Abstract
In traditional folk medicine, Xanthoxylum plants are referred to as ‘toothache trees’ because their anesthetic or counter-irritant properties render them useful in the treatment of pain. Psychophysical studies have identified hydroxy-α-sanshool as the compound most responsible for the unique tingling and buzzing sensations produced by Szechuan peppercorns or other Xanthoxylum preparations. Although it is generally agreed that sanshool elicits its effects by activating somatosensory neurons, the underlying cellular and molecular mechanisms remain a matter of debate. Here we show that hydroxy-α-sanshool excites two types of sensory neurons, including small-diameter unmyelinated cells that respond to capsaicin (but not mustard oil) as well as large-diameter myelinated neurons that express the neurotrophin receptor TrkC. We found that hydroxy-α-sanshool excites neurons through a unique mechanism involving inhibition of pH- and anesthetic-sensitive two-pore potassium channels (KCNK3, KCNK9 and KCNK18), providing a framework for understanding the unique and complex psychophysical sensations associated with the Szechuan pepper experience.
Somatosensation, or the sense of touch, is the process whereby we detect changes in ambient temperature or pressure. This sensory modality is mediated by subsets of primary afferent neurons that detect chemical, thermal or mechanical stimuli over a range of stimulus intensities. Generally speaking, pain-producing (noxious) stimuli are detected by neurons (referred to as nociceptors) that have small- to medium-diameter somata that correspond to unmyelinated C and lightly myelinated Aδ nerve fibers. In contrast, innocuous stimuli, such as light touch, are detected by large-diameter neurons corresponding to more heavily myelinated Aα or Aβ fibers1. These main groups of somatosensory neurons can be further subdivided based on their expression of numerous molecular markers or their specific functional (biophysical or pharmacological) characteristics.
A key goal in understanding somatosensation is to elucidate the contribution of sensory neuron subtypes to specific psychophysical sensations. In this regard we, and others, have exploited the power of folk medicine and natural products to probe somatosensory mechanisms and identify functionally and molecularly distinct classes of somatosensory neurons2–4. In particular, pungent plant-derived irritants, such as capsaicin, mustard oil and menthol, have been used to define nociceptor subtypes and the receptors that mediate painproducing thermal or inflammatory responses in vivo5,6. This approach has been extremely fruitful in identifying cellular and molecular mechanisms contributing to nociception and the detection of painful stimuli. Thus, it is interesting to ask whether other plant-derived compounds, perhaps those eliciting milder somatosensory percepts, could be useful in identifying neuronal subtypes that contribute to the detection of non-noxious stimuli. In this regard, we have focused our attention on natural products produced by Xanthoxylum plants, such as the Chinese prickly ash, from which Szechuan peppercorns are harvested.
Szechuan peppers or related plants have been exploited for their medicinal and culinary properties in both traditional Asian and Native American cultures7,8. In contrast to the intense, burning pain associated with ‘hot’ chili peppers of the Capsicum family, Szechuan peppers elicit a wholly unique sensation that is best described as a tingling paresthesia or numbing9,10, suggestive of an interaction with neurons involved in tactile sensation and innocuous touch11. Hydroxy-α-sanshool (sanshool) is the active ingredient in Szechuan peppers, and although there has been some preliminary analysis of its effects on cultured sensory neurons9,11,12, its cellular and molecular site of action remains enigmatic. For example, sanshool was initially proposed to activate subsets of primary afferent fibers that respond to cooling, heat or light touch11, whereas a more recent study suggests that most, if not all, sensory neurons respond to sanshool. Furthermore, two studies reach different conclusions as to the involvement of specific molecular targets, most notably the capsaicin receptor (TRPV1), in this response10,12.
Here, we take a multifaceted approach to elucidate the cellular and molecular basis of sanshool action. We show that sanshool activates a constellation of sensory neurons that include specific subpopulations of small- and large-diameter cells, which together represent a unique subset of nociceptors and presumptive light-touch receptors. Moreover, we find that sanshool excites these neurons by inhibiting background potassium conductances. Specifically, we identify three members of the pH-sensitive two-pore KCNK channel family as being molecular targets for sanshool action. Notably, these channels are also targeted by volatile anesthetics, perhaps accounting for the numbing properties elicited by sanshool compounds and for the use of Xanthoxylum extracts in traditional folk medicine for treating toothache and other types of orofacial pain.
RESULTS
Sanshool activates a unique subset of somatosensory neurons
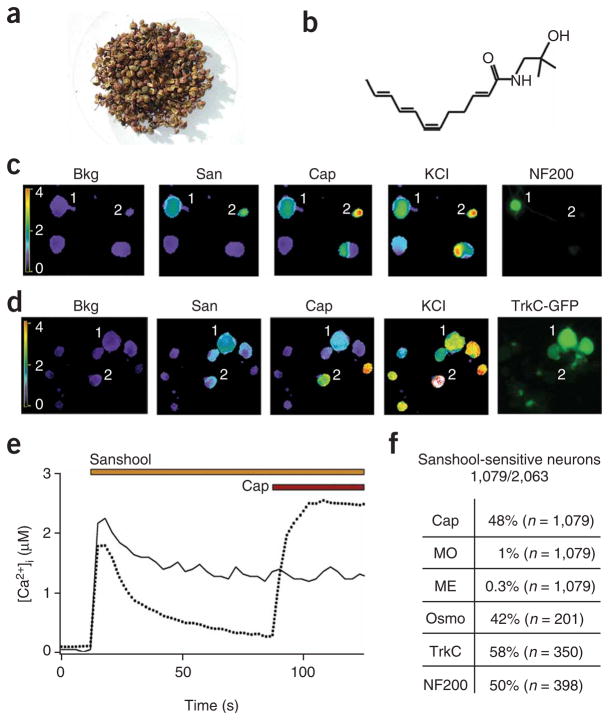
We first purified sanshool from Szechuan pepper (Fig. 1a,b and Supplementary Fig. 1 online) and asked whether it excites a specific subpopulation of sensory neurons. Neurons were cultured from trigeminal or dorsal root ganglia of the mouse and responses assessed using live-cell calcium imaging as a functional readout (Fig. 1c–f). We found that sanshool (100 μM) serves as an excitatory agent for a specific subgroup (52.3%; n = 2,063) of sensory neurons that could be further categorized into two main classes based on expression of molecular markers13. One class corresponds to a subset of smalldiameter, unmyelinated neurons that express the capsaicin receptor, TRPV1; the other class corresponds to a subset of large-diameter, myelinated neurons that show NF-200 immunoreactivity and express the neurotrophin (NT-3) receptor TrkC (Fig. 1c,d). We also examined the pharmacological properties of these sanshool-sensitive classes using three additional natural-product agonists—capsaicin, mustard oil and menthol—that define cells expressing excitatory TRPV1, TRPA1 and TRPM8 channels, respectively5,14. Consistent with our histological results, we found that the sanshool-sensitive small-diameter neurons were also activated by capsaicin, but not by mustard oil or menthol (Fig. 1e,f). In contrast, the sanshool-sensitive, large-diameter cells were not activated by any of these irritants, but did respond to osmotic stimuli (Fig. 1f). Based on these characteristics, we conclude that sanshool sensitivity is exhibited by both presumptive nociceptors and low-threshold mechanoreceptors. Notably, sanshool sensitivity now provides a functional marker for the subset of capsaicin-sensitive neurons that do not respond to mustard oil (the TRPV1-positive, TRPA1-negative population) and that represent ~50% of capsaicinexcitable cells15.
Figure 1.
Hydroxy-α-sanshool excites a subset of presumptive nociceptors and mechanoreceptors. (a) Szechuan peppers are the spicy berries of Xanthoxylum piperitum, a species of prickly ash found in China and Japan. (b) Structure of hydroxy-α-sanshool, the main pungent compound from Xanthoxylum plants. (c) Cultured sensory neurons were exposed to sanshool (San; 100 μM) followed by capsaicin (Cap; 1 μM) and subsequently 140 mM potassium chloride (KCl) and were analyzed by calcium imaging. No response to sanshool was observed in the absence of extracellular calcium, demonstrating that the calcium signal is due to influx (data not shown). After calcium imaging, neurons were fixed and probed for NF200 reactivity by immunohistochemistry. Responses to sanshool were observed in NF200- positive, capsaicin-insensitive neurons (cell 1) as well as NF200-negative, capsaicin-sensitive neurons (cell 2). (d) Cultured DRG sensory neurons from TrkC-GFP mice were treated and analyzed as in c. Sanshool responses were observed in TrkC-positive, capsaicin-insensitive neurons (cell 1) as well as TrkC-negative, capsaicin-sensitive neurons (cell 2). (e) Calcium imaging shows that some sanshool-sensitive cells are capsaicin sensitive, whereas others are not, corresponding to small- and large-diameter neurons, respectively (average sizes = 18.0 and 35.7 μm; average response from 15 representative cells). (f) Quantitative analysis of concordance between sanshool sensitivity and other histological or pharmacological attributes. Cells showing sensitivity to sanshool (100 μM) were examined for activation by capsaicin (Cap; 1 μM), mustard oil (MO; 100 μM), menthol (ME; 500 μM) or hypo-osmotic (Osmo; 226 mOsm) stimuli, as well as for expression of TrkC or neurofilament (NF200) immunoreactivity. Note the high (>40%) preponderance of capsaicin or hypo-osmotic sensitivity among sanshoolsensitive neurons, as compared to the relatively low (≤1%) sensitivity to mustard oil or menthol. Moreover, many (≥50%) of sanshool-positive cells were myelinated (NF200 positive) and/or TrkC positive.
Sanshool inhibits a pH-sensitive K+ leak conductance
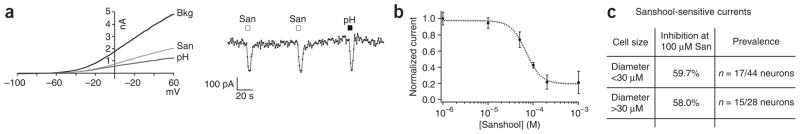
We next used whole-cell voltage-clamp recording methods to determine the nature of the ionic current(s) underlying sanshool-evoked depolarization of these neurons. Application of sanshool led to a dosedependent inhibition of an outwardly rectifying background leak current (Fig. 2a). Moreover, the sanshool-evoked block was reversible and showed no sensitization in response to repetitive application. Inhibition was observed in a subset of both small- and large-diameter trigeminal neurons, with a half-maximal inhibitory concentration (IC50) of 69.5 ± 5.3 μM (Fig. 2b). The extent of inhibition (at 100 μM sanshool) was equivalent for each size class (59.7 ± 2.6% and 58.0 ± 2.4%, respectively; n = 6–7; Fig. 2c). Replacement of extracellular Na+ or Ca2+ with NMDG or EGTA, respectively, had no effect on this sanshool-inhibited current, demonstrating that it is not mediated by influx of these cations. In contrast, replacement of extracellular Na+ with 135 mM K+ resulted in a shift of the reversal potential from −78.5 ± 6.2 mV to −3.8 ± 2.9 mV (data not shown). In addition, sanshool-sensitive currents were not observed when K+ was replaced with Cs+. Taken together, these data show that sanshool depolarizes sensory neurons by inhibiting a background K+ channel.
Figure 2.
Sanshool inhibits pH-sensitive background potassium channels in sensory neurons. (a) Representative whole-cell voltage-clamp recording from a cultured trigeminal neuron subjected to a voltage ramp (+60 mV to −100 mV, 100 ms; applied every 2 s). Current-voltage relationship before (Bkg) or after application of sanshool (100 μM) or low pH (pH 6.5) (left). Average current recorded at −60 mV in response to sanshool or low pH (right). (b) Dose-response curve of sanshool-evoked inhibition of background potassium conductance in sensory neurons (holding potential = –60 mV) recorded in extracellular Ringer’s solution (IC50 = 69.5 ± 5.3 μM; n = 3–7 cells per point). (c) Summary of sanshool-sensitive currents measured in small- and large-diameter neurons.
We further found that the sanshool-sensitive current was unaltered by classical K+ channel blockers, such as tetraethylammonium (TEA) or 4-aminopyridine (4-AP) (data not shown), but could also be inhibited by extracellular protons (Fig. 2a). Both KCNQ and KCNK channels fit this pharmacological profile16–18, suggesting that sanshool targets one or more members of these K+ channel families. The KCNQ inhibitors linopiridine (50 μM) and XE-991 (50 μM) neither triggered calcium influx nor altered sanshool-evoked calcium responses in cultured sensory neurons (data not shown). Thus, we conclude that KCNK channels are the likely targets of sanshool action. Indeed, members of this two-pore K+ channel subfamily (KCNK2, KCNK3, KCNK9, KCNK10 and KCNK18) have been proposed to set the resting membrane potential of primary afferent sensory neurons, with KCNK18 (TRESK) having the predominant role19–21.
Sanshool targets members of the KCNK K+ channel family
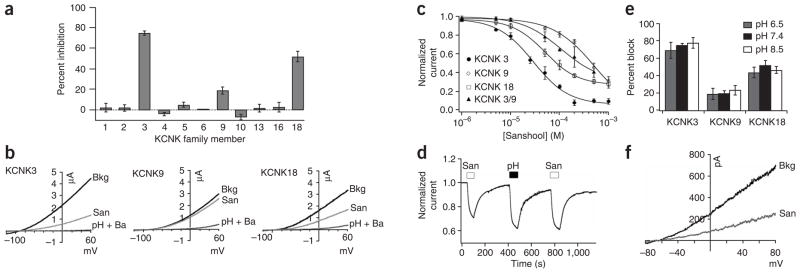
To determine whether KCNK channels show sanshool sensitivity, we expressed each family member in Xenopus laevis oocytes and asked whether bath-applied sanshool suppressed basal K+ currents in these cells (Fig. 3a). Only three subtypes showed significant inhibition, including KCNK3, KCNK9 and KCNK18 (also known as TASK-1, TASK-3 and TRESK, respectively; Fig. 3a,b), all of which are targeted by volatile and local anesthetics17,18,22–27. The greatest potency was observed with KCNK3 and KCNK18 (IC50 = 30.3 ± 4.9 and 50.2 ± 1.9 μM, respectively), whereas KCNK9 was much less sensitive (IC50 = 450 ± 30.1 μM) (Fig. 3c). KCNK3 and KCNK9 are capable of forming functional heteromeric complexes28, and we found that such heteromeric channels showed intermediate sanshool sensitivity, with an IC50 = 252 ± 31 μM (Fig. 3c). As observed in cultured neurons (Fig. 2a), sanshool-evoked inhibition of cloned KCNK channels was fully reversible (Fig. 3d). Moreover, although KCNK channels are strongly regulated by protons, changes in extracellular pH did not affect the sanshool sensitivity of KCNK3, KCNK9 or KCNK18 (Fig. 3e), demonstrating that their effects are not additive. Analysis of inside-out patches excised from KCNK18-expressing HEK293 cells showed that sanshool is fully capable of inhibiting this channel in a membranedelimited manner, whether applied to the inside or the outside surface of the membrane (Fig. 3f). Taken together, these results demonstrate that a subset of pH-sensitive KCNK channels is directly inhibited by hydroxy-α-sanshool.
Figure 3.
Sanshool inhibits KCNK3, KCNK9 and KCNK18. (a) Xenopus oocytes expressing a given KCNK family member were subjected to two-electrode voltage-clamp analysis and the percent suppression of leak current was determined after bath application of purified sanshool (100 μM) (n = 5–8 cells per channel). (b) Representative traces of sanshool-evoked inhibition of KCNK3 (left), KCNK9 (middle) and KCNK18 (right) in oocytes (holding potential = −80 mV). Inhibition by extracellular protons (pH 6.5) and barium (2 mM) is shown for comparison. (c) Dose-response curves of sanshool-evoked inhibition at 0 mV of KCNK3, KCNK9, KCNK18 or KCNK3/KCNK9 heteromers, recorded in Xenopus oocytes (n = 5–8 cells per point). (d) Representative current recorded from Xenopus oocytes expressing KCNK18 in response to sanshool (100 μM) or low pH (pH 6.5) (holding potential = −60mV, n = 5). (e) Sanshool-evoked inhibition of KCNK channels at pH 6.5, pH 7.4 and pH 8.5 (n = 4–10 oocytes per condition). (f) Inhibition of KCNK18 currents by sanshool (100 μM) applied to an inside-out patch from transfected HEK293 cells (n = 7). Seals were obtained with extracellular Ringer’s solution in both pipette and bath. After excision of the patch, bath solution was replaced with high-potassium Ringer’s (see Methods). Sanshool has no effect on background currents observed in vector-transfected control cells (not shown).
Szechuan pepper extracts also contain hydroxy-β-sanshool, but this isomer plays a minor role in eliciting the tingling psychophysical effects of Xanthoxylum plants10. Indeed, we found that application of hydroxy-β-sanshool (100 μM–1 mM) did not excite sensory neurons (Supplementary Fig. 2a online). Among cloned KCNK channels, only KCNK3 showed significant inhibition by hydroxy-β-sanshool (Supplementary Fig. 2b–e), suggesting that this subtype does not mediate sanshool sensitivity in primary afferent neurons. Furthermore, these results are consistent with the finding that hydroxy-α-sanshool is the principal pungent agent in Szechuan peppercorns.
Excitatory channels are not targets of sanshool action
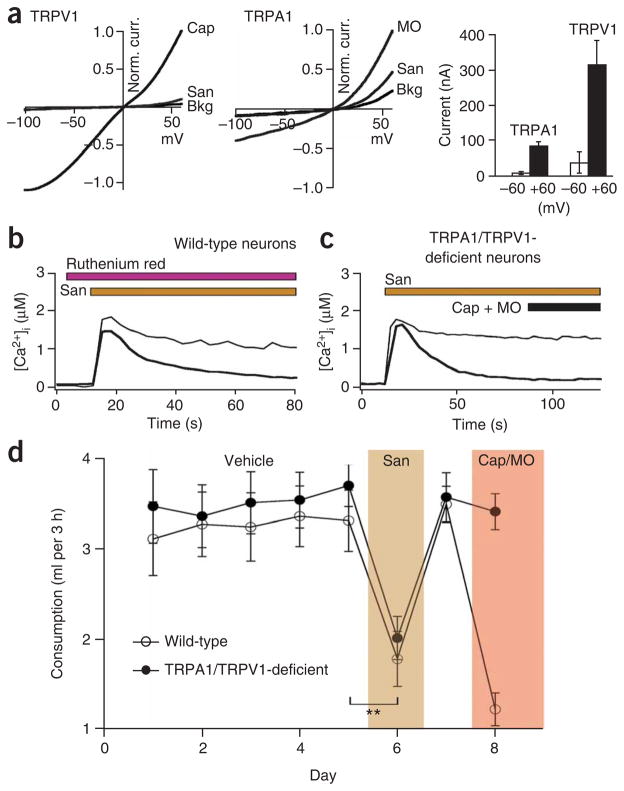
The idea that sanshool depolarizes neurons by blocking an outward K+ current is somewhat unexpected because a recent report has suggested that excitability is achieved through activation of inward currents. Specifically, TRPV1 and TRPA1 have been proposed to serve as sanshool receptors12. In light of our findings, we carried out a more comprehensive analysis of sanshool specificity by examining its effects on a range of other heterologously expressed potassium channels as well as excitatory ion channels known to be present on sensory neurons, including members of the Kv, Kir, TRP, ASIC/MDEG, P2X and 5-HT3 families (Supplementary Fig. 3 online). No significant actions were observed with any of these candidates, except for minor and somewhat anomalous effects on TRPV1 and TRPA1 at very high (1 mM) sanshool concentrations. In these cases, sanshool activated an outward current that was blocked by ruthenium red (a nonselective TRP channel inhibitor), but inward currents were extremely small (<1% of maximal response to capsaicin or mustard oil) and therefore unlikely to account for neuronal excitation (Fig. 4a and data not shown). One surprising finding was that pluronic acid, which was previously used to enhance sanshool solubility, was itself an activator of TRPA1; this may account for the previous suggestion12 that sanshool activates cloned or native TRPA1 channels and induces nocifensive behavior (Supplementary Fig. 3). To avoid such nonspecific actions in our experiments, we used DMF and/or β-cyclodextrin to increase sanshool solubility, as these agents have no effect on sensory neurons or cloned channels (Supplementary Fig. 4 online).
Figure 4.
TRPA1 and TRPV1 are not required for sanshool sensitivity. (a) Sanshool elicits small, but detectable, currents in TRPV1- (left) or TRPA1-expressing (middle) Xenopus oocytes but only at positive membrane potentials. Bar graph (right) shows summary of sanshool (1 mM)-evoked currents recorded at +60 versus −60 mV holding potential (n = 5). (b) Sanshool-evoked calcium influx in trigeminal sensory neurons is not blocked by ruthenium red (10 μM), a blocker of TRPV1 and TRPA1 channels (n = 114). (c) Sanshool-evoked calcium influx is normal in neurons cultured from mice deficient in both TRPV1 and TRPA1 (n = 108). (d) Consumption of water containing sanshool (1 mM) was significantly decreased in TRPA1 TRPV1–deficient mice (closed circles), as well as in their wild-type littermates (open circles). In contrast, only wild-type animals showed decreased consumption of water containing capsaicin and mustard oil. **P o 0.01, one-way ANOVA; n = 10 animals per genotype.
Additional observations further suggest that the physiological actions of sanshool are unlikely to be mediated by activation of TRPV1 or TRPA1. For example, we found that crude Szechuan pepper extracts or purified sanshool (over a wide concentration range: 0.01–1 mM) does not excite TRPA1-positive (mustard oil–sensitive) sensory neurons and activates only a subset of TRPV1-positive (capsaicinsensitive) cells (Fig. 1d and Supplementary Fig. 5 online). Furthermore, sanshool-evoked neuronal responses were not affected by ruthenium red (Fig. 4b) or by the TRPV1-selective antagonist capsezapine (data not shown). Perhaps most significantly, sanshool-evoked responses were not altered (in prevalence or magnitude) in sensory neurons cultured from mutant mice lacking both TRPV1 and TRPA1 channels (Fig. 4c). Finally, these double-mutant mice showed the same aversion to sanshool-infused water as their wild-type littermates in a timed drinking test: both wild-type and TRPV1 TRPA1 double-mutant mice drank sanshool-containing (1 mM) water for 5–15 s, but then became agitated, moved quickly around the cage and rubbed their faces, reflecting a gradual onset of sanshool-elicited irritancy of the oral cavity. In contrast, when provided with capsaicin- and mustard oillaced water, wild-type animals showed a characteristically rapid onset of drinking cessation and irritancy29,30, to which TRPV1 TRPA1 double-mutant mice were completely insensitive (Fig. 4d and SupplementaryMovie 1 online). Although these findings contrast with the conclusions of one earlier study12, they are consistent with other work showing that sanshool and capsaicin sensitivity are non-overlapping at the cellular level11 and that activation of cloned TRPV1 channels by sanshool is insufficiently robust to account for its pungency9.
CNS neurons expressing KCNK3 and KCNK9 are sanshool sensitive
Taken together, our data suggest that KCNK channels, and not TRP or other excitatory channels, are the physiological targets of sanshool action. To further test this hypothesis, we asked whether cerebellar granule neurons (CGN), which lack sensory TRP channels but express KCNK channels as their major background K+ conductance28,31–33, are sanshool sensitive. Indeed, we observed marked and robust calcium responses in most, if not all, of these neurons after bath application of sanshool (100 μM) (Fig. 5a). In contrast, cultured hippocampal neurons, in which KCNQ rather than KCNK channels account for primary background K+ conductance34, were insensitive to sanshool (data not shown).
Figure 5.
Sanshool excites CNS neurons that express KCNK3, KCNK9 or KCNK18. (a) Representative calcium response of cultured cerebellar granule neurons (CGNs) in response to sanshool (100 μM; left). Representative electrophysiological response of a CGN to sanshool (100 μM) or protons (pH 6.5) during whole-cell voltage-clamp recording (holding potential = −60 mV) (right). (b) Comparison of sanshool-evoked calcium responses by CGNs cultured for 2 d (top) versus 7 d (bottom) (days in vitro, d.i.v.). (c) Quantitative PCR analysis of sanshool-sensitive KCNK transcripts from CGN cultured for 7 d.i.v. versus 2 (n = 3–4).
As observed with sensory neurons, sanshool-evoked responses in CGNs were not altered by classical K+ channel inhibitors (TEA, 4-AP) or by ruthenium red (data not shown). Moreover, CGNs were insensitive to capsaicin and mustard oil, ruling out expression of functional TRPV1 or TRPA1 channels by these cells (data not shown). These results were corroborated by whole-cell voltage-clamp recordings, in which sanshool inhibited a pH-sensitive background K+ current (43% at 200 μM) (Fig. 5a). Taken together, these results support the idea that KCNK channels serve as physiological targets for sanshool action in both sensory and CNS neurons.
Sanshool sensitivity correlates with KCNK subtype expression
While examining CGNs in culture, we found that these cells are insensitive to sanshool during the first 2 d in culture, but develop sensitivity by day 7 (Fig. 5b). In fact, previous studies have shown that background potassium conductances change significantly during the first week ofCGNculture32. We therefore asked whether the acquisition of sanshool sensitivity corresponds with a temporal change in the expression of one or more KCNK channel subtypes. Quantitative PCR analysis revealed a marked increase in the expression of KCNK3 and KCNK9 transcripts (17.4 ± 4.4 and 8.6 ± 1.3-fold, respectively) from the second to the seventh day of culture (Fig. 5c, Supplementary Fig. 6). In contrast, expression of KCNK18 did not change significantly (1.2 ± 0.5-fold increase) during this time. Thus, we conclude that sanshool sensitivity of CGNs is likely mediated by KCNK3 and/or KCNK9, but not KCNK18. This is consistent with previous studies demonstrating that KCNK3 and KCNK9 account for the bulk of background K+ current in these cells28,31,33.
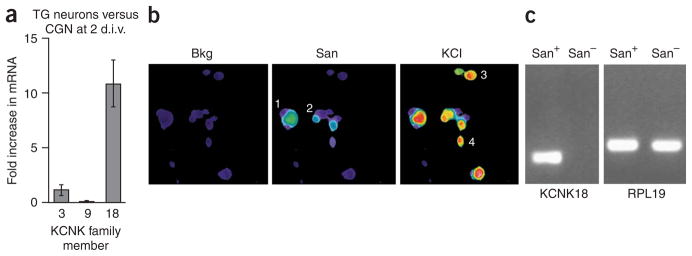
Finally, we assessed the profile of KCNK expression in trigeminal sensory ganglia using quantitative PCR (Fig. 6a, Supplementary Fig. 6). When compared to CGNs on day 2 (when they are sanshool insensitive), trigeminal neurons have similar levels of KCNK3 and lower levels of KCNK9, indicating that these subtypes do not contribute to the sanshool sensitivity of trigeminal neurons. In contrast, KCNK18 transcript levels were significantly more abundant (13.1 ± 1.2-fold) in trigeminal ganglia than in CGNs at day 2 or 7, suggesting that this subtype has an important role in mediating sanshool sensitivity of primary sensory neurons. Indeed, among sanshool-sensitive KCNK subtypes, KCNK18 contributes significantly to the background K+ current in cultured DRG neurons20. Moreover, in situ hybridization histochemistry suggests that KCNK18 transcripts are expressed by a significant fraction of primary sensory neurons21. However, this analysis is likely to overestimate the prevalence of KCNK18 expression because of the high sequence conservation among KCNK subtypes, with consequent hybridization of in situ probes to numerous KCNK transcripts. We therefore used a more stringent PCR-based analysis to correlate KCNK18 gene expression with sanshool sensitivity among trigeminal neurons. We collected cytoplasmic contents from small groups (2–3) of cells that were either sanshool sensitive or sanshool insensitive as determined by calcium imaging (Fig. 6b) and subjected them to RT-PCR using KCNK18-specific primers. These results clearly showed that the expected KCNK18 fragment was amplified from all sanshool-responsive neurons, but was not observed in any sanshoolinsensitive samples (Fig. 6c).
Figure 6.
Sanshool excites sensory neurons that express KCNK3, KCNK9 or KCNK18. (a) Quantitative PCR analysis of sanshoolsensitive KCNK expression in cultured trigeminal (TG) sensory neurons versus CGN cultured for 2 d.i.v. (n = 3–4). (b) Representative calcium imaging experiment used to identify sanshoolsensitive cells. Cells 1 and 2 are sanshoolsensitive, whereas cells 3 and 4 are insensitive. (c) Representative PCR analysis of KCNK18 expression in sanshool-positive and sanshoolnegative sensory neurons. Lane 1 contains a sample amplified from cDNA prepared from cells 1 and 2; lane 2 contains a sample amplified from cDNA prepared from cells 3 and 4 (see above). n = 6 samples for sanshool-sensitive neurons and 3 for sanshool-insensitive neurons; each sample contained 2–3 cells. Note the presence of control RPL19 product in all samples (right).
DISCUSSION
Our findings elucidate the molecular mechanism through which pungent agents of Xanthoxylum plants mediate their unique psychophysical effects, akin to the experience of touching one’s tongue to the terminals of a 9-V battery. This sensation is rather distinct from that elicited by other pungent natural products, such as those derived from chili peppers or wasabi, which produce a more acute and painful irritation that is also associated with local tissue inflammation and pain hypersensitivity. Moreover, our data suggest that sanshool is unique among pungent agents in that its excitatory actions are mediated by inhibition of pH-sensitive background potassiumchannels, rather than through the more familiar mechanism involving direct activation of an excitatory TRP channel2. Indeed, such functional differences may contribute to perceived differences in the pungency (onset or intensity) of these natural-product irritants. More broadly, the mechanism of sanshool-evoked K+ channel inhibition differs from that of most chemosensory agents, which involve G protein–coupled receptor signaling pathways35,36.
Differences in pungency perception and other neurally mediated effects may also reflect the activation of overlapping, but distinct, subpopulations of primary afferent sensory neurons. For example, capsaicin activates a subgroup of small-diameter sensory neurons, of which approximately 50% are also activated by mustard oil. This latter component of dually responsive neurons also express proinflammatory neuropeptides (CGRP and substance P), accounting for both the acute pain and the neurogenic inflammatory actions commonly associated with exposure to chili peppers, wasabi or garlic. In contrast, we show that sanshool excites only the subset of capsaicin-sensitive neurons that are mustard oil insensitive, thereby excluding most of the peptidergic nociceptors. This is consistent with the fact that exposure to sanshool is not associated with intense pain, neurogenic inflammation or hyperalgesia.
We also found that sanshool activates large-diameter, TrkC-positive, myelinated neurons, which are generally associated with proprioception and the detection of non-noxious mechanical stimuli, such as light touch or vibration37. It is therefore notable that the majority of TrkC-positive, osmotically sensitive neurons are also sensitive to sanshool, consistent with the idea that sanshool elicits its tingling and buzzing sensation by activating a cohort of touch-sensitive fibers. Sanshool therefore serves as the first pungent natural product with which to identify this specific cohort of mechanosensitive primary afferent neurons.
At the molecular level, sanshool has been proposed to activate neurons by opening excitatory ion channels9,11,12, and we were therefore surprised to find that it depolarizes neurons by inhibiting background potassium channels. Aside from our direct electrophysiological characterization of sanshool-sensitive membrane currents in sensory neurons, our examination of various cloned channels also rules out a significant involvement of members of the TRP channel family that have been suggested to play a role in this response, including receptors for capsaicin, mustard oil and menthol (TRPV1, TRPA1 and TRPM8, respectively). Rather, our data demonstrate that a subset of pH-sensitive two-pore K+ channels, namely KCNK3, KCNK9 and KCNK18, are the molecular targets of sanshool action. Indeed, sanshool now provides a new pharmacological tool for discriminating among two-pore K+ channel subtypes, one that is more selective in its action compared to rutheniumred, zinc, protons and anesthetics, all of which target multiple KCNK subtypes as well as other ion channels.
Our data clearly show that KCNK3 and KCNK18 are the principal sanshool-sensitive subtypes in sensory neurons, whereas in CGNs, KCNK3 and KCNK9 predominate, thereby accounting for the bulk of cellular sensitivity to sanshool. Our pharmacological results with hydroxy-β-sanshool indicate that KCNK3 homomeric channels are not primary contributors to the excitatory effects of sanshool, where KCNK18 or KCNK3/KCNK9 heteromeric complexes must account for these actions in sensory neurons or CGNs, respectively. Further analysis of the relative contributions of these channels to sanshool sensitivity must await the development of additional subtype-selective antagonists or the generation of triple KCNK3-, KCNK9- and KCNK18-deficient mice, because animals lacking any single KCNK subtype show compensatory upregulation of related channels31,38. Similarly, the analysis of such animals will show whether additional ion channels or receptors contribute to sanshool sensitivity at the behavioral level.
Xanthoxylum plants have been exploited for centuries as natural analgesics to alleviate acute and chronic pain. Fruits of these plants have also been use extensively in the kitchen because of their unique pungent qualities. In contrast to the familiar burning or irritating pain elicited by chili peppers or mustard extracts, the sensorial experience produced by Szechuan peppercorns is more generally described as “tingling and numbing,” “mild electric shock” or a “pins and needles” effect9,11. These psychophysical percepts are in many ways consistent with the cellular and molecular sites that we identify as sanshool targets. Thus, the numbing qualities of sanshoolmay resemble the effects of anesthetics on KCNK3, KCNK9 and KCNK18. The tactile component may result from the activation of large-diameter, touch-sensitive fibers, whereas the pungent or irritant qualities may involve excitation of nonpeptidergic, capsaicin-sensitive nociceptors. The identification of sanshool-sensitive channels and sensory neuron subtypes represents an essential first step in understanding mechanisms underlying this unique pungency and its relationship to tactile sensitivity.
METHODS
Pharmacological reagents
Capsaicin and capsazepine were purchased from Tocris. All other chemicals were purchased from Sigma. Hydroxy-α-sanshool (2E,6Z,8E,10E)-2′-hydroxyl-N-isobutyl-2,6,8,10-dodecatetraenamide) was purified as follows: dried seeds from Zanthoxylum piperitum (50 g; San Francisco Herb Company) were ground to a fine powder and extracted twice, each time with 1 liter diethyl ether for 24 h at 4 °C. Extracts were combined and filtered, solvent removed in vacuo, and the residue further dried on high vacuum overnight to yield 4.25 g of crude material. This was further purified by flash chromatography (1:2 ethyl acetate/hexanes; 230–400-mesh silica gel, Selecto Scientific) followed by preparative HPLC (30–100% methanol gradient over 40 min; 10 ml min−1; COMBI-A C18 preparatory column, Peeke Scientific; absorbance monitored at 215 and 245 nm) to give 55.2 mg of sanshool (0.1% yield; pale brown solid). As indicated by NMR spectra, this material consists predominantly of hydroxy-α-sanshool. LC-ESI-MS [MH]+ m/z for hydroxy-α-sanshool, C16H25NO2: calculated, 264.38; observed, 264.48. Pure hydroxy-β-sanshool was isolated using an Xterra C-18 column (Waters) on a Parallex Flex (Biotage) preparative HPLC instrument with a solvent gradient of 20–70% acetonitrile/water at a flow rate of 20 ml min−1 for 60 min. Injections were monitored at 254 nm and each fraction lyophilized and assessed by 1H NMR.
1H spectra were recorded on a Varian 400 spectrometer at 400 MHz. Chemical shifts were reported as parts per million (p.p.m.) downfield from an internal tetramethylsilane standard (d = 0.0 for 1H NMR) or from solvent references. Low-resolution electrospray ionization mass spectra (EI+-MS) were recorded on a Waters Micromass ZQ 4000 spectrometer. LC-MS (MS: EI+) was performed on a Waters Alliance HT LC-MS with a flow rate of 0.2 ml min−1 (monitored at 215 nm and 245 nm) using an Xterra MS C18 column (Waters). Analytical thin-layer chromatography was performed with silica gel 60 F254 glass plates (EM Science). Purified hydroxy-α-sanshool was dissolved in dimethylformamide (DMF at a concentration of 100 mM and stored at −80 °C. 200 μM β-cyclodextrin was added to all solutions containing 1 mM sanshool to increase solubility.
Neuronal cell culture, calcium imaging and electrophysiology
Preparation of mouse trigeminal or dorsal root ganglion neurons and ratiometric calcium imaging were carried out as previously described15. Extracellular Ringer’s solution contained 140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM D-glucose and 10 mM sodium HEPES (pH 7.4). Extracellular Ringer’s was supplemented with 1 μM tetrodotoxin citrate (Tocris) for voltage-clamp recordings. High-potassium Ringer’s solution, used for excised patch recordings, contained 150 mM KCl, 5 mM NaCl, 1 mM CaCl2, 1 mM EGTA, 2 mM MgCl2 and 10 mM sodium HEPES (pH 7.4). Primary cultures of mouse CGN were prepared from postnatal day 7 (P7) mouse cerebellum as described39. Whole-cell patch-clamp recordings were made at 7–9 d.i.v. in an extracellular solution containing 145 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 5 mM D-glucose, 25 mM sucrose, 5 mM HEPES (all from Sigma) and 1 μM tetrodotoxin citrate (Tocris), pH 7.3. Internal pipette solution containing 150 mM potassium methyl sulfate, 10 mM KCl, 4 mM NaCl, 10 mM HEPES, 0.4 mM tetrasodium GTP and 4 mM dimagnesium ATP, pH 7.25, was used for all recordings. Agonist was applied via a local perfusion barrel system (Automate Scientific). For calcium imaging, cells were loaded with 10 μM Fura-2- AM (Molecular Probes) at 22–25 °C for 60 min in extracellular Ringer’s solution. Cells were illuminated using a xenon light source and filter wheel (Lambda LS and Lambda-10, Sutter Instruments) for 300 ms, alternately at 350 nm and 380 nm (band-pass filters from Chroma Technology). Fluorescence emission at >480 nm (long-pass filter from Chroma Technology) was captured with an intensified CCD camera (Hamamatsu) and was digitized, background corrected and analyzed with the MetaFluor imaging system (Molecular Devices). Background-corrected 340/380 ratio images were collected every 3 s. Intracellular calcium concentration ([Ca2+]i)was determined from the relationship [Ca2+]i = K*(R – Rmin)/(Rmax – R), where R is the F340/F380 ratio, Rmin and Rmax are the ratios at 0 Ca2+ and saturating Ca2+ (10 mM), respectively, and K* is the apparent dissociation constant40.
For oocyte expression, cDNAs were linearized and transcribed with T7 RNA polymerase (mMessage mMachine, Ambion). Two-electrode voltage-clamp analysis was performed 1–5 d after cRNA injection. Currents were recorded in ND96 (96 mM NaCl, 2 mM KCl, 0.3 mM CaCl2, 1 mM MgCl2 and 5 mM HEPES, pH 7.4). For recordings at pH 6.5 and pH 8.5, solutions were adjusted using HCl or NaOH, respectively. Similar results were obtained when using 2-(N-morpholino)ethanesulfonic acid (MES) as a high-pH buffer. TRPV1- expressing cells were analyzed in calcium-free solution (120 mM CsCl, 1 mM EGTA, 10 mM HEPES, 2 mM MgCl2, pH 7.4), and Kir2.1 and Kir3.1/3.4 were analyzed using high-potassium solutions (20 mM NaCl, 78 mM KCl, 1 mM CaCl2, 2 mM MgCl2, pH 7.4, for Kir2.1 and 2 mM NaCl, 96 mM KCl, 1 mM CaCl2, 2 mM MgCl2, pH 7.4, for Kir3.1/3.4). Because heterologously expressed KCNK1 does not form functional homomeric channels, a mutant (K274E) that shows basal activity41 was expressed in these experiments. Currents were recorded with a GeneClamp 500B amplifier and Digidata 1322A interface and acquired with pClamp software (Axon Instruments).
Immunohistochemistry
After calcium imaging, neurons were fixed in PBS containing 4% formaldehyde at 4 °C for 10 min, then washed with PBS and permeabilized in PBS containing 0.1% Triton X-100 for 5 min at 22–25 °C. Samples were then incubated with 10% horse serum and 0.1% (vol/vol) Triton X-100 for 1 h, followed by anti-NF200 mouse monoclonal antibody (1:500 dilution, Sigma) overnight at 4 °C. Samples were washed three times, for 10 min each, with PBS containing 0.3% Triton X-100, and then incubated with Alexa Fluor 488 secondary antibody (Molecular Probes) at room temperature for 30 min, washed three times for 10 min each with PBS and visualized by indirect immunofluorescence.
Mice and behavior
Mice (20–35 g) were housed with 12 h/12 h light/dark cycle at 21 °C. All experiments were performed according to the policies and recommendations of the International Association for the Study of Pain and approved by the University of California, San Francisco, Institutional Animal Care and Use Committee. TRPV1 TRPA1 double-mutant mice were generated by crossing TRPV1−/− and TRPA1−/− animals15,29; resulting TRPV1+/− TRPA1+/− progeny were then crossed to yield wild-type and double-knockout siblings for analysis. TrkC-GFP mice have been described elsewhere42.
Sanshool sensitivity was assessed with an aversive drinking test as previously described29. On days 1–5 and 7, mice were allowed to drink for 3 h per day from a bottle containing 0.125% saccharine plus vehicle (1:200 DMF) in water. On day 6, this solution was supplemented with sanshool extract (3%, described above) or purified hydroxy-α-sanshool. 100 μM sanshool was not adequate to elicit robust avoidance responses, which we ascribe to issues such as long-term stability, light sensitivity, solubility of sanshool in drinking containers, and tissue access. We therefore used 1 mM sanshool in these behavioral tests. Notably, 100 μM or 1 mM sanshool, and crude extracts, do not elicit different spectra of sensory neuron responses (see Supplementary Fig. 5). On day 8, the drinking solution was supplemented with 1 μM capsaicin and 100 μMmustard oil. All drinking solutions were presented to both wild-type and TRPV1 TRPA1 double-knockout mice (n = 10 per genotype). Volumes consumed were measured each day.
PCR
RNA was isolated from trigeminal ganglia or whole brains of P0 mice with Trizol (Invitrogen). First-strand cDNA was transcribed using murine Moloney leukemia virus reverse transcriptase and poly dT(12–18) primers. After PCR amplification, resulting cDNAs were blunt-cloned into the EcoRV site of the pMO vector. All sequences were verified by DNA sequencing. KCNK family members were PCR amplified using specific primer pairs for each gene (Supplementary Table 1).
For quantitative PCR (qPCR) experiments, total RNA samples were isolated from cultured trigeminal ganglion neurons or CGNs and reverse transcribed as described above. qPCR experiments were performed using Sybr Green reagents and Applied Biosystems 7300 Real-Time PCR system. All experiments were performed in triplicate. Amplification of ribosomal protein L19 (RPL19) transcripts was used as a standard and for normalization of all qPCR reactions. 100–250-bp fragments were amplified using specific primers for each gene (Supplementary Table 2).
For RT-PCR analysis of individual sensory neurons, cells were examined for sanshool sensitivity by calcium imaging and 2–3 cells in each category were aspirated into a large-diameter glass electrode filled with lysis buffer (50 mM Tris-Cl, pH 8.3, 75 mM KCl, 3 mM MgCl2, 5 U μl−1 RNasin (Promega)) and flash frozen. Reverse transcription was performed using both murine Moloney leukemia virus and avian reverse transcriptases at 37 °C for 1 h. The product was diluted 1:5 and used as the template for PCR experiments.
Supplementary Material
Acknowledgments
We are grateful to S. Yost (University of California, San Francisco) and D. Kim (Rosalind Franklin University) for providing KCNK6 and KCNK16 cDNAs, respectively; H. Haeberle and E. Lumpkin (University of California, San Francisco, and Baylor College of Medicine) for TrkC-GFP mice; A. Tzingounis and A. Priel for helpful discussion; and J. Poblete for expert technical assistance. This work was supported by grants from the NIH (D.J. and R.A.N.), a Burroughs Welcome Fund Career Award in Biomedical Sciences (D.M.B.) and an NSF Graduate Research Fellowship (A.D.M.).
Footnotes
Note: Supplementary information is available on the Nature Neuroscience website.
AUTHOR CONTRIBUTIONS
D.M.B. and Y.M.S. designed and carried out cellular physiology and histological studies involving native and cloned ion channels; D.M.B. designed and carried out behavioral experiments; D.M.B., Y.M.S. and A.D.M. carried out experiments involving analysis of CGNs; J.L.G. and J.A.Z. designed and effected purification procedures and chemical analysis of sanshool compounds; P.R.T. contributed to gene cloning and electrophysiological analysis; D.M.B., Y.M.S. and D.J. wrote the manuscript; R.A.N. and D.J. provided advice and guidance throughout.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/
References
- 1.Meyer RA, Ringkamp M, Campbell JN, Raja SN. Peripheral mechanisms of cutaneous nociception. In: McMahon SB, Koltzenburg M, editors. Textbook of Pain. Elsevier; Philadelphia: 2006. pp. 3–34. [Google Scholar]
- 2.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 3.Fields HL. Pain. McGraw-Hill; New York: 1987. [Google Scholar]
- 4.Snyder SH. Opiate receptors and internal opiates. Sci Am. 1977;236:44–56. doi: 10.1038/scientificamerican0377-44. [DOI] [PubMed] [Google Scholar]
- 5.Julius D. From peppers to peppermints: natural products as probes of the pain pathway. Harvey Lect. 2005;101:89–115. [PubMed] [Google Scholar]
- 6.Woolf CJ, Ma Q. Nociceptors–noxious stimulus detectors. Neuron. 2007;55:353–364. doi: 10.1016/j.neuron.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 7.Foster S, Duke JA. Eastern/Central Medicinal Plants and Herbs. Houghton-Mifflin; New York: 2000. [Google Scholar]
- 8.McGee H. On Food and Cooking: The Science and Lore of the Kitchen. Scribner; New York: 2004. [Google Scholar]
- 9.Sugai E, et al. Pungent qualities of sanshool-related compounds evaluated by a sensory test and activation of rat TRPV1. Biosci Biotechnol Biochem. 2005;69:1951–1957. doi: 10.1271/bbb.69.1951. [DOI] [PubMed] [Google Scholar]
- 10.Sugai E, Morimitsu Y, Kubota K. Quantitative analysis of sanshool compounds in Japanese pepper (Xanthoxylum piperitum DC.) and their pungent characteristics. Biosci Biotechnol Biochem. 2005;69:1958–1962. doi: 10.1271/bbb.69.1958. [DOI] [PubMed] [Google Scholar]
- 11.Bryant BP, Mezine I. Alkylamides that produce tingling paresthesia activate tactile and thermal trigeminal neurons. Brain Res. 1999;842:452–460. doi: 10.1016/s0006-8993(99)01878-8. [DOI] [PubMed] [Google Scholar]
- 12.Koo JY, et al. Hydroxy-alpha-sanshool activates TRPV1 and TRPA1 in sensory neurons. Eur J Neurosci. 2007;26:1139–1147. doi: 10.1111/j.1460-9568.2007.05743.x. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi K, et al. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with aδ/c-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- 14.Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- 15.Bautista DM, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 16.Delmas P, Brown DA. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat Rev Neurosci. 2005;6:850–862. doi: 10.1038/nrn1785. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein SA, Bockenhauer D, O’Kelly I, Zilberberg N. Potassium leak channels and the KCNK family of two-P-domain subunits. Nat Rev Neurosci. 2001;2:175–184. doi: 10.1038/35058574. [DOI] [PubMed] [Google Scholar]
- 18.Lesage F, Lazdunski M. Molecular and functional properties of two-pore-domain potassium channels. Am J Physiol Renal Physiol. 2000;279:F793–F801. doi: 10.1152/ajprenal.2000.279.5.F793. [DOI] [PubMed] [Google Scholar]
- 19.Alloui A, et al. TREK-1, a K+ channel involved in polymodal pain perception. EMBO J. 2006;25:2368–2376. doi: 10.1038/sj.emboj.7601116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang D, Kim D. TREK-2 (K2P10.1) and TRESK (K2P18.1) are major background K+ channels in dorsal root ganglion neurons. Am J Physiol Cell Physiol. 2006;291:C138–C146. doi: 10.1152/ajpcell.00629.2005. [DOI] [PubMed] [Google Scholar]
- 21.Dobler TM, et al. TRESK two-pore-domain K+ channels constitute a significant component of background potassium currents in murine DRG neurones. J Physiol (Lond) 2007;585:867–879. doi: 10.1113/jphysiol.2007.145649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duprat F, et al. TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J. 1997;16:5464–5471. doi: 10.1093/emboj/16.17.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim Y, Bang H, Kim D. TASK-3, a new member of the tandem pore K+ channel family. J Biol Chem. 2000;275:9340–9347. doi: 10.1074/jbc.275.13.9340. [DOI] [PubMed] [Google Scholar]
- 24.Sano Y, et al. A novel two-pore domain K+ channel, TRESK, is localized in the spinal cord. J Biol Chem. 2003;278:27406–27412. doi: 10.1074/jbc.M206810200. [DOI] [PubMed] [Google Scholar]
- 25.Liu C, Au JD, Zou HL, Cotten JF, Yost CS. Potent activation of the human tandem pore domain K channel TRESK with clinical concentrations of volatile anesthetics. Anesth Analg. 2004;99:1715–1722. doi: 10.1213/01.ANE.0000136849.07384.44. [DOI] [PubMed] [Google Scholar]
- 26.Patel AJ, et al. Inhalational anesthetics activate two-pore-domain background K+ channels. Nat Neurosci. 1999;2:422–426. doi: 10.1038/8084. [DOI] [PubMed] [Google Scholar]
- 27.Talley EM, Bayliss DA. Modulation of TASK-1 (Kcnk3) and TASK-3 (Kcnk9) potassium channels: volatile anesthetics and neurotransmitters share a molecular site of action. J Biol Chem. 2002;277:17733–17742. doi: 10.1074/jbc.M200502200. [DOI] [PubMed] [Google Scholar]
- 28.Kang D, Han J, Talley EM, Bayliss DA, Kim D. Functional expression of TASK-1/TASK-3 heteromers in cerebellar granule cells. J Physiol (Lond) 2004;554:64–77. doi: 10.1113/jphysiol.2003.054387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caterina MJ, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 30.Kwan KY, et al. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 31.Brickley SG, et al. TASK-3 two-pore domain potassium channels enable sustained high-frequency firing in cerebellar granule neurons. J Neurosci. 2007;27:9329–9340. doi: 10.1523/JNEUROSCI.1427-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han J, Truell J, Gnatenco C, Kim D. Characterization of four types of background potassium channels in rat cerebellar granule neurons. J Physiol (Lond) 2002;542:431–444. doi: 10.1113/jphysiol.2002.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Millar JA, et al. A functional role for the two-pore domain potassium channel TASK-1 in cerebellar granule neurons. Proc Natl Acad Sci USA. 2000;97:3614–3618. doi: 10.1073/pnas.050012597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peters HC, Hu H, Pongs O, Storm JF, Isbrandt D. Conditional transgenic suppression of M channels in mouse brain reveals functions in neuronal excitability, resonance and behavior. Nat Neurosci. 2005;8:51–60. doi: 10.1038/nn1375. [DOI] [PubMed] [Google Scholar]
- 35.Bargmann CI. Comparative chemosensation from receptors to ecology. Nature. 2006;444:295–301. doi: 10.1038/nature05402. [DOI] [PubMed] [Google Scholar]
- 36.Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444:288–294. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- 37.McMahon SB, Bennett DLH, Bevan S. Inflammatorymediators and modulators of pain. In: McMahon SB, Koltzenburg M, editors. Textbook of Pain. Elsevier; Philadelphia: 2006. pp. 49–72. [Google Scholar]
- 38.Aller MI, et al. Modifying the subunit composition of TASK channels alters the modulation of a leak conductance in cerebellar granule neurons. J Neurosci. 2005;25:11455–11467. doi: 10.1523/JNEUROSCI.3153-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kato AS, et al. New transmembrane AMPA receptor regulatory protein isoform, gamma- 7, differentially regulates AMPA receptors. J Neurosci. 2007;27:4969–4977. doi: 10.1523/JNEUROSCI.5561-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Almers W, Neher E. The Ca signal from fura-2 loaded mast cells depends strongly on the method of dye-loading. FEBS Lett. 1985;192:13–18. doi: 10.1016/0014-5793(85)80033-8. [DOI] [PubMed] [Google Scholar]
- 41.Feliciangeli S, et al. Does sumoylation control K2P1/TWIK1 background K+ channels? Cell. 2007;130:563–569. doi: 10.1016/j.cell.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 42.Funfschilling U, et al. TrkC kinase expression in distinct subsets of cutaneous trigeminal innervation and nonneuronal cells. J Comp Neurol. 2004;480:392–414. doi: 10.1002/cne.20359. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.