJ, Wuertzer CA, Gainetdinov R, Caron M, Di Monte DA, Federoff HJ. Behavioral and neurochemical effects of wild-type and mutated human a-synuclein in transgenic mice. Exp. Neurol. 2002a;175:35–48. doi: 10.1006/exnr.2002.7882. [DOI] [PubMed] [Google Scholar]
- Richfield EK, Thiruchelvam MJ, Cory-Slechta DA, Wuertzer CA, Gainetdinov RA, Caron MG, Di Monte DA, Federoff HJ. Behavioral and neurochemical effects of wild-type and mutated human a-synuclein in transgenic mice. Exp. Neurol. 2002b;175:35–48. doi: 10.1006/exnr.2002.7882. [DOI] [PubMed] [Google Scholar]
- Robertson DC, Schmidt O, Ninkina N, Jones PA, Sharkey J, Buchman VL. Developmental loss and resistance to MPTP toxicity of dopaminergic neurones in substantia nigra pars compacta of gamma-synuclein, alpha-synuclein and double alpha/gamma-synuclein null mutant mice. J Neurochem. 2004;89:1126–1136. doi: 10.1111/j.1471-4159.2004.02378.x. [DOI] [PubMed] [Google Scholar]
- Schlüter OM, Forna F, Alessandríe MG, Takamoric S, Geppertb M, Jahn R, Südhof TC. Role of a-synuclein in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism in mice. Neuroscience. 2003;118:985–1002. doi: 10.1016/s0306-4522(03)00036-8. [DOI] [PubMed] [Google Scholar]
- Sen S, West AB. The Therapeutic Potential of LRRK2 and Alpha-synuclein in Parkinson's Disease. Antioxid Redox Signal. 2009 doi: 10.1089/ars.2009.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu A, Wersinger C, Vernier P. alpha-Synuclein regulation of the dopaminergic transporter: a possible role in the pathogenesis of Parkinson's disease. FEBS Lett. 2004a;565:1–5. doi: 10.1016/j.febslet.2004.03.063. [DOI] [PubMed] [Google Scholar]
- Sidhu A, Wersinger C, Vernier P. Does alpha-synuclein modulate dopaminergic synaptic content and tone at the synapse? FASEB J. 2004b;18:637–647. doi: 10.1096/fj.03-1112rev. [DOI] [PubMed] [Google Scholar]
- Specht CG, Schoepfer R. Deletion of the alpha-synuclein locus in a subpopulation of C57BL/6J inbred mice. BMC Neuroscience. 2001;2:11. doi: 10.1186/1471-2202-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Goedert M. The alpha-synucleinopathies: Parkinson's disease, dementia with Lewy bodies, and multiple system atrophy. Ann N Y Acad Sci. 2000;920:16–27. doi: 10.1111/j.1749-6632.2000.tb06900.x. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Tan EK, Tan C, Shen H, Chai A, Lum SY, Teoh ML, Yih Y, Wong MC, Zhao Y. Alpha synuclein promoter and risk of Parkinson's disease: microsatellite and allelic size variability. Neurosci Lett. 2003;336:70–72. doi: 10.1016/s0304-3940(02)01178-3. [DOI] [PubMed] [Google Scholar]
- Thiruchelvam M, Powers J, Cory-Slechta DA, Richfield EK. Risk factors for dopaminergic neuron loss in human α-synuclein transgenic mice. Eur. J. of Neuroscience. 2004;19:845–854. doi: 10.1111/j.0953-816x.2004.03139.x. [DOI] [PubMed] [Google Scholar]
- Vartianinen S, Pehkonen P, Lakso M, Nass R, Wong G. Identification of gene expression changes in transgenic C. elegans overexpressing human a-synuclein. 2006 doi: 10.1016/j.nbd.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Hayashi S, Kakita A, Yamada M, Toyoshima Y, Yoshimoto M, Takahashi H. Accumulation of ?-synuclein/NACP is a cytopathological feature common to Lewy body disease and multiple system atrophy. Acta Neuropathologica. 1998;36:445–452. doi: 10.1007/s004010050918. [DOI] [PubMed] [Google Scholar]
- Walker SJ, Grant KA. Peripheral blood alpha-synuclein mRNA levels are elevated in cynomolgus monkeys that chronically self-administer ethanol. Alcohol. 2006;38:1–4. doi: 10.1016/j.alcohol.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Wersinger C, Jeannotte A, Sidhu A. Attenuation of the norepinephrine transporter activity and trafficking via interactions with alpha-synuclein. European Journal of Neuroscience. 2006a;24:3141–3152. doi: 10.1111/j.1460-9568.2006.05181.x. [DOI] [PubMed] [Google Scholar]
- Wersinger C, Rusnak M, Sidhu A. Modulation of the trafficking of the human serotonin transporter by human alpha-synuclein. European Journal of Neuroscience. 2206b;24:55–64. doi: 10.1111/j.1460-9568.2006.04900.x. [DOI] [PubMed] [Google Scholar]
- Yavich L, Tanila H, Vepsalainen S, Jakala P. Role of alpha-Synuclein in presynaptic dopamine recruitment. Journal of Neuroscience. 2004;24:11165–11170. doi: 10.1523/JNEUROSCI.2559-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Lia X, Liua G, Hana J, Zhangb C, Lia Y, Xua S, Liuc C, Gaoc Y, Yangb H, Uédaa K, Chan P. Extensive nuclear localization of a-synuclein in normal rat brain neurons revealed by a novel monoclonal antibody. Neuroscience. 2007;145:539–555. doi: 10.1016/j.neuroscience.2006.12.028. [DOI] [PubMed] [Google Scholar]
- Yu S, Yuo X, Li Y, Zhang C, Zhou M, Zhang YA, Ueda K, Chan P. Inhibition of tyrosine hydroxylase expression in a-synuclein-transfected dopaminergic neuronal cells. Neuroscience Letters. 2004;67:34–39. doi: 10.1016/j.neulet.2004.05.118. [DOI] [PubMed] [Google Scholar]
- Zaghloul KA, Blanco JA, Weidemann CT, McGill K, Jaggi JL, Baltuch GH, Kahana MJ. Human substantia nigra neurons encode unexpected financial rewards. Science. 2009;323:1496–1499. doi: 10.1126/science.1167342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziolkowska B, Gieryk A, Bilecki W, Wawrzczak-Bargiela A, Wedzony K, Chocyk A, Danielson PE, Thomas EA, Hilbush BS, Sutcliffe JG, et al. Regulation of alpha-Synuclein expression in limbic and motor brain regions of morphine-treated mice. Journal of Neuroscience. 2005;25:4996–5003. doi: 10.1523/JNEUROSCI.4376-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziolkowska B, Gieryk A, Wawrzczak-Bargiela A, Krowka T, Kaminska D, Korkosz A, Bienkowski P. alpha-Synuclein expression in the brain and blood during abstinence from chronic alcohol drinking in mice. Neuropharmacology. 2008;54:1239–1246. doi: 10.1016/j.neuropharm.2008.04.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure. Fluorescence-based qRT-PCR amplification plots used for genotyping. All mice were tested in duplicate using adjacent wells. A. Mouse genomic NMDA NR1 receptor amplification plot for ten mice (5 hαSYN Tg+, 5 nTg) using the VIC detector, demonstrating all the mice express the NMDA NR1 receptor, each well contains a similar amount of DNA, and crosses the CT at about the same cycle. B. Transgenic hαSYN amplification plot using the same ten mice as A and the FAM detector, demonstrating the difference between hαSYN Tg+ and nTg mice in the same screen. Five mice express hαSYN and cross CT, whereas 5 did not. C. Mouse genomic NMDA NR1 receptor amplification plot for two mice demonstrating similar amounts of genomic DNA used per well and corresponding to the same mice used in D. D. The mouse with the lower CT pair was a homozygote with two alleles of hαSYN compared to the hemizygote mouse with one allele and the higher CT. CT values are normalized to the CT values for the NMDA receptor gene using the ΔΔCT method described in the text. E. Transgenic hαSYN SNP amplification plot for a single compound hemizygote mouse with one hm2αSYN allele and one hwαSYN allele. The hwαSYN transgene was detected in the VIC channel (higher CT) whereas the hm2αSYN was detected in the FAM channel (lower CT). F. Mouse αSYN amplification plot for multiple B6JOla mice using the FAM detector. Only the B6J(nTg) positive control mouse demonstrated amplification and curve crosses a threshold, whereas all B6JOla mice failed to cross a threshold and demonstrated the spontaneous deletion.