Abstract
Objectives
To evaluate the concordance between the subjective and objective methods of sleep assessment in patients with fibromyalgia (FMS) and to delineate factors associated with discrepancy between the two sleep assessment methods.
Methods
Seventy-five patients with FMS completed a 7 day home assessment protocol. They wore an Actigraphic device at all times. In the morning, they used the electronic diary (ED) to record the subjective report of sleep from the previous night and current severity of the FMS-related symptoms.
Results
On average, the two assessment methods yielded a 73 absolute minute difference per night per patient. About the half of the nights, sleep duration was underestimated. Approximately 20% of the nights had greater than 2 hour difference between the two methods. Factors related to this large discrepancy were 1) objective indicator of restless sleep, 2) subjective report of difficulty falling asleep, and 3) report of fatigue at the time of reporting. FMS-related symptoms were related to subjective report of poor sleep but not to objective sleep data.
Discussion
Misestimation of sleep appears common in FMS patients, particularly when their sleep quality is poor. Careful considerations for evaluating the severity of patients’ sleep complaints are critical in adequate management of sleep disturbance that is commonly reported by FMS patients.
Keywords: Fibromyalgia, Sleep, Actigraph, Electronic Diary
Introduction
Fibromyalgia syndrome (FMS) is a prevalent, chronic pain conditions, characterized by the presence of generalized body pain and hyperalgesic responses to specific tender points 1. It is estimated that 3-5% of the US population is afflicted with FMS 2. FMS patients typically complain of various functional co-morbidities, including chronic fatigue, mood disturbance, headaches, functional disturbance and sleep disturbance 3, 4. Sleep disturbance in FMS has been of particular interest because 1) sleep disturbance is one of the most common complaints in FMS 5 and 2) the experimental studies have shown that FMS-like symptoms develop following repeated deprivation of slow wave sleep 6, 7.
There are several methods for assessing sleep. The “gold standard” of sleep measure is to use polysomnography. Earlier electrocephalographic (EEG) data showed the presence of alpha wave intrusion during the delta wave sleep 8, leading to a hypothesis that FMS might be a variant of a sleep disorder. However, subsequent research failed to confirm that the alpha intrusion is a universal phenomenon in FMS 9, and a recent study 10 showed no difference in the sleep architectures between FMS patients and healthy controls.
Unfortunately, polysomnographic evaluations are labor and cost intensive and are not always feasible. Recently, as an alternative objective method to assess sleep, some studies have used actigraphic devices. These are small, wrist watch sized devices that patients are typically asked to wear at all times. They continuously record 3-dimensional movements, and sleep scores can be derived using the pre-determined algorithm. Thus far, literature seems to be equivocal as to whether FMS is associated with actigraphically obtained sleep disturbance. Landis et al. 11 have found no difference in actigraphically obtained sleep parameters of FMS women relative to healthy women, although FMS women showed significant relationship between several actigraphic indicators of poor sleep and fatigue. Korszun et al 12 on the other hand, have shown the significantly poorer sleep, specifically greater activity levels during night, for FMS patients compared to healthy controls.
Overall, however, the most common method to assess the quality of sleep is self-report. Such measures include standardized instruments such as the Pittsburgh Sleep Quality Index (PSQI) 13 and questionnaires that include sleep-related questions such as Fibromyalgia Impact Questionnaire 14. Based upon the PSQI, FMS patients reported an average of 6.5 hours of sleep per night and elevated level of unrestorative feeling 15. FMS patients reported significantly greater sleep problems than the healthy controls 16. Alternatively sleep may be self-monitored on the daily basis, using a format such as a written sleep diary or electronic device. The advantage of these methods is that they allow longitudinal assessment and time-series analyses of sleep and relevant factors in FMS. Sequential time series study has shown the dynamic interaction of sleep and pain in FMS 17; pain seems to worsen after poor sleep and in turn, increased pain seems to lead to poor sleep. Similarly, negative emotional response seems to follow a night of poor sleep 18.
One of the concerns about the subjective report of sleep is validity. Generally the association between the subjective and objective sleep measures appears to be modest 19. There is some evidence suggesting that subjective sleep data poorly agree with objectively assessed sleep data for people with sleep disturbance. For example, poor concordance between the subjective and objective reports of sleep duration among insomniacs has been repeatedly demonstrated 20-25. Similarly, the study of twins with chronic fatigue syndrome (CFS) showed significantly greater problems with sleep in their subjective reports among the CFS patients compared to their healthy co-twins, although the polysomnographic data failed to show such differences between the twins 26. A recent study 27 evaluating the quality of sleep in older chronic pain patients relative to age matched controls without sleep complaints found significant differences in the subjective sleep reports between the groups; however, with an exception of sleep duration, the groups did not differ in the actigraphically obtained measures of sleep quality.
The lack of concordance between the subjective and objective methods of sleep assessment may impose a significant clinical dilemma for those who treat FMS. Sleep is one of the most significant impairments for FMS patients and the initiation and direction of sleep therapy for these patients typically depends upon their subjective report of their sleep quality. The objectives of the present study were to 1) examine the concordance between the subjective and objective (actigraphic) methods of sleep assessment in FMS patients over a week and 2) delineate factors associated with a high degree of discrepancy between the two sleep assessment methods. We used the nightly sleep as a unit of analysis, because this will take into account the potential intrasubject variability of sleep quality over time that may be common among people with disturbed sleep 28.
METHODS
The research protocol was approved by the Institutional Review Board at the University of Utah. All subjects provided written consent prior to entering the study.
Participants
A total of 75 FMS patients, who were recruited to be a part of a larger clinical study, were included in this study. The participants were mostly females (n=73, 97%) and 45.56 years old on average (SD=10.79 years), with the average FMS duration of 13.08 years (SD=10.94). General background information of the patients is shown in Table 1. Their FMS classification by the American College of Rheumatology 1 must be met and was confirmed by the standardized tender point examination 29 conducted by a nurse practitioner and the use of Pain Drawing 30 to assess the presence of widespread pain.
Table 1.
Background Information (n=75)
| Age | 45.56 (SD=10.79) |
| Pain Duration in Years | 13.08 (SD=10.94) |
| Sex (female) | 97% |
| Race (white) | 96% |
| Education (> HS) | 97% |
| Marital (married) | 67% |
| Employment | |
| Working full time | 24% |
| Working part time | 11% |
| Un employed due to pain | 7% |
| Pain Onset (insidious) | 53% |
| Medications | |
| NSAIDs | 79% |
| Opioid analgesics | 39% |
| Tricyclics | 12% |
| SSRI/SNRI | 51% |
| Antieplectic | 21% |
| Muscle relaxant | 25% |
| Benzodiazepine | 27% |
| Nonbenzo sedatives | 35% |
Procedures
Objective Assessment of Sleep: Actigraph Sleep Assessment
Each participant was asked to continuously wear a Micro Mini Motionlogger Actigraph (Ambulatory Monitoring, Ardsley, NY, USA) on his/her nondominant wrist, a wristwatch like device that measures three-dimensional movement, for 7 days. The device was set up during the first visit to our study center by Research Assistant. Once it was set up, a subject could not modify the setting. The actigraph value was not visible and thus, the subject was blind to the actigraph data. Each subject continuously wore the device once the set-up was completed, and the sleep data were derived from the night of the visit and for 7 consecutive nights. Epoch length was determined at one minute, using the zero-crossing mode. Sleep was defined if the probability of sleep exceeds the criterion in the epoch, based upon the algorithm defined in Sadeh et al.31. The software Action series (Ambulatory Monitoring Inc.) calculates common sleep parameters (total sleep time, sleep efficiency, sleep onset latency, # nocturnal awaking, and time awake after sleep onset), using the algorithm developed by Cole et al.32. Actigraph data has shown high agreement with polysomnographic data (91-93%) 31 as well as minute-by-minute agreement with EEG 32.
Subjective Method: Electronic Diary Assessment
Each participant was asked to carry a Palm Pilot device, PalmOne Zire34 (Palm Inc., Sunnyvale, CA) that was used for Electronic Diary (ED) for 7 consecutive days. Each PalmOne was custom-programmed (TikiSoft, Orange, CA) to disable all the functions except for the ED questions and prompting intervals.
A day was divided into three epochs, and there was one random audible prompt in each epoch. Epochs were defined as first (time of wakening - 11:59 am), second (noon-4:59pm) and third (5:00pm - 8:59pm). Participants were asked to use an alarm clock programmed into the Palm Pilot to indicate the period when they have been sleeping. The audible tone lasted 60 seconds. If not answered within this time, the tone would be repeated 5 minutes later, and if not answered again, 5 minutes later. Failure to answer this sequence of 3 requests for data entry produced a missing entry for that epoch. If the prompt occurred at an inconvenient moment, participants were able to delay the assessment for up to 20 minutes. Because data entry requests were signaled randomly and participants were unable to predict the exact timing of the signal, the allowance of 20 minute delay appeared reasonable to minimize the intrusiveness of the ED. Since the ED was a random sampling procedure, this delay likely would not undermine the quality of the data.
ED questions were presented one at a time in a fixed order on a liquid crystal display. Participants were asked to respond to each question by scrolling across fixed response options with backward and forward arrows and then pressing an “enter” button to save the response and its time stamp on an “Electronically Erasable Programmable Read-Only Memory” data pack. Within an epoch the responders could move backward to change their response to a prior question; however, once they completed the responses for that assessment epoch and saved them, participants could not access the data, nor could they access data from prior assessment epochs.
At each ED epoch, participants were asked to rate their present levels for 7 FMS-related problems. Additionally, 5 questions relevant to sleep were included in the morning assessment epoch. The questions are listed in Table 2. Although there are three ED assessments a day, we were only interested in the morning data because only the AM epoch has the sleep related questions. Based upon the Q1 and Q2, we calculated self-reported sleep duration for the night. Participants returned to the laboratory at the end of the 7-day trial period with their actigraph and ED devices. The Research Assistant (RA) then uploaded data from the device to a desk-top computer via a serial link. Data were automatically entered into a spreadsheet file for subsequent analysis.
Table 2.
Palm Electronic Diary Questions
|
Statistical Analyses
Given that the majority of subjects both under- and over-reported their subjective sleep durations and that there was no nightly pattern effect on the directions, the differences between the two methods were converted into the absolute minutes per night. Next, we divided the nights into two categories: One group having less than 2 hours discrepancy and the other with greater than 2 hour discrepancy. This yielded 100 out of 525 nights that had greater than 2 hour discrepancy between the two methods (19%). A series of analyses of covariance (ANCOVAs) were performed to identify sleep and FMS-related symptoms that were associated with greater than 2 hour discrepancy, with the use of hypnotic medications as a covariance. Then, based upon the results of the ANCOVAs, we conducted a discriminant analysis to evaluate the strengths of the factors that predicted the discrepancy between the two methods. Relationships among Actigraph sleep data, ED sleep data, and FMS-related symptoms were evaluated with correlational analyses.
Results
Number of Assessment Points
All 75 patients completed the 7 night Actigraphic assessment, yielding a total of 525 sleep data. There were three missing assessment points for the ED sleep and pain data (522 data total points). There was one additional missing score for the ED fatigue data (521 data points) and two additional missing scores for the rest of the ED data (520 data points). The mean values from the Actigraphically derived sleep measures and ED measures are listed in Table 3.
Table 3.
Mean Values Sleep and FMS-related values
| Actigraph | |
| Sleep Duration | 449.14 min (105.26) |
| Sleep Latency | 12.43 min (16.84) |
| Sleep Efficiency | 90.97% (8.10) |
| Waking after Sleep Onset | 44.36 min (42.12) |
| Mean Sleep Episode | 80.50 min (89.28) |
| Activity during Sleep | 55.64 (17.55) |
| Electric Diary | |
| Sleep Duration | 496.42 min (123.71) |
| How difficult was it to fall asleep? | 3.37 (1.73) |
| How many times did you wake up during the night? | 3.25 (2.23) |
| How refreshed did you feel when you woke up? | 1.96 (1.27) |
| Pain | 3.15 (1.22) |
| Fatigue | 4.07 (1.34) |
| Emotional Distress | 2.23 (1.52) |
| Stiffness | 3.70 (1.31) |
| Feeling Relaxed | 2.82 (1.37) |
| Can control pain | 2.88 (1.33) |
| Can be active | 2.42 (1.22) |
Discrepancy between ED- and Actigraph Derived Sleep Data
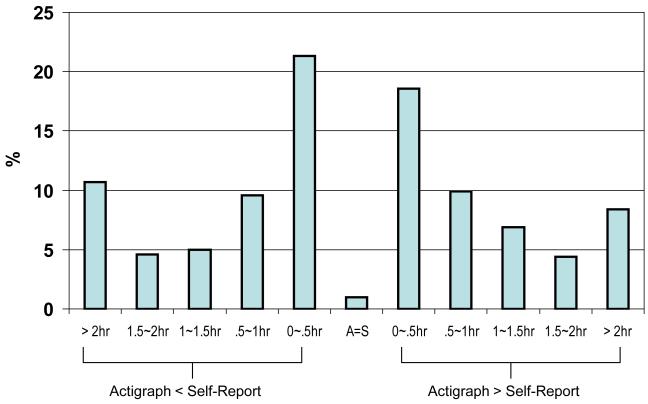
Overall, there was a significant difference between Actigraphically obtained sleep duration (M=449.13 min, SD=105.42) and ED based sleep duration (M=496.42, SD=123.71) (t(522)=9.47, p<001). Sleep duration was longer for the ED method for 51% of the cases. Five cases (1%) had a complete match, and the actigraphically derived sleep duration was longer for 48% of the cases. Figure 1 shows the range of discrepancy, both under- and over-estimation. The directionality of the discrepancy between the two methods appears to be equally divided.
Figure 1.
Range of discrepancy in the estimation of sleep duration between the two methods
In order to assure that there was no night effect on the direction of the discrepancy, we examined the number of subjects over- and under reporting their sleep durations each night. As can be seen in Table 4, the SD>Actigraph and SD<Actigraph were fairly evenly distributed across nights; X2(12)=11.55 NS.
Table 4.
Number of subjects under- and over-reporting sleep duration per night
| Nights | ED=Actigraph | ED>Actigraph | ED<Actigraph |
|---|---|---|---|
| 1 | 1 | 28 | 45 |
| 2 | 0 | 39 | 36 |
| 3 | 0 | 41 | 34 |
| 4 | 1 | 38 | 36 |
| 5 | 2 | 37 | 36 |
| 6 | 0 | 43 | 32 |
| 7 | 1 | 40 | 33 |
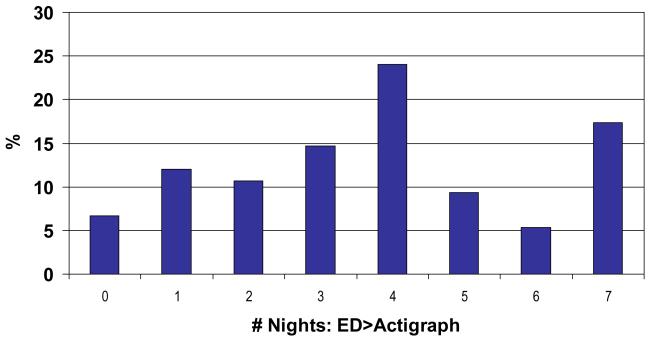
Another possible effect to consider is subject-specific. That is to say whether there were certain individuals who were prone to misestimate whereas there were others who were consistently accurate in their subjective estimation of their sleep durations. Figure 2 shows the distributions of subjects over the number of nights the sleep duration minutes were longer for their ED data relative to the Actigraph data. The majority of the subjects seemed to have had mixed results, that is, they had longer ED sleep duration for some nights and longer Actigraph sleep duration for other nights.
Figure 2.
Distributions of subjects (%) reporting longer sleep duration than the Actigraph data by night
When the absolute difference in minutes between the two methods (ie, either under- or over-estimation), the discrepancy was 73 minutes per night on average across 7 nights (SD=84.44, 64.91~101.32). The repeated ANOVA yielded no significant difference across nights F(6,67)=.72, or any time trends (Linear F(1,72)=.004, Quadratic F(1,72)=3.45, Cubic F(1,72)=.19, all NS).
Another potential subject-specific factor that may influence sleep is the use of certain medications, listed in Table 1. Two classes of medications we were of particularly interest: hypnotics and SSRI/SNRI, both of which may impact the quantity and quality of their sleep. Hypnotics include both benzodiazepine and non- benzodiazepine types. Thirty eight (51%) of the participants were taking at least one type of hypnotic. The mean absolute discrepancy minutes between the two sleep measures were 86.06 (SD=58.93) for those taking hypnotics and 60.50 (SD=49.04) for those who were not taking hypnotics. The difference was significant (F(1,73)=4.16, p<.05). The difference between those taking SSRI/SNRI (M=78.74, SD=53.73) and those who were not taking SSRI/SNRI (M=68.02, SD=57.33) did not reach statistical significance (F(1,73)=.70). Given these results, the use of hypnotics was used as a covariate in the following series of univariate analyses.
Factors Related to Large Discrepancy between ED- and Actigraph- derived Sleep Data
As noted earlier, the nights were divided into two groups based upon whether the subjects had greater than two hour discrepancy or not. The mean values of the Actigraph and ED data for the two groups are shown in Table 5. In general, the discrepancy greater than 2 hour was related to the indicators of poor sleep, such as poorer sleep efficiency (F(1,520)=12.09 (p<.001), minutes waking after sleep onset (F(1,520)=13.10, p<.001), greater activity during sleep F(1,520)=6.01, p<.02), shorter minutes per sleep episode (F(1,520)=9.85, p<.002), greater ED reported difficulty falling asleep(F(1,519)=8.89, p<.004, and greater ED reported number of times waken during night (F(1,519)=4.87, p<.03). The discrepancy greater than two hours were also related to worse FMS-related conditions reported in the morning after the assessed night, such as pain F(1,519)=4.23, p<.05), fatigue (F(1,518)=1417, p<.001), emotional distress (F(1,518)=6.88, p<.01), less relaxed (F(1,518)=7.84, p<.005), and less able to be active (F(1,518)=6.69, p<.01).
Table 5.
Actigraph and ED data values: < 2 hr discrepancy vs > 2 hr discrepancy between the two methods
| Variables | < 2 hr | > 2r |
|---|---|---|
| Actigraph Data | ||
| Sleep Duration (min) | 446.97 (95.66) | 458.29 (139.60) |
| Sleep Latency (min) | 11.81 (15.49) | 15.02 (21.61) |
| Sleep Efficiency (%)** | 91.56 (7.87) | 88.54 (8.60) |
| Wake after Sleep Onset (min)** | 41.18 (40.27) | 57.51 (46.89) |
| Activity during Sleep* | 54.69 (17.11) | 59.66 (18.89) |
| Mean Sleep Episode (min)** | 86.57 (95.82) | 53.75 (43.00) |
| ED Data | ||
| Sleep Duration (min) | 500.18 (99.72) | 480.51 (194.91) |
| Difficulty Falling Asleep1** | 3.49 (1.67) | 2.87 (1.88) |
| # Waken during night* | 3.14 (2.22) | 3.68 (2.20) |
| How Refreshed upon Waking | 2.00 (1.25) | 1.77 (1.33) |
| Pain* | 3.10 (1.18) | 3.37 (1.35) |
| Fatigue** | 3.96 (1.33) | 4.52 (1.27) |
| Emotional Distress* | 2.14 (1.50) | 2.64 (1.57) |
| Stiffness | 3.66 (1.29) | 3.86 (1.36) |
| Feeling Relaxed2* | 2.90 (1.35) | 2.47 (1.39) |
| Can Control Pain | 2.93 (1.31) | 2.66 (1.37) |
| Can Be Active* | 2.49 (1.20) | 2.14 (1.27) |
p<.05,
p<.001
1: 0=Very difficult, 6=Not difficult at all
2: 0=Not relaxed at all, 6=Very relaxed
In order to integrate the results of the univariate comparisons, a discriminant analysis was conducted to evaluate which variables predicted the high degree of discrepancy between the two sleep measures. All variables with the significant F values were included in the analysis. The loading of .3 indicates significant association with the high degree of the discrepancy 33. The results revealed that three factors showed significant association with the dependent variable: Actigraphically obtained minutes awake after sleep onset (.59), ED fatigue (.50), and ED report of how difficult it was to fall asleep (−.41). All others yielded the loadings of less than .30. All together, 63.4% of the nights with less than 2 hour discrepancy and 62.6% of the nights with greater than 2 hour discrepancy were correctly classified.
Relationships between Actigraph Sleep Data and ED Sleep Data
We performed the correlational analyses between actigraphically obtained data (sleep duration, sleep efficiency, latency, # wake after sleep onset, activity during night, and mean sleep episode) and subjective ED sleep data (sleep duration, # awakenings, difficulty falling asleep, and feeling of refreshed upon wakening). There was a significant association between sleep duration between the two methods, r(523)=.51, p<.001. No other significant association was observed.
Relationships between Sleep and FMS-Related Problems
Correlations between the sleep data and FMS-related problems are shown in Table 6. For the Actigraph sleep data, none of the sleep parameters show significant association with the FMS-related problems. On the other hand, some of the ED sleep measures were significantly related to FMS-related problems. Most notably, ED report of how refreshed subjects felt upon wakening was significantly related to all FMS-related problems; the less refreshed, the greater pain, fatigue, emotional distress, stiffness and the less relaxed, having control over pain and being able to be active. The number of reported times subjects woke during night was also related to greater pain, reduced feeling of relaxation and less ability to control pain.
Table 6.
Correlations between Sleep and FMS-related Conditions: Actigraph Sleep vs ED Sleep data
| FMS-Related Problems | |||||||
|---|---|---|---|---|---|---|---|
| Pain | Fatigue | Emotional Distress |
Stiffness | Relaxed | Pain Control | Activeness | |
| Actigraph | |||||||
| Sleep Duration (min) | −.05 | −.02 | −.08 | −.01 | .08 | .06 | .01 |
| Sleep Latency (min) | −.04 | .02 | .00 | −.04 | −.02 | .05 | .09 |
| Sleep Efficiency (%) | −.01 | .00 | −.13 | −.03 | .08 | .02 | .03 |
| Wake after Sleep Onset (min) | .01 | −.01 | .13 | .04 | −.07 | −.03 | −.04 |
| Activity during Sleep | .00 | −.01 | .05 | −.03 | .01 | .04 | .07 |
| Mean Sleep Episode (min) | −.01 | −.01 | −.11 | .01 | −.01 | .02 | .02 |
| ED | |||||||
| Sleep Duration (min) | .03 | .01 | .02 | .08 | −.01 | .03 | −.03 |
| Difficulty Falling Asleep1 | −.07 | −.12 | −.08 | −.06 | .16* | .11 | .06 |
| # Waken during night | .18* | .13 | .13 | .13 | −.25* | −.25* | −.13 |
| How Refreshed upon Waking | −.42* | −.62* | −.32* | −.39* | .43* | .51* | .56* |
p<.005
1: 0=Very difficult, 6=Not difficult at all
Discussion
The results from the present study suggest that the misestimation of sleep in FMS is fairly common. The direction and the extent of the difference in duration between the two methods vary greatly. Unlike the insomniacs who are more likely to underestimate their sleep duration 22, our patients had the near equal distribution of underestimation and overestimation of their sleep. The mismatch was not related to specific nights or specific subjects, suggesting that the misestimation of sleep is not likely to be explained by personal trait or repeated nightly measures. Many patients under- or over-estimated their sleep duration for some nights, on average 73 minutes per night. A sizable number of nights (20%) had differences of greater than 2 hours between the two methods. The results from the further analysis suggests that the patients were more likely to misestimate their sleep when sleep was restless according to the actigraphic data, with subjective report of long sleep latency and when a person felt tired on the morning after.
Interestingly, those who were taking hypnotic medications, either benzodiazepine or non benzodiazepine types, exhibited significantly greater discrepancy, by about 26 minutes on average, compared to those who were not taking hypnotic medication. This seems rather counterintuive, as hypnotics are typically used to aid sleep management. However, hypnotics are known to have residual sedation 34; and the morning drowsiness may have potentially impacted people’s ability to estimate their sleep. Future research is needed to address how the use of commonly used hypnotic may influence people’s sleep perception.
It is important to remember, however, that although our results showed the discrepancy between the subjective sleep reports and the actigraphically obtained sleep data, their sleep parameters typically fell into the normal range. For the most part, our subjects did not exhibit seriously aberrant sleep patterns. It is possible that some of these discrepancies may reflect a form of sleep misperception (“paradoxical insomnia”) in which a person complains of insomnia without any objective signs of sleep deprivation 35. The misperception can also be positive (ie, overestimation) 36. Currently whether paradoxical insomnia is related to the sleep complaints of FMS patients is not known. It would be an important line of research as it could direct the therapeutic effort to target perception (e.g., cognitive-behavioral approach), rather than sleep architecture per se (medications).
The results from the correlational analyses are rather discouraging. The self-reported sleep quality showed very little relationship with the objective actigraph sleep data, except for sleep duration. Furthermore, whereas the FMS related symptoms/problems showed no relations to the objectively obtained sleep data, they were significantly related to the subjective report of how refreshed they felt upon waking and to lesser extent, the subjective estimation of restlessness of their sleep during the previous night.
There are a few possible explanations for these results. First, monomethod bias could account for the significant relationship between the subjective sleep report and the FMS symptoms because both were assessed in the morning with the ED method. The monomethod bias means that the observed variance is more attributable to the measurement method than the constructs that the measurement is supposed to assess, and this is a major threat to the validity of research 37. Many potential sources of monomethod bias, including personal, measurement item, and environmental context factors, have been suggested (see 38 for details). Interestingly, however, others studying insomniacs using the multimethod approach 23 have suggested that poor mood adversely impacts a person’s ability to estimate sleep duration,. Thus, the demonstrated association between subjective poor sleep and FMS symptoms may go beyond the monomethod bias.
Another possibility, although it may not be totally independent from the monomtheod bias discussed above, is the anchoring effect. When a person tries to judge how well he/she has slept in the previous night, he/she may anchor his/her answer to how he/she feels right now. If she feels achy, tired and distressed, she may deduce that her sleep must not have been good. Alternatively, she may attribute the increase in the symptom severity to poor sleep. This raises a question as to how well can a person accurately recall the quality of sleep from the previous night.
Alternatively, the correlations may reflect prominent aspects of the disorder itself. The highest correlation was observed between fatigue and feeling of not refreshed upon wakening. Both are common and prominent fibromyalgia symptoms. Yet we may consider another possibility that people may have a natural tendency to correlate poor sleep quality and symptoms. That is, when we do not feel well, we may make greater relational attribution to sleep disturbance than actually deserved. For example, people with obsessive compulsive disorder (OCD) shows high accuracy in recalling their OCD symptoms but tend to overestimate the relationship between those symptoms and sleep 39. One way to rule this out is to better understand the relationship between objectively obtained sleep quality and FMS symptoms. Currently, literature on how objective sleep duration may be related to FMS symptoms is scarce. From the sleep architecture perspectives, Stage II length has been shown to be associated with an pain scores 40. Alpha-intrusion during Stage IV, however did not show the relationship with myalgic scores. On the other hand, research using the time-series method has reported increased pain severity following a night of poor sleep 17.
Our results impose significant clinical implications. For clinicians, patients’ self-report of sleep duration and quality drives their evaluation and treatment of the sleep complaints, which often accompany their FMS. Our results suggest that patients’ report of their sleep may be less reliable after a night of poor sleep quality, particularly those who take hypnotic medications. This is particularly disturbing as sleep complaints in clinical settings are assessed with subjective report of poor sleep. How can we be sure whether what they are reporting is “true” sleep problem vs. potential measurement error vs. fatigue that may or may not be related to the actual sleep quality? Furthermore, literature suggests that sedatives typically used for insomnia are ineffective for improving mood, sleep or other symptoms of FMS 41-43. Could the lack of efficacy be attributable to the problem of assessing sleep disturbance in FMS?
When a clinician finds patients’ sleep to be recalcitrant to treatment, it would be critical to evaluate whether treatment failure is due to lack of efficacy of that particular regimen or due to inadequate assessment of sleep. It would be ideal to confirm patients’ sleep complaints with the objective method; however, it often is not practical or feasible in a clinical setting. There are, however, some considerations we can entertain to improve clinical assessment of sleep disturbance for FMS patients in a clinic. One way may be to consider using an instrument with the proven psychometric properties. One of the most commonly used sleep questionnaires is the PSQI. The PSQI have been validated against the diagnoses of sleep disorders 13. The correlations between the PSQI and the polysomnographic data are not nearly as strong as the one between the PSQI and self-reported sleep logs, although the PSQI was modestly related to some of the polysomnographic data: % Stage II, sleep efficiency, and sleep duration excluding Stage I 44. Moreover, recently tested instruments to assess sleep disturbance and sleep-related impairments have also shown good psychometric properties 45. These items were derived from a large number of sleep related items in the effort to improve sleep outcome measures, as a part of the Patient-Reported Outcomes Information System (PROMIS) effort of the National Institute of Health Roadmap initiative. They contain a reasonable number of items and may be quite feasible to incorporate in clinical practice.
Further research efforts to delineate how clinicians direct their treatment plan for sleep complaints is urgently needed. Accumulation of empirical evidence should also lead to the development of clinical practice guidelines on how we should treat self-report of sleep in the management of sleep complaints in chronic pain patients. Another line of research that may be worthwhile is to evaluate how we could effectively use sleep diaries. This may sound contradictory as the results of the present study using the ED sleep data raised this very question. The key here, however, is to use the dairy over time. The results of the present study showed the discrepancy between the subjective and objective data; however, they also suggest that most patients are not discrepant all the time. Thus, collecting sleep data over, say 2 weeks or so, could provide a better and hopefully more accurate sampling of patient’s true sleep data. Repeated assessment also has an advantage of reducing the measurement error 46. A sleep diary is also easily implemented particularly for those who find the use of the standardized instruments, although desirable, impractical because of the time and effort involved in administering, scoring, and interpreting the results.
There are limitations of the present study that need to be discussed. In this study, our participants were not screened specifically for sleep disorders. Thus, we were unable to delineate how dyssomnia and parasomnia may interact with fibromyalgia pain, and possibly contribute to the nightly variations of their sleep quality. Indeed, a recent study 47 suggests that the presence of sleep disorders in patients with chronic fatigue syndrome may significantly impact the agreement/discrepancy between objective and subjective sleep measures. Another limitation of the study is our reliance on actigraphy for obtaining “objective” sleep measures. Although past research has shown good agreement between actigraphic data and polysomnographic data in healthy individuals 48, 49, the validity of actigraphically obtained sleep data in any chronic pain populations is yet to be tested. This is particularly important as the accuracy of actigraphic sleep data may show some decline as sleep difficulty, which is common in chronic pain, become more problematic 50. Finally, our sample demographic may suggest a certain degree of homogeneity; our subjects were predominantly middle aged female, Caucasian and well educated. Although fibromyalgia is far more common in women and this profile may be fairly typical of those who volunteer to clinical studies, fibromyalgia affects people with a range of racial/ethnic and economic status 51, 52. Although the FMS-related symptoms and complaints in our sample appear typical of FMS patients in clinical research settings, whether our results can be generalized to all fibromyalgia needs to be tested in a more heterogeneous study sample.
In summary, FMS patients seem to commonly misestimate the duration and quality of their sleep. The difference between self-reported sleep duration and actigraphically obtained duration is particularly large when their sleep is restless, they feel that it was difficult to fall a sleep, and when they are tired at the time of reporting. FMS-related symptoms are mostly related to subjective report of poor sleep but not to objective sleep data. Since clinical evaluation of sleep regimen for FMS patients typically relies upon subjective sleep report, clinicians need to be aware of the pitfalls of the subjective report and consider ways to improve the accuracy of their assessment. Future research should also address the illness-related, personal, environmental, and biological factors that may be relevant to the misestimation of sleep, in order to help develop reliable and valid sleep assessment methods that can be readily used in a busy clinical practice to treat FMS patients.
Acknowledgment
The preparation of this manuscript was supported by the NIAMS grant (R01AR4888) to the first author. The authors would like to thank Reiko Mitsunaga RN, Diana Mayer, Sarah Featherstone, and Mark Herrera JD, for their technical and administrative assistance for the project.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33(2):160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 2.White KP, Speechley M, Harth M, Ostbye T. The London Fibromyalgia Epidemiology Study: the prevalence of fibromyalgia syndrome in London, Ontario. J Rheumatol. 1999;26(7):1570–1576. [PubMed] [Google Scholar]
- 3.Baumstark K, Buckelew S. Fibromyalgia: clinical signs; research findings; treatment implications; and future directions. Ann Beh Med. 1992;14:282–291. [Google Scholar]
- 4.Yunus M, Masi AT, Calabro JJ, Miller KA, Feigenbaum SL. Primary fibromyalgia (fibrositis): clinical study of 50 patients with matched normal controls. Semin Arthritis Rheum. 1981;11(1):151–171. doi: 10.1016/0049-0172(81)90096-2. [DOI] [PubMed] [Google Scholar]
- 5.Schaefer KM. Sleep disturbances and fatigue in women with fibromyalgia and chronic fatigue syndrome. J Obstet Gynecol Neonatal Nurs. 1995 Mar-Apr;24(3):229–233. doi: 10.1111/j.1552-6909.1995.tb02467.x. [DOI] [PubMed] [Google Scholar]
- 6.Lentz MJ, Landis CA, Rothermel J, Shaver JL. Effects of selective slow wave sleep disruption on musculoskeletal pain and fatigue in middle aged women. J Rheumatol. 1999 Jul;26(7):1586–1592. [PubMed] [Google Scholar]
- 7.Moldofsky H, Scarisbrick P, England R, Smythe H. Musculosketal symptoms and non-REM sleep disturbance in patients with “fibrositis syndrome” and healthy subjects. Psychosom Med. 1975;37(4):341–351. doi: 10.1097/00006842-197507000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Branco J, Atalaia A, Paiva T. Sleep cycles and alpha-delta sleep in fibromyalgia syndrome. J Rheumatol. 1994 Jun;21(6):1113–1117. [PubMed] [Google Scholar]
- 9.Carette S, Oakson G, Guimont C, Steriade M. Sleep electroencephalography and the clinical response to amitriptyline in patients with fibromyalgia. Arthritis Rheum. 1995 Sep;38(9):1211–1217. doi: 10.1002/art.1780380906. [DOI] [PubMed] [Google Scholar]
- 10.Chervin RD, Teodorescu M, Kushwaha R, et al. Objective measures of disordered sleep in fibromyalgia. J Rheumatol. 2009 Sep;36(9):2009–2016. doi: 10.3899/jrheum.090051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landis CA, Frey CA, Lentz MJ, Rothermel J, Buchwald D, Shaver JL. Self-reported sleep quality and fatigue correlates with actigraphy in midlife women with fibromyalgia. Nurs Res. 2003 May-Jun;52(3):140–147. doi: 10.1097/00006199-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Korszun A, Young EA, Engleberg NC, Brucksch CB, Greden JF, Crofford LA. Use of actigraphy for monitoring sleep and activity levels in patients with fibromyalgia and depression. J Psychosom Res. 2002 Jun;52(6):439–443. doi: 10.1016/s0022-3999(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 13.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989 May;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 14.Burckhardt CS, Clark SR, Bennett RM. The fibromyalgia impact questionnaire: development and validation. J Rheumatol. 1991;18(5):728–733. [PubMed] [Google Scholar]
- 15.Theadom A, Cropley M, Humphrey KL. Exploring the role of sleep and coping in quality of life in fibromyalgia. J Psychosom Res. 2007 Feb;62(2):145–151. doi: 10.1016/j.jpsychores.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Theadom A, Cropley M. Dysfunctional beliefs, stress and sleep disturbance in fibromyalgia. Sleep Med. 2008 May;9(4):376–381. doi: 10.1016/j.sleep.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Affleck G, Urrows S, Tennen H, Higgins P, Abeles M. Sequential daily relations of sleep, pain intensity, and attention to pain among women with fibromyalgia. Pain. 1996 Dec;68(2-3):363–368. doi: 10.1016/s0304-3959(96)03226-5. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton NA, Affleck G, Tennen H, et al. Fibromyalgia: the role of sleep in affect and in negative event reactivity and recovery. Health Psychol. 2008 Jul;27(4):490–497. doi: 10.1037/0278-6133.27.4.490. [DOI] [PubMed] [Google Scholar]
- 19.Palermo TM, Toliver-Sokol M, Fonareva I, Koh JL. Objective and subjective assessment of sleep in adolescents with chronic pain compared to healthy adolescents. Clin J Pain. 2007 Nov-Dec;23(9):812–820. doi: 10.1097/AJP.0b013e318156ca63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Den Berg JF, Van Rooij FJ, Vos H, et al. Disagreement between subjective and actigraphic measures of sleep duration in a population-based study of elderly persons. J Sleep Res. 2008 Sep;17(3):295–302. doi: 10.1111/j.1365-2869.2008.00638.x. [DOI] [PubMed] [Google Scholar]
- 21.Carskadon MA, Dement WC, Mitler MM, Guilleminault C, Zarcone VP, Spiegel R. Self-reports versus sleep laboratory findings in 122 drug-free subjects with complaints of chronic insomnia. Am J Psychiatry. 1976 Dec;133(12):1382–1388. doi: 10.1176/ajp.133.12.1382. [DOI] [PubMed] [Google Scholar]
- 22.Edinger JD, Fins AI. The distribution and clinical significance of sleep time misperceptions among insomniacs. Sleep. 1995 May;18(4):232–239. doi: 10.1093/sleep/18.4.232. [DOI] [PubMed] [Google Scholar]
- 23.Edinger JD, Fins AI, Glenn DM, et al. Insomnia and the eye of the beholder: are there clinical markers of objective sleep disturbances among adults with and without insomnia complaints? J Consult Clin Psychol. 2000 Aug;68(4):586–593. [PubMed] [Google Scholar]
- 24.Means MK, Edinger JD, Glenn DM, Fins AI. Accuracy of sleep perceptions among insomnia sufferers and normal sleepers. Sleep Med. 2003 Jul;4(4):285–296. doi: 10.1016/s1389-9457(03)00057-1. [DOI] [PubMed] [Google Scholar]
- 25.Trajanovic NN, Shapiro CM, Ong A. Atypical presentation of NREM arousal parasomnia with repetitive episodes. Eur J Neurol. 2007 Aug;14(8):947–950. doi: 10.1111/j.1468-1331.2007.01866.x. [DOI] [PubMed] [Google Scholar]
- 26.Watson NF, Kapur V, Arguelles LM, et al. Comparison of subjective and objective measures of insomnia in monozygotic twins discordant for chronic fatigue syndrome. Sleep. 2003 May 1;26(3):324–328. doi: 10.1093/sleep/26.3.324. [DOI] [PubMed] [Google Scholar]
- 27.Lunde LH, Pallesen S, Krangnes L, Nordhus IH. Characteristics of sleep in older persons with chronic pain: a study based on actigraphy and self-reporting. Clin J Pain. 2010 Feb;26(2):132–137. doi: 10.1097/AJP.0b013e3181b61923. [DOI] [PubMed] [Google Scholar]
- 28.Edinger JD, Marsh GR, McCall WV, Erwin CW, Lininger AW. Sleep variability across consecutive nights of home monitoring in older mixed DIMS patients. Sleep. 1991 Feb;14(1):13–17. [PubMed] [Google Scholar]
- 29.Okifuji A, Turk DC, Sinclair JD, Starz TW, Marcus DA. A standardized manual tender point survey. I. Development and determination of a threshold point for the identification of positive tender points in fibromyalgia syndrome. J Rheumatol. 1997;24(2):377–383. [PubMed] [Google Scholar]
- 30.Margolis RB, Tait RC, Krause SJ. A rating system for use with patient pain drawings. Pain. 1986 Jan;24(1):57–65. doi: 10.1016/0304-3959(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 31.Sadeh A, Aster J, Urbach D, Lavie P. Actigraphcally based automatic bedtime sleep-wake scoring: validity and clinical application. J Ambulat Monit. 1989;2:209–216. [Google Scholar]
- 32.Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15(5):461–469. doi: 10.1093/sleep/15.5.461. [DOI] [PubMed] [Google Scholar]
- 33.Hair J, Anderson R, Tatham R, Black W. Multivariate Data Analysis with Readings. 3rd ed Macmillan; New York, NY: 1992. [Google Scholar]
- 34.Ebert B, Wafford KA, Deacon S. Treating insomnia: Current and investigational pharmacological approaches. Pharmacol Ther. 2006 Dec;112(3):612–629. doi: 10.1016/j.pharmthera.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 35.McCall WV, Edinger JD. Subjective total insomnia: an example of sleep state misperception. Sleep. 1992 Feb;15(1):71–73. [PubMed] [Google Scholar]
- 36.Trajanovic NN, Radivojevic V, Kaushansky Y, Shapiro CM. Positive sleep state misperception - a new concept of sleep misperception. Sleep Med. 2007 Mar;8(2):111–118. doi: 10.1016/j.sleep.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 37.Campbell DT, Fiske DW. Convergent and discriminant validation by the multitrait-multimethod matrix. Psychol Bull. 1959 Mar;56(2):81–105. [PubMed] [Google Scholar]
- 38.Podsakoff PM, MacKenzie SB, Lee JY, Podsakoff NP. Common method biases in behavioral research: a critical review of the literature and recommended remedies. J Appl Psychol. 2003 Oct;88(5):879–903. doi: 10.1037/0021-9010.88.5.879. [DOI] [PubMed] [Google Scholar]
- 39.Gloster AT, Richard DC, Himle J, et al. Accuracy of retrospective memory and covariation estimation in patients with obsessive-compulsive disorder. Behav Res Ther. 2008 May;46(5):642–655. doi: 10.1016/j.brat.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 40.Burns JW, Crofford LJ, Chervin RD. Sleep stage dynamics in fibromyalgia patients and controls. Sleep Med. 2008 Aug;9(6):689–696. doi: 10.1016/j.sleep.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 41.Drewes AM, Andreasen A, Jennum P, Nielsen KD. Zopiclone in the treatment of sleep abnormalities in fibromyalgia. Scand J Rheumatol. 1991;20(4):288–293. doi: 10.3109/03009749109096802. [DOI] [PubMed] [Google Scholar]
- 42.Moldofsky H, Lue FA, Mously C, Roth-Schechter B, Reynolds WJ. The effect of zolpidem in patients with fibromyalgia: a dose ranging, double blind, placebo controlled, modified crossover study. J Rheumatol. 1996;23(3):529–533. [PubMed] [Google Scholar]
- 43.Russell IJ, Fletcher EM, Michalek JE, McBroom PC, Hester GG. Treatment of primary fibrositis/fibromyalgia syndrome with ibuprofen and alprazolam. A double-blind, placebo-controlled study. Arthritis Rheum. 1991;34(5):552–560. doi: 10.1002/art.1780340507. [DOI] [PubMed] [Google Scholar]
- 44.Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002 Sep;53(3):737–740. doi: 10.1016/s0022-3999(02)00330-6. [DOI] [PubMed] [Google Scholar]
- 45.Buysse DJ, Yu L, Moul DE, et al. Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments. Sleep. 2010 Jun 1;33(6):781–792. doi: 10.1093/sleep/33.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Donaldson G. Patient-reported outcomes and the mandate of measurement. Qual Life Res. 2008 Dec;17(10):1303–1313. doi: 10.1007/s11136-008-9408-4. [DOI] [PubMed] [Google Scholar]
- 47.Creti L, Libman E, Baltzan M, Rizzo D, Bailes S, Fichten CS. Impaired sleep in chronic fatigue syndrome: how is it best measured? J Health Psychol. 2010 May;15(4):596–607. doi: 10.1177/1359105309355336. [DOI] [PubMed] [Google Scholar]
- 48.Blood ML, Sack RL, Percy DC, Pen JC. A comparison of sleep detection by wrist actigraphy, behavioral response, and polysomnography. Sleep. 1997 Jun;20(6):388–395. [PubMed] [Google Scholar]
- 49.Jean-Louis G, Kripke DF, Cole RJ, Assmus JD, Langer RD. Sleep detection with an accelerometer actigraph: comparisons with polysomnography. Physiol Behav. 2001 Jan;72(1-2):21–28. doi: 10.1016/s0031-9384(00)00355-3. [DOI] [PubMed] [Google Scholar]
- 50.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003 May 1;26(3):342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 51.McBeth J, Jones K. Epidemiology of chronic musculoskeletal pain. Best Pract Res Clin Rheumatol. 2007 Jun;21(3):403–425. doi: 10.1016/j.berh.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 52.Wolfe F, Anderson J, Harkness D, et al. Work and disability status of persons with fibromyalgia. J Rheumatol. 1997 Jun;24(6):1171–1178. [PubMed] [Google Scholar]