Abstract
Cyto-nuclear shuttling of β-catenin is at the epicenter of the canonical Wnt pathway and mutations in genes that result in excessive nuclear accumulation of β-catenin are the driving force behind the initiation of many cancers. Recently, Naked Cuticle homolog 1 (Nkd1) has been identified as a Wnt-induced intracellular negative regulator of canonical Wnt signaling. The current model suggests that Nkd1 acts between Disheveled (Dvl) and β-catenin. Here, we employ the zebrafish embryo to characterize the cellular and biochemical role of Nkd1 in vivo. We demonstrate that Nkd1 binds to β-catenin and prevents its nuclear accumulation. We also show that this interaction is conserved in mammalian cultured cells. Further, we demonstrate that Nkd1 function is dependent on its interaction with the cell membrane. Given the conserved nature of Nkd1, our results shed light on the negative feedback regulation of Wnt signaling through the Nkd1-mediated negative control of nuclear accumulation of β-catenin.
Introduction
Wnt signaling is involved in many aspects of development and again in the homeostasis of certain stem cells in the adult, such as those found in hematopoietic, hair follicle and intestinal crypt stem cells [1], [2], [3], [4]. Consequently, dysregulation of Wnt signaling during development or in the adult can cause disease [1], [5], [6]. Under normal conditions, canonical or Wnt/β-catenin signaling is initiated upon Wnt ligands binding to their transmembrane receptors. This activates Disheveled (Dvl), which then inhibits the constitutively active destruction complex consisting of Axin, GSK3β and APC. Inhibition of this destruction complex results in cytoplasmic accumulation of β-catenin, which then translocates into the nucleus to activate its transcriptional program. Two consistent, and perhaps universal targets of Wnt signaling are the negative feedback regulators Naked Cuticle Homolog 1 (Nkd1) and Axin2 [7], [8], [9], [10].
Whereas it has been demonstrated that Axin2 functions similarly to Axin1, by binding to β-catenin and inducing its degradation [11], [12], the mechanism by which Nkds antagonize Wnt signaling is less understood. In vertebrates, there are two Nkd homologues: Nkd1 and Nkd2, which are equally similar to Naked Cuticle sharing approximately 45% amino acid identity and we previously demonstrated that both can inhibit canonical Wnt/β-catenin and non-canonical Wnt/PCP signaling [9]. But there are significant expression and functional differences between the two homologues to strongly suggest that Nkd1 is the Naked Cuticle orthologue. First, nkd1 expression recapitulates many, if not all, of the known Wnt signaling events during development [9], [10], [13] and is upregulated in cancers that are known to have activating mutations in the Wnt/β-catenin pathway [14], [15]. In contrast, nkd2 expression does not appear to be under the control of Wnt signaling [9], [13], [16]. Functionally, Nkd2 can regulate Wnt signaling specifically by targeting Dvl1 for degradation [17]. But Nkd2 also has a role in escorting TGFa to the basolateral surface of polarized epithelial cells [18], [19], [20], [21]. In contrast, Nkd1 does not share these functions with Nkd2 [20] and thus far Nkd1 appears specific for Wnt signaling [14], [22].
Previous work in Drosophila has established that Nkd acts between Dvl and β-catenin, and its inhibitory activity is dependent on intact and active Wnt signaling [23]. Subsequent work has suggested that Nkd has a nucleo-cytoplasmic role, shuttling either Dvl or other signaling components out of the nucleus [24]. In both Drosophila and in mammalian in vitro assays, Nkd was found to interact physically with the PDZ domain of Dvl [22], [23] and Dvl binds Nkd/Nkd1 in at least two domains: a conserved region encompassing the EF-hand domain and a region in the C-terminal half of Nkd1 [10], [20], [22]. In Drosophila, the EF-Hand of Nkd alone is required for binding to Dvl [25], but curiously, Nkd mutant proteins lacking the EF-Hand are capable of rescuing nkd-/- mutants to adulthood [26]. This suggests that in Drosophila the interaction between Nkd and Dvl is dispensable for Nkd activity. Previously we demonstrated that Nkd2 is myristoylated. This modification targets Nkd2 to the plasma membrane and is required for its proper function [20]. While the myristoylation sequence is conserved in Nkd1, it is not conserved in fly Nkd. Nevertheless, fly and mosquito Nkds share a unique N-terminal sequence that confers functional properties and membrane association to Nkd [27].
Further insight into Nkd1 function was recently gained using an integrated physical and functional screen to identify Wnt inhibitors [28]. Using this approach, it was found that Nkd1 interacts with Axin and this interaction is required to antagonize Wnt signaling. In this screen, it was also found that Nkd1 had a significant interaction with β-catenin but it is unknown if this is dependent on Axin or vice versa [28]. These recent findings imply that Nkd1 is not acting solely at the level of Dvl and that its interactions with other components of the Wnt/β-catenin pathway are likely involved in the ability of Nkd1 to inhibit Wnt signaling.
To probe further the functional significance of the Nkd1-β-catenin interaction in vivo, we employed the zebrafish model. At dome stage, which is approximately 4.3 hours post fertilization (hpf), the zebrafish blastula contains approximately 4000 to 8000 pluripotent cells that have yet to undergo differentiation or gastrulation [29]. At this stage, the embryo has been undergoing active zygotic transcription for about 1.3 hours [30], but is only initiating the first zygotic canonical Wnt/β-catenin signaling event, via Wnt8, along the ventro-lateral margin [9], [31], [32]. While endogenous canonical Wnt/β-catenin signaling is only occurring along the ventro-lateral margin, the entire blastula at this stage is responsive to canonical Wnt/β-catenin signaling [33], [34]. Furthermore, the non-canonical Wnt/PCP pathway is not yet active at this stage and so we took advantage of these canonical Wnt/β-catenin-responsive cells to delineate the function of Nkd1 in antagonizing canonical Wnt/β-catenin signaling, hereafter referred to as Wnt signaling. Using this system and overexpression assays, we demonstrate that Nkd1 interacts with β-catenin and acts to prevent β-catenin from accumulating within the nucleus. These results provide insight into the negative feedback mechanism of endogenous Wnt signaling in an in vivo model, specifically at the level of cyto-nuclear distribution of β-catenin.
Results
Myristoylation sequence is required for plasma membrane localization
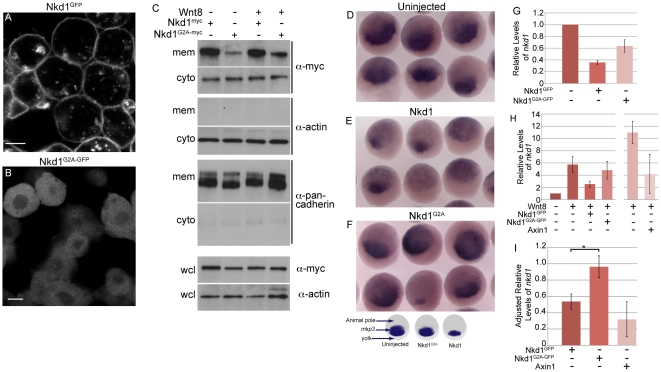
The N-terminal myristoylation sequence is highly conserved between vertebrate Nkd homologues and it has been previously demonstrated that human Nkd2 is myristoylated [9], [20]. Thus, we wanted to determine if zebrafish Nkd1 is plasma membrane bound and if this is myristoylation sequence dependent. Immunohistochemical analysis of mosaically overexpressed Nkd1GFP (C-terminal GFP tag) showed enriched protein expression at the plasma membrane at dome stage (4.3 hpf) (Fig. 1A). In addition, there were also large and small GFP positive puncta within the cytoplasm, reminiscent of Dvl puncta (Fig. 1A) [35], [36]. To confirm that Nkd1 is plasma membrane localized, zebrafish blastula (4.3 hpf) overexpressing Nkd1myc (C-terminal myc tag) alone or with Wnt8 were homogenized and the lysate was fractionated to isolate the plasma membrane and cytoplasmic fractions (Fig. 1C). Consistent with the immunohistochemistry data, we found Nkd1myc to be enriched within the plasma membrane fraction, although some Nkd1myc was also detected in the cytoplasmic fraction (Fig. 1C). Co-injection of wnt8 RNA did not appear to alter the overall levels of plasma membrane or cytoplasmic Nkd1myc. To determine if the above subcellular distribution of Nkd1 was dependent on myristoylation, we mutated the second amino acid, glycine, to alanine (G2A) to generate Nkd1G2A-GFP or Nkd1G2A-myc. This mutation abolishes the myristoylation activity of human Nkd2 [20]. In contrast to Nkd1GFP, Nkd1G2A-GFP was no longer plasma membrane-enriched and no longer formed the cytoplasmic puncta as assayed by immunohistochemistry (Fig. 1B). Instead, Nkd1G2A-GFP became evenly distributed within the cytoplasm and possibly in the nucleus as well. Fractionation experiments confirmed the loss of plasma membrane association, as there was dramatically less plasma membrane enrichment of Nkd1G2A-myc relative to Nkd1myc (Fig. 1C). The fractionation data also revealed that a portion of Nkd1G2A-myc still segregated with the plasma membrane fraction, which was not obvious by immunohistochemistry (Fig. 1 B,C).
Figure 1. Plasma membrane localized Nkd1 is required for antagonizing Wnt signaling.
nkd1GFP (A) or nkd1G2A-GFP (B) mRNA was injected into 1 of 4 blastomeres and allowed to develop until dome stage (4.3 hpf). Embryos were viewed live with confocal microscopy. (C) In a similar experiment, embryos injected at the one cell stage with either nkd1myc or nkd1G2A -myc in combination with wnt8, were collected at dome stage and fractionated into cytoplasmic (cyto) or plasma membrane (mem) fractions. Western blots of fractions were probed with anti-myc. This blot was also probed with anti-actin or anti-pancadherin to determine purity of fractions. Each lane represents the equivalent of 1 embryo from an average of 10 embryos. To determine total amount of protein, a portion of the pre-fractionated whole cell lysate (wcl) was western blotted and probed with anti-myc and anti-actin as a loading control. For the wcl, each lane represents the equivalent of 0.75 of an embryo from an average of 10 embryos. (D, E, F) Embryos were injected at the one cell stage with either nkd1 (E) or nkd1G2A (F) RNA and harvested, along with uninjected controls (D), at sphere stage (3.8 hpf). Embryos were processed for whole mount in situ hybridization using a mkp3 anti-sense probe [38]. The schematic below (F) summarizes the whole mount in situ hybridization data, identifies the different regions of the embryo and the domain of mkp3 expression in the dorsal organizer region. (G) Embryos were injected at the 1 cell stage with nkd1GFP or nkd1G2A-GFP, harvested at dome stage and total RNA was isolated and reversed transcribed. qRT-PCR was performed using endogenous nkd1 as readout. The 5′ untranslated region of nkd1 was used as a target, the sequence of which was not incorporated into the expression vectors. Values are relative to uninjected embryos which was arbitrarily set to 1 (N = 3). (H) Quantification of endogenous nkd1 levels by qRT-PCR in the presence of Wnt8. (N = 6 for Wnt8; Wnt8+Nkd1GFP; Wnt8+Nkd1G2A-GFP). The Wnt8+Axin experiments (N = 3) were done independently of the others, which showed higher induction of nkd1 by Wnt8 alone, compared to the other experiments and relative to uninjected. (I) To adjust for the differences between experiments described in (H), the ratio of endogenous levels of nkd1 was determined for Wnt8+Nkd1GFP:Wnt8 (N = 6), Wnt8+Nkd1G2A-GFP:Wnt8 (N = 6) and Wnt8+Axin1:Wnt8 (N = 3) for each individual experiment and then averaged. (* = Students t-test, p = 0.0252). A level of one indicates no effect. Error bars represent standard error. Scale bar in A,B represents 10 µm.
We also noticed that the levels of cytoplasmic plus membrane Nkd1G2A proteins did not add up to the levels detected in the whole cell lysate when compared to wild-type Nkd1 by Western blot analysis. We generated a Nkd1G2P mutant form and observed that it was also unstable in our fractionation experiments (not shown), even though a proline at the N-terminal end of proteins is predicted to be stable [37]. This suggests that the fractionation procedure is unfavorable to Nkd1G2A/P stability. In general, we found that Nkd1 was more stable than Nkd1G2A (TVR submitted) and so more Nkd1G2A RNA was injected to compensate for this difference.
Nkd1 Myristoylation is required to antagonize Wnt activity
To ascertain if the myristoylation sequence of Nkd1 was required for antagonizing Wnt activity, we overexpressed Nkd1 and Nkd1G2A and assayed for mkp3 expression by whole mount in situ hybridization at sphere stage (3.8. hpf). At this stage, mkp3 is a direct target of the maternal Wnt/β-catenin pathway [38] but shortly after becomes regulated by FGF signaling [38]. We observed that Nkd1G2A had very little effect on mkp3 expression in contrast to Nkd1 overexpression, which led to a reduction in mkp3 expression (Fig. 1 D–F). This was confirmed using quantitative RT-PCR (qRT-PCR). Total RNA from embryos injected with either nkd1GFP or nkd1G2A-GFP RNA was assayed for endogenous nkd1 expression at dome stage (4.3 hpf) as readout of active Wnt signaling (Fig. 1G). Consistent with the whole mount in situ hybridization data, we observed that Nkd1GFP could reduce the expression of a Wnt target gene, while a myristoylation-deficient form of Nkd1 (Nkd1G2A) was less effective at doing so.
To determine if Nkd1G2A could prevent the activation of target genes induced by excess Wnt signaling, we performed qRT-PCR on embryos co-injected with wnt8 and nkd1GFP or nkd1G2A-GFP RNAs. For comparison we also co-injected wnt8 and axin1 RNA, the latter encoding a potent inhibitor of Wnt signaling (Fig. 1H,I). We observed that Nkd1GFP and Axin1 reduced the effect of ectopic Wnt8 on endogenous nkd1 expression, with Nkd1GFP having slightly less influence than Axin1. In contrast, Nkd1G2A-GFP was markedly less active in this assay (Fig. 1H,I). Therefore, we conclude that myristoylation of Nkd1 is required for its ability to antagonize Wnt signaling, suggesting that plasma membrane association of Nkd1 is critical for its activity. The latter conclusion is similar to results found in Drosophila where removal of the N-terminal sequence of Nkd abrogated both its plasma membrane localization and its ability to function. This N-terminal-deleted Nkd isoform was also much less stable [27]. This argues for conservation of function, as both fly and vertebrate Nkd/Nkd1 appear to require association with the plasma membrane for proper activity in antagonizing Wnt signaling.
Nkd1 binding to Dvl2 is independent of myristoylation
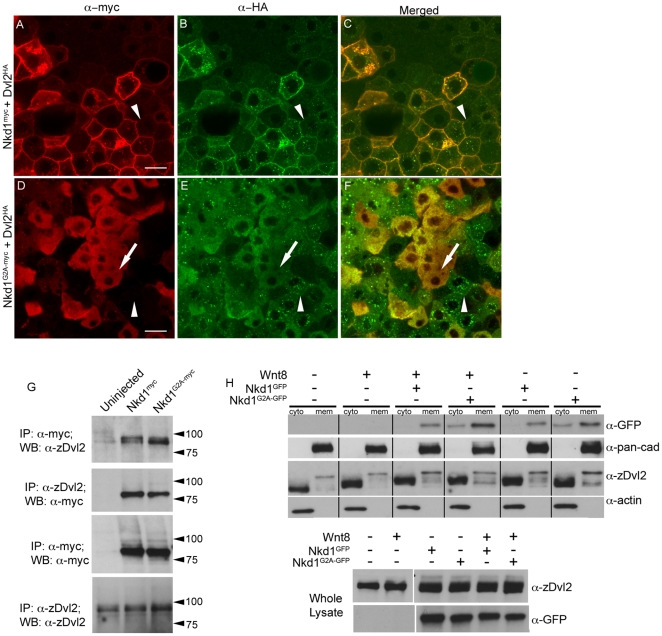
Previous studies have demonstrated that Nkds bind Dvl proteins [10], [22], [23], [39]. Therefore, we wanted to determine if Dvl binding was dependent on Nkd1 myristoylation. Immunohistochemistry of blastula (4.3 hpf) overexpressing Nkd1myc and Dvl2HA in a mosaic fashion showed that Dvl2HA co-localized with Nkd1myc at the plasma membrane but also in cytoplasmic puncta (Fig. 2A–C). It is possible that Nkd1myc stabilized Dvl2HA at the plasma membrane, as adjacent cells expressing Dvl2HA alone exhibited low plasma membrane staining (arrowheads in Fig. 2A–C). In contrast, immunohistochemistry with Dvl2HA and Nkd1G2A-myc demonstrated that cells co-expressing both Dvl2HA and Nkd1G2A-myc, but not Dvl2HA alone, have lost the Dvl2HA puncta staining (compare arrows and arrowheads, Fig. 2D–F), arguing that Nkd1G2A-myc can still bind to Dvl2HA and dissociate Dvl2HA puncta.
Figure 2. Nkd1 co-localizes with Dvl2.
(A–F) Dvl2HA mRNA was injected at the one cell stage followed by injection of nkd1myc or nkd1G2A-myc RNA into 1 of 4 blastomeres at the 4-cell stage. Embryos were harvested at 50% epiboly (5.25 hpf) and processed for immunohistochemistry using anti-myc probes (red) or anti-HA probes (green). Arrowheads in A-F identify a cell that is positive for Dvl2HA but negative for Nkd1myc (A–C) or Nkd1G2A-myc (D–F). Arrow in D–F indentifies a cell that is positive for both Dvl2HA and Nkd1G2A-myc. (G–H) Nkd1 interacts with Dvl2. (G) Embryos were injected at the one cell stage with nkd1myc or nkd1G2A-myc and harvested at dome stage (4.3 hpf) for co-immunoprecipations. Lysates were incubated with anti-zDvl2 or anti-myc antibodies for immunoprecipitation. Immunoprecipitates were western blotted and probed with anti-zDvl2 or anti-myc antibodies (WB). Each lane represents the equivalent of 17.5 embryos. (H) nkd1GFP or nkd1G2A-GFP RNAs were co-injected with wnt8 RNA at the one cell stage. At 4.3 hpf, embryos were harvested and fractionated to isolate cytoplasmic (cyto) and plasma membrane (mem) fractions. Anti-actin and anti-pan-cadherin antibodies determined the purity of the cytoplasmic and plasma membrane fractions, respectively. A portion of the whole cell lysate was recovered prior to fractionation (lower panel) to determine loading controls. (Note: the high percentage gel precluded observing Dvl mobility shifts induced by Wnt8 in the whole cell lysate). The predicted MW of Nkd1myc is 61 kD, but the observed MW is closer to 75 kD. We speculate that this is likely due to post-translational modifications of Nkd1. Each lane represents the equivalent of 1 embryo, averaged from 10 embryos. Scale bar represents 20 µm.
To confirm that Nkd1myc interacts with endogenous Dvl2 and to determine if Nkd1G2A-myc can also bind to endogenous Dvl2, we performed co-immunoprecipitation assays. Consistent with the immunohistochemistry data, we observed that Nkd1 could interact with Dvl2 independent of its myristoylation, but the molecular weights of DshHA were different between Nkd1myc and Nkd1G2A-myc (Fig. 2G). We observed Nkd1myc immunoprecipitated with Dvl2 isoforms that migrate with different mobilities, when compared to those complexed with Nkd1G2A-myc (Fig. 2G). Nkd1GFP interacted with both slow and fast migrating isoforms of Dvl2, while Nkd1G2A-GFP interacted preferentially with the fast migrating isoform. To pursue the effect of Nkd1 on Dvl2 further, we co-injected RNAs encoding nkd1GFP or nkd1G2A -GFP with wnt8 RNA and fractionated the embryo lysates to observe the plasma membrane and cytoplasmic forms of Dvl2. In the cytoplasmic fractions, Dvl2 undergoes a characteristic mobility shift due to phosphorylation in the presence of Wnt8 [40], [41], [42], [43], which was also observed for the two Nkd1 constructs (Fig. 2H). In agreement with the co-immunoprecipitation experiment, we also observed different banding patterns in the plasma membrane fractions of Dvl2 between Nkd1GFP and Nkd1G2A-GFP, which appear independent of the addition of Wnt8 (Fig. 2H). Specifically, we observed an increase in intensity of the slower and intermediate migrating bands in the presence of Nkd1GFP, but an increase in a fast migrating band of Dvl2 in the presence of Nkd1G2A-GFP when compared to uninjected or wnt8 RNA alone injected embryos (Fig. 2H).
The differences in plasma membrane vs cytoplasmic levels of Dvl2 observed by western blot analysis did not exactly correlate with the immunohistochemical data (Fig. 2) as we did not see an increase in endogenous Dvl2 in the membrane fraction by western, while we observed increased membrane localization with exogenous Dvl2HA by immunohistochemistry. This may be due to fundamental differences between ectopic and endogenous Dvl2, or that membrane association of Dvl2 is lost during the fractionation process. Nonetheless and taken together, this data suggests that Nkd1GFP modifies, interacts with or stabilizes different isoforms of Dvl2. While Nkd1G2A-GFP also interacts with Dvl2, it is doing so differently than Nkd1GFP. This is consistent with the ability of Nkd1, but not Nkd1G2A, to inhibit ectopic Wnt8 signaling (Fig. 1I).
Nkd1 does not alter cytoplasmic levels of β-catenin
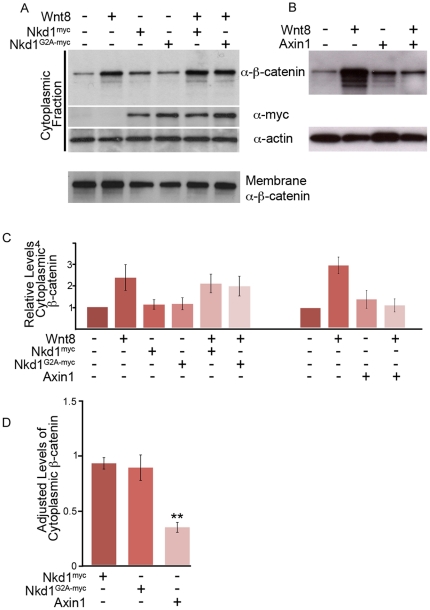
Thus far, we have determined that plasma membrane localization of Nkd1 is required for its activity to antagonize Wnt signaling. We have also demonstrated that Nkd1 binds to and somehow modifies, interacts with, or stabilizes different Dvl2 isoforms. These specific interactions and modifications also appear to occur at the plasma membrane. A model based on genetic epistasis studies suggests that Nkd1 acts between Dvl and β-catenin [23], with the simplest and most attractive model being that Nkd1 acts at the level of Dvl, somehow binding to and inactivating Dvl [10], [23], [24], [26], [27], [44]. If Nkd1 is indeed antagonizing Wnt signaling at the level of Dvl, then Nkd1 should prevent the accumulation of cytoplasmic β-catenin induced by Wnt8. To test this model, we co-injected nkd1 or nkd1G2A RNAs (either with myc or GFP tags) with or without wnt8 RNA, isolated the cytoplasmic fraction and assayed for β-catenin by Western blot (Fig. 3A). Inconsistent with the model described above, we observed no decrease in cytoplasmic β-catenin levels in the presence of Nkd1 or Nkd1G2A with or without Wnt8 (Fig. 3A,C,D). To determine that this is qualitatively different than Axin, we next compared the effect of Nkd1 to Axin1. Consistent with the known function of Axin1, we observed reduced cytoplasmic levels of β-catenin induced by Wnt8 in the presence of Axin1 (Fig. 3B,D). These experiments were repeated 6 times with similar results (Fig. 3C; Table 1). Taken together, we conclude that while the constitutively active Axin1 destruction complex destabilizes cytoplasmic β-catenin, Nkd1 does not appear to antagonize the Wnt pathway at this level, arguing that Nkd1 may instead be acting downstream of stabilized cytoplasmic β-catenin.
Figure 3. Nkd1 does not affect cytoplasmic levels of β-catenin.
(A,B) Embryos were injected at the one stage with wnt8, nkd1myc or nkd1G2A -myc (A) or axin1 (B) and harvested at dome stage (4.3 hpf). Lysates were fractionated into plasma membrane (not shown) and cytosolic fractions, Western blotted and probed with anti-β-catenin, anti-myc and anti-actin antibodies. Each lane represents, on average, the equivalent of one embryo averaged from 10 embryos. (C) The average levels of cytosolic β-catenin from 6 independent experiments (Wnt8+/- Nkd1myc/Nkd1G2A-myc) or from 3 independent experiments (Wnt8+/-Axin1) were determined. Each lane was first normalized to actin levels and then compared to uninjected embryos, which was arbitrarily set to 1. D) To adjust for the differences between experiments described in (C), the ratio of cytoplasmic levels of β-catenin was determined for Wnt8+Nkd1myc:Wnt8 (N = 6), Wnt8+Nkd1G2A-myc:Wnt8 (N = 6) and Wnt8+Axin1:Wnt8 (N = 3) for each individual experiment and then averaged. (**-Students t-test, p = 0.0002). A level of one indicates no effect. Error bars represent standard error.
Table 1. Relative cytoplasmic levels of β-catenin.
| Injection | Average | Ttest p-values |
| Uninj | 1.0 | |
| Wnt81 | 2.4 | 0.05 † |
| Nkd1myc | 1.1 | 0.61 † |
| Nkd1G2A-myc | 1.2 | 0.60†† |
| Wnt8+Nkd1myc | 2.1 | 0.72†† |
| Wnt8+Nkd1G2A-myc | 2.0 | 0.61†† |
| Wnt82 | 3.2 | 0.02 † |
| Axin1 | 1.4 | 0.38 † |
| Wnt8+Axin1 | 1.1 | 0.04†† |
Averages were taken from 6 (Nkd1myc or Nkd1G2A-myc co-injections) or 3 (Axin1 co-injections) independent experiments and first normalized to actin levels. Uninjected levels were set to 1.00 and other levels are relative to uninjected.
Nkd1myc and Nkd1G2A-myc co-injection series.
Axin1 co-injection series.
-Students t-test (2 tailed, equal variance) compared to uninjected;
-Students t-test (2 tailed, equal variance) compared to Wnt8 injected.
Nkd1 prevents Wnt8-induced nuclear accumulation of β-catenin
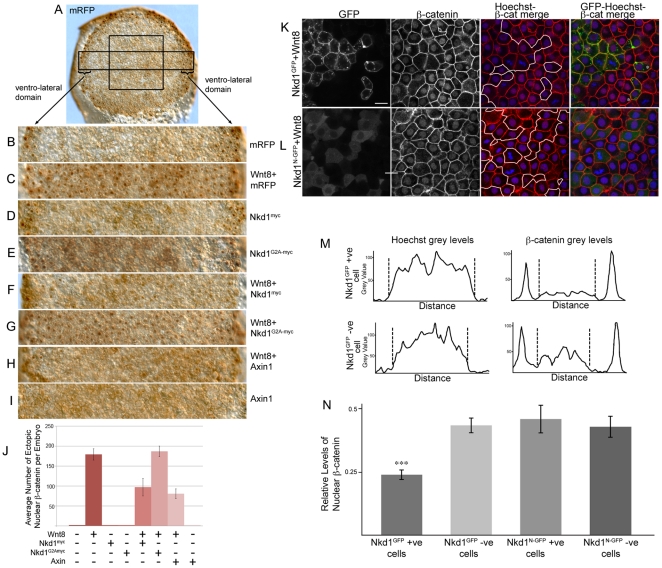
As Nkd1 does not significantly alter the levels of cytoplasmic β-catenin, we wanted to determine if Nkd1 altered the levels of nuclear β-catenin. To test this we overexpressed Wnt8 with or without Nkd1myc or Nkd1G2A-myc and performed whole mount immunohistochemistry against β-catenin at dome stage (4.3 hpf). GFP mock-injected embryos displayed nuclear β-catenin around the perimeter of the embryo as a result of endogenous ventro-lateral Wnt8 activity (Fig. 4A,B) [32]. However, there was a complete absence of nuclear β-catenin in cells at the animal pole at this stage (Fig. 4A,B,J). Injection of wnt8 RNA alone induced nuclear β-catenin in the majority of cells in the animal pole region at this stage (Fig. 4C,J). Injection of nkd1myc or nkd1G2A-myc RNA alone had only a modest effect on ventro-lateral nuclear β-catenin, consistent with our previous observations (Fig. 4D,E,J) [9]. In contrast, injection of axin1 RNA resulted in significantly fewer ventro-lateral cells with nuclear β-catenin (Fig. 4J). When RNAs encoding wnt8 and nkd1myc were co-injected, there was a substantial reduction in both ectopic and ventro-lateral nuclear β-catenin (Figs. 4F,J). In contrast, ectopic Nkd1G2A-myc was unable to prevent the accumulation of nuclear β-catenin induced by Wnt8 overexpression (Fig. 4G,J). This data was consistent across numerous embryos and experiments (Fig. 4J and Table 2). Based on these results, we suggest that Nkd1 functions upstream of nuclear accumulation of β-catenin and potentially functions by preventing nuclear accumulation of β-catenin.
Figure 4. Nkd1 inhibits accumulation of nuclear β-catenin.
(A–I) Embryos were injected at the one cell stage with mRFP (A,B); wnt8+mRFP (C); nkd1myc (D); nkd1G2A -myc (E); wnt8+nkd1myc (F); wnt8+nkd1G2A -myc (G); wnt8+axin1 (H); and axin1 alone (I). At dome stage (4.3 hpf) embryos were collected and processed for whole-mount immunohistochemistry with anti-β-catenin. The expression of nuclear β-catenin around the ventro-lateral margin of the embryo is due to endogenous Wnt8 activity. Note that Axin1 is also sufficient to prevent nuclear localization of β-catenin induced by endogenous Wnt8 activity. The box in (A) represents the size and position of analysis for (J). The rectangle in (A) depicts the regions shown in (B–I) which encompasses the ventro-lateral margins on either side of the embryo. (J) Blind counts of the number of cells expressing ectopic nuclear β-catenin was quantified (Table 2). Error bars represent standard error. To confirm the effect of Nkd1 on nuclear β-catenin, wnt8 RNA was co-injected with nkd1GFP (K) or nkd1N -GFP RNA (L) into 1 of 4 blastomeres, harvested at dome stage and processed for endogenous β-catenin staining. Regions of mosaic expression were chosen for analysis. Clones of Nkd1GFP or Nkd1G2A-GFP positive clones are outlined in white. Black and white images of GFP and β-catenin are shown for contrast. See also Fig. S1 for the complete set of images. (M-N) Quantification of the differences in nuclear β-catenin from six cells (Wnt8+Nkd1GFP) or 10 cells (Wnt8+Nkd1N-GFP) cells each for GFP positive/Hoechst positive and juxtaposed GFP negative/Hoechst positive cells. The graphs in (M) represents the two cells identified by asterisks in the right hand-most image of (K). The dashed lines delineate the nuclear grey levels. (N) The level of nuclear β-catenin was adjusted using the Hoechst staining. Only the grey values located between the dashed lines were evaluated. (p value = 0.0002). Error bars represent std error. Scale bar represents 20 µM.
Table 2. Nuclear β-catenin counts.
| Injection | Ave # cells with Nuclear β-catenin | number of embryos | Ttest p-values* |
| mGFP | 2.00 | 6 | |
| Wnt8 | 179.22 | 9 | |
| Nkd1myc | 2.00 | 6 | |
| Nkd1G2A-myc | 2.00 | 6 | |
| Wnt8+Nkd1myc | 97.00 | 6 | 0.002794 |
| Wnt8+Nkd1G2A-myc | 187.00 | 10 | 0.346332 |
| Wnt8+Axin | 80.67 | 6 | 0.000144 |
| Axin | 2.00 | 6 |
Nuclear β-catenin counts from the central square shown in figure 4. * Students t-test (2 tailed, equal variance) compared to Wnt8 injected.
To test further the hypothesis that Nkd1 functions at the level of nuclear β-catenin accumulation, we co-injected RNAs encoding wnt8 and nkd1GFP or wnt8 and nkd1N -GFP (an N-terminal GFP fusion and more stable form of myristoylation deficient Nkd1) into 1 of 4 blastomeres at the 4-cell stage. We reasoned that if Nkd1GFP somehow affects nuclear β-catenin, we would predict GFP positive cells to have reduced levels of nuclear β-catenin, compared to juxtaposed, GFP negative cells, which would have elevated levels of nuclear β-catenin due to the non-cell autonomous effects of Wnt8. Accordingly, we observed a decrease in nuclear β-catenin in Nkd1GFP positive cells, compared to juxtaposed and surrounding cells that were GFP negative (Fig. 4K,M,N). As the nucleus of every cell is not in the same plane of focus, we stained the nuclei with Hoechst, a general DNA marker, and adjusted the level of β-catenin staining intensity according the Hoechst staining intensity (Fig. 4M, S1). The Hoechst staining confirmed that nuclei in the Nkd1GFP positive cells were visible and that they had significantly less β-catenin staining when compared to juxtaposed GFP negative cells (Fig. 4N, S1).
In contrast, Nkd1N-GFP was much less efficient at reducing the levels of nuclear β-catenin (Fig. 4L,N). We conclude that myristoylated Nkd1 likely acts downstream of stabilized cytoplasmic β-catenin, preventing its nuclear accumulation.
Nkd1 physically interacts with β-catenin
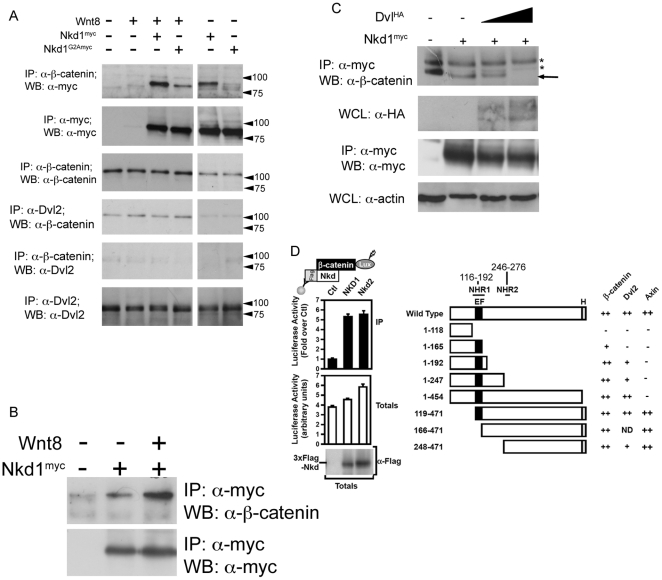
β-catenin is at the center of the canonical Wnt signaling cascade and the nucleo-cytoplasmic shuttling of this protein is the focus of recent research [45], [46]. We found that while Nkd1 is enriched at the plasma membrane with cytoplasmic puncta, it does not appear to alter cytoplasmic levels of β-catenin (Fig. 3A). Instead our studies suggest that Nkd1 prevents the nuclear accumulation of β-catenin. Therefore, we wanted to determine if there was a physical interaction between Nkd1myc and β-catenin. By co-immunoprecipitation, we observed a strong interaction between Nkd1myc and β-catenin, but a much weaker interaction between Nkd1G2A-myc and β-catenin (Fig. 5A). This suggests that Nkd1 myristoylation and its association with the plasma membrane is important for binding to β-catenin, although we do not know if this a direct or indirect interaction. We also observed an increase in Nkd1-β-catenin interaction in the presence of Wnt8 (Fig. 5B); however, it is unclear if this is a result of increased levels of cytoplasmic β-catenin due to excess Wnt8, or if Wnt signaling alters the affinity of Nkd1 for β-catenin, or both. Furthermore, these experiments cannot distinguish which pool of β-catenin (plasma membrane or cytoplasmic) interacts with which pool of Nkd1 (plasma membrane or cytoplasmic puncta).
Figure 5. Nkd1 interacts with β-catenin.
To determine if Nkd1 and β-catenin interact, wnt8 +/− nkd1myc or nkd1G2A -myc was injected at the 1 cell stage and harvested at dome stage for immunoprecipitation with anti-β-catenin (A). Each lane represents the equivalent of 17.5 embryos. Note, this experiment was part of a larger experiment involving the co-immunoprecipitations shown in Fig. 2 (B) and as such share some of the same controls. (B) nkd1myc +/− wnt8 was injected into 1 cell stage embyros and harvested at dome stage, immunoprecipitated with anti-myc and western blotted with anti-β-catenin. The interaction between Nkd1 and β-catenin is much stronger in the presence of exongenous Wnt. Slight non-specific binding of the anti-myc antibody is observed in the control lane. A no primary antibody control showed no banding pattern (not shown). C) nkd1myc was co-injected with increasing doses of dshHA (0pg, 125pg and 250pg) harvested at dome stage, immunoprecipitated with anti-myc and western blotted with anti-β-catenin. The astericks identify non-specific banding patterns. D) The interaction of luciferase-tagged β-catenin (β-catenin-Lux) with wild type Nkd1 and Nkd2 (left panel) and Nkd1 mutants (right panel) was tested using the LUMIER assay in HEK293T cells. Numbers above Wild Type Nkd1 refer to amino acid positions in human Nkd1. Axin data has been previously reported [28] and is shown here for completeness. Data are shown as the mean of 2 samples +/− standard deviation (left panel) and interaction intensities of less than 50% (−), 50–75% (+) and >75% (++) of wild type are indicated (right panel). clarity we refer to NHR1 as Naked Homology Region 1 (116–192aa), which encompasses the EF hand (143–154aa). The NHR1 region has also been referred to as NH2 [56] as well as EFX [10].
Since Nkd1 also binds to Dvl2, we were curious if we could observe a ternary complex between Nkd1, Dvl2 and β-catenin. Immunoprecipitating Dvl2 we detected a low background level of co-immunoprecipitated β-catenin that did not change in the presence of Wnt, Nkd1myc or Nkd1G2A-myc, whereas the reverse IP suggested little or no interaction (Fig. 5A). Even though Nkd1 was found to interact with both Dvl2 and β-catenin (Figs. 2G, 5A), this suggests that Dvl2, Nkd1 and β-catenin may not co-exist in a ternary complex and further that Dvl2 and β-catenin may compete for binding to Nkd1. To test this, we co-injected increasing amounts of Dvl2HA and observed a dramatic reduction in the levels of β-catenin that immunoprecipitated with Nkd1myc with increasing amounts of Dvl2HA (Fig. 5C).
Using human Nkd1 deletion mutants [28], we mapped the regions of Nkd1 that are required to bind β-catenin. We found that β-catenin binds to Nkd1 in a conserved region encompassing the EF-hand (NHR1 domain), as well as in a region C-terminal to this, which includes a second region of conserved homology (NHR2) [9]. Further, we found that Dvl2 binds to Nkd1 in a partially overlapping region with its β-catenin-binding site (Fig. 5D, S2). Previously we demonstrated that Axin1 binds to Nkd1 via the C-terminal poly histidine tail on Nkd1 (Fig. 5D) [28]. Our current analysis demonstrates that this region is not required for binding to either Dvl2 or β-catenin suggesting that Axin1 binding to Nkd1 is not a requirement for Nkd1 binding to Dvl2 or β-catenin.
These mapping results support previous findings. Using just the EF-hand, Wharton et al., found this region was sufficient to bind Dvl [10], while Yan et al., found Dvl still bound Nkd in the absence of the EF-hand [22] and we previously found that Dvl binds a domain in the C-terminal region on Nkd2 [20]. Therefore, Dvl2 (and β-catenin) bind to regions encompassing the EF-hand and a separate domain C-terminal to the conserved EF-hand domain, which includes the NHR2 domain. Fine interaction mapping of these regions is currently underway, which will determine more precisely where Dvl2 and β-catenin bind Nkd1 and if these two proteins bind to the same or juxtaposed regions of Nkd1. Thus, we conclude that the Nkd1-β-catenin interaction is conserved between zebrafish and mammals. Moreover, our data also suggests that Dvl2 and β-catenin likely compete for binding to Nkd1.
Discussion
Activation of canonical Wnt signaling is well characterized and is considered to proceed through the formation of Wnt-induced signalosomes [47], [48]. What is less well understood is how this pathway controls its own activity to limit the amount of Wnt signaling. Here we show that Nkd1, a Wnt-inducible negative-feedback regulator limits Wnt signaling by preventing nuclear accumulation of β-catenin. This work furthers our understanding of the role Dvl plays in this process. We have found that Nkd1 interacts with both a slow and fast migrating form of Dvl2 correlating with phosphorylated and unphosphorylated forms of Dvl2, respectively [40], [41], [42], [43], [49]. In the presence of Nkd1, exogenous Dvl also becomes enriched at the plasma membrane. Thus, it appears that plasma membrane localization of Nkd1, via myristoylation, is required for its association with a slower migrating (phosphorylated) form of Dvl. This is supported by our observations that, in the absence of myristoylation, Nkd1G2A interacts preferentially with a faster migrating form of Dvl and both remain uniformly distributed within the cytoplasm. Alternatively, the binding of Nkd1, but not Nkd1G2A, to Dvl, may lead to Dvl phosphorylation, but this does not account for the observation that Nkd1 interacts with both a phosphorylated and unphosphorylated form of Dvl.
Thus far, this data fits well with Nkd1 antagonism occuring at the level of Dvl, simply inhibiting Dvl in the signalosome. However, this model cannot account for the lack of effect of Nkd1 on the levels of cytoplasmic β-catenin. While Axin can effectively reduce the levels of cytoplasmic β-catenin induced by Wnt8, Nkd1 cannot. In fact, we often observed slight (but not significant) increases in cytoplasmic β-catenin in the presence of Nkd1, without Wnt stimulation. Further, we observed that Nkd1 physically interacts with β-catenin and that the strength of this interaction is dependent on an intact myristoylation sequence in Nkd1. This suggests that Nkd1 needs to associate with the plasma membrane before it can interact with β-catenin. Also, we have determined that β-catenin and Dvl overlap in Nkd1 binding domains, that they likely compete for binding to Nkd1, and that Wnt signaling may promote binding between Nkd1 and β-catenin. Finally, our observation that Nkd1 inhibits accumulation of nuclear β-catenin suggests that this effect of Nkd1 takes place in the cytoplasm.
Recently, Axin1 and Axin2 were also found to interact with Nkd1 through the C-terminal poly-histidine tail of Nkd1 [28], but it is not yet clear if this interaction is occurring in the cytoplasm or at the plasma membrane or both. Axin has been found to form both plasma membrane localized and intracellular puncta, but these two domains act opposite to one another with respect to Wnt signaling. Wnt ligands induce the formation of plasma membrane-localized Axin clusters which also contain Dvl and LRP5/6 proteins forming a Wnt signalosome [48], while cytoplasmic Axin clusters co-localize with APC and β-catenin and constitute the constitutively active destruction complex[50]. Also, it is well documented that these clusters are aggregates of Wnt signaling components and not true vesicles [36], [48]. Thus, as Nkd1 binds to Axin [28], Dvl [10], [22], [23], [39] (this study) and β-catenin [28] (this study), it is likely that the observed Nkd1 puncta are also part of a Wnt signalosome and/or destruction complex, although this requires formal testing.
In Drosophila, Nkd was also tested for interactions with Axin and β-catenin by yeast-2-hybrid but no interaction was detected [23]. Since we found that membrane localization of Nkd1 is critical for its interaction with β-catenin, it is possible that the yeast-2-hybrid environment could not recapitulate a possible membrane requirement for Drosophila Nkd to interact with β-catenin. This is supported by the finding that fly Nkd function is also dependent on its interaction with the membrane [27]. Alternatively, the interaction between Nkd/Nkd1 and β-catenin requires an intermediate protein, which would be available in vivo, but not available in a yeast-2-hybrid assay.
Myristoylation of Nkd1 is critical for its activity and to understand this further, we generated Nkd1 chimeras to target Nkd1 to the membrane in the absence of myristoylation to determine if these chimera's could recapitulate wild type Nkd1 activity. Tagging Nkd1 with alternate membrane localization motifs (N- or C-terminal Plekstrin Homology domain, a N-terminal signal sequence with transmembrane motif, and a C-terminal CAAX domain) could not recapitulate Nkd1 activity and in all cases could not localize, or only weakly localize, to the plasma membrane (TVR, LS-K, RJC unpublished data). This suggests that myristoylation is specifically effective at bringing Nkd1 to the membrane.
The exact mechanism of Nkd1 action is still unknown, but may involve the nuclear import machinery. In Drosophila it was found that Nkd Cuticle binds to Importin alpha3 via its ARM repeats and that this region in Nkd is critical for its function [24]. Interestingly, Importin alpha3 shares significant homology to β-catenin specifically in the ARM-repeats and we are currently testing the hypothesis that Nkd1 and β-catenin interact through the β-catenin ARM repeats. Fly Nkd also contains nuclear localization sequences and is observed in the nucleus. While these nuclear localization sequences are conserved between fly and mosquito Nkd proteins, they are not conserved in vertebrate Nkds. Furthermore, suppression of nuclear export in Drosophila cells did not result in nuclear accumulation of Drosophila Nkd [26], arguing against a role for Nkd in the nucleus. While we can not rule out a role for vertebrate Nkd1 in the nucleus, the absence of conserved nuclear localization sequences and the presence of a myristoyl moiety suggests that, at least in zebafish blastula cells, Nkd1 is unlikely to have a significant role in the nucleus.
Our observations that Nkd1 has a more dramatic effect on ectopic Wnt signaling compared with endogenous Wnt signaling appears to reflect an evolutionarily conserved phenomenon. In fly, Nkd is only required during early segmentation of the Drosophila embryo and not during other aspects of development involving Wg signaling [51]. In vertebrates knockout of both Nkd1 and Nkd2 has no overt phenotype [52]. We have also knocked down zebrafish Nkd1 and Nkd2 and observed modest, but significant, changes in Wnt signaling [9], and overexpression of Nkd, Nkd1 or Nkd2 by itself does not have a dramatic or consistent phenotype in wild-type embryos [9], [51]. Only under compromised Wnt signaling conditions do we observe Nkd1 function, either by overexpression of Wnt or by genetic manipulation that results in reduced or ectopic Wnt signaling [9] (this study and TVR, LS-K, RJC unpublished). This is entirely consistent with observations in Drosophila [23], [51] and suggests that there is a threshold of Wg/Wnt signaling upon which Nkd functions. We predict that this threshold would be lower in a Wg/Wnt sensitized background. This could explain the subtle loss of function phenotype in the Nkd1/Nkd2 double knockout mouse if this threshold is never perturbed under normal physiological conditions, or why there are not additional nkd-/- phenotypes in the developing Drosophila embryo.
In conclusion, we have demonstrated that in zebrafish blastula cells, Nkd1 prevents nuclear accumulation of β-catenin and that this function of Nkd1 is dependent on its plasma membrane localization via myristoylation. Furthermore, we also found Nkd1 to interact with β-catenin and that Dvl2 and β-catenin may compete for binding to Nkd1. The functional significance of Nkd1 interacting with β-catenin, Dvl2 and Axin is currently under investigation.
Materials and Methods
Quantitative RT-PCR
Total RNA from embryos was prepared using Trizol (Gibco). cDNA was synthesized with Superscript II (Invitrogen). Quantitative RTPCR (qRT-PCR) was performed using IQ Syber green (BioRad). Three replicates were performed for each experiment. Sequences of primers were as follows. β-Actin Forward: 5′-ATGCCCCTCGTGCTGTTTT-3′; β-Actin Reverse: 5′-TCTGTCCCATGCCAACCAT-3′; Nkd1-5′UTR Forward 5′- AAGCCGCGCGCTCTTC-3′; Nkd1-5′UTR Reverse 5′- GAAACATCAGAAACAAAAAACAATCC-3′; Axin2 Forward: 5′-ATGCCCCTCGTGCTGTTTT-3′; Axin2 Reverse 5′-TCTGTCCCATGCCAACCAT-3′. Absolute quantification was determined using a standard curve generated by β-actin. Relative quantitative RT-PCR (qRT-PCR) was determined by normalizing to uninjected.
Constructs
Nkd1G2A constructs were generated by site directed mutagenesis. Constructs were cloned into the ClaI site of pCS2+myc or pCS2+GFP. Mutagenesis primers are as follows Nkd1G2A For 5′- GCAGGATCCCATCGATGATGGCTAAACTTCATTCC-3′; Nkd1G2A Rev 5′- GGAATGAAGTTTAGCCATCATCGATGGGATCCTGC-3′; Orientation and sequence integrity were confirmed by sequencing. pCS2+Axin1 was kindly provided by Toshio Hirano [33]. pCS2+Wnt8 was kindly provided by Randall Moon [31], [53].
mRNA injections
Capped mRNA was synthesized and purified using Ambion's mMessage mMachine kit following their protocol. mRNA was diluted in RNase free water, and its quantity and quality was analyzed by spectrophotometer and RNase free gel analysis. For all of the experiments, except where noted, 800 pg of nkd1 myc, nkd1GFP; nkd1N-GFP; or 1200 pg nkd1G2A-myc or nkd1G2A-GFP; 25 pg wnt8; 100 pg dsh2-HA; 400 pg axin1 in 1% phenol red were pressure injected into the yolk cell of one- to two-cell-stage embryos. For immunohistochemistry on nuclear β-catenin, a mixture of 25 pg wnt8 and 100 pg of nkd1GFP or nkd1N-GFP was pressure injected into one of four blastomeres.
Fractionations
Ten embryos were harvested at dome stage (4.3 hpf), mechanically dechorionated and deyolked in 0.3X Danieau. Deyolked animal caps were lysed by trituration in TKM buffer (50 mM Tris-HCl 7.5; 25 mM KCl; 5 mM MgCl2; 1 mM EGTA; 0.02% Na Azide) containing Complete® Protease Inhibitor (Roche). Lysates were centrifuged at 1000g for 5 min at 4°C to pellet nuclei, large membrane fragments and whole cells. The resulting lysate was further fractionated into crude plasma membrane and cytoplasmic fractions by centrifugation at 100,000g for 1 hr at 4°C.
Immunoprecipitations
Cells were lysed through an 18 guage needle in modified Rubenfelds buffer [54] (modification: 0.3% NP-40 replaced 1% Triton-X) containing Complete Protease Inhibitior® (Roche). Primary antibodies consisted of (monoclonal anti-β-catenin, Sigma; monoclonal anti-myc 9E10, Vanderbilt Antibody Core; polyclonal anti-Dvl2 generated against the zebrafish Dvl2 peptide HSSGSTRSDGEKKRRGPKSVSE, corresponding to amino acids 573–592 and conjugated on the C-terminus with a Cysteine (Covance).
Western Analysis
Monoclonal anti-myc 9E10 1∶2000; monoclonal anti-β-catenin, 1∶1000; polyclonal anti-GFP (Torrey Pines) 1∶1000, polyclonal anti-pan-cadherin (Abcam) 1∶1000, monoclonal anti-actin (Sigma) 1∶2000; polyclonal anti-Dvl2 1∶5000; anti-HA (Vanderbilt Antibody Core) 1∶1000.
Immunohistochemistry
Primary antibodies: mouse anti-myc 1∶500; Rat anti-HA 1∶200; Rabbit anti-zDvl2 1∶1000 or 1∶5000; mouse anti-β-catenin 1∶250). Secondary antibodies: Cy2 Donkey anti-Rat 1∶200; Cy3 Goat anti-mouse 1∶200; Cy3 Goat anti-Rabbit 1∶200; Cy2 Goat anti-Rabbit 1∶200; AlexaFluor 594 1∶200; syto59 1∶10,000.
Nuclear β-catenin
Whole mount immunhistochemistry: Nuclear β-catenin staining was carried out using the Vectastain Elite ABC kit (Vectastain Laboratories) along with Avidin D (blocking kit, Vectastain Laboratories) and ImmPact DAB (Vectastain Laboratories).
Immunohistochemistry: To quantify the changes in nuclear β-catenin, 6 cells Nkd1GFP positive and 6 juxtaposted GFP negative cells, and 10 cells for Nkd1N-GFP positive and juxtaposed GFP negative cells were analyzed. Grey scales for Nuclear Hoechst and nuclear β-catenin where calculated using Image J. They grey levels of signal intensity were measured across the cell for both the Hoechst staining and the β-catenin staining. For each cell, the average level of β-catenin in the nuclei was normalized to its Hoechst staining. The adjusted averages were then plotted and compared by students T-test.
Nkd1 Interaction Mapping
Immunoprecipitation (IP) and immunoblotting (IB) were carried out using an anti-flag antibody (Sigma #F3165; 1∶1000 IP, 1∶3000 IB). LUMIER experiments were performed as previously described [28], [55].
Supporting Information
Nkd1 inhibits nuclear β-catenin. (A) Whole embryo analysis of nuclear β-catenin staining as per Fig. 4.) (B) Immunohistochemistry of Nkd1GFP and Nkd1N-GFP co-injections with wnt8. Injections are labeled along the left side, staining is identified along the top.
(TIF)
Dvl2 and β-catenin bind to similar domains on Nkd1. HEK293T cells were transfected with luciferase-tagged β-catenin (left panel) or Dvl2 (right panel) and the indicated 3XFlag-Nkd1 constructs. Cell lysates were subject to anti-Flag immunoprecipitation and the presence of β-catenin or Dvl2 was assessed by luciferase assay. Luciferase activity of the immunoprecipitate was normalised to that of the total cell lysate (left panel) or plotted separately (right panel). Data are the average of at least 4 independent experiments expressed as % wild type +/− SEM (left panel) or as the mean of 2 samples +/− standard deviation of a representative experiment (right panel).
(TIF)
Acknowledgments
We would like to thank members of the Solnica-Krezel, Coffey, Attisano and Van Raay labs for support and valuable comments.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: TVR acknowledges support from the University of Guelph and the Canadian Foundation for Innovation; LA acknowledges support from the Canadian Cancer Society; LSK acknowledges the support of National Institutes of Health (NIH) RO1 grant GM55101; RJC acknowledges the support of NCI CA46413. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 2.Behrens J, Lustig B. The Wnt connection to tumorigenesis. Int J Dev Biol. 2004;48:477–487. doi: 10.1387/ijdb.041815jb. [DOI] [PubMed] [Google Scholar]
- 3.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 4.Fuchs E. The tortoise and the hair: slow-cycling cells in the stem cell race. Cell. 2009;137:811–819. doi: 10.1016/j.cell.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones S, Chen WD, Parmigiani G, Diehl F, Beerenwinkel N, et al. Comparative lesion sequencing provides insights into tumor evolution. Proc Natl Acad Sci U S A. 2008;105:4283–4288. doi: 10.1073/pnas.0712345105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 7.Chang JL, Chang MV, Barolo S, Cadigan KM. Regulation of the feedback antagonist naked cuticle by Wingless signaling. Dev Biol. 2008;321:446–454. doi: 10.1016/j.ydbio.2008.05.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Van Raay TJ, Coffey RJ, Solnica-Krezel L. Zebrafish Naked1 and Naked2 antagonize both canonical and non-canonical Wnt signaling. Dev Biol. 2007;309:151–168. doi: 10.1016/j.ydbio.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wharton KA, Jr, Zimmermann G, Rousset R, Scott MP. Vertebrate proteins related to Drosophila Naked Cuticle bind Dishevelled and antagonize Wnt signaling. Dev Biol. 2001;234:93–106. doi: 10.1006/dbio.2001.0238. [DOI] [PubMed] [Google Scholar]
- 11.Behrens J, Jerchow BA, Wurtele M, Grimm J, Asbrand C, et al. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998;280:596–599. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- 12.Chia IV, Costantini F. Mouse axin and axin2/conductin proteins are functionally equivalent in vivo. Mol Cell Biol. 2005;25:4371–4376. doi: 10.1128/MCB.25.11.4371-4376.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmidt C, Otto A, Luke G, Valasek P, Otto WR, et al. Expression and regulation of Nkd-1, an intracellular component of Wnt signalling pathway in the chick embryo. Anat Embryol (Berl) 2006;211:525–534. doi: 10.1007/s00429-006-0102-4. [DOI] [PubMed] [Google Scholar]
- 14.Guo J, Cagatay T, Zhou G, Chan CC, Blythe S, et al. Mutations in the human naked cuticle homolog NKD1 found in colorectal cancer alter Wnt/Dvl/beta-catenin signaling. PLoS One. 2009;4:e7982. doi: 10.1371/journal.pone.0007982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan D, Wiesmann M, Rohan M, Chan V, Jefferson AB, et al. Elevated expression of axin2 and hnkd mRNA provides evidence that Wnt/beta-catenin signaling is activated in human colon tumors. Proc Natl Acad Sci U S A. 2001;98:14973–14978. doi: 10.1073/pnas.261574498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lei H, Juan AH, Kim MS, Ruddle FH. Mouse naked cuticle 2 (mNkd2) as a direct transcriptional target of Hoxc8 in vivo. J Exp Zool A Ecol Genet Physiol. 2007;307:1–6. doi: 10.1002/jez.a.327. [DOI] [PubMed] [Google Scholar]
- 17.Hu T, Li C, Cao Z, Van Raay TJ, Smith JG, et al. Myristoylated Naked2 antagonizes Wnt-beta-catenin activity by degrading Dishevelled-1 at the plasma membrane. J Biol Chem. 2010;285:13561–13568. doi: 10.1074/jbc.M109.075945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao Z, Li C, Higginbotham JN, Franklin JL, Tabb DL, et al. Use of fluorescence-activated vesicle sorting for isolation of Naked2-associated, basolaterally targeted exocytic vesicles for proteomics analysis. Mol Cell Proteomics. 2008;7:1651–1667. doi: 10.1074/mcp.M700155-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding W, Li C, Hu T, Graves-Deal R, Fotia AB, et al. EGF receptor-independent action of TGF-alpha protects Naked2 from AO7-mediated ubiquitylation and proteasomal degradation. Proc Natl Acad Sci U S A. 2008;105:13433–13438. doi: 10.1073/pnas.0806298105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li C, Franklin JL, Graves-Deal R, Jerome WG, Cao Z, et al. Myristoylated Naked2 escorts transforming growth factor alpha to the basolateral plasma membrane of polarized epithelial cells. Proc Natl Acad Sci U S A. 2004;101:5571–5576. doi: 10.1073/pnas.0401294101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li C, Hao M, Cao Z, Ding W, Graves-Deal R, et al. Naked2 acts as a cargo recognition and targeting protein to ensure proper delivery and fusion of TGF-alpha containing exocytic vesicles at the lower lateral membrane of polarized MDCK cells. Mol Biol Cell. 2007;18:3081–3093. doi: 10.1091/mbc.E07-02-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan D, Wallingford JB, Sun TQ, Nelson AM, Sakanaka C, et al. Cell autonomous regulation of multiple Dishevelled-dependent pathways by mammalian Nkd. Proc Natl Acad Sci U S A. 2001;98:3802–3807. doi: 10.1073/pnas.071041898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rousset R, Mack JA, Wharton KA, Jr, Axelrod JD, Cadigan KM, et al. Naked cuticle targets dishevelled to antagonize Wnt signal transduction. Genes Dev. 2001;15:658–671. doi: 10.1101/gad.869201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan CC, Zhang S, Rousset R, Wharton KA., Jr Drosophila Naked cuticle (Nkd) engages the nuclear import adaptor Importin-alpha3 to antagonize Wnt/beta-catenin signaling. Dev Biol. 2008;318:17–28. doi: 10.1016/j.ydbio.2008.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rousset R, Wharton KA, Jr, Zimmermann G, Scott MP. Zinc-dependent interaction between dishevelled and the Drosophila Wnt antagonist naked cuticle. J Biol Chem. 2002;277:49019–49026. doi: 10.1074/jbc.M203246200. [DOI] [PubMed] [Google Scholar]
- 26.Waldrop S, Chan CC, Cagatay T, Zhang S, Rousset R, et al. An unconventional nuclear localization motif is crucial for function of the Drosophila Wnt/wingless antagonist Naked cuticle. Genetics. 2006;174:331–348. doi: 10.1534/genetics.106.061853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan CC, Zhang S, Cagatay T, Wharton KA., Jr Cell-autonomous, myristyl-independent activity of the Drosophila Wnt/Wingless antagonist Naked cuticle (Nkd). Dev Biol. 2007;311:538–553. doi: 10.1016/j.ydbio.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller BW, Lau G, Grouios C, Mollica E, Barrios-Rodiles M, et al. Application of an integrated physical and functional screening approach to identify inhibitors of the Wnt pathway. Mol Syst Biol. 2009;5:315. doi: 10.1038/msb.2009.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan L, Collodi P. Zebrafish embryonic stem cells. Methods Enzymol. 2006;418:64–77. doi: 10.1016/S0076-6879(06)18004-0. [DOI] [PubMed] [Google Scholar]
- 30.Kane DA, Kimmel CB. The zebrafish midblastula transition. Development. 1993;119:447–456. doi: 10.1242/dev.119.2.447. [DOI] [PubMed] [Google Scholar]
- 31.Kelly GM, Greenstein P, Erezyilmaz DF, Moon RT. Zebrafish wnt8 and wnt8b share a common activity but are involved in distinct developmental pathways. Development. 1995;121:1787–1799. doi: 10.1242/dev.121.6.1787. [DOI] [PubMed] [Google Scholar]
- 32.Ramel MC, Lekven AC. Repression of the vertebrate organizer by Wnt8 is mediated by Vent and Vox. Development. 2004;131:3991–4000. doi: 10.1242/dev.01277. [DOI] [PubMed] [Google Scholar]
- 33.Shimizu T, Yamanaka Y, Ryu SL, Hashimoto H, Yabe T, et al. Cooperative roles of Bozozok/Dharma and Nodal-related proteins in the formation of the dorsal organizer in zebrafish. Mech Dev. 2000;91:293–303. doi: 10.1016/s0925-4773(99)00319-6. [DOI] [PubMed] [Google Scholar]
- 34.Ryu SL, Fujii R, Yamanaka Y, Shimizu T, Yabe T, et al. Regulation of dharma/bozozok by the Wnt pathway. Dev Biol. 2001;231:397–409. doi: 10.1006/dbio.2000.0150. [DOI] [PubMed] [Google Scholar]
- 35.Schwarz-Romond T, Merrifield C, Nichols BJ, Bienz M. The Wnt signalling effector Dishevelled forms dynamic protein assemblies rather than stable associations with cytoplasmic vesicles. J Cell Sci. 2005;118:5269–5277. doi: 10.1242/jcs.02646. [DOI] [PubMed] [Google Scholar]
- 36.Schwarz-Romond T, Fiedler M, Shibata N, Butler PJ, Kikuchi A, et al. The DIX domain of Dishevelled confers Wnt signaling by dynamic polymerization. Nat Struct Mol Biol. 2007;14:484–492. doi: 10.1038/nsmb1247. [DOI] [PubMed] [Google Scholar]
- 37.Hwang CS, Shemorry A, Varshavsky A. N-terminal acetylation of cellular proteins creates specific degradation signals. Science. 2010;327:973–977. doi: 10.1126/science.1183147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsang M, Maegawa S, Kiang A, Habas R, Weinberg E, et al. A role for MKP3 in axial patterning of the zebrafish embryo. Development. 2004;131:2769–2779. doi: 10.1242/dev.01157. [DOI] [PubMed] [Google Scholar]
- 39.Angers S, Thorpe CJ, Biechele TL, Goldenberg SJ, Zheng N, et al. The KLHL12-Cullin-3 ubiquitin ligase negatively regulates the Wnt-beta-catenin pathway by targeting Dishevelled for degradation. Nat Cell Biol. 2006;8:348–357. doi: 10.1038/ncb1381. [DOI] [PubMed] [Google Scholar]
- 40.Hino S, Michiue T, Asashima M, Kikuchi A. Casein kinase I epsilon enhances the binding of Dvl-1 to Frat-1 and is essential for Wnt-3a-induced accumulation of beta-catenin. J Biol Chem. 2003;278:14066–14073. doi: 10.1074/jbc.M213265200. [DOI] [PubMed] [Google Scholar]
- 41.Peters JM, McKay RM, McKay JP, Graff JM. Casein kinase I transduces Wnt signals. Nature. 1999;401:345–350. doi: 10.1038/43830. [DOI] [PubMed] [Google Scholar]
- 42.McKay RM, Peters JM, Graff JM. The casein kinase I family in Wnt signaling. Dev Biol. 2001;235:388–396. doi: 10.1006/dbio.2001.0308. [DOI] [PubMed] [Google Scholar]
- 43.Gao ZH, Seeling JM, Hill V, Yochum A, Virshup DM. Casein kinase I phosphorylates and destabilizes the beta-catenin degradation complex. Proc Natl Acad Sci U S A. 2002;99:1182–1187. doi: 10.1073/pnas.032468199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wharton KA. Runnin' with the dvl: proteins that associate with dsh/dvl and their significance to wnt signal transduction. Dev Biol. 2003;253:1–17. doi: 10.1006/dbio.2002.0869. [DOI] [PubMed] [Google Scholar]
- 45.Li FQ, Mofunanya A, Harris K, Takemaru K. Chibby cooperates with 14-3-3 to regulate beta-catenin subcellular distribution and signaling activity. J Cell Biol. 2008;181:1141–1154. doi: 10.1083/jcb.200709091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phelps RA, Chidester S, Dehghanizadeh S, Phelps J, Sandoval IT, et al. A two-step model for colon adenoma initiation and progression caused by APC loss. Cell. 2009;137:623–634. doi: 10.1016/j.cell.2009.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cong F, Schweizer L, Varmus H. Wnt signals across the plasma membrane to activate the beta-catenin pathway by forming oligomers containing its receptors, Frizzled and LRP. Development. 2004;131:5103–5115. doi: 10.1242/dev.01318. [DOI] [PubMed] [Google Scholar]
- 48.Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, et al. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science. 2007;316:1619–1622. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- 49.Klimowski LK, Garcia BA, Shabanowitz J, Hunt DF, Virshup DM. Site-specific casein kinase 1epsilon-dependent phosphorylation of Dishevelled modulates beta-catenin signaling. FEBS J. 2006;273:4594–4602. doi: 10.1111/j.1742-4658.2006.05462.x. [DOI] [PubMed] [Google Scholar]
- 50.Faux MC, Coates JL, Catimel B, Cody S, Clayton AH, et al. Recruitment of adenomatous polyposis coli and beta-catenin to axin-puncta. Oncogene. 2008;27:5808–5820. doi: 10.1038/onc.2008.205. [DOI] [PubMed] [Google Scholar]
- 51.Zeng W, Wharton KA, Jr, Mack JA, Wang K, Gadbaw M, et al. naked cuticle encodes an inducible antagonist of Wnt signalling. Nature. 2000;403:789–795. doi: 10.1038/35001615. [DOI] [PubMed] [Google Scholar]
- 52.Zhang S, Cagatay T, Amanai M, Zhang M, Kline J, et al. Viable mice with compound mutations in the Wnt/Dvl pathway antagonists nkd1 and nkd2. Mol Cell Biol. 2007;27:4454–4464. doi: 10.1128/MCB.00133-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Erter CE, Wilm TP, Basler N, Wright CV, Solnica-Krezel L. Wnt8 is required in lateral mesendodermal precursors for neural posteriorization in vivo. Development. 2001;128:3571–3583. doi: 10.1242/dev.128.18.3571. [DOI] [PubMed] [Google Scholar]
- 54.Rubinfeld B, Souza B, Albert I, Muller O, Chamberlain SH, et al. Association of the APC gene product with beta-catenin. Science. 1993;262:1731–1734. doi: 10.1126/science.8259518. [DOI] [PubMed] [Google Scholar]
- 55.Barrios-Rodiles M, Brown KR, Ozdamar B, Bose R, Liu Z, et al. High-throughput mapping of a dynamic signaling network in mammalian cells. Science. 2005;307:1621–1625. doi: 10.1126/science.1105776. [DOI] [PubMed] [Google Scholar]
- 56.Katoh M. Molecular cloning, gene structure, and expression analyses of NKD1 and NKD2. Int J Oncol. 2001;19:963–969. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Nkd1 inhibits nuclear β-catenin. (A) Whole embryo analysis of nuclear β-catenin staining as per Fig. 4.) (B) Immunohistochemistry of Nkd1GFP and Nkd1N-GFP co-injections with wnt8. Injections are labeled along the left side, staining is identified along the top.
(TIF)
Dvl2 and β-catenin bind to similar domains on Nkd1. HEK293T cells were transfected with luciferase-tagged β-catenin (left panel) or Dvl2 (right panel) and the indicated 3XFlag-Nkd1 constructs. Cell lysates were subject to anti-Flag immunoprecipitation and the presence of β-catenin or Dvl2 was assessed by luciferase assay. Luciferase activity of the immunoprecipitate was normalised to that of the total cell lysate (left panel) or plotted separately (right panel). Data are the average of at least 4 independent experiments expressed as % wild type +/− SEM (left panel) or as the mean of 2 samples +/− standard deviation of a representative experiment (right panel).
(TIF)