Abstract
Coccidioidomycosis is an endemic disease of the Western hemisphere. The cases in non-endemic areas are mostly imported. Determining a history of exposure is critical for performing the diagnosis of coccidioidomycosis, especially for cases occurring in a non-endemic area. In this study, a 71-year-old Chinese male presented to our hospital with chronic cough and malaise, and was found to have a mass in the middle lobe of right lung. He had been visiting Arizona, USA for four months before admission. Pulmonary coccidioidomycosis was confirmed by the results of histopathological examination after lobectomy. Typical microscopic features of Coccidioides are definitely diagnostic, but need be differentiated from other budding yeast infection or carcinoma histopathologically.
Keywords: Coccidioidomycosis, Imported disease, Pulmonary fungal infection, Endemic disease
1. Introduction
Coccidioidomycosis is a primary pulmonary fungal infection caused by inhalation of Coccidioides spores in an immunocompetent host. An aggressive disseminated coccidioidomycosis involving the lung, skin, bones, joints, and central nervous system occurs rarely, but it is associated with high mortality. Coccidioidomycosis is an endemic disease, which has long been identified in the southwestern USA, northwestern part of Mexico, and some areas of Brazil and Argentina in South America. The rare cases in non-endemic areas are mostly imported, and suspected based on the evidence of endemic exposure (Desai et al., 2001). We herein present the detailed description of an imported coccidioidomycosis case with a typical exposure history in an epidemic region.
2. Case presentation
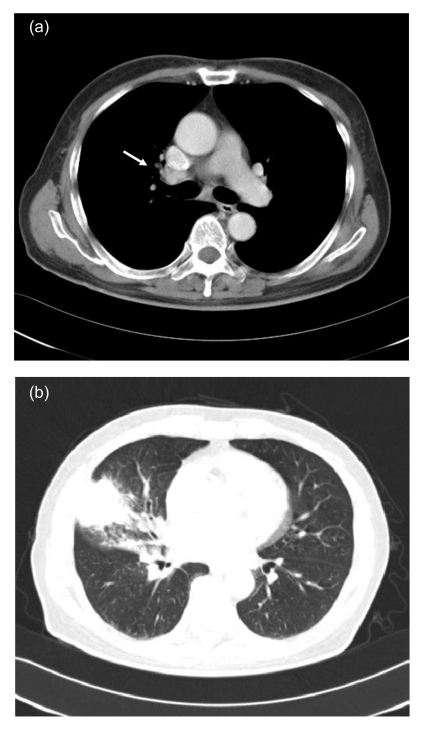
A 71-year-old Chinese male was admitted with complaint of chronic cough and malaise for two months. He had been in Tucson, Arizona, USA, visiting for four months right before the symptoms occurred. He had transient low-grade fever, but denied hemoptysis, night sweats, skin rashes, or headache. His past medical history included four years of primary hypertension and three years of coronary heart disease. He did not smoke or abuse drugs. Physical examination revealed no abnormalities. Lab tests showed an elevated erythrocyte sedimentation rate (ESR) of 46 mm/h, while complete blood count (CBC), eosinophil count, serum chemistry, and tumor biomarkers were all in normal range. Human immunodeficiency virus (HIV) antibody was found to be negative. Sputum culture showed normal flora growth. Chest computed tomographic (CT) scan revealed an irregular-margined opacity measuring 3.0 cm×3.8 cm in diameter in the sub-pleura region of right middle lobe. Right hilar and mediastinal lymphadenopathy was noted (Fig. 1). Lung cancer was considered as the most likely diagnosis. Subsequent bronchoscopy and brush cytology were negative. The patient received a procedure of right middle lobe and lower lobectomy on the eighth day after admission.
Fig. 1.
Chest computed tomographic (CT) scan
(a) Mediastinal window showing right hilar lymphadenopathy (white arrow); (b) Lung window showing a solid mass in the right middle lobe
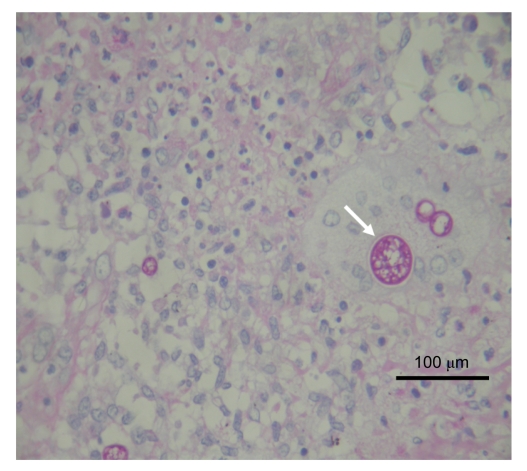
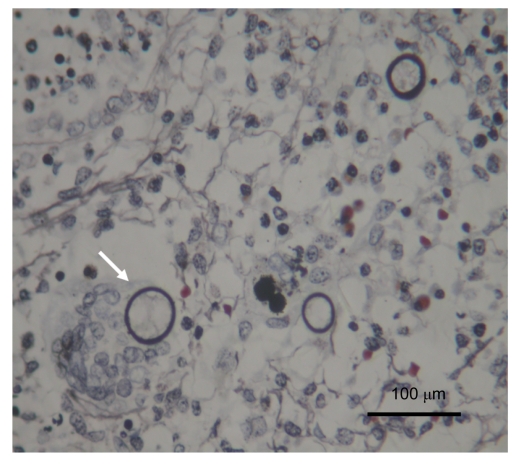
Histopathological examination of the surgically obtained lung specimen revealed a solid mass of 5.0 cm×4.0 cm×1.5 cm in the right middle lobe. Microscopic examination showed focal necrotic granulomatous inflammation with multinucleated giant cells containing fungal spherules and infiltrations of massive neutrophils, eosinophils, and lymphocytes. Some other free and typical large, encapsulated spherules with endosporulation measuring 40 µm in diameter could be seen in the surrounding tissues (Fig. 2). Periodic acid-Shiff (PAS) and periodic acid silver methenamine (PASM) stains were all positive (Figs. 3 and 4), while acid fast stain was negative. The final diagnosis thus was confirmed pathologically as pulmonary Coccidioides infection. The patient was totally free from discomfort in the course of two years follow-up after the lobectomy.
Fig. 2.
Hematoxylin and eosin (H&E) staining of fungal encapsulated spherules with endosporulation (white arrows), measuring 20–40 µm in diameter, found in the surrounding tissues and multinucleated giant cells
Fig. 3.
Periodic acid-Schiff (PAS) staining of multiple fungal encapsulated spherules with endosporulation (white arrow)
Fig. 4.
Periodic acid silver methenamine (PASM) staining of multiple capsule of fungal spherules known as Coccidioides (white arrow)
3. Discussion
Coccidioidomycosis is known as a fungal parasitic infection, initially recognized as a human disease in Argentina in 1892. The Coccidioides genus is considered dimorphic and thought to have two species, Coccidioides immitis and Coccidioides posadasii. These two organisms are genetically different, but they cannot be distinguished in terms of morphology, clinical features, or immune response (Desai et al., 2001; Laniado-Laborin, 2007; Saubolle et al., 2007). Dimorphism in Coccidioides is characterized by the productions of septate hyphae and thick-walled arthroconidia as the fungal spores are in external warm semiarid soil or laboratory conditions. The spores then convert into large spherules, ranging from 20 to 200 µm in diameter, which subsequently develop progeny endospores after being inhaled into the host’s lungs. The sac of larger spherules containing the endospores may rupture in vivo, whereupon new spherules will develop. Symptomatic infection and immune response occur during the specific fungal parasitic cycle of life (Taxy and Kodros, 2005; Gaidici and Saubolle, 2009). The natural endemic region for Coccidioides spp. lies exclusively in arid or semiarid areas of the western hemisphere (Laniado-Laborin, 2007). Therefore, coccidioidomycosis is an endemic disease (Hector and Laniado-Laborin, 2005).
The life zone of Coccidioides spp. is in the hot deserts of the southwestern USA: Texas, Arizona, Nevada, New Mexico, and much of central and southern California. Meanwhile, many cases are recorded annually in northwestern Mexico and some areas of Brazil and Argentina in South America (Laniado-Laborin, 2007; Saubolle et al., 2007). The incidence rate of the disease was dramatically increasing in the last decade in USA, especially in California during the years 2000 to 2007. The recent increases in reported coccidioidomycosis are probably due to local environmental and climatic changes, increased construction activity, growing numbers of immunocompromised patients, immigration of unexposed persons from non-epidemic areas, and higher awareness of this disease among healthcare providers (CDC, 2009).
Other cases have also been reported separately in many regions and countries far away from the endemic areas, including the northeastern states of USA (Desai et al., 2001), the Netherlands (Indhirajanti et al., 2009), Poland (Batura-Gabryel and Brajer, 2008), India (Verghese et al., 2002), Japan (Futsuki et al., 2000; Kishi et al., 2008), and Hong Kong of China (Kwok et al., 2009). All these cases were clearly connected with past traveling activities or residential history in the disease-epidemic region, which is supposed to be an important factor for diagnosis (Indhirajanti et al., 2009).
There have been reports of separated cases of Coccidioides infection in mainland China since 1985. Among these cases, interestingly, some were without travel history and diagnosed barely on histopathological examination (Lan et al., 2010). However, whether there are other possible routes of infection remain unknown.
Coccidioidomycosis typically is acquired via inhalation of aerosolized spores or arthroconidia from the soil. Under most circumstances, person-to-person or person-to-animal transmission does not occur (Desai et al., 2001; Saubolle et al., 2007; Gaidici and Saubolle, 2009). Recently, however, the fungal infection transmission to humans from contaminated inanimate objects in an endemic area has been reported in a series of 23 cases in Ohio, USA, which is believed not to belong to endemic states in USA (Desai et al., 2001). And still there have been a few reports of the transmission by tissue transplantation to recipients from donors (Saubolle et al., 2007) or by cat-bite (Gaidici and Saubolle, 2009).
The suspected diagnosis of Coccidioides infection can be confirmed by the following laboratory tests: (1) histopathological examination, (2) pathogen isolation and culture, (3) immunological antibody screening, and (4) fungal nucleic acid detection by molecular biological techniques (Saubolle et al., 2007).
Among these diagnostic methods, pathogen culture is performed when the specific organism infection is suspected, but is also considered to result in potential laboratory transmission without necessary protection. Serological antibody screening and polymerase chain reaction (PCR) assay for the investigation of a specific sequence in the DNA of the agents are not routinely available in laboratories especially in the non-epidemic regions. Histopathological examination on the lesion tissues obtained through biopsy is considered to be more valuable and easier to perform. Identification of endospore-containing spherules differing in diameter from 20 to 200 μm without budding under microscopy is definitely diagnostic of coccidioidomycosis. Predominantly small immature coccidioidal spherules abutting each other need to be differentiated from budding yeast forms of Blastomyces, Histoplasma, Cryptococcus, or Candida. Commonly used histological stains, such as the hematoxylin-eosin (H&E) stain, are able to distinguish typical morphological structures of spherules, but are not considered to be as sensitive as the PAS stain and the Grocott-methenamine silver (GMS) stain. Special stains (PAS, GMS) for fungal glycogen components are more valuable for thick cell wall staining; hence they can aid in the identification of the presence of low numbers of spherules (Bialek et al., 2005; Saubolle et al., 2007). Histopathological manifestations of chronic active pyogranulomatous inflammation and reactive atypical pneumocytes may also be mistaken for tuberculosis and malignancy, respectively. Lee et al. (2008) reported a case of pulmonary coccidioidomycosis misdiagnosed as carcinoma. They emphasized that an examination of the specimen using fluorescent antibody or nucleic acid testing or cultures for fungi is diagnostic in the absence of morphologically typical Coccidioides infection under microscopy (Taxy and Kodros, 2005; Lee et al., 2008).
Based on all the diagnostic strategies we mentioned above, determining a history of exposure is critical for performing the diagnosis of coccidioidomycosis, especially in a non-endemic area. If there is a lack of exposure history or typically microscopic features, then further examination of special specimen staining, organism isolation and culture, specific humoral antibodies, or nucleic acid detection is definitely necessary for differentiation and confirming the diagnosis.
The patient in our case study, who had been traveling and temporally residing in Arizona for four months, provided us further information about his gardening work during his visit. Considering the exposure of endemic soil contact and the detection of typical histopathological findings of the large size of spherules and presence of endospores, an imported pulmonary coccidioidomycosis was confirmed.
The majority of human cases (60%) result from asymptomatic infection and are self-limited. Post-infected patients are likely to have lifelong immunity (Galgiani et al., 2005; Hector and Laniado-Laborin, 2005; Saubolle et al., 2007). Patients who present with severe pneumonia or disseminated disease soon after infection and those with risk factors (e.g., weight loss, night sweats, multiple infiltrates in lungs, persistent hilar adenopathy, high titer of antibody, victims of African or Filipino descent, and pregnancy) warrant antifungal therapy. In general, the more rapidly progressive a coccidioidal infection is, the more likely amphotericin B should be selected for initial therapy. Sub-acute or chronic presentations are more likely to be treated initially with an azole (ketoconazole, fluconazole, and itraconazole). Newly available antifungal medicines for the treatment of refractory coccidioidal infections are voriconazole and caspofungin (Galgiani et al., 2005). Surgical intervention is suitable for diagnostic dilemmas involving nodular disease, symptomatic nonresponsive cavitary disease, or complications of disease (e.g., effusion, pneumothorax, empyema, and hemoptysis). Resection should result in symptom resolution and long-term freedom from recurrence (Jaroszewski et al., 2009).
The choice of surgical lobectomy in our case remained controversial before the diagnosis was made, and it was thought to be a relatively aggressive treatment option. Less invasive diagnostic procedures such as specifc fungal culture, CT-guided needle biopsy, or transbronchoscopic biopsy should have been adopted first. Lack of knowledge of the rare endemic diesease and presence of otherwise typical manifestations of elderly lung cancer in the case presented here were responsible for the decisions made. Hence, we provide a detail case study of typical, imported pulmonary coccidioidomycosis to increase awareness of this disease in non-endemic areas.
References
- 1.Batura-Gabryel H, Brajer B. Coccidioidomycosis in a 38-year-old man: a case report. Pol Arch Med Wewn. 2008;118(6):387–390. [PubMed] [Google Scholar]
- 2.Bialek R, Gonzalez GM, Begerow D, Zelck UE. Coccidioidomycosis and blastomycosis: advances in molecular diagnosis. FEMS Immunol Med Microbiol. 2005;45(3):355–360. doi: 10.1016/j.femsim.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 3.CDC (Centers for Disease Control and Prevention) Increase in coccidioidomycosis—California, 2000–2007. MMWR Morb Mortal Wkly Rep. 2009;58(5):105–109. [PubMed] [Google Scholar]
- 4.Desai SA, Minai OA, Gordon SM, O′Neil B, Wiedemann HP, Arroliga AC. Coccidioidomycosis in non-endemic areas: a case series. Respir Med. 2001;95(4):305–309. doi: 10.1053/rmed.2000.1039. [DOI] [PubMed] [Google Scholar]
- 5.Futsuki Y, Nagashima S, Yamamoto Y, Araki J, Asai S, Sawatari K, Maesaki S, Kohno S. An imported case of primary pulmonary coccidioidomycosis. Kansenshogaku Zasshi. 2000;74(7):580–584. doi: 10.11150/kansenshogakuzasshi1970.74.580. (in Japanese) [DOI] [PubMed] [Google Scholar]
- 6.Gaidici A, Saubolle MA. Transmission of coccidioidomycosis to a human via a cat bite. J Clin Microbiol. 2009;47(2):505–506. doi: 10.1128/JCM.01860-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galgiani JN, Ampel NM, Blair JE, Catanzaro A, Johnson RH, Stevens DA, Williams PL. Coccidioidomycosis. Clin Infect Dis. 2005;41(9):1217–1223. doi: 10.1086/496991. [DOI] [PubMed] [Google Scholar]
- 8.Hector RF, Laniado-Laborin R. Coccidioidomycosis—a fungal disease of the Americas. PLoS Med. 2005;2(1):e2. doi: 10.1371/journal.pmed.0020002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Indhirajanti S, Maartense E, Posthuma EF, Pannekoek BJ, Vreede RW. Pulmonary coccidioidomycosis: import illness and the importance of travel history. Neth J Med. 2009;67(10):353–355. [PubMed] [Google Scholar]
- 10.Jaroszewski DE, Halabi WJ, Blair JE, Coakley BJ, Wong RK, Parish JM, Vaszar LT, Kusne S, Vikram HR, DeValeria PA, et al. Surgery for pulmonary coccidioidomycosis: a 10-year experience. Ann Thorac Surg. 2009;88(6):1765–1772. doi: 10.1016/j.athoracsur.2009.07.075. [DOI] [PubMed] [Google Scholar]
- 11.Kishi K, Fujii T, Takaya H, Miyamoto A, Kurosaki A, Kohno T, Yoshimura K. Pulmonary coccidioidomycosis found in healthy Japanese individuals. Respirology. 2008;13(2):252–256. doi: 10.1111/j.1440-1843.2008.01232.x. [DOI] [PubMed] [Google Scholar]
- 12.Kwok HK, Chan JW, Li IW, Chu SY, Lam CW. Coccidioidomycosis as a rare cause of pneumonia in non-endemic areas: a short exposure history should not be ignored. Respirology. 2009;14(4):617–620. doi: 10.1111/j.1440-1843.2008.01407.x. [DOI] [PubMed] [Google Scholar]
- 13.Lan F, Tong YZ, Huang H, Xiong WN, Xu YJ, Xiong SD. Primary pulmonary coccidioidomycosis in China. Respirology. 2010;15(4):722–725. doi: 10.1111/j.1440-1843.2010.01747.x. [DOI] [PubMed] [Google Scholar]
- 14.Laniado-Laborin R. Expanding understanding of epidemiology of coccidioidomycosis in the western hemisphere. Ann N Y Acad Sci. 2007;1111(1):19–34. doi: 10.1196/annals.1406.004. [DOI] [PubMed] [Google Scholar]
- 15.Lee CH, Wilcox L, Chorneyko K, McIvor A. Coccidioides immitis: two cases of misidentified mycosis. Can Respir J. 2008;15(7):377–379. doi: 10.1155/2008/540562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saubolle MA, McKellar PP, Sussland D. Epidemiologic, clinical, and diagnostic aspects of coccidioidomycosis. J Clin Microbiol. 2007;45(1):26–30. doi: 10.1128/JCM.02230-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taxy JB, Kodros S. Musculoskeletal coccidioidomycosis: unusual sites of disease in a nonendemic area. Am J Clin Pathol. 2005;124(5):693–696. doi: 10.1309/KRNYU4RN7Q12WEYD. [DOI] [PubMed] [Google Scholar]
- 18.Verghese S, Arjundas D, Krishnakumar KC, Padmaja P, Elizabeth D, Padhye AA, Warnock DW. Coccidioidomycosis in India: report of a second imported case. Med Mycol. 2002;40(3):307–309. doi: 10.1080/mmy.40.3.307.309. [DOI] [PubMed] [Google Scholar]