Abstract
Background
Evidence suggests that higher levels of vitamin D and calcium are associated with greater lung function and that vitamin D is inversely associated with atopic sensitisation. It is unknown whether the associations of vitamin D and calcium with lung function are independent of each other or mediated by atopic sensitisation.
Objective
To study the associations of 25-hydroxyvitamin D [25(OH)D] and ionised calcium levels with lung function and specific allergen sensitisation in adolescents (12-19 years) and adults (20-59 years) and to assess whether the associations with lung function are due to altered atopic sensitisation.
Methods
Cross-sectional analysis of the data from the third National Health and Nutrition Examination Survey.
Results
25(OH)D levels were positively associated with forced vital capacity in adolescents [0.035(95 %CI: 0.007-0.064) standard deviations;SD in model adjusted for multiple confounders]. This association and the previously reported association between higher serum levels of 25(OH)D and better lung function in adults were independent of serum calcium levels, which were not associated with lung function. In adults, calcium was associated with sensitisation to grass allergens [OR per SD,1.17(1.03-1.32), 1.15(1.01-1.31) and 1.18 (1.06-1.32) for white oak, Bermuda grass and short ragweed, respectively] and peanut OR 1.21 (95%CI 1.02-1.43) after adjusting for age, gender and race/ethnicity, but these associations attenuated towards the null after adjusting for additional confounders. The associations were independent of 25(OH)D levels, which were not associated with allergen sensitisation.
Conclusions
Circulating levels of 25(OH)D are positively associated with lung function and this does not appear to be driven by allergen sensitisation or influenced by calcium levels.
Keywords: vitamin D, calcium, allergy, spirometry, the third National Health and Nutrition Examination Survey
INTRODUCTION
Atopy is characterised by a tendency to mount immunoglobulin E (IgE)-mediated, type I hypersensitivity reactions to allergens. It is strongly associated with allergic diseases, including asthma. In many Western countries 10-20 percent of the population are affected by atopy and the prevalence of atopic diseases in children and adults has increased in these countries from the 1970s (Asher et al., 2006; Upton et al., 2000). Although there is evidence of genetic heritability (Swarr and Hakonarson, 2010), little is known about modifiable risk factors for atopy that might be used in prevention.
Vitamin D is a key regulator of calcium homeostasis, and biological evidence suggests that vitamin D and calcium might be implicated in atopic diseases and respiratory health, (Hughes and Norton, 2009; Litonjua, 2009; Hirota et al., 2007; Chhabra et al., 1999) though epidemiological evidence is limited and inconsistent (Devereux et al., 2007; Laaksi et al., 2007; Ginde et al., 2009; Erkkola et al., 2009; Kunisaki et al., 2010; Hypponen et al., 2004; Hypponen et al., 2009; Searing et al., 2010; Miyake et al., 2010). Differences in how vitamin D exposure and outcomes were assessed or differences in the confounders that were taken into account in different studies may have contributed to differences between them. Wheezing and associated reduced lung function are amongst the wide range of symptoms associated with atopy and there is some evidence of positive association between vitamin D (measured as serum 25-hydroxyvitamin D [25(OH)D] and lung function (Devereux et al., 2007; Searing et al., 2010; Brehm et al., 2009; Black and Scragg, 2005; Sutherland et al., 2010), although a null association has also been reported (Devereux et al., 2009).
Previous studies in the US third National Health and Nutrition Examination Survey (NHANES III) have shown positive association between serum calcium and forced expiratory volume in one second (FEV1) (McKeever et al., 2008) but not with atopy (McKeever et al., 2004). In addition, higher serum 25(OH)D levels were associated with better lung function in the same study (Black and Scragg, 2005), but it is unknown whether the associations of 25(OH)D were independent of calcium and vice versa.
Our study adds to these previous papers by: (i) examining whether the associations of 25(OH)D and calcium are independent of each other: (ii) examining the association of 25(OH)D and calcium with specific allergen sensitisation: (iii) studying the association between serum calcium and FVC and (iv) comparing whether the magnitudes and/or directions of these associations differ between adolescents and adults. The previous lung function studies have included participants older than 17 years only (McKeever et al., 2008) or older than 20 years (Black and Scragg, 2005). The association between calcium and atopy was studied in 6-16 year olds and 17-59 year olds (McKeever et al., 2004).
METHODS
Sample
Data from the third US NHANES III were used. NHANES III, conducted between 1988 and 1994, is a survey of the civilian non-institutionalized population of the United States. Data and documentation are available at http://www.cdc.gov/nchs/nhanes/nh3rrm.htm. Serum 25(OH)D and calcium measurements were available for participants aged ≥12 years and skin prick allergen responses for participants up to age 59 years (with a random 50 percent of 20-59 year olds having these tests). Thus, we included participants aged 12-59 years, examining adolescents (12-19 years) and adults (20-59 years) separately. Figure 1 shows the flow of participants who attended mobile examination clinics and final analyses samples for the studies in adolescents and adults. We only included individuals with complete data on exposures, outcome and all covariables in any analyses meaning that the final sample sizes were 2446 and 2315 adolescents for associations with lung function and allergy tests, respectively and 8049 and 3656 adults for associations with lung function and allergy tests, respectively. The NHANES III study was approved by NHANES Institutional Review Board and documented consent was obtained from participants.
Figure 1.
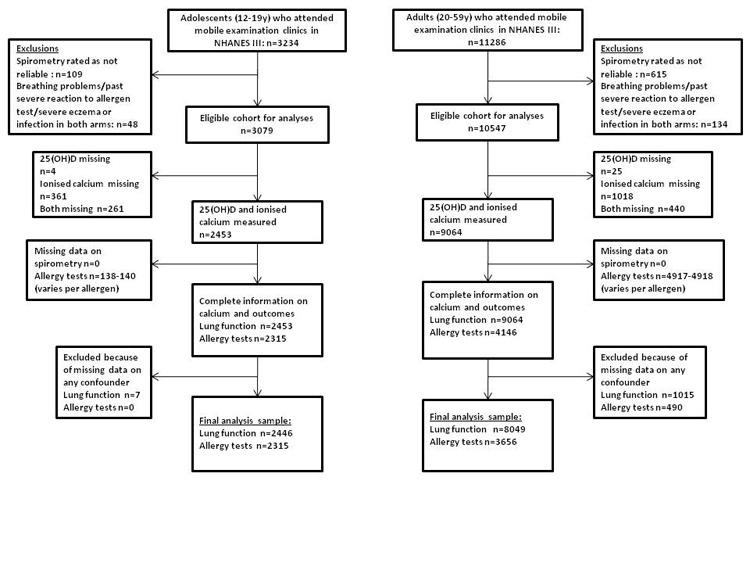
Flowchart of adolescent and adult participants
Exposures
Serum levels of 25(OH)D and calcium were measured on venous samples. 25(OH)D was assayed with INCSTAR 25-OH-D I25 radioimmunoassay (Diasorin, Stillwater, MN, USA) and serum calcium with NOVA 7 analyzer (Nova Biomedical, Waltham, MA, USA). Ionised calcium (adjusted for serum pH) was used in these analyses.
Outcomes
Lung function and allergy tests were conducted at mobile examination centres (MECs). Lung function [FEV1 and forced vital capacity (FVC)] was assessed with a Ohio 827 rolling seal spirometer (Ohio Medical Instrument Company, Cincinnati, OH USA) according to American Thoracic Society criteria (1987) (American Thoracic Society, 1987).
The allergen panel consisted of 10 common allergens (Dermatophagoides farinae, cat, German cockroach, short ragweed, perennial rye, Alternaria alternata, Bermuda grass, Russian thistle, white oak and peanut) and a negative (glycerinated diluent) control. Hypersensitivity reactions were evaluated 15 minutes after administering the allergens on separate sites of examinee's forearms with prick-puncture technique. A positive test response was defined as maximum weal diameter≥3mm for allergen and <3mm for negative control. Atopy was defined as a positive response to one or more allergens. Individuals with severe eczema or signs of skin infection on both arms, those with previous severe response to allergen testing or participants with signs of breathing difficulties were excluded from the allergy tests. We appreciate that these might include individuals with most severe atopy, but due to small numbers (39 adolescents; 111 adults) it was not possible to examine this as an outcome by itself in both adolescents and adults.
Potential confounders
The following were considered potential confounding factors due to their known or plausible associations with exposures and outcomes: age, gender, race/ethnicity, height, health status, socioeconomic position, outdoor activity and smoking.
Height and weight were measured using standard research procedures at the MECs and were used to calculate body mass index (BMI). Physicians' impressions of health status (Poor/Fair/Good/Very good/Excellent) at the MECs were used as an indicator of general health. Data on self-reported race/ethnicity, smoking, pets, socioeconomic background, outdoor physical activity and history of respiratory/allergic diseases (emphysema, chronic bronchitis, asthma and hay fever) were obtained during interview. Family poverty-income ratio (below/equal to or above 1) was used as indicator of socioeconomic position. Outdoor activity during past month for adults was derived from the interview data according to method by Scragg and Camargo (Scragg and Camargo, Jr., 2008). These data were not collected from those aged 18 or younger. In adolescents, we adjusted for pubertal stage, which was assessed by Tanner scales (Tanner JM, 1962). Among adults, smoking status was defined as never, former (has smoked ≥100 cigarettes but not currently smoking), and current smoker. Pack-years of smoking were calculated using answers to 12 tobacco questions and included assessment of current number of cigarettes smoked, maximum number of cigarettes smoked, and periods during which a current smoker did not smoke. Due to the skewed distribution, a categorical variable for pack-years was derived (0, 0.1-4.9, 5-9.9, 10-19.9 and ≥20 pack-years). Because of missing data on pack-years (22% of the adults), in our main analyses we adjusted for smoking status only and in sensitivity analyses we examined the effect of adjusting for pack-years in those with these data. In adolescents, smoking status was classified as current smoker or non-smoker currently due to low frequency of ex-smokers (n=67, 6.3% of those with smoking data available). Because 61.7% of adolescents did not answer the smoking questionnaire, we did not include smoking as a covariable in the main analyses in adolescents but conducted a sensitivity analysis in those with smoking data available to see if adjusting for smoking affected the associations.
Statistical analyses
Statistical analyses were conducted with Stata 11.0 (Stata Corp LP, College Station, TX USA), using the svy procedure to account for cluster effects and sampling weights from MECs (lung function) and allergy subsamples (allergen sensitisation). A more detailed description of recommended statistical methodology is available in (Landis et al., 1982). The associations of 25(OH)D and calcium levels with lung function were analyzed with multivariable linear regression. The association with atopy and sensitisation to allergens were assessed with multivariable logistic regression. Serum calcium, 25(OH)D and spirometry measurements were analyzed as z-scores.
RESULTS
The weighted mean (standard error) 25(OH)D and ionised serum calcium levels among adolescents were 80.3 (1.80) nmol/l and 1.25 (0.003) mmol/l. Among adults, the weighted mean 25(OH)D was 75.0 (1.00) nmol/l and weighted mean calcium level was 1.24 (0.003) mmol/l. The correlation coefficient r for serum 25(OH)D and ionised calcium was 0.02 (P=0.34) in adolescents and 0.04 (P=0.0009) in adults. There was no statistical evidence that any associations differed between males and females (P for interaction ≥0.13). Therefore, results are presented for males and females combined.
Supplementary Tables 1-4 summarise the age- and gender-adjusted characteristics of adolescents and adults across quartiles of serum 25(OH)D and ionised calcium. The proportion of females, non-Hispanic blacks and persons from households with poverty/income ratio below 1 decreased across the quartiles of 25(OH)D in adolescents, while the proportion of non-Hispanic whites increased across the quartiles. Adolescents in the higher quartiles had lower Tanner scores. Among adults, mean age and BMI decreased across quartiles of 25(OH)D, and mean serum level of ionised calcium and height increased across the quartiles. The proportion of men, non-Hispanic whites, those reporting outdoor physical activity during past month, and those who had a pet increased across the quartiles of 25(OH)D. The proportion of non-Hispanic blacks, individuals with fair health status and individuals from households with poverty-income ratio below 1 decreased across the quartiles of 25(OH)D in adults.
The proportion of men increased linearly across the quartiles of ionised serum calcium in adults. Mean age and number of pack-years smoked decreased across the calcium quartiles in adults. Among the 12-19 year olds, mean BMI increased across the quartiles. In the adult group, mean serum 25(OH)D increased across the quartiles, while the proportion of Mexican-American and Other ethnicities decreased across the quartiles.
With respect to outcomes, in these age- and gender-adjusted models 25(OH)D was associated positively with FEV1 and FVC and inversely with responsiveness to German cockroach allergen in both age groups. In adults, 25(OH)D was inversely associated with the prevalence of atopy, and in adolescents, decreased responsiveness to ragweed, Bermuda grass and perennial rye. Serum ionised calcium was positively associated with sensitisation to short ragweed, Bermuda grass, white oak and peanut allergens in adults only. Ionised calcium was not associated with any outcomes in adolescents, or with other outcomes in adults. Where associations occurred they appeared linear across the quartiles.
Tables 1 and 2 show multivariable associations of 25(OH)D and calcium with lung function in adolescents and adults respectively. In adolescents (Table 1), 25(OH)D was positively associated with FVC in the confounder adjusted model, but not with FEV1. Additional adjustment for pubertal stage (n=1566) did not alter the associations presented (results available from authors). In adults (Table 2) serum 25(OH)D levels were positively associated with both FEV1 and FVC after adjusting for all potential confounders (Model 2). Ionised serum calcium was not associated lung function in adolescents or adults (Tables 1-2). With additional mutual adjustment (associations of 25(OH)D adjusted for ionised calcium and vice versa) the associations remained unchanged (Model 3 in Tables 1-2).
Table 1.
Association of circulating 25(OH)D and ionised calcium (n2446) with lung function in 12-19 year olds.
| Mean difference in respiratory function (SD) per 1 SD change in 25(OH)D (95%CI) |
Mean difference in respiratory function (SD) per 1 SD change in ionised serum calcium (95%CI) |
||||||
|---|---|---|---|---|---|---|---|
| SD | Model 11 | Model22 | Model33 | Model 11 | Model22 | Model33 | |
| FEV1 | 753 | 0.009 (−0.028 to 0.046) |
0.009 (−0.028 to 0.046) |
0.008 (−0.028 to 0.045) |
0.010 (−0.024 to 0.045) |
0.010 (−0.024 to 0.045) |
0.010 (−0.024 to 0.044) |
| FVC | 901 | 0.036 (0.007 to 0.065) |
0.036 (0.007 to 0.065) |
0.035 (0.007 to 0.064) |
0.007 (−0.024 to 0.038) |
0.007 (−0.024 to 0.038) |
0.005 (−0.025 to 0.034) |
FEV1 forced expiratory volume in 1 second; FVC forced vital capacity
Model 1 is adjusted for age, ethnicity/race, gender and height
Model 2 as Model 1, plus additional adjustment for BMI, poverty index, household pets (cat/dog/bird), general health and diagnosed asthma/hay fever/chronic bronchitis
Model 3 as Model 2, plus additional adjustment for calcium in 25(OH)D analysis and 25(OH)D in calcium analysis
Table 2.
Association of circulating 25(OH)D and ionised calcium(n=8049) with lung function in 20-59 year olds.
| Mean difference in respiratory function(SD) per 1 SD change in 25(OH)D(95%CI) |
Mean difference in respiratory function(SD) per 1 SD change in ionised serum calcium(95%CI) |
||||||
|---|---|---|---|---|---|---|---|
| SD | Model 11 | Model22 | Model33 | Model 11 | Model22 | Model33 | |
| FEV1 | 821 | 0.034 (0.016 to 0.052) |
0.023 (0.004 to 0.041) |
0.023 (0.005 to 0.041) |
0.006 (−0.012 to 0.025) |
0.002 (−0.018 to 0.021) |
0.000 (−0.019 to 0.019) |
| FVC | 1006 | 0.043 (0.024 to 0.061) |
0.032 (0.015 to 0.049) |
0.032 (0.015 to 0.049) |
0.004 (−0.018 to 0.025) |
−0.001 (−0.022 to 0.020) |
−0.003 (−0.024 to 0.018) |
FEV1 forced expiratory volume in 1 second; FVC forced vital capacity
Model 1 is adjusted for age, ethnicity/race, gender and height
Model 2 as Model 1, plus additional adjustment for BMI, poverty index, household pets(cat/dog/bird), general health, outdoor activity during last month, smoking and diagnosed asthma/emphysema/hay fever/chronic bronchitis
Model 3 as Model 2, plus additional adjustment for calcium in 25(OH)D analysis and 25(OH)D in calcium analysis
Tables 3 and 4 summarise the multivariable associations of 25(OH)D and calcium levels with previously diagnosed respiratory or allergic diseases and atopic outcomes in adolescents and adults. No associations were observed in adolescents (Table 3) and serum 25(OH)D levels were not associated with allergic diseases or allergen sensitisation in adults, apart from slightly decreased sensitisation to German cockroach allergen (Table4), Higher calcium levels were associated with increased sensitisation to short ragweed, Bermuda grass, white oak and peanut allergens, when adjusted for age, gender, race/ethnicity and height (model 1). With adjustment for additional confounders (Model 2) and 25(OH)D (Model3) the associations with sensitisation to short ragweed and Bermuda grass allergen remained, but other associations were attenuated to the null.
Table 3.
Association of circulating 25(OH)D and ionised calcium levels with previously diagnosed respiratory/allergic diseases (asthma, chronic bronchitis or hay fever) (n=2446) and allergy test results (n=2317) in 12-19 year olds
| Odds ratio for each allergen outcome per 1SD change in 25(OH)D (95%CI) | Odds ratio for each allergen outcome per 1SD change in ionised serum calcium (95%CI) | ||||||
|---|---|---|---|---|---|---|---|
| Prev (%) |
Model 11 | Model22 | Model33 | Model 11 | Model22 | Model33 | |
| Respiratory disease |
16.3 | 0.92 (0.76-1.13) | 0.92 (0.76-1.13) | 0.92 (0.75-1.12) | 1.09 (0.91-1.29) | 1.09 (0.91-1.29) | 1.09 (0.92-1.30) |
| Atopy | 60.6 | 1.05 (0.89-1.24) | 1.06 (0.90-1.26) | 1.06 (0.90-1.26) | 1.03 (0.89-1.19) | 1.01 (0.88-1.17) | 1.01 (0.87-1.16) |
| D.farinae | 31.1 | 0.96 (0.79-1.18) | 0.97 (0.80-1.17) | 0.97 (0.80-1.18) | 0.98 (0.83-1.15) | 0.96 (0.81-1.14) | 0.96 (0.81-1.14) |
| Cat | 18.4 | 0.93 (0.78-1.10) | 0.93 (0.78-1.11) | 0.94 (0.78-1.12) | 0.94 (0.76-1.18) | 0.93 (0.73-1.18) | 0.93 (0.73-1.18) |
| German cockroach | 32.9 | 0.90 (0.77-1.05) | 0.92 (0.79-1.08) | 0.92 (0.79-1.08) | 1.03 (0.85-1.26) | 1.03 (0.83-1.27) | 1.03 (0.84-1.27) |
| Short ragweed | 31.6 | 0.92 (0.79-1.06) | 0.94 (0.81-1.08) | 0.93 (0.81-1.08) | 1.06 (0.91-1.23) | 1.04 (0.90-1.22) | 1.05 (0.90-1.22) |
| Bermuda grass | 23.1 | 0.95 (0.80-1.14) | 0.96 (0.79-1.16) | 0.96 (0.79-1.16) | 0.98 (0.80-1.21) | 0.97 (0.79-1.20) | 0.98 (0.79-1.20) |
| Perennial rye | 31.6 | 0.96 (0.81-1.14) | 0.96 (0.81-1.14) | 0.96 (0.82-1.14) | 1.00 (0.87-1.14) | 0.99 (0.86-1.14) | 0.99 (0.86-1.14) |
| Russian thistle | 18.6 | 1.06 (0.90-1.25) | 1.08 (0.91-1.29) | 1.08 (0.91-1.30) | 0.95 (0.78-1.16) | 0.94 (0.76-1.17) | 0.94 (0.75-1.18) |
| White oak | 13.5 | 0.99 (0.83-1.18) | 1.01 (0.83-1.24) | 1.01 (0.83-1.24) | 1.03 (0.80-1.33) | 1.02 (0.76-1.38) | 1.02 (0.76-1.38) |
| A. alternata | 18.2 | 1.06 (0.89-1.26) | 1.09 (0.90-1.32) | 1.09 (0.90-1.32) | 1.08 (0.86-1.35) | 1.07 (0.83-1.38) | 1.06 (0.82-1.38) |
| Peanut | 10.6 | 0.92 (0.74-1.15) | 0.93 (0.74-1.17) | 0.93 (0.74-1.17) | 0.99 (0.82-1.21) | 0.98 (0.81-1.18) | 0.98 (0.81-1.19) |
Prev: Prevalence
Model 1 is adjusted for age, ethnicity/race, gender and height
Model 2 as Model 1, plus additional adjustment for BMI, poverty index, household pets (cat/dog/bird), general health and (for other outcomes than respiratory disease) diagnosed asthma/hay fever/chronic bronchitis
Model 3 as Model 2, plus additional adjustment for calcium in 25(OH)D analysis and 25(OH)D in calcium analysis
Table 4.
Association of circulating 25(OH)D and ionised calcium levels with previously diagnosed respiratory/allergic diseases(asthma, chronic bronchitis or hay fever, n=8049) and allergy test results (n=3656) in 20-59 year olds
| Odds ratio for each allergen outcome per 1SD change in 25(OH)D (95%CI) | Odds ratio for each allergen outcome per 1SD change in ionised serum calcium (95%CI) | ||||||
|---|---|---|---|---|---|---|---|
| Prev (%) | Model 11 | Model22 | Model33 | Model 11 | Model22 | Model33 | |
| Respiratory disease |
16.1 | 0.96 (0.85-1.09) | 0.96 (0.85-1.09) | 0.96 (0.84-1.09) | 1.04 (0.95-1.15) | 1.04 (0.94-1.15) | 1.04 (0.94-1.16) |
| Atopy | 57.8 | 0.97 (0.85-1.11) | 0.98 (0.86-1.12) | 0.98 (0.85-1.12) | 1.06 (0.97-1.15) | 1.03 (0.95-1.13) | 1.03 (0.95-1.13) |
| D.farinae | 29.0 | 1.01 (0.88-1.16) | 1.02 (0.89-1.18) | 1.02 (0.89-1.17) | 1.04 (0.93-1.17) | 1.03 (0.91-1.16) | 1.03 (0.92-1.15) |
| Cat | 18.1 | 1.07 (0.91-1.27) | 1.08 (0.91-1.27) | 1.07 (0.91-1.27) | 1.09 (0.98-1.22) | 1.04 (0.92-1.16) | 1.03 (0.92-1.16) |
| German cockroach | 32.3 | 0.89 (0.79-1.01) | 0.89 (0.79-1.01) | 0.89 (0.78-1.01) | 1.05 (0.95-1.15) | 1.04 (0.94-1.15) | 1.05 (0.95-1.16) |
| Short ragweed | 29.2 | 1.04 (0.91-1.19) | 1.05 (0.92-1.21) | 1.05 (0.91-1.20) | 1.15 (1.01-1.31) | 1.14 (1.01-1.29) | 1.15 (1.02-1.30) |
| Bermuda grass | 20.8 | 0.96 (0.83-1.10) | 0.95 (0.82-1.10) | 0.94 (0.81-1.09) | 1.18 (1.06-1.32) | 1.14 (1.01-1.29) | 1.15 (1.02-1.30) |
| Perennial rye | 28.5 | 0.98 (0.87-1.12) | 0.97 (0.85-1.12) | 0.97 (0.85-1.12) | 1.06 (0.97-1.16) | 1.02 (0.94-1.12) | 1.03 (0.94-1.12) |
| Russian thistle | 17.7 | 0.92 (0.80-1.07) | 0.91 (0.78-1.06) | 0.91 (0.78-1.06) | 1.10 (0.96-1.27) | 1.06 (0.91-1.24) | 1.07 (0.91-1.24) |
| White oak | 13.5 | 1.03 (0.88-1.21) | 1.02 (0.87-1.20) | 1.02 (0.87-1.19) | 1.17 (1.03-1.32) | 1.11 (0.98-1.26) | 1.11 (0.98-1.26) |
| A. alternata | 13.5 | 1.04 (0.90-1.21) | 1.03 (0.87-1.21) | 1.03 (0.88-1.22) | 0.99 (0.86-1.15) | 0.94 (0.81-1.08) | 0.93 (0.81-1.08) |
| Peanut | 9.8 | 1.01 (0.86-1.18) | 1.00 (0.86-1.16) | 0.99 (0.85-1.16) | 1.19 (1.02-1.39) | 1.15 (0.98-1.34) | 1.15 (0.98-1.34) |
Prev: Prevalence
Model 1 is adjusted for age, ethnicity/race, gender and height
Model 2 as Model 1 plus additional adjustment for BMI, poverty index, household pets (cat/dog/bird), general health, outdoor activity during last month, smoking and (for other outcomes than respiratory disease) diagnosed asthma/emphysema/hay fever/chronic bronchitis
Model 3 as Model 2, plus additional adjustment for calcium in 25(OH)D analysis and 25(OH)D in calcium analysis
Sensitivity analyses are shown in supplementary Tables 5-12. In the subgroups of adolescents with smoking data and adults with data on pack-years associations that were the equivalent of Models 1-3 for the whole eligible sample (Tables 1-4) were markedly different in inconsistent ways, suggesting that analyses in these subgroups may be affected by selection bias. Adjusting for smoking in adolescents and pack-years in adults did not markedly alter associations in these subgroups.
DISCUSSION
We showed that the previously reported positive association of 25(OH)D with FVC and FEV1 in adults (Black and Scragg, 2005) is independent of serum calcium levels, and not due decreased risk of allergen sensitisation or respiratory diseases, as serum 25(OH)D levels were not associated with these outcomes. In addition, the association between 25(OH)D levels and FVC, but not with FEV1 was observed in adolescents, whereas a previous publication using NHANES III data had only explored this association in adults (Black and Scragg, 2005). As with adults, this association was independent of calcium and allergen sensitisation. We did not find an association between ionised serum calcium and lung function in either adolescents or adults, although a previous study reported a positive association between ionised calcium and FEV1 among NHANES III participants age≥17 years (McKeever et al., 2008). That previous study did not address the association between FVC and calcium. The difference between our results and those of that previous NHANES study are likely to be due to differences in age ranges and numbers of participants included in the analyses of the two papers. We were interested in exploring whether associations differed between adolescents (defined as those aged 12-19) and adults, who had data on allergen sensitisation (20-59 year old). By contrast McKeever et al. were exploring associations in adults only (defined as anyone aged 17 years or older), consequently they had larger numbers. McKeever et al. also categorised calcium in a different way to the way we have used this variable. When we repeat our analyses using calcium in the same way as that used by McKeever et al. we still find no strong statistical evidence of an association in either of the age groups we examined, by contrast we can perfectly replicate McKeevers results in those aged 17 years and above.
Vitamin D and lung function
Several mechanisms may link higher vitamin D levels to better lung function. The positive association of 25(OH)D with lung function might be the result of vitamin D protecting against atopic asthma, but we found no evidence that higher levels of 25(OH)D were associated with decreased allergic responsiveness to common allergens. FEV1 is primarily used as a measure of intrathoracic airway obstruction, as seen in asthma, in clinical and epidemiological settings, although changes in total lung capacity (TLC) will affect both FEV1 and FVC. FVC is a dynamic representation of the difference between TLC and residual volume and, in healthy subjects, it is likely to vary with TLC and reflect lung growth. It is therefore possible that the positive association of 25(OH)D with FVC in adolescents reflects the established role of vitamin D in skeletal growth (Holick, 2005), i.e. higher 25(OH)D is related to greater height via beneficial effects on skeletal development and greater height is associated with greater lung volume. However, all associations were adjusted for height and so this is unlikely to fully explain the association. In adults 25(OH)D was positively associated with both FEV1 and FVC, supporting a role beyond the relation to skeletal development.
High consumption of antioxidants have been associated with lung function,(e.g (Gilliland et al., 2003)) so it is possible that the association of vitamin D is mediated via its antioxidative properties. Alternative mechanisms might be the regulation of immune response, cellular differentiation and/or proliferation by vitamin D (Bikle, 2009; van Etten et al., 2008). Vitamin D receptor is present in human bronchial smooth muscle cells, epithelial cells lining the respiratory tract, activated lymphocytes and antigen-presenting cells(van Etten et al., 2008; Bosse et al., 2007), which support the local functions of vitamin D in the lungs and in the immune system. Therefore, it could be that vitamin D regulates the growth of bronchial smooth muscle cells or protects from the lung inflammation by promoting the expression of antimicrobial peptides and through anti-inflammatory mechanisms (Bikle, 2009; van Etten et al., 2008). Alterations in calcium homeostasis are known to occur in asthma sufferers (Chhabra et al., 1999), so it would be tempting to speculate this as a possible mechanism, although in our study circulating levels of ionised calcium were not associated with lung function, However, despite the lack of association between serum calcium levels and lung function, local calcaemic effects such as alterations in intracellular calcium signalling could still relate to the mechanism.
Ionised calcium and atopy
Our results suggest that variation in ionised serum calcium is more robustly associated with allergic response to common allergens in adults than is variation in vitamin D. In adults positive associations were found with sensitisation to grass allergens and peanut, although apart from short ragweed and Bermuda grass, these associations were attenuated towards the null after adjusting for confounders. Experimental studies suggest that calcium is involved in allergic response: bronchial smooth muscle contraction, mast cells granulation and histamine release from mast cells are calcium-dependent (Hirota et al., 2007; Alm, 1984) and effects of vitamin D on the immune system depend on adequate calcium levels (Cantorna et al., 1999). Both intra- and extracellular calcium levels have been shown to regulate the histamine release (Alm, 1984), so one would perhaps expect to see a disruption of calcium homeostasis in atopy, although intracellular calcium levels are strictly controlled and thus unlikely to be affected by normal variation in serum calcium levels. In addition, the calcium-binding motifs in allergens, especially of grass origin, are necessary for IgE binding and the chelation of calcium ions from sera of allergic patients lead to decreased allergen-IgE binding or prevented this occurring (Suphioglu et al., 1997; Ledesma et al., 2002). To our knowledge only one previous study has examined the association of circulating calcium with allergic response and that cross-sectional study found no association (McKeever et al., 2004). The positive associations we have found here in adults require further exploration.
Study strengths and limitations
The main strengths of this study are its sample size, examination of associations with serum measurements of 25(OH)D and calcium rather than dietary reports, examination with objectively assessed outcomes of lung function and allergic response to common allergens, comparison of associations in adolescents and adults and the ability to adjust for a wide range of potential confounding factors. In adults we were only able to adjust for smoking status (current/ex/never) for our main analyses and, whilst sensitivity analyses suggested that adjustment for pack-years in the subgroup with these data did not result in greater attenuation compared with smoking status, there was some evidence that selection bias may have influenced this subgroup analysis. Similarly, in adolescents we were unable to adjust for smoking in the main analysis and those with smoking data appeared to be a selected subgroup.
Because this study is cross-sectional, we cannot rule out reverse causality as an explanation for the observed associations. Further exploration in prospective studies are warranted, though we are unaware of any studies that currently have measurements of serum 25(OH)D and calcium with later objectively measured lung function and response to common allergens. NHANES III has single measurements of calcium and 25(OH)D, which may be inadequate to reflect long-term status (Schram et al., 2007), although a single measurement may be a useful biomarker of season-specific vitamin D status over a longer time (Hofmann et al., 2010) and serum calcium levels are normally maintained at relatively narrow limits within individuals (Parfitt, 1987). Other studies, including those finding associations with bone phenotypes (Jesudason et al., 2002), have used single measurements of 25(OH)D. A further limitation is the lack of data on IgE and parathyroid hormone levels.
Conclusions
Our findings contribute to the emerging literature on a possible role of vitamin D and calcium with lung function and atopy. We showed that the positive cross-sectional association between serum 25(OH)D levels and lung function is independent of variation in ionised calcium levels and does not appear to be driven by increased allergen sensitisation. By contrast we report a novel positive association between serum levels of ionised calcium and grass allergen sensitisation in adults, which is independent of a wide-range of potential confounding factors and of vitamin D.
Supplementary Material
Acknowledgments
FUNDING: Work on this study is funded by an UK Medical Research Council (MRC) Grant (G0701603), which also pays AMT's salary. The MRC and the University of Bristol provide core funding for the MRC Centre of Causal Analyses in Translational Epidemiology (G0600705). DW is funded by a Wellcome Trust 4-year PhD studentship in Molecular, genetic and lifecourse epidemiology [WT083431MA].
The views expressed in this paper are those of the authors and not necessarily those of any funding body or others whose support is acknowledged. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
ABBREVIATIONS
- 25(OH)D
25-hydroxyvitamin D
- BMI
body mass index
- CI
confidence interval
- FEV1
forced expiratory volume in one second
- FVC
forced vital capacity
- IgE
immunoglobulin E
- MEC
mobile examination center
- NHANES III
the third National Health and Nutrition Examination Survey
- SD
standard deviation
- TLC
total lung capacity
Footnotes
COMPETING INTERESTS: None
Supplementary information is available at The European Journal of Clinical Nutrition's website.
REFERENCES
- Alm PE. Histamine liberators and the mechanisms of mediator release. Acta Otolaryngol. Suppl. 1984;414:102–107. doi: 10.3109/00016488409122889. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society Standardization of spirometry--1987 update. Official statement of American Thoracic Society. Respir. Care. 1987;32:1039–1060. [PubMed] [Google Scholar]
- Asher MI, Montefort S, Bjorksten B, Lai CK, Strachan DP, Weiland SK, Williams H. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–743. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- Bikle D. Nonclassic actions of vitamin D. J. Clin. Endocrinol. Metab. 2009;94:26–34. doi: 10.1210/jc.2008-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin d and pulmonary function in the third national health and nutrition examination survey. Chest. 2005;128:3792–3798. doi: 10.1378/chest.128.6.3792. [DOI] [PubMed] [Google Scholar]
- Bosse Y, Maghni K, Hudson TJ. 1alpha,25-dihydroxy-vitamin D3 stimulation of bronchial smooth muscle cells induces autocrine, contractility, and remodeling processes. Physiol Genomics. 2007;29:161–168. doi: 10.1152/physiolgenomics.00134.2006. [DOI] [PubMed] [Google Scholar]
- Brehm JM, Celedon JC, Soto-Quiros ME, Avila L, Hunninghake GM, Forno E, Laskey D, Sylvia JS, Hollis BW, Weiss ST, Litonjua AA. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am. J Respir. Crit Care Med. 2009;179:765–771. doi: 10.1164/rccm.200808-1361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantorna MT, Humpal-Winter J, DeLuca HF. Dietary calcium is a major factor in 1,25-dihydroxycholecalciferol suppression of experimental autoimmune encephalomyelitis in mice. J Nutr. 1999;129:1966–1971. doi: 10.1093/jn/129.11.1966. [DOI] [PubMed] [Google Scholar]
- Chhabra SK, Khanduja A, Jain D. Increased intracellular calcium and decreased activities of leucocyte Na+,K+-ATPase and Ca2+-ATPase in asthma. Clin Sci. (Lond) 1999;97:595–601. [PubMed] [Google Scholar]
- Devereux G, Litonjua AA, Turner SW, Craig LC, McNeill G, Martindale S, Helms PJ, Seaton A, Weiss ST. Maternal vitamin D intake during pregnancy and early childhood wheezing. Am. J Clin Nutr. 2007;85:853–859. doi: 10.1093/ajcn/85.3.853. [DOI] [PubMed] [Google Scholar]
- Devereux G, Wilson A, Avenell A, McNeill G, Fraser WD. A case-control study of vitamin D status and asthma in adults. Allergy. 2009 doi: 10.1111/j.1398-9995.2009.02220.x. [DOI] [PubMed] [Google Scholar]
- Erkkola M, Kaila M, Nwaru BI, Kronberg-Kippila C, Ahonen S, Nevalainen J, Veijola R, Pekkanen J, Ilonen J, Simell O, Knip M, Virtanen SM. Maternal vitamin D intake during pregnancy is inversely associated with asthma and allergic rhinitis in 5-year-old children. Clin Exp. Allergy. 2009;39:875–882. doi: 10.1111/j.1365-2222.2009.03234.x. [DOI] [PubMed] [Google Scholar]
- Gilliland FD, Berhane KT, Li YF, Gauderman WJ, McConnell R, Peters J. Children's lung function and antioxidant vitamin, fruit, juice, and vegetable intake. Am. J Epidemiol. 2003;158:576–584. doi: 10.1093/aje/kwg181. [DOI] [PubMed] [Google Scholar]
- Ginde AA, Mansbach JM, Camargo CA., Jr. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch. Intern. Med. 2009;169:384–390. doi: 10.1001/archinternmed.2008.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota S, Helli P, Janssen LJ. Ionic mechanisms and Ca2+ handling in airway smooth muscle. Eur. Respir. J. 2007;30:114–133. doi: 10.1183/09031936.00147706. [DOI] [PubMed] [Google Scholar]
- Hofmann JN, Yu K, Horst RL, Hayes RB, Purdue MP. Long-term variation in serum 25-hydroxyvitamin D concentration among participants in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cancer Epidemiol Biomarkers Prev. 2010;19:927–931. doi: 10.1158/1055-9965.EPI-09-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick MF. The influence of vitamin D on bone health across the life cycle. J Nutr. 2005;135:2726S–2727S. doi: 10.1093/jn/135.11.2726S. [DOI] [PubMed] [Google Scholar]
- Hughes DA, Norton R. Vitamin D and respiratory health. Clin. Exp. Immunol. 2009;158:20–25. doi: 10.1111/j.1365-2249.2009.04001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hypponen E, Berry DJ, Wjst M, Power C. Serum 25-hydroxyvitamin D and IgE - a significant but nonlinear relationship. Allergy. 2009;64:613–620. doi: 10.1111/j.1398-9995.2008.01865.x. [DOI] [PubMed] [Google Scholar]
- Hypponen E, Sovio U, Wjst M, Patel S, Pekkanen J, Hartikainen AL, Jarvelinb MR. Infant vitamin d supplementation and allergic conditions in adulthood: northern Finland birth cohort 1966. Ann. N. Y. Acad. Sci. 2004;1037:84–95. doi: 10.1196/annals.1337.013. [DOI] [PubMed] [Google Scholar]
- Jesudason D, Need AG, Horowitz M, O'Loughlin PD, Morris HA, Nordin BE. Relationship between serum 25-hydroxyvitamin D and bone resorption markers in vitamin D insufficiency. Bone. 2002;31:626–630. doi: 10.1016/s8756-3282(02)00866-9. [DOI] [PubMed] [Google Scholar]
- Kunisaki KM, Niewoehner DE, Singh RJ, Connett JE. Vitamin D Status and Longitudinal Lung Function Decline in the Lung Health Study. Eur Respir. J. 2010 doi: 10.1183/09031936.00146509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laaksi I, Ruohola JP, Tuohimaa P, Auvinen A, Haataja R, Pihlajamaki H, Ylikomi T. An association of serum vitamin D concentrations < 40 nmol/L with acute respiratory tract infection in young Finnish men. Am. J. Clin. Nutr. 2007;86:714–717. doi: 10.1093/ajcn/86.3.714. [DOI] [PubMed] [Google Scholar]
- Landis JR, Lepkowski JM, Eklund SA, Stehouwer SA. A statistical methodology for analyzing data from a complex survey: the first National Health and Nutrition Examination Survey. Vital Health Stat. 1982;2:1–52. [PubMed] [Google Scholar]
- Ledesma A, Gonzalez E, Pascual CY, Quiralte J, Villalba M, Rodriguez R. Are Ca2+-binding motifs involved in the immunoglobin E-binding of allergens? Olive pollen allergens as model of study. Clin Exp. Allergy. 2002;32:1476–1483. doi: 10.1046/j.1365-2745.2002.01493.x. [DOI] [PubMed] [Google Scholar]
- Litonjua AA. Childhood asthma may be a consequence of vitamin D deficiency. Curr. Opin. Allergy Clin Immunol. 2009;9:202–207. doi: 10.1097/ACI.0b013e32832b36cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeever TM, Lewis SA, Smit H, Burney P, Britton J, Cassano PA. Serum nutrient markers and skin prick testing using data from the Third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2004;114:1398–1402. doi: 10.1016/j.jaci.2004.08.006. [DOI] [PubMed] [Google Scholar]
- McKeever TM, Lewis SA, Smit HA, Burney P, Cassano PA, Britton J. A multivariate analysis of serum nutrient levels and lung function. Respir. Res. 2008;9:67. doi: 10.1186/1465-9921-9-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake Y, Sasaki S, Tanaka K, Hirota Y. Dairy food, calcium and vitamin D intake in pregnancy, and wheeze and eczema in infants. Eur Respir. J. 2010;35:1228–1234. doi: 10.1183/09031936.00100609. [DOI] [PubMed] [Google Scholar]
- Parfitt AM. Bone and plasma calcium homeostasis. Bone. 1987;8(Suppl 1):S1–S8. [PubMed] [Google Scholar]
- Schram MT, Trompet S, Kamper AM, de Craen AJ, Hofman A, Euser SM, Breteler MM, Westendorp RG. Serum calcium and cognitive function in old age. J Am. Geriatr. Soc. 2007;55:1786–1792. doi: 10.1111/j.1532-5415.2007.01418.x. [DOI] [PubMed] [Google Scholar]
- Scragg R, Camargo CA., Jr. Frequency of leisure-time physical activity and serum 25-hydroxyvitamin D levels in the US population: results from the Third National Health and Nutrition Examination Survey. Am. J Epidemiol. 2008;168:577–586. doi: 10.1093/aje/kwn163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searing DA, Zhang Y, Murphy JR, Hauk PJ, Goleva E, Leung DY. Decreased serum vitamin D levels in children with asthma are associated with increased corticosteroid use. J Allergy Clin Immunol. 2010;125:995–1000. doi: 10.1016/j.jaci.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suphioglu C, Ferreira F, Knox RB. Molecular cloning and immunological characterisation of Cyn d 7, a novel calcium-binding allergen from Bermuda grass pollen. FEBS Lett. 1997;402:167–172. doi: 10.1016/s0014-5793(96)01520-7. [DOI] [PubMed] [Google Scholar]
- Sutherland ER, Goleva E, Jackson LP, Stevens AD, Leung DY. Vitamin D Levels, Lung Function and Steroid Response in Adult Asthma. Am. J Respir. Crit Care Med. 2010;181:699–704. doi: 10.1164/rccm.200911-1710OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarr DT, Hakonarson H. Unraveling the complex genetic underpinnings of asthma and allergic disorders. Curr Opin Allergy Clin Immunol. 2010;10:434–442. doi: 10.1097/ACI.0b013e32833da71d. [DOI] [PubMed] [Google Scholar]
- Tanner JM. Growth at adolescence. Blackwell Scientific Publications; Oxford: 1962. [Google Scholar]
- Upton MN, McConnachie A, McSharry C, Hart CL, Smith GD, Gillis CR, Watt GC. Intergenerational 20 year trends in the prevalence of asthma and hay fever in adults: the Midspan family study surveys of parents and offspring. BMJ. 2000;321:88–92. doi: 10.1136/bmj.321.7253.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Etten E, Stoffels K, Gysemans C, Mathieu C, Overbergh L. Regulation of vitamin D homeostasis: implications for the immune system. Nutr. Rev. 2008;66:S125–S134. doi: 10.1111/j.1753-4887.2008.00096.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


