Abstract
Objectives
To estimate risk factors for premature infants not receiving antenatal steroids in a population-based cohort and to determine whether the gains of a quality improvement collaborative project on antenatal steroid administration were sustained long-term.
Methods
Clinical data for premature infants born in 2005-2007 were obtained from the California Perinatal Quality Care Collaborative, which collects data on more than 90% of neonatal admissions in California. Eligible infants had a birth weight of less than 1500 grams or gestational age less than 34 weeks born at a Collaborative hospital. These data were linked to administrative data from California Vital Statistics. Socio-demographic and medical risk factors for not receiving antenatal steroids were determined. We also examined the effect of birth hospital participation in a prior quality improvement collaborative project. A random effects logistic regression model was used to determine independent risk factors.
Results
Of 15,343 eligible infants, 23.1% did not receive antenatal steroids in 2005-2007. Hispanic mothers (25.6%), mothers younger than age 20 (27.6%), and those without prenatal care (52.2%) were less likely to receive antenatal steroids. Mothers giving birth vaginally (26.8%) and mothers with a diagnosis of “fetal distress” (26.5%) were also less likely to receive antenatal steroids. Rupture of membranes prior to delivery, and multiple gestations were associated with higher likelihood of antenatal steroid administration. Hospitals who participated in a quality improvement collaborative in 1999-2000 had higher rates of antenatal steroid administration (85% vs. 69%, p < 0.0001).
Conclusion
A number of eligible mothers do not receive antenatal steroids. Quality improvement initiatives to improve antenatal steroid administration could target specific high-risk groups.
Introduction
It has been known for more than 35 years that exposure to antenatal steroids (ANS) decreases the incidence of neonatal respiratory distress syndrome and other morbidities in premature infants.(1-2) Therefore, it is the recommendation of obstetric and pediatric societies to routinely administer ANS to pregnant women when delivery is expected prior to 34 weeks gestation.(3-5) Nonetheless, only 75-85% of eligible women receive ANS.(6-7)
A recent study of 790 infants from a single region in France identified risk factors for not receiving ANS.(6) These included medical factors such as preterm birth associated with maternal bleeding, as well as socio-demographic factors such as young maternal age. In their cohort of 790 premature neonates, 19.4% did not receive ANS.(6) A similar population based study has not been performed in the United States. Furthermore, the impact of socio-demographic factors may be different in a system with universal health care coverage and potential relevant factors in the United States, such as prenatal care and payment source, have yet to be investigated.(6)
Our objective was to estimate risk factors associated with lack of ANS administration in a contemporary data set. We also wanted to evaluate whether active participation in a quality improvement (QI) collaborative, the California Perinatal Quality Care Collaborative (CPQCC), was associated with sustained improvement in care over time.
Methods
Study Sample
The CPQCC collects maternal and neonatal clinical data prospectively for infants born at 128 member California hospitals, using an expanded version of the Vermont Oxford Dataset.(8-9) Membership is offered to any hospital in California that provides neonatal intensive care. In the study period of January 2005 to December 2007, greater than 90% of California’s neonatal intensive care admissions were cared for in CPQCC hospitals. Clinical data from CPQCC were linked to administrative data from the California Vital Records using a linkage strategy developed with the support of March of Dimes.(10)
The study population included 17,467 hospitalized newborns with a birth weight less than 1,500 grams or gestational age less than 34 weeks born at a CPQCC hospital during the study period. We excluded the following patients: infants whose antenatal steroid administration status was unknown (n = 529), and infants for whom linkage between CPQCC and Vital Statistics could not be established (n = 535). We also excluded infants who died in the delivery room (n = 1,041), as these infants may have been born in circumstances in which there was a decision for non-resuscitation, and therefore ANS may not have been considered. Ninety percent of delivery room deaths occurred in infants whose gestational age was 25 weeks or lower. We further excluded infants whose birth weight was larger than the 99th percentile for gestational age, based on previous norms as these infants were likely to have a miscoded gestational age (n = 19).(11) The number of participating hospitals that had eligible patients was 94 in 2005, 108 in 2006, and 113 in 2007.
We identified 15,343 eligible patients during the study period. We evaluated patient characteristics, including maternal demographics (age, race / ethnicity, prenatal care), obstetric conditions (“fetal distress”, diabetes, hypertension, bleeding, non-vertex presentation, premature rupture of membranes (rupture prior to delivery), prolonged rupture of membranes (defined as > 18 hours of rupture), multiple gestations, mode of delivery), infant characteristics (sex, birth weight, gestational age at birth), birth year, and insurance status. Gestational age was the best estimate available, with the following hierarchy: 1. obstetric measures, based on last menstrual period, obstetrical parameters, or prenatal ultrasound as recorded in the maternal chart; 2. neonatologists’s estimate based on physical or neurologic examination, combined physical and gestational age examination (Ballard / Dubowitz), or examination of the lens. Data regarding the ultimate method used for estimating gestational age were not recorded.
We also evaluated the impact of prior QI activity, as determined by active group participation in a prior CPQCC QI collaborative from 1999 to 2000 focused on improving ANS administration rates.(7) The dissemination process for the collaborative was developed by the CPQCC Perinatal Quality Improvement Panel and key components included a QI toolkit made available to all members of the collaborative, and webcasts and workshops which were open to all members, but selectively attended. For our analysis, we considered those member hospitals that attended some or all of the webcasts and workshops as QI participants.
To assess the impact of level of neonatal care, we used the California Children’s Services (CCS) classification of neonatal intensive care units (NICUs) into three levels, based on published guidelines by the American Academy of Pediatrics (AAP).(12) Regional NICUs provide mechanical ventilation and major surgery without restriction (equivalent to AAP levels IIIC and IIID); community NICUs provide unrestricted care and ventilation to infants of all gestational ages, but are limited to surgery of only stable infants or those with a patent ductus arteriosus (equivalent to AAP levels IIIA and IIIB); intermediate NICUs provide care to a variably restricted population, ventilate only up to a specified number of hours and refer all complicated cases to a higher level of care.(12) There are a small number of hospitals in California who are not classified by CCS.
The primary outcome of interest was ANS administration, defined as any dose of ANS given prior to delivery. CPQCC records ANS administration as a yes / no variable and does not record the exact timing of administration before time of birth.
We conducted univariable and multivariable analyses to examine associations with ANS administration. For univariable analyses, the Mann-Whitney test was used for continuous variables and chi-square test for categorical variables. For multivariable analysis, nonlinear mixed regression models were performed with PROC NLMIXED in SAS version 9.2 (SAS Institute Inc, Cary, North Carolina), with individual hospitals modeled as a random effect. Forward stepwise selection was used to determine the optimal model. Mean ANS administration rates were compared between QI participants and non-participants, both crude rates and after risk adjustment accounting for the significant factors determined from multivariable analysis. We derived ANS rates for each hospital as predicted random effects using mixed effects logistic regression modeling that adjusted for significant variables from the stepwise selection. The strength of a random effects model is to account for factors that are not determined even after risk adjustment for known risk factors and allow attribution to being clustered at or cared for at a specific hospital. We fit the model using PROC NLMIXED which calculates predicted random effects as posterior modes. Such predictions tend to “shrink” the crude rates; further description of the relationship of predicted random effects and crude estimates are available elsewhere.(13)
The development of a strategy to link data from CPQCC and California Vital Records was facilitated by a grant from the March of Dimes. This study was reviewed by the institutional review boards of University of California, San Francisco and Stanford University.
Results
During 2005-2007, there were 15,343 eligible infants born at CPQCC hospitals, with an overall ANS administration rate of 76.9%. Infants whose mothers received ANS were more likely to have had lower birth weight (1220 vs. 1350 grams, p < 0.0001) and deliver at an earlier gestational age (28.9 vs. 29.7 weeks, p < 0.0001). Further characteristics of the study population and univariable analysis are shown in Table 1. In unadjusted analysis, infants of mothers who were younger or Hispanic were less likely to receive ANS. Female infants and those born from multiple gestations were more likely to receive ANS. Private insurance patients had higher ANS rates (80.3%) compared to other payers (71.5-73.7%, p < 0.0001).
Table 1.
Antenatal steroid administration in preterm infants in the California Perinatal Quality Care Collaborative.
| Antenatal steroid administration | |||
|---|---|---|---|
| Yes | No | P | |
| Birth Year | |||
| 2005 | 3658 (76.7%) | 1109 (23.3%) | 0.0088 |
| 2006 | 3950 (75.7%) | 1271 (24.3%) | |
| 2007 | 4186 (78.2%) | 1169 (21.8%) | |
| Maternal age (years) | |||
| < 20 | 994 (72.4%) | 378 (27.6%) | < 0.0001 |
| 20 – 29 | 4720 (75.4%) | 1539 (24.6%) | |
| 30 – 39 | 5297 (79.3%) | 1382 (20.7%) | |
| >= 40 | 783 (75.8%) | 250 (24.2%) | |
| Prenatal care | |||
| Yes | 11496 (78.1%) | 3223 (21.9%) | < 0.0001 |
| No | 298 (47.8%) | 326 (52.2%) | |
| Maternal race / ethnicity | |||
| Non-Hispanic White | 3655 (80.2%) | 905 (19.8%) | < 0.0001 |
| Native American | 32 (86.5%) | 5 (13.5%) | |
| Asian / Pacific Islander | 1250 (78.5%) | 343 (21.5%) | |
| Black | 1441 (77.4%) | 421 (22.6%) | |
| Hispanic | 5112 (74.4%) | 1763 (25.6%) | |
| Other | 217 (71.6%) | 86 (28.4%) | |
| Fetal distress | |||
| Yes | 2303 (73.5%) | 831 (26.5%) | < 0.0001 |
| No | 9491 (77.7%) | 2718 (22.3%) | |
| Maternal diabetes | |||
| Yes | 1132 (80.5%) | 274 (19.5%) | 0.0007 |
| No | 10662 (76.5%) | 3275 (23.5%) | |
| Maternal hypertension | |||
| Yes | 3079 (81.7%) | 689 (18.3%) | < 0.0001 |
| No | 8111 (75.0%) | 2703 (25.0%) | |
| Maternal bleeding / placental abruption / placenta previa | |||
| Yes | 1708 (74.1%) | 597 (25.9%) | 0.0006 |
| No | 10086 (77.4%) | 2952 (22.6%) | |
| Malpresentation / breech | |||
| Yes | 2714 (78.8%) | 732 (21.2%) | 0.0028 |
| No | 9080 (76.3%) | 2817 (23.7%) | |
| Membrane rupture prior to delivery | |||
| Yes | 3798 (79.7%) | 968 (20.3%) | < 0.0001 |
| No | 7996 (75.6%) | 2581 (24.4%) | |
| Prolonged (> 18 hours) rupture prior to delivery | |||
| Yes | 2090 (89.9%) | 234 (10.1%) | < 0.0001 |
| No | 9704 (74.5%) | 3315 (25.5%) | |
| Multiple gestation | |||
| Yes | 3770 (82.8%) | 785 (17.2%) | < 0.0001 |
| No | 8022 (74.4%) | 2764 (25.6%) | |
| Sex | |||
| Female | 5798 (77.7%) | 1662 (22.3%) | 0.018 |
| Male | 5993 (76.1%) | 1881 (23.9%) | |
| Mode of delivery | |||
| Cesarean section | 8816 (78.2%) | 2459 (21.8%) | < 0.0001 |
| Vaginal | 2972 (73.2%) | 1088 (26.8%) | |
| CCS level | |||
| Community | 7075 (74.5%) | 2423 (25.5%) | < 0.0001 |
| Intermediate | 513 (71.2%) | 208 (28.8%) | |
| Non-classified | 577 (84.0%) | 110 (16.0%) | |
| Regional | 3629 (81.8%) | 808 (18.2%) | |
| Payment source | |||
| MediCal / Other government / Indian Health | 4888 (73.7%) | 1742 (26.3%) | < 0.0001 |
| Private insurance | 6297 (80.3%) | 1540 (19.7%) | |
| Self-pay or other | 442 (71.5%) | 176 (28.5%) | |
The following categories do not add up to the total n = 15,343 of the total cohort due to missing or unknown values: maternal race, hypertension, multiple gestation, mode of delivery, and payment source.
After 30 weeks gestational age, a mother was much less likely to receive antenatal steroids, with the ANS administration ranging from 80 to 85% from 24 to 30 weeks and then decreasing steadily from 78% at 31 weeks to 39% at 34 weeks gestational age (Figure 1). Factors associated with not receiving antenatal steroids in multivariable analysis are shown in Table 2. Obstetrical conditions, in which timing of delivery may have been a factor such as fetal distress and vaginal delivery (vs. cesarean), were associated with non-ANS administration when adjusting for maternal age, race / ethnicity, prenatal care, birth year, and NICU level. On the other hand, mothers who had rupture of membranes of any duration prior to delivery were more likely to receive ANS. Infants with higher birth weight and those born at later gestational age were less likely to receive ANS. Patients without prenatal care and those born at non-Regional hospitals were also less likely to receive ANS.
Figure 1.
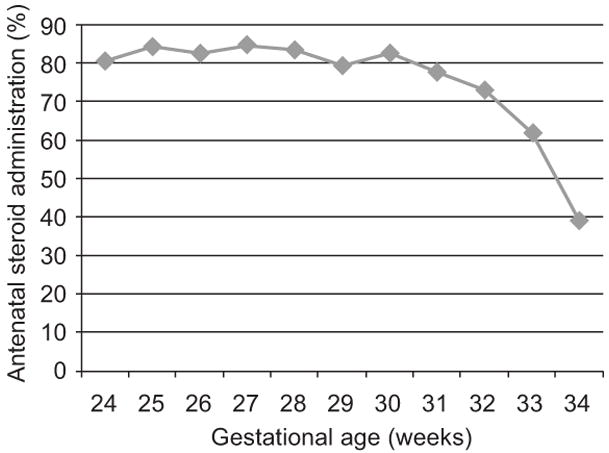
Antenatal steroid administration by gestational age.
Table 2.
Multivariable analysis of risk factors for non-administration of antenatal steroids.
| Odds ratio (95% confidence interval) | |
|---|---|
| Socio-demographic factors | |
| Prenatal care | 0.27 (0.23, 0.33) |
| Race / Ethnicity | |
| Non-Hispanic White | 1.00 (reference) |
| Native American | 0.51 (0.18, 1.44) |
| Asian / Pacific Islander | 1.15 (0.98, 1.36) |
| Black | 1.16 (0.99, 1.36) |
| Hispanic | 1.16 (1.04, 1.30) |
| Other | 1.59 (1.16, 2.18) |
| Maternal age (years) | |
| < 20 | 0.95 (0.82, 1.11) |
| 20-29 | 1.00 (reference) |
| 30-39 | 0.96 (0.87, 1.06) |
| >= 40 | 1.23 (1.03, 1.47) |
| Clinical factors | |
| Prolonged membrane rupture prior to delivery | 0.31 (0.27, 0.37) |
| Maternal hypertension | 0.54 (0.48, 0.60) |
| Multiple gestation | 0.62 (0.56, 0.69) |
| Membrane rupture prior to delivery | 0.85 (0.77, 0.95) |
| Birth weight (increase of 100 grams) | 1.02 (1.00, 1.03) |
| Male sex | 1.03 (0.95, 1.12) |
| Gestational age (increase of 1 week) | 1.12 (1.09, 1.14) |
| Fetal distress | 1.28 (1.15, 1.42) |
| Vaginal delivery (vs. Cesarean) | 1.30 (1.17, 1.45) |
| Birth year | |
| 2005 | 1.00 (reference) |
| 2006 | 1.00 (0.90, 1.11) |
| 2007 | 0.80 (0.72, 0.90) |
| California Children’s Services level | |
| Regional | 1.00 (reference) |
| Community | 1.71 (1.16, 2.51) |
| Intermediate | 1.75 (1.03, 2.97) |
| Non-classified | 1.07 (0.58, 1.99) |
Factors listed are those that remained after stepwise selection. Non-significant factors were: maternal diabetes, maternal bleeding, malpresentation / breech, and payment source.
Hospital rates of ANS administration varied widely (Figure 2). During the years of the study, there were 11 hospitals which had participated in the previous CPQCC quality improvement collaborative caring for 3,111 infants. Forty-five percent of these were Regional hospitals. The remaining 107 hospitals, 11% of which were Regional hospitals, cared for 12,232 infants. Infants born at QI participant hospitals were more likely to receive ANS than those born at hospitals which did not participate, with unadjusted rates for QI participants of 85% vs. non-participants 69% (p < 0.0001). After risk adjustment for risk factors identified in Table 2 and modeling individual hospital as a random effect, QI participants also had higher ANS administration than non-participants (58% vs. 49%, p = 0.028). This method of modeling allowed for risk adjusted comparison of hospitals accounting for clustering at the hospital level and can result in “shrunken” estimates, which was consistent with the present analysis.
Figure 2.
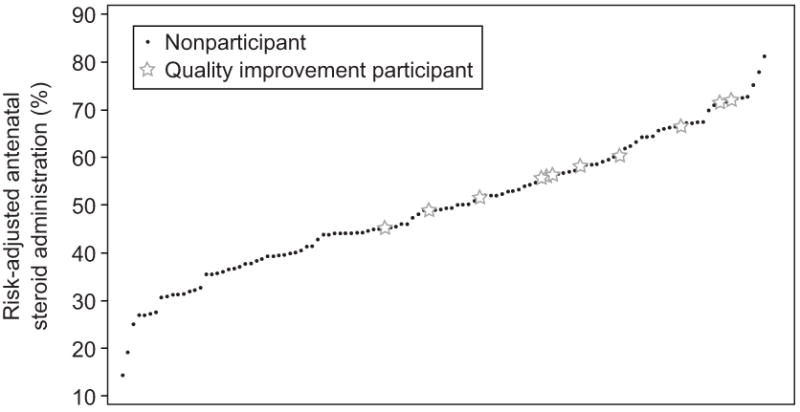
Adjusted rates of antenatal steroid administration by hospital. Rates were risk adjusted using a random effects logistic regression model. Adjusted rates for the majority of hospitals were lower than actual crude rates.
Discussion
In this study of premature infants born in CPQCC hospitals, we found that contrary to well established guidelines, 23.1% of mothers of infants with birth weight < 1500 grams or born < 34 weeks gestation did not receive ANS. Moreover, this phenomenon was disproportionately evident among more vulnerable women (i.e. no prenatal care, Hispanic women). Mothers giving birth after 30 weeks gestation were less likely to receive ANS. Additionally, we found wide variation in the use of ANS among different hospitals, which was evident even after risk adjustment. We also found that participating in webcasts and workshops in a quality improvement collaborative several years prior to the study period was associated with higher ANS administration.
Our findings are consistent with existing literature indicating 15-25% of eligible infants are not exposed to ANS and suggest specific targets for reducing inequalities in care.(6-7) Through a new data linkage strategy, we were able to combine neonatal clinical data from CPQCC with maternal clinical and socio-demographic data from California Vital Records. In unadjusted analyses, younger mothers were at higher risk for not receiving ANS, as has been described in other studies.(6, 14) There were also differences by race / ethnicity; however, after risk adjustment, the most prominent factors associated with not receiving ANS were neonatal level of care and lack of prenatal care (Table 2). Mothers in this higher risk cohort may have greater benefit from ANS, considering that their infants may be more vulnerable to being medically underserved or subject to disparities in access to care.
We also found obstetrical conditions such as vaginal birth increased the likelihood of not receiving ANS. Although the lack of ANS in these cases may be related to the urgency of delivery, they still represent lost opportunities for improvement in quality of care. Although optimal neonatal benefit for ANS occurs after 24 hours of exposure prior to delivery, there is still likely to be some benefit if delivery occurs prior to this time.(15-16) Furthermore, 10.1% of mothers with prolonged rupture of membranes (> 18 hours) did not receive ANS. More than 80% of prolonged rupture of membranes was accounted for by infants born prior to 32 weeks gestation in this cohort. The American College of Obstetricians and Gynecologists recommends ANS for all infants up to 32 weeks gestation, even in the setting of premature membrane rupture, so lack of sufficient time could not have accounted for this finding in our cohort.(3, 28)
In addition to socio-demographic and medical risk factors, we found that the rate of non-administration of ANS increased rapidly in infants delivering between 30 and 34 weeks gestation, with a six-fold increase in adjusted odds of not receiving ANS at 34 weeks. The reasons for this are unclear. It may represent a difference in the imminence of birth on maternal presentation at later gestational ages, or it may reflect reduced attention to the potential adverse consequences of “moderately preterm” birth. There has been recent interest in the increased risk of morbidity and mortality in late preterm infants (34 and 37 weeks gestation).(18-21) The recognition that degree of prematurity presents something akin to a dose-dependent risk should also lead to closer attention of the “moderately preterm” infant born from 30 to 34 weeks gestation. These infants are at even higher risk than late preterm infants for respiratory and other morbidities, yet we found that the ANS rates in this population were markedly lower than infants born at younger gestational ages.(22) Given the evidence of benefit of ANS to 34 6/7 weeks, there is opportunity to improve care for mothers with threatened preterm birth at these intermediate gestational ages.(23-25)
Risk adjusted hospital-specific ANS rates for eligible infants varied widely (Figure 2), indicating substantial variation that cannot be explained by hospital characteristics. Considering that there remained large hospital variation in practice after risk adjustment suggests that there are substantial opportunities for improving ANS rates at many facilities.
The evidence that ANS administration reduces the risk of neonatal death, respiratory distress syndrome, and other morbidities in premature infants has led to it being the standard of care for at least the past decade.(3-5,16, 23) Due to the evidence supporting its use, ANS administration increased dramatically in the 1990’s, from 23.8% in 1991 to 71.6% in 1999 in the Vermont Oxford Network.(26) Although the causes of this rise have not been studied in detail, directed quality improvement interventions have been used successfully to increase ANS rates.(7, 27-28)
One such effort occurred within CPQCC from 1999 to 2000. Details of the dissemination strategy have been described previously.(7) An ANS toolkit was developed and disseminated. A series of interactive workshops and webcasts were then provided. Although the toolkit was available to all CPQCC members, not all hospitals participated in the workshops. ANS rates increased from 76% in 1998 to 86% in 2001.(7)
The sustainability of QI initiatives is an increasing area of scrutiny. There have been a dearth of studies on long-term effects of quality improvement collaborative efforts, and specifically none which have looked at the sustainability of efforts to improve ANS administration.(29) For our analysis, we considered those hospitals which sent representatives to participate in the workshops and webcasts as the quality intervention participants. We found that the efforts of this group were associated with long-term change; participating hospitals had higher rates of ANS administration than other hospitals 5 to 7 years after the end of the intervention. Furthermore, there was evidence of holding the gains of the previous collaborative with ANS rates in the QI participants remaining at 85%.
Limitations of our study include those related to the obstetrical data collected. ANS administration is categorized by the CPQCC as any dose of ANS given prior to delivery, as opposed to a complete course. Therefore, our reported rate of ANS administration likely overestimates the rate of the more optimal complete ANS course. We were unable to determine if some mothers may have been admitted to the hospital just prior to delivery, without enough time for ANS administration. Finally, the comparison of ANS rates by quality improvement collaborative participation was not the result of a randomized controlled design. There may be some degree of selection bias in that hospitals that participated may have other factors relevant to their success. Nevertheless, the higher ANS administration rates in quality improvement participants are encouraging, as it suggests that the collaborative effort was associated with a sustained improvement at those hospitals. As quality improvement becomes a higher priority in medicine, further research should focus on not just the effectiveness of quality improvement, but its sustainability.
We found that there is still a concerning proportion of eligible patients at risk for not receiving ANS. It may be the case that relatively “easy” patients to identify and treat such as those who are followed through prenatal care with identification of high risk conditions are generally being treated appropriately. On the other hand, mothers who present without prenatal care and / or mothers who present suddenly without warning with imminent delivery may be at highest risk for not receiving ANS. There also may be some complacency toward ANS administration at later, yet still eligible, gestational ages. Quality improvement initiatives to target such mothers may be the next priority in further efforts to increase ANS rates. Our study showed that a collaborative effort by CPQCC may have had a lasting change on participating hospitals, an encouraging result in this era of quality improvement.
Acknowledgments
Supported by NIH/NCRR/OD UCSF-CTSI grant number KL2 RR024130. Data management was funded in part by a Community Grant from the March of Dimes California Chapter. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or the March of Dimes.
The authors thank Chengshi Jin and John M. Neuhaus for assistance with statistical consultation and data analysis.
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
References
- 1.Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972 Oct;50(4):515–25. [PubMed] [Google Scholar]
- 2.Crowley P. Prophylactic corticosteroids for preterm birth. Cochrane Database Syst Rev. 2000;2:CD000065. doi: 10.1002/14651858.CD000065. [DOI] [PubMed] [Google Scholar]
- 3.ACOG Committee Opinion No. 402: Antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2008 Mar;111(3):805–7. doi: 10.1097/AOG.0b013e318169f722. [DOI] [PubMed] [Google Scholar]
- 4.ACOG committee opinion. Antenatal corticosteroid therapy for fetal maturation. Number 210, October 1998 (Replaces Number 147, December 1994). Committee on Obstetric Practice. American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet. 1999 Mar;64(3):334–5. [PubMed] [Google Scholar]
- 5.Miracle X, Di Renzo GC, Stark A, Fanaroff A, Carbonell-Estrany X, Saling E. Guideline for the use of antenatal corticosteroids for fetal maturation. J Perinat Med. 2008;36(3):191–6. doi: 10.1515/JPM.2008.032. [DOI] [PubMed] [Google Scholar]
- 6.Burguet A, Ferdynus C, Thiriez G, Bouthet MF, Kayemba-Kays S, Sanyas P, et al. Very preterm birth: who has access to antenatal corticosteroid therapy? Paediatr Perinat Epidemiol. 2010 Jan;24(1):63–74. doi: 10.1111/j.1365-3016.2009.01090.x. [DOI] [PubMed] [Google Scholar]
- 7.Wirtschafter DD, Danielsen BH, Main EK, Korst LM, Gregory KD, Wertz A, et al. Promoting antenatal steroid use for fetal maturation: results from the California Perinatal Quality Care Collaborative. J Pediatr. 2006 May;148(5):606–12. doi: 10.1016/j.jpeds.2005.12.058. [DOI] [PubMed] [Google Scholar]
- 8.Vermont Oxford Network Database Manual of Operations for Infants Born in 2009. [9-8-2010]; Release 13.2 2008. Revised April 2009. www.vtoxford.org/tools/2009ManualofOperationswithindex13_2.pdf.
- 9.CPQCC Network Database 2008 Member Instructions for Electronic Data Submission. Version 01.08. www.cpqcc.org2008.
- 10.California Perinatal Quality Care Collaborative. [9-2-2010]; http://cpqcc.org/research.
- 11.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003 Jul 8;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stark AR. Levels of neonatal care. Pediatrics. 2004 Nov;114(5):1341–7. doi: 10.1542/peds.2004-1697. [DOI] [PubMed] [Google Scholar]
- 13.McCulloch CE, Searle SR, Neuhaus JM. Generalized, Linear, and Mixed Models. Wiley-Interscience; Hoboken, NJ: Ch. 13. Prediction; pp. 303–19. [Google Scholar]
- 14.Foix-L’Helias L, Marret S, Ancel PY, Marchand L, Arnaud C, Fresson J, et al. Impact of the use of antenatal corticosteroids on mortality, cerebral lesions and 5-year neurodevelopmental outcomes of very preterm infants: the EPIPAGE cohort study. BJOG. 2008 Jan;115(2):275–82. doi: 10.1111/j.1471-0528.2007.01566.x. [DOI] [PubMed] [Google Scholar]
- 15.Gates S, Brocklehurst P. Decline in effectiveness of antenatal corticosteroids with time to birth: real or artefact? BMJ. 2007 Jul 14;335(7610):77–9. doi: 10.1136/bmj.39225.677708.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crowley P, Chalmers I, Keirse MJ. The effects of corticosteroid administration before preterm delivery: an overview of the evidence from controlled trials. Br J Obstet Gynaecol. 1990 Jan;97(1):11–25. doi: 10.1111/j.1471-0528.1990.tb01711.x. [DOI] [PubMed] [Google Scholar]
- 17.ACOG Practice Bulletin No. 80: premature rupture of membranes. Clinical management guidelines for obstetrician-gynecologists. Obstet Gynecol. 2007 Apr;109(4):1007–19. doi: 10.1097/01.AOG.0000263888.69178.1f. [DOI] [PubMed] [Google Scholar]
- 18.Jain L. Morbidity and mortality in late-preterm infants: more than just transient tachypnea! J Pediatr. 2007 Nov;151(5):445–6. doi: 10.1016/j.jpeds.2007.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chyi LJ, Lee HC, Hintz SR, Gould JB, Sutcliffe TL. School outcomes of late preterm infants: special needs and challenges for infants born at 32 to 36 weeks gestation. J Pediatr. 2008 Jul;153(1):25–31. doi: 10.1016/j.jpeds.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 20.McIntire DD, Leveno KJ. Neonatal mortality and morbidity rates in late preterm births compared with births at term. Obstet Gynecol. 2008 Jan;111(1):35–41. doi: 10.1097/01.AOG.0000297311.33046.73. [DOI] [PubMed] [Google Scholar]
- 21.Raju TN. Epidemiology of late preterm (near-term) births. Clin Perinatol. 2006 Dec;33(4):751–63. doi: 10.1016/j.clp.2006.09.009. abstract vii. [DOI] [PubMed] [Google Scholar]
- 22.Marret S, Ancel PY, Marpeau L, Marchand L, Pierrat V, Larroque B, et al. Neonatal and 5-year outcomes after birth at 30-34 weeks of gestation. Obstet Gynecol. 2007 Jul;110(1):72–80. doi: 10.1097/01.AOG.0000267498.95402.bd. [DOI] [PubMed] [Google Scholar]
- 23.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;3:CD004454. doi: 10.1002/14651858.CD004454.pub2. [DOI] [PubMed] [Google Scholar]
- 24.Colin AA, McEvoy C, Castile RG. Respiratory morbidity and lung function in preterm infants of 32 to 36 weeks’ gestational age. Pediatrics. 2010 Jul;126(1):115–28. doi: 10.1542/peds.2009-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joseph KS, Nette F, Scott H, Vincer MJ. Prenatal corticosteroid prophylaxis for women delivering at late preterm gestation. Pediatrics. 2009 Nov;124(5):e835–43. doi: 10.1542/peds.2009-0905. [DOI] [PubMed] [Google Scholar]
- 26.Horbar JD, Badger GJ, Carpenter JH, Fanaroff AA, Kilpatrick S, LaCorte M, et al. Trends in mortality and morbidity for very low birth weight infants, 1991-1999. Pediatrics. 2002 Jul;110(1 Pt 1):143–51. doi: 10.1542/peds.110.1.143. [DOI] [PubMed] [Google Scholar]
- 27.Leviton LC, Goldenberg RL, Baker CS, Schwartz RM, Freda MC, Fish LJ, et al. Methods to encourage the use of antenatal corticosteroid therapy for fetal maturation: a randomized controlled trial. JAMA. 1999 Jan 6;281(1):46–52. doi: 10.1001/jama.281.1.46. [DOI] [PubMed] [Google Scholar]
- 28.Horbar JD, Carpenter JH, Buzas J, Soll RF, Suresh G, Bracken MB, et al. Collaborative quality improvement to promote evidence based surfactant for preterm infants: a cluster randomised trial. BMJ. 2004 Oct 30;329(7473):1004. doi: 10.1136/bmj.329.7473.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schouten LM, Hulscher ME, van Everdingen JJ, Huijsman R, Grol RP. Evidence for the impact of quality improvement collaboratives: systematic review. BMJ. 2008 Jun 28;336(7659):1491–4. doi: 10.1136/bmj.39570.749884.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]


