Abstract
MK-801, a non-competitive NMDA-R antagonist, has been utilized in the analysis of mammalian learning and memory. The zebrafish is a novel vertebrate study species that has been proposed for the analysis of the mechanisms of learning and memory. Although learning paradigms have been developed for this species, psychopharmacological characterization of its behavioral responses is rudimentary. Before one attempts the analysis of the effects of MK-801 on learning and memory in zebrafish, one needs to know whether this drug affects motor function, perception and/or motivation, factors that may influence performance in learning tasks. Here we conduct dose response analyses investigating the effects of 0, 2, 20 and 100 µM MK-801 administered 24 hours or 30 minutes before the behavioral test, or during the test. We analyze responses in the open tank to measure motor and posture patterns, in the light dark paradigm to evaluate visual perception, and in a group preference task to attempt to quantify motivation. Our results show a significant performance alteration only in the highest (100 µM) dose groups. These fish spent more time on the bottom of their tank, showed elevated erratic movement, increased their clockwise and counterclockwise turning frequency, and reduced the time spent near a shoal stimulus, behavioral alterations that also depended upon the timing of drug administration. Thus, using the current delivery procedures and outbred zebrafish population, the highest dose that may not lead to significant performance deficits is 20 µM, a concentration we propose to use in a future learning study in zebrafish.
Keywords: dizocilpine, MK-801, NMDA-R, motivation, motor function, perception, zebrafish
1. INTRODUCTION
The zebrafish is a small (4 cm long) freshwater teleost that inhabits slowly moving streams and small lakes of the Indian subcontinent [8]. It has been exported and bred for the pet trade for a long time and has also been well studied in the laboratory. For example, for the past three decades the zebrafish has been in the forefront of developmental biology and genetics [17]. As a result of the accumulated genetic information and the development of a sophisticated genetics tool set, numerous scientific fields have taken notice of this fish. Among these is behavioral brain research [10, 28]. Briefly, several authors have argued that the zebrafish represents an excellent compromise between system complexity (it is a vertebrate) and practical simplicity (it is small, easy to keep and breed in large numbers) (11, 18]. There are some drawbacks, however, when one wants to study mechanisms of brain function and behavior using this species. The number of behavior and/or brain related studies of the zebrafish is orders of magnitude less than those devoted to rodents, for example [11,28]. Most recently, however, several papers have appeared that started to ameliorate this problem. For example, behavioral characteristics of this species have been increasingly explored [5] and analysis of the psychopharmacological properties of zebrafish has also been started [19].
Learning and memory have been well studied and numerous molecular players and neurobiological mechanisms involved in these processes have already been revealed [32]. However, despite the large number of studies devoted to the understanding of the biological mechanisms of these brain functions, a lot remains to be discovered. The zebrafish has been proposed as a potentially useful laboratory tool for the analysis of these mechanisms [29]. In addition to, or in combination with, molecular genetic approaches, psychopharmacological tools have been particularly useful in this research [9]. However, little is known about the psychopharmacological characteristics of zebarfish. For example, only very few studies have investigated the function of N-Methyl-D-Aspartate Receptor (NMDA-R) in zebrafish.
NMDA-R is a ligand (glutamate) and voltage gated calcium/sodium ion channel that is believed to play fundamental roles in learning and memory [32]. As a coincidence detector of presynaptic activation (glutamate release) and postsynaptic activation (depolarization), it is argued to be crucial for processes including long-term potentiation (LTP) and long-term depression (LTD), synapse level phenomena proposed to underlie associative learning and memory [32]. Importantly, NMDA-R and several genes corresponding to its subunits have been identified in zebarfish [7,24] and the nucleotide sequence of its genes has been found highly similar (approaching 90 % identity in some cases) to those of mammalian genes [7]. It is thus expected that the zebrafish NMDA-R functions similarly to the mammalian receptor [22,5] and that psychopharmacological tools developed for the mammalian receptor work with zebrafish NMDA-R [27].
MK-801 (dizocilpine, or (+)-5-methyl-10,11- dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine) is a non-competitive NMDA-R antagonist that has been utilized in learning and memory research, mostly using rodents and other mammalian species [33,35]. In addition to its learning and memory altering properties [35], it is also known that MK-801 has many “side effects”, i.e. it can potentially alter behavioral performance other than learning and memory itself [34]. Performance alterations in a learning paradigm may be mistaken to learning or memory deficits, thus it is important that one tries to dissociate these effects [13].
The psychopharmacology of MK-801 has started to be explored with zebrafish. For example, hyperactivity induced by this drug was recently shown to be reversible by administration of antipsychotic drugs [27]. The effects of MK-801 on circling and place preference have also been explored [31]. The effect of MK-801 on learning performance of zebrafish has also started to be explored. One study demonstrated that when administered after training, MK-801 inhibited learning performance (consolidation of memory) in a one-trial avoidance task [3] while another study showed that it did not have such an inhibitory effect in an active avoidance task [36]. Given the potentially complex psychopharmacological profile of MK-801 and the above controversies, we decided to expand on these findings and attempted to identify potential performance altering properties of MK-801 that may impede behavior of zebrafish in learning paradigms. Briefly, the goal of the current study is to help us select a dose that elicits no such performance impairing effects, a concentration that may be appropriate for the analysis of learning and memory in zebrafish.
Performance factors that can alter behavior in learning tasks may be classified into three distinct categories: motor function, perception and motivation (e.g. Gerlai, 2001 [13]). We have developed a learning task for zebrafish in which the test fish are expected to actively swim in a maze to a target [29]. The target is a group of conspecifics placed in a separate tank [1]. This tank is visible from inside the test maze of the experimental fish. We have shown previously that zebrafish are highly social [26,20,21] and are motivated to join a shoal and that this motivation is sufficiently strong to support good learning performance [1]. In the current paper we utilize behavioral tests that can potentially tap into performance characteristics required for the above learning task. We investigate whether MK-801 can disrupt motor function (important for navigating through the maze), visual perception (i.e. whether the drug can disrupt the ability to see the target stimulus), and motivation (i.e. whether the drug can reduce/alter shoaling tendencies) in zebrafish. Last, we also study the effect of the timing of drug delivery and study how MK-801 may influence behavioral performance when administered 24 hours or 30 minutes before the behavioral test, or during the test. Analysis of the effects of MK-801 administered at these time points we hope will help us in future learning studies aimed at dissociating temporally distinct mechanisms associated with acquisition, consolidation and recall of memory in zebrafish.
2. METHODS
2.1 Animals and Housing
The experimental and stimulus subjects were all short-fin wild type (SF) zebrafish (Danio rerio), which were of the second filial generation that originated from breeders purchased in a local pet store (Big Al’s Aquarium Services, Mississauga, Ontario, Canada). SF zebrafish are a genetically uncharacterized heterogeneous stock whose advantage over genetically well defined standard zebrafish strains is the expected lack of strain specific idiosyncratic features, i.e. absence of inbreeding induced genetic drift (random fixation of alleles). Briefly, in addition to being robust and good breeders these fish are expected to possess behavioral characteristics typical of wild type zebrafish. Young sexually mature adults (6–8 months of age, approximately 50–50% males and females) were tested in the behavioral paradigms. Importantly, each fish was tested in only one behavioral paradigm and only once, a between subject experimental design. Fish were bred, raised and maintained in house (University of Toronto Mississauga Vivarium) in 2.8 l acrylic aquaria (15 fish per tank) on a high density rack system (Aquaneering Inc., San Diego, CA, USA), which provided multistage filtration (mechanical filter, a fluidized glass bed biological filter, activated carbon chemical filter, and a fluorescent UV light sterilizing unit). 10% of the water was replaced daily with deionized water supplemented with 60mg/l Instant Ocean Sea Salt (the system water). The water temperature was maintained at 26 ± 2°C and the aquaria were illuminated by fluorescent light tubes from the ceiling of the holding room (lights turned on at 08:00 h and off at 20:00 h). Fish were fed a mixture of ground flake food (4 parts, Tetramin Tropical Flakes, Tetra, USA) and powdered spirulina (1 part, Jehmco Inc., Lambertville, NJ, USA).
2.2 MK-801 Dosing procedures
We employed four concentrations of MK-801 hydrogen maleate (M107, Sigma-Aldrich) dissolved in system water: 0 µM, 2 µM (674 ng/ml), 20 µM and 100µM. We administered the drug at three different time points: (a) fish received the drug during the 30 min long behavioral session, (b) fish received the drug for a 30 min period immediately before the behavioral session, and (c) fish received the drug for 30 min 24 h before the behavioral session. A particular fish received only one dose and only once, i.e. the experimental design was a 4 (concentration) × 3 (timing) between subject design. The chosen concentrations were based upon a previous study on zebrafish [31], the mammalian literature as well as on our own pilot experiments. The rationale for the different time points of administration was as follows. Detailed ADME (absorption, distribution, metabolism and excretion) information has not been obtained for MK-801 in zebrafish. The pharmacokinetic and pharmacodynamic properties of this drug are unknown for this species. From a previous study with zebnrafish (e.g. Swain et al., 2004, [31]) and from the mammalian literature, however, it appears reasonable to assume that a 30 min drug exposure should allow the drug to reach the brain and occupy a significant amount of NMDA-R, thus we employed the 30 min prior to test dosing. MK-801 may reach the brain sooner and/or may have direct immediate peripheral effects. For example, perhaps unlikely, but the drug may irritate the gills or the skin and thus induce behavioral responses without a central (brain) action (fish are swum in MK-801 solution and passively uptake this water soluble drug). Therefore, we explored if MK-801 affects behavior if it is present in the water only during behavioral testing. Last, learning trials often need to be performed repeatedly. For example, in the plus maze, we trained fish across multiple days [29] and thus it is important to know whether a drug administered 24 hours before could have a residual effect on performance tested at a subsequent trial the following day, hence the 24 hour prior to test drug administration. In all cases, MK-801 was administered using a 2 liter rectangular exposure tank in which the single fish received the corresponding drug solution for 30 min and absorbed the drug (via the skin, gills and orally) while swimming in the solution.
Three separate behavioral tests were performed, each on a set of naïve fish. In each of the three behavioral experiments 12 groups of fish (3 drug exposure time points × 4 concentrations) were tested, and each of these groups had a sample size (n) equaling 8, i.e. a total of 386 fish were analyzed. This test battery consisted of the following behavioral paradigms: open tank, light-dark preference, and group preference.
2.3 Recording of behavior: General procedures
Two 13W fluorescent light bulbs illuminated the experimental tanks from above. In each test a single fish was monitored at a time. Once the fish completed the tests, they were netted from the experimental apparatus and returned to a collecting tank. All experiments were conducted with the experimenter absent from the testing room, and occurred during the light phase of the light/dark cycle, i.e. in between 1000 and 1600 h. All of the behavioral experiments were videotaped from the side using a digital hard drive video camera (JVC Everio GZ-MG500, Yokohama, Japan). The digital video-files were later transferred to a computer, replayed and scored using the event recorder application Observer Color Pro 5.0 (Noldus, Wageningen, The Netherlands).
2.4 The open tank test
Motor responses were assessed in a novel empty, i.e. open, 2L (21cm × 10cm × 10cm) tank made of Plexiglas. The behavioral recording session was 30 min long. Exposure to a novel environment as well as handling by the experimenter is an inherent part of any experiment and is expected to induce passive and active avoidance reactions [e.g. 11,14]. In a novel open tank thus zebrafish may perform numerous motor and posture patterns [12,4] including Freezing (complete immobility, only the eyes or the pectoral fins may move, fish is usually either at the surface or on the bottom most often in close proximity to an object or the wall of the tank), Erratic movement (zig zagging, fast movement whose direction changes rapidly), Creeping (very slow movement during which only the pectoral fins propel the fish forward), Thrashing on the side (forceful swimming with the use of the caudal fin while physically in contact with the side wall of the tank), Thrashing on the bottom (as in the previous behavior but on the bottom glass), Floating (fish is stationary and maintains its position by synchronously opening and closing its pectoral, anal, dorsal and caudal fins), Foraging (opening the mouth and picking up visible objects), Sinking (similar to freezing but the fish changes its position in the water column without moving any of its fins), Swimming (fast straight line locomotion with the use of the caudal fin), Tilting (deviation from the horizontal position), Jumping (single and fast leap in one direction) or Circling (completion of a 360 degree turn) (for further details and definitions see 16,4,15]. These motor patterns were easily observed in the small experimental tank (good fish to tank size ratio) from the side view of the camera. The duration of time fish performed a motor pattern (expressed as the percent of time relative to total session length) was measured for the first 10 of these patterns whereas the frequency (number of occurrences) was recorded and analyzed for the last 2 motor patterns. In addition, fish exposed to MK-801 may occupy the water column differently compared to control and may spend increasing amount of time on the bottom or in the upper water layer. These responses were measured by placing a transparency on the video-monitor during replay that divided the tank to upper, middle and bottom third layer. The duration of time spent in each of these layers was quantified but only bottom and middle layer dwell times are analyzed and presented here as upper layer dwell time would be redundant (can be calculated by subtracting the bottom and middle layer times from 100%). Last, locomotory activity was also quantified in the open tank. A transparency was placed in front of the video-monitor and the 2 vertical and two horizontal lines drawn on it divided the tank into 3×3 square segments on the side view. The total number of entries to these segments was counted and used as a measure of general swimming activity. Entry to a segment was defined when at least half of the body of the fish entered it. In summary, in the open field a broad array of behavioral responses were analyzed, which we expected to allow detailed characterization of potential motor response altering effects of MK-801.
2.5 Light vs. dark preference
The novel open tank test is expected to allow quantification of numerous motor and posture patterns and may also be sensitive to measuring changes in fear. The task is not particularly sensitive to perception, however, as the fish can use any modalities to detect the novel aspect of the task (for example, visual, olfactory, tactile, lateral line, temperature cues). Most learning paradigms, including the one we intend to employ [29], require zebrafish to attend to visual cues. Thus we wanted to test whether vision may be affected by MK-801 treatment. The relevance of this point is underscored by the fact that NMDA-R has been found not only in the brain but also in the retina of zebrafish (Cox et al., 2005). Here we employed a light-dark preference task [16]. Zebrafish were placed singly in a 2L test tank (identical in dimensions to the one used in the open tank test). Half of this tank was covered with black nylon sheets on all four sides, while the other half was left transparent. There was no physical barrier between the two sides, and the fish could swim freely in the entire test tank. Behavior was recorded for 30 minutes. Preference for the light vs. the dark side was quantified by measuring the amount of time spent in the two compartments of the tank.
2.6 Group Preference
In several appetitive, i.e. positively reinforced, learning tasks food has been used as reward. We have also successfully utilized food as a motivator for zebrafish in a learning task [29]. However, we also noticed that food poses some issues given that zebrafish can satiate fast and can remain satiated with food for prolonged periods of time. We have therefore developed a novel motivator, the sight of conspecifics, which we argued would represent a stable reinforcer that may not satiate as fast as food and whose presentation may be controlled (precise visual stimulus) better than that of food rewards [1]. Zebrafish are highly social and seek out and prefer to stay close to their conspecifics [26, 21, 1]. MK-801 may alter shoaling responses if it impairs vision or the motivation to join a shoal (social behavior) in zebrafish. To test whether different concentrations of MK-801 may have such an effect, we performed the group preference task. Experimental zebrafish were placed in a tank identical in dimensions to the ones used in the above experiments. On one side of the test tank there was an empty stimulus tank and on the other side the stimulus tank contained 5 zebrafish, the stimulus fish, which were identical in size and color to the test fish (stimulus fish were of the same population and age as test fish). The two stimulus side tanks were obscured from the test tank by lowering removable white plastic barriers until the start of the recording session. The side on which the stimulus fish vs. the empty tank was presented varied randomly across test fish but for any given test fish the stimulus location remained constant throughout the behavioral recording session. The session started with the removal of the white plastic barriers and it lasted for 30 min.
The behavior of the experimental fish was video-recorded and the recordings later replayed for quantification of responses. During replay, a transparency was placed on the monitor on which vertical lines divided the test tank into three imaginary compartments, stimulus fish side, middle, and opposite side. Preference for conspecifics was quantified by measuring the amount of time the experimental fish spent in the side closest to the stimulus fish.
2.7 Statistical analysis
Analysis of data was performed using SPSS version 14 written for the PC. Separate variance analyses (ANOVAs) were performed for each of the three dosing time periods to investigate the effect of different concentrations of MK801 (4 levels). In case of significant ANOVA term, a post hoc multiple comparison test, Tukey Honestly Significant Difference (HSD) test was performed. In all comparisons the null hypothesis was rejected in case of p < 0.05. The variance homogeneity and normal distribution criteria of parametric statistical tests were ignored (but the former was met and the latter cannot be tested in small samples), as parametric tests, such as ANOVA and Tukey HSD, are insensitive to the violation of these criteria in case of equal sample sizes.
3. RESULTS
3.1 Open tank
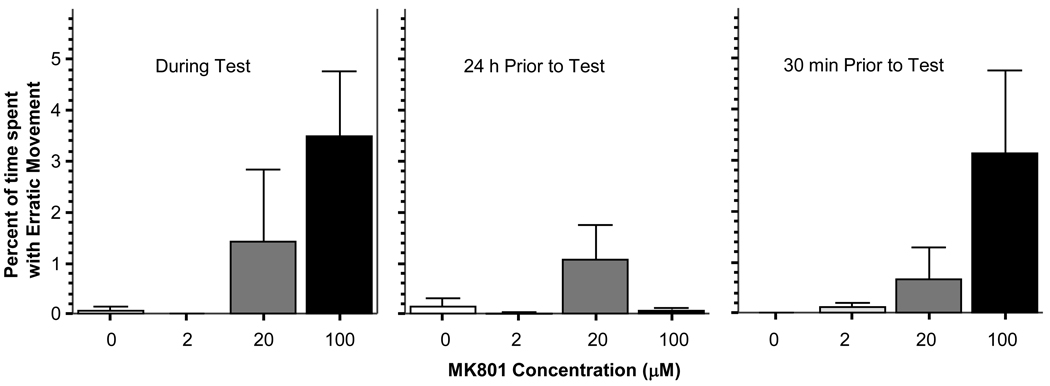
In the open tank test, MK-801 had no significant effect on the percent of time fish performed Creeping, Thrashing on the side, Thrashing on the bottom, Floating, Foraging, Freezing, Sinking, Swimming, Tilting or on the frequency of these motor patterns. Also, no significant effect on the frequency of jumping was detected. Similarly, MK801 was found not to significantly alter general locomotor activity. Furthermore, the above lack of significant drug effects were detected for all drug exposure time points, i.e. when MK-801 was administered during the behavioral test, 24 hours before the test or for 30 min immediately preceding the test (data not shown). However, an apparent MK-801 effect was found for the percent of time fish performed Erratic movement (figure 1) when the drug was administered during the test (ANOVA F(3, 28) = 2.952, p = 0.05), a result that bordered significance but could not be confirmed with the post hoc Tukey HSD test. This latter test found no significant differences between any dose groups (i.e. p > 0.05). The effect of MK-801 was also found non-significant on Erratic movement when the drug was administered 24 h before the test (ANOVA F(3, 28) = 1.963, p > 0.10). Similarly, despite the apparent trend (figure 1), no significant drug effect was found when MK-801 was administered for 30 min immediately preceding behavioral testing (ANOVA F(3, 28) = 2.843, p > 0.05).
Figure 1.
MK-801 induces an apparent increase of Erratic movement (measured as the percent of time fish spent with this behavior) in the open tank when the drug is administered during the test or for a 30 min period just before the test. Mean ± Standard Error are shown. Note that the results do not reach statistical significance (i.e. p > 0.05).
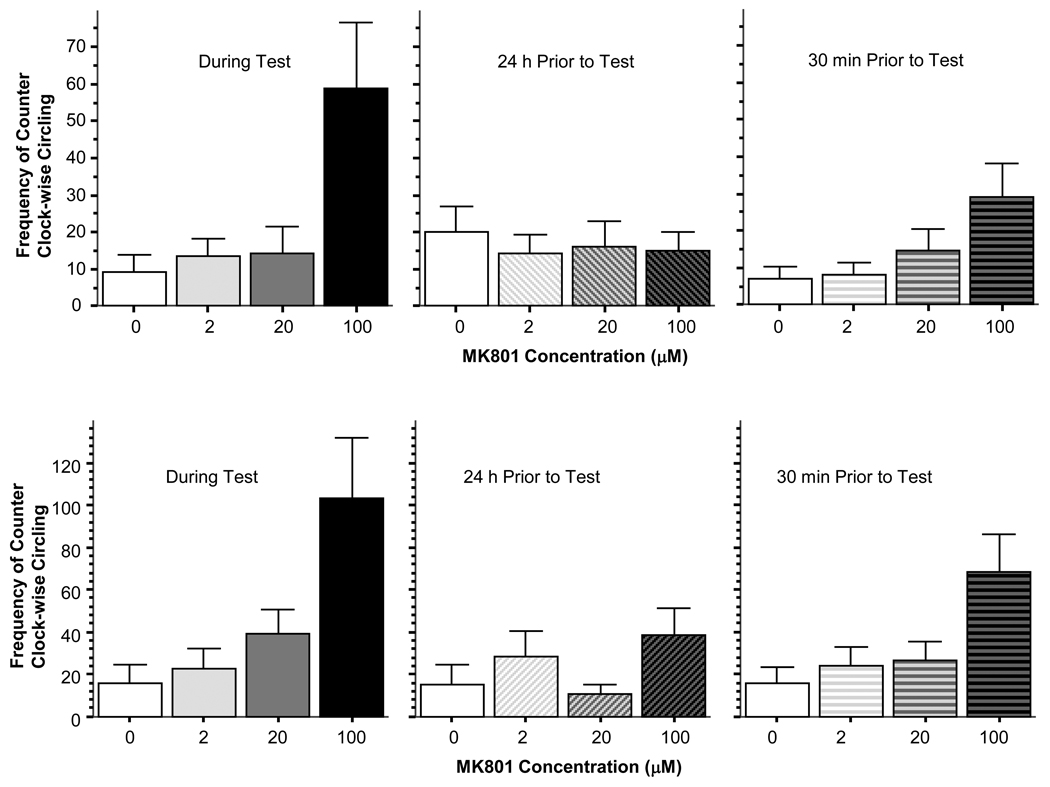
The only behavioral response that was significantly affected by MK-801 in the open field was circling (figure 2). The frequency of clock-wise circling was significantly altered when the drug was given during the test (ANOVA F(3, 28) = 5.483, p < 0.01) and Tukey HSD confirmed that this effect was due to the significant increase in circling in the highest dose group (the 100 µM MK-801 exposed fish) which differed (p < 0.05) from all other concentration groups, while the other dose groups did not significantly differ from each other. MK-801 administered 24 h prior to test did not have such an effect (ANOVA F(3, 28) = 0.176, p > 0.90) suggesting that the drug washed out within less then 24 hours. MK-801 also significantly affected clock-wise circling when the drug administration started 30 min prior to testing and ended just before testing (ANOVA F(3, 28) = 2.993, p < 0.05). Tukey HSD confirmed this finding and showed that the highest dose group (100 µM MK-801) differed (p < 0.05) from the lowest dose group (0 µM MK-801) while other group comparisons showed no significant differences. The results for the frequency of counter clock-wise circling were very similar to the above. MK-801 administered during the test significantly increased counter clock-wise circling (ANOVA F(3, 28) = 5.898, p < 0.01) in the highest dose group as compared to all other dose groups (Tukey HSD), but these other dose groups did not differ from each other. When administered 24 h before the test, MK-801 was not effective (ANOVA F(3, 28) =1.531, p > 0.20). However, when given 30 min prior and up to the start of the test, MK-801 also significantly increased counter clock-wise circling (ANOVA F(3, 28) = 4.242, p < 0.05) in the highest dose group, which was significantly different (p < 0.05) from all other dose groups, and again these latter dose groups did not differ from each other (Tukey HSD).
Figure 2.
The frequency of clock-wise (upper graphs) and counter clock-wise circling (lower graphs) is significantly increased in the open tank by administering MK-801 during the test or during the 30 min period immediately prior to the test. Mean ± Standard Error are shown. Note that the drug effect is not significant when MK-801 was administered 24 hours before the test. Also note that the significant drug effect is due to the significantly increased circling response in the highest (100 µM) dose groups.
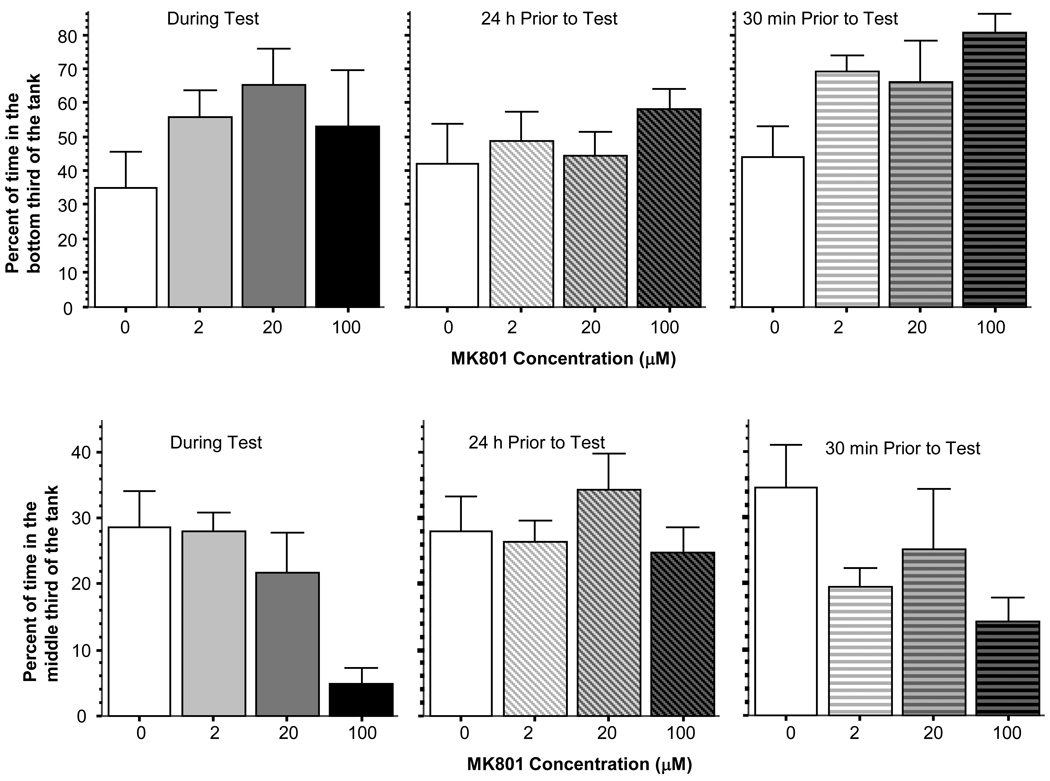
Analysis of the vertical location of the fish in the open tank also showed that fish did not significantly change their position in any dose groups except the highest and also that this effect could only be found for certain time of drug exposures (figure 3). For example, MK-801 did not significantly affect the percent of time in the bottom third of the tank when the drug was given during the test (ANOVA F(3, 28) = 1.160, p > 0.30) or 24 hours prior the test (ANOVA F(3, 28) = 0.676, p > 0.55). However, when MK-801 was administered for the 30 min period immediately preceding the test, it significantly increased the percent of time on the bottom (ANOVA F(3, 28) = 3.397, p < 0.05) but only in the highest dose group, which significantly ((p < 0.05) differed from all other dose groups while these other dose groups did not differ from each other (Tukey HSD). Percent of time in the middle layer of the water (vertical position) was affected by MK-801 slightly differently. In this measure, MK-801 was effective when administered during the test (ANOVA F(3, 28) = 5.956, p < 0.01). Tukey HSD confirmed this result and revealed that the highest dose group significantly (p < 0.05) differed from all other three dose groups whereas these latter dose groups were not different from each other. MK-801 administered 24 hours before (ANOVA F(3, 28) = 0.809, p > 0.45) or 30 min before the test (ANOVA F(3, 28) = 2.070, p > 0.10) was ineffective.
Figure 3.
The position of experimental zebrafish in the water column (time spent in the bottom third of the tank, upper graph; and time spent in the middle third of the tank) in the open tank is significantly affected by MK-801 treatment but the effect is dependent upon when the drug was administered. Mean ± Standard Error are shown. Note that MK-801 led to a significant increase of bottom dwell time only in the highest dose group when the drug was delivered for the 30 min period preceding the test (upper right graph) and also led to significant reduction of middle-layer dwell time also only in the highest dose group when the drug was administered during the test (bottom left graph). Also note that the drug had no effect when it was administered 24 h before the test.
3.2 Light vs. dark preference
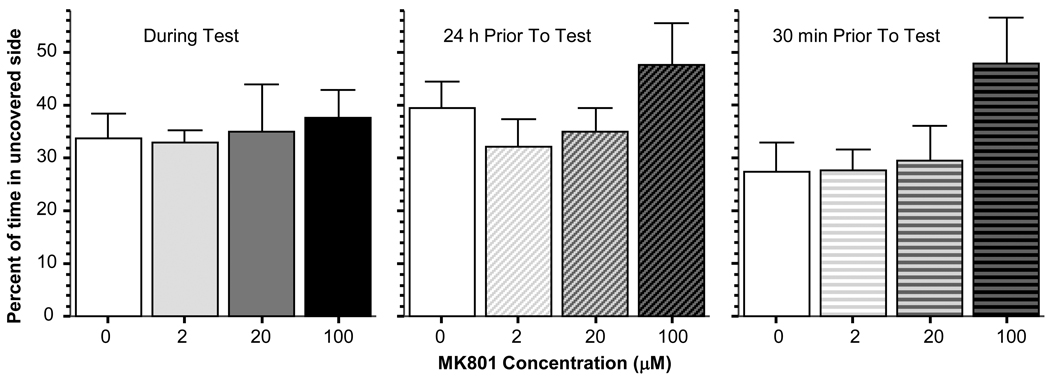
The light-dark preference task has been routinely used with rodents, nocturnal species that prefer the dark compartment to the lighted one in the laboratory. Zebrafish may show a similar response depending upon the level of illumination or the exact parameters of the test [11]. The paradigm is expected to be sensitive to vision as well as fear. For example, blind subjects or subjects without the motivation to avoid perceived danger (e.g. the well illuminated side of the tank where predators may potentially see the target prey) may chose at random chance. Zebrafish tended to prefer the dark compartment of the tank (figure 4) in the current study but this preference was not significantly altered (reduced or enhanced) by MK-801 treatment. That is, ANOVA’s showed no drug treatment effects for any one of the three timing methods of dosing (MK-801 administered during the test F(3, 28) = 0.127, p > 0.90; MK-801 administered 24 hours prior to the test F(3, 28) = 1.381, p > 0.25; MK-801 administered for the 30 min period preceding the test ANOVA F(3, 28) = 2.448, p > 0.05), although a notable trend is observable in figure 4 for this last drug delivery timing with the highest dose apparently approaching chance (50%) performance.
Figure 4.
MK-801 administration resulted in no alteration in light vs. dark choice in a two compartment choice tank in zebrafish. Choice is expressed as percent of time spent in the uncovered (well illuminated) side. Mean ± Standard Error are shown. Note that although the effect is not significant, MK-801 appeared to increase percent of time in the uncovered side in the highest dose group when the drug was administered 24 hours or 30 min prior to the test.
3.3 Group preference task
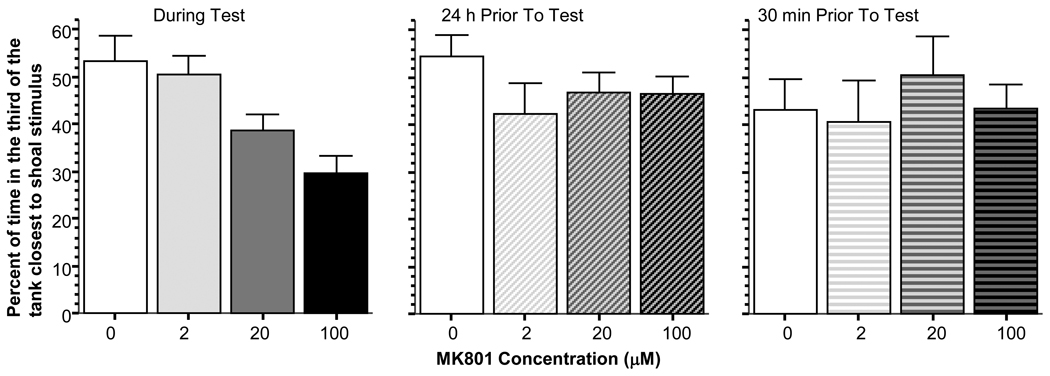
As explained above, we have previously identified a robust reinforcing stimulus expected to provide a stable motivation for zebrafish in associative learning tasks, the sight of conspecifics [1]. If the innate and species-typical motivation of zebrafish to form groups is altered by drug treatment, a conspecific stimulus-reinforced learning task may detect performance deficits despite the absence of learning or memory disruption. The group preference task was designed to investigate this question, i.e. whether MK-801 at the 0–100 µM dose range has such performance altering property. Briefly, MK-801 was not found to significantly affect the time experimental zebrafish spent in the third of the test tank closest to the stimulus fish when the drug was administered 24 hours prior to the test (ANOVA F(3, 28) = 1.132, p > 0.35) or when the drug was delivered for the 30 min period preceding the test (ANOVA F(3, 28) = 0.335, p > 0.80) (also see figure 5). However, when MK-801 was given during the test, it exerted a significant dose dependent effect (ANOVA F(3, 28) = 6.996, p = 0.001). A post hoc Tukey HSD test confirmed this finding and showed that fish in the highest dose group (100 µM MK-801) spent significantly (p < 0.05) less time near the stimulus fish as compared to the fish in the control and 2µM dose groups, while differences between the other groups were non-significant.
Figure 5.
MK-801 administration reduced shoaling tendencies in the highest dose group when the drug was delivered during the test but not when it was administered 24 hours or 30 min before the test. Shoaling is expressed as percent of time spent in the third of the tank closest to the conspecific stimulus fish. Mean ± Standard Error are shown.
4. DISCUSSION
MK-801 is one of the few psychopharmacological tools that have already been utilized in the analysis of zebrafish behavior and brain function [27,31,3,36]. The goal of the current paper was to expand on this knowledge and investigate aspects of the effects of this drug most relevant for future learning studies we, and others, may want to conduct. Given that in our learning paradigms active exploration of the test environment, visual perception of the conditioned and unconditioned stimuli, and group preference (shoaling, i.e. motivation to perform a behavioral response to the US) are required, we focused on these performance factors and investigated whether MK-801 administration may alter them. Briefly, MK-801 in the currently employed dose range (0–100 µM) appeared fairly safe, led to no increased mortality or morbidity, and rarely resulted in significant behavioral changes. There were some notable exceptions to this, however.
Most of the motor and posture patterns of zebrafish remained unaltered by MK-801 in the open tank. However, the percent of time fish moved erratically appeared to increase when the drug was administered during the test or for the 30 min period that preceded the test. This increase was not significant but the pattern of dose responses showed a tendency that may need to be kept in mind when the drug is utilized in learning studies. Erratic movement is often observed in the context of pain or fear inducing stimuli [11,14,25,30,2,] thus increased levels of this behavior may imply elevated fear or anxiety, which would be contradictory to the suggested anxiolytic properties of higher doses of MK-801 found in mammals [6]. Alternatively, these results may be due to moderately increased pain or irritation that one may speculate could result from the direct peripheral effects of immersion in the drug solution. Last, it is also possible that the erratic behavior observed is a direct motor response to MK-801, i.e. do not imply changes in underlying emotional states or pain reactions, a hypothesis that is in line with the known ataxic gate inducing effects of higher doses of this drug demonstrated in mammals [6].
Potential changes in erratic movement in response to MK-801 exposure have not been described before, but another behavioral response, increased circling has been reported [31]. This latter study employed a small (13 cm diameter) cylinder to measure circling in zebrafish. It is notable, however, that the geometry of this test chamber was likely to force straight line locomotory responses (including swimming or thrashing) into circling. Also notably, in this latter study the fish were placed in deionized water instead of salt reconstituted system water. Zebrafish are well adapted in nature to soft (low dissolved salt) waters [8] but they may respond adversely to deionized water (Gerlai personal observation). Thus the lack of dissolved salts during drug exposure and testing could have contributed to the abnormalities observed in Swain et al. (2004). Nevertheless, our results do confirm that circling is increased by MK-801. We found the highest (100 µM) MK-801 dose but not the lower ones (20 µM, and 2 µM) to elevate the frequency of circling (both clock- and counter clock-wise). The increase was most dramatic when the drug was present during the test but was also found significant when it was administered for 30 min immediately before the test. These results suggest that even in the larger rectangular tank, circling may be induced by MK-801. The behavioral and neurobiological mechanisms underlying this drug response is unknown at this point.
The location of the experimental fish relative to the bottom of the open tank also changed in response to MK801 treatment but again, only the highest dose group showed a significant response and only when the drug was either present during the test (reduced time in the middle layer) or when it was administered during the 30 min period preceding the test (increased bottom layer dwell time). Overall, these and the other above discussed results suggest that only the highest (100 µM) MK-801 concentration may affect motor and posture patterns and even this dose is quite well tolerated in zebrafish. Also important to note that even the highest dose of MK-801 had no detectable behavioral effects in the open tank 24 h after it was administered suggesting that this period is long enough for a washout and also that any changes the drug may have caused after a single exposure to it are not permanent and have reversed.
Last, it is also notable that analysis of the behavior of MK-801 exposed fish in the open tank revealed no significant alterations in activity levels of the fish. MK-801 has been shown to induce hyperactivity in mammals [23], but a previous study with zebrafish found that at a low dose (2 µM) it induces hypo- whereas at a high dose (200 µM) hyper-activity and that at 20 µM it does not have locomotor activity altering effects[31]. Another study [27], on the other hand, found a significant hyperactivity inducing effect of MK-801 at the 20 µM concentration. The reasons for these controversial findings are unclear at this point. It is possible that the salt constitution and concentration of the vehicle (the fish water) and/or the genetic composition of the zebrafish population (strain) used, which all differed across these studies, may explain the discrepancies.
In the light dark choice task we found no significant MK-801 effect, although the highest concentration (100 µM) of MK801 when administered for the 30 min period preceding the test had an effect on the behavior of zebrafish that bordered significance. These latter fish made their choice close to chance, i.e. apparently stopped avoiding the uncovered well illuminated side of the tank, a response that may be interpreted as an anxiolytic or fear reducing effect of the drug or, alternatively, as indication of impaired visual perception. It is important to note that light-dark choice paradigms have been controversial in the zebrafish literature [11,16]. Some authors find zebrafish to prefer lighter side while others detect the opposite and find dark preference in zebrafish, which again may be the result of a number of factors including methodological differences and/or genetically different zebrafish populations used For example, some studies employ a completely covered “cave” like dark compartment vs. an open illuminated opposite side (as in the current work), while others test the choice between a dark background vs. light background under identical illumination levels, yet others argue that the relative level of illumination and even the level of hunger may influence the choice zebrafish make. Irrespective of what zebrafish really prefer, it appears that MK-801 did not robustly alter performance in the light-dark paradigm, i.e. neither visual perception nor levels of fear may be grossly altered by the drug.
Last, we explored whether the response to conspecifics may be altered by MK-801. This test is expected to be sensitive to alterations in both vision (the presence of conspecifics can only be detected using visual perception), and/or motivation (group forming or shoaling). MK-801 turned out to be relatively innocuous in this task too and only the highest dose (100 µM) and only when delivered during the test significantly reduced the shoaling response. We therefore conclude that lower doses of MK-801 are unlikely to alter vision and the motivation to swim close to conspecifics.
Overall, the above results suggest that the highest safe dose that is unlikely to affect motor function, visual perception, and/or motivation to respond to conspecifics in the short fin heterogeneous stock of zebrafish is 20 µM, and MK-801 at this dose will not affect these crucial performance factors irrespective of whether the drug is present during or if it has been administered prior to the behavioral test. We therefore suggest that 20 µM may be an appropriate concentration for the analysis of the potential learning and memory impairing properties of MK-801 in zebrafish.
ACKNOWLEDGMENTS
The authors thank Rajesh Krisnannair, Madiha Sheikh, and Waqqas Shams for their technical help. Supported by NSERC (Canada) and NIH/NIAAA (USA) grants to R.G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Al-Imari L, Gerlai R. Conspecifics as reward in associative learning tasks for zebrafish (Danio rerio) Behav. Brain Res. 2008;189:216–219. doi: 10.1016/j.bbr.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Bass SLS, Gerlai R. Zebrafish (Danio rerio) responds differentially to stimulus fish: The effects of sympatric and allopatric predators and harmless fish. Behav. Brain Res. 2008;186:107–117. doi: 10.1016/j.bbr.2007.07.037. [DOI] [PubMed] [Google Scholar]
- 3.Blank M, Guerim LD, Cordeiro RF, Vianna MR. A one-trial inhibitory avoidance task to zebrafish: rapid acquisition of an NMDA-dependent long-term memory. Neurobiol Learn Mem. 2009;92:529–534. doi: 10.1016/j.nlm.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Blaser R, Gerlai R. Behavioral phenotyping in Zebrafish: Comparison of three behavioral quantification methods. Behavior Research Methods. 2006;38:456–469. doi: 10.3758/bf03192800. [DOI] [PubMed] [Google Scholar]
- 5.Brennan CH. Zebrafish behavioural assays of translational relevance for the study of psychiatric disease. Rev Neurosci. 2011 doi: 10.1515/RNS.2011.006. (in press) [DOI] [PubMed] [Google Scholar]
- 6.Clineschmidt BV, Martin GE, Bunting PR, Papp NL. Central sympathomimetic activity of (+)-5-methyl-10,11-dihydro-5H-dibenzo [d]cyclohepten- 5,10-immine(MK-801), a substance with potent anticonvulsant, central sympathomimetic, and apparent anxiolytic properties. Drug Dev Res. 1982;2:135–145. [Google Scholar]
- 7.Cox JA, Kucenas S, Voigt MM. Molecular characterization and embryonic expression of the family of N-methyl-D-aspartate receptor subunit genes in the zebrafish. Dev Dyn. 2005;234:756–766. doi: 10.1002/dvdy.20532. [DOI] [PubMed] [Google Scholar]
- 8.Engeszer RE, Patterson LB, Rao AA, Parichy DM. Zebrafish in the Wild: A review of natural history and new notes from the field. Zebrafish. 2007;4:21–40. doi: 10.1089/zeb.2006.9997. [DOI] [PubMed] [Google Scholar]
- 9.Frankland PW, Ohno M, Takahashi E, Chen AR, Costa RM, Kushner SA, Silva AJ. Pharmacologically regulated induction of silent mutations (PRISM): combined pharmacological and genetic approaches for learning and memory. Neuroscientist. 2003;9:104–109. doi: 10.1177/1073858403252225. [DOI] [PubMed] [Google Scholar]
- 10.Gerlai R. High-throughput Behavioral Screens: the First Step towards Finding Genes Involved in Vertebrate Brain Function Using Zebrafish. Molecules. 2010;15:2609–2622. doi: 10.3390/molecules15042609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerlai R. Zebrafish antipredatory responses: A future for translational research? Behav. Brain Res. 2010;207:223–231. doi: 10.1016/j.bbr.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerlai R. Zebra fish: An uncharted behavior genetic model. Behav. Genet. 2003;33:461–468. doi: 10.1023/a:1025762314250. [DOI] [PubMed] [Google Scholar]
- 13.Gerlai R. Behavioral tests of hippocampal function: Simple paradigms, complex problems. Behav. Brain Res. 2001;125:269–277. doi: 10.1016/s0166-4328(01)00296-0. [DOI] [PubMed] [Google Scholar]
- 14.Gerlai R, Fernandes Y, Pereira T. Zebrafish (Danio rerio) responds to the animated image of a predator: Towards the development of an automated aversive task. Behav. Brain Res. 2009;201:318–324. doi: 10.1016/j.bbr.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerlai R, Prajapati S, Ahmad F. Differences in acute alcohol induced behavioral responses among zebrafish populations. Alcoholism: Clinical and Experimental Research. 2008;32:1763–1773. doi: 10.1111/j.1530-0277.2008.00761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerlai R, Lahav M, Guo S, Rosenthal A. Drinks like a fish: Zebra fish (Danio rerio) as a behavior genetic model to study alcohol effects. Pharmacol. Biochem. Behav. 2000;67:773–782. doi: 10.1016/s0091-3057(00)00422-6. [DOI] [PubMed] [Google Scholar]
- 17.Grunwald DJ, Eisen JS. Timeline: Headwaters of the zebrafish—emergence of a new model vertebrate. Nat Rev Genet. 2002;3:717–724. doi: 10.1038/nrg892. [DOI] [PubMed] [Google Scholar]
- 18.Guo S. Linking genes to brain, behavior and neurological diseases: what can we learn from zebrafish? Genes Brain Behav. 2004;3:63–74. doi: 10.1046/j.1601-183x.2003.00053.x. [DOI] [PubMed] [Google Scholar]
- 19.Levin ED. Zebrafish assessment of cognitive improvement and anxiolysis: Filling the gap between in vitro and rodent models for drug development. Rev Neurosci. 2011 doi: 10.1515/RNS.2011.009. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller N, Gerlai R. Oscillations in Shoal Cohesion in Zebrafish (Danio rerio) Behav. Brain Res. 2008;193:148–151. doi: 10.1016/j.bbr.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller N, Gerlai R. Quantification of Shoaling Behaviour in Zebrafish (Danio rerio) Behav. Brain Res. 2007;184:157–166. doi: 10.1016/j.bbr.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Nam RH, Kim W, Lee CJ. NMDA receptor-dependent long-term potentiation in the telencephalon of the zebrafish. Neurosci Lett. 2004;370:248–251. doi: 10.1016/j.neulet.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 23.Ouagazzal A, Nieoullon A, Amalric M. Locomotor activation induced by MK-801 in the rat: postsynaptic interactions with dopamine receptors in the ventral striatum. Eur J Pharmacol. 1994;251:229–236. doi: 10.1016/0014-2999(94)90404-9. [DOI] [PubMed] [Google Scholar]
- 24.Pan Y, Mo K, Razak Z, Westwood JT, Gerlai R. Chronic Alcohol Exposure Induced Gene Expression Changes in the Zebrafish Brain. Behav. Brain Res. 2010;216:66–76. doi: 10.1016/j.bbr.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parra KV, Adrian JC, Jr, Gerlai R. The synthetic substance hypoxanthine 3-N-oxide elicits alarm reactions in zebrafish (Danio rerio) Behav. Brain Res. 2009;205:336–341. doi: 10.1016/j.bbr.2009.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saverino C, Gerlai R. The social zebrafish: Behavioral responses to conspecific, heterospecific, and computer animated fish. Behav. Brain Res. 2008;191:77–87. doi: 10.1016/j.bbr.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seibt KJ, Oliveira Rda L, Zimmermann FF, Capiotti KM, Bogo MR, Ghisleni G, Bonan CD. Antipsychotic drugs prevent the motor hyperactivity induced by psychotomimetic MK-801 in zebrafish (Danio rerio) Behav Brain Res. 2010;214:417–422. doi: 10.1016/j.bbr.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 28.Sison M, Cawker J, Buske C, Gerlai R. Fishing for genes of vertebrate behavior: Zebra fish as an upcoming model system. Lab Animal. 2006;35:33–39. doi: 10.1038/laban0506-33. [DOI] [PubMed] [Google Scholar]
- 29.Sison M, Gerlai R. Associative learning in zebrafish (Danio rerio) in the plus maze. Behav. Brain Res. 2009;207:99–104. doi: 10.1016/j.bbr.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Speedie N, Gerlai R. Alarm substance induced behavioral responses in zebrafish (Danio rerio) Behav. Brain Res. 2008;188:168–177. doi: 10.1016/j.bbr.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swain HA, Sigstad C, Scalzo FM. Effects of dizocilpine (MK-801) on circling behavior, swimming activity, and place preference in zebrafish (Danio rerio) Neurotoxicol Teratol. 2004;26:725–729. doi: 10.1016/j.ntt.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 32.Sweatt D. Mechanisms of Memory. Amsterdam: Academic Press & Elsevier; 2010. p. 450. [Google Scholar]
- 33.Takahashi E, Niimi K, Itakura C. Impairment of spatial short-term memory following acute administration of the NMDA receptor antagonist in heterozygous rolling Nagoya mice carrying the Ca V 2.1 alpha1 mutation. Behav Brain Res. 2010;213:121–125. doi: 10.1016/j.bbr.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 34.Vanderschuren LJ, Schoffelmeer AN, Mulder AH, De Vries TJ. Dizocilpine (MK801): use or abuse? Trends Pharmacol Sci. 1998;19:79–81. doi: 10.1016/s0165-6147(97)01164-4. [DOI] [PubMed] [Google Scholar]
- 35.Venable N, Kelly PH. Effects of NMDA receptor antagonists on passive avoidance learning and retrieval in rats and mice. Psychopharmacology (Berl) 1990;100:215–221. doi: 10.1007/BF02244409. [DOI] [PubMed] [Google Scholar]
- 36.Xu X, Scott-Scheiern T, Kempker L, Simons K. Active avoidance conditioning in zebrafish (Danio rerio) Neurobiol Learn Mem. 2007;87:72–77. doi: 10.1016/j.nlm.2006.06.002. [DOI] [PubMed] [Google Scholar]