Abstract
Lipoic acid (LA) is a naturally occurring fatty acid that exhibits anti-oxidant and anti-inflammatory properties and is being pursued as a therapeutic for many diseases including multiple sclerosis, diabetic polyneuropathy and Alzheimer’s disease. We previously reported on the novel finding that racemic LA (50:50 mixture of R and S LA) stimulates cAMP production, activates prostanoid EP2 and EP4 receptors and adenylyl cyclases (AC), and suppresses activation and cytotoxicity in NK cells. In this study we present evidence that furthers our understanding of the mechanisms of action of LA. Using various LA derivatives, dihydrolipoic acid (DHLA), S,S-dimethyl lipoic acid (DMLA) and lipoamide (LPM), we discovered that only LA is capable of stimulating cAMP production in NK cells. Furthermore, there is no difference in cAMP production after stimulation with either R-LA, S-LA or racemic LA. Competition and synergistic studies indicate that LA may also activate AC independent of the EP2 and EP4 receptors. Pretreatment of PBMCc with KH7 (a specific peptide inhibitor of soluble AC) and the calcium inhibitor (Bapta) prior to LA treatment resulted in reduced cAMP levels, suggesting that soluble AC and calcium signaling mediate LA stimulation of cAMP production. In addition, pharmacological inhibitor studies demonstrate that LA also activates other G- protein coupled receptors, including histamine and adenosine, but not the beta adrenergic receptors. These novel findings provide information to better understand the mechanisms of action of LA, which can help facilitate the use of LA as a therapeutic for various diseases.
Keywords: G protein coupled receptors, adenylyl cyclase, cAMP, lipoic acid
1. Introduction
Lipoic acid (LA) is an eight carbon sulfur containing small molecule that is produced in most prokaryotic and eukaryotic organisms, including plants and animals. It is synthesized de novo from octanoic acid by lipoic acid synthase [1]. It is important as a biological co-factor for the pyruvate dehydrogenase (PDH) complex, which is involved in transforming pyruvate into acetyl-CoA that is then utilized in metabolism. Exogenous sources of LA are obtained through the diet from ingestion of natural food products and supplements. Commercially available LA comes as a racemic mixture of R and S enantiomers (50:50 ratio). R-LA is produced naturally while S-LA is a byproduct of biosynthesis. Numerous studies are available demonstrating the beneficial effects of LA. As an antioxidant, LA and its reduced form, dihydrolipoic acid (DHLA), have been shown to protect against peroxynitrite induced tissue damage [2] by acting as a scavenger of reactive oxygen and nitrogen species. LA also acts as a chelator of transition and heavy metals and is able to help replenish endogenous antioxidants such as glutathione, ascorbate and vitamin E [3]. More recently, LA has been shown to have anti-inflammatory properties. LA inhibits expression of adhesion molecules such as VCAM-1 and ICAM-1 needed for immune cell migration [4, 5] and downregulates surface CD4 expression on blood mononuclear cells [6]. We first reported on the novel finding that LA stimulates production of the immunomodulator cAMP in peripheral mononuclear cells, purified T lymphocytes and natural killer cells [7, 8]. In addition, we discovered that LA inhibits NK cell activation and cytolytic function [7]. However, the biochemical mechanisms that mediate the effects of LA have not yet been fully elucidated.
cAMP is a ubiquitous small molecule second messenger that is involved in the transduction of signals from the extracellular environment into the cell. Activation of the cAMP-dependent signaling pathway regulates many aspects of biology and physiology, including proliferation, migration, apoptosis and gene expression [9–11]. In addition, cAMP is a key modulator of the inflammatory process [11]. Utilization of cAMP elevating agents in numerous studies have demonstrated reduction in monocyte and neutrophil mobility, reduction in the release of histamine, leukotrienes, reactive oxygen species, cytokines and chemokines, and inhibition of lymphocyte proliferation, activation and function [7, 12–15]. Thus, cAMP and other members of the signaling pathway have been therapeutic targets in many diseases with an inflammatory component, such as cardiovascular disease, rheumatoid arthritis and Alzheimer’s disease [16, 17].
Changes in cAMP levels are transient and are regulated by adenylyl cyclases (ACs) and phosphodiesterases (PDEs). ACs generate cAMP from ATP while PDEs degrade cAMP into AMP and Pi. There are two pools of ACs responsible for cAMP synthesis, transmembrane ACs (tmACs) and soluble ACs (sACs) [18, 19]. tmACs are tethered to the plasma membrane and are regulated by heterotrimeric G-proteins coupled to transmembrane receptors (GPCR), including the prostanoid EP2 and EP4 receptors, histamine, adenosine and β-adrenergic receptors. Upon receptor binding by ligands such as hormones, neurotransmitters, chemokines and growth factors, the G-protein subunits dissociate and activate tmACs to produce cAMP (reviewed by [19]). sACs are widely expressed in mammalian cells, lack transmembrane spanning domains, are not sensitive to G-proteins, and are regulated by bicarbonate and calcium [18, 20–24].
In this paper, we present novel evidence that LA, not DHLA or other synthetic derivatives, stimulates cAMP production, and that there are no significant differences between R-LA and S-LA in their ability to stimulate cAMP. In addition we demonstrate that LA stimulates a second pool of ACs (soluble ACs) and also identify the histamine and adenosine receptors as new mediators of LA stimulated cAMP production.
2. Methods and materials
2.1. Materials and Reagents
RPMI, high glucose DMEM, Lipofectamine 2000, fetal calf serum and all other tissue culture reagents were purchased from Invitrogen (Carlsbad, CA). The EasySep NK cell negative purification kit was purchased from Stem Cell Technologies Inc. (Vancouver, British Columbia). The cAMP kits were purchased from BioAssay Designs (Ann Arbor, MI). Lipoic acid, Bapta-am, PGE2, histamine, isoproterenol, 5′-(N-ethylcarboxamido) adenosine (NECA), famotidine, propranolol and alloxazine were obtained from Sigma (St. Louis, MO). The BCA protein assay kit was obtained from Pierce Biotechnology (Rockford, IL). HEK 293 EBNA cell line was purchased from ATCC (Manassas, VA). KH7 was purchased from ChemDiv Inc (San Diego, CA).
2.2. Cell culture
Human peripheral blood mononuclear cells (PBMCs) were obtained from source leukocytes (buffy coat) from the Red Cross in Portland, OR (approval #VACARR) or apheresis products purchased from Keybiologics (Memphis, TN). For buffy coats, enriched leukocytes were subjected to ficoll purification (Amersham) and centrifugation at 1400 rpm with the brake turned off for 30 minutes to remove contaminating red blood cells and platelets. The interface was collected, washed with RPMI 1640 and centrifuged at 1300 rpm for 10 minutes. The supernatant was decanted and the cells were subjected to two more wash steps. Cells were resuspended in freezing medium (RPMI + 25% FCS + 12% DMSO) and stored in liquid nitrogen for future use. For apheresis products, blood were split into conical tubes, diluted with 4× volume with 1× PBS (no Ca2+ or Mg2+), and centrifuged at 200 × g for 15 min at RT. Supernatants were decanted, cells were resuspended in a small volume by flicking the tube, and fresh PBS (50 ml) were added. Cells were centrifuged at 200 × g for 15 min at RT. This wash step was repeated once more. Cells were then subjected to ficoll gradient purification as described previously for buffy coats.
Previously frozen PBMCs were thawed and NK cells purified using the EasySep negative purification kit following the manufacture’s protocol. Briefly, human NK cell enrichment cocktail (50 μl/ml cells) was added to PBMCs (2 × 107 cells/ml) in PBS + 2% FBS and 1 mM EDTA and incubated at RT for 10 min. EasySep magnetic microparticles (100 μl/ml cells) were added to the cell mixture and incubated at RT for 5 min. The total suspension was brought up to 2.5 mls with buffer and placed into a magnet for 2.5 minutes. The supernatant containing the purified NK cells was then collected.
HEK 293 EBNA cells were maintained in a humidified 5% CO2 atmosphere chamber at 37°C in high glucose DMEM supplemented with 10% fetal bovine serum (FBS). Media was changed every three days. Cells were used for receptor transfection and competition assays.
2.3. Cyclic AMP assay
LA and derivative studies
1–2 × 105 NK cells in 500 μl RPMI were treated with 0, 25, 50, 75 and 100 μg/ml LA for 1 minute at RT. Samples were centrifuged at 1300 rpm for 1 minute and supernatants were then decanted. Cells were lysed with the addition of 400 μl 0.1 M HCl and boiling for 10 minutes. Samples were centrifuged at 1300 rpm and 100 μl was used for cAMP assays following the manufacture’s protocol. The absorbance was measured at 405 nm using a colorimetric 96-well plate reader (SpectraMax). Results in pmol/ml were then divided by the protein concentration to obtain pmol of cAMP per milligram of protein. Raw values were then normalized to the value obtained for the 100 μg/ml LA sample to obtained relative cAMP values in percentages.
Enantiomer studies
2 × 106 PBMCs were treated for 1 minute at RT with racemic (50:50 mixture of R and S LA), R, S or a homemade concoction of 50:50 R and S LA. Samples were centrifuged for 5 min, supernatants were decanted and pellets were resuspended and lysed in 0.1 M HCl and boiling for 10 minutes. Samples were used in cAMP assays as previously described.
Synergistic studies
1 × 105 NK cells or 2 × 106 PBMCs were either not treated or treated with 100 μg/ml LA, 10 μM PGE2, or a combination of LA and PGE2 for 1 min at RT. Samples were then treated as previously described.
Inhibitor studies
2 × 106 PBMCs were pre-incubated with DMSO vehicle control, 10 or 25 μM KH7, 10 μM Bapta-am, or 25–100 μM 2,5′ dideoxyadenosine (ddAdo) for 30 minutes at 37°C and then stimulated with 100 μg/ml LA or 25 μM forskolin for 1 minute. Samples were centrifuged and prepared as described above. KH7 is a small molecule specific inhibitor of sAC and is inert toward tmACs in vitro and in whole cells at concentrations up to 300 μM [25]. Bapta-am is a membrane permeable calcium chelator and ddAdo is a p-site inhibitor of transmembrane adenylyl cyclase (tmAC).
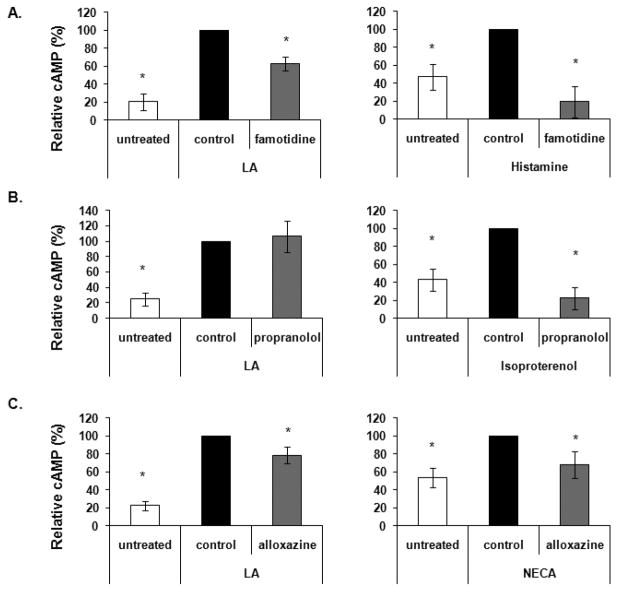
Separately, cells were pre-incubated with vehicle control (DMSO for propranolol and alloxazine, methanol for famotidine), 25 μM famotidine, 25 μM propranolol or 100 μM alloxazine for 30 min at 37°C then stimulated with either 100 μg/ml LA, 100 μM each histamine, isoproterenol or NECA for 1 min at RT. Cells were centrifuged, lysed and treated as described previously. Famotidine, propranolol, and alloxazine block the histamine H2, β adrenergic and adenosine A2 receptors, respectively, while histamine, isoproterenol and NECA are agonists of the receptors.
2.4. Bicinchoninic acid (BCA) assay
Depending on the experiment, varying volumes of supernatants (10–60 μl) from cAMP assays were used to determine total protein concentrations using the BCA assay kit (Pierce, Rockford, IL) following the manufacturer’s protocol. Absorbance readings were measured at 562 nm. Bovine serum albumin (BSA) standards were prepared in 0.1 M HCl at concentrations ranging from 0–1 mg/ml. Protein concentrations for unknown samples were extrapolated from the standard curve using Softmax Pro software (Molecular Devices, Sunnyvale, CA).
2.5. Receptor transfection
HEK293 EBNA cells were seeded into a T75 and transfected at approximately 40% confluence 24 hours later using Lipofectamine 2000. Briefly, 20 μg pcDNA3 EP2 or pcDNA3 EP4 receptor DNA were combined with 1.5 ml Opti-mem and incubated at room temperature (RT) for 5 minutes (DNA mixture). Simultaneously, 90 μl lipofectamine and 3 mls Opti-mem were mixed and incubated for 5 minutes at RT (lipofectamine mixture). The DNA and lipofectamine mixtures were combined, incubated at RT for 30 minutes and added to flask in a dropwise fashion. High glucose DMEM supplemented with 10% fetal bovine serum (FBS) was added to each flask. Cells were incubated at 37°C, 5% CO2 for 72 hours prior to experimentation.
2.6. Competition assay
Competition binding using 3HPGE2 were performed using a modified protocol published by Sugimoto et al. and Fujino et al. [26, 27]. Previously transfected cells were trypsinized and resuspended in MES buffer (10 mM MES pH 6, 0.4 mM EDTA, and 10 mM MnCl2. Cells (100 μl) were transferred to 96-well plates and treated with 50 μl of varying concentrations of LA or PGE2. 2.5 nM 3HPGE2 (50 μl) was then added to each well. The reaction (total volume is 200 μl) is incubated at 37°C for 1 hour. Samples were filtered through Whatman GF/C glass filters to terminate the incubation and then washed five times with ice-cold MES buffer. Radioactivity was measured by liquid scintillation counting. Relative 3HPGE2 binding was determined by normalizing all values to maximum 3HPGE2 radioactivity. Curves were fitted using one-site competition curve in Prism using the equation Y = Bottom + (Top−Bottom)/1 + 10X−LogEC50. The equation describes the competition of a ligand for receptor binding, which is identical to the sigmoid dose-response curve with HILLSLOPE = −1.0.
2.7. Statistical analysis
The data were analyzed using EXCEL 2007. Statistics were performed using Student’s t-test and were considered significant at a p value of ≤ 0.05. All treatments were performed independently at least 3 times.
3. Results
3.1. Stimulation of cAMP production by LA and its derivatives
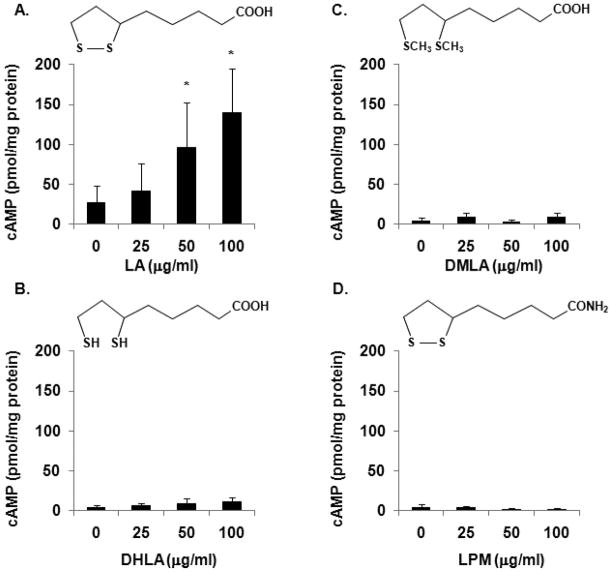
LA has been shown to exhibit anti-inflammatory properties in animal models of experimental autoimmune encephalomyelitis (EAE) and in human cells in vitro [4, 6–8, 28–31]. In an effort to determine the biochemical mechanisms that mediate the anti-inflammatory effects of LA, we discovered that LA stimulates cAMP production in immune cells [7, 8]. cAMP is a signaling molecule that has immunomodulatory effects. It is not known, however, if DHLA and other LA derivatives, dimethyl lipoic acid (DMLA), or α lipoamide (LPM) can also stimulate cAMP production in NK cells. DHLA is the reduced form of LA whereby the disulfide bond is broken to form a pair of thiol groups (Fig. 1). DMLA contains 2 methyl groups in the opened pentane ring while the carboxy group was exchanged with an amine group to generate LPM. DMLA and LPM were used to elucidate the relative contributions of the ring structure and the carboxyl group, respectively, to cAMP production. To test this, purified human NK cells were either not treated or treated with 25, 50 or 100 μg/ml LA, DHLA, DMLA or LPM for 1 minute at RT and cAMP level was assayed as described in methods and materials. In figure 1, we present the average data of 3 independent experiments. Due to donor variability (raw baseline cAMP values ranged from 1–72 pmol/mg protein while the maximal values were 42–230 pmol/mg protein depending on the donors being used). Consistent with our previous report, LA was effective at stimulating cAMP production (Fig. 1A). The average cAMP values compared to unstimulated control demonstrate that 25, 50, and 100 μg/ml LA induced 1.7, 4.5, and 8 fold increases in cAMP production, respectively. In contrast, the same concentrations of DHLA, DMLA and LPM failed to stimulate cAMP production (Fig. 1B-C). These data demonstrate that the disulfide pentane ring and carboxy group are necessary for LA to stimulate cAMP production.
Fig. 1. LA, but not its derivatives, stimulates cAMP production.
Purified human NK cells were treated with 25, 50 or 100 μg/ml LA (A), DHLA (B), DMLA (C) or LPM (D) for 1 min. Samples were pelleted by centrifugation at 13,000 rpm for 1 min at RT. The supernatants were decanted and the cells were lysed in 0.1 M HCl with boiling for 10 min. Samples were centrifuged and supernatants were used to measure cAMP levels via ELISAs from Assay Designs (Ann Arbor, MI). N = 3 independent experiments, 3 donors in duplicate. * indicates statistical significance using t-test compared to untreated control, p < 0.05.
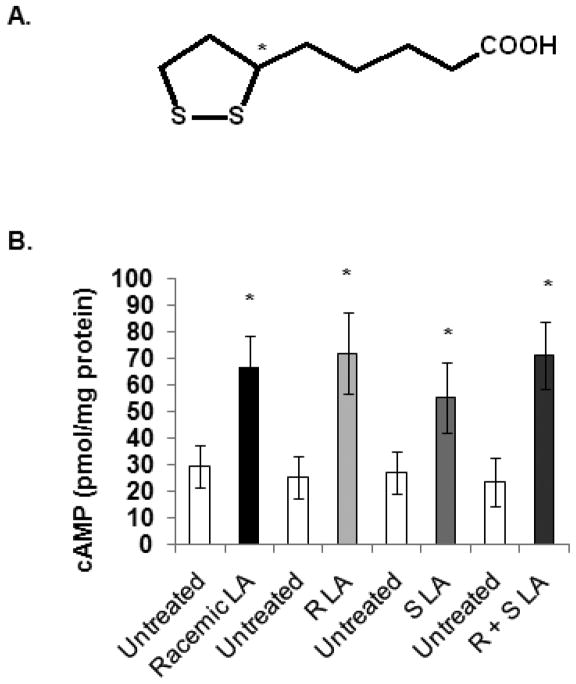
3.2. Stimulation of cAMP production by R and S LA
Two LA enantiomers exist (R and S) as a result of a chiral center at the C6 position (Fig. 2A). R-LA is found in nature while S-LA is a byproduct of synthesis. Several reports have demonstrated differences in absorbance and activity of R and S-LA in cells, animals and humans [32–38]. However, evidence is also available showing no differences in the function of R and S-LA [39]. Thus, we determined if there is a difference between R and S-LA in their ability to stimulate cAMP. PBMCs were treated with either 100 μg/ml racemic LA, R-LA, S-LA or a homemade mixture of R and S-LA for 1 min at RT. Cells were centrifuged and processed for cAMP analysis. As illustrated in figure 2B, treatment with racemic LA, R-LA, S-LA and R+S LA all resulted in increased cAMP production (n= 7 independent experiments, 5 donors, p < 0.05). The average fold-change increases in cAMP compared to untreated controls were 2.28, 2.86, 2.05 and 3.03 folds. Thus there were no significant differences in the ability of racemic LA, R-LA and R + S LA to stimulate cAMP production. Although there was a decreasing trend in cAMP levels in S-LA treated samples compared to R-LA, the result was not statistically significant (p = 0.232). This may be attributed to donor differences since R and S-LA behaved similarly in some donors while R-LA was more efficient than S-LA and vice versa in other donors. Similar observations were made in NK cells (data not shown). Collectively, the data suggest that racemic LA, R-LA, and S-LA behave similarly in their ability to stimulate cAMP production. As such, we used only racemic LA for subsequent experiments.
Fig. 2. Stimulation of cAMP production by R and S LA.
(A) Schematic of the chemical structure of LA indicating a chiral center at the C6 position (*). (B) Human PBMC were either not treated or treated with 100 μg/ml racemic LA, R-LA, S-LA or mixture of R + S LA for 1 min. Samples were pelleted by centrifugation at 13,000 rpm for 1 min at RT. The supernatants were decanted and the cells were lysed in 0.1 M HCl with boiling for 10 min. Samples were centrifuged and supernatants were used to measure cAMP levels via ELISAs from Assay Designs (Ann Arbor, MI). N = 3 independent experiments, 3 donors in duplicate. * indicates statistical significance using t-test compared to untreated control, p < 0.05.
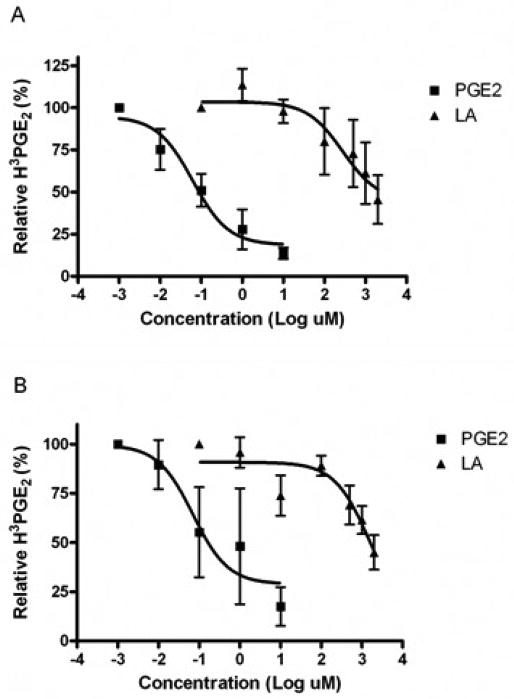
3.3. LA is less effective at binding of the prostanoid EP2 and EP4 receptors than PGE2
LA has a lipid-like backbone and is rapidly absorbed, suggesting that passive diffusion is the mechanism by which LA crosses the cell membrane [40]. However, there is evidence demonstrating the ability of LA to stimulate various signal transduction pathways and activate transcription factors [7, 8, 41, 42]. We first reported on the novel observation that LA activates the transmembrane G protein coupled prostanoid receptors, EP2 and EP4. Here, we determined if LA is a competitor of PGE2 for receptor binding. EP2 and EP4 receptor DNA were transfected into HEK 293 EBNA cells for 72 hours, incubated with PGE2 or LA and 3HPGE2 for 1 hour, washed and transferred to Whatman filters to separate free from bound radioactivity. Untransfected cells do not produce cAMP in response to PGE2 or LA [7, 26], suggesting that the receptors are not expressed. As such, we did not observe any 3HPGE2 binding activity (data not shown). In figure 3, we present the average data for three independent experiments where percent 3HPGE2 binding was calculated by dividing counts per minute (CPM) values for each data point by maximum 3HPGE2 CPM. Sigmoidal binding curves were then generated using the one site competition equation in GraphPad Prism. The top of the curve is a plateau at a value equal to radioligand binding in the absence of the competing unlabeled drug. The bottom of the curve is a plateau equal to nonspecific binding. As illustrated, PGE2 and LA were both able to compete for binding of the EP2 and EP4 receptors with 3HPGE2, however, the LA curve is further to the right indicating that LA binds with less affinity. The EC50 of PGE2 is 0.062 and 0.07 μM for EP2 and EP4, respectively. The EC50 for LA is dramatically higher at 0.282 and 1.57 M, respectively. The data indicate that LA is a much weaker competitor of 3HPGE2 for EP2 and EP4 binding than PGE2. This is consistent with our previous report showing greater amounts of cAMP in HEK 293 EBNA cells treated with PGE2 than LA [7].
Fig. 3. LA is less effective at binding of the prostanoid EP2 and EP4 receptors compared to PGE2.
HEK 293 EBNA cells were transfected in a T-75 flask at ~40% confluence with 20 μg EP2 (A) or EP4 (B) DNA using lipofectamine 2000 (InVitrogen). Cells were incubated at 37°C, 5% CO2 for 72 hours. Cells were harvested with trypsinization and rinsed in MES buffer. Cells were then incubated with 3HPGE2 and either LA or PGE2 for 1 hour at 37°C. Radioactivity was measured by liquid scintillation counting. Relative 3HPGE2 binding was determined by normalizing all values to maximum 3HPGE2 radioactivity. N = 3 independent experiments in duplicate.
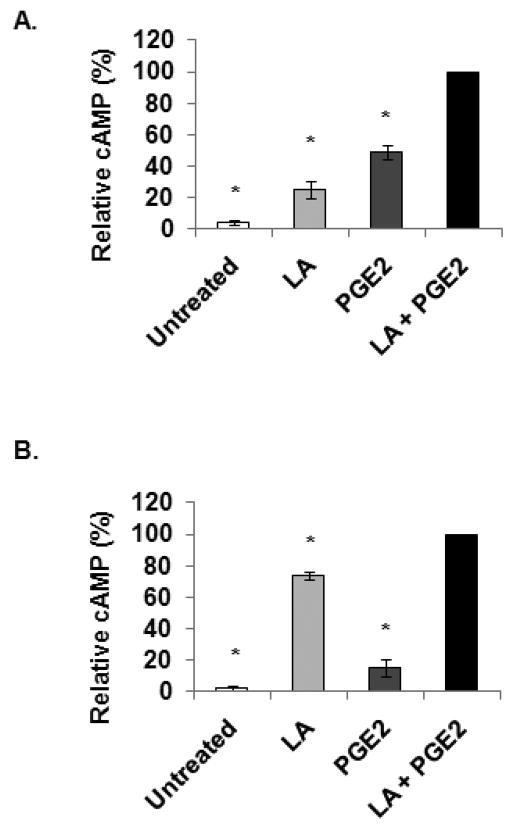
3.4. LA and PGE2 synergistically elevated cAMP levels
Competition and cAMP data obtained in transfected HEK 293 EBNA cells indicate that LA is less effective at activating the EP2/EP4 receptors than PGE2. This led to the hypothesis that LA is using additional pathways to stimulate cAMP production in immune cells. In figures 4 through 6, we present data in support of this hypothesis. First, we conducted studies to determine if LA and PGE2 have a synergistic effect on cAMP production. We conducted preliminary studies to determine the optimum concentrations of LA and PGE2 to use and found that 100 μg/ml LA and 10 μM PGE2 were sufficient to elicit maximal cAMP production (data not shown). Subsequently, PBMCs and NK cells were either not treated or treated with 100 μg/ml LA, 10 μM PGE2, or a combination of LA and PGE2. Figure 4A represents cAMP data (percent of maximum, LA + PGE2) obtained for PBMCs. The average basal level was 4.5% of maximum. Treatment with LA, PGE2, or LA + PGE2 resulted in 5.53, 10.77, and 22 fold increases, respectively, in cAMP compared to untreated controls. Treatment with LA + PGE2 resulted in significantly more cAMP than in either LA or PGE2 treatments alone. We next examined the effects of combined LA and PGE2 treatment in NK cells (Fig. 4B). Basal cAMP levels were at 2% of maximum while treatment resulted in 40, 7.4 and 48.7 fold increases in cAMP with LA, PGE2 or LA + PGE2 treatments, respectively. Interestingly, incubation with LA produced more cAMP in NK cells than in PBMCs and, conversely, PGE2 produced less cAMP in NK cells than PBMCs. However, in agreement with data obtained for PBMCs, NK cells treated with the combination of LA and PGE2 resulted in more cAMP produced than with either LA or PGE2 alone. Taken together, these data suggest that NK cells have different amounts/types/levels of receptors than PBMCs, and confirm that LA is utilizing mechanisms in addition to the EP2/EP4 receptors to stimulate cAMP production.
Fig. 4. LA and PGE2 synergistically elevated cAMP levels.
Human PBMC (A) and purified NK cells (B) were either not treated or treated with 100 μg/ml racemic LA, 10 μM PGE2 or LA + PGE2 for 1 min. Samples were pelleted by centrifugation at 13,000 rpm for 1 min at RT. The supernatants were decanted and the cells were lysed in 0.1 M HCl with boiling for 10 min. Samples were centrifuged and supernatants were used to measure cAMP levels via ELISAs from Assay Designs (Ann Arbor, MI). N = 3 independent experiments, 3 donors in duplicate. * indicates statistical significance using t-test compared to LA + PGE2 treated samples, p < 0.05. Data presented here are averages of normalized cAMP values in percent of maximal stimulation. The following are actual values in pmol/mg protein starting with untreated: PBMC-32.1, 144.4, 286.2, 608.2; NK cells-23.7, 1033.4, 239.5, 1395.8.
Fig. 6. Identification of other GPCRs that are activated by LA.
PBMCs were pretreated with vehicle control, 25 μM famotidine (A), 25 μM propranolol (B) or 100 μM alloxazine (C) for 30 min then stimulated with either 100 μg/ml LA or 100 μM each histamine, isoproterenol or NECA for 1 or 5 min depending on the agonist. Samples were pelleted by centrifugation at 13,000 rpm for 1 min at RT. The supernatants were decanted and the cells were lysed in 0.1 M HCl with boiling for 10 min. Samples were centrifuged and supernatants were used to measure cAMP levels via ELISAs from Assay Designs (Ann Arbor, MI). N = minimum of 3 independent experiments, 3 donors in duplicate. * indicates statistical significance using t-test compared to stimulated control, p ≤ 0.05. Data presented here are averages of normalized cAMP values in percent of maximal stimulation. The following are actual values in pmol/mg protein starting with untreated: (A) 3.9, 28.8, 17.8, 3.9, 10.1, 1.0; (B) 4.1, 20.3, 22.5, 4.13, 12.14, 1.49; (C) 15.17, 76.7, 58.9, 37.6, 56.4, 46.1.
3.5. Soluble adenyly cyclase mediates LA stimulated cAMP production
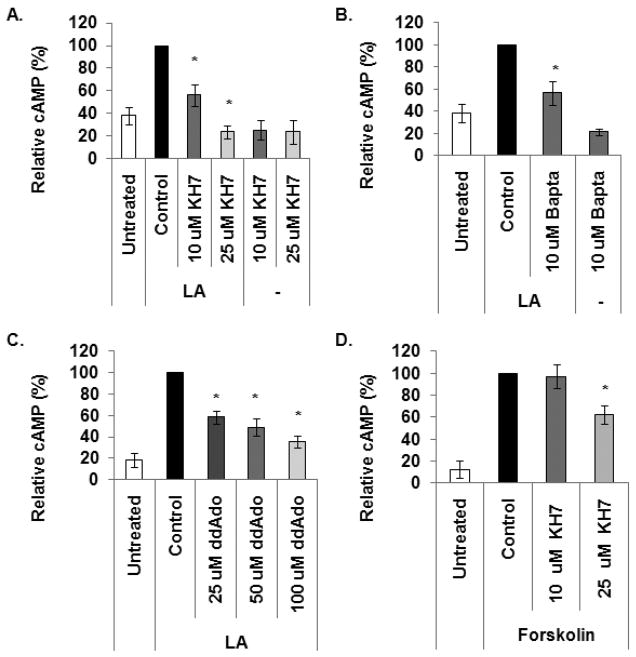
Two distinct types of adenylyl cyclases exist within the cell, transmembrane (tmACs) and the more recently discovered soluble (sACs) adenylyl cyclases [25, 43, 44]. Our data indicates that LA activates tmACs upon binding to the EP2/4 receptors. Here, we examined if LA also activates sACs to generate cAMP via mechanisms independent of the EP receptors. PBMCs were pretreated with vehicle (DMSO), 10 or 25 μM KH7 for 30 minutes prior to treatment with 100 μg/ml LA for 1 minute. cAMP levels were measured as described in methods and materials. KH7 is an inhibitor specific to sAC and is inert to tmACs at concentrations up to 300 μM [25]. As illustrated in Fig. 5A, pretreatment with 10 and 25 μM KH7 resulted in an average reduction of 44% and 80%, respectively, compared to DMSO control. PBMCs were also treated with 10 or 25 μM KH7 in the absence of LA to determine the effects of KH7 on basal cAMP levels (Fig. 5A). KH7, at both concentrations, resulted in modest decreases in basal cAMP levels compared to untreated control, however, the difference is not statistically significant.
Fig. 5. Both soluble and transmembrane adenylyl cyclases mediate LA stimulated cAMP production.
(A) 2 × 106 PBMCs were pretreated with vehicle control (DMSO) or KH7 for 30 minutes prior to stimulation with 100 μg/ml LA for 1 min. Data presented here are averages of normalized cAMP values in percent of maximal stimulation. (B) Cells were pretreated with 10 μM Bapta for 30 minutes prior to stimulation with LA for 1 min. (C) Cells were pretreated with different concentrations of ddAdo for 30 min and stimulated with LA for 1 min. (D) Cells were pretreated with KH7 for 30 min and stimulated with 25 μM forskolin for 1 min. After treatment, samples were pelleted by centrifugation at 13,000 rpm for 1 min at RT. The supernatants were decanted and the cells were lysed in 0.1 M HCl with boiling for 10 min. Samples were centrifuged and supernatants were used to measure cAMP levels via ELISAs from Assay Designs (Ann Arbor, MI). N = 4 independent experiments, 4 donors in duplicate. * indicates statistical significance using t-test compared to LA or forskolin stimulated control, p < 0.05. Data presented here are averages of normalized cAMP values in percent of maximal stimulation. The following are actual values in pmol/mg protein starting with untreated: (A) 11.76, 34.2, 16.7, 6.57, 6.2, 5.4; (B) 11.25, 34, 21.1, 6.5; (C) 14.5, 115.2, 62.2, 43.5, 32.1; (D) 8.58, 120.52, 124.19, 83.19.
Since sAC are activated by calcium, we determined if calcium mediates LA activation of sAC to generate cAMP. PBMCs were pretreated with 10 μM of the membrane permeable calcium chelator, Bapta, for 30 minutes prior to treatment with 100 μg/ml LA for 1 minute. Bapta has a 105-fold greater affinity for Ca2+ than Mg2+ [45, 46]. Pretreatment with Bapta resulted in approximately 44% reduction in cAMP level compared to DMSO control (Fig. 5B). Bapta treatment slightly inhibited production of basal cAMP levels, however, the difference is not statistically significant (p=0.225).
We previously published data showing that 100 μM ddAdo, a p-site inhibitor of transmembrane AC (tmAC), reduced cAMP levels by approximately 75%; and cAMP production was completely inhibited with 250 μM ddAdo. The data indicated that tmAC mediates LA stimulated cAMP levels [7]. However, the data presented here show complete inhibition of cAMP production in PBMCs treated with 25 μM KH7 suggesting that cAMP response to LA is exclusively due to sAC. To reconcile these contrasting data, we treated PBMCs with lower concentrations of ddAdo. It is reported that ddAdo concentrations higher than 50 μM becomes less specific towards tmACs [25]. We treated cells with 25, 50 and 100 μM ddAdo for 30 minutes prior to stimulation with LA. Figure 5C shows that pretreatment with 25, 50, or 100 μM ddAdo resulted in reduction in cAMP levels by 42, 52 and 62%, respectively. This data suggests that tmACs also mediate cAMP production in PBMCs after treatment with LA and that it is not exclusively due to sAC.
Even though Stessin et al. [25] reported that KH7 is selective for sAC at concentrations up to 300 μM, our data (Fig. 5A and C) suggest that KH7 is less specific in PBMCs at 25 μM. To test this, we pretreated PBMCs with 10 or 25 μM KH7 for 30 minutes prior to stimulation with 25 μM forskolin, which activates tmACs to stimulate cAMP production. sAC does not respond to forskolin stimulation [18, 20], therefore KH7 should not have an effect on forskolin stimulated cAMP production. Figure 5D shows that 10 μM KH7 had no effect on cAMP synthesis while pretreatment with 25 μM KH7 reduced forskolin induced cAMP levels by approximately 40%. This indicates that 25 μM KH7 is less specific to sAC in PBMCs leading to inhibition of both sAC and tmACs, thus explaining the complete inhibition of LA stimulated cAMP production observed in Fig. 5A. Since 10 μM KH7 had no effect on tmACs, the partial reduction in cAMP levels (Fig. 5A) in response to LA stimulation is due to inhibition of sAC. This, taken together with the partial inhibition in cAMP levels observed with pretreatment with 25 and 50 μM ddAdo (Fig. 5C), indicates that LA activates both sAC and tmACs in PBMCs.
3.6. Identification of other GPCRs that are activated by LA
It is estimated that approximately 1% of the mammalian genome codes for GPCRs, and hundreds are predicted to exist [47]. Aside from the prostanoid EP receptors, some of the most studied are the histamine, adenosine and β-adrenergic receptors. Together, these receptors are responsible for regulating many physiological functions, including muscle contraction/relaxation, mast cell chemotaxis, immune cell proliferation and cytokine production, neurotransmitter release and calcium mobilization. Here we identified other GPCRs that are activated by LA and narrowed our focus by concentration on these receptors. PBMCs were pre-incubated with vehicle control or antagonists of the histamine, β-adrenergic or adenosine receptors (25 μM famotidine, 25 μM propranolol or 100 μM alloxazine, respectively) for 30 minutes at 37°C. Cells were then treated with 100 μg/ml LA, or as positive controls, 100 μM histamine, isoproterenol or NECA, which are agonists for the histamine, β-adrenergic and adenosine receptors, respectively. As shown in Fig. 6A, pretreatment with 25 μM famotidine reduced LA stimulated cAMP levels by approximately 38% (N = 4 independent experiments). Famotidine treatment inhibited histamine stimulated cAMP levels by almost 81%. Pre-incubation with propranolol decreased cAMP levels by ~78% in isoproterenol stimulated controls, but had no effect on LA treatment (Fig. 6B, N = 3 independent experiments). In Fig. 6C, alloxazine reduced LA and NECA stimulated cAMP level by approximately 22% and 32% respectively (N = 5 independent experiments). The data suggest that LA activates the histamine and adenosine, but not the β-adrenergic receptors. Taken together with figures 4 and 5, these data provide evidence that LA stimulates cAMP levels using mechanisms in addition to stimulation of the EP receptors.
4. Discussion
Commercially available LA supplements are being used as alternative and/or complementary therapeutics for diseases such as Alzheimer’s disease, diabetic polyneuropathy and atherosclerosis. The therapeutic benefit of LA is assumed to be related to its antioxidant properties, when in fact, little is known about the biochemical and cellular mechanisms that mediate the effects of LA in vivo. Toward this end, we first reported the novel finding that LA stimulates the production of cAMP [7, 8], a signaling molecule with potent anti-inflammatory properties. In this study, we expand our initial findings. We discovered that only LA, not its reduced form, DHLA or the derivatives DMLA or LPM, are able to stimulate cAMP production in NK cells (Fig. 1). This is consistent with our data in T cells suggesting that this is not a cell type specific event [8]. The inability of DHLA to stimulate cAMP supports the hypothesis that stimulation of cAMP production is occurring independent of the conversion of LA to DHLA. Furthermore, this notion is strengthen by data obtained from DMLA, whereby the molecule cannot be reduced due to the presence of two methyl groups attached to the sulfur molecule. Taken together with data showing no elevation in cAMP levels after LPM treatment, the results indicate that the closed disulfide pentane ring and carboxy group are necessary for LA to stimulate cAMP production. Given the importance of cAMP as a second messenger involved in the regulation of a large number of genes, we believe the ability of LA to stimulate cAMP may be critical to its therapeutic effects.
We also discovered that there is no significant difference between the R and S isomers of LA or between R-LA and racemic LA in increasing cAMP levels (Fig. 2). There is no consensus on whether or not R-LA is biologically more active than S-LA. A study on the stereoselectivity and specificity of LA for the pyruvate dehydrogenase complex and its component enzymes demonstrated more selectivity for R-LA than S-LA, with R-LA reacting 24 times faster in binding reactions while S-LA exhibited inhibitory effects on R-LA, reducing its biological activity [48]. Similarly, treatment of adipocytes in culture showed higher glucose uptake with R-LA than either S-LA or the racemic mixture [49]. R-LA was more effective at enhancement of aortic flow in rat heart during hypoxia than S-LA [37]. Smith et al. reported that racemic and S-LA were less effective than R-LA in protecting against tertiary butyl hydroperoxide damaged C6 glioma cells [50]. However, the authors reported that all forms of LA protected against hydrogen peroxide toxicity. Investigating lipid peroxidation in both nerve and brain, Nickander et al. found that R and S-LA both reduced peroxidation, and that there was no difference between the two enantiomers [51]. Both R and S-LA protected brain tissue against ischemic damage with similar potency [39]. Other investigators have also reported similar potencies between R and S-LA [52, 53]. As discussed here, the evidence for different or similar functional behavior for R and S-LA are equally compelling. It is unclear what the reason is for these differences, but studies by Smith et al. suggest that whether or not R and S-LA behave in a similar fashion may be dependent on the cell type and/or treatment variables under investigation [50].
The generation of cAMP is historically believed to be due to activation of adenylyl cyclases by ligand-receptor binding of GPCRs, subsequent dissociation of G proteins and activation of tmACs. Our previous data indicate that LA activates tmACs after binding to the prostanoid EP2/EP4 receptors [7, 8]. Here, we determined that LA is a weak competitor of 3HPGE2 for binding of these receptors in transfected HEK 293 EBNA cells (Fig. 3). Although LA and PGE2 are both hydrophobic and can exist in multiple conformers, which likely allow them to bind the same receptors, they are structurally very different. These differences may explain the different binding affinities observed. As discussed by Gether et al., the inactive conformation of GPCRs are stabilized by constraining intramolecular interactions that have been evolutionarily conserved to maintain the receptor preferentially in an inactive conformation in the absence of agonists [54]. Receptor activation requires disruption of these intramolecular interactions by ligands. Ligand-receptor binding occurs by intermolecular forces, such as ionic bonds, hydrogen bonds and Van der Waals forces, which lead to conformational changes in the tertiary structure of the receptor, allowing the receptor to more readily convert from the inactive to active state. The type of interaction is determined by the specific amino acid residues involved in binding [55]. PGE2 has two hydroxyl groups in addition to the carboxylic tail. LA, on the other hand, only has a carboxylic tail. Thus, PGE2 is able to form more interactions with the residues located inside the binding pockets of the EP2/EP4 receptors than LA, which may contribute to more efficient disruption of the native intramolecular interactions.
While LA binds with lower affinity to the EP receptors, LA routinely induces greater cAMP production than PGE2. We also determined that LA and PGE2 have synergistic effects on cAMP production in PBMCs and NK cells. These data suggest two possibilities: 1) LA and PGE2 in combination exhibit cooperative binding behaviors to enhance cAMP production or 2) LA stimulates other pathways in addition to activation of the EP receptors. We will first address the former possibility. Cooperative interactions could occur through binding of identical sites or between multiple independent sites. Binding studies and information obtained from the crystallized structures of rhodopsin, β-adrenergic and adenosine receptors indicate that GPCRs contain a single binding pocket for endogenous ligands [54, 56–59]. However, these studies do not exclude the possibility that other allosteric sites exist on these receptors. While our binding data did not fit to a two binding site competition model, further studies would be necessary to confirm if LA is allosterically binding to a site unique from the PGE2 binding site on the EP receptors. Alternatively, LA may be stimulating additional pathways. The synergistic effects of LA and PGE2 are likely attributable to LA activating other pathways in addition to the EP receptors. The following sections will discuss the evidence to support this theory.
Recent identification of a second pool of ACs (sACs) presented a possible explanation for the effects of LA on cAMP production in vivo. By blocking sAC activation using the specific inhibitor KH7, we discovered that sACs mediate LA stimulation of cAMP production (Fig. 5). This is the first data showing that sAC mediates cAMP production in PBMCs and NK cells (data not shown) and that sAC is activated by LA. Our data is consistent with reports by Stessin et al. showing reduction in cAMP synthesis in PC12 cells treated with nerve growth factor [25]. Similar results were found in human neutrophils treated with ionomycin [60]. sAC was initially characterized in mammalian sperm, but is now known to be expressed in a variety of tissues including brain, liver, heart, spleen, epithelial cells and neutrophils [21, 60]. In addition to regulating sperm function, sAC is implicated in mediating neuronal differentiation and fast migration [25, 61], tumor necrosis factor (TNF) signal transduction to inhibit TNF induced hydrogen peroxide release [60], and regulation of ciliary beat frequency in the airway [62]. sAC is localized at multiple, subcellular compartments throughout the cell [22] suggesting that there may be independently modulated cAMP signaling microdomains, which contribute to the specificity of cAMP signaling [63]. It will be interesting to see if the cAMP signaling microdomains determine the specificity of cellular responses to LA treatment.
We next identified other GPCRs that may be activated by LA. We narrowed our research by focusing on some of the most studied receptors, including histamine, adenosine and β-adrenergic receptors, specifically the subtypes that are coupled to stimulatory G-proteins and cAMP signaling. Using pharmacological inhibitors to prevent receptor binding, we discovered that LA activates histamine and adenosine, but not β-adrenergic receptors (Fig. 6). The histamine receptors play important roles in regulating inflammation. Activation of H2 receptors on peripheral monocytes potently suppresses interleukin (IL)-12 and stimulates IL-10 production [64], which may shift from Th1 to Th2 immune response. In autoimmune disorders such as arthritis and multiple sclerosis (MS), activation of Th1 is thought to be pathogenic while Th2 response is protective. This may explain why LA treatment is effective in the animal model of arthritis and MS. However, the shift to a Th2 response is implicated in promoting allergic reactions and tumorigenesis [64–66]. Whether LA promotes a shift to Th2 immune response, and whether or not this has detrimental consequences remain to be elucidated.
Activation of the adenosine receptors have also been shown to be important in inflammation. Takahashi et al. showed that treatment with adenosine resulted in inhibition of IL-18 induced intercellular adhesion molecule (ICAM)-1 expression in monocytes and production of the proinflammatory cytokines IL-12, TNF-α and interferon (IFN)-γ by PBMC [67]. Adenosine has also been shown to inhibit cytotoxic activity and cytokine production in NK cells [68, 69]. Similarly, LA treatment has been shown to reduce ICAM-1 and vascular cell adhesion molecule (VCAM)-1 expression in spinal cords and stimulated murine brain endothelial cells [4], inhibit VCAM-1 and endothelial adhesion of human monocytes [5], down modulate CD4 expression from human T cells [6] and inhibit IFN-γ production and cytotoxic activity in human NK cells [7]. Our data suggest that activation of the adenosine receptors may mediate the anti-inflammatory effects of LA. It will be exciting to test this hypothesis in the future.
In summary, we provide novel evidence that LA, not its derivatives, is responsible for stimulating cAMP production, weakly competes for EP2/EP4 binding, and activates sAC, histamine and adenosine receptors. These data indicate that LA utilizes many mechanisms to generate cAMP. Activation of the histamine receptor suggests that LA supplementation may not be completely without long term side effects and that some cautionary approaches may be necessary to prevent potential problems such as the development of allergies and tumors. These data provide a foundation for future studies to determine the mechanisms by which LA can be beneficial in human health and identify any potential long term issues.
Acknowledgments
We would like to thank Sarah Fiedler for helpful critique of the manuscript. This research was supported by the Department of Veterans Affairs Biomedical Laboratory Research & Development Service (D.W.C. and D.N.B.), NIH Grant P50AT00066-01 (D.N.B.), the Nancy Davis Center Without Walls (D.N.B.) and the Collins Medical Trust (S.S.). We would also like to thank Dr. David Carlson of GeroNova (Carson City, Nevada) who provided R-LA for these studies.
Funding sources: This research was supported by the Department of Veterans Affairs Biomedical Laboratory Research & Development Service (D.W.C. and D.N.B.), NIH Grant P50AT00066-01 (D.N.B.), The National MS Society CRG CA1055-A-3 (D.N.B.) the Laura Fund for Innovation in Multiple Sclerosis Research (D.N.B.), the Nancy Davis Center Without Walls (D.N.B.), and the Collins Medical Trust (S.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morikawa T, Yasuno R, Wada H. Do mammalian cells synthesize lipoic acid? Identification of a mouse cDNA encoding a lipoic acid synthase located in mitochondria. FEBS Lett. 2001;498:16–21. doi: 10.1016/s0014-5793(01)02469-3. [DOI] [PubMed] [Google Scholar]
- 2.Whiteman M, Tritschler H, Halliwell B. Protection against peroxynitrite-dependent tyrosine nitration and alpha 1-antiproteinase inactivation by oxidized and reduced lipoic acid. FEBS Lett. 1996;379:74–6. doi: 10.1016/0014-5793(95)01489-6. [DOI] [PubMed] [Google Scholar]
- 3.Maitra I, Serbinova E, Trischler H, Packer L. Alpha-lipoic acid prevents buthionine sulfoximine-induced cataract formation in newborn rats. Free Radic Biol Med. 1995;18:823–9. doi: 10.1016/0891-5849(94)00195-p. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhary P, Marracci GH, Bourdette DN. Lipoic acid inhibits expression of ICAM-1 and VCAM-1 by CNS endothelial cells and T cell migration into the spinal cord in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2006;175:87–96. doi: 10.1016/j.jneuroim.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Kunt T, Forst T, Wilhelm A, Tritschler H, Pfuetzner A, Harzer O, et al. Alpha-lipoic acid reduces expression of vascular cell adhesion molecule-1 and endothelial adhesion of human monocytes after stimulation with advanced glycation end products. Clin Sci (Lond) 1999;96:75–82. [PubMed] [Google Scholar]
- 6.Marracci GH, Marquardt WE, Strehlow A, McKeon GP, Gross J, Buck DC, et al. Lipoic acid downmodulates CD4 from human T lymphocytes by dissociation of p56(Lck) Biochem Biophys Res Commun. 2006;344:963–71. doi: 10.1016/j.bbrc.2006.03.172. [DOI] [PubMed] [Google Scholar]
- 7.Salinthone S, Schillace RV, Marracci GH, Bourdette DN, Carr DW. Lipoic acid stimulates cAMP production via the EP2 and EP4 prostanoid receptors and inhibits IFN gamma synthesis and cellular cytotoxicity in NK cells. J Neuroimmunol. 2008;199:46–55. doi: 10.1016/j.jneuroim.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schillace RV, Pisenti N, Pattamanuch N, Galligan S, Marracci GH, Bourdette DN, et al. Lipoic acid stimulates cAMP production in T lymphocytes and NK cells. Biochem Biophys Res Commun. 2007;354:259–64. doi: 10.1016/j.bbrc.2006.12.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen CH, Zhang DH, LaPorte JM, Ray A. Cyclic AMP activates p38 mitogen-activated protein kinase in Th2 cells: phosphorylation of GATA-3 and stimulation of Th2 cytokine gene expression. J Immunol. 2000;165:5597–605. doi: 10.4049/jimmunol.165.10.5597. [DOI] [PubMed] [Google Scholar]
- 10.Brosens JJ, Gellersen B. Death or survival--progesterone-dependent cell fate decisions in the human endometrial stroma. J Mol Endocrinol. 2006;36:389–98. doi: 10.1677/jme.1.02060. [DOI] [PubMed] [Google Scholar]
- 11.Schillace RV, Carr DW. The role of protein kinase A and A-kinase anchoring proteins in modulating T-cell activation: progress and future directions. Crit Rev Immunol. 2006;26:113–31. doi: 10.1615/critrevimmunol.v26.i2.20. [DOI] [PubMed] [Google Scholar]
- 12.Moore AR, Willoughby DA. The role of cAMP regulation in controlling inflammation. Clin Exp Immunol. 1995;101:387–9. doi: 10.1111/j.1365-2249.1995.tb03123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joshi PC, Zhou X, Cuchens M, Jones Q. Prostaglandin E2 suppressed IL-15-mediated human NK cell function through down-regulation of common gamma-chain. J Immunol. 2001;166:885–91. doi: 10.4049/jimmunol.166.2.885. [DOI] [PubMed] [Google Scholar]
- 14.Kuklina EM, Shirshev SV. Role of cAMP-dependent signal transduction in the control of T lymphocyte activation. Biochemistry (Mosc) 2000;65:629–39. [PubMed] [Google Scholar]
- 15.Roder JC, Argov S, Klein M, Petersson C, Kiessling R, Andersson K, et al. Target-effector cell interaction in the natural killer cell system. V. Energy requirements, membrane integrity, and the possible involvement of lysosomal enzymes. Immunology. 1980;40:107–16. [PMC free article] [PubMed] [Google Scholar]
- 16.McPhee I, Gibson LC, Kewney J, Darroch C, Stevens PA, Spinks D, et al. Cyclic nucleotide signalling: a molecular approach to drug discovery for Alzheimer’s disease. Biochem Soc Trans. 2005;33:1330–2. doi: 10.1042/BST0331330. [DOI] [PubMed] [Google Scholar]
- 17.Osadchii OE. Myocardial phosphodiesterases and regulation of cardiac contractility in health and cardiac disease. Cardiovasc Drugs Ther. 2007;21:171–94. doi: 10.1007/s10557-007-6014-6. [DOI] [PubMed] [Google Scholar]
- 18.Buck J, Sinclair ML, Schapal L, Cann MJ, Levin LR. Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proc Natl Acad Sci U S A. 1999;96:79–84. doi: 10.1073/pnas.96.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neves SR, Ram PT, Iyengar R. G protein pathways. Science. 2002;296:1636–9. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, et al. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science. 2000;289:625–8. doi: 10.1126/science.289.5479.625. [DOI] [PubMed] [Google Scholar]
- 21.Sinclair ML, Wang XY, Mattia M, Conti M, Buck J, Wolgemuth DJ, et al. Specific expression of soluble adenylyl cyclase in male germ cells. Mol Reprod Dev. 2000;56:6–11. doi: 10.1002/(SICI)1098-2795(200005)56:1<6::AID-MRD2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 22.Zippin JH, Chen Y, Nahirney P, Kamenetsky M, Wuttke MS, Fischman DA, et al. Compartmentalization of bicarbonate-sensitive adenylyl cyclase in distinct signaling microdomains. Faseb J. 2003;17:82–4. doi: 10.1096/fj.02-0598fje. [DOI] [PubMed] [Google Scholar]
- 23.Jaiswal BS, Conti M. Calcium regulation of the soluble adenylyl cyclase expressed in mammalian spermatozoa. Proc Natl Acad Sci U S A. 2003;100:10676–81. doi: 10.1073/pnas.1831008100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Litvin TN, Kamenetsky M, Zarifyan A, Buck J, Levin LR. Kinetic properties of “soluble” adenylyl cyclase. Synergism between calcium and bicarbonate. J Biol Chem. 2003;278:15922–6. doi: 10.1074/jbc.M212475200. [DOI] [PubMed] [Google Scholar]
- 25.Stessin AM, Zippin JH, Kamenetsky M, Hess KC, Buck J, Levin LR. Soluble adenylyl cyclase mediates nerve growth factor-induced activation of Rap1. J Biol Chem. 2006;281:17253–8. doi: 10.1074/jbc.M603500200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujino H, West KA, Regan JW. Phosphorylation of glycogen synthase kinase-3 and stimulation of T-cell factor signaling following activation of EP2 and EP4 prostanoid receptors by prostaglandin E2. J Biol Chem. 2002;277:2614–9. doi: 10.1074/jbc.M109440200. [DOI] [PubMed] [Google Scholar]
- 27.Sugimoto Y, Negishi M, Hayashi Y, Namba T, Honda A, Watabe A, et al. Two isoforms of the EP3 receptor with different carboxyl-terminal domains. Identical ligand binding properties and different coupling properties with Gi proteins. J Biol Chem. 1993;268:2712–8. [PubMed] [Google Scholar]
- 28.Marracci GH, Jones RE, McKeon GP, Bourdette DN. Alpha lipoic acid inhibits T cell migration into the spinal cord and suppresses and treats experimental autoimmune encephalomyelitis. J Neuroimmunol. 2002;131:104–14. doi: 10.1016/s0165-5728(02)00269-2. [DOI] [PubMed] [Google Scholar]
- 29.Marracci GH, McKeon GP, Marquardt WE, Winter RW, Riscoe MK, Bourdette DN. Alpha lipoic acid inhibits human T-cell migration: implications for multiple sclerosis. J Neurosci Res. 2004;78:362–70. doi: 10.1002/jnr.20255. [DOI] [PubMed] [Google Scholar]
- 30.Morini M, Roccatagliata L, Dell’Eva R, Pedemonte E, Furlan R, Minghelli S, et al. Alpha-lipoic acid is effective in prevention and treatment of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2004;148:146–53. doi: 10.1016/j.jneuroim.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 31.Schreibelt G, Musters RJ, Reijerkerk A, de Groot LR, van der Pol SM, Hendrikx EM, et al. Lipoic acid affects cellular migration into the central nervous system and stabilizes blood-brain barrier integrity. J Immunol. 2006;177:2630–7. doi: 10.4049/jimmunol.177.4.2630. [DOI] [PubMed] [Google Scholar]
- 32.Carlson DA, Smith AR, Fischer SJ, Young KL, Packer L. The plasma pharmacokinetics of R-(+)-lipoic acid administered as sodium R-(+)-lipoate to healthy human subjects. Altern Med Rev. 2007;12:343–51. [PubMed] [Google Scholar]
- 33.Hagen TM, Vinarsky V, Wehr CM, Ames BN. (R)-alpha-lipoic acid reverses the age-associated increase in susceptibility of hepatocytes to tert-butylhydroperoxide both in vitro and in vivo. Antioxid Redox Signal. 2000;2:473–83. doi: 10.1089/15230860050192251. [DOI] [PubMed] [Google Scholar]
- 34.Harrison EH, McCormick DB. The metabolism of dl-(1,6–14C)lipoic acid in the rat. Arch Biochem Biophys. 1974;160:514–22. doi: 10.1016/0003-9861(74)90428-7. [DOI] [PubMed] [Google Scholar]
- 35.Hermann R, Wildgrube HJ, Ruus P, Niebch G, Nowak H, Gleiter CH. Gastric emptying in patients with insulin dependent diabetes mellitus and bioavailability of thioctic acid-enantiomers. Eur J Pharm Sci. 1998;6:27–37. doi: 10.1016/s0928-0987(97)00065-1. [DOI] [PubMed] [Google Scholar]
- 36.Teichert J, Kern J, Tritschler HJ, Ulrich H, Preiss R. Investigations on the pharmacokinetics of alpha-lipoic acid in healthy volunteers. Int J Clin Pharmacol Ther. 1998;36:625–8. [PubMed] [Google Scholar]
- 37.Zimmer G, Beikler TK, Schneider M, Ibel J, Tritschler H, Ulrich H. Dose/response curves of lipoic acid R-and S-forms in the working rat heart during reoxygenation: superiority of the R-enantiomer in enhancement of aortic flow. J Mol Cell Cardiol. 1995;27:1895–903. doi: 10.1016/0022-2828(95)90012-8. [DOI] [PubMed] [Google Scholar]
- 38.Streeper RS, Henriksen EJ, Jacob S, Hokama JY, Fogt DL, Tritschler HJ. Differential effects of lipoic acid stereoisomers on glucose metabolism in insulin-resistant skeletal muscle. Am J Physiol. 1997;273:E185–91. doi: 10.1152/ajpendo.1997.273.1.E185. [DOI] [PubMed] [Google Scholar]
- 39.Wolz P, Krieglstein J. Neuroprotective effects of alpha-lipoic acid and its enantiomers demonstrated in rodent models of focal cerebral ischemia. Neuropharmacology. 1996;35:369–75. doi: 10.1016/0028-3908(95)00172-7. [DOI] [PubMed] [Google Scholar]
- 40.Jones W, Li X, Qu ZC, Perriott L, Whitesell RR, May JM. Uptake, recycling, and antioxidant actions of alpha-lipoic acid in endothelial cells. Free Radic Biol Med. 2002;33:83–93. doi: 10.1016/s0891-5849(02)00862-6. [DOI] [PubMed] [Google Scholar]
- 41.Salinthone S, Yadav V, Bourdette DN, Carr DW. Lipoic acid: a novel therapeutic approach for multiple sclerosis and other chronic inflammatory diseases of the CNS. Endocr Metab Immune Disord Drug Targets. 2008;8:132–42. doi: 10.2174/187153008784534303. [DOI] [PubMed] [Google Scholar]
- 42.Shay KP, Moreau RF, Smith EJ, Smith AR, Hagen TM. Alpha-lipoic acid as a dietary supplement: molecular mechanisms and therapeutic potential. Biochim Biophys Acta. 2009;1790:1149–60. doi: 10.1016/j.bbagen.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pavan B, Biondi C, Dalpiaz A. Adenylyl cyclases as innovative therapeutic goals. Drug Discov Today. 2009;14:982–91. doi: 10.1016/j.drudis.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 44.Sunahara RK, Taussig R. Isoforms of mammalian adenylyl cyclase: multiplicities of signaling. Mol Interv. 2002;2:168–84. doi: 10.1124/mi.2.3.168. [DOI] [PubMed] [Google Scholar]
- 45.Harrison SM, Bers DM. The effect of temperature and ionic strength on the apparent Ca-affinity of EGTA and the analogous Ca-chelators BAPTA and dibromo-BAPTA. Biochim Biophys Acta. 1987;925:133–43. doi: 10.1016/0304-4165(87)90102-4. [DOI] [PubMed] [Google Scholar]
- 46.Tsien RY, Pozzan T, Rink TJ. T-cell mitogens cause early changes in cytoplasmic free Ca2+ and membrane potential in lymphocytes. Nature. 1982;295:68–71. doi: 10.1038/295068a0. [DOI] [PubMed] [Google Scholar]
- 47.Hur EM, Kim KT. G protein-coupled receptor signalling and cross-talk: achieving rapidity and specificity. Cell Signal. 2002;14:397–405. doi: 10.1016/s0898-6568(01)00258-3. [DOI] [PubMed] [Google Scholar]
- 48.Loffelhardt S, Bonaventura C, Locher M, Borbe HO, Bisswanger H. Interaction of alpha-lipoic acid enantiomers and homologues with the enzyme components of the mammalian pyruvate dehydrogenase complex. Biochem Pharmacol. 1995;50:637–46. doi: 10.1016/0006-2952(95)00175-y. [DOI] [PubMed] [Google Scholar]
- 49.Estrada DE, Ewart HS, Tsakiridis T, Volchuk A, Ramlal T, Tritschler H, et al. Stimulation of glucose uptake by the natural coenzyme alpha-lipoic acid/thioctic acid: participation of elements of the insulin signaling pathway. Diabetes. 1996;45:1798–804. doi: 10.2337/diab.45.12.1798. [DOI] [PubMed] [Google Scholar]
- 50.Smith JR, Thiagaraj HV, Seaver B, Parker KK. Differential activity of lipoic acid enantiomers in cell culture. J Herb Pharmacother. 2005;5:43–54. [PubMed] [Google Scholar]
- 51.Nickander KK, McPhee BR, Low PA, Tritschler H. Alpha-lipoic acid: antioxidant potency against lipid peroxidation of neural tissues in vitro and implications for diabetic neuropathy. Free Radic Biol Med. 1996;21:631–9. doi: 10.1016/0891-5849(96)00172-4. [DOI] [PubMed] [Google Scholar]
- 52.Dimpfel W, Spuler M, Pierau FK, Ulrich H. Thioctic acid induces dose-dependent sprouting of neurites in cultured rat neuroblastoma cells. Dev Pharmacol Ther. 1990;14:193–9. [PubMed] [Google Scholar]
- 53.Podda M, Tritschler HJ, Ulrich H, Packer L. Alpha-lipoic acid supplementation prevents symptoms of vitamin E deficiency. Biochem Biophys Res Commun. 1994;204:98–104. doi: 10.1006/bbrc.1994.2431. [DOI] [PubMed] [Google Scholar]
- 54.Gether U, Asmar F, Meinild AK, Rasmussen SG. Structural basis for activation of G-protein-coupled receptors. Pharmacol Toxicol. 2002;91:304–12. doi: 10.1034/j.1600-0773.2002.910607.x. [DOI] [PubMed] [Google Scholar]
- 55.Ma B, Shatsky M, Wolfson HJ, Nussinov R. Multiple diverse ligands binding at a single protein site: a matter of pre-existing populations. Protein Sci. 2002;11:184–97. doi: 10.1110/ps.21302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: subtypes and signaling. Annu Rev Pharmacol Toxicol. 2001;41:661–90. doi: 10.1146/annurev.pharmtox.41.1.661. [DOI] [PubMed] [Google Scholar]
- 57.Jaakola VP, Griffith MT, Hanson MA, Cherezov V, Chien EY, Lane JR, et al. The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science. 2008;322:1211–7. doi: 10.1126/science.1164772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oliveira L, Paiva AC, Vriend G. A low resolution model for the interaction of G proteins with G protein-coupled receptors. Protein Eng. 1999;12:1087–95. doi: 10.1093/protein/12.12.1087. [DOI] [PubMed] [Google Scholar]
- 59.Stillman BA, Audoly L, Breyer RM. A conserved threonine in the second extracellular loop of the human EP2 and EP4 receptors is required for ligand binding. Eur J Pharmacol. 1998;357:73–82. doi: 10.1016/s0014-2999(98)00522-6. [DOI] [PubMed] [Google Scholar]
- 60.Han H, Stessin A, Roberts J, Hess K, Gautam N, Kamenetsky M, et al. Calcium-sensing soluble adenylyl cyclase mediates TNF signal transduction in human neutrophils. J Exp Med. 2005;202:353–61. doi: 10.1084/jem.20050778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Young JJ, Mehdi A, Stohl LL, Levin LR, Buck J, Wagner JA, et al. “Soluble” adenylyl cyclase-generated cyclic adenosine monophosphate promotes fast migration in PC12 cells. J Neurosci Res. 2008;86:118–24. doi: 10.1002/jnr.21458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schmid A, Sutto Z, Nlend MC, Horvath G, Schmid N, Buck J, et al. Soluble adenylyl cyclase is localized to cilia and contributes to ciliary beat frequency regulation via production of cAMP. J Gen Physiol. 2007;130:99–109. doi: 10.1085/jgp.200709784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zippin JH, Farrell J, Huron D, Kamenetsky M, Hess KC, Fischman DA, et al. Bicarbonate-responsive “soluble” adenylyl cyclase defines a nuclear cAMP microdomain. J Cell Biol. 2004;164:527–34. doi: 10.1083/jcb.200311119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Elenkov IJ, Webster E, Papanicolaou DA, Fleisher TA, Chrousos GP, Wilder RL. Histamine potently suppresses human IL-12 and stimulates IL-10 production via H2 receptors. J Immunol. 1998;161:2586–93. [PubMed] [Google Scholar]
- 65.Chen Q, Daniel V, Maher DW, Hersey P. Production of IL-10 by melanoma cells: examination of its role in immunosuppression mediated by melanoma. Int J Cancer. 1994;56:755–60. doi: 10.1002/ijc.2910560524. [DOI] [PubMed] [Google Scholar]
- 66.Karttunen RA, Karttunen TJ, Yousfi MM, el-Zimaity HM, Graham DY, el-Zaatari FA. Expression of mRNA for interferon-gamma, interleukin-10, and interleukin-12 (p40) in normal gastric mucosa and in mucosa infected with Helicobacter pylori. Scand J Gastroenterol. 1997;32:22–7. doi: 10.3109/00365529709025058. [DOI] [PubMed] [Google Scholar]
- 67.Takahashi HK, Iwagaki H, Hamano R, Wake H, Kanke T, Liu K, et al. Effects of adenosine on adhesion molecule expression and cytokine production in human PBMC depend on the receptor subtype activated. Br J Pharmacol. 2007;150:816–22. doi: 10.1038/sj.bjp.0707126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lokshin A, Raskovalova T, Huang X, Zacharia LC, Jackson EK, Gorelik E. Adenosine-mediated inhibition of the cytotoxic activity and cytokine production by activated natural killer cells. Cancer Res. 2006;66:7758–65. doi: 10.1158/0008-5472.CAN-06-0478. [DOI] [PubMed] [Google Scholar]
- 69.Raskovalova T, Lokshin A, Huang X, Jackson EK, Gorelik E. Adenosine-mediated inhibition of cytotoxic activity and cytokine production by IL-2/NKp46-activated NK cells: involvement of protein kinase A isozyme I (PKA I) Immunol Res. 2006;36:91–9. doi: 10.1385/IR:36:1:91. [DOI] [PubMed] [Google Scholar]