Abstract
We performed experiments to determine the effect of PKR activation on respiratory syncytial virus (RSV) replication. We first determined that RSV infection activates PKR which induces the phosphorylation of eIF2α, resulting in the formation of host stress granules. We used RNA interference to decrease endogenous PKR levels. RSV replication was not altered in cells deficient for PKR expression. However, RSV-mediated stress granule formation was significantly reduced in PKR-knockdown cells. As an alternative method to block PKR activation, we used treatment with the kinase inhibitor 2-aminopurine (2-AP). We observed that 2-AP treatment significantly reduced viral replication. We also treated PKR-knockdown cells with 2-AP and inoculated with RSV. Under these conditions, 2-AP treatment diminished viral replication in the absence of PKR expression. These results suggest that PKR activation has a minimal effect on RSV replication and that the antiviral effect of 2-AP during RSV infection likely occurs via a PKR-independent mechanism.
Keywords: Respiratory syncytial viruses, eIF-2 kinase, 2-Aminopurine, Paramyxovirinae, RNA viruses
Introduction
Respiratory syncytial virus (RSV) infection is a leading cause of serious viral lower respiratory tract illness in both infants and the elderly worldwide. RSV is a member of the Paramyxoviridae family and belongs to the Pneumovirinae subfamily. The RSV genome consists of a single-stranded, negative-sense RNA molecule that encodes 11 proteins. The viral nucleoprotein (N), phosphoprotein (P), and the large polymerase protein (L) make up the ribonucleoprotein complex that is necessary for viral transcription and replication. Each of these replication complex proteins is found, along with genomic viral RNA, in discrete cytoplasmic granules often termed viral inclusion bodies (Garcia et al., 1993; Carromeu et al., 2007; Lindquist et al., 2010). These inclusion bodies are thought to represent sites of viral replication. While transcription of viral proteins likely occurs immediately after entry into host cells, detectable replication of the viral genome appears to begin several hours after inoculation. Following accumulation of sufficient amounts of viral proteins, a transition occurs in the RSV replication program from one dominated by transcription of viral genes to one in which replication of the full-length genome predominates. As is the case for all negative-sense single-stranded RNA viruses, for replication to occur, the virus must first make a full-length antigenome intermediate RNA, which then serves as template for the production of new RNA genomes (Cowton et al., 2006).
PKR is activated by double-stranded RNA (dsRNA) during viral infection and often is associated with antiviral host cell responses. Upon binding to dsRNA, PKR dimerizes and is autophosphorylated, resulting in activation of the protein. Phosphorylated PKR is one of the four known kinases that regulates the activation of the translation initiation factor eukaryotic initiation factor 2 (eIF2α), which also include PKR-like ER-localized eIF2α kinase (PERK), heme-regulated inhibitor (HRI) kinase, and general control nonrepressed 2 (GCN2) kinase. Upon binding to eIF2α, activated PKR phosphorylates eIF2α at serine 51. Phosphorylated eIF2α is incapable of delivering initiator Met-tRNA to host translation complexes, resulting in a reduction of protein synthesis in virus-infected cells. eIF2α phosphorylation also leads to the formation of host stress granules, which are host RNA cytoplasmic granules that contain mRNA, translation factors, and mRNA-binding proteins. In addition to activating eIF2α, PKR also functions in the activation of other proteins such as STAT1, p53, and NFκB (Garcia et al., 2007).
Many viruses deploy mechanisms to prevent PKR activation, presumably to inhibit the type I interferon response or to ensure that the host translation machinery remains sufficiently active for viral protein synthesis. Viral strategies to prevent PKR activation include expression of viral products that interact directly with PKR and prevent activation, expression of viral proteins that bind and sequester dsRNA, or activation of host proteins that inhibit or counteract PKR activation (Garcia et al., 2007). In virtually every case studied to date, the activation of PKR in virus-infected cells is associated with induction of an antiviral state. However, activation of PKR during hepatitis C virus (HCV) infection may enhance viral replication because of the resulting lack of synthesis of specific antiviral interferon-stimulated gene products during infection (Garaigorta and Chisari, 2009).
RSV infection leads to increased levels of total PKR in cells during infection (Groskreutz et al., 2006). RSV infection also can induce the phosphorylation and activation of PKR (Groskreutz et al., 2010). These studies suggest a direct interaction between PKR and the RSV nucleoprotein (N).
In the current study, we sought to determine the functional role of PKR expression and activation in response to RSV infection using two well-established methods of PKR inhibition. The data reveal that 2-aminopurine (2-AP), a previously defined PKR chemical inhibitor, reduced RSV replication. Unexpectedly PKR protein knockdown by shRNA did not affect RSV replication. However, we did observe a drastic decrease in stress granule formation in PKR-knockdown cells. These results suggest that PKR expression and activation have little effect on RSV replication in cultured cells, and that the PKR inhibitor 2-AP likely inhibits RSV replication in a manner independent of PKR.
Results
RSV induces the activation of PKR and eIF2α
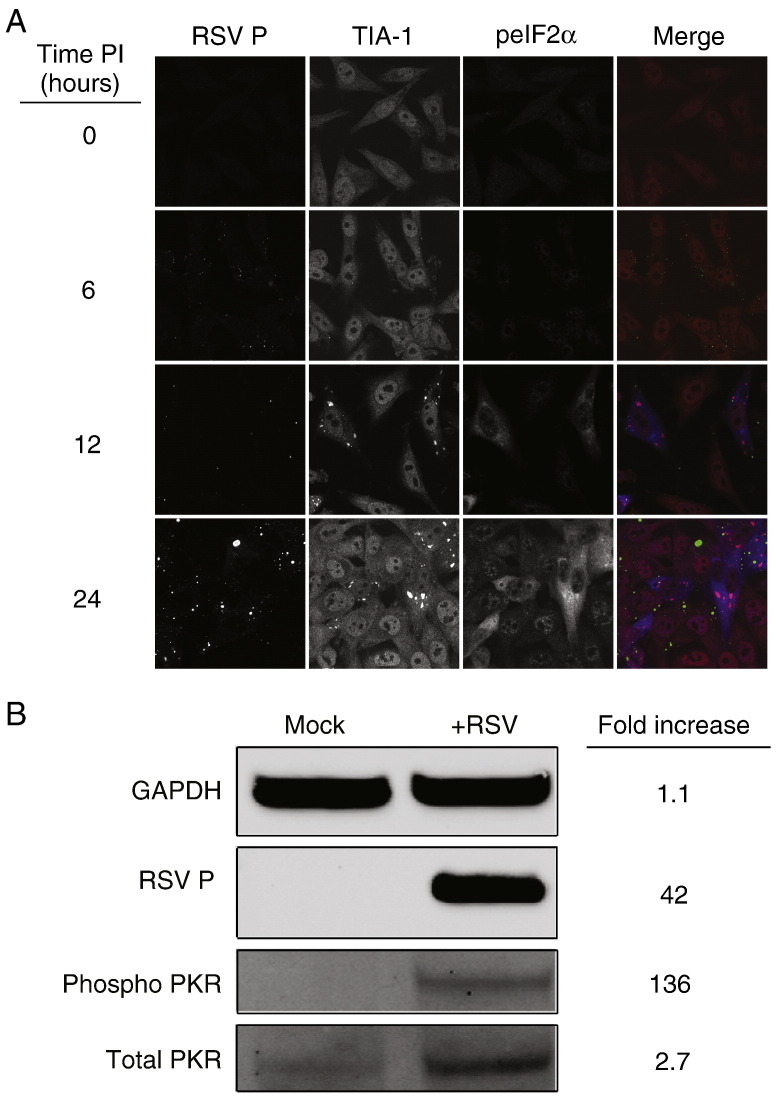
RSV infection potently induces stress granule formation (Lindquist et al., 2010). We sought here to determine the mechanism by which RSV initiates this process. Typically, stress granules form following induction of one of many types of stress-related pathways that result in a common downstream event, phosphorylation of the translation initiation factor, eIF2α (Kedersha and Anderson, 2007). Therefore, we examined cells inoculated with RSV (MOI = 1.0 pfu/cell) for the presence of phosphorylated eIF2α using indirect immunofluorescence. We observed a striking increase in eIF2α phosphorylation beginning 12 h post-inoculation that was concomitant with the appearance of stress granules and continued throughout infection (Fig. 1A).
Fig. 1.
PKR and eIF2α are phosphorylated during RSV infection. (A) HEp-2 cells were infected with RSV (MOI = 1.0 pfu/cell) for the indicated times, fixed, and processed for immunofluorescence. Anti-RSV P monoclonal antibody was used to localize viral protein and appears green in the merge panel. Anti-TIA-1 antibody was used to detect stress granule formation and appears red in the merge panel. Phosphorylated eIF2α (peIF2α) appears blue in the merge panel. (B) HEp-2 cells were mock-inoculated or inoculated with RSV (MOI = 1.0 pfu/cell) for 24 h. Cell lysates were analyzed by immunoblots for GAPDH, RSV P, phosphorylated PKR, or total PKR. Relative protein densities comparing RSV-infected cells to mock-infected cells were quantified using Li-Cor Odyssey imaging software.
We next sought to determine the mechanism by which RSV mediates the phosphorylation of eIF2α. There are four well-defined kinases capable of eIF2α phosphorylation: protein kinase RNA (PKR), a kinase activated by double-stranded RNA; PKR-like ER-localized eIF2α kinase (PERK); general control nonrepressed 2 (GCN2); and heme-regulated inhibitor kinase (HRI) (Proud, 2005). We reasoned that PKR was likely responsible for eIF2α phosphorylation during RSV infection, as it is known that RSV infection activates PKR (Groskreutz et al., 2010). In cells inoculated with RSV (MOI = 1.0 pfu/cell) for 24 h, we observed phosphorylated PKR and higher expression of total PKR in comparison to mock-inoculated cells (Fig. 1B), consistent with previous reports.
Knockdown of PKR expression does not affect RSV replication
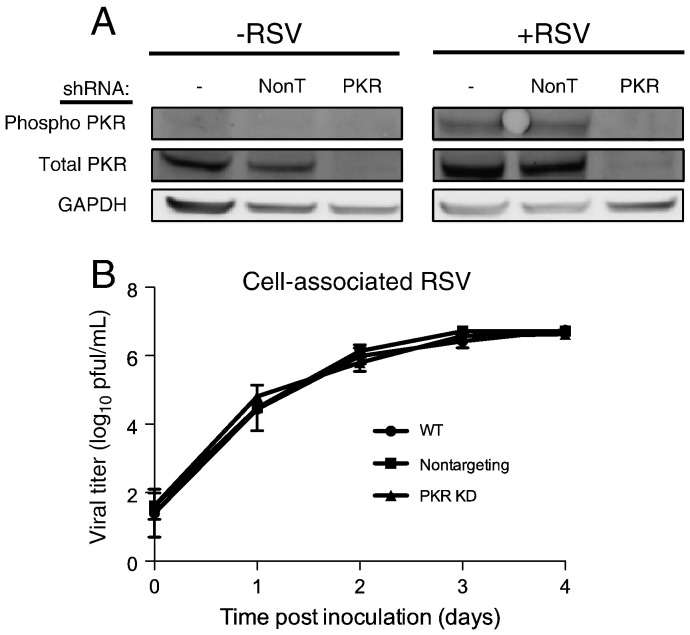
We investigated what effects inhibition of PKR signaling might have on RSV replication. HEp-2 cells were transduced with shRNA lentiviral constructs specific for PKR or a non-targeting shRNA control. We selected for cells stably expressing the shRNA constructs and we compared PKR levels by immunoblotting in wild-type cells that were not transduced or cells transduced with PKR-targeting or non-targeting shRNA. Cells expressing the PKR shRNA construct exhibited an approximate 90% decrease in total PKR protein levels (Fig. 2A) in comparison to wild-type cells or those expressing a non-targeting shRNA. Wild-type, non-targeting, or PKR-knockdown cells were inoculated with RSV (MOI = 1.0 pfu/cell) and incubated for 24 h. Immunoblot analysis of cell lysates did not demonstrate an increase in PKR phosphorylation in the RSV-inoculated PKR-knockdown cells in comparison to wild-type or non-targeting shRNA cells. We next quantified the viral titers over a time course for each cell type. Cells were inoculated with RSV (MOI = 0.1 pfu/cell) and incubated for 0–4 days. Cell-associated virus was harvested for each time point, and titers were determined by plaque assay. Viral titers in the PKR-knockdown cells did not differ significantly from those in either wild-type cells or non-targeting shRNA-expressing cells (Fig. 2B). These results indicate that inhibition of PKR activation does not affect RSV replication.
Fig. 2.
PKR knockdown does not affect RSV replication. (A) Wild-type HEp-2 cells or HEp-2 cells transduced with non-targeting shRNA (NonT) or PKR-specific shRNA were mock-inoculated or inoculated with RSV (MOI = 1.0 pfu/cell) for 24 h. Cell lysates were analyzed by immunoblotting for total PKR, phosphorylated PKR, or GAPDH. (B) Each cell type from (A) was inoculated with RSV (MOI = 0.1 pfu/cell) for the indicated times. Titers of cell-associated virus were determined for each time point by plaque assay. Error bars indicate standard deviations.
Knockdown of PKR prevents formation of RSV-induced stress granules during infection
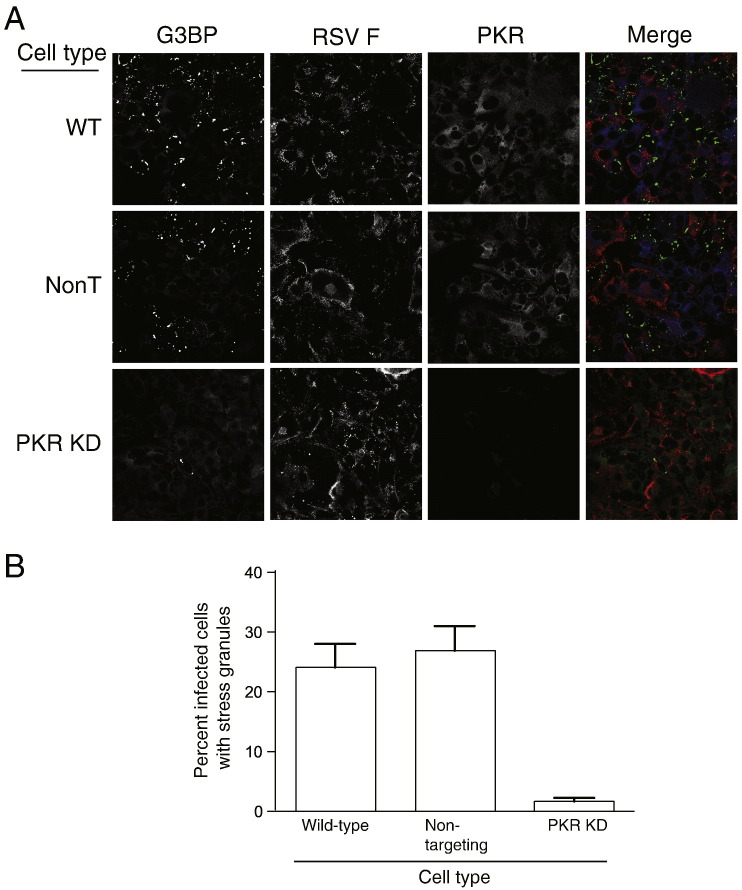
We next determined whether PKR is the kinase responsible for stress granule formation during RSV infection. We infected wild-type, shRNA non-targeting, or PKR-knockdown cells with RSV for 48 h (MOI = 5.0 pfu/cell). We then examined cells for the presence of stress granules using indirect immunofluorescence (Fig. 3A). In wild-type and shRNA non-targeting cells, we observed robust stress granule formation in infected cells. However, in PKR-knockdown cells, stress granule formation was substantially diminished. We quantified the number of RSV-infected cells that formed stress granules for each cell type. Our results show that while approximately 25–30% of infected wild-type and shRNA non-targeting cells form stress granules, stress granules form in only 2% of infected PKR-knockdown cells (Fig. 3B). When each cell type was treated with sodium arsenite, a drug that induces stress granules via HRI kinase rather than PKR, we observed an equivalent frequency of stress granule formation (data not shown). These results confirm that PKR is required for stress granule formation following RSV infection.
Fig. 3.
PKR expression is necessary for RSV-induced stress granule formation. (A) Wild-type cells or HEp-2 cells transduced with non-targeting shRNA (NonT) or PKR-specific shRNA were inoculated with RSV (MOI = 5.0 pfu/cell) for 48 h, fixed, and processed for immunofluorescence. Anti-G3BP monoclonal antibody was used to detect stress granules and appears green in the merge panel. Anti-RSV F antibody was used to localize viral protein and appears red in the merge panel. PKR appears blue in the merge panel. (B) For each cell type from (A), the percentage of infected cells containing stress granules per high-powered field (HPF) was determined. A total of 20 HPFs were counted for each cell type. Error bars indicate SEM.
The chemical inhibitor 2-AP prevents PKR signaling and reduces RSV infection
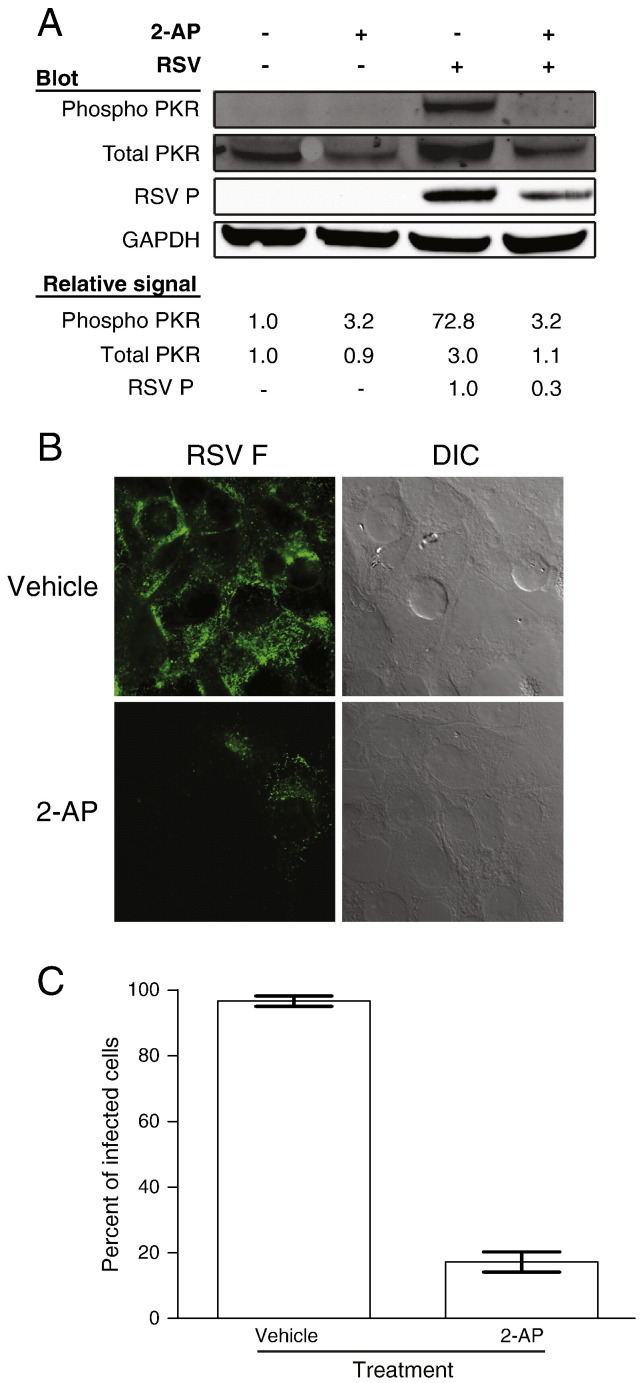
To confirm that PKR activation is dispensable for RSV replication, we sought another method to inhibit activation of the kinase. The nucleotide analog 2-AP prevents PKR activation (Hu and Conway, 1993). We first tested whether 2-AP treatment alters RSV-mediated PKR activation. HEp-2 epithelial cells were pretreated with 10 mM 2-AP or vehicle for 2 h. We then inoculated cells with RSV (MOI = 1.0 pfu/cell) in the presence of 2-AP or vehicle control for 1 h. The inoculum was removed and replaced with medium containing 2-AP or vehicle, and the infection was allowed to proceed for 24 h. Cell lysates were harvested for immunoblotting to assess the level of total or phosphorylated PKR. The phosphorylation of PKR in RSV-infected cells treated with 2-AP was greatly reduced in comparison to vehicle-treated cells (Fig. 4A). In addition, we did not observe an increase in total PKR levels in the presence of 2-AP that occurs during RSV infection following virus inoculation. As a control, we compared 2-AP-treated and vehicle-treated cells in the absence of infection and found no changes in the levels of PKR or phosphorylated PKR. These results indicate that 2-AP inhibits PKR activation during RSV infection. We next assessed the effect of 2-AP treatment on viral protein expression using an immunoblotting assay for RSV P protein as a surrogate marker. Relative amounts of total PKR, phosphorylated PKR, RSV P, and GAPDH were quantified using Odyssey imaging analysis software. Interestingly, we observed a 70% decrease in the amount of RSV P protein in 2-AP-treated cells in comparison to vehicle-treated cells. This result suggests that treatment with 2-AP inhibits viral protein synthesis.
Fig. 4.
Treatment with 2-AP prevents PKR activation during RSV infection. (A) HEp-2 cells were pretreated with 10 mM 2-AP or vehicle. Cells then were mock-inoculated or inoculated with RSV (MOI = 1.0 pfu/cell) for 24 h in the presence of 2-AP or vehicle. Cell lysates were analyzed by immunoblots for GAPDH, RSV P, phosphorylated PKR, or total PKR. Relative protein densities were quantified using Li-Cor Odyssey imaging software. The relative amount of RSV P protein expression was quantified for cells infected with RSV and treated with either vehicle or 2-AP. Protein levels were standardized to RSV-infected and vehicle-treated cells. The relative expression of PKR and phosphorylated PKR were quantified for each condition and compared to that of mock-infected cells treated with vehicle. (B) HEp-2 cells were pretreated with 2-AP or vehicle, inoculated with RSV (MOI = 1.0 pfu/cell) for 24 h in the presence of 2-AP or vehicle, and processed for immunofluorescence. Anti-RSV F antibody was used to detect virus-infected cells. Differential interference contrast (DIC) was used to define the periphery of each cell. (C) The percentage of cells per HPF that stained positively for RSV F protein in images from (B) was determined. Error bars indicate SEM.
To directly determine the effect of 2-AP treatment on RSV infection, we used indirect immunofluorescence to detect viral proteins in infected cells. Cells were pretreated with 2-AP or vehicle and inoculated with RSV (MOI = 1.0 pfu/cell). After 24 h, cells were fixed and stained for the RSV F protein as a marker for infection. RSV-infected cells per high-powered field (HPF) were quantified for each treatment. We observed an approximately 80% decrease in the percent of cells that stained positively for RSV F protein following 2-AP treatment (Fig. 4B and C). Thus, 2-AP treatment diminishes RSV infectivity.
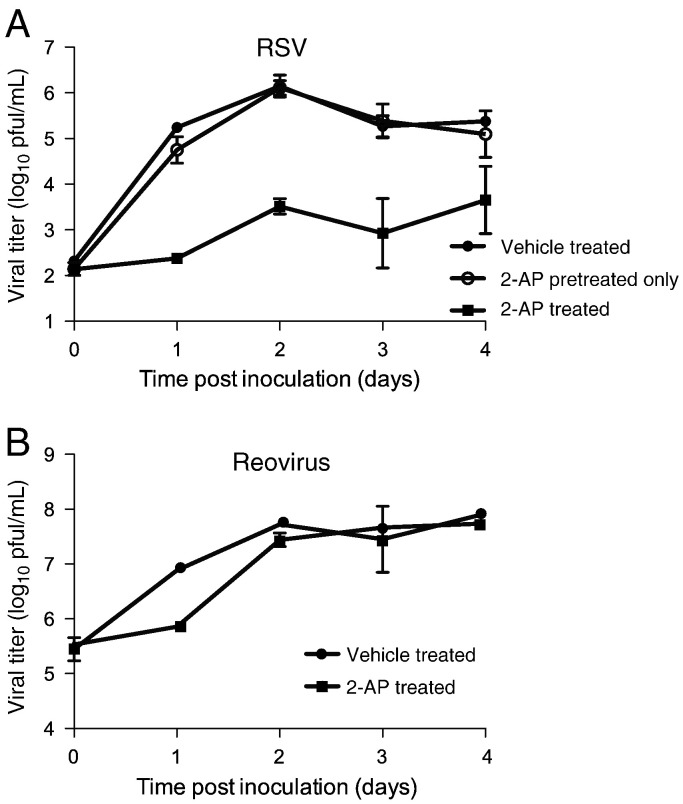
To determine the effects of 2-AP treatment on the capacity of RSV to complete an infectious cycle, cells were pretreated with vehicle or 2-AP and inoculated with RSV (MOI=0.1 pfu/cell). After a 1-h adsorption, the inoculum was replaced with medium containing vehicle or 2-AP. Titers of cell-associated virus were determined at 24-h intervals for 0–4 days. The results show that 2-AP treatment mediated an approximately 1000-fold decrease in viral titer throughout the time course of infection (Fig. 5A). To determine if 2-AP treatment affects viral entry, cells were pretreated with 2-AP and then inoculated with RSV (MOI = 0.1 pfu/cell). The inoculum then was replaced with medium containing vehicle control only and incubated for 0–4 days. In contrast to cells incubated with 2-AP throughout infection, cells that had only been pretreated with 2-AP exhibited no change in viral titer when compared to vehicle-treated cells. These results suggest that 2-AP does not affect viral entry but rather a later step in the viral life cycle. To determine whether the effects of 2-AP treatment on RSV were virus-specific, we performed a similar experiment using reovirus. Reovirus replication is unaffected by PKR knockdown (Zhang and Samuel, 2007). In addition, the reovirus σ3 protein inhibits PKR activation by competitively binding dsRNA targets (Sherry, 2009). In contrast to our results with RSV, 2-AP treatment only affected reovirus replication during the first 24 h after infection. After 24 h, we did not observe a significant difference in viral titers between 2-AP-treated or vehicle-treated cells, indicating that 2-AP treatment is much less inhibitory for reovirus replication in comparison to the effect on RSV (Fig. 5B).
Fig. 5.
Treatment with 2-AP inhibits RSV replication. (A) HEp-2 cells were (i) pretreated with vehicle, inoculated with RSV (MOI = 0.1 pfu/cell), and incubated in medium containing vehicle; (ii) pretreated with 2-AP, inoculated, and incubated in medium containing vehicle; or (iii) pretreated with 2-AP, inoculated, and incubated in medium containing 2-AP. (B) HEp-2 cells were incubated with vehicle or 2-AP and inoculated with reovirus (MOI = 5.0 pfu/cell), in the presence of 2-AP or vehicle. Viral titers were determined at the intervals shown by plaque assay for (A) and (B). Error bars indicate standard deviations.
2-AP inhibits RSV replication in the absence of PKR
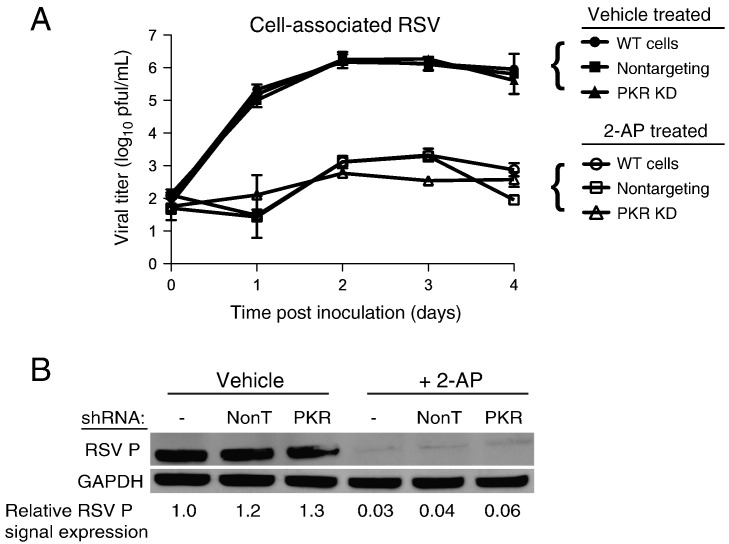
We next tested whether the effect on viral replication observed following 2-AP treatment requires PKR. We quantified the effect on viral titers in RSV-inoculated wild-type, non-targeting shRNA, or PKR-knockdown cells treated with 2-AP or vehicle. Cells were inoculated with RSV (MOI = 0.1 pfu/cell) and incubated for 0–4 days. Cells were collected for each time point, and titers of cell-associated virus were determined by plaque assay. Treatment of cells with 2-AP once again mediated a 1000-fold reduction in viral titers throughout the time course (Fig. 6A). Remarkably, 2-AP inhibited RSV replication in each cell type regardless of whether PKR was expressed or induced.
Fig. 6.
Treatment with 2-AP inhibits RSV replication independent of PKR. (A) Wild-type HEp-2 cells or HEp-2 cells transduced with non-targeting shRNA (NonT) or PKR-specific shRNA were pretreated with 10 mM 2-AP or vehicle. Cells were inoculated with RSV (MOI = 0.1 pfu/cell) in the presence of 2-AP or vehicle for the indicated intervals. Titers of cell-associated virus were determined for each time point by plaque assay. (B) Each cell type from (A) was pretreated with 10 mM 2-AP or vehicle and inoculated with RSV (MOI = 1.0 pfu/cell) for 24 h in the presence of 2-AP or vehicle. Cell lysates were subjected to immunoblotting for RSV P protein and GAPDH. The relative amount of RSV P protein was quantified for each cell type and compared to that in wild-type cells treated with vehicle. Error bars indicate standard deviations.
We next treated wild-type, non-targeting shRNA, or PKR-knockdown cells with 2-AP or vehicle and inoculated the cells with RSV (MOI = 1.0 pfu/cell). Following incubation for 24 h, we harvested the cell lysates and used immunoblots to detect total PKR, phosphorylated PKR, or RSV P. The results show that 2-AP inhibited RSV P production in each cell type, independent of PKR levels (Fig. 6B). Taken together, these data indicate that inhibition of PKR activation does not affect RSV replication. Furthermore, the decrease in RSV replication by 2-AP appears to be mediated via a PKR-independent mechanism.
Discussion
Since RSV induces the expression and activation of PKR, we hypothesized that activation of this dsRNA detector would mediate an antiviral effect during RSV infection. Surprisingly, we found that knockdown of PKR protein levels does not affect RSV replication. However, treatment with the kinase inhibitor 2-AP (which inhibits PKR) effectively blocks RSV replication regardless of PKR expression. This PKR-independent effect on RSV replication suggests that induction of other PKR-like kinases, which are 2-AP sensitive, enhances RSV replication. The identity of such kinases is not known.
Our results are unexpected for two main reasons. First, PKR initiates a cascade of events resulting in phosphorylation of eIF2α and stress granule formation. These events are associated with stalled translation initiation complexes, which might be expected to diminish the capacity of host cells to support viral replication. Second, induction of PKR is often associated with an antiviral state. For example, both vesicular stomatitis virus and influenza virus replicate to higher titers in PKR−/− mice in comparison to wild-type mice (Balachandran et al., 2000). Similarly, PKR−/− mice succumb to infection at a higher frequency when infected with Bunyamwera virus in comparison to wild-type mice (Streitenfeld et al., 2003). However, there are cases, in which PKR has a minimal effect on viral replication. Severe acute respiratory syndrome coronavirus replication is unaffected in cells treated with phosphorodiamidate morpholino oligomers specific for PKR mRNA (Krahling et al., 2009). Similarly, treatment of cells with PKR siRNAs has little effect on adenovirus or reovirus replication (Zhang and Samuel, 2007) or rotavirus protein synthesis (Rojas et al., 2010). Rift Valley fever virus replication is not altered in PKR−/− mouse embryo fibroblast cells (Habjan et al., 2009). However, PKR effects are not always antagonistic to viral infection. Knockdown of PKR by shRNA results in significantly decreased HCV RNA levels in cells treated with exogenous interferon and infected with HCV (Garaigorta and Chisari, 2009). Interestingly, levels of other proteins involved in the antiviral response are increased, suggesting that HCV induces PKR activation to inhibit host translation and prevent the expression of interferon-stimulated genes. We predicted a similar function for PKR during RSV infection since previous reports have demonstrated that 2-AP treatment leads to a dose-dependent increase in IFN-α production in RSV-infected cells (Hornung et al., 2004).
Although RSV is a single-stranded RNA virus, it produces multiple dsRNA intermediates during replication that are capable of activating PKR. PKR activation is likely required for eIF2α phosphorylation and subsequent stress granule formation during RSV infection (Lindquist et al., 2010; Hanley et al., 2010). Other studies have reported that RSV induces robust PKR activation during infection, but eIF2α phosphorylation is limited (Groskreutz et al., 2010). Phosphorylation of eIF2α may have been dampened by increased activity of protein phosphatase 2A (PP2A) during infection. In contrast, we observed extensive eIF2α phosphorylation during RSV infection, predominantly in cells that had been infected with RSV and in which stress granules had formed (Fig. 1). Since phosphorylation of eIF2α frequently precedes stress granule formation (Kedersha and Anderson, 2007), our results are not surprising given the extensive stress granule formation induced by RSV (Lindquist et al., 2010). It is possible that PP2A activity is not sufficient to reverse eIF2α phosphorylation in RSV-infected cells that form stress granules. Further experiments are required to assess PP2A activity in such cells.
Experiments using PKR-knockout mice show that RSV RNA levels increase during infection, suggesting a classic role for PKR as an antiviral molecule. In PKR-knockout mice, RSV infection induces lower levels of several cytokines, including TNF-α, IFN-β, and RANTES, among others, in comparison to wild-type mice. These results, when combined with histological analysis of mouse lung tissue, suggest that functional PKR leads to enhanced pathogenesis in RSV-infected animals (Minor et al., 2010).
Our previous data show that RSV replication is reduced in cells in which stress granule formation is inhibited by knockdown of the stress granule-related Ras-GAP SH3 domain-binding protein (G3BP) (Lindquist et al., 2010). In the current study, we found that RSV replication is unaffected in PKR-knockdown cells that similarly are deficient for stress granule formation. One possible explanation for this discrepancy is that knockdown of G3BP has effects in addition to inhibiting stress granule formation. Thus, viral replication might be inhibited by an alternative effect of the G3BP knockdown. Another possibility is that in PKR-knockdown cells, PKR signaling does not occur, preventing the activation of intrinsic antiviral responses to the virus. Thus, the potential pro-viral function for which stress granules may normally be required would not be necessary, allowing the virus to replicate efficiently.
The drug 2-AP has been cited as a specific inhibitor of PKR activation (Hu and Conway, 1993; Loving et al., 2006; Silva et al., 2004) and used in a number of studies examining the role of PKR during viral infection. However, 2-AP also may inhibit activation of tumor suppressor p53 during genotoxic stress (Huang et al., 2003), interfere with cell cycle progression (Andreassen and Margolis, 1994), and inhibit mitogen-activated protein kinase activation (Thorburn et al., 1994). Although PKR activation has been implicated in each of these processes (Garcia et al., 2006), it is possible that 2-AP inhibits multiple kinases. Our results demonstrate that while 2-AP is efficient at blocking PKR activation during RSV infection, its inhibitory effect on RSV replication is PKR-independent. Therefore, the reliability of 2-AP as a PKR-specific inhibitor is suspect, given that the drug mediates non-PKR-dependent effects. Defining the other targets of 2-AP might lend insight into the complex perturbations that occur in host gene function during RSV infection.
Materials and methods
Cells
HEp-2 cells (ATCC CCL-23) were maintained in OPTI-MEM I medium (Invitrogen) containing 5% (v/v) fetal calf serum (Sigma), 2 mM L-glutamine (Mediatech), 2.5 μg/mL amphotericin B (Mediatech), 100 µg/mL streptomycin (Mediatech), and 100 I.U./mL penicillin (Mediatech). L929 cells were grown in either suspension or monolayer cultures in Joklik's modified Eagles minimal essential medium (SMEM, Lonza) containing 5% (v/v) fetal bovine serum (FBS, Invitrogen), 2 mM L-glutamine (Invitrogen), 100 U/mL of penicillin, 100 mg/mL of streptomycin (Invitrogen), and 250 μg/mL amphotericin B (Sigma).
Viruses
A suspension of RSV wild-type strain A2 prepared in HEp-2 cells (1 × 106 pfu/mL) was used to infect HEp-2 cell monolayer cultures. Infectious virus was adsorbed to the cells for 1 h in a 37 °C incubator in 5% CO2. Following adsorption, the inoculum was removed and fresh medium added. Cells then were incubated at 37 °C in 5% CO2 for the duration of the infection period. Reovirus strain T1L is a laboratory stock.
Determination of viral titers
For RSV plaque assays, cell-associated virus was harvested at indicated times by scraping infected HEp-2 cell culture monolayers into 1.0 mL of medium. Virus then was released from harvested cells as described (Utley et al., 2008). Viral titers were determined using plaque assays as described (Murphy et al., 1990). For reovirus, titers were determined by plaque assay using L929 cells. Reovirus-infected HEp-2 cells were scraped into the medium, frozen at − 80 °C and thawed at room temperature twice prior to determination of viral titer in the suspension by plaque assay (Virgin et al., 1988). Reovirus yields were calculated according to the following formula: log10 yield t = log10 (pfu/cell)t − log10 (pfu/cell)t 0, where t is the time postinfection and t 0 is the time at inoculation after absorption.
2-Aminopurine (2-AP) treatment
We generated a 150 mM stock solution of 2-AP (Sigma A3509) diluted in phosphate-buffered saline (PBS) and glacial acetic acid (Fisher A-38-500). Glacial acetic acid was diluted at a ratio of 1:200 in PBS. For each use, the solution was incubated at 60 °C for re-suspension. HEp-2 cells were pretreated with 10 mM 2-AP diluted in medium or mock-treated with vehicle (glacial acetic acid in PBS). Cells then were infected with RSV in the presence of 10 mM 2-AP or vehicle for 1 h. Following infection, the inoculum was removed and replaced with fresh medium containing 10 mM 2-AP or vehicle and incubated for the duration of infection.
Fixation and immunostaining
Cells were fixed with 3.7% (w/v) formaldehyde in PBS for 10 min at room temperature. Cells were permeabilized with 0.2% (w/v) Triton X-100 and 3.7% paraformaldehyde in PBS for 10 min at room temperature. Following fixation, cells were blocked in 5% (w/v) BSA in PBS for 1 h followed by addition of primary antibody for 1 h. Cells then were washed three times in PBS and species-specific IgG Alexa Fluor (Molecular Probes) was added at a dilution of 1:1000 in block solution for detection of primary antibodies. Cells were washed 3 times in PBS and fixed on glass slides using Prolong Antifade kit (Molecular Probes). Images were obtained using a Zeiss inverted LSM510 confocal microscope equipped with a 40× Plan-Neofluar oil objective lens.
A polyclonal anti-TIA-1 (sc-1751) antibody was obtained from Santa Cruz and used for immunostaining. Monoclonal anti-phosphorylated eIF2α (1090-1) and anti-PKR (1511-1) antibodies were obtained from Epitomics. An anti-RSV F protein humanized mouse monoclonal antibody (palivizumab; MedImmune) was used to identify RSV F protein. Anti-RSV P protein (clone 3_5) monoclonal antibody was a kind gift of Ewa Bjorling and Earling Norrby.
shRNA reagents
Lentiviral shRNA particles were obtained from Dharmacon for human GAPDH (S-001000-01), a non-targeting control (S-005000-01), and a set of three PKR SMARTvector shRNA constructs (SK-003527-00-10). HEp-2 cells were plated in 96-well plates and transduced with individual lentiviral constructs according to the manufacturer's protocol. For selection of each target, cells containing integrated lentivirus sequences were selected using puromycin (5 μg/mL) diluted in medium. Medium containing puromycin was replaced every 3 days until resistant colonies were observed.
Immunoblotting
HEp-2 cells were grown in 6-well plates and harvested for protein. Cells were lysed using lysis buffer (50 mM Tris–HCl, 150 mM NaCl, 1% Triton X-100, pH 8.0) containing 0.5% (v/v) of protease inhibitor cocktail (Sigma) and 1.0% (v/v) of phosphatase inhibitors (Sigma). Lysates were separated in 4–12% NuPAGE Bis–Tris gels (Invitrogen) and transferred to nitrocellulose membranes using an iBlot dry-blotting system (Invitrogen). Membranes were blocked for 1 h using Odyssey blocking buffer (Li-Cor) diluted 1:1 in PBS. Primary antibodies were diluted in blocking buffer and incubated overnight at 4 °C. Membranes then were washed four times in Tris buffered saline with 0.2% Tween (TBST) for 5 min each. Li-Cor IRDye 680CW or IRDye 800CW secondary antibodies were diluted 1:5000 in blocking buffer and added to each membrane for 1 h. Membranes were washed four times in TBST. Bands were imaged and quantified using the Odyssey Infrared Imaging System. Total PKR or phosphorylated PKR were detected using monoclonal antibodies from Epitomics (1511-1 and 2283-1, respectively). GAPDH was detected using a monoclonal antibody from Millipore (MAB374).
Acknowledgments
Confocal microscopy imaging experiments were performed in the Vanderbilt Cell Imaging Shared Resources (supported by NIH grants CA68485, DK20593, DK58404, HD15052, DK59637, and EY08126). This work was supported by a Burroughs Wellcome Fund Clinical Scientist Award in Translation Research (J.E.C.), and by a grant from the March of Dimes. Additional support was provided by NIH grants T32 HLO7751 and F32 A180108 (B.M.) and R01 AI 32539 (T.S.D.) and the Elizabeth B. Lamb Center for Pediatric Research.
References
- Andreassen P.R., Margolis R.L. Microtubule dependency of p34cdc2 inactivation and mitotic exit in mammalian cells. J. Cell Biol. 1994;127:789–802. doi: 10.1083/jcb.127.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran S., Roberts P.C., Brown L.E., Truong H., Pattnaik A.K., Archer D.R., Barber G.N. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity. 2000;13:129–141. doi: 10.1016/s1074-7613(00)00014-5. [DOI] [PubMed] [Google Scholar]
- Carromeu C., Simabuco F.M., Tamura R.E., Farinha Arcieri L.E., Ventura A.M. Intracellular localization of human respiratory syncytial virus L protein. Arch. Virol. 2007;152:2259–2263. doi: 10.1007/s00705-007-1048-4. [DOI] [PubMed] [Google Scholar]
- Cowton V.M., McGivern D.R., Fearns R. Unravelling the complexities of respiratory syncytial virus RNA synthesis. J. Gen. Virol. 2006;87:1805–1821. doi: 10.1099/vir.0.81786-0. [DOI] [PubMed] [Google Scholar]
- Garaigorta U., Chisari F.V. Hepatitis C virus blocks interferon effector function by inducing protein kinase R phosphorylation. Cell Host Microbe. 2009;6:513–522. doi: 10.1016/j.chom.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J., Garcia-Barreno B., Vivo A., Melero J.A. Cytoplasmic inclusions of respiratory syncytial virus-infected cells: formation of inclusion bodies in transfected cells that coexpress the nucleoprotein, the phosphoprotein, and the 22K protein. Virology. 1993;195:243–247. doi: 10.1006/viro.1993.1366. [DOI] [PubMed] [Google Scholar]
- Garcia M.A., Gil J., Ventoso I., Guerra S., Domingo E., Rivas C., Esteban M. Impact of protein kinase PKR in cell biology: from antiviral to antiproliferative action. Microbiol. Mol. Biol. Rev. 2006;70:1032–1060. doi: 10.1128/MMBR.00027-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M.A., Meurs E.F., Esteban M. The dsRNA protein kinase PKR: virus and cell control. Biochimie. 2007;89:799–811. doi: 10.1016/j.biochi.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Groskreutz D.J., Babor E.C., Monick M.M., Varga S.M., Hunninghake G.W. Respiratory syncytial virus limits alpha subunit of eukaryotic translation initiation factor 2 (eIF2alpha) phosphorylation to maintain translation and viral replication. J. Biol. Chem. 2010;285:24023–24031. doi: 10.1074/jbc.M109.077321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groskreutz D.J., Monick M.M., Powers L.S., Yarovinsky T.O., Look D.C., Hunninghake G.W. Respiratory syncytial virus induces TLR3 protein and protein kinase R, leading to increased double-stranded RNA responsiveness in airway epithelial cells. J. Immunol. 2006;176:1733–1740. doi: 10.4049/jimmunol.176.3.1733. [DOI] [PubMed] [Google Scholar]
- Habjan M., Pichlmair A., Elliott R.M., Overby A.K., Glatter T., Gstaiger M., Superti-Furga G., Unger H., Weber F. NSs protein of rift valley fever virus induces the specific degradation of the double-stranded RNA-dependent protein kinase. J. Virol. 2009;83:4365–4375. doi: 10.1128/JVI.02148-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley L.L., McGivern D.R., Teng M.N., Djang R., Collins P.L., Fearns R. Roles of the respiratory syncytial virus trailer region: effects of mutations on genome production and stress granule formation. Virology. 2010;406:241–252. doi: 10.1016/j.virol.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V., Schlender J., Guenthner-Biller M., Rothenfusser S., Endres S., Conzelmann K.K., Hartmann G. Replication-dependent potent IFN-alpha induction in human plasmacytoid dendritic cells by a single-stranded RNA virus. J. Immunol. 2004;173:5935–5943. doi: 10.4049/jimmunol.173.10.5935. [DOI] [PubMed] [Google Scholar]
- Hu Y., Conway T.W. 2-Aminopurine inhibits the double-stranded RNA-dependent protein kinase both in vitro and in vivo. J. Interferon Res. 1993;13:323–328. doi: 10.1089/jir.1993.13.323. [DOI] [PubMed] [Google Scholar]
- Huang S., Qu L.K., Cuddihy A.R., Ragheb R., Taya Y., Koromilas A.E. Protein kinase inhibitor 2-aminopurine overrides multiple genotoxic stress-induced cellular pathways to promote cell survival. Oncogene. 2003;22:3721–3733. doi: 10.1038/sj.onc.1206490. [DOI] [PubMed] [Google Scholar]
- Kedersha N., Anderson P. Mammalian stress granules and processing bodies. Meth. Enzymol. 2007;431:61–81. doi: 10.1016/S0076-6879(07)31005-7. [DOI] [PubMed] [Google Scholar]
- Krahling V., Stein D.A., Spiegel M., Weber F., Muhlberger E. Severe acute respiratory syndrome coronavirus triggers apoptosis via protein kinase R but is resistant to its antiviral activity. J. Virol. 2009;83:2298–2309. doi: 10.1128/JVI.01245-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist M.E., Lifland A.W., Utley T.J., Santangelo P.J., Crowe J.E., Jr. Respiratory syncytial virus induces host RNA stress granules to facilitate viral replication. J. Virol. 2010 doi: 10.1128/JVI.00260-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loving C.L., Brockmeier S.L., Ma W., Richt J.A., Sacco R.E. Innate cytokine responses in porcine macrophage populations: evidence for differential recognition of double-stranded RNA. J. Immunol. 2006;177:8432–8439. doi: 10.4049/jimmunol.177.12.8432. [DOI] [PubMed] [Google Scholar]
- Minor R.A., Limmon G.V., Miller-DeGraff L., Dixon D., Andrews D.M., Kaufman R.J., Imani F. Double-stranded RNA-activated protein kinase regulates early innate immune responses during respiratory syncytial virus infection. J. Interferon Cytokine Res. 2010;30:263–272. doi: 10.1089/jir.2009.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B.R., Sotnikov A.V., Lawrence L.A., Banks S.M., Prince G.A. Enhanced pulmonary histopathology is observed in cotton rats immunized with formalin-inactivated respiratory syncytial virus (RSV) or purified F glycoprotein and challenged with RSV 3–6 months after immunization. Vaccine. 1990;8:497–502. doi: 10.1016/0264-410x(90)90253-i. [DOI] [PubMed] [Google Scholar]
- Proud C.G. eIF2 and the control of cell physiology. Semin. Cell Dev. Biol. 2005;16:3–12. doi: 10.1016/j.semcdb.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Rojas M., Arias C.F., Lopez S. Protein kinase R is responsible for the phosphorylation of eIF2alpha in rotavirus infection. J. Virol. 2010;84:10457–10466. doi: 10.1128/JVI.00625-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry B. Rotavirus and reovirus modulation of the interferon response. J. Interferon Cytokine Res. 2009;29:559–567. doi: 10.1089/jir.2009.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva A.M., Whitmore M., Xu Z., Jiang Z., Li X., Williams B.R. Protein kinase R (PKR) interacts with and activates mitogen-activated protein kinase kinase 6 (MKK6) in response to double-stranded RNA stimulation. J. Biol. Chem. 2004;279:37670–37676. doi: 10.1074/jbc.M406554200. [DOI] [PubMed] [Google Scholar]
- Streitenfeld H., Boyd A., Fazakerley J.K., Bridgen A., Elliott R.M., Weber F. Activation of PKR by Bunyamwera virus is independent of the viral interferon antagonist NSs. J. Virol. 2003;77:5507–5511. doi: 10.1128/JVI.77.9.5507-5511.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorburn J., Frost J.A., Thorburn A. Mitogen-activated protein kinases mediate changes in gene expression, but not cytoskeletal organization associated with cardiac muscle cell hypertrophy. J. Cell Biol. 1994;126:1565–1572. doi: 10.1083/jcb.126.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utley T.J., Ducharme N.A., Varthakavi V., Shepherd B.E., Santangelo P.J., Lindquist M.E., Goldenring J.R., Crowe J.E., Jr. Respiratory syncytial virus uses a Vps4-independent budding mechanism controlled by Rab11-FIP2. Proc. Natl Acad. Sci. USA. 2008;105:10209–10214. doi: 10.1073/pnas.0712144105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin H.W.t., Bassel-Duby R., Fields B.N., Tyler K.L. Antibody protects against lethal infection with the neurally spreading reovirus type 3 (Dearing) J. Virol. 1988;62:4594–4604. doi: 10.1128/jvi.62.12.4594-4604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Samuel C.E. Protein kinase PKR plays a stimulus- and virus-dependent role in apoptotic death and virus multiplication in human cells. J. Virol. 2007;81:8192–8200. doi: 10.1128/JVI.00426-07. [DOI] [PMC free article] [PubMed] [Google Scholar]