SUMMARY
Insulin-like growth factor (IGF) signaling is essential for achieving optimal body size during fetal development, whereas, in the adult, IGFs are associated with aging and age-related diseases. However, it is unclear as to what extent lifespan is influenced by events that occur during development. Here we provide direct evidence that the exceptional longevity of mice with altered IGF signaling is not linked to prenatal programming of body size. Mice null for pregnancy-associated plasma protein-A (PAPP-A), an IGF binding protein proteinase that increases local IGF bioavailability, are 60–70% the size of their wild-type littermates at birth and have extended median and maximum lifespan of 30–40%. In this study, PAPP-A−/− mice whose body size was normalized during fetal development through disruption of IgfII imprinting, did not lose their longevity advantage. Adult-specific moderation of IGF signaling through PAPP-A inhibition may present a unique opportunity to improve lifespan without affecting important aspects of early life physiology.
Keywords: pregnancy-associated plasma protein-A, insulin-like growth factor longevity, mouse model, body size, fetal development
Extended longevity is associated with small body size in several species of mice, dogs, and humans (Miller et al. 1999, Harper et al. 2004, Swindell et al. 2008, Patronek et al. 1997, Rollo 2002, Samaras et al. 2003). This negative correlation between body size and lifespan occurs as a consequence of the effect of insulin-like growth factor (IGF) signaling on these two traits via antagonistic pleiotropy, i.e., genes that are beneficial early in life can be detrimental later in life (Bartke 2008, Harper et al. 2006, Sutter et al. 2007, van Heemst et al. 2005, Bonafe et al. 2003, Suh et al. 2008, Williams 1957).
Thus, IGF signaling is essential for achieving optimal body size during fetal development, whereas, in the adult, IGFs are associated with aging and age-related diseases (Bartke 2008, D'Ercole 1996). However, it is unclear as to what extent lifespan is influenced by events that occur during development. Mice null for pregnancy-associated plasma protein-A (PAPP-A), an IGF binding protein proteinase that increases local IGF bioavailability, are 60–70% the size of their wild-type litter mates at birth and have extended median and maximum lifespan of 30–40% with diminished occurrence of age-related diseases (Conover & Bale 2007). The proportional dwarfism of PAPP-A−/− mice is due to the loss of enhanced bioavailability of IGF-II, the predominant fetal IGF, during a critical period in early embryogenesis (Conover et al. 2004) IgfII is an imprinted gene closely associated with a reciprocally imprinted downstream gene, H19. Mutation of H19 results in relaxation of IgfII imprinting and expression of IGF-II from both alleles. This mutation rescues the dwarf phenotype of PAPP-A−/− mice (Conover & Bale 2005). We took advantage of this model to determine whether PAPP-A−/− mice whose body size was normalized during fetal development would retain their longevity phenotype.
Characterization of the strains of mice used in this study, which carry targeted mutations in the Pappa and H19 loci, have been detailed in previous publications (Conover & Bale 2007, Conover et al. 2004, Conover & Bale 2005). Mice receiving the H19 mutation from the maternal allele (H19m−/+) have approximately two-fold increased levels of IGF-II in the embryo due to expression from both the maternal and paternal alleles, with consequent fetal overgrowth (Leighton et al. 1995). In contrast, paternal transmission of the mutation has no phenotypic consequences for the progeny. The Pappa gene is not imprinted, and both males and females with homozygous deletion (PAPP-A−/−) are 60–70% of the body weight of wild-type and heterozygous littermates (Conover et al. 2004). Accordingly, paternally-transmitted female H19 heterozygous mice were first crossed with PAPP-A−/− males. Of these offspring, females inheriting the H19 mutant allele and heterozygous for Pappa deletion were then mated to male PAPP-A−/− mice to generate embryos belonging to four genotypes designated as Control (H19+/+PAPP-A+/−), H19 mutant (H19m−/+PAPP-A+/−), PAPP-A knock-out (KO; H19+/+PAPP-A−/−), and double-mutant (H19m−/+PAPP-A−/−), the latter being the `rescue' of the dwarf phenotype. These studies were approved by the Institutional Animal Care and Use Committee of Mayo Clinic.
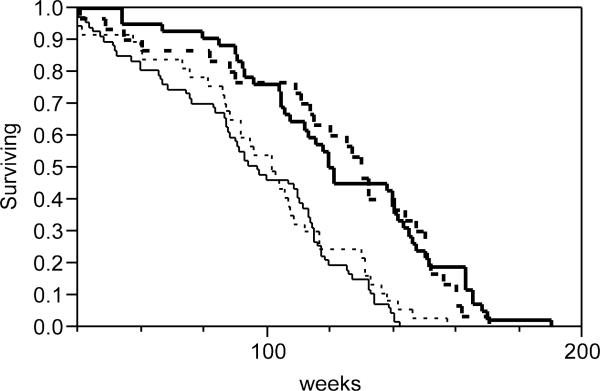
Survival data were collected on 176 Control, H19 mutant, PAPP-A KO and double-mutant mice that were housed in a specific pathogen-free barrier facility throughout their life (Fig. 1). Longevity was increased approximately 30% in PAPP-A KO mice compared to Control mice (P < 0.0001, log-rank test). Lifespan parameters for H19 mutant mice were similar to those of Control mice. Importantly, double-mutant mice with the rescued dwarf phenotype had significantly increased lifespan compared to Control mice (P < 0.0001). Median and maximum lifespan data for the four groups of mice are summarized in Table 1. For the double-mutant mice, median lifespan was increased 35% and maximum lifespan was increased 20%. Body weights of 6-month-old mice are presented in Table 2. Differences in adult weights reflected the birth weights of the four genotypes, reported previously (Conover et al. 2004). Thus, ΔH19 mutants were 20–30% larger and PAPP-A KO mice were 25–30% smaller than Control mice. The weights of double-mutant mice were not significantly different from Controls.
Figure 1.
Survival distribution of Control (thin solid line), H19 mutant (thin dashed line), PAPP-A KO (thick solid line) and double-mutant (thick dashed line) mice.
Table 1.
Longevity analyses.
| Lifespan (weeks) | |||
|---|---|---|---|
| N |
Median |
Maximuma |
|
| Control | 67 | 95 | 137 |
| H19 mutant | 37 | 98 (3%) | 144 (5%) |
| PAPP-A KO | 42 | 120 (26%) | 174 (27%) |
| Double-mutant | 30 | 128 (35%) | 164 (20%) |
Data, presented in weeks with % increase over Control given in parentheses, were calculated from the survival data in Figure 1 (see supplemental table).
Average age of last decile of surviving mice.
Table 2.
Body weights at 6 months
| Grams |
% of Control |
|
|---|---|---|
| Males | ||
| Control | 31.7 ± 1.26 | 100 |
| H19 mutant | 40.8 ± 3.20* | 129 |
| PAPP-A KO | 21.1 ± 1.64* | 67 |
| Double-mutant | 28.5 ± 0.89 | 90 |
| Females | ||
| Control | 26.9 ± 1.42 | 100 |
| H19 mutant | 32.8 ± 1.66* | 122 |
| PAPP-A KO | 20.2 ± 1.13* | 75 |
| Double-mutant | 25.0 ± 1.40 | 93 |
Data are presented as mean ± SEM of 9–11 mice.
P < 0.05 versus Control.
There have been several studies suggesting that early body weight can predict longevity in mice (Miller et al. 2002, Harper et al. 2004, Swindell et al. 2008). Furthermore, spontaneous and targeted disruption of IGF signaling has been associated with small size and increased lifespan in mice, dogs, and humans (Bartke 2008, Harper et al. 2006, Sutter et al. 2007, van Heemst et al. 2005, Bonafe et al. 2003, Suh et al. 2008). An understanding of this relationship is not only biologically important, it also has therapeutic implications. However, from these models it is difficult to distinguish primary effects of reduced IGF-I signaling during postnatal growth from possible consequences of the reduced body size programmed during fetal development. Thus, we bred for a new mouse model that allowed determination of the contribution of body size to lifespan. PAPP-A KO mice are born as proportional dwarfs and have an extended lifespan without secondary endocrine abnormalities, altered metabolism, or caloric restriction (Conover et al. 2004, Conover & Bale 2007, Conover et al. 2008). PAPP-A KO mice born normal-sized through disruption of IgfII imprinting and that were also without secondary endocrine abnormalities, altered metabolism, or caloric restriction (data not shown), lived 30–40% longer than Control mice or mice with the H19 mutation but without loss of PAPP-A. Thus, body size is not itself a critical determinant of lifespan regulation via reduced IGF-I signaling.
IGF-II is essential for optimal embryonic growth but does not affect postnatal growth in mice. H19 mutant mice, in spite of increased body size, had a similar lifespan to that of Controls. In agreement, Moerth et al. (2007). showed that elevated levels of IGF-II in the postnatal period did not rescue the body and skeletal growth defects in the absence of IGF-I. Together, these data indicate that reduced IGF-I signaling postnatally is a more important determinant of longevity than the reduced IGF-II signaling prenatally that affects body size.
PAPP-A is an attractive target for anti-aging strategies since it is a relatively accessible enzyme whose activity would be amenable to pharmacologic intervention. The findings of this study suggest the possibility of using PAPP-A inhibitors to extend lifespan and improve long-term health independent of many of the detrimental pleiotropic effects that alteration of IGF signaling can have on physiology.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by of the National Institutes of Health (RO1 AG0287141) and The Ellison Medical Foundation.
REFERENCES
- Bartke A. Impact of reduced insulin-like growth factor-1/insulin signaling on aging in mammals: novel findings. Aging Cell. 2008;7:285–290. doi: 10.1111/j.1474-9726.2008.00387.x. [DOI] [PubMed] [Google Scholar]
- Bonafe M, et al. Polymorphic variants of insulin-like growth factor I (IGF-I) receptor and phosphoinositide 3-kinase genes affect IGF-I plasma levels and human longevity: cues for an evolutionarily conserved mechanism of life span control. J. Clin. Endocrinol Metab. 2003;88:3299–3304. doi: 10.1210/jc.2002-021810. [DOI] [PubMed] [Google Scholar]
- Conover CA, et al. Metalloproteinase pregnancy-associated plasma protein A is a critical growth regulatory factor during fetal development. Development. 2004;131:1187–1194. doi: 10.1242/dev.00997. [DOI] [PubMed] [Google Scholar]
- Conover CA, Bale LK. Disruption of insulin-like growth factor-II imprinting during embryonic development rescues the dwarf phenotype of mice null for pregnancy-associated plasma protein-A. J. Endocrinol. 2005;186:325–331. doi: 10.1677/joe.1.06259. [DOI] [PubMed] [Google Scholar]
- Conover CA, Bale LK. Loss of pregnancy-associated plasma protein A extends lifespan in mice. Aging Cell. 2007;6:727–729. doi: 10.1111/j.1474-9726.2007.00328.x. [DOI] [PubMed] [Google Scholar]
- Conover CA, et al. Metabolic consequences of pregnancy-associated plasma protein-A deficiency in mice: exploring possible relationship to the longevity phenotype. J. Endocrinol. 2008;198:599–605. doi: 10.1677/JOE-08-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ercole AJ. Insulin-like growth factors and their receptors in growth. Endocrinol. Metab. Clinics N. Am. 1996;25:573–590. doi: 10.1016/s0889-8529(05)70341-8. [DOI] [PubMed] [Google Scholar]
- Harper JM, et al. Body weight, hormones and T cell subsets as predictors of life span in genetically heterogeneous mice. Mech. Ageing Dev. 2004;125:382–390. doi: 10.1016/j.mad.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Harper JM, et al. Genetic modulation of hormone levels and life span in hybrids between laboratory and wild-derived mice. J. Gerontol. Biol. Sci. 2006;61A:1019–1029. doi: 10.1093/gerona/61.10.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton PA, et al. Disruption of imprinting caused by deletion of the H19 gene region in mice. Nature. 1995;375:34–39. doi: 10.1038/375034a0. [DOI] [PubMed] [Google Scholar]
- Miller RA, et al. Big mice die young: early life body weight predicts longevity in genetically heterogeneous mice. Aging Cell. 2002;1:22–29. doi: 10.1046/j.1474-9728.2002.00006.x. [DOI] [PubMed] [Google Scholar]
- Moerth C, et al. Postnatally elevated levels of insulin-like growth factor (IGF)-II fail to rescue the dwarfism of IGF-I-deficient mice except kidney weight. Endocrinology. 2007;148:441–451. doi: 10.1210/en.2006-0385. [DOI] [PubMed] [Google Scholar]
- Patronek GJ, et al. Comparative longevity of pet dogs and humans: implications for gerontology research. J. Gerontol. A Biol. Sci. Med. Sci. 1997;52A:B171–B178. doi: 10.1093/gerona/52a.3.b171. [DOI] [PubMed] [Google Scholar]
- Rollo CD. Growth negatively impacts the life span of mammals. Evol. Dev. 2002;4:55–61. doi: 10.1046/j.1525-142x.2002.01053.x. [DOI] [PubMed] [Google Scholar]
- Samaras TT, et al. Is height related to longevity? Life Sci. 2003;72:1781–1802. doi: 10.1016/s0024-3205(02)02503-1. [DOI] [PubMed] [Google Scholar]
- Suh Y, et al. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc. Natl. Acad. Sci. USA. 2008;105:3438–3442. doi: 10.1073/pnas.0705467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter NB, et al. A single IGF1 allele is a major determinant of small size in dogs. Science. 2007;316:112–115. doi: 10.1126/science.1137045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindell WR, et al. How long will my mouse live? Machine learning approaches for prediction of mouse life span. J. Gerontol. Biol. Sci. 2008;63A:895–906. doi: 10.1093/gerona/63.9.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heemst D, et al. Reduced insulin/IGF-1 signalling and human longevity. Aging Cell. 2005;4:79–85. doi: 10.1111/j.1474-9728.2005.00148.x. [DOI] [PubMed] [Google Scholar]
- Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.