Abstract
Circulating levels of soluble lectin-like oxidized low-density lipoprotein receptor-1 (sLOX-1) play an important role in the development and progression of atherosclerosis. We hypothesized that the inflammatory marker C-reactive protein (CRP) might stimulate sLOX-1 release by activating tumor necrosis factor-α converting enzyme (TACE). Macrophages differentiated from THP-1 cells were stimulated with TNF-α and further treated with CRP in the absence or presence of specific inhibitors or small interfering RNA (siRNA). Our results showed that CRP increased sLOX-1 release from activated macrophages in a dose-dependent manner and that these effects were regulated by Fc γ receptor II (FcγRII)-mediated p47phox phosphorylation, reactive oxygen species (ROS) production, and TACE activation. CRP also enhanced sLOX-1 release from macrophages derived from peripheral blood mononuclear cells (PBMC) of patients with acute coronary syndrome (ACS). Pretreatment with antibody against FcγRII or with CD32 siRNA, p47phox siRNA, apocynin, N-acetylcysteine, tumor necrosis factor-α protease inhibitor 1 (TAPI-1) or TACE siRNA attenuated sLOX-1 release induced by CRP. CRP also elevated serum sLOX-1 levels in a rabbit model of atherosclerosis. Thus, CRP might stimulate sLOX-1 release, and the underlying mechanisms possibly involved FcγRII-mediated p47phox phosphorylation, ROS production, and TACE activation.
Keywords: lectin-like oxidized low density lipoprotein receptor-1, tumor necrosis factor-α converting enzyme, C-reactive protein, acute coronary syndrome
An abundant increase in inflammatory factors leads to acute inflammatory diseases, such as acute coronary syndrome (ACS) (1). C-reactive protein (CRP), the prototypic marker of inflammation, is one of the strongest predictors of cardiovascular events (2). Recent studies have demonstrated that CRP is present in atherosclerotic plaques and plays a pivotal role in promoting atherogenesis by regulating the expression and release of inflammatory cytokines (3–5).
Lectin-like oxidized low density lipoprotein receptor-1 (LOX-1), a type II membrane glycoprotein acting as a receptor for oxidized low-density lipoprotein, mediates vascular dysfunction (3). LOX-1 is expressed on the cell surface and can be proteolytically cleaved at its extracellular domain and released as a soluble form (sLOX-1) (6). sLOX-1 level reflects increased oxidative stress of vascular walls and has been identified as a novel marker for early diagnosis of ACS (7, 8). However, the exact mechanisms of sLOX-1 release from the cell membrane are poorly understood.
Tumor necrosis factor-α converting enzyme (TACE), a disintegrin and metalloproteinase, mediates the release of growth factors, receptors, and adhesion molecules (9). TACE is synthesized in a latent form and activated by reactive oxygen species (ROS) before reaching the cell membrane (10, 11). Studies report that CRP can upregulate ROS production by activating NAPDH oxidase in macrophages (12). However, it is still unknown whether CRP could stimulate sLOX-1 release by activating TACE.
In the present study, we hypothesized that CRP could stimulate sLOX-1 release from macrophages via Fc γ receptor II (FcγRII)-mediated p47phox phosphorylation, ROS production, and subsequent TACE activation, and we provided experimental evidence to validate our hypothesis.
MATERIALS AND METHODS
Reagents
Trypsin, penicillin/streptomycin solution (P/S), fetal bovine serum (FBS), RPMI 1640, and Opti-MEM were from Life Technologies. LOX-1 ELISA kit was from R&D Systems. Recombinant human CRP, also from R&D Systems, is endotoxin-free (endotoxin level < 0.1 endotoxin units/1 µg protein) and azide-free. The protein extraction kits were from Millipore or Biovision.
Cell culture and treatment
THP-1 cells purchased from the American Type Culture Collection were grown in RPMI 1640 medium with 10% FBS and 1% P/S. For monocyte differentiation, cells were seeded in culture plates at 2 × 106 cells/1 ml per well and allowed to adhere and differentiate overnight at 37°C in the presence of 100 nM phorbol myristate acetate (PMA, Sigma). Macrophages derived from THP-1 cells were treated with TNF-α (5 ng/ml) for 12 h in serum-free medium and were then divided into control and CRP treatment groups. In addition, macrophages in CRP treatment group were pretreated with siRNA oligonucleotides 48 h before CRP treatment while inhibitors, including N-acetylcysteine (NAC, 10 mM, Sigma), apocynin (Apo, 10 mM, Sigma) and tumor necrosis factor-α protease inhibitor 1 (TAPI-1, 100 µM, Peptides International Inc.), phenylmethyl sulfonylfluoride (PMSF, 3 mM, Sigma), were added 1 h before CRP treatment. To identify the effect of the CRP to be specific, polymixin B sulfate (10 µg/ml, Amerson) and boiled CRP (25 µg/ml) were used. Cell viability was >95% in all experiments.
Peripheral blood samples were obtained from six normal subjects and six patients with ACS after their informed consent. Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll density gradient centrifugation and cultured in RPMI 1640 medium with 20% autologous serum at 37°C in 5% CO2 for seven days to induce differentiation into macrophages. Then macrophages from PBMCs were stimulated similarly to the macrophages from THP-1 cells but without TNF-α treatment. Cell-free supernatants were harvested and kept at −80°C for cytokine measurements. The experiment was approved by the Committee of Shandong University.
In vivo experiment
Twenty New Zealand white rabbits, 8 weeks old, underwent balloon-induced endothelial injury in the abdominal aorta after being anesthetized with 3% pentobarbital sodium (30 mg/kg). They were fed a 0.5% cholesterol diet for 12 weeks to establish an animal model of atherosclerosis as described previously (13). At the end of week 12, rabbits were fasted for 24 h and randomly divided into group A (n = 10) and group B (n = 10). Group A received intravenous injection of human recombinant CRP (3 mg/kg), and group B received intravenous injection of saline. Blood samples were obtained 6 h after injection, and levels of human CRP in the serum were measured by a highly sensitive nephelometric assay. The experiment complied with the Animal Management Rule of the Ministry of Public Health, People's Republic of China (documentation 55, 2001) and was approved by the Animal Care Committee of Shandong University.
ELISA
sLOX-1 in the culture supernatants or serum was measured by ELISA assay according to the manufacturer's instruction. The level of sLOX-1 in the culture supernatants was measured from 2 × 106 cells/1 ml per well.
RNA interference
Macrophages were washed with Opti-MEM and incubated for 2 h with Opti-MEM medium before being transfected with siRNA oligonucleotides (Santa Cruz Biotechnology). The cells were incubated with siRNA of TACE (200 nM), p47phox (100 nM), CD32 (100 nM), CD64 (100 nM) or control (200/100 nM) using Lipofectamine 2000 (5 µL/ml, Invitrogen) as the transfection reagent. After 12 hr transfection, the Opti-MEM medium was replaced with fresh RPMI 1640 (containing 10% FBS/20% autologous serum), and the samples were incubated for another 36 h.
Determination of intracellular ROS production
Intracellular ROS generation was measured by use of 2′, 7′-dichlorodihydrofluorescein diacetate (DCF-DA, Sigma), a cell-permeable indicator for ROS. After cells were treated with TNF-α (5 ng/ml) for 12 h, they were incubated with antibody against CD32 (3 µg/ml) or with NAC (10 mM), Apo (10 mM) for 1 h, or with siRNA oligonucleotides for 48 h, followed by CRP (25 µg/ml) challenge for the indicated times (10 min to 1 h), then loaded with DCF-DA (10 µM) for 10 min at 37°C. ROS-mediated fluorescence was detected by use of a fluorescence microplate reader (Varioskan Flash, Thermo Scientific) with excitation 498 nm and emission 520 nm.
Measurement of TACE activity
TACE activity of cell lysates was measured by use of the Sensolyte 520 TACE activity assay kit (Anaspec) according to the manufacturer's instruction. Briefly, after macrophages were collected in assay buffer, incubated for 10 min at 4°C and centrifuged at 2500 g for 10 min at 4°C, the supernatants were collected. Equal amounts of cell lysates were incubated with 50 µl TACE substrate for 30 min at 37°C, and changes in fluorescence were monitored by the fluorescence microplate reader with excitation 490 nm and emission 520 nm. Fluorescence quenching was used to calculate percentage activity with the appropriate control values.
Western blot analysis
Extracts containing cytoplasmic, membrane, or total proteins were separately prepared according to the manufacturer's instructions. Equal amounts of cytosolic, membrane, or total protein extracts were separately subjected to Western analysis with antibodies against LOX-1 (1:250, R&D Systems), TACE (1:200, Abcam), phosphorylated p47phox (1:200, Syd Labs), CD32 (1:200, Santa Cruz Biotechnology), CD64 (1:200, Santa Cruz Biotechnology), Gαs (1:400, Santa Cruz Biotechnology), and β-actin (1:1000, Santa Cruz Biotechnology). The antigen-antibody complexes were detected by enhanced chemiluminescence. All blots were probed with Gαs or β-actin as a loading control, and densitometric analysis was performed with an image analyzer (AlphaImager 2200, Alpha).
Real-time PCR
Total RNA was isolated from macrophages by use of TRIzol Reagent (Invitrogen) and treated with DNase (Ambion) to remove contaminating genomic DNA. cDNA was prepared from 500 ng RNA by use of PrimeScriptTM Reverse Transcriptase (Takara Bio Inc.) according to the manufacturer's instructions. Real-time PCR reactions involved the SYBR Green method for 45 cycles with a LightCycler (Roche), and a melt curve analysis was performed after each reaction to verify that primer dimers were absent. Data analysis was performed with LightCycler Software 4.0 (Roche) and the 2−ΔΔCT method was used to assess the relative mRNA expression level normalized to that of GAPDH. The sequences of primers were shown in Table 1.
TABLE 1.
Primer sequences
| Gene | Primer Sequence | Product Length |
|---|---|---|
| TACE | Sense 5′-ACCTGA AGAGCTTGTTCATCGAG-3′ Antisense 5′-CCATGAAGTGTTCCGATAGATGTC-3′ |
190bp |
| LOX-1 | Sense 5′-TTACTCTCCATGGTGGTGCC-3′ Antisense 5′-AGCTTCTTCTGCTTGTTGCC-3′ |
193bp |
| GAPDH | Sense 5′-AAGGTGAAGGTCGGAGTCAAC-3′ Antisense 5′-GGGGTCATTGATGGCAACAATA -3′ |
102bp |
Statistical analysis
All experiments were repeated at least three times. Data are presented as mean ± SD. Data analysis was performed with one-way ANOVA followed by Newman-Keuls posthoc test or unpaired Student's t-test. P < 0.05 was considered statistically significant.
RESULTS
CRP stimulated sLOX-1 release from macrophages activated by TNF-α
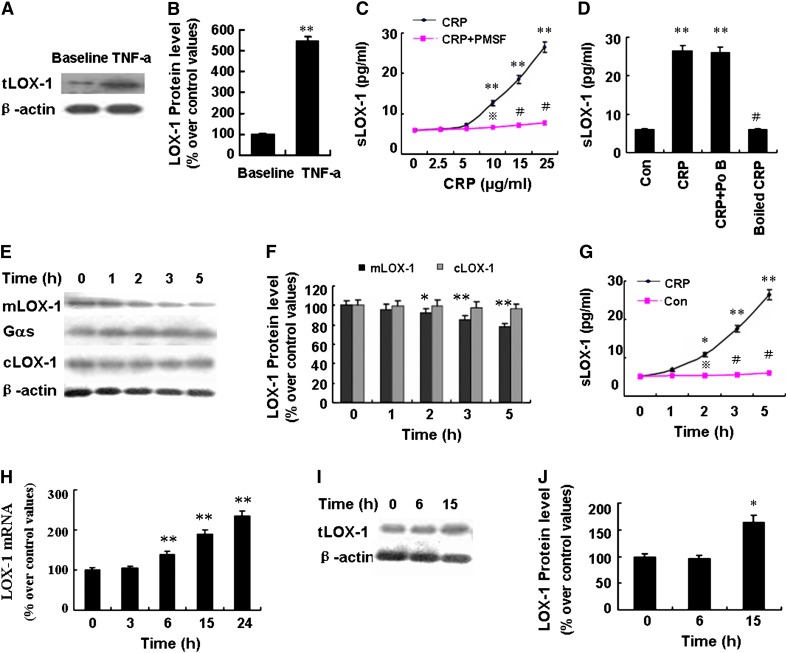
The LOX-1 protein expression of macrophages was low at baseline and was significantly upregulated after TNF-α (5 ng/ml) treatment for 12 h (Fig. 1A, B). Incubating macrophages with CRP (2.5∼25 µg/ml) for a further 5 h after TNF-α treatment resulted in dose-dependent increase in sLOX-1 levels, with a stepwise and significant increase from the dose of 10 µg/ml (Fig. 1C). However, exposure of macrophages to CRP (25 µg/ml) without TNF-α stimulation did not affect the sLOX-1 level, which was too low to be detectable. Moreover, boiled CRP and polymixin B sulfate produced no effects on the sLOX-1 levels induced by CRP (Fig. 1D). Furthermore, CRP (25 µg/ml) treatment for 5 h caused a time-dependent decrease in membrane-bound LOX-1 (mlOX-1) levels and increase in sLOX-1 levels (Fig. 1E–G), but the cytoplasmic LOX-1 (cLOX-1) protein levels were unaffected (Fig. 1E, F). Pretreating activated macrophages with PMSF (3 mM), an inhibitor of serine protease, could attenuate the sLOX-1 increase induced by CRP (Fig. 1C). Furthermore, CRP (25 µg/ml) treatment for 6 h significantly upregulated LOX-1 mRNA expression but had no effect on the LOX-1 protein expression (Fig. 1H–J), possibly due to the fact that 6 h of CRP treatment were too short for LOX-1 protein to enhance expression. These results indicated that CRP specifically induced sLOX-1 release from activated macrophages but that this effect could be blocked by protease inhibitor.
Fig. 1.
Effect of CRP on LOX-1 release in macrophages derived from THP-1 cells activated by TNF-α. A: Western blot analysis of LOX-1 expression in cell lysates at baseline and after stimulation with TNF-α (5 ng/ml) for 12 h. B: Quantity analysis of A. **P < 0.01 versus baseline. C: Cells were treated with CRP (0∼25 µg/ml) in the presence or absence of PMSF (3 mM) for 5 h, and soluble LOX-1 (sLOX-1) levels in supernatant were determined by ELISA. **P < 0.01 versus CRP (0 µg/ml), P < 0.05, #P < 0.01 versus CRP (similar dose). D: Cells were treated with CRP (25 µg/ml) in the presence or absence of Polymixin B sulfate (Po B, 10 µg/ml) or treated with boiled CRP (25 µg/ml) for 5 h, and sLOX-1 levels were determined by ELISA. **P < 0.01 versus control group (Con, without CRP stimulation), #P < 0.01 versus CRP. E: Cells were treated with CRP (25 µg/ml) for 0-5 h, and membrane LOX-1 (mlOX-1) and cytoplasmic LOX-1 (cLOX-1) protein levels were determined by Western blot analysis. F: Quantity analysis of E and the relative expression of mlOX-1 and cLOX-1 protein were normalized to Gαs protein or β-actin. *P < 0.05, **P < 0.01 versus 0 h. G: Cells were treated with CRP (25 µg/ml) for 0-5 h, and sLOX-1 levels were determined by ELISA. *P < 0.05, **P < 0.01 versus 0 h, P < 0.05, #P < 0.01 versus CRP (similar time point). H: Cells were treated with CRP (25 µg/ml) for 0-24 h, and LOX-1 mRNA was analyzed by real-time PCR (RT-PCR). **P < 0.01 versus 0 h. I: Cells were treated with CRP (25 µg/ml) for 0-15 h, and total LOX-1 (tLOX-1) protein level of cell lysates was determined by Western blot analysis. J: Quantity analysis of I. *P < 0.05 versus 0 h. Data are means ± SEM of three different experiments.
TACE regulated sLOX-1 release from activated macrophages induced by CRP
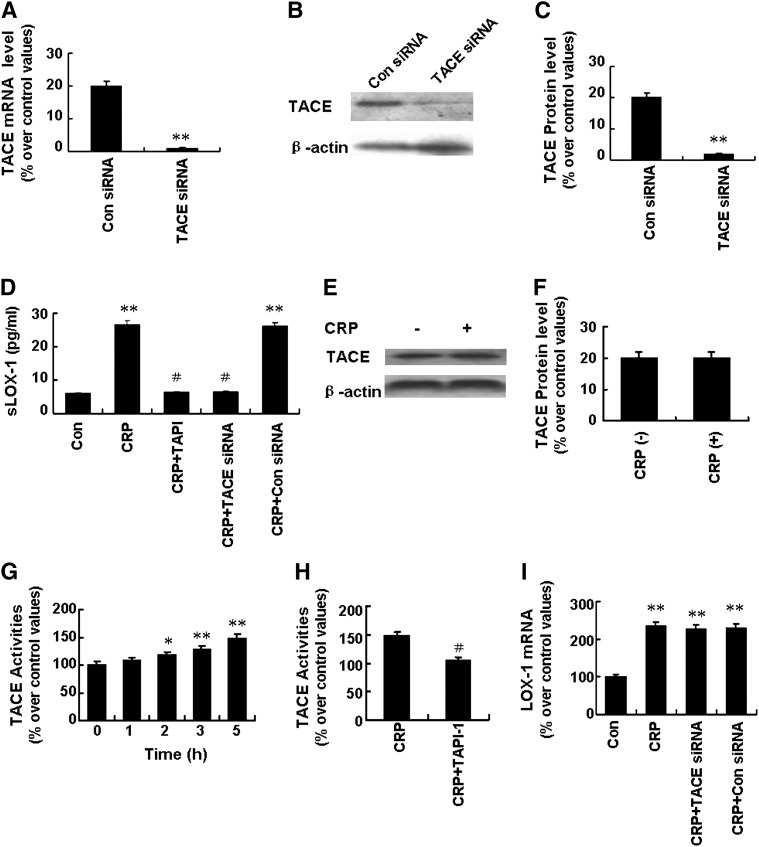
It has been reported that LOX-1 can be cleaved from the cell surface by proteases and released as sLOX-1 (6) and that TACE mediates processing membrane-bound inflammatory factors to active soluble forms (10). To determine whether TACE was involved in sLOX-1 release, we designed loss-of-function experiments with the TACE inhibitor TAPI-1 (100 µM) and TACE siRNA (200 nM). TACE siRNA successfully knocked down the expression of TACE at both mRNA and protein levels (Fig. 2A–C). As shown in Fig. 2D, pretreatment with TAPI-1 and TACE siRNA effectively prevented the increase of sLOX-1 levels induced by CRP. Treatment with CRP (25 μg/ml) for 5 h had no effect on the TACE protein expression; however, it enhanced TACE activity in a time-dependent manner, with 47.5% increase in activity after 5 h treatment (Fig. 2E–G). Furthermore, the activity of TACE induced by CRP was attenuated by pretreatment with TAPI-1 (100 µM) (Fig. 2H). Thus, TACE might be involved in sLOX-1 release from activated macrophages induced by CRP. On the other hand, TACE siRNA had no effect on LOX-1 mRNA expression levels (Fig. 2H).
Fig. 2.
Effects of TACE on sLOX-1 release from activated macrophages induced by CRP. Macrophages were stimulated with TNF-α (5 ng/ml) for 12 h. A, B: Cells were incubated with control siRNA (Con siRNA, 200 nM) or TACE siRNA (200 nM) for 48 h. Then TACE mRNA expression was determined by RT-PCR (A) and TACE protein expression by Western blot analysis (B). C: Quantity analysis of B. **P < 0.01 versus Con siRNA. D: Cells were incubated with TAPI-1 (100 µM) for 1 h or with TACE siRNA (200 nM) for 48 h, then with CRP (25 µg/ml) for 5 h. After that, sLOX-1 level was determined by ELISA. **P < 0.01 versus Con, #P < 0.01 versus CRP. E: Cells were incubated with CRP (25 µg/ml) for 5 h, and total TACE protein expression was determined by Western blot analysis. F: Quantity analysis of E. G: Cells were incubated with CRP (25 µg/ml) for 0-5 h, and TACE activity was determined. *P < 0.05, **P < 0.01 versus 0 h. H: Cells were incubated with CRP (25 µg/ml) in the presence or absence of TAPI-1 (100 µM) for 5 h, and then TACE activity was determined. #P < 0.01 versus CRP. I: Cells were incubated with CRP (25 µg/ml) in the presence or absence of TACE siRNA (200 nM) for 24 h, and LOX-1 mRNA expression was determined by RT-PCR. **P < 0.01 versus Con. Data are means ± SEM of three different experiments.
CRP stimulating sLOX-1 release from activated macrophages was mediated by ROS generated from NADPH oxidase
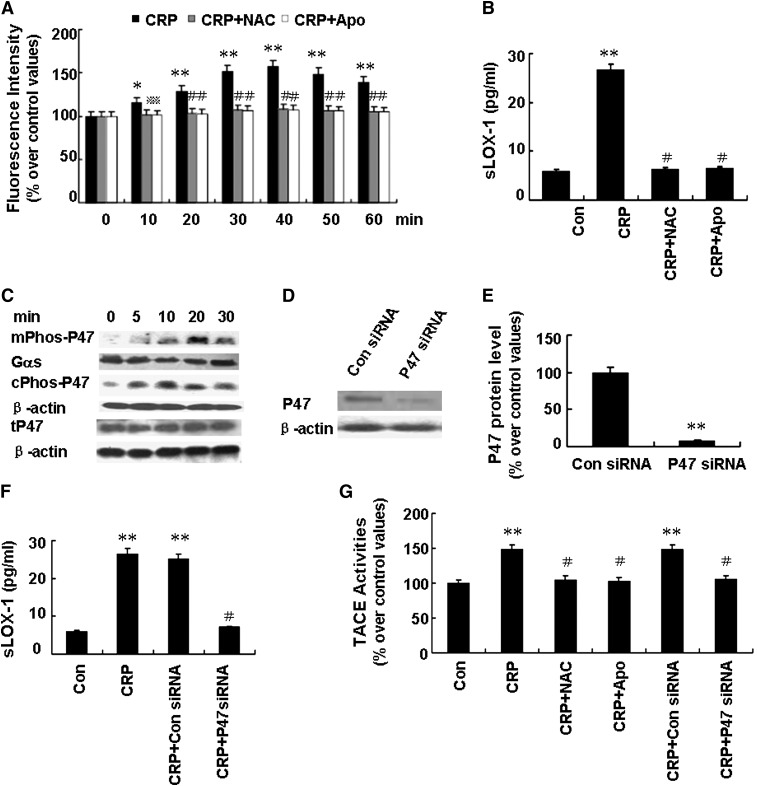
Previous studies showed that TACE was synthesized in a latent form and activated before reaching the cell surface membrane (10). In airway epithelial cells, ROS was reported to activate TACE by oxidating a crucial cysteine thiol (14). We observed that exposure of macrophages to CRP (25 µg/ml) led to a time-dependent increase of intracellular ROS generation, with a maximal response at 40 min (Fig. 3A). Moreover, pretreatment with ROS scavenger NAC (10 mM) significantly decreased sLOX-1 levels induced by CRP (Fig. 3B). Similarly, pretreatment with the NADPH oxidase inhibitor Apo (10 mM) markedly reduced ROS production and sLOX-1 levels induced by CRP (Fig. 3A, B). In contrast, exposure of unactivated macrophages to Apo or NAC did not affect basal DCF fluorescence (P > 0.05).
Fig. 3.
Effects of ROS generated from NADPH oxidase on sLOX-1 release from activated macrophages induced by CRP. Macrophages were stimulated with TNF-α (5 ng/ml) for 12 h. A: Cells were incubated with NAC (10 mM) or Apo (10 mM) for 1 h, and then with CRP (25 µg/ml) for 0-60 min, and intracellular ROS generation was measured. *P < 0.05, **P < 0.01 versus 0 min, *P < 0.05 versus CRP (10 min), #P < 0.01 versus CRP (at similar time point). B: Cells were incubated with NAC (10 mM) or Apo (10 mM) for 1 h, and then with CRP (25 µg/ml) for 5 h, and sLOX-1 levels were determined by ELISA. **P < 0.01 versus Con, #P < 0.01 versus CRP. C: Cells were incubated with CRP (25 µg/ml) for 0-30 min, and then the protein levels of phosphorylated p47phox of the membrane fraction (mPhosp-P47) or cytoplasmic fraction (cPhosp-P47) or total p47phox (tP47) were determined by Western blot analysis. D: Cells were incubated with p47phox siRNA (100 nM) for 48 h, and then p47phox (P47) protein expression was determined by Western blot analysis. E: Quantity analysis of D. **P < 0.01 versus Con siRNA. F: Cells were incubated with p47phox siRNA (100 nM) or Con siRNA (100 nM) for 48 h, and then incubated with CRP (25 µg/ml) for 5 h. The sLOX-1 level was determined by ELISA. **P < 0.01 versus Con, #P < 0.01 versus CRP. G: Cells were incubated with NAC (10 mM) or Apo (10 mM) for 1 h or p47phox siRNA for 48 h, and then incubated with CRP (25 µg/ml) for 5 h. Then TACE activity was determined. *P < 0.05, **P < 0.01 versus Con, #P < 0.01 versus CRP; Data are means ± SEM of three different experiments.
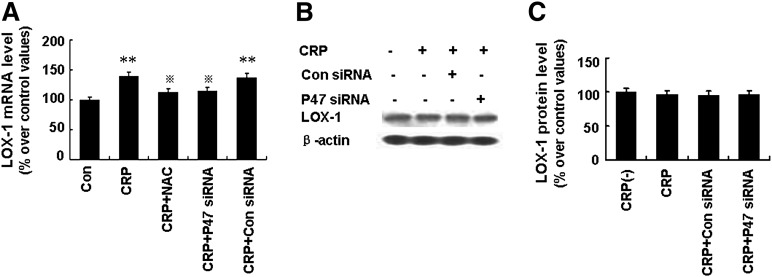
NADPH oxidase is a multi-component protein whose activation depends on phosphorylation of the cytosolic subunit p47phox and its translocation to the cell membrane (15). As shown in Fig. 3C, CRP (25 µg/ml) led to a time-dependent increase in the phosphorylation and translocation of p47phox to the cell membrane, with a maximal response at 20 min. We also found that p47phox siRNA successfully knocked down the expression of p47phox protein (Fig. 3D, E) and that pretreatment with p47phox siRNA (100 nM) successfully decreased sLOX-1 levels induced by CRP (Fig. 3F). Furthermore, pretreatment with NAC (10 mM), Apo (10 mM), and p47phox siRNA (100 nM) effectively attenuated TACE activity induced by CRP (Fig. 3G). Thus, sLOX-1 release induced by CRP was mediated by ROS generated from NADPH oxidase. However, incubation with p47phox siRNA partially suppressed LOX-1 mRNA levels but had no effect on LOX-1 protein expression after 6 h CRP treatment (Fig. 4A–C), suggesting that the pathway by which ROS and p47phox enhance LOX-1 mRNA expression is probably different from the pathways by which ROS and p47phox increase sLOX-1 release.
Fig. 4.
Effects of ROS generated from NADPH oxidase on LOX-1 expression on activated macrophages induced by CRP. Macrophages were stimulated with TNF-α (5 ng/ml) for 12 h. A: Cells were incubated with NAC (10 mM) or Apo (10 mM) for 1 h or p47phox siRNA (100 nM) for 48 h, and then incubated with CRP (25 µg/ml) for 6 h. Then LOX-1 mRNA was determined. **P < 0.01 versus Con, # versus CRP. B: Cells were incubated with p47phox siRNA (100 nM) for 48 h, and then incubated with CRP (25 µg/ml) for 6 h. LOX-1 protein levels was determined by Western blot analysis. C: Quantity analysis of B. Data are means ± SEM of three different experiments.
sLOX-1 release from activated macrophages induced by CRP was mediated by FcγRII
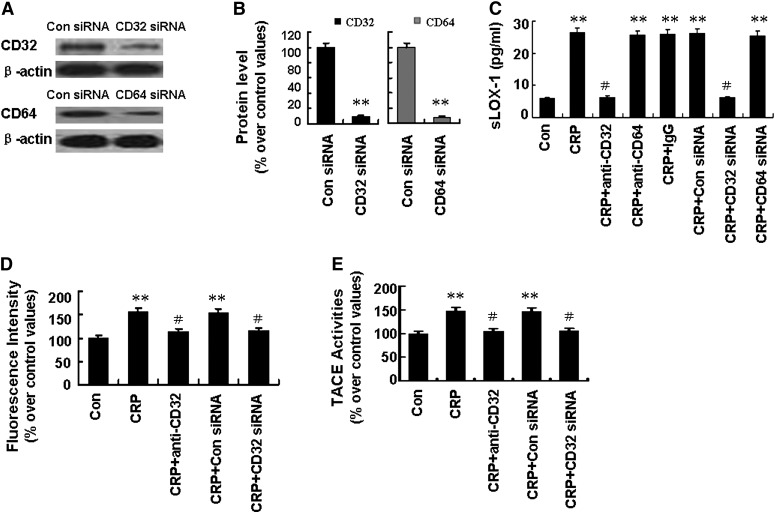
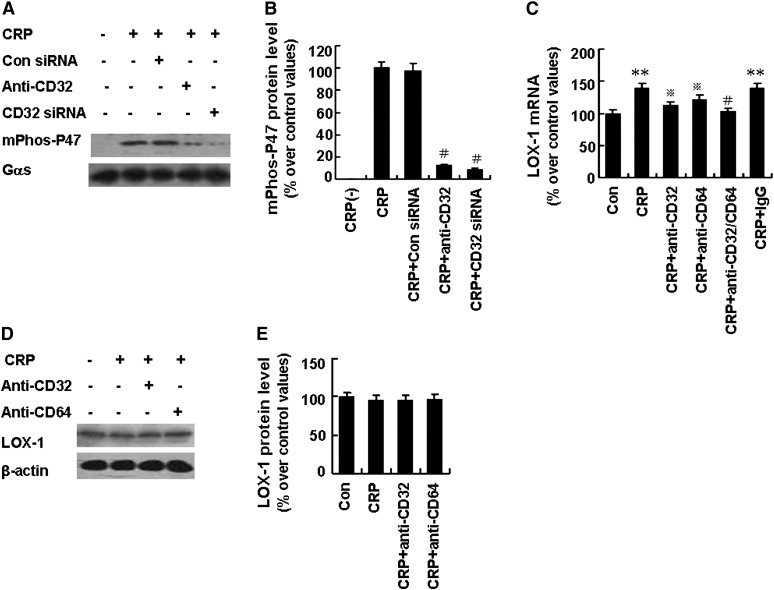
CRP has been reported to bind to the family of FcγRs in human macrophages, including FcγRI (CD64) and FcγRII (CD32). Our results demonstrated that FcγR siRNA effectively abolished FcγR protein expression (Fig. 5A, B). Pretreatment of activated macrophages with antibody against CD32 (3 µg/ml) or with CD32 siRNA (100 nM) effectively prevented sLOX-1 release induced by CRP (Fig. 5C). However, pretreatment of activated macrophages with antibody against CD64 (3 µg/ml) or IgG (3 µg/ml) or with CD64 siRNA (100 nM) did not affect sLOX-1 release induced by CRP (Fig. 5C). Moreover, ROS production, TACE activation, and p47phox protein phosphorylation and translocation were all suppressed by pretreatment with antibody against CD32 (3 µg/ml) or with CD32 siRNA (100 nM) (Fig. 5D, E and Fig. 6A, B). In addition, antibodies against both CD32 and CD64 showed significant suppression of LOX-1 mRNA expression (Fig. 6C) but exerted no effects on LOX-1 protein expression within 6 h of CRP treatment (Fig. 6D, E).
Fig. 5.
Effects of FcγRII on sLOX-1 release from activated macrophages induced by CRP. Macrophages were stimulated with TNF-α (5 µg/ml) for 12 h. A: Cells were incubated with FcγRII (CD32) siRNA (100 nM), FcγRI (CD64) siRNA (100 nM), or Con siRNA for 48 h, then CD32 and CD64 protein expression was determined by Western blot analysis. B: Quantity analysis of A. **P < 0.01 versus Con siRNA. C: Cells were incubated with antibodies against CD32, CD64, or IgG (all 3 µg/ml) for 1 h and incubated with CD32 siRNA (100 nM) or CD64 siRNA (100 nM) for 48 h, and then treated with CRP (25 µg/ml) for another 5 h. sLOX-1 level was determined by ELISA. **P < 0.01 versus Con, #P < 0.01 versus CRP. D: Macrophages were incubated with antibody against CD32 (3 µg/ml) for 1 h or with CD32 siRNA (100 nM) for 48 h, then treated with CRP (25 µg/ml) for 40 min. Then intracellular ROS generation was measured. **P < 0.01 versus Con, #P < 0.01 versus CRP. E: Macrophages were incubated with antibody against CD32 (3 µg/ml) for 1 h, and then treated with CRP (25 µg/ml) for 5 h. Then TACE activity was determined. **P < 0.01 versus Con, #P < 0.01 versus CRP. Data are means ± SEM of three different experiments.
Fig. 6.
Effects of FcγRII on p47phox phosphorylation and LOX-1 expression from activated macrophages induced by CRP. Macrophages were stimulated with TNF-α (5 µg/ml) for 12 h. A: Macrophages were incubated with antibody against CD32 (Anti-CD32, 3 µg/ml) for 1 h or CD32 siRNA (100 nM) for 48 h, then treated with CRP (25 µg/ml) for 20 min. Phosphorylated p47phox level of membrane fraction (mPhos-P47) was determined by Western blot analysis. B: Quantity analysis of A. #P < 0.01 versus CRP. C: Cells were incubated with the antibodies against CD32, CD64, IgG or CD32 and CD64 (all 3 µg/ml) for 1 h, then treated with CRP (25 µg/ml) for 6 h. LOX-1 mRNA level was determined by RT-PCR. **P < 0.01 versus Con, P < 0.05 versus CRP. D: Cells were incubated with the antibodies against CD32 or CD64 (both 3 µg/ml) for 1 h, then treated with CRP (25 µg/ml) for 6 h. LOX-1 protein level was determined by Western blot analysis. E: Quantity analysis of D. Data are means ± SEM of three different experiments.
CRP regulated sLOX-1 release from macrophages derived from PBMCs of ACS patients
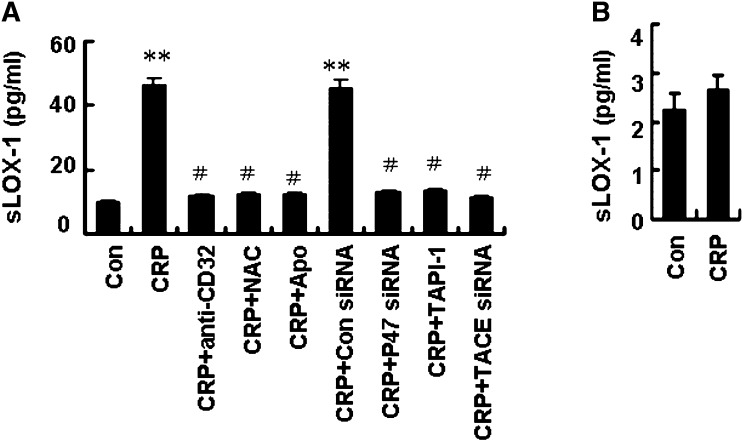
Having established the role of CRP in activated macrophages derived from THP-1 cells, we further examined the effects of CRP on macrophages derived from PBMCs of normal subjects and ACS patients. We observed that CRP enhanced sLOX-1 release from macrophages derived from PBMCs of ACS patients (Fig. 7A); in addition, pretreatment with antibody against CD32 (3 µg/ml), or with NAC (10 mM), Apo (10 mM), p47phox siRNA (100 nM), TAPI-1 (100 µM), and TACE siRNA (200 nM) attenuated sLOX-1 release induced by CRP. However, CRP had no effect on sLOX-1 release from the macrophages of normal subjects (Fig. 7B). Therefore, a signaling pathway of FcγRII-NADPH oxidase (p47phox)-ROS-TACE was involved in sLOX-1 release from activated macrophages induced by CRP (Fig. 8).
Fig. 7.
Effects of CRP on LOX-1 release from macrophages in patients with ACS. A: Macrophages derived from monocytes of ACS patients were preincubated with NAC (10 nM), Apo (10 nM), antibody against CD32 (3 µg/ml) or TAPI-1 (100 µM) for 1 h, P47phox siRNA (100 nM) or TACE siRNA (200 nM) for 48 h, then treated with CRP (25 µg/ml) for 5 h. sLOX-1 levels were determined by ELISA. B: Effects CRP on LOX-1 release from macrophages of normal subjects. Data are means ± SEM of six different ACS patients. **P < 0.01 versus Con, #P < 0.01 versus CRP.
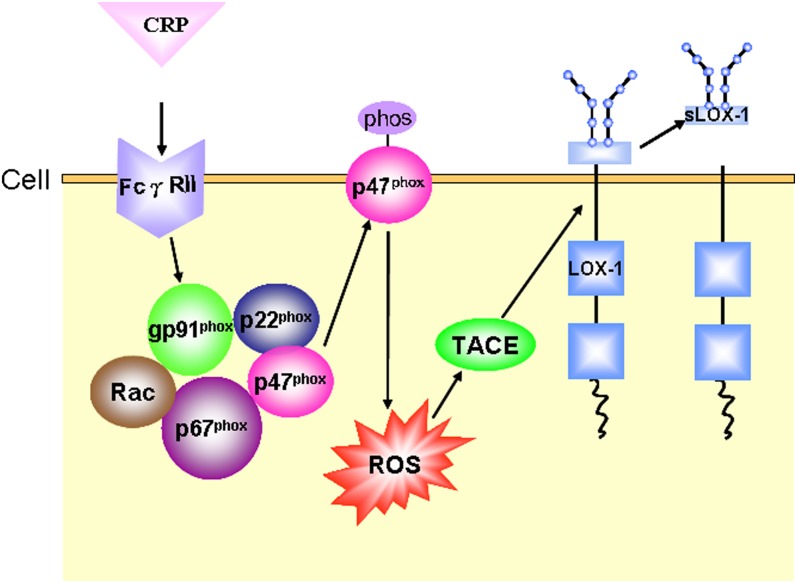
Fig. 8.
Schematic illustration of the pathway of CRP stimulating sLOX-1 release from macrophage membrane. Arrows indicate the pathway involved in sLOX-1 release induced by CRP stimulation.
CRP elevated sLOX-1 levels in vivo in a rabbit model of atherosclerosis
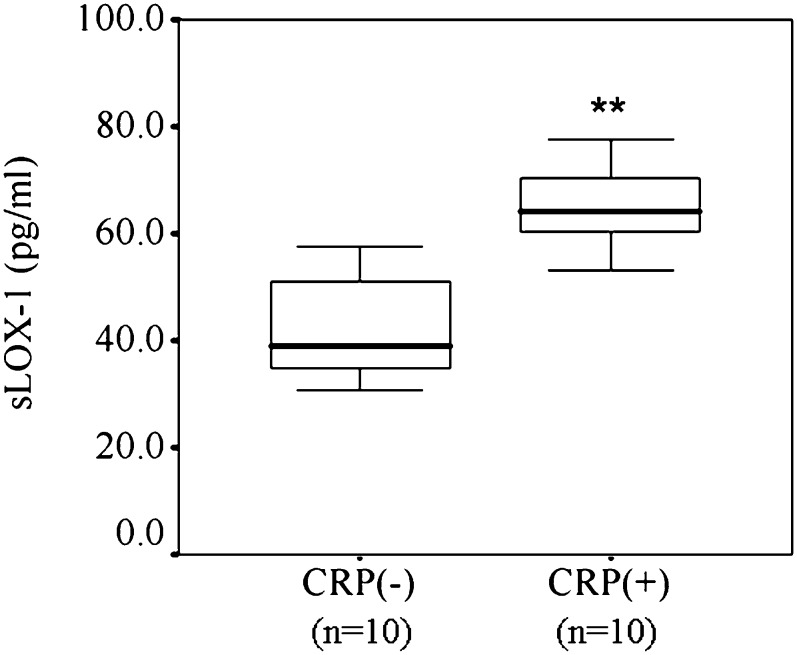
We further examined whether CRP enhanced sLOX-1 levels in vivo by use of a rabbit model of atherosclerosis. Serum levels of human CRP were higher in group A than in group B (16 ± 4 μg/ml versus undetected, P < 0.001). Likewise, circulating sLOX-1 levels were significantly higher in group A than in group B (Fig. 9); median circulating sLOX-1 level increased 1.68-fold: 64.3 pg/ml (interquartile range 59.9-70.7 pg/ml) in group A versus 39.1 pg/ml (34.0-52.0 pg/ml) in group B (P < 0.01).
Fig. 9.
Effects of CRP on circulating sLOX-1 levels in a rabbit model of atherosclerosis. sLOX-1 levels were measured by ELISA 6 h after human recombinant CRP (3 mg/kg) intravenous injection. Median, interquartile ranges, and outliers are indicated by the middle lines, boxes, and outer bars, respectively. **P < 0.01 versus CRP (−). The difference was statistically significant (P < 0.001). n = 10 in each group.
DISCUSSION
Because circulating levels of sLOX-1 play a critical role in the development and progression of atherosclerotic lesions and CRP has been established as an important biomarker of the prognosis of ACS, we tested our hypothesis that CRP might stimulate sLOX-1 release by activating TACE. Our in vitro and in vivo results revealed that CRP might cleave and release sLOX-1 from activated macrophages and that the potential mechanisms might involve FcγRII-mediated p47phox phosphorylation and translocation, ROS production, and subsequent TACE activation (Fig. 8).To the best of our knowledge, this is the first report to link CRP, LOX-1 cleavage, and TACE activation.
In addition to being a predictor of cardiovascular risk, CRP may contribute to the progression of atherosclerosis by directly stimulating vascular cells to synthesize and secrete various inflammatory factors (4, 5, 16). In the current study, we presented evidence that CRP stimulated sLOX-1 release dose-dependently from activated macrophages derived from THP-1 or macrophages derived from PBMCs of ACS patients. We also showed that CRP elevated the serum sLOX-1 levels in atherosclerotic rabbits in vivo. In clinical practice, most patients with stable coronary artery disease have CRP levels <10 μg/ml, and the CRP cutoff value for identifying patients at high risk of cardiovascular events has been defined as 3 μg/ml (17, 18). Recent guidelines recommend that measurement of CRP can be useful in the selection of patients for statin therapy (19)
LOX-1 as a receptor for oxidized LDL mediates vascular dysfunction and promotes foam cell formation. Studies of human atherosclerotic plaques showed that LOX-1 was primarily produced by endothelial cells and abundant in macrophages and smooth muscle cells in advanced atherosclerotic plaques or after inflammatory factor stimulation (20, 21). In this study, we found that TNF-α enhanced LOX-1 expression in macrophages. LOX-1 is expressed on the cell surface and can be proteolytically cleaved at its extracellular domain and released as sLOX-1 (6). Recent clinical studies found that circulating sLOX-1 levels were elevated and had prognostic significance in patients with ACS (8, 22), as elevated sLOX-1 levels reflected increased oxidative stress and inflammatory status in vascular walls (23, 24). The inflammatory factor interleukin (IL)18 can stimulate release of sLOX-1 from cell membrane (25). Our data demonstrated that CRP enhanced the release of sLOX-1 from activated macrophages in vitro and increased circulating sLOX-1 levels in vivo, which agreed with previous studies showing sLOX-1 levels correlated significantly with CRP levels in ACS patients (26). Recent studies have shown that CRP may act a ligand for LOX-1 and that the interaction of CRP with LOX-1 enhances endothelial inflammation (27, 28). Thus, when the CRP concentration reaches a high level, it may stimulate secretion of inflammatory factors and directly impair endothelial function. In addition, when CRP binds with LOX-1 or promotes sLOX-1 release, it may act synergistically with LOX-1 or sLOX-1 to accelerate vascular inflammation and endothelial dysfunction.
In general, soluble inflammatory factors can be generated by two different mechanisms: splicing out the exon encoding the transmembrane region or proteolytic cleavage of the membrane-bound receptor (29, 30). LOX-1 structurally belongs to the C-type lectin family and consists of an extracellular lectin-like domain, a single transmembrane domain, and a short cytoplasmic tail. Thus, CRP can be proteolytically cleaved at its extracellular domain and released as sLOX-1 (31). LOX-1 can be cleaved at two different sites, Lys89-Ser90 and Arg86-Ser87, in the extracellular domain (32). Our data suggest that the enzymes involved in the release of sLOX-1 are PMSF-sensitive, as PMSF could inhibit the release of sLOX-1 from activated macrophages induced by CRP (Fig. 1). TACE has been found to shed membrane-bound inflammatory factors into active soluble forms, including tumor necrosis factor (TNF)-α, TNFR, epidermal growth factor receptor, and transforming growth factor-α (10, 33, 34). In the current study, pretreatment with the TACE inhibitor TAPI-1 or with TACE siRNA abolished the release of sLOX-1 induced by CRP, which indicated that TACE was involved in the process of sLOX-1 release from CRP-stimulated macrophages.
TACE is synthesized in a latent form and activated before reaching the cell-surface membrane (10). TACE activation depends on posttranslational processing, in which ROS oxidating a crucial cysteine thiol group leads to full enzymic activity (11). In the current study, pretreating activated macrophages with the ROS scavenger NAC abolished sLOX-1 release and TACE activation induced by CRP, which indicated that ROS production induced by CRP fully governed CRP-mediated TACE activity and sLOX-1 release from activated macrophages. These observations were consistent with other reports that ROS could stimulate the release of TGF-α via activating TACE (14).
The cytoplasmic NADPH oxidase complex has been implicated as the main source of cellular ROS in the expression and release of inflammatory factors (35). A role for ROS derived from NADPH oxidase in TACE activation has been reported for inflammatory factor IL-8 release (36). CRP could attenuate endothelial nitric oxide synthase activity and induce endothelial dysfunction, which was mediated by ROS generated from NADPH oxidase of endothelial cells (37, 38). In the current study, we observed that the ROS scavenger NAC, NADPH oxidase inhibitor Apo and p47phox siRNA could reduce the release of sLOX-1 and TACE activation induced by CRP in activated macrophages. Thus, NADPH oxidase was involved in CRP-mediated ROS production, TACE activation and subsequent sLOX-1 release in activated macrophages.
CRP binds to both FcγRI (CD64) and FcγRII (CD32) in human macrophages (39, 40). FcγRs modulate the activation of NADPH oxidase in neutrophils and mediate intracellular ROS generation in macrophages (41, 42). CRP could activate NADPH oxidase and upregulate ROS generation through FcγRs in human aortic endothelial cells (43). We found that pretreatment with antibody against CD32 or CD32 siRNA (but not with antibody against CD64 or CD64 siRNA) decreased sLOX-1 release. Thus, it was FcγRII rather than FcγRI that was involved in the sLOX-1 release from activated macrophages induced by CRP. Our results agreed with previous findings that CRP enhanced macrophage lipoprotein lipase expression through CD32 rather than CD64 (44). Moreover, ROS production, TACE activation, and phosphorylation of p47phox induced by CRP were all attenuated by pretreatment with antibody to CD32 or with CD32 siRNA. Thus, FcγRII was involved in CRP-induced NADPH oxidase activation, TACE activation, and subsequent sLOX-1 release in activated macrophages.
In conclusion, we demonstrated that CRP could stimulate sLOX-1 release from activated macrophages in vitro and elevate sLOX-1 levels in a rabbit model of atherosclerosis in vivo. These data provided further evidence for the role of CRP in ACS.
Footnotes
Abbreviations:
- ACS
- acute coronary syndrome
- Apo
- apocynin
- CRP
- C-reactive protein
- DCF-DA
- 2′,7′-dichlorodihydrofluorescein diacetate
- FcγR
- Fc gamma receptor
- IL
- interleukin
- LOX-1
- lectin-like oxidized low-density lipoprotein receptor-1
- NAC
- N-acetylcysteine
- PBMC
- peripheral blood mononuclear cell
- PMSF
- phenylmethyl sulfonylfluoride
- ROS
- reactive oxygen species
- siRNA
- small interfering RNA
- TACE
- tumor necrosis factor-α converting enzyme
- TAPI-1
- tumor necrosis factor-α protease inhibitor 1
- TNF-α
- tumor necrosis factor-α
This work was supported by the National 973 Basic Research Program of China (Nos. 2009CB521900 and 2011CB503906), the National High-Tech Research and Development Program of China (No. 2006AA02A406), the State Program of National Natural Science Foundation of China for Innovative Research Group (No. 81021001), the State Key Program of National Natural Science Foundation of China (No. 60831003), and the National Natural Science Foundation of China (Nos. 30700301, 30971096, 30972809, and 81000126).
REFERENCES
- 1.Libby P., Theroux P. 2005. Pathophysiology of coronary artery disease. Circulation. 111: 3481–3488. [DOI] [PubMed] [Google Scholar]
- 2.De Servi S., Mariani M., Mariani G., Mazzone A. 2005. C-reactive protein increase in unstable coronary disease cause or effect? J. Am. Coll. Cardiol. 46: 1496–1502. [DOI] [PubMed] [Google Scholar]
- 3.Li L., Roumeliotis N., Sawamura T., Renier G. 2004. C-reactive protein enhances LOX-1 expression in human aortic endothelial cells: relevance of LOX-1 to C-reactive protein-induced endothelial dysfunction. Circ. Res. 95: 877–883. [DOI] [PubMed] [Google Scholar]
- 4.Khreiss T., Jozsef L., Potempa L. A., Filep J. G. 2005. Loss of pentameric symmetry in C-reactive protein induces interleukin-8 secretion through peroxynitrite signaling in human neutrophils. Circ. Res. 97: 690–697. [DOI] [PubMed] [Google Scholar]
- 5.Singh U., Devaraj S., Dasu M. R., Ciobanu D., Reusch J., Jialal I. 2006. C-reactive protein decreases interleukin-10 secretion in activated human monocyte-derived macrophages via inhibition of cyclic AMP production. Arterioscler. Thromb. Vasc. Biol. 26: 2469–2475. [DOI] [PubMed] [Google Scholar]
- 6.Navarra T., Del Turco S., Berti S., Basta G. 2010. The lectin-like oxidized low-density lipoprotein receptor-1 and its soluble form: cardiovascular implications. J. Atheroscler. Thromb. 17: 317–331. [DOI] [PubMed] [Google Scholar]
- 7.Kume N., Mitsuoka H., Hayashida K., Tanaka M., Kominami G., Kita T. 2010. Soluble lectin-like oxidized LDL receptor-1 (sLOX-1) as a sensitive and specific biomarker for acute coronary syndrome--comparison with other biomarkers. J. Cardiol. 56: 159–165. [DOI] [PubMed] [Google Scholar]
- 8.Hayashida K., Kume N., Murase T., Minami M., Nakagawa D., Inada T., Tanaka M., Ueda A., Kominami G., Kambara H., et al. 2005. Serum soluble lectin-like oxidized low-density lipoprotein receptor-1 levels are elevated in acute coronary syndrome: a novel marker for early diagnosis. Circulation. 112: 812–818. [DOI] [PubMed] [Google Scholar]
- 9.Huovila A. P., Turner A. J., Pelto-Huikko M., Karkkainen I., Ortiz R. M. 2005. Shedding light on ADAM metalloproteinases. Trends Biochem. Sci. 30: 413–422. [DOI] [PubMed] [Google Scholar]
- 10.Black R. A., Rauch C. T., Kozlosky C. J., Peschon J. J., Slack J. L., Wolfson M. F., Castner B. J., Stocking K. L., Reddy P., Srinivasan S., et al. 1997. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 385: 729–733. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Z., Oliver P., Lancaster J. J., Schwarzenberger P. O., Joshi M. S., Cork J., Kolls J. K. 2001. Reactive oxygen species mediate tumor necrosis factor alpha-converting, enzyme-dependent ectodomain shedding induced by phorbol myristate acetate. FASEB J. 15: 303–305. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi S., Inoue N., Ohashi Y., Terashima M., Matsui K., Mori T., Fujita H., Awano K., Kobayashi K., Azumi H., et al. 2003. Interaction of oxidative stress and inflammatory response in coronary plaque instability: important role of C-reactive protein. Arterioscler. Thromb. Vasc. Biol. 23: 1398–1404. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L., Liu Y., Lu X. T., Wu Y. L., Zhang C., Ji X. P., Wang R., Liu C. X., Feng J. B., Jiang H., et al. 2009. Traditional Chinese medication Tongxinluo dose-dependently enhances stability of vulnerable plaques: a comparison with a high-dose simvastatin therapy. Am. J. Physiol. Heart Circ. Physiol. 297: H2004–H2014. [DOI] [PubMed] [Google Scholar]
- 14.Shao M. X., Nadel J. A. 2005. Dual oxidase 1-dependent MUC5AC mucin expression in cultured human airway epithelial cells. Proc. Natl. Acad. Sci. USA. 102: 767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serezani C. H., Aronoff D. M., Jancar S., Peters-Golden M. 2005. Leukotriene B4 mediates p47phox phosphorylation and membrane translocation in polyunsaturated fatty acid-stimulated neutrophils. J. Leukoc. Biol. 78: 976–984. [DOI] [PubMed] [Google Scholar]
- 16.Ridker P. M., Stampfer M. J., Rifai N. 2001. Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA. 285: 2481–2485. [DOI] [PubMed] [Google Scholar]
- 17.Ridker P. M. 2003. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 107: 363–369. [DOI] [PubMed] [Google Scholar]
- 18.Ridker P. M., Cook N. 2004. Clinical usefulness of very high and very low levels of C-reactive protein across the full range of Framingham Risk Scores. Circulation. 109: 1955–1959. [DOI] [PubMed] [Google Scholar]
- 19.Greenland P., Alpert J. S., Beller G. A., Benjamin E. J., Budoff M. J., Fayad Z. A., Foster E., Hlatky M. A., Hodgson J. M., Kushner F. G., et al. 2010. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 56: e50–e103. [DOI] [PubMed] [Google Scholar]
- 20.Aoyama T., Sawamura T., Furutani Y., Matsuoka R., Yoshida M. C., Fujiwara H., Masaki T. 1999. Structure and chromosomal assignment of the human lectin-like oxidized low-density-lipoprotein receptor-1 (LOX-1) gene. Biochem. J. 339: 177–184. [PMC free article] [PubMed] [Google Scholar]
- 21.Kataoka H., Kume N., Miyamoto S., Minami M., Moriwaki H., Murase T., Sawamura T., Masaki T., Hashimoto N., Kita T. 1999. Expression of lectinlike oxidized low-density lipoprotein receptor-1 in human atherosclerotic lesions. Circulation. 99: 3110–3117. [DOI] [PubMed] [Google Scholar]
- 22.Kume N., Mitsuoka H., Hayashida K., Tanaka M., Kita T. 2010. Soluble lectin-like oxidized low-density lipoprotein receptor-1 predicts prognosis after acute coronary syndrome - a pilot study. Circ. J. 74: 1399–1404. [DOI] [PubMed] [Google Scholar]
- 23.Kamezaki F., Yamashita K., Tasaki H., Kume N., Mitsuoka H., Kita T., Adachi T., Otsuji Y. 2009. Serum soluble lectin-like oxidized low-density lipoprotein receptor-1 correlates with oxidative stress markers in stable coronary artery disease. Int. J. Cardiol. 134: 285–287. [DOI] [PubMed] [Google Scholar]
- 24.Tan K. C., Shiu S. W., Wong Y., Leng L., Bucala R. 2008. Soluble lectin-like oxidized low density lipoprotein receptor-1 in type 2 diabetes mellitus. J. Lipid Res. 49: 1438–1444. [DOI] [PubMed] [Google Scholar]
- 25.Mitsuoka H., Kume N., Hayashida K., Inui-Hayashiada A., Aramaki Y., Toyohara M., Jinnai T., Nishi E., Kita T. 2009. Interleukin 18 stimulates release of soluble lectin-like oxidized LDL receptor-1 (sLOX-1). Atherosclerosis. 202: 176–182. [DOI] [PubMed] [Google Scholar]
- 26.Lubrano V., Del Turco S., Nicolini G., Di Cecco P., Basta G. 2008. Circulating levels of lectin-like oxidized low-density lipoprotein receptor-1 are associated with inflammatory markers. Lipids. 43: 945–950. [DOI] [PubMed] [Google Scholar]
- 27.Shih H. H., Zhang S., Cao W., Hahn A., Wang J., Paulsen J. E., Harnish D. C. 2009. CRP is a novel ligand for the oxidized LDL receptor LOX-1. Am. J. Physiol. Heart Circ. Physiol. 296: H1643–H1650. [DOI] [PubMed] [Google Scholar]
- 28.Fujita Y., Kakino A., Nishimichi N., Yamaguchi S., Sato Y., Machida S., Cominacini L., Delneste Y., Matsuda H., Sawamura T. 2009. Oxidized LDL receptor LOX-1 binds to C-reactive protein and mediates its vascular effects. Clin. Chem. 55: 285–294. [DOI] [PubMed] [Google Scholar]
- 29.Hooper N. M., Karran E. H., Turner A. J. 1997. Membrane protein secretases. Biochem. J. 321: 265–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rose-John S., Heinrich P. C. 1994. Soluble receptors for cytokines and growth factors: generation and biological function. Biochem. J. 300: 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sawamura T., Kume N., Aoyama T., Moriwaki H., Hoshikawa H., Aiba Y., Tanaka T., Miwa S., Katsura Y., Kita T., et al. 1997. An endothelial receptor for oxidized low-density lipoprotein. Nature. 386: 73–77. [DOI] [PubMed] [Google Scholar]
- 32.Murase T., Kume N., Kataoka H., Minami M., Sawamura T., Masaki T., Kita T. 2000. Identification of soluble forms of lectin-like oxidized LDL receptor-1. Arterioscler. Thromb. Vasc. Biol. 20: 715–720. [DOI] [PubMed] [Google Scholar]
- 33.Hinkle C. L., Mohan M. J., Lin P., Yeung N., Rasmussen F., Milla M. E., Moss M. L. 2003. Multiple metalloproteinases process protransforming growth factor-alpha (proTGF-alpha). Biochemistry. 42: 2127–2136. [DOI] [PubMed] [Google Scholar]
- 34.Sunnarborg S. W., Hinkle C. L., Stevenson M., Russell W. E., Raska C. S., Peschon J. J., Castner B. J., Gerhart M. J., Paxton R. J., Black R. A., et al. 2002. Tumor necrosis factor-alpha converting enzyme (TACE) regulates epidermal growth factor receptor ligand availability. J. Biol. Chem. 277: 12838–12845. [DOI] [PubMed] [Google Scholar]
- 35.Jiang F., Zhang Y., Dusting G. J. 2011. NADPH oxidase-mediated redox signaling: roles in cellular stress response, stress tolerance, and tissue repair. Pharmacol. Rev. 63: 218–242. [DOI] [PubMed] [Google Scholar]
- 36.Koff J. L., Shao M. X., Ueki I. F., Nadel J. A. 2008. Multiple TLRs activate EGFR via a signaling cascade to produce innate immune responses in airway epithelium. Am. J. Physiol. Lung Cell. Mol. Physiol. 294: L1068–L1075. [DOI] [PubMed] [Google Scholar]
- 37.Singh U., Devaraj S., Vasquez-Vivar J., Jialal I. 2007. C-reactive protein decreases endothelial nitric oxide synthase activity via uncoupling. J. Mol. Cell. Cardiol. 43: 780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hein T. W., Singh U., Vasquez-Vivar J., Devaraj S., Kuo L., Jialal I. 2009. Human C-reactive protein induces endothelial dysfunction and uncoupling of eNOS in vivo. Atherosclerosis. 206: 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tron K., Manolov D. E., Rocker C., Kachele M., Torzewski J., Nienhaus G. U. 2008. C-reactive protein specifically binds to Fcgamma receptor type I on a macrophage-like cell line. Eur. J. Immunol. 38: 1414–1422. [DOI] [PubMed] [Google Scholar]
- 40.Heyman B. 2003. Feedback regulation by IgG antibodies. Immunol. Lett. 88: 157–161. [DOI] [PubMed] [Google Scholar]
- 41.Daniel D. S., Dai G., Singh C. R., Lindsey D. R., Smith A. K., Dhandayuthapani S., Hunter R. L., Jr, Jagannath C. 2006. The reduced bactericidal function of complement C5-deficient murine macrophages is associated with defects in the synthesis and delivery of reactive oxygen radicals to mycobacterial phagosomes. J. Immunol. 177: 4688–4698. [DOI] [PubMed] [Google Scholar]
- 42.Davidson-Moncada J. K., Lopez-Lluch G., Segal A. W., Dekker L. V. 2002. Involvement of protein kinase D in Fc gamma-receptor activation of the NADPH oxidase in neutrophils. Biochem. J. 363: 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh U., Devaraj S., Jialal I. 2005. C-reactive protein decreases tissue plasminogen activator activity in human aortic endothelial cells: evidence that C-reactive protein is a procoagulant. Arterioscler. Thromb. Vasc. Biol. 25: 2216–2221. [DOI] [PubMed] [Google Scholar]
- 44.Maingrette F., Li L., Renier G. 2008. C-reactive protein enhances macrophage lipoprotein lipase expression. J. Lipid Res. 49: 1926–1935. [DOI] [PubMed] [Google Scholar]