Abstract
Meta-cognitive strategy training may be used to augment inpatient rehabilitation to promote active engagement and subsequent benefit for individuals with cognitive impairments after stroke. We examined the feasibility of administering a form of meta-cognitive strategy training, Cognitive Orientation to daily Occupational Performance, during inpatient rehabilitation. We trained an individual with cognitive impairments after right hemisphere stroke to identify performance problems, set self-selected goals, develop plans to address goals, and evaluate performance improvements. To assess feasibility, we examined the number of meta-cognitive training sessions attended, the number of self-selected goals, and changes in goal-related performance. We also examined changes in rehabilitation engagement and disability. The participant used the meta-cognitive strategy to set 8 goals addressing physically-oriented, instrumental, and work-related activities. Mean improvement in Canadian Occupational Performance Measure Performance Scale scores was 6.1. Pittsburgh Rehabilitation Participation Scale scores (measuring rehabilitation engagement) improved from 3.2 at admission to 4.9 at discharge. Functional Independence Measure scores (measuring disability) improved from 68 at admission, to 97 at discharge. Performance Assessment of Self-care Skills scores improved from 1.1 at admission to 2.9 at discharge. The results indicate that meta-cognitive strategy training was feasible during inpatient rehabilitation and warrants further evaluation to determine its effectiveness.
Keywords: Strategy Training, Cognition, Rehabilitation, Stroke, Brain Injury
Approximately one-third to one-half of individuals demonstrate cognitive impairments within the first 3 months after stroke (Patel, Coshall, Rudd, & Wolfe, 2003; Pohjasvaara et al., 2002; Serrano, Domingo, Rodriguez-Garcia, & del Ser, 2007; Tatemichi et al., 1994). These individuals can benefit from rehabilitation, but do not benefit as much as individuals without cognitive impairments (Rabadi, Rabadi, Edelstein, & Peterson, 2008). In fact, cognitive impairments immediately after stroke are one of the most robust predictors of several negative outcomes, including prolonged hospitalization, institutionalization, chronic disability in activities of daily living (ADL) and poor quality of life (Patel et al, 2003; Heruti et al., 2002; Nys et al., 2007).
Cognitive impairments may directly facilitate disability because they impede the ability to perform basic and complex ADLs. Cognitive impairments may also indirectly facilitate disability by prohibiting full engagement in and benefit from rehabilitation (Skidmore et al., 2010). Hence, individuals with impairment in various cognitive domains, by the very nature of their impairment, may have difficulty attending to, recalling, and assimilating information. They may also have difficulty consistently following through with rehabilitation recommendations. As a result of this poor “engagement” and “follow-through,” individuals with cognitive impairments may be left with greater ADL disability than individuals without cognitive impairments. If rehabilitation practitioners modified our approach to help individuals with cognitive impairments engage in and benefit from rehabilitation programs, we may be able to reduce ADL disability among these individuals.
Meta-cognitive strategy training shows promise as a class of interventions that may help individuals with cognitive impairments actively engage in, and even “steer” their rehabilitation programs (Kennedy et al., 2008; McEwen, Polatajko, Davis, Huijbregts, & Ryan, 2010). This training teaches individuals a meta-cognitive strategy that can be used to increase awareness of impaired skills or processes (through self-assessment and self-monitoring), develop goals and plans to address areas of disability, and improve their ability to perform desired activities (thus reducing disability). Previous studies have reported improved awareness of cognitive impairments, improved ability to verbally articulate and implement the meta-cognitive strategy, reductions in performance errors and inappropriate behaviors during laboratory tasks, and improved performance of skilled behaviors among individuals with chronic traumatic brain injury and stroke (Cicerone, 2002; Dawson et al., 2009; McEwen, Polatajko, Huijbregts, & Ryan, 2010; Von Cramon, Matthes-Von Cramon, & Mai, 1991; Webster & Scott, 1983). Recent evidence from 3 case studies suggests that these cognitive strategies may be effective in helping individuals with cognitive impairments after traumatic brain injury reduce disability in self-selected activities in their home and community (Dawson et al., 2009). Therefore, meta-cognitive strategy training is a promising approach for individuals with cognitive impairments (Cicerone et al., 2005; Geusgens et al., 2007).
While the evidence supporting the effectiveness of meta-cognitive strategy training for individuals with chronic stroke and brain injury is encouraging, no studies have examined this approach in individuals with cognitive impairments early in the acute phase of recovery (i.e., during inpatient rehabilitation). Providing meta-cognitive strategy training during the acute phase may be optimal given the evidence that supports the benefits of early intervention to augment natural recovery occurring during this phase (Ashley & Persel, 1999; Wood, McCrea, Wood, & Merriam, 1999; Duncan et al., 2005; Ottawa Panel, 2006). However, there may be barriers to providing such training to individuals with cognitive impairments during this critical time in recovery. Individuals may be too impaired, fatigued, or over-whelmed to engage in and apply meta-cognitive strategy training during inpatient rehabilitation. Further, previously tested meta-cognitive strategy training programs may require adjustments in dosing (more frequent sessions in a more concentrated period of time) to be implemented in inpatient rehabilitation, and we do not know whether it is feasibile to offer meta-cognitive strategy training with this intensity in individuals with cognitive impairments after acute stroke.
Therefore, the purpose of the present report is to examine the feasibility of administering meta-cognitive strategy training to an individual with cognitive impairments who was simultaneously engaged in inpatient rehabilitation after acute stroke. Specifically, we were interested in determining whether an individual with cognitive impairments after acute stroke would tolerate daily meta-cognitive strategy training sessions provided in addition to usual inpatient rehabilitation training sessions, and be able to learn and apply the meta-cognitive strategy during the inpatient rehabilitation length of stay. A secondary purpose was to describe rehabilitation engagement and ADL disability in the individual who participated in the meta-cognitive strategy training.
Methods
Participant
All procedures were approved by the University Institutional Review Board. We recruited the study participant from the inpatient stroke rehabilitation service at an academic health center. To qualify for the study, the participant had to meet the following criteria: 1) diagnosis of acute stroke, 2) impairment in executive functions [as indicated by score of 11 or greater on the Executive Interview (Royall, Mahurin, & Gray, 1992), a gross measure of several domains of executive function], and 3) able to provide written informed consent. The participant also had to demonstrate the absence of 1) pre-existing disabling neurological conditions, 2) pre-existing cognitive impairments, 3) current significant immediate memory impairment (as indicated by a score greater than 2 standard deviations below age-corrected scores on the first 3 trials of the Hopkins Verbal Learning Test-Revised; Shapiro, Benedict, Schretlen, & Brandt, 1999), 4) current severe aphasia (as indicated by a score of 2 or lower on the Boston Diagnostic Aphasia Examination, 3rd Edition; Goodglass, Kaplan, & Barressi, 2001), 5) current major depressive, bipolar or psychotic disorder (as measured by the PRIME-MD (Spitzer et al., 1999) and the Mini-International Neuropsychiatric Interview (Sheehan, 1998), and 6) active alcohol or substance abuse within the past 6 months.
The participant we recruited was a 31 year old college-educated European American male who sustained a mild to moderately severe embolic stroke. MRI findings revealed an embolic “shower” in the right posterior cerebral artery territory and a focal infarct in the right pons. Prior to this stroke, the participant was a healthy and active vocational youth pastor with an otherwise unremarkable medical history. On rehabilitation admission, the participant was 7 days post stroke and demonstrated mild impairments in attention and severe impairments in visuospatial function (visual attention and visuospatial relations) and delayed memory (Table 1). He demonstrated no impairments in immediate memory or language. Despite the participant's demonstration of impairment in executive functions as measured by the Executive Interview (score=11), the participant demonstrated very mild impairments in set-shifting and inhibition as measured by the DKEFS subtests. He demonstrated poor awareness of deficits (i.e., signficantly over-estimating his abilities to complete self-care and mobility tasks), but did not demonstrate visual or body neglect. Despite the poor awareness of deficits, we considered the participant to be a good candidate for the intervention given his ability to verbalize and demonstrate an understanding of the intervention process. The participant demonstrated moderate impairments in postural control, and severe motor impairment in his non-dominant left arm and hand, leg and foot. In addition, he reported a mild level of depressive symptoms and minimal apathy.
Table 1.
Clinical Assessment Findings in the Study Participant
| Baseline | Discharge | |
|---|---|---|
| National Institutes of Health Stroke Scale | 7 | 3 |
| Repeatable Battery of Neuropsychological Status* | ||
| Attention Index Score | 79 | |
| Visuospatial/Constructional Index Score | 62 | |
| Language Index Score | 112 | |
| Immediate Memory Index Score | 112 | |
| Delayed Memory Index Score | 52 | |
| Delis-Kaplan Executive Function System* | ||
| Trail Making, Condition 4 Scaled Score | 9 | |
| Trail Making, Condition 5 Scaled Score | 12 | |
| Color-Word Interference, Inhibition Scaled Score | 13 | |
| Color-Word Interference, Inhibition/Switching Scaled Score | 11 | |
| Inhibition/Switching vs. Inhibition Contrast Score | 8 | |
| Chedoke-McMaster Assessment | ||
| Posture Score | 4 | 5 |
| Arm Score | 1 | 4 |
| Hand Score | 1 | 4 |
| Leg Score | 2 | 5 |
| Foot Score | 1 | 5 |
| Hamilton Rating Scale for Depression, Total Score | 13 | 6 |
| Apathy Evaluation Scale, Total Score | 26 | 28 |
| Pittsburgh Rehabilitation Participation Scale, Mean Score | 3.2 | 4.9 |
| Functional Independence Measure, Total Score | 68 | 97 |
| Performance Assessment of Self-care Skills, Mean Score | 1.1 | 2.9 |
These tools were not re-administered at rehabilitation discharge.
Intervention
We utilized the Cognitive Orientation to daily Occupational Performance (CO-OP) intervention approach as the framework for our meta-cognitive strategy training (Dawson et al., 2009; McEwen et al., 2010; Polatajko & Mandich, 2004). The hallmark of the CO-OP approach is its emphasis on “client-driven” processes where the focus is on the clients identifying problems, setting goals, problem solving adaptations, and evaluating their own performance. Thus, individuals acquire a process that not only helps them identify, set, and address their own goals, but also provides the skills to facilitate self-monitoring and self-directed learning during rehabilitation despite their cognitive impairments. Briefly, the CO-OP approach contains 5 active ingredients: self-selected activity-based goals, dynamic performance analysis, meta-cognitive strategy training, guided discovery, and caregiver training (Figure 1).
Figure 1.
Cognitive Orientation to daily Occupational Performance (CO-OP)
We administered the CO-OP intervention in one 45 minute session per day, 5 days per week, for the length of the inpatient rehabilitation stay (14 days). The daily CO-OP session was in addition to the participant's usual inpatient rehabilitation program (5–6 hours of occupational, physical and speech therapy per weekday, and 2–3 hours of therapy on Saturdays). An occupational therapist with expertise in stroke rehabilitation intervention, who was also trained in the delivery of the CO-OP intervention, delivered all CO-OP intervention sessions.
The first CO-OP session was provided on the first full day of inpatient rehabilitation, and focused on introducing the meta-cognitive strategy and identifying initial goals. The therapist introduced the CO-OP meta-cognitive strategy (Goal-Plan-Do-Check) using workbook materials created for the present study, based on previously standardized materials (Polatajko & Mandich, 2004). To facilitate goal identification, the therapist used the procedure outlined by the Canadian Occupational Performance Measure (COPM; Law et al., 1998). Briefly this process involved asking the participant to describe a typical day prior to the onset of rehabilitation, identify activities that were difficult for him, and then rate the importance of these activities (COPM Importance Scale: 10=extremely important, 1= not important at all). The participant was then asked to prioritize these activities and identify 3 precisely defined activity-based goals that he would like to address in the course of his inpatient rehabilitation program. The first 3 goals identified by the participant were 1) Concentrate on praying for 30 minutes every day, 2) Complete one set of 10 modified push-ups using both arms, and 3) Make a peanut butter and jelly sandwich without assistance (he usually packed lunches for his children).
After identifying 3 goals, the participant rated his performance on each of the 3 activities (COPM Performance Scale: 10=able to do it very well, 1=not able to do it). In addition, the participant used the COPM Performance Scale to set a performance-related goal for each of the 3 activities. Next, the participant applied the meta-cognitive strategy to address the first of the 3 goals using workbook materials. The therapist assisted the participant in this process by using dynamic performance analysis. Specifically, she asked the participant to identify the steps necessary to complete the activity, and the areas where “breakdowns” in performance occurred. By the end of the first session, the participant had a specific goal and plan for addressing his first activity-based goal the next day.
The second CO-OP session began with a review of the CO-OP meta-cognitive strategy, and the application of the meta-cognitive strategy to the first goal. The participant reviewed the goal and the plan, described the execution of the plan (“do”), and evaluated the execution of the plan (“check). Through guided discovery, the participant identified those aspects of the plan that “worked” and those that “didn't work” and made changes to the plan to improve performance of the activity to meet his goal. After each execution of the plan, the participant rated his performance using the COPM Performance Scale, producing iterative scores for each activity.
The remaining CO-OP sessions repeated this process, reviewing performance of identified activities, and applying the meta-cognitive strategy to improve performance. After the second session, the participant focused on 2 goals per session. Once the initial 3 goals met the participant's criteria using the COPM Performance Scale (established a priori), the participant identified and addressed 5 additional goals during his inpatient rehabilitation length of stay. The therapist used guided discovery to facilitate the participant's ability to review his goals and related plans, assess whether the plans worked, and determine whether modifications to the plan were necessary. This process was aided by workbook materials that asked leading questions to facilitate the participant's own problem solving skills (i.e., What was the goal? What was the plan? Did you do the plan? Did the plan work? Does the plan need modification? What modifications will you use?). At the end of each session, the therapist encouraged the participant to summarize the content of the session and to describe the plan going forward. This process continued until the end of the inpatient rehabilitation program.
The participant's spouse participated in these first 3 sessions, observing the dynamic performance analysis process, and demonstrating the ability to learn and use guided discovery skills modeled by the therapist.
Measures
Descriptive measures
We used several measures, all of which have well-established psychometric properties, to describe the type and severity of impairments sustained after stroke. To characterize the extent of stroke severity, we used the National Institutes of Health Stroke Scale (NIHSS; Brott et al., 1989). The NIHSS assesses the type and severity of neurological impairment in levels of consciousness, eye movement, strength, coordination, vision, sensation, articulation, and language.
To characterize cognitive function, we used the Repeatable Battery Assessment of Neuropsychological Status (RBANS; Randolph, Teirney, Mohr, & Chase, 1998) and selected subtests of the Delis-Kaplan Executive Functioning System (DKEFS; Delis, Kaplan, & Kramer, 2001). The RBANS measures several cognitive domains, including attention, immediate memory, delayed memory, visuospatial perception, and language. The DKEFS assesses several domains of executive function. For the present study, we used scores from selected DKEFS subtests: the Trail Making Test and the Color-Word Interference Test. Due to the short interval between rehabilitation admission and rehabilitation discharge, we did not anticipate substantive changes on the RBANS and the DKEFS. Thus, we made an a priori decision not to repeat administration of the RBANS and DKEFS at rehabilitation discharge.
To describe the range and severity of motor impairment, we used 5 scales from the Chedoke McMaster Assessment Impairment Inventory (Gowland et al., 1993). The Chedoke-McMaster Assessment Impairment Inventory classifies the stage of motor recovery after stroke using 7-point ordinal scales that address postural control, arm, hand, leg, and foot impairment. To describe mood and motivational status, we used the Hamilton Rating Scale for Depression (Hamilton, 1960) and the Apathy Evaluation Scale (Marin, Biedrzycki, & Firinciogullari, 1991). The Hamilton Rating Scale for Depression is a structured interview that rates 21 symptoms and severity of depression with a 3 – 5 point ordinal scale. The Apathy Evaluation Scale rates 18 symptoms of apathy (i.e., lack of interest in activities or social participation) on a 4-point ordinal scale.
Outcome measures
We measured rehabilitation engagement during each therapy session throughout the inpatient rehabilitation length of stay using the Pittsburgh Rehabilitation and Participation Scale (Lenze et al., 2004). This scale is a valid and reliable scale that requires rehabilitation practitioners to characterize a participant's active engagement during a given rehabilitation session. Scores are assigned based on the proportion of prescribed activities in which participants actively participated, given levels of interest, effort, direction following, and completion. Each session is scored 1 (no engagement, refusal) to 6 (excellent engagement). Scores were generated by members of the clinical team (i.e., occupational therapy, physical therapy, and speech and language pathology) who were trained in using the Pittsburgh Rehabilitation and Participation Scale. We examined average daily scores (i.e., averaged across all sessions each day; range 1 – 6) to describe changes in rehabilitation engagement over the length of stay.
We measured ADL disability at rehabilitation admission and discharge using the Functional Independence Measure (FIM; Stineman et al., 1996) and the Performance Assessment of Self-care Skills (PASS; Holm & Rogers, 1999). The FIM is a valid and reliable performance observation tool designed to measure rehabilitation outcomes, and is the industry-standard for measuring ADL disability attributed to stroke. The FIM assesses 18 tasks in 6 functional domains (self-care, sphincter control, transfers, locomotion, communication and social cognition) using a scale of 1 (dependent) to 7 (independent) for each item. We examined the total FIM scores (range 7 – 126) collected at rehabilitation admission and discharge to describe changes is basic ADL disability.
The PASS is a valid and reliable performance observation tool that was developed by occupational therapy researchers at the University of Pittsburgh to evaluate a spectrum of activities deemed critical to independent living in the community. Compared to the FIM, which focuses on basic activities of daily living, the PASS also includes instrumental ADL, scoring observed performance on a 4-point scale from 0 (dependent) to 3 (independent). The PASS has been used in a variety of clinical populations, including stroke (Skidmore, Rogers, Chandler, & Holm, 2006; Skidmore, Rogers, Chandler, & Holm, 2007). We examined mean PASS scores (range 0 – 3) collected at rehabilitation admission and discharge.
Data Analyses
To assess the feasibility of administering meta-cognitive strategy training to a participant with cognitive impairments while engaged in inpatient rehabilitation we examined: 1) the number of training sessions attended by the participant during the inpatient rehabilitation length of stay, 2) the number activity-based goals set by the participant, and 3) change in performance of activities identified by the participant. To describe rehabilitation engagement and ADL disability in the participant receiving the meta-cognitive strategy training, we examined 1) change in therapist reported engagement in the rehabilitation process, and 2) change in measures of ADL disability.
Results
The participant's inpatient rehabilitation length of stay was 14 calendar days, with 11 therapy days (including 1 Saturday). Over the course of that time, the participant demonstrated reductions in overall neurological impairment, motor impairment, and depressive symptoms (Table 1). Levels of apathy remained relatively stable.
During the inpatient rehabilitation length of stay, the participant attended all 10 scheduled CO-OP sessions. In the course of these sessions, the participant identified 8 activity-based goals. The mean improvement in COPM Performance Scale scores for the 8 goals was 6.1 (out of a possible 10 points), and the participant met his own target performance quality rating for 4 of the 8 goals (Table 2).
Table 2.
Participant's Performance Scale Ratings for Self-Selected Goals and Performance
| Performance |
||||
|---|---|---|---|---|
| Activity | Goal Rating | Initial Rating | Final Rating | Goal Met |
| Praying | 10 | 1 | 10 | Yes |
| Completing Push-Ups | 8 | 1 | 8 | Yes |
| Making Lunches | 10 | 1 | 10 | Yes |
| Making Coffee | 10 | 5 | 10 | Yes |
| Manage Daily Schedule | 10 | 2 | 9 | No |
| Reading a Book Chapter | 10 | 7 | 8 | No |
| Play Football with Children | 10 | 1 | 8 | No |
| Complete Work-Related Calls | 10 | 4 | 8 | No |
|
| ||||
| Total Goals Met | 4/8 | |||
Note. Participant set goals and scored performance using the Canadian Occupational Performance Measure Performance Scale (10=able to do it very well; 1=not able to do it).
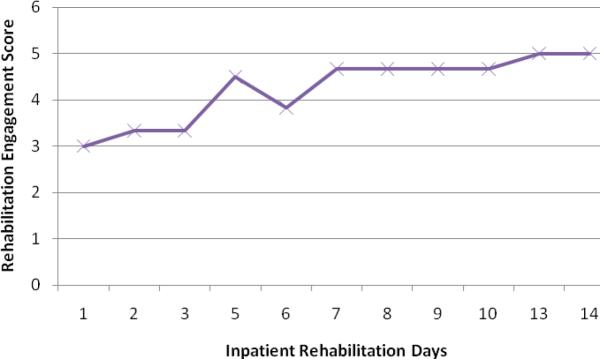
During the first 3 days of inpatient rehabilitation, the average rehabilitation engagement score was 3.2 (Figure 2), with a “3” indicating that the participant “engaged in most or all of activities, but did not provide maximal effort or finish most activities; required much encouragement.” During the last 3 days of inpatient rehabilitation, the average rehabilitation engagement score was 4.9, with a “5” indicating that the participant “engaged in all activities with maximal effort and finished all activities, but passively followed directions.” We consider this change clinically meaningful, suggesting that rehabilitation practitioners recognized more active engagement in the rehabilitation process on the part of the participant as inpatient rehabilitation progressed.
Figure 2.
Average Daily Rehabilitation Engagement Scores for Length of Stay
On rehabilitation admission, FIM and PASS testing indicated that the participant required moderate assistance with most basic ADL (FIM Total Score=68), and physical assistance to perform selected instrumental ADL tasks (PASS Mean Score=1.1; Table 1). At rehabilitation discharge, the participant required supervision or minimal assistance basic ADL (FIM Total Score=97) and only occasional verbal support to perform selected instrumental ADL tasks (PASS Mean Score=2.9). The changes in ADL disability on both measures were clinically meaningful (Beninato et al., 2006; Holm & Rogers, 1999).
Anecdotally, the participant, the inpatient rehabilitation team, and the participant's spouse all reported satisfaction with the meta-cognitive strategy training. Although we did not solicit feedback using formal qualitative methods, each entity provided the following statements at the conclusion of the program. Without prompting, the participant stated, “I like doing COOP because I get to be creative. I identify the activity, set the goal and come up with the plan. It comes from my head, and then I get to try [the activity].” When asked “Do you have any feedback for research team regarding the CO-OP program?” a member of the inpatient rehabilitation team stated, “I feel like I saw a change in [the participant]. At first it was hard to get him to focus on his therapy. And then, it seemed like he `took charge' asking me if he could practice certain activities [related to his CO-OP goals] during the therapy session.” When asked the same question, the participant's spouse stated, “He likes the [CO-OP] process. Early on it helped him feel better about himself, helping him take control of his life after his stroke. The [CO-OP] process has helped him resume many activities that are important to him.”
Discussion
To our knowledge, this is the first application of meta-cognitive strategy training during inpatient rehabilitation after acute stroke. Despite mild impairments in attention and executive functions, and severe impairments in visuospatial functional and delayed memory, the participant demonstrated the ability to learn and apply the meta-cognitive strategy to his daily activities. Thus, based on our experiences, the meta-cognitive strategy training appears to be feasible for implementation during inpatient rehabilitation.
In addition, the participant demonstrated clinically meaningful changes in rehabilitation engagement and ADL disability while receiving meta-cognitive strategy training. Although we cannot conclude from the present study that the meta-cognitive strategy training caused these changes, our observations in the present study suggest hypotheses that might be tested in future studies. For example, a previous study has demonstrated that the presence of cognitive impairments, particularly impairments in executive functions, is a strong predictor of poor rehabilitation engagement in usual inpatient rehabilitation (Skidmore et al., 2010). Through meta-cognitive strategy training we may be able to equip these individuals with tools to help them more actively engage in the rehabilitation process. By increasing the level of active engagement in inpatient rehabilitation, we may be providing individuals with cognitive impairments opportunities to enhance natural recovery and maximize reductions in ADL disability. These hypotheses warrant careful examination through future clinical trials.
While we cannot generalize our experiences to comment on the effects of the training on rehabilitation engagement or disability outcomes, we learned several things that can be used to improve the next implementation of this meta-cognitive strategy training. First, successful implementation of the meta-cognitive strategy training required careful coordination with the entire clinical rehabilitation team, including the attending physiatrist, nursing director and therapy team members. For example, the participant's plan to address the first goal required that he coordinate with his nurses and therapists to identify 30 minutes of uninterrupted time to pray. Second, the nature and scope of the activity-based goals was associated with the number of goals identified and addressed during inpatient rehabilitation. In the present study, the participant identified fairly simple activities, and set his goals to be easily achievable. For example, the participant's first goal was achieved within the first two sessions, and then the participant decided to move to a new goal. The next 2 goals were addressed over 6 sessions. Third, the type and number of goals seems to be less important than the repetitive practice applying the meta-cognitive strategy to each goal. The meta-cognitive strategy carried over into inpatient rehabilitation sessions, as evidenced by the participant telling the one of his inpatient therapists that he would like to set up an exercise program that he could work on in the evening by himself. This action was a byproduct of the meta-cognitive strategy training, in which the participant reportedly used the “goal-plan-do-check” process to develop and implement the program. Finally, the short turn around between CO-OP sessions seemed to produce opportunities to reinforce learning through repeated exposure to the meta-cognitive strategy training in a concentrated time period.
Our experiences with this single case study provided valuable insights into the feasibility of introducing meta-cognitive strategy training to individuals with cognitive impairments in inpatient rehabilitation after stroke. Our experiences also raised several questions to be considered in the design of future clinical trials. First, it remains to be seen whether the addition of meta-cognitive strategy training in inpatient rehabilitation adds benefit and reduces disability. An experimental study examining differences in rehabilitation engagement and ADL disability between individuals who receive the meta-cognitive strategy training and individuals who receive an attention control intervention may address this question. Second, while the participant reported that he continued to use the meta-cognitive strategy after inpatient rehabilitation, the benefit of this extended use is unknown. Long-term follow-up would help to address these questions. Based on our experiences with this single case, we designed a clinical study examining the efficacy of meta-cognitive strategy training, compared to an attention control intervention, for improving rehabilitation engagement and reducing ADL disability over time. Data collection for this study is currently underway.
In conclusion, we conducted a case study to examine the feasibility of implementing meta-cognitive strategy training in concert with inpatient rehabilitation after stroke. Our preliminary experiences suggest that this approach is feasible in this setting and warrants further study to determine whether it improves rehabilitation engagement and reduces disability among individuals with cognitive impairments after acute stroke.
References
- Ashley M, Persel C. Traumatic brain injury recovery rates in post-acute rehabilitation of traumatic brain injury: spontaneous recovery or treatment? Journal of Rehabilitation Outcomes and Measurement. 1999;3:15–21. [Google Scholar]
- Beninato M, Gill-Body KM, Salles S, Stark PC, Black-Schaffer RM, Stein J. Determination of the minimal clinically important difference in the FIM instrument in patients with stroke. Archives of Physical Medicine & Rehabilitation. 2006;87:32–39. doi: 10.1016/j.apmr.2005.08.130. [DOI] [PubMed] [Google Scholar]
- Brott T, Adams HP, Olinger CP, Marler JR, Barsan WG, Biller J, Hertzberg V. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- Cicerone KD. Remediation of `working attention' in mild traumatic brain injury. Brain Injury. 2002;16:185–195. doi: 10.1080/02699050110103959. [DOI] [PubMed] [Google Scholar]
- Cicerone K, Dahlberg C, Malec J, Langenbahn D, Felicetti T, Kneipp S, Catanese J. Evidence-based cognitive rehabilitation: updated review of the literature from 1998 through 2002. Archives of Physical Medicine and Rehabilitation. 2005;86:1681–1692. doi: 10.1016/j.apmr.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Dawson D, Gaya A, Hunt A, Levine B, Lemsky C, Polatajko H. Using the cognitive orientation to occupational performance (CO-OP) with adults with executive dysfunction following traumatic brain injury. Canadian Journal of Occupational Therapy. 2009;76:74–86. doi: 10.1177/000841740907600209. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan EF, Kramer JH. Delis-Kaplan Executive Function System (DKEFS) Harcourt Assessment Inc.; London: 2001. [Google Scholar]
- Duncan PW, Zorowitz R, Bates B, Choi JY, Glasberg JJ, Graham GD, Katz RC, Lamberty K, Reker D. Management of adult stroke rehabilitation care: a clinical practice guideline. Stroke. 2005;36:e100–143. doi: 10.1161/01.STR.0000180861.54180.FF. [DOI] [PubMed] [Google Scholar]
- Geusgens C, Winkens I, van Heugten C, Jolles J, van den Heuvel W. Occurrence and measurement of transfer in cognitive rehabilitation: a critical review. Journal of Rehabilitation Medicine. 2007;39:425–439. doi: 10.2340/16501977-0092. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E, Barressi B. The assessment of aphasia and related disorders. 3rd ed Lippincott Williams & Wilkins; Philadelphia, PA: 2001. [Google Scholar]
- Gowland C, Stratford P, Ward M, Moreland J, Torresin W, Van Hullenaar S, Plews N. Measuring physical impairment and disability with the Chedoke-McMaster Stroke Assessment. Stroke. 1993;24:58–63. doi: 10.1161/01.str.24.1.58. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heruti RJ, Lusky A, Danker R, Ring H, Dolgopiat M, Barell V, Adunsky A. Rehabilitation outcome of elderly patients after a first stroke: effect of cognitive status at admission on the functional outcome. Archives of Physical Medicine Rehabilitation. 2002;83:742–729. doi: 10.1053/apmr.2002.32739. [DOI] [PubMed] [Google Scholar]
- Holm MB, Rogers JC. Functional assessment: the Performance Assessment of Self-Care Skills (PASS) In: Hemphill BJ, Holm MB, Rogers JC, editors. Functional assessment: the Performance Assessment of Self-Care Skills (PASS), Assessments in occupational therapy mental health: an integrative approach. Slack, Inc; Thorofare, NJ: 1999. pp. 117–124. [Google Scholar]
- Kennedy MRT, Coelho C, Turkstra L, Ylvisaker M, Sohlberg MM, Yorkston K, Kan PF. Intervention for executive functions after traumatic brain injury: a systematic review, meta-analysis and clinical recommendations. Neuropsychological Rehabilitation. 2008;18:257–299. doi: 10.1080/09602010701748644. [DOI] [PubMed] [Google Scholar]
- Law M, Baptiste S, Carswell A, McColl MA, Polatajko H, Pollock N. Canadian Occupational Performance Measure. 2nd ed. Rev. CAOT Publications ACE; Ottawa, ON: 1998. [Google Scholar]
- Lenze EJ, Munin MC, Quear T, Dew MA, Rogers JC, Begley AE, Reynolds CF. The Pittsburgh Rehabilitation Participation Scale: reliability and validity of a clinician-rated measure of participation in acute rehabilitation. Archives of Physical Medicine & Rehabilitation. 2004;85:380–384. doi: 10.1016/j.apmr.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the apathy evaluation scale. Psychiatry Research. 1991;38:143–162. doi: 10.1016/0165-1781(91)90040-v. [DOI] [PubMed] [Google Scholar]
- McEwen SE, Polatajko HJ, Davis JA, Huijbregts MPH, Ryan JD. “There's a real plan here, and I am responsible for that plan”: participant experiences with a novel cognitive-based treatment approach for adults living with chronic stroke. Disability & Rehabilitation. 2010;32:541–550. doi: 10.3109/09638280903180189. [DOI] [PubMed] [Google Scholar]
- McEwen SE, Polatajko HJ, Huijbregts MPH, Ryan JD. Exploring a cognitive-based treatment approach to improve motor skill performance in chronic stroke: results of three single case experiments. Brain Injury. 2009;23:1041–1053. doi: 10.3109/02699050903421107. [DOI] [PubMed] [Google Scholar]
- Nys G, van Zandvoort M, de Kort P, Jansen B, de Haan E, Kappelle L. Cognitive disorders in acute stroke: prevalence and clinical determinants. Cerebrovascular Diseases. 2007;23:408–416. doi: 10.1159/000101464. [DOI] [PubMed] [Google Scholar]
- Ottawa Panel Evidence-based clinical practice guidelines for post-stroke rehabilitation. Topics in Stroke Rehabilitation. 2006;13:1–269. doi: 10.1310/3TKX-7XEC-2DTG-XQKH. [DOI] [PubMed] [Google Scholar]
- Patel M, Coshall C, Rudd AG, Wolfe CD. Natural history of cognitive impairment after stroke and factors associated with its recovery. Clinical Rehabilitation. 2003;17:158–166. doi: 10.1191/0269215503cr596oa. [DOI] [PubMed] [Google Scholar]
- Pohjasvaara T, Leskela M, Vataja R, Kalska H, Ylikoski R, Hietanen M. Post-stroke depression, executive dysfunction and functional outcome. European Journal of Neurology. 2002;9:269–275. doi: 10.1046/j.1468-1331.2002.00396.x. [DOI] [PubMed] [Google Scholar]
- Polatajko HJ, Mandich A. Enabling occupation in children: the Cognitive Orientation to daily Occupational Performance (CO-OP) approach. CAOT Publications; Ottawa, ON: 2004. [Google Scholar]
- Rabadi MH, Rabadi FM, Edelstein L, Peterson M. Cognitively impaired stroke patients do benefit from admission to an acute rehabilitation unit. Archives of Physical Medicine & Rehabilitation. 2008;89:441–448. doi: 10.1016/j.apmr.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): Preliminary clinical validity. Journal of Clinical Experimental Neuropsychology. 1998;20:310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- Royall DR, Mahurin RK, Gray KF. Bedside assessment of executive cognitive impairment: the executive interview. JAGS. 1992;40:1221–1226. doi: 10.1111/j.1532-5415.1992.tb03646.x. [DOI] [PubMed] [Google Scholar]
- Serrano S, Domingo J, Rodriguez-Garcia E, del Ser T. Frequency of cognitive impairment without dementia in patients with stroke: a two-year follow-up study. Stroke. 2007;38:105–110. doi: 10.1161/01.STR.0000251804.13102.c0. [DOI] [PubMed] [Google Scholar]
- Shapiro AM, Benedict RH, Schretlen D, Brandt J. Construct and concurrent validity of the Hopkins Verbal Learning Test-Revised. Clinical Neuropsychology. 1999;13:348–358. doi: 10.1076/clin.13.3.348.1749. [DOI] [PubMed] [Google Scholar]
- Sheehan DV. The Mini-International Neuropsychiatric Interview (M.I.N.I): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59:22–57. [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Kroenke K, Linzer M, deGruy FV, Hahn SR, Johnson JG. Utility of a new procedure for diagnosing mental disorders in primary care: the Prime-MD 1000 study. JAMA. 1994;272:1749–1756. [PubMed] [Google Scholar]
- Skidmore ER, Rogers JC, Chandler LS, Holm MB. Developing empirical models to enhance stroke rehabilitation. Disability and Rehabilitation. 2006;28:1027–1034. doi: 10.1080/09638280500494728. [DOI] [PubMed] [Google Scholar]
- Skidmore ER, Rogers JC, Chandler LS, Holm MB. Associations between neurobehavior and disability after stroke. Archives of Physical Medicine & Rehabilitation. 2007;88:e11–e12. [Google Scholar]
- Skidmore ER, Whyte EM, Holm MB, Becker JT, Butters MA, Dew MA, Lenze EJ. Cognitive and affective predictors of rehabilitation participation after stroke. Archives of Physical Medicine Rehabilitation. 2010;91:203–207. doi: 10.1016/j.apmr.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stineman MG, Shea JA, Jette A, Tassoni CJ, Ottenbacher KJ, Fiedler R, Granger CV. The Functional Independence Measure: tests of scaling assumptions, structure and reliability across 20 diverse impairment categories. Archives of Physical Medicine & Rehabilitation. 1996;77:1101–1108. doi: 10.1016/s0003-9993(96)90130-6. [DOI] [PubMed] [Google Scholar]
- Tatemichi TK, Desmond DW, Stern Y, Paik M, Sano M, Bagiella E. Cognitive impairment after stroke: frequency, patterns, and relationship to functional abilities. Journal of Neurology, Neurosurgery, and Psychiatry. 1994;57:202–207. doi: 10.1136/jnnp.57.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Cramon D, Matthes-von Cramon G, Mai N. Problem-solving deficits in brain-injured patients: a therapeutic approach. Neuropsychological Rehabilitation. 1991;1:45–64. [Google Scholar]
- Webster J, Scott R. The effects of self-instructional training on attentional deficits following head injury. Clinical Neuropsychology. 1983;5:69–74. [Google Scholar]
- Wood R, McCrea J, Wood L, Merriam R. Clinical and cost effectiveness of post-acute neurobehavioral rehabilitation. Brain Injury. 1999;13:69–88. doi: 10.1080/026990599121746. [DOI] [PubMed] [Google Scholar]