Abstract
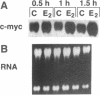
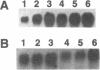
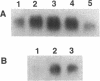
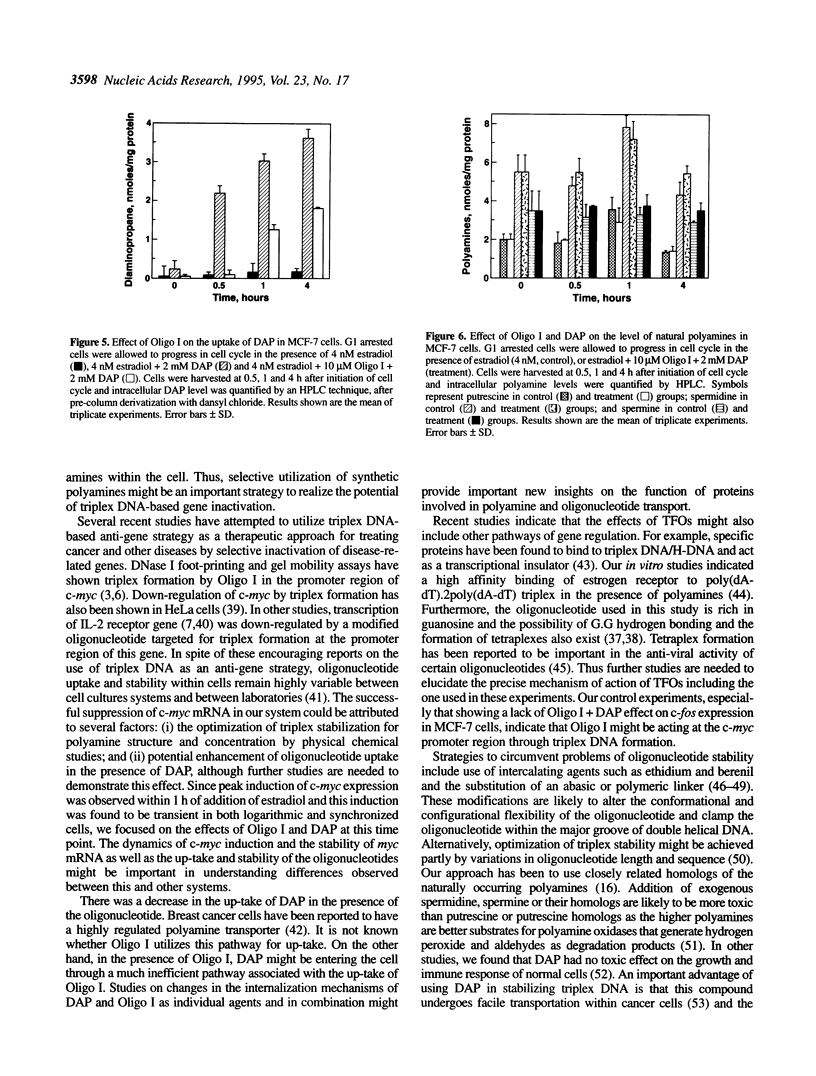
Polyamines are excellent stabilizers of triplex DNA. Recent studies in our laboratory revealed a remarkable structural specificity of polyamines in the induction and stabilization of triplex DNA. 1,3-Diaminopropane (DAP) showed optimum efficacy amongst a series of synthetic diamines in stabilizing triplex DNA. To utilize the potential of this finding in developing an anti-gene strategy for breast cancer, we treated MCF-7 cells with a 37mer oligonucleotide to form triplex DNA in the up-stream regulatory region of the c-myc oncogene in the presence of DAP. As individual agents, the oligonucleotide and DAP did not downregulate c-myc mRNA in the presence of estradiol. Complexation of the oligonucleotide with 2 mM DAP reduced c-myc mRNA signal by 65% at 10 microM oligonucleotide concentration. In contrast, a control oligonucleotide had no significant effect on c-myc mRNA. The expression of c-fos oncogene was not significantly altered by the triplex forming oligonucleotide (TFO). DAP was internalized within 1 h of treatment; however, it had no significant effect on the level of natural polyamines. These data indicate that selective utilization of synthetic polyamines and TFOs might be an important strategy to develop anti-gene-based therapeutic modalities for breast cancer.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beato M. Transcriptional control by nuclear receptors. FASEB J. 1991 Apr;5(7):2044–2051. doi: 10.1096/fasebj.5.7.2010057. [DOI] [PubMed] [Google Scholar]
- Berthois Y., Katzenellenbogen J. A., Katzenellenbogen B. S. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2496–2500. doi: 10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume S. W., Gee J. E., Shrestha K., Miller D. M. Triple helix formation by purine-rich oligonucleotides targeted to the human dihydrofolate reductase promoter. Nucleic Acids Res. 1992 Apr 11;20(7):1777–1784. doi: 10.1093/nar/20.7.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celano P., Baylin S. B., Giardiello F. M., Nelkin B. D., Casero R. A., Jr Effect of polyamine depletion on c-myc expression in human colon carcinoma cells. J Biol Chem. 1988 Apr 25;263(12):5491–5494. [PubMed] [Google Scholar]
- Cheng A. J., Van Dyke M. W. Oligodeoxyribonucleotide length and sequence effects on intermolecular purine-purine-pyrimidine triple-helix formation. Nucleic Acids Res. 1994 Nov 11;22(22):4742–4747. doi: 10.1093/nar/22.22.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. H., Morris D. R., Coffino P. Sequestered end products and enzyme regulation: the case of ornithine decarboxylase. Microbiol Rev. 1992 Jun;56(2):280–290. doi: 10.1128/mr.56.2.280-290.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubik D., Dembinski T. C., Shiu R. P. Stimulation of c-myc oncogene expression associated with estrogen-induced proliferation of human breast cancer cells. Cancer Res. 1987 Dec 15;47(24 Pt 1):6517–6521. [PubMed] [Google Scholar]
- Durland R. H., Kessler D. J., Gunnell S., Duvic M., Pettitt B. M., Hogan M. E. Binding of triple helix forming oligonucleotides to sites in gene promoters. Biochemistry. 1991 Sep 24;30(38):9246–9255. doi: 10.1021/bi00102a017. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faaland C. A., Laskin J. D., Thomas T. J. Inhibition of epidermal growth factor-stimulated EGF receptor tyrosine kinase activity in A431 human epidermoid carcinoma cells by polyamines. Cell Growth Differ. 1995 Feb;6(2):115–121. [PubMed] [Google Scholar]
- Feuerstein B. G., Szöllösi J., Basu H. S., Marton L. J. alpha-Difluoromethylornithine alters calcium signaling in platelet-derived growth factor-stimulated A172 brain tumor cells in culture. Cancer Res. 1992 Dec 15;52(24):6782–6789. [PubMed] [Google Scholar]
- Heby O., Persson L. Molecular genetics of polyamine synthesis in eukaryotic cells. Trends Biochem Sci. 1990 Apr;15(4):153–158. doi: 10.1016/0968-0004(90)90216-x. [DOI] [PubMed] [Google Scholar]
- Hélène C., Toulmé J. J. Specific regulation of gene expression by antisense, sense and antigene nucleic acids. Biochim Biophys Acta. 1990 Jun 21;1049(2):99–125. doi: 10.1016/0167-4781(90)90031-v. [DOI] [PubMed] [Google Scholar]
- Ing N. H., Beekman J. M., Kessler D. J., Murphy M., Jayaraman K., Zendegui J. G., Hogan M. E., O'Malley B. W., Tsai M. J. In vivo transcription of a progesterone-responsive gene is specifically inhibited by a triplex-forming oligonucleotide. Nucleic Acids Res. 1993 Jun 25;21(12):2789–2796. doi: 10.1093/nar/21.12.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler D. J., Pettitt B. M., Cheng Y. K., Smith S. R., Jayaraman K., Vu H. M., Hogan M. E. Triple helix formation at distant sites: hybrid oligonucleotides containing a polymeric linker. Nucleic Acids Res. 1993 Oct 11;21(20):4810–4815. doi: 10.1093/nar/21.20.4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessard M., Zhao C., Singh S. M., Poulin R. Hormonal and feedback regulation of putrescine and spermidine transport in human breast cancer cells. J Biol Chem. 1995 Jan 27;270(4):1685–1694. [PubMed] [Google Scholar]
- Mayfield C., Miller D. Effect of abasic linker substitution on triplex formation, Sp1 binding, and specificity in an oligonucleotide targeted to the human Ha-ras promoter. Nucleic Acids Res. 1994 May 25;22(10):1909–1916. doi: 10.1093/nar/22.10.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield C., Miller D. Effect of abasic linker substitution on triplex formation, Sp1 binding, and specificity in an oligonucleotide targeted to the human Ha-ras promoter. Nucleic Acids Res. 1994 May 25;22(10):1909–1916. doi: 10.1093/nar/22.10.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel D., Chatelain G., Herault Y., Harper F., Brun G. H-DNA can act as a transcriptional insulator. Cell Mol Biol Res. 1993;39(2):131–140. [PubMed] [Google Scholar]
- Mondovì B., Riccio P., Agostinelli E. The biological functions of amine oxidases and their reaction products: an overview. Adv Exp Med Biol. 1988;250:147–161. doi: 10.1007/978-1-4684-5637-0_14. [DOI] [PubMed] [Google Scholar]
- Morgan A. R., Wells R. D. Specificity of the three-stranded complex formation between double-stranded DNA and single-stranded RNA containing repeating nucleotide sequences. J Mol Biol. 1968 Oct 14;37(1):63–80. doi: 10.1016/0022-2836(68)90073-9. [DOI] [PubMed] [Google Scholar]
- Moser H. E., Dervan P. B. Sequence-specific cleavage of double helical DNA by triple helix formation. Science. 1987 Oct 30;238(4827):645–650. doi: 10.1126/science.3118463. [DOI] [PubMed] [Google Scholar]
- O'Malley B. The steroid receptor superfamily: more excitement predicted for the future. Mol Endocrinol. 1990 Mar;4(3):363–369. doi: 10.1210/mend-4-3-363. [DOI] [PubMed] [Google Scholar]
- Orson F. M., Thomas D. W., McShan W. M., Kessler D. J., Hogan M. E. Oligonucleotide inhibition of IL2R alpha mRNA transcription by promoter region collinear triplex formation in lymphocytes. Nucleic Acids Res. 1991 Jun 25;19(12):3435–3441. doi: 10.1093/nar/19.12.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E. Polyamine metabolism and its importance in neoplastic growth and a target for chemotherapy. Cancer Res. 1988 Feb 15;48(4):759–774. [PubMed] [Google Scholar]
- Pilch D. S., Breslauer K. J. Ligand-induced formation of nucleic acid triple helices. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9332–9336. doi: 10.1073/pnas.91.20.9332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter C. W., Bergeron R. J. Spermidine requirement for cell proliferation in eukaryotic cells: structural specificity and quantitation. Science. 1983 Mar 4;219(4588):1083–1085. doi: 10.1126/science.6823570. [DOI] [PubMed] [Google Scholar]
- Postel E. H., Flint S. J., Kessler D. J., Hogan M. E. Evidence that a triplex-forming oligodeoxyribonucleotide binds to the c-myc promoter in HeLa cells, thereby reducing c-myc mRNA levels. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8227–8231. doi: 10.1073/pnas.88.18.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando R. F., Ojwang J., Elbaggari A., Reyes G. R., Tinder R., McGrath M. S., Hogan M. E. Suppression of human immunodeficiency virus type 1 activity in vitro by oligonucleotides which form intramolecular tetrads. J Biol Chem. 1995 Jan 27;270(4):1754–1760. doi: 10.1074/jbc.270.4.1754. [DOI] [PubMed] [Google Scholar]
- Scaria P. V., Shafer R. H. Binding of ethidium bromide to a DNA triple helix. Evidence for intercalation. J Biol Chem. 1991 Mar 25;266(9):5417–5423. [PubMed] [Google Scholar]
- Scaria P. V., Will S., Levenson C., Shafer R. H. Physicochemical studies of the d(G3T4G3)*d(G3A4G3).d(C3T4C3) triple helix. J Biol Chem. 1995 Mar 31;270(13):7295–7303. doi: 10.1074/jbc.270.13.7295. [DOI] [PubMed] [Google Scholar]
- Singh A. B., Thomas T. J., Thomas T., Singh M., Mann R. A. Differential effects of polyamine homologues on the prevention of DL-alpha-difluoromethylornithine-mediated inhibition of malignant cell growth and normal immune response. Cancer Res. 1992 Apr 1;52(7):1840–1847. [PubMed] [Google Scholar]
- Spencer C. A., Groudine M. Control of c-myc regulation in normal and neoplastic cells. Adv Cancer Res. 1991;56:1–48. doi: 10.1016/s0065-230x(08)60476-5. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Thomas T. J., Bloomfield V. A., Canellakis Z. N. Differential effects on the B-to-Z transition of poly(dG-me5dC).poly(dG-me5dC) produced by N1- and N8-acetyl spermidine. Biopolymers. 1985 Apr;24(4):725–729. doi: 10.1002/bip.360240411. [DOI] [PubMed] [Google Scholar]
- Thomas T. J., Bloomfield V. A. Ionic and structural effects on the thermal helix-coil transition of DNA complexed with natural and synthetic polyamines. Biopolymers. 1984 Jul;23(7):1295–1306. doi: 10.1002/bip.360230713. [DOI] [PubMed] [Google Scholar]
- Thomas T. J., Messner R. P. Structural specificity of polyamines in left-handed Z-DNA formation. Immunological and spectroscopic studies. J Mol Biol. 1988 May 20;201(2):463–467. doi: 10.1016/0022-2836(88)90155-6. [DOI] [PubMed] [Google Scholar]
- Thomas T., Kiang D. T. A twenty-two-fold increase in the relative affinity of estrogen receptor to poly (dA-dC).poly (dG-dT) in the presence of polyamines. Nucleic Acids Res. 1988 May 25;16(10):4705–4720. doi: 10.1093/nar/16.10.4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T., Kiang D. T. Additive growth-inhibitory effects of DL-alpha-difluoromethylornithine and antiestrogens on MCF-7 breast cancer cell line. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1338–1345. doi: 10.1016/s0006-291x(87)80279-6. [DOI] [PubMed] [Google Scholar]
- Thomas T., Kiang D. T., Jänne O. A., Thomas T. J. Variations in amplification and expression of the ornithine decarboxylase gene in human breast cancer cells. Breast Cancer Res Treat. 1991 Nov;19(3):257–267. doi: 10.1007/BF01961162. [DOI] [PubMed] [Google Scholar]
- Thomas T., Nandi U. S., Podder S. K. Enzymatic degradation and circular dichroism of deoxyguanosine oligonucleotides. Indian J Biochem Biophys. 1984 Aug;21(4):227–231. [PubMed] [Google Scholar]
- Thomas T., Thomas T. J. Estradiol control of ornithine decarboxylase mRNA, enzyme activity, and polyamine levels in MCF-7 breast cancer cells: therapeutic implications. Breast Cancer Res Treat. 1994 Feb;29(2):189–201. doi: 10.1007/BF00665680. [DOI] [PubMed] [Google Scholar]
- Thomas T., Thomas T. J. Regulation of cyclin B1 by estradiol and polyamines in MCF-7 breast cancer cells. Cancer Res. 1994 Feb 15;54(4):1077–1084. [PubMed] [Google Scholar]
- Thomas T., Thomas T. J. Structural specificity of polyamines in modulating the binding of estrogen receptor to potential Z-DNA forming sequences. J Recept Res. 1993;13(8):1115–1133. doi: 10.3109/10799899309063267. [DOI] [PubMed] [Google Scholar]
- Thomas T., Trend B., Butterfield J. R., Jänne O. A., Kiang D. T. Regulation of ornithine decarboxylase gene expression in MCF-7 breast cancer cells by antiestrogens. Cancer Res. 1989 Nov 1;49(21):5852–5857. [PubMed] [Google Scholar]
- Tonkinson J. L., Stein C. A. Patterns of intracellular compartmentalization, trafficking and acidification of 5'-fluorescein labeled phosphodiester and phosphorothioate oligodeoxynucleotides in HL60 cells. Nucleic Acids Res. 1994 Oct 11;22(20):4268–4275. doi: 10.1093/nar/22.20.4268. [DOI] [PMC free article] [PubMed] [Google Scholar]