Abstract
Study Design
Histological analysis of intervertebral disc (IVD) in three types of transgenic mice.
Objectives
To investigate the role of Wnt/β-catenin signaling in regulation of IVD development and organization.
Summary of Background Data
β-catenin dependent Wnt signaling is one of the central regulators in cartilage development during limb skeletal formation. Little is known, however, about the physiological relevance of this signaling pathway to IVD development and organization.
Methods
Temporal-spatial distribution of Wnt/β-catenin signaling activity was examined in IVD using Wnt/β-catenin reporter (TOPGAL) mice. The structural changes in the mouse IVD components such as the nucleus pulposus (NP), endplate (EP), annulus fibrosus (AF), and the growth plate (GP) of the vertebral body were analyzed following transient activation of Wnt/β-catenin signaling or deletion of β-catenin in the mice.
Results
Activity of Wnt/β-catenin signaling was high in EP, AF and GP in the embryonic stages and decreased at the postnatal stage; it was undetectable in the embryonic NP but up-regulated after birth. The transient activation of Wnt/β-catenin signaling caused severe deterioration of the GP and the AF, whereas deficiency of β-catenin accelerated bone formation in between EP and GP.
Conclusion
The findings in this study suggest that proper regulation of Wnt/β-catenin signaling is required for development and organization of IVD.
Keywords: Wnt, β-catenin, intervertebral disc
Introduction
The intervertebral disc (IVD) is a unique structure comprised of annulus fibrosus (AF) and nucleus pulposus (NP). The IVD is sandwiched between two adjacent cartilaginous endplates (EP). These tissues have major differences in cellular distribution and extracellular matrix organization, and cooperatively maintain IVD architecture and function 1–3. Previous studies have reported that several growth factor proteins, such as fibroblast growth factors, insulin like growth factor, bone morphogenetic proteins (BMPs) and transforming growth factor-β(TGF-β), are synthesized by disc cells and affect matrix synthesis and other cellular activities in these cells. 4,5. However, the molecular mechanisms underlying regulation of the IVD cell function and organization of the IVD structures have not been fully elucidated yet.
The Wnt family of proteins govern a wide range of biological processes, including initial embryonic development, tissue organization, self renewal, cell proliferation and differentiation, and pathological processes such as tumorigenesis 6–8. Wnt signaling is transmitted by three major pathways: β-catenin, JNK, and calcium-dependent pathways 7. Wnt/β-catenin signaling, also known as canonical Wnt signaling, is dependent on sequential molecular events involving β-catenin: (i) the inhibition of β-catenin phosphorylation; (ii) an increase in cytoplasmic β-catenin content; (iii) the stimulation of nuclear translocation of β-catenin; (iv) the interaction of β-catenin with lymphoid enhancer factor/T cell factor (LEF/TCF); and (v) finally, stimulation of the target gene transcription 9. We have shown that Wnt/β-catenin signaling has a central role in the regulation of cartilage and joint development during development 10–12. We have also found that this signaling has a strong influence on cartilage matrix metabolism 13. In this study, we hypothesized that the Wnt/β-catenin pathway plays an important role in IVD function and that imbalances in this signaling could lead to IVD organization impairment. To test this hypothesis, we first determined the location and the degree to which Wnt/β-catenin signaling is active in mouse vertebrae by using Wnt reporter mice. We then analyzed the consequences of manipulating this signaling activity on the organization of the IVD in the mouse.
Materials and Methods
Transgenic (TG) mice
Wnt/β-catenin signaling in IVDs was monitored using Wnt reporter TOPGAL mice that harbor LEF/TCF binding sites and a minimal promoter of c-fos linked to β-galactosidase (Tg(Fos-lacZ)34Efu/J, The Jackson Laboratory, Bar Harbor, Maine) 14. To activate Wnt/β-catenin signaling, we used inducible transgenic mice for β-catenin signaling (Col11 CA-β-catER mice, C57BL/6J) that we developed 15. These mice have a constitutively active N-terminally-truncated β-catenin linked to a modified estrogen receptor ligand binding domain (ER) (ΔNβ-catenin-ERTM, provided by UK London Research Institution) 16 under control of collagen 11α2 (Col11a2) promoter/enhancer (provided by Osaka University) 17. This allows induction of β-catenin signaling by tamoxifen (TX). Tamoxifen, at a dose of 200 µg/20 µl/mouse, was peritoneally injected into 2-weeks-old transgenic mice for seven days. The TX stock solution was prepared in 99% ethanol at a concentration of 100 mg/ml at 55 °C and was diluted to 10 mg/ml with corn oil prior to use. Control mice received the same amount of ethanol (10%) in corn oil (90%). Three wild type (WT) mice and three of TG mice were sacrificed one day after the TX injection series was completed. Eight WT and 11 TG mice were sacrificed four weeks after the TX injection. Two WT and two TG control mice were sacrificed four weeks after they had received the control injections.
Mice conditionally deficient in β–catenin were created by mating β–catenin floxed mice (β–cateninfl/fl) possessing loxP sites in introns 1 and 6 in the β–catenin gene (6.129-Ctnnb1tmKem/KnwJ line from The Jackson Laboratory) with Col2a1-CreER mice 18. Genotyping of the β–catenin allele was carried out according to protocols developed by The Jackson Laboratory. To induce recombination of β–catenin gene, we injected TX intraperitoneally three times daily at a dose of 200 µg/20 µl/mouse from P5 to P7. Five Col2a1-CreER/β–cateninfl/fl mice and five of their littermates of β–cateninfl/fl were used. The efficiency of TX-induced Cre recombinase activity was confirmed in compound transgenic mouse from crosses of Col2a1-CreER mouse and RosaR26R mouse (obtained from The Jackson Laboratory). Littermates that did not harbor Col2a1-CreER were used as control.
Histological, histochemical and in situ hybridization analyses
Lumber vertebrae (L1 to L6) were dissected after perfusion fixation with 4% paraformaldehyde, decalcified with 12.5% EDTA for 3–5 days and embedded in paraffin. Serial sections (5 µm thick) were subjected to staining with hematoxylin and eosine (H-E), Safranin O or Alcian blue. To evaluate cell proliferation, the mice received an intraperitoneal injection of BrdU (Invitrogen, Carlsbad, CA) (150 µg/ml in phosphate buffered saline) two hours before sacrifice. Longitudinal sections were incubated with anti-BrdU antibodies (1:200, Roche Diagnostics, Indianapolis, IN) followed by incubation with Alaxofluor 488-anti mouse IgG (Invitrogen).
For detection of β-galactosidase activity, frozen sections (10 µm thick) were incubated with β-galactosidase substrate (X-Gal, Millipore, Billerica, MA) according to the manufacturer’s protocol. Gene expression of the trasngene or type 2 collagen was analyzed by in situ hybridization using 35S-labled riboprobes (CA-β–catER) or digoxigenin-labeled riboprobes (collagen 2α1) 19. A probe for the transgene CA-β–catER was designed to recognize the C-terminal end of β–catenin plus the N-terminal part of ER domain.
Micro CT scan
Vertebrae were fixed in buffered 4% paraformaldehyde overnight at 4°C, rinsed and subjected to micro-computed tomography (µCT) using a µCT40 SCANCO Medical system (Southeastern, PA, USA). Samples were scanned at 45 kV and 177 µA, 12 µm scanning thickness and medium resolution by using a 20.5 mm holder. Two-dimensional slice images were selected and used to generate three-dimensional reconstructions with filter width sigma=0.8, support level=1.0 and threshold=244. The same values were used to analyze WT and TG mouse samples. Axial images were rotated at specific angles to generate frontal views of vertebral bodies to acquire inter-sliced images.
Results
Wnt/β-catenin signaling in IVD
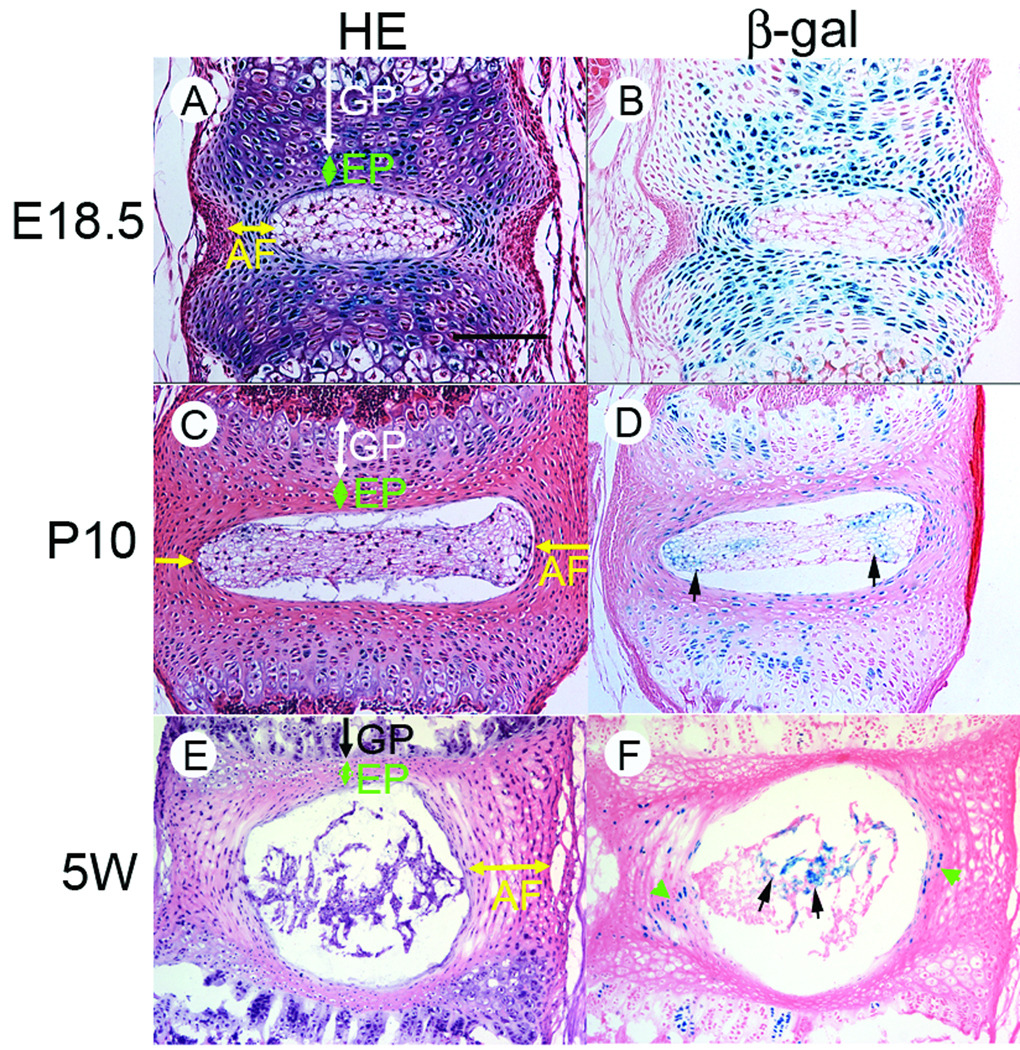
To examine temporal-spatial activity of Wnt/β-catenin signaling in the IVD during formation and maturation of the IVD structure, lumbar vertebral discs were dissected from the Wnt/β-catenin reporter mice (TOPGAL mice) at embryonic age 18.5 days, post-natal day10 (P10) and 5 weeks-old (5W), and stained for β-galactosidase activity (Figure 1). At E18.5, the overall IVD structures were established (Figure 1A), but the border between the GP and EP was not as distinct as those seen in the two older ages (Figures 1C and 1E). In addition, the lamellar structure of the AF was still immature at E18.5 (Figure 1A, AF). At E18.5 the signal for Wnt/β-catenin signaling was strong in the GP, EP and AF, but was barely detectable in the NP (Figure 1B). In these tissues, the reporter activity weakened, but it was still clearly observed in AF, EP and GP (Figure 1D), while the activity had appeared in NP cells (Figure 1D, arrows) at P10. At 5-weeks old, the reporter activity was further weakened in the AF and EP and only detected in the AF (Figure 1F, arrow heads). The NP cells exhibited stronger signal at 5-weeks old (Figure 1F, arrows). The appearance of TOPGAL reporter-positive cells in the NP was consistently observed in the mouse samples examined. These findings suggest that Wnt/β-catenin signaling activity and localization dynamically change during establishment of IVD structures at embryonic and postnatal stages.
Figure 1.
Wnt/β-catenin signaling activity in the IVD. Vertebrae were dissected form TOPGAL mice at E18.5, post-natal Day 10 (P10) or 5-weeks old (5W). IVD tissue sections were subjected to Hematoxylin-Eosin (HE) or β-galactosidase activity (β-gal) staining. A bar represents 150 µm for A–D and 300 µm for E and F.
Excess activation of Wnt/β-catenin signaling in IVDs
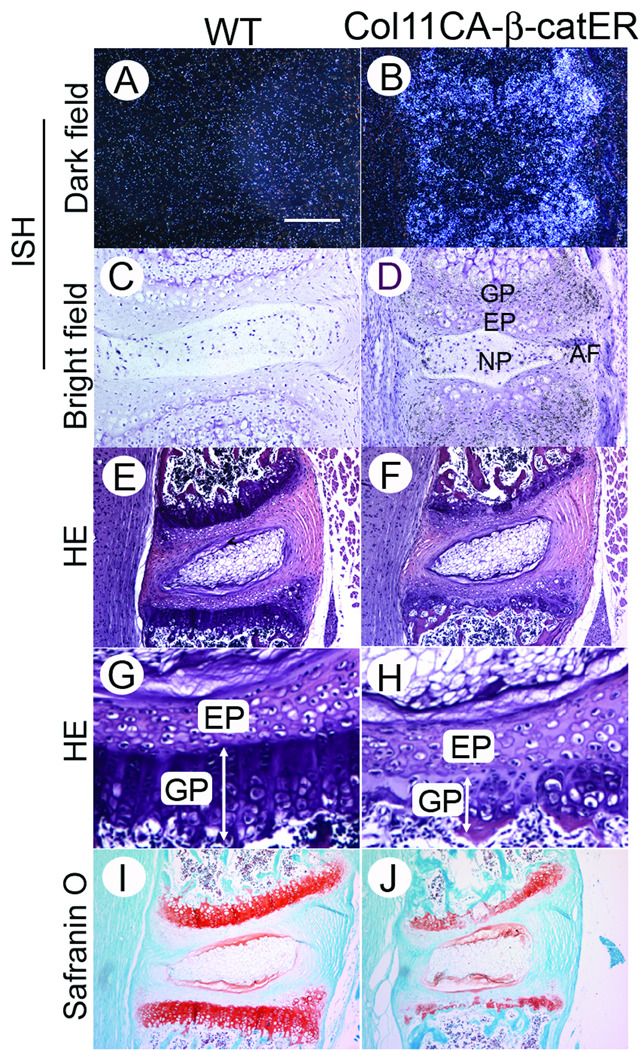
To study the significance of Wnt/β-catenin signaling during IVD organization, we first asked how up-regulation of this signaling affects IVD development and organization (Figure 2). We have recently developed transgenic mice that harbor tamoxifen-inducible11 constitutive active β-catenin under the control of collagen 11α promoter/enhancer 17. This mouse (Col11 CA-β-catER mice) allows us to activate Wnt/β-catenin signaling specific in cartilage by tamoxifen injection. To address the location where the constitutive active form of β-catenin transgene is expressed in IVD, we performed in situ hybridization for this transgene. The transgene was highly expressed in the AF, EP and GP and weakly in NP cells (Figure 2B and D), indicating that we could activate Wnt/β-catenin signaling by tamoxifen injection. We gave seven daily tamoxifen intraperitoneal injection at 2 weeks of age and sacrificed the mice on the day following completion of the injection schedule. Structural changes in lumbar IVDs were examined histologically (Figures 2E–2J). A severe disorganization of the GP occurred in the tamoxifen-treated Col11 CA-β-catER mice; the total height was shortened with an irregular cell alignment (Figure 2H, GP) and the protoeglycan content was decreased in growth plate (Figure 2J) compared with that of the control mice (received corn-oil only) (Figures 2E, 2G and 2I). In contrast, the endplate, AF and NP of both tamoxifen-treated WT and TG mice did not show major change (Figures 2E–2H).
Figure 2.
Expression of the transgene and degeneration of the vertebral GP in tamoxifen-treated Col11 CAβ-catER mouse. A–D, Tissue sections of the tails from 6-weeks-old Col11 CAβ-catER mouse (B and D) and their WT littermates (A and C) were prepared and subjected to in situ hybridization for CAβ-catER transgene. E–J, Col11 CAβ-catER mice (F,H and J) and the WT littermates (E,G and I) received seven daily injections of tamoxifen (200 µg/20 µl/day). On the day following completion of the injections, the vertebrae were dissected and the tissue sections stained with HE (E–H) or Safranin O (I and J). The bar represents 150 °m for A–D, 300 µm for E, F, I and J, 75 µm for G and H.
Wnt/β-catenin signaling on growth plate intervertebral disc structure
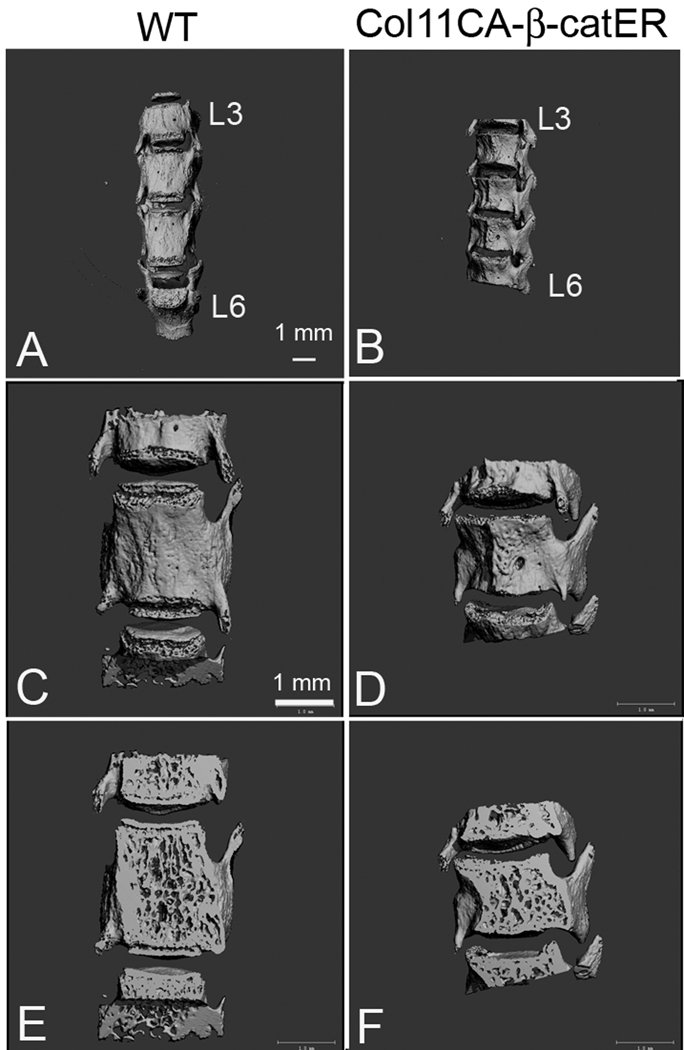
To examine the consequences of transient over activation of Wnt/β-catenin signaling on growth plate intervertebral disc structure, mice described above were kept for 4 weeks following the tamoxifen or control injection. The tamoxifen-injected transgenic mice showed growth retardation while the same tamoxifen treatment did not induce such effect in the WT mice. For the structure of vertebral bodies, we performed the micro CT scan of the lumbar vertebrae (from the third vertebral body (L3) to the sixth vertebral body (L6)). The heights of the vertebral bodies were clearly shorter in the tamoxifen-injected TG mice than in those of the control WT mice (Figure 3). This suggests that the growth plate was affected, resulting in shortened vertebral body length.
Figure 3.
MicroCT images of the vertebrae in tamoxifen-treated Col11 CAβ-catER mouse. Col11 CAβ-catER mice (B, D and F) and the WT littermates (A, C and E) received seven daily injections of tamoxifen (200 µg/20 µl/day). Four weeks after the treatment, the third lumber vertebrate to the sixth lumber vertebrate were analyzed by MicroCT (A and B, L3–L6). C–F were the magnified L5 views of three dimensional (C and D) and slice (E and F) images.
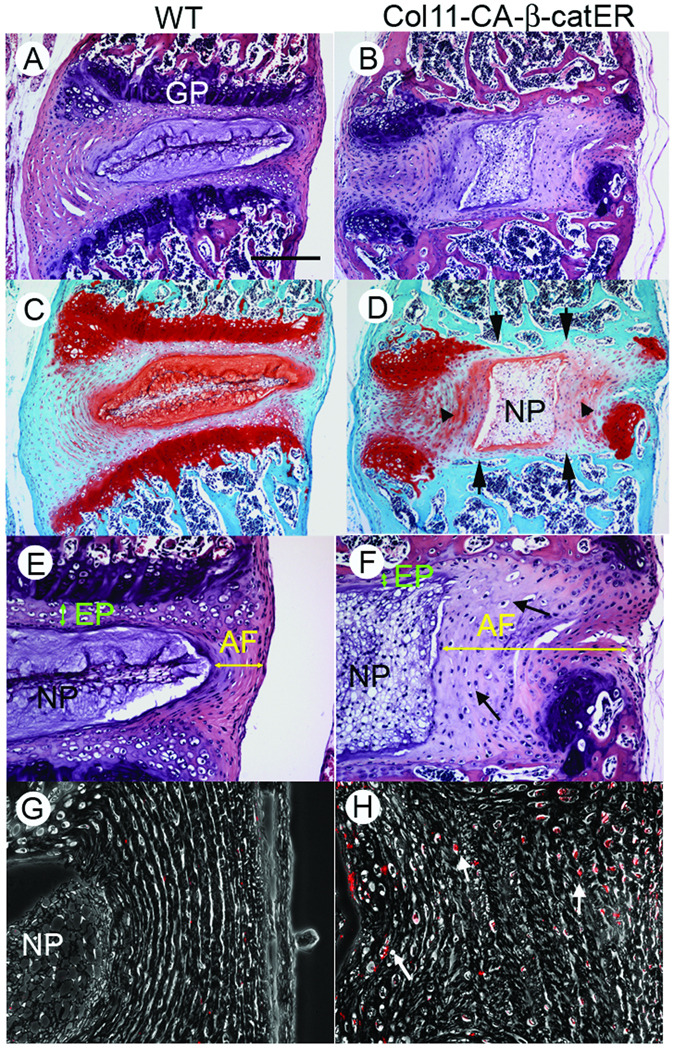
Histological analyses shown in Figure 4 revealed that the IVD architecture was severely deteriorated in the tamoxifen-treated Col11 CA-β-catER mouse; the GP structure was almost completely destroyed (Figure 4D, arrows), the AF lamella became very irregular and contained spherical cells (Figure 4F, yellow arrows) and the NP tissue was enlarged, but had a much lower content of protegolycan (Figure 4D, NP). To study the cell proliferation activity, the mice received BrdU injection 2h before sacrificed. The transgenic AF showed higher labeling index of BrdU (Figure 4H, red dots, arrows), indicating the AF cells increased proliferating activity in the transgenic mice. These findings indicate that an excess activation of Wnt/β-catenin signaling brings serious deformity of the IVD structure with disruption of the GP, activation of AF cell proliferation, derangement of AF lamellae structures and depletion of proteoglycan in the NP.
Figure 4.
Disorganization of the IVD structure in tamoxifen-treated Col11 CAβ-catER mouse. Col11 CAβ-catER mice (B, D, F, H and J) and their WT littermates (A, C, E, G and I) received seven daily injections of tamoxifen (200 µg/20 µl/day). Four weeks after the treatment, the vertebrae were dissected. The tissue sections were prepared and subjected to HE (A, B, E and F) or Safranin O (C and D) staining, immunostaining for anti-BrdU antibody (G and H). A bar represents 300 µm for A–D, 150 µm for E and F, and 75 µm for G and H.
Deletion of β-catenin in IVDs
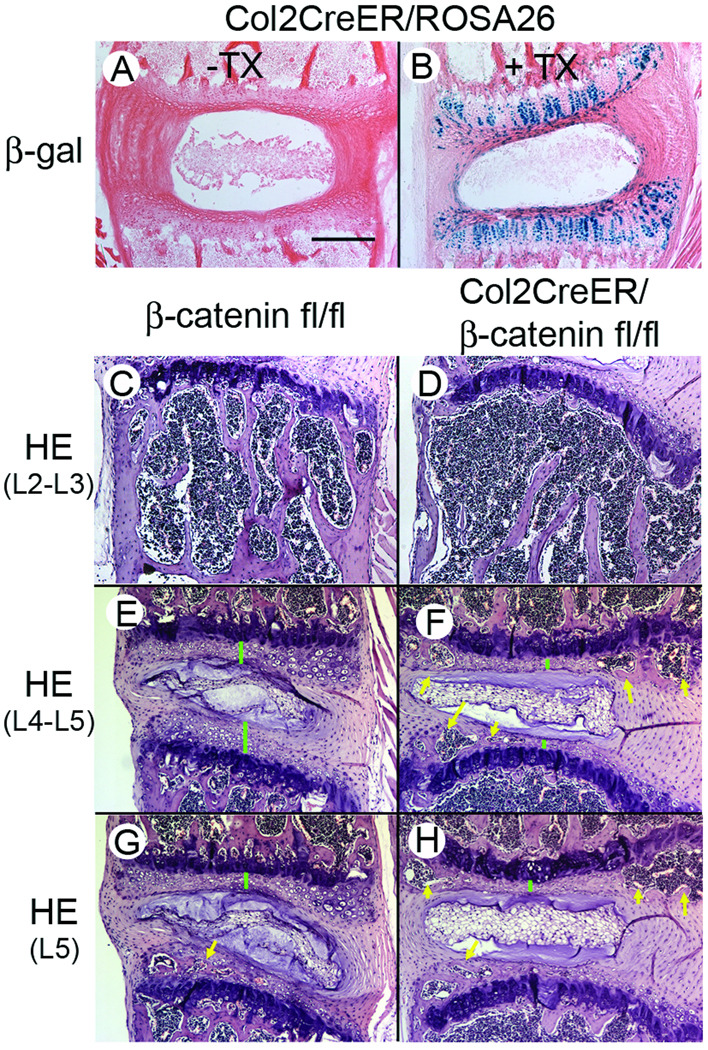
To elucidate the requirement for Wnt/β-catenin signaling in IVD organization and maintenance, we induced a conditional deletion of β-catenin from the GP and EP by tamoxifen injection into compound transgenic mice (Col2a1-CreER/β-cateninfl/fl) that harbor floxed β-catenin and Col2a1-CreER (Figure 5). When Cre recombinase cleaves the floxed site in the β-catenin gene in response to tamoxifen, the β-catenin gene is ablated, resulting in silencing of β-catenin expression. To determine which IVD cell type shows Cre recombinase activity following tamoxifen treatment under the control of collagen 2a1(Col2a1) promoter/enhancer, we administrated three daily injections of tamoxifen into 5 day-old neonatal compound TG mice with Col2a1-CreER/RosaR26R and harvested the vertebrae three days after the course of tamoxifen injection was completed. The RosaR26R mouse harbors a Cre recombinase sensitive β-galactosidase gene. When Cre recomibnase is activated by tamoxifen, β-galacatosidase gene is constitutively activated. Thus we could evaluate the location where the Cre recombinase is active as determined by β-galactosidase activity staining. The β-galactodsidase activity was found in GP, EP and inner AF cells (arrows), but not in outer AF cells (Figure 5B). We performed the same tamoxifen injection protocol in the Col2a1-CreER/β-cateninfl/fl mice and β-catnine fl/fl without Col2a1-CreER mice as control. At 6 weeks after the TX injection, the lumber vertebrae were harvested and subjected to histological inspection. We found distinct differences in the histological features of vertebral bodies between the Col2a1-CreER/β-cateninfl/fl (Figure 5D) and the control mice (β-catnine fl/fl without Col2a1-CreER) (Figure 5C). The volume of trabecular bone was greatly reduced and the cortical bone was also much thinner in the Col2a1-CreER/β-cateninfl/fl (Figure 5D). Despite the less bony tissues in the vertebral bodies, we found a greater frequency and wider sphere of bone formation in the EP of the Col2a1-CreER/β-cateninfl/fl compared to the control mice (β-catnine fl/fl without Col2a1-CreER). All the tamoxifen-injected Col2a1-CreER/β-catefl/fl mice that we examined had bone formation in the EP of L2 and L3 (Figure 5F, arrows) while the control mice (β-catenin fl/fl without Col2a1-CreER) had little bone in the EP in the same level of lumbar IVDs (Figure 5E). Bone formation was also appreciably more obvious in IVDs of L4 and L5 in Col2a1-CreER/β-catenin fl/fl mice (Figure 5H, arrows) compared with control mice (Figure 5G). In addition, the height of EP was reduced under the deficiency of β-catenin (Figure 5E vs 5F, green bars). A similar tendency was found in the all five Col2a1-CreER/β-catenin fl/fl mice that we had examined. The GP did not show clear changes in the Col2a1-CreER/β-catefl/fl mice (Figures 5E–5H).
Figure 5.
Cre recombinase activity in tamoxifen-treated Col2a1-CreER mouse and acceleration of bone formation in the IVD of tamoxifen-treated Col2a1-CreER/β-cateninfl/fl mouse. Col2a1-CreER (A), Col2a1-CreER/Rosa26A (B), β-catenin fl/fl (C, E and G) or Col2a1-CreER/β-cateninfl/fl (D, F and H) mice received three daily injections of tamoxifen (200 µg/20 µl/day) when they were 5-days-old. The vertebrae were dissected at ages of 5-weeks-old (A and B) or 9-weeks-old (C–H). The tissues sections were subjected to β-galactosidase activity staining (A and B) or HE staining. Green bars represent EP and yellow arrows show bone formation. Bar, 300 µm.
Discussion
Studies by our group and others have indicated that Wnt/β-catenin signaling is a major regulator of cartilage development and maintenance of the appendicular skeletons 10–12,20–22. In this study, we have demonstrated that the activity and localization of Wnt/β-catenin signaling dynamically changes during IVD development and structure maturation. At an embryonic stage, the signaling activities were found in the AF as well as cartilaginous elements such as the GP and the EP, but lower in the NP. The signaling activity weakened with maturation of the IVD structure, but appeared in NP cells at older age. It is suggested that Wnt/β-catenin signaling plays important roles in regulation of function of GP and EP chondrocytes and AF cells during IVD development, and that proper control of this signaling would be critical to establish IVD structure and to support maturation of IVD organization.
The responsiveness to Wnt/β-catenin signaling and its action are very different among the GP, AF and EP. The most severe abnormality associated with excessive activation of Wnt/β-catenin signaling was found in the GPs. These effects included rapid decrease in proteoglycan content, loss of hypertrophic chondrocytes and consequent disruption of the entire GP. The AF structure was disorientated with an up-regulation of cell proliferation, but the change in the EP was milder. These findings suggest that GP is very sensitive to this signaling and undergoes irreversible degenerative changes while AF cells are also responsive to this signaling but induce anabolic reaction following removal of the signaling stimulus. In contrast, the EP cells is likely resistant with excess activity of Wnt/β-catenin signaling. We have also observed remarkable changes in NP after activation of Wnt/β-catenin signaling that include enlargement of NP and loss of proteoglaycan. However, this abnormality would be results from degeneration and disorganization of GP and AF since our experimental system does not directly modulate Wnt/β-catenin signaling in NP.
Abnormality of the EP induced by the β-catenin deficiency indicates an indispensable role of Wnt/β-catenin signaling in maintenance of the EP. We detected the signaling activity in inner AF, but not in the EP at later stages, suggesting that the maintenance of EP only requires a very low level of Wnt/β-catenin signaling activity that was not detectable by our analytical methodologies, or is indirectly executed by other molecule(s) produced in inner AF cells or other cells. Further investigation is required to understand which molecule works as a downstream of Wnt/β-catenin signaling to regulate EP function. The single nucleotide polymorphism (SNP) in Wisp1 has been recently demonstrated to be associated with spinal osteoarthritis 23. Wisp 1 has been initially found to be a target molecule of Wnt 1 in tumorgenesis24. The SNP of Wisp 1 at 3’ UTR region shows a clear relation with endplate sclerosis, but not with osteophyte formation or disc narrowing 23. Further, up-regulation of Wisp-1 has been found in human osteoarthritis cartilage 25. It would be very interesting to study whether a decrease in Wnt/β-catenin signaling activity would be related to IVD degeneration, such as endplate sclerosis/calcification.
In summary, our data suggest that Wnt/β-catenin signaling supports organization of the annulus fibrosus and endplates, and that control of this signaling is critical for maintenance of intervertebral disc structures during development.
Key Points
Wnt/β-catenin signaling is active in the IVD.
Excess activation of Wnt/β-catenin signaling results in disorganization of IVD during development.
Deficiency of β-catenin leads to precautious bone formation in endplate.
Acknowledgements
This publication is made possible by NIAMS, NIH (AR046000 and AR050507) and NIA, NIH (AG025868). We thank Drs. S. MacKem (NCI, NIH), F. Watt, (UK London Res. Inst.) and N. Tsumaki (Osaka University) for providing Col2 α1Cre-ER mice, CA-β-catER and Col11α2 promotor/enhancer, respectively. We also thank Ms. J. Williams, A. Hargett and D. Pilchak for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Freemont AJ. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology (Oxford) 2009;48:5–10. doi: 10.1093/rheumatology/ken396. [DOI] [PubMed] [Google Scholar]
- 2.Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine. 2006;31:2151–2161. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- 3.Moore RJ. The vertebral endplate: disc degeneration, disc regeneration. Eur Spine J. 2006;15(Suppl 3):S333–S337. doi: 10.1007/s00586-006-0170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masuda K, An HS. Prevention of disc degeneration with growth factors. Eur Spine J. 2006;15(Suppl 3):S422–S432. doi: 10.1007/s00586-006-0149-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sobajima S, Kim JS, Gilbertson LG, et al. Gene therapy for degenerative disc disease. Gene Ther. 2004;11:390–401. doi: 10.1038/sj.gt.3302200. [DOI] [PubMed] [Google Scholar]
- 6.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 7.Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281:22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- 8.Huang H, He X. Wnt/beta-catenin signaling: new (and old) players and new insights. Curr Opin Cell Biol. 2008;20:119–125. doi: 10.1016/j.ceb.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 10.Enomoto-Iwamoto M, Kitagaki J, Koyama E, et al. The Wnt antagonist Frzb-1 regulates chondrocyte maturation and long bone development during limb skeletogenesis. Dev Biol. 2002;251:142–156. doi: 10.1006/dbio.2002.0802. [DOI] [PubMed] [Google Scholar]
- 11.Koyama E, Shibukawa Y, Nagayama M, et al. A distinct cohort of progenitor cells participates in synovial joint and articular cartilage formation during mouse limb skeletogenesis. Dev Biol. 2008;316:62–73. doi: 10.1016/j.ydbio.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamamura Y, Otani T, Kanatani N, et al. Developmental regulation of Wnt/beta-catenin signals is required for growth plate assembly, cartilage integrity, and endochondral ossification. J Biol Chem. 2005;280:19185–19195. doi: 10.1074/jbc.M414275200. [DOI] [PubMed] [Google Scholar]
- 13.Yuasa T, Otani T, Koike T, et al. Wnt/beta-catenin signaling stimulates matrix catabolic genes and activity in articular chondrocytes: its possible role in joint degeneration. Lab Invest. 2008;88:264–274. doi: 10.1038/labinvest.3700747. [DOI] [PubMed] [Google Scholar]
- 14.DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- 15.Yuasa T, Kondo N, Yasuhara R, et al. Transient Activation of Wnt/beta-catenin signaling induces abnormal growth plate closure and articular cartilage thickening in postnatal mice. Am J Pathol. 2009;175:1993–2003. doi: 10.2353/ajpath.2009.081173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lo Celso C, Prowse DM, Watt FM. Transient activation of beta-catenin signalling in adult mouse epidermis is sufficient to induce new hair follicles but continuous activation is required to maintain hair follicle tumours. Development. 2004;131:1787–1799. doi: 10.1242/dev.01052. [DOI] [PubMed] [Google Scholar]
- 17.Tsumaki N, Kimura T, Matsui Y, et al. Separable cis-regulatory elements that contribute to tissue- and site-specific alpha 2(XI) collagen gene expression in the embryonic mouse cartilage. J Cell Biol. 1996;134:1573–1582. doi: 10.1083/jcb.134.6.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura E, Nguyen MT, Mackem S. Kinetics of tamoxifen-regulated Cre activity in mice using a cartilage-specific CreER(T) to assay temporal activity windows along the proximodistal limb skeleton. Dev Dyn. 2006;235:2603–2612. doi: 10.1002/dvdy.20892. [DOI] [PubMed] [Google Scholar]
- 19.Koyama E, Leatherman JL, Shimazu A, et al. Syndecan-3, tenascin-C, and the development of cartilaginous skeletal elements and joints in chick limbs. Dev Dyn. 1995;203:152–162. doi: 10.1002/aja.1002030204. [DOI] [PubMed] [Google Scholar]
- 20.Akiyama H, Lyons JP, Mori-Akiyama Y, et al. Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev. 2004;18:1072–1087. doi: 10.1101/gad.1171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Day TF, Guo X, Garrett-Beal L, et al. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8:739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 22.Mak KK, Chen MH, Day TF, et al. Wnt/beta-catenin signaling interacts differentially with Ihh signaling in controlling endochondral bone and synovial joint formation. Development. 2006;133:3695–3707. doi: 10.1242/dev.02546. [DOI] [PubMed] [Google Scholar]
- 23.Urano T, Narusawa K, Shiraki M, et al. Association of a single nucleotide polymorphism in the WISP1 gene with spinal osteoarthritis in postmenopausal Japanese women. J Bone Miner Metab. 2007;25:253–258. doi: 10.1007/s00774-007-0757-9. [DOI] [PubMed] [Google Scholar]
- 24.Xu L, Corcoran RB, Welsh JW, et al. WISP-1 is a Wnt-1- and beta-catenin-responsive oncogene. Genes Dev. 2000;14:585–595. [PMC free article] [PubMed] [Google Scholar]
- 25.Blom AB, Brockbank SM, van Lent PL, et al. Involvement of the Wnt signaling pathway in experimental and human osteoarthritis: Prominent role of Wnt-induced signaling protein 1. Arthritis Rheum. 2009;60:501–512. doi: 10.1002/art.24247. [DOI] [PubMed] [Google Scholar]