Abstract
Objective To compute the burden of cancer attributable to current and former alcohol consumption in eight European countries based on direct relative risk estimates from a cohort study.
Design Combination of prospective cohort study with representative population based data on alcohol exposure.
Setting Eight countries (France, Italy, Spain, United Kingdom, the Netherlands, Greece, Germany, Denmark) participating in the European Prospective Investigation into Cancer and Nutrition (EPIC) study.
Participants 109 118 men and 254 870 women, mainly aged 37-70.
Main outcome measures Hazard rate ratios expressing the relative risk of cancer incidence for former and current alcohol consumption among EPIC participants. Hazard rate ratios combined with representative information on alcohol consumption to calculate alcohol attributable fractions of causally related cancers by country and sex. Partial alcohol attributable fractions for consumption higher than the recommended upper limit (two drinks a day for men with about 24 g alcohol, one for women with about 12 g alcohol) and the estimated total annual number of cases of alcohol attributable cancer.
Results If we assume causality, among men and women, 10% (95% confidence interval 7 to 13%) and 3% (1 to 5%) of the incidence of total cancer was attributable to former and current alcohol consumption in the selected European countries. For selected cancers the figures were 44% (31 to 56%) and 25% (5 to 46%) for upper aerodigestive tract, 33% (11 to 54%) and 18% (−3 to 38%) for liver, 17% (10 to 25%) and 4% (−1 to 10%) for colorectal cancer for men and women, respectively, and 5.0% (2 to 8%) for female breast cancer. A substantial part of the alcohol attributable fraction in 2008 was associated with alcohol consumption higher than the recommended upper limit: 33 037 of 178 578 alcohol related cancer cases in men and 17 470 of 397 043 alcohol related cases in women.
Conclusions In western Europe, an important proportion of cases of cancer can be attributable to alcohol consumption, especially consumption higher than the recommended upper limits. These data support current political efforts to reduce or to abstain from alcohol consumption to reduce the incidence of cancer.
Introduction
Alcohol consumption is thought to account for a substantial number of deaths worldwide, with Europe and America showing the highest alcohol attributable fractions of 6.5% and 5.6%, respectively.1 Chronic diseases, especially cancer, contribute markedly to this burden. In 2007 the International Agency for Research on Cancer (IARC) added two of the most common cancers—female breast and colorectal cancer—to the list of cancers causally related to alcohol, which previously consisted of oral cavity, pharynx, larynx, oesophagus, and liver cancer.2 Although alcohol consumption is a major risk factor for cancer incidence,3 and Europe is among the regions with the highest per capita alcohol consumption, detailed information on the fractions of cancer that are attributable to alcohol consumption based on direct empirical evidence for the different cancer sites is sparse,4 5 and systematic and comparable estimations across European countries are lacking. Moreover, previous estimates of the alcohol attributable fractions refer to the burden from current alcohol consumption but do not consider the risk of former alcohol consumption. Also, in 2007 the World Cancer Research Fund/American Institute for Cancer Research published recommendations on the maximum recommended daily alcohol consumption.6 We do not know how much of the burden of incidence of cancer is attributable to alcohol and occurs because of consumption higher than the recommended upper limit.
We estimated the total (current and former alcohol consumption) and partial (alcohol consumption higher than the recommended upper limit) alcohol attributable fractions for the incidence of total and specific cancers related to alcohol in eight European countries based on hazard rate ratios from the European Prospective Investigation into Cancer and Nutrition (EPIC) study and linked those alcohol attributable fractions to incidences of cancer to estimate the annual absolute number of cancer cases attributable to alcohol in these countries.
Methods
Study population
The EPIC study is a multicentre prospective cohort study that, from 1992 to 2000, recruited about 520 000 randomly selected men and women aged mainly 35-70 from 10 European countries.7 8 Eligible participants were selected from the general population, except in France, where selection was based on members of the health insurance system or state school employees, and in Utrecht (the Netherlands), where selection was based on women attending screening for breast cancer. Participants gave informed consent and completed questionnaires on diet and lifestyle. The present analyses included participants free from cancer at recruitment and who were not in the top or bottom 1% of the ratio of energy requirement to energy expenditure (n=478 478). Participants with incomplete information on alcohol consumption at recruitment or in the past (n=114 481) and missing dietary information (n=9) were excluded, leaving 363 988 men and women from France, Italy, Spain, the Netherlands, United Kingdom, Greece, Germany, and Denmark. France and Utrecht enrolled only women. As we wanted to consider the risk of cancer incidence associated with former alcohol consumption, we had to exclude the centres of Norway, Sweden, Bilthoven, and Naples because they did not have information on past consumption.
Alcohol consumption at recruitment (in grams per day) was measured with a validated dietary questionnaire assessing frequency and portion size of beer/cider, wine, spirits, and fortified wine covering the 12 months before recruitment.9 10 11 Consumption in the past was assessed as self reported consumption of beer, wine, and spirits at the ages of 20, 30, 40, and 50. Based on consumption in the past and at recruitment we distinguished between never (no consumption in the past and no consumption at recruitment), former (consumption in the past but no consumption at recruitment), and lifetime consumers (consumption in the past and at recruitment). For lifetime consumers, consumption as applied in this analysis reflects the past years’ consumption before recruitment.
We obtained information on incidence of cancer through record linkage with regional cancer registers in countries with passive follow-up (Denmark, Italy, the Netherlands, Spain, UK) or by a combination of methods including medically verified self reports of the participant or the next of kin, cancer or pathology registers, health insurance records, or death certificates in countries with active follow-up (France, Germany, Greece). The follow-up ended between 2002 and 2005,12 and loss to follow-up was relatively low, with <2% in all countries irrespective of active or passive follow-up. We investigated cancers with a causal association to alcohol consumption3 (colorectal (C18-21, ICD-O (international classification of diseases-oncology, 2nd revision), upper aerodigestive tract (C00-10, C12-15, C32), liver (C22), female breast (C50)), as well as total cancer (C00-C80, except C44 skin cancer) and alcohol related cancers combined (upper aerodigestive tract, colorectal, liver, and, for women, female breast cancer). ICD codes of cancer end points were in accordance with the GLOBOCAN-2008 cancer definitions.13
Statistical analysis
We combined hazard rate ratios derived from the EPIC study with representative data on alcohol consumption from the general population. Cox proportional hazard regressions were applied to compute hazard rate ratios14 during a mean follow-up time of 8.8 years for alcohol consumption among lifetime consumers per 12 g/day increment (equivalent to one drink of any alcoholic beverage) and for former compared with never consumers and incidence of first primary cancer. Age was used as the underlying time variable with entry and exit time defined as the participant’s age at recruitment and age at diagnosis of cancer or at censoring, respectively. Results on some single cancer outcomes have been published earlier.15 16 17 18 19 20 We used updated information and recomputed hazard rate ratios for these sites. To control for age and variations in study procedures across the EPIC centres we stratified the analyses by age (in 1 year categories) and centre.
All models were run separately for men and women, and included the following potential confounders, which were measured at recruitment: smoking (never; past <10 years ago, ≥10 years ago; current <15, 15-25, or ≥25 cigarettes/day, other (cigars, pipe, cigarettes with missing dose)) and smoking duration (<10, 10-<20, 20-<30, 30-<40, ≥40 years, missing (4.1%)); education (higher education/university, technical school, secondary school, primary school, none or missing (1.2%)); physical activity (inactive, moderately inactive, moderately active, active)21; body mass index (BMI; kg/m²); consumption (g/day) of meat and meat products, fish, fruits and vegetables; fibre, and non-alcohol energy intake (kJ/day); and, for women, menopausal status (premenopausal, postmenopausal/surgical, perimenopausal), age at menarche (<13, 13/14, >14 years, missing (30.8%)), and whether she had ever breast fed (yes, no, or missing (32.9%)), ever used oral contraceptives (yes, no, or missing (31.6%)), and ever used hormone replacement therapy (yes, no, or missing (30.6%)).
Restricted cubic spline regressions (knots: p10, p50, p90 and p25, p50, p75) did not indicate deviation from linearity of associations between alcohol and risk of cancer among lifetime consumers, except for liver cancer in men (P<0.01 for non-linearity). We therefore used regression coefficients (β) as risk functions to express the risk for cancer incidence per 1 g/day increment in alcohol consumption among current lifetime consumers. For simplicity and for comparability we also used this approach for liver cancer in men.
We tested effect modification by smoking for the site specific cancers by including product terms of smoking status (never, former, current smoker) with alcohol (g/day) and performing the likelihood ratio test between nested models. There was indication for an effect modification by smoking for upper aerodigestive tract and liver cancer in men and for colorectal cancer in women (P<0.1).
Heterogeneity of hazard rate ratios across centres was examined by the meta-analytic approach22 23 and by including interaction terms of centre and alcohol (g/day) in the models and applying likelihood ratio tests between nested models. There was significant heterogeneity across centres for liver cancer in men and colorectal cancer in women. Reasons for heterogeneity are unknown. Reduction in alcohol consumption could have been the consequence of pre-diagnostic diseases (such as liver cirrhosis) or positive results on screening (such as for liver function markers or colon polyps) performed in some but not all EPIC countries. This could lead to reverse causation2 24 25 and thus to attenuation of the association between alcohol and cancer in these centres, resulting in heterogeneity of the association across centres. This speculation was supported by the fact that the heterogeneity was no longer present when we excluded the first four years of follow-up.
All statistical tests were two sided with significance at the 5% level.
Alcohol attributable fractions
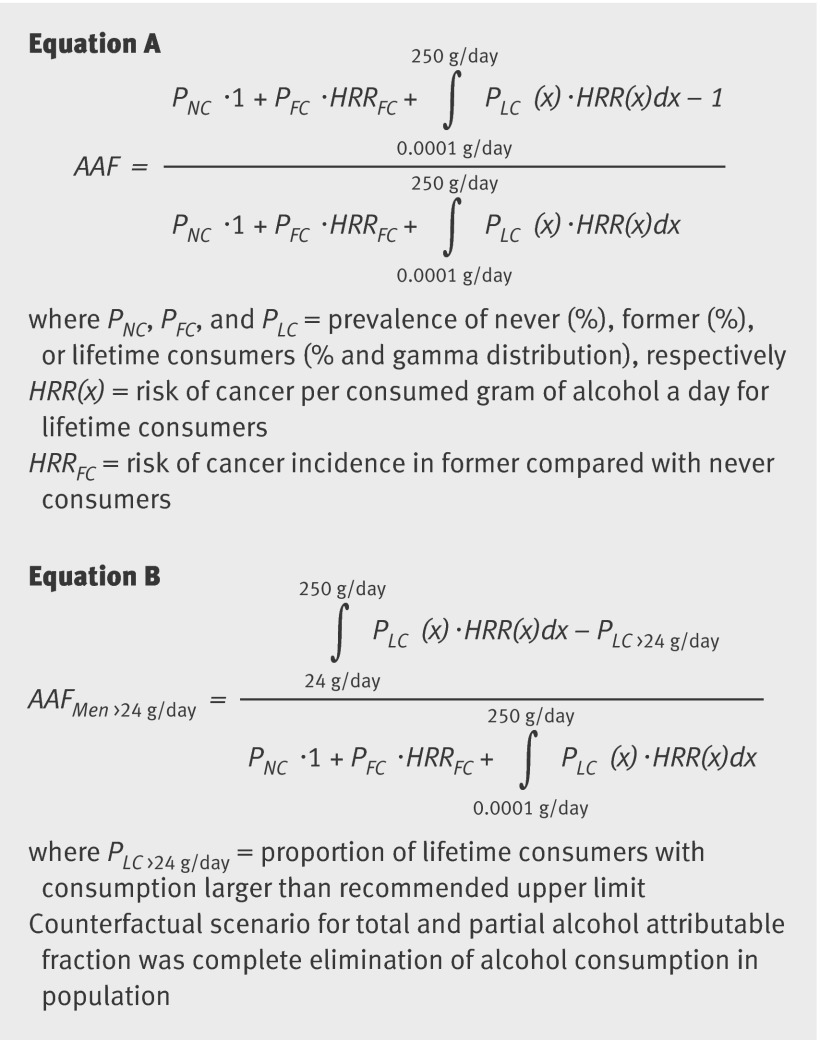
The computation of alcohol attributable fractions requires not only the information on relative risks for alcohol consumption (such as hazard rate ratios) but also information on the distribution of alcohol consumption within the general population. We computed alcohol exposure data from the general population following an algorithm (triangulation)26 that combined information of alcohol consumption from survey data as reported by the World Health Organization27 and per capita consumption28 for each country and separately for men and women aged ≥15. These sex and country specific data on alcohol exposure were modelled as gamma function29 in current consumers of alcohol by applying a formula30 based on the triangulated population mean alcohol intake (combining alcohol survey information with data on per capita alcohol consumption).26 These gamma functions were shown to fit and best model the right skewed distribution of alcohol consumption on the population level. Furthermore, this exhibits the clear advantage of using alcohol consumption continuously, as the estimation of alcohol attributable fractions based on alcohol categories might lose valuable information. We also obtained information on the proportions of never and former consumers of alcohol from WHO, which used the GENACIS survey as source of information, except for Greece, for which data were derived from a national survey on licit and illicit drug use.31 We then calculated the country and sex specific alcohol attributable fractions reflecting the burden of cancer incidence associated with total alcohol consumption on the population level (equation A, fig 1).
Fig 1 Equations for computation of alcohol attributable fractions
We also computed the part of the alcohol attributable fraction (partial alcohol attributable fraction) that reflects the burden of cancer incidence associated with alcohol consumption higher than the recommended upper limit6 of two standard drinks a day (>24 g/day) for men and one standard drink a day (>12 g/day) in women (equation B, fig 1).
For adjusted risk estimates, the prevalence of exposure among cases rather than among the general population should be used,32 33 34 35 36 which is hardly feasible because no exposure information in cases from the general population is available. We therefore performed sensitivity analyses by simulating the distribution of alcohol exposure among cases by using the alcohol exposure information in cancer cases in the EPIC study and shifting this curve towards the alcohol exposure information of the general population (by deriving weights by dividing the gamma distribution of the general population by the gamma distribution of the EPIC participants). Doing so, we simulated the alcohol exposure distribution of cases as if they originated from the general population. As expected, the estimated mean alcohol consumption among cancer cases was higher than among the general population. Alcohol attributable fractions were stratified by sex but not by country because of sparse numbers of cases in some countries. This was also why we applied this approach as a sensitivity analysis and not as our main approach.
As smoking seemed to modify the association between alcohol and cancers of the upper aerodigestive tract and liver in men and colorectal cancer in women, we also computed hazard rate ratios for alcohol intake (continuous, per 12 g/day and for former v never consumers) and cancer incidence among never smokers. For upper aerodigestive tract and liver cancer the number of cases became low in men. We therefore computed the hazard rate ratios in never smokers for men and women combined with additional adjustment for sex. Alcohol attributable fractions were recomputed by replacing the smoking adjusted hazard rate ratios from the total EPIC cohort by the hazard rate ratios in never smokers.
We used 10 000 Monte-Carlo simulated alcohol attributable fractions, considering the uncertainty of the hazard rate ratios, to compute the variances, standard errors, and corresponding 95% confidence intervals of the alcohol attributable fractions. We estimated the absolute number of alcohol attributable cancer cases by multiplying the alcohol attributable fractions with the total number of incidental cancer cases from 2008 derived from the GLOBOCAN 2008 project.13
The analyses were performed with SAS, version 9.2, and R, version 2.9.1.
Results
Across the countries investigated, alcohol consumption followed a north south gradient with Greece and Spain having the highest proportions of never and former consumers, and Denmark and Germany having the highest proportion of lifetime consumers (table 1). This gradient was also seen for the proportions of alcohol consumption higher than the recommended upper limit with Greece and Spain showing the lowest and Germany and Denmark the highest proportions.
Table 1.
Proportions of never, former, and lifetime consumers of alcohol and mean alcohol consumption in lifetime consumers in general adult population aged 15 years or older
| Country | Never consumers (%) | Former consumers (%) | Lifetime consumers (%) | Mean* (SD) g/day | % drinking over daily recommended upper limit† |
|---|---|---|---|---|---|
| Men | |||||
| Denmark | 0.6 | 2.5 | 96.9 | 32.9 (38.7) | 42.6 |
| Germany | 1.3 | 2.6 | 96.1 | 34.9 (41.0) | 43.8 |
| Greece | 6.7 | 9.3 | 84.0 | 27.4 (32.3) | 32.4 |
| Italy | 5.8 | 3.9 | 90.3 | 30.1 (35.3) | 37.3 |
| Spain | 9.5 | 23.4 | 67.1 | 33.0 (38.8) | 29.5 |
| UK | 8.9 | 1.5 | 89.7 | 35.2 (41.4) | 41.1 |
| Total‡ | 5.6 | 6.2 | 88.2 | 33.2 (39.0) | 39.0 |
| Women | |||||
| Denmark | 0.9 | 7.0 | 92.1 | 17.5 (21.5) | 41.0 |
| France | 3.3 | 7.1 | 89.6 | 16.7 (20.6) | 38.7 |
| Germany | 2.0 | 2.6 | 95.3 | 18.1 (22.3) | 43.5 |
| Greece | 21.0 | 19.0 | 60.0 | 14.4 (17.9) | 23.4 |
| Italy | 19.4 | 6.0 | 74.6 | 12.4 (15.6) | 25.8 |
| Netherlands | 16.7 | 19.1 | 64.1 | 15.4 (18.1) | 27.1 |
| Spain | 24.7 | 31.6 | 43.8 | 13.4 (16.7) | 16.1 |
| UK | 15.2 | 2.9 | 81.9 | 17.6 (20.6) | 37.7 |
| Total‡ | 11.7 | 9.0 | 79.3 | 15.9 (19.7) | 33.2 |
*Mean alcohol consumption computed among lifetime consumers.
†>24 g/day in men; >12 g/day in women.
‡Weighted average by using population size data of population from each country.
Among male and female lifetime consumers, the risk for all the cancers we included increased with each additional drink a day (table 2). Former consumption compared with never was associated with a considerably higher risk for total and alcohol related cancer in men. We could not compute the risk for former consumers of alcohol and upper aerodigestive tract and liver cancer in men because of low number of cases in those who had never consumed alcohol. Hence, we computed alcohol attributable fractions for upper aerodigestive tract and liver cancer in men based on the hazard rate ratio for former alcohol consumption and total cancer.
Table 2.
Adjusted hazard rate ratios (HRRs)* (95% confidence intervals) per 12 g/day increment for lifetime consumers and for former versus never consumers (reference category) of alcohol
| Cancer site | Continuous (per 12 g/day)† | Former consumers‡ | |||
|---|---|---|---|---|---|
| No of cases | HRR (95% CI) | No of cases | HRR (95% CI) | ||
| Men | |||||
| Total cancer | 5726 | 1.03 (1.02 to 1.04) | 403 | 1.54 (1.20 to 1.98) | |
| Alcohol related | 1235 | 1.10 (1.07 to 1.12) | 91 | 3.72 (1.81 to 7.65) | |
| Upper aerodigestive tract | 272 | 1.17 (1.12 to 1.23) | — | 1.54§ (1.20 to 1.98) | |
| Colorectum | 859 | 1.05 (1.02 to 1.09) | 53 | 2.19 (0.99 to 4.83) | |
| Liver | 104 | 1.13 (1.04 to 1.22) | — | 1.54§ (1.20 to 1.98) | |
| Women | |||||
| Total cancer | 12 467 | 1.03 (1.01 to 1.05) | 776 | 1.10 (1.01 to 1.20) | |
| Alcohol related | 6671 | 1.05 (1.03 to 1.07) | 354 | 1.04 (0.92 to 1.19) | |
| Upper aerodigestive tract | 113 | 1.25 (1.10 to 1.42) | 9 | 0.65 (0.27 to 1.56) | |
| Colorectum | 1245 | 1.04 (0.99 to 1.09) | 79 | 1.05 (0.79 to 1.40) | |
| Liver | 54 | 1.09 (0.89 to 1.33) | 10 | 2.28 (0.89 to 5.85) | |
| Breast | 5259 | 1.05 (1.02 to 1.07) | 256 | 1.03 (0.88 to 1.20) | |
*Adjusted for smoking (dose and duration); education; physical activity; BMI; consumption of meat, fish, fruit and vegetables; fibre, and non-alcoholic energy intake (kJ/day); and, for women additionally, menopausal status, age at menarche, breast feeding, oral contraceptive use, hormone replacement therapy.
†Among lifetime consumers (102 648 men, 216 149 women). Log linear estimates used to establish risk functions.
‡Reference category=never consumers of alcohol.
§When there were no or a limited number of cases in reference category of never consumers, HRR for total cancer was used.
If we assume causality, these estimates would translate into 10% (95% confidence interval 7% to 13%) of total cancer in men (table 3) and 3.0% (1% to 5%) of total cancer in women (table 4) being attributable to alcohol consumption in these selected European countries. In both sexes the alcohol attributable fraction was highest for cancer of the upper aerodigestive tract (44% (31% to 56%) in men; 25% (5% to 46%) in women), followed by liver cancer (33% (11% to 54%) and 18% (−3% to 38%), respectively). Alcohol consumption was associated with 17% (10% to 25%) of cases of colorectal cancer in men and 4% (−1% to 10%) in women. Also, 5% (2% to 8%) of cases of breast cancer in women could be associated with total alcohol consumption. The alcohol attributable fractions varied across countries because of the differences in alcohol exposure, with relatively high alcohol attributable fractions for Spanish men compared with men in other countries. Confidence intervals of the alcohol attributable fractions, however, overlapped for all countries in both men and women.
Table 3.
Proportion of cancer cases attributable to alcohol use in men aged ≥15 years. Figures are percentages (95% confidence interval)
| Cancer site | Denmark | Germany | Greece | Italy | Spain | UK | Total |
|---|---|---|---|---|---|---|---|
| Total cancer | 8 (5 to 12) | 9 (5 to 12) | 10 (7 to 12) | 8 (5 to 11) | 15 (13 to 17) | 8 (5 to 11) | 10 (7 to 13) |
| Alcohol related | 29 (22 to 35) | 30 (23 to 37) | 33 (29 to 36) | 28 (23 to 33) | 46 (44 to 49) | 27 (21 to 34) | 32 (27 to 38) |
| Upper aerodigestive tract | 45 (32 to 57) | 47 (34 to 60) | 37 (26 to 47) | 40 (29 to 52) | 41 (31 to 51) | 45 (32 to 58) | 44 (31 to 56) |
| Colorectum | 15 (7 to 24) | 16 (7 to 25) | 18 (12 to 23) | 15 (8 to 22) | 28 (23 to 32) | 14 (5 to 23) | 17 (10 to 25) |
| Liver | 34 (10 to 57) | 35 (11 to 59) | 28 (10 to 45) | 30 (9 to 51) | 32 (15 to 49) | 33 (10 to 57) | 33 (11 to 54) |
Table 4.
Proportion of cancer cases attributable to alcohol use in women aged ≥15 years. Figures are percentages (95% confidence interval)
| Cancer site | Denmark | France | Germany | Greece | Italy | Netherlands | Spain | UK | Total |
|---|---|---|---|---|---|---|---|---|---|
| Total cancer | 3 (1 to 5) | 3 (1 to 5) | 3 (1 to 5) | 3 (2 to 4) | 2 (1 to 3) | 3 (2 to 5) | 4 (3 to 5) | 3 (1 to 5) | 3 (1 to 5) |
| Alcohol related | 7 (4 to 10) | 6 (4 to 9) | 7 (4 to 10) | 4 (3 to 6) | 4 (2 to 6) | 5 (3 to 7) | 4 (3 to 5) | 6 (3 to 9) | 5 (3 to 8) |
| Upper aerodigestive tract | 32 (9 to 55) | 30 (8 to 52) | 35 (11 to 59) | 15 (−1 to 31) | 18 (4 to 32) | 17 (0 to 34) | 5 (−8 to 18) | 30 (9 to 51) | 25 (5 to 46) |
| Colorectum | 5 (−2 to 12) | 5 (−1 to 11) | 6 (−2 to 13) | 4 (0 to 7) | 3 (−1 to 7) | 4 (0 to 8) | 3 (1 to 6) | 5 (−2 to 11) | 4 (−1 to 10) |
| Liver | 18 (−8 to 44) | 17 (−7 to 42) | 15 (−16 to 46) | 24 (12 to 36) | 13 (−3 to 29) | 24 (11 to 38) | 31 (24 to 38) | 13 (−13 to 39) | 18 (−3 to 38) |
| Breast | 6 (3 to 10) | 6 (3 to 9) | 7 (3 to 10) | 4 (2 to 6) | 4 (2 to 6) | 4 (2 to 6) | 3 (2 to 4) | 5 (2 to 8) | 5 (2 to 8) |
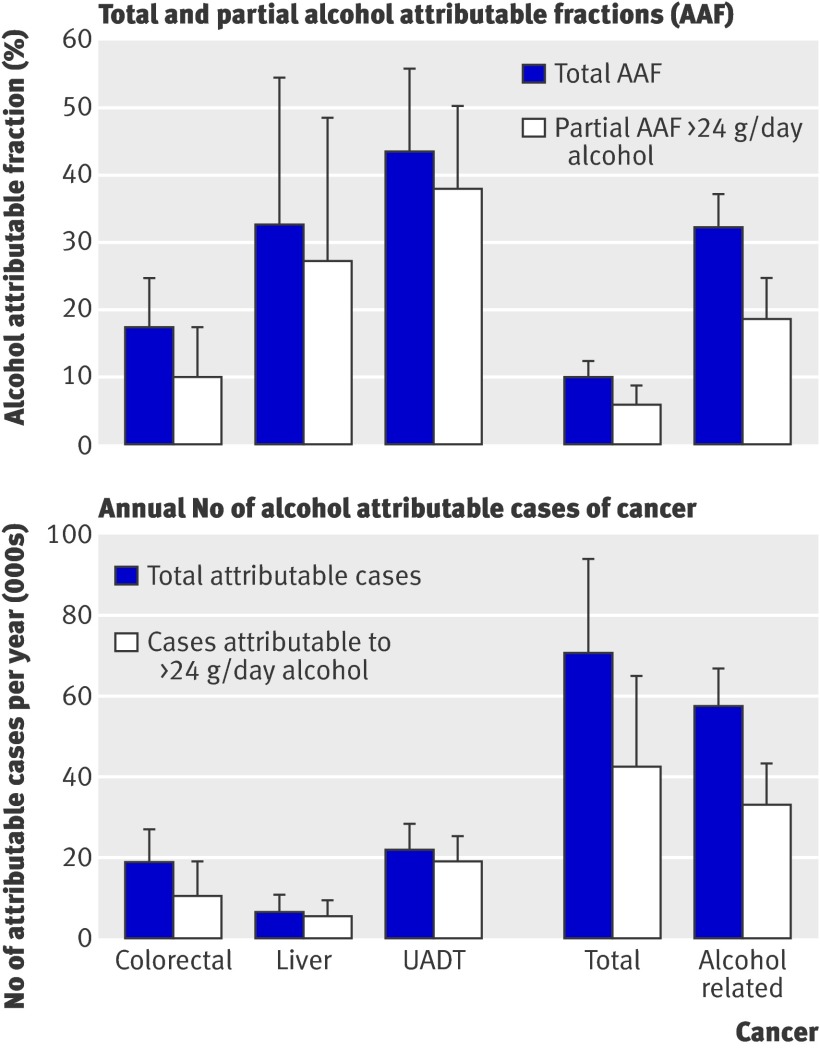
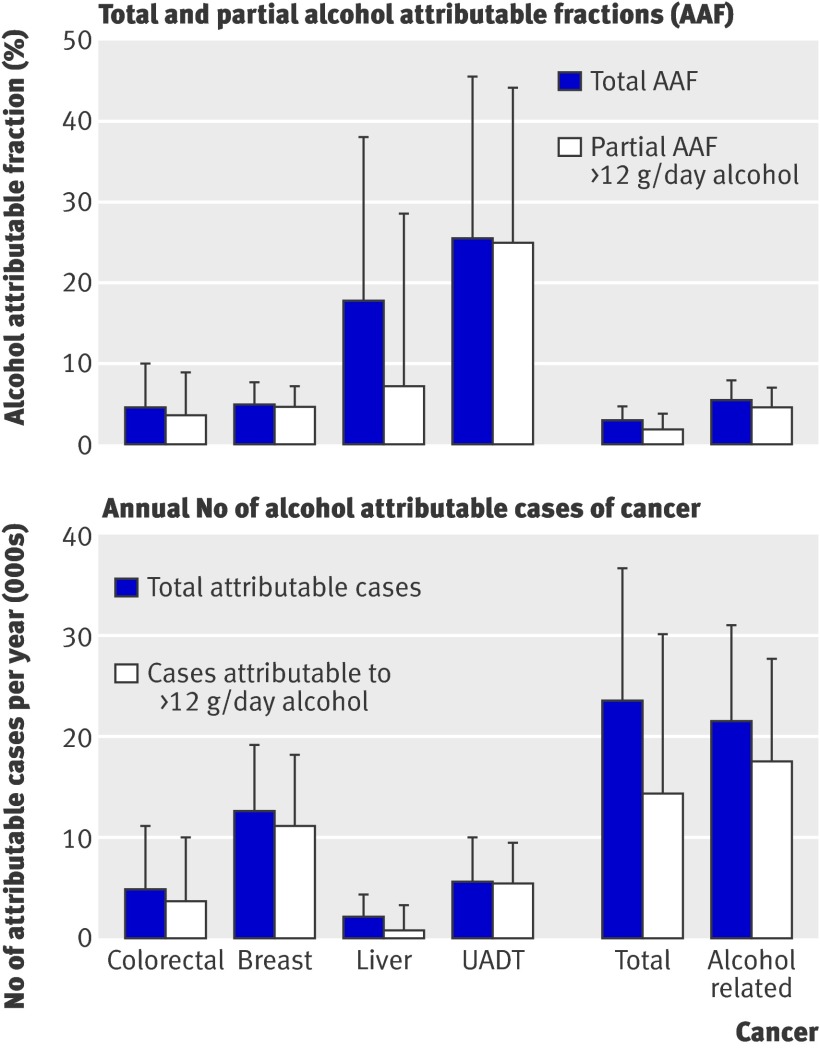
Partial attributable fractions for alcohol consumption higher than two drinks a day in men accounted for 10% of colorectal cancer, 27% of liver cancer, and 38% of upper aerodigestive tract cancer (fig 2), which accounted for 57% to 87% of the total alcohol attributable fractions. The proportion of cancer associated with alcohol consumption higher than the recommended upper limit did not vary much by country in men, except for Greece and Spain, where partial alcohol attributable fractions were somewhat lower because of the lower proportions of men consuming more than two drinks a day. In women, partial alcohol attributable fractions accounted for 3% of colorectal cancer, 4% of breast cancer, 7% of liver cancer, and 25% of upper aerodigestive tract cancer (fig 3), which accounted for 40% to 98% of the total alcohol attributable fractions. For all cancers investigated in women, the partial alcohol attributable fraction was lowest in Spain, Greece, and Italy and highest in Germany, Denmark, and the UK. When we compared total with partial alcohol attributable fractions, a substantial part (40-98%) of the incidence of alcohol attributable cancer occurred because of alcohol consumption higher than the recommended upper limit in both men and women. The remaining part of the total alcohol attributable fraction (2-60%) was associated with consumption of less than the recommended upper limit and former consumption. In men, about three in 100 alcohol related cancer cases were associated with alcohol consumption of ≤24 g/day and more than 18 in 100 were associated with alcohol consumption >24 g/day. In women one in 100 alcohol attributable cancer cases was associated with alcohol consumption of ≤12 g/day and about four in 100 associated with alcohol consumption >12 g/day.
Fig 2 Total and partial alcohol attributable fractions with 95% confidence intervals and corresponding number of cases of cancer with 95% confidence intervals in men in selected EPIC countries (Italy, Spain, UK, Greece, Germany, Denmark) in 2008. UADT=upper aerodigestive tract
Fig 3 Total and partial alcohol attributable fractions with 95% confidence intervals and corresponding number of cases of cancer with 95% confidence intervals in women in selected EPIC countries (France, Italy, Spain, UK, Netherlands, Greece, Germany, Denmark) in 2008. UADT=upper aerodigestive tract
In terms of total numbers of cases of alcohol related cancer, and if we accept that there is a causal association between alcohol consumption and occurrence of cancer, in 2008, 33 037 of 178 578 alcohol related cancer cases in men and 17 470 of 397 043 alcohol related cancer cases in women were associated with alcohol consumption of more than two (one for women) drinks a day. Cancer of the upper aerodigestive tract accounted for the highest number of alcohol attributable cases in men (22 022 cases), with Germany showing most cases (table 5). In women, breast cancer contributed most to the number of alcohol attributable cancer cases with 12 589 cases (fig 2, table 5 ). The numbers of total alcohol attributable cancer cases varied considerably by country, mainly because of different population sizes in the investigated countries but also because of varying alcohol attributable fractions across the countries.
Table 5.
Total number* of alcohol attributable cancer cases for general population in 2008 in selected countries
| Cancer site | Denmark | France | Germany | Greece | Italy | Netherlands | Spain | UK | Total |
|---|---|---|---|---|---|---|---|---|---|
| Men | |||||||||
| Total | 1313 | NA | 22 388 | 1968 | 14 556 | NA | 18 173 | 12 564 | 71 519 |
| Alcohol related | 996 | NA | 17 967 | 1107 | 12 332 | NA | 14 295 | 9299 | 57 596 |
| Upper aerodigestive tract | 445 | NA | 7864 | 351 | 7045 | NA | 4236 | 5133 | 22 022 |
| Colorectum | 348 | NA | 6053 | 318 | 4206 | NA | 4602 | 2936 | 18 836 |
| Liver | 64 | NA | 1797 | 178 | 2414 | NA | 1170 | 700 | 6465 |
| Women | |||||||||
| Total | 503 | 4415 | 6561 | 524 | 3254 | 1282 | 2996 | 3791 | 23 307 |
| Alcohol related | 459 | 4 717 | 7240 | 293 | 2076 | 939 | 1376 | 4058 | 21 520 |
| Upper aerodigestive tract | 146 | 1 359 | 1698 | 39 | 528 | 217 | 395 | 1589 | 5499 |
| Colorectal | 116 | 923 | 1771 | 59 | 744 | 221 | 411 | 787 | 4919 |
| Liver | 13 | 269 | 376 | 78 | 529 | 29 | 442 | 169 | 2020 |
| Breast | 259 | 2 956 | 4193 | 177 | 1734 | 550 | 680 | 2498 | 12 589 |
NA=not applicable.
*Numbers refer to total burden of incident cancer cases associated with current and former alcohol consumption in population. Sum of cases for single countries might not exactly add to total number of cases because of country specific estimation of numbers and separate estimation for all countries combined.
The sensitivity analysis using the alcohol consumption data in the cancer cases only had similar results to those in tables 3 and 4 (data not shown). The maximum deviation was 3 percentage points in men for alcohol related cancers (29% v 32%) and 2 percentage points in women for upper aerodigestive tract cancer (23% v 25%).
Given there is a causal association between alcohol consumption and risk of cancer in people who have never smoked, sensitivity analyses with the hazard rate ratios of never smokers indicated noticeable differences compared with alcohol attributable fractions that were based on hazard rate ratios adjusted for smoking from the total cohort, particularly for liver cancer, for which the alcohol attributable fraction in men who had never smoked (AAFSens) was 78% compared with 33% in the total population, and for upper aerodigestive tract cancer, for which the AAFSens was 14% compared with 44%. The alcohol attributable fractions for colorectal cancer in women differed by 3 percentage points with AAFSens 1% v 4%, which was, however, within the confidence interval computed for the alcohol attributable fraction based on estimates of the total cohort.
Discussion
If we assume causality, our analysis shows that about 10% of total cancer in men and 3% in women could be attributed to current and former alcohol consumption in the European countries included in this study. In relative terms, the alcohol attributable fraction of cancer incidence was highest for cancer of the upper aerodigestive tract, followed by liver cancer. The highest absolute number of alcohol attributable cancer cases in men was found for upper aerodigestive tract and in women for breast cancer. Furthermore, a substantial part of the alcohol attributable cancer cases were associated with consumption of more than two or one standard drinks per day for men and women, respectively.
Comparison with other studies
Few previous studies have reported on alcohol attributable mortality37 38 39 40 41 42 43 or incidence4 5 44 45 of cancer. Published estimates for alcohol attributable incidence of cancer in Europe38 and France44 were of similar magnitude to our estimates. For women both higher5 45 and lower44 alcohol attributable fractions for single cancer sites were reported. Differences could emerge because we considered also the risk of cancer associated with former consumption of alcohol, or because of the risk functions used by one study,44 which were not derived for men and women separately,46 or because of the different application of alcohol exposure data. One British study used population means of alcohol consumption,5 while we applied gamma distributions, which better represent the right skewness of the data on alcohol consumption. Because of the various methods used to compute the alcohol attributable fractions in previous studies and because of limited data, no comparable estimates across the European countries on the alcohol attributable burden of cancer have been available until now.
Besides the total burden, we also quantified the burden of cancer incidence associated with exceeding the recommended maximal daily limit of alcohol. We found that a substantial part of this incidence was associated with consumption above the recommended upper limit, indicating the potential for cancer prevention merely by adhering to the current recommendations. For cancer sites with markedly higher risks for former compared with never consumers—such as liver cancer —a noticeable part of the total alcohol attributable fractions was associated with former consumption. That also explains why for those cancers the partial alcohol attributable fractions associated with consumption above the recommended upper limit were lower than for cancers with less strong risk estimates in former consumers, such as breast cancer. Alcohol consumption below the recommended upper limit accounted for a modest part of the total alcohol attributable fraction of alcohol related cancers, with at least three in 100 cases of cancer in men and one in 100 cases in women. This shows that following the current recommendation would not eliminate alcohol attributable cancer incidence completely. In contrast, for all cause mortality alcohol consumption is often shown to be associated with a lower risk for up to four drinks a day in men and two drinks a day in women.47 This lower risk is probably because of the lower risk of death from cardiovascular disease, especially coronary heart disease and ischaemic stroke.48 49 50 Heavy alcohol consumption above the recommended upper limit, however, was shown to be not related to48 or detrimental for46 cardiovascular diseases, whereas for cancer, as shown by many studies46 51 including ours, there is no sensible limit below which the risk of cancer is decreased. Therefore, even though light to moderate alcohol consumption might decrease the risk for cardiovascular disease and mortality, the net effect of alcohol is harmful.1 Thus, alcohol consumption should not be recommended to prevent cardiovascular disease or all cause mortality.
Sensitivity analysis
Smoking, known to be closely related to alcohol consumption, could be a potential synergistic risk factor, particularly for cancer of the upper aerodigestive tract. A possible synergistic effect modification of smoking on the risk of alcohol and cancer could lead to an overestimation of the alcohol attributable fraction.52 We observed a substantially higher alcohol attributable fraction for liver cancer and a considerably lower alcohol attributable fraction for upper aerodigestive tract cancer in men when we applied the hazard rate ratios for alcohol consumption among never smokers. Potential effect modification by smoking was also indicated for colorectal cancer in women, for which the alcohol attributable fraction computed by using hazard rate ratios among never smokers was lower than the overall alcohol attributable fraction, but within the 95% confidence interval. In the groups of never smokers the number of cases of cancer was limited in the EPIC study, which led to a limited power to assess the association between the consumption of alcohol and risk of cancer in this subgroup. This could be one explanation why the alcohol attributable fractions differed from the originally computed overall alcohol attributable fractions. We could also have overestimated the alcohol attributable fraction for upper aerodigestive tract cancer in men because of the effect of smoking. However, a recent pooled analysis of 17 European and American case-control studies investigating people who had ever consumed alcohol compared with those who had never consumed alcohol in relation to head and neck cancer in people who had never smoked,4 53 estimated a population attributable fraction of 29.5% in men and 31.5% women. These estimates are of similar magnitude to our estimates based on results from the total EPIC study population, suggesting the estimates of our overall alcohol attributable fractions to be valid. Regarding liver cancer, smoking is a recognised causal risk factor.3 Thus, there is no plausible explanation for the substantial higher alcohol attributable fraction for liver cancer based on risk estimates computed in those who had never smoked. Therefore, the limited power because of low numbers of cases and the resulting imprecise point estimates of the hazard rate ratios in never smokers is the most plausible explanation for the considerably higher alcohol attributable fraction of liver cancer.
Advantages and limitations
Our results are limited by the underlying data quality to generate the relative risk functions and by the data on alcohol exposure. The risk estimates were adjusted for several confounders—for example, dietary factors or extensive adjustment for smoking, the main confounder for the association between alcohol and risk of cancer. Different confounding in the various countries we investigated is unlikely to play a major role because the relative risk estimates were observed to be relatively homogenous across the countries. Also, the relative risk estimates are in line with results from meta-analyses24 46 54 and with the evaluation of the carcinogenicity of alcohol,26 which supports the validity of our effect estimates for alcohol consumption and risk of cancer. Loss to follow-up, though low, could have led to underestimation of the risk of cancer, as high risk or exposed people are more likely to be lost to follow-up. Recall bias is unlikely to have had an impact on the relative risk estimates as the exposure information was assessed before the cancer occurred. Also, under-reporting of alcohol consumption because of social desirability should not have affected our risk estimates, assuming that under-reporting was independent of later case and non-case status and that this did not change the ranking of the study participants. As the EPIC study population is a convenience sample, the transferability of study results to the general population could be questioned. Selective participation, however, should not impair aetiological conclusions expressed as relative risk estimates because these effect measures are both internally and externally valid.8 55
The data on alcohol consumption were representative for the countries investigated, and they were quantified comparably across all countries.27 Thus, the exposure information is highly comprehensive and comparable, resulting in directly comparable alcohol attributable fractions across the selected countries. Comparison of previous country specific alcohol attributable fraction estimates was impeded as the studies used different methods and strategies to compute the alcohol attributable burden of cancer incidence.4 5 44 45 As the prevalence of alcohol exposure differs across the European countries, however, we would also expect the burden of alcohol attributable cancer incidence to differ across the countries, which is of potential interest for public health policy makers.
We have provided a systematic and comparable overview of the alcohol attributable cancer incidence for several European countries, and presented alcohol attributable fractions for causally related cancer sites based on empirical original data for both the total (current and former) alcohol consumption as well as for alcohol consumption higher than the recommended upper limit. We considered the risk from former alcohol consumption and could thus capture, in contrast with previous studies, the full burden of cancer incidence associated with alcohol consumption. The gamma distributions we used for current alcohol consumption overcome the limitation of using categorical risk estimates and categories of proportions of alcohol consumption. This is of particular value because the risk of cancer increases linearly and alcohol consumption follows a strongly right skewed distribution. The attributable burden of cancer incidence associated with consuming above the recommended upper limit illustrates the potential of avoidable cancer incidence, if the recommendations of the WCRF/AICR6 are followed. Until now, it was only speculated that reducing alcohol consumption to two drinks a day in men and one drink a day in women would be beneficial in terms of incidence of cancer. We have now computed quantitative measures, both relative (alcohol attributable fraction) and absolute (total number of cancer cases), for the burden associated with alcohol consumption above the recommended upper limit.
Conclusions and policy implications
In conclusion, if we assume causality between alcohol consumption and overall and specific cancer incidence, a considerable proportion of the most common and most lethal cancers is attributable to former and current alcohol consumption in the selected European countries, especially to consumption above the recommended upper limit. This strongly underlines the necessity to continue and to increase efforts to reduce alcohol consumption in Europe,56 both on the individual and the population level.
What is already known on this topic
Alcohol consumption has been causally related to cancers of the oral cavity, pharynx, larynx, oesophagus, liver, colorectum, and female breast
Current estimates of the alcohol attributable burden of these cancers refer mostly to current alcohol consumption and to Europe as a whole, and do not include the risk associated with previous alcohol consumption
What this study adds
If we assume causality between alcohol consumption and cancer, about 10% of all cancer cases in men and 3% of all cancer cases in women are attributable to current and former alcohol consumption in the investigated European countries
For cancers that are causally related to alcohol consumption, the proportions were 32% in men and 5% in women, with a substantial part (40-98%) being attributable to current alcohol consumption above the recommended upper limit of two drinks a day in men and one drink a day in women
Contributors: All authors had full access to all of the data (including statistical reports and tables) and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors MS, MMB, TP, HB, JR, TK, and ER were responsible for study concept and design. HB, TJK, K-TK, PP, AB, MD, M-DC, MJS, LR, AA, PV, RT, FB, DP, AT, RK, FC-C, KO, AMT, AO, CD, M-CB-R, VB, DZ, SR, CvG, NW, MJ, NS, DR, PAW, and ER acquired the data. MS, MMB, HB, TP, JR, TK, GG, PB, NEA, and PP analysed and interpreted the data. MS, MB, TP, HB, JR, TK, NEA, KO, PP, and PB drafted the manuscript, which was critically revised for important intellectual content by all the authors. MS, JR, TK, GG, TP, and MMB were responsible for the statistical analysis. MMB, JR, and HB supervised the study. MS is guarantor.
Funding: The work was performed (partly) within the coordinated action EPIC (SP23-CT-2005-006438), which has received research funding from the Community’s Sixth Framework Programme, as well as by the “Europe Against Cancer” Programme of the European Commission (SANCO); Deutsche Krebshilfe; German Cancer Research Center; German Federal Ministry of Education and Research; Danish Cancer Society; Health Research Fund (FIS) of the Spanish Ministry of Health (grant No: Network RCESP C03/09); Spanish Regional Governments of Andalucia, Asturias, Basque Country, Murcia and Navarra; ISCIII, Red de Centros RETIC(RD06/0020) (grant No: C03/09); Cancer Research UK; Medical Research Council, UK; Stroke Association, UK; British Heart Foundation; Department of Health, UK; Food Standards Agency, UK; Wellcome Trust, UK; Italian Association for Research on Cancer (AIRC); Compagnia di San Paolo; Progetto Integrato Oncologia-PIO, Regione Toscana; Dutch Ministry of Public Health, Welfare and Sports; National Cancer Registry of the Netherlands; Greek Ministry of Health and Social Solidarity; Hellenic Health Foundation and Stavros Niarchos Foundation. The funders were independent of the research of the presented study.
Role of sponsors: The sponsors had no input in the design, the conduct, the analysis, or the interpretation of the study, and did not influence the manuscript preparation.
Competing interests: All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: The EPIC study was approved by the IARC ethical committee and by the local ethics committees relevant for each study centre. All participants gave informed consent.
Data sharing: No additional data available.
Cite this as: BMJ 2011;342:d1584
References
- 1.Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 2009;373:2223-33. [DOI] [PubMed] [Google Scholar]
- 2.Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, et al. Carcinogenicity of alcoholic beverages. Lancet Oncol 2007;8:292-3. [DOI] [PubMed] [Google Scholar]
- 3.Secretan B, Straif K, Baan R, Grosse Y, El Ghissassi F, Bouvard V, et al. A review of human carcinogens—part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol 2009;10:1033-4. [DOI] [PubMed] [Google Scholar]
- 4.Hashibe M, Brennan P, Chuang SC, Boccia S, Castellsague X, Chen C, et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol Biomarkers Prev 2009;18:541-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen NE, Beral V, Casabonne D, Kan SW, Reeves GK, Brown A, et al. Moderate alcohol intake and cancer incidence in women. J Natl Cancer Inst 2009;101:296-305. [DOI] [PubMed] [Google Scholar]
- 6.World Cancer Research Fund/American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. WCRF/AICR, 2007.
- 7.Riboli E, Hunt KJ, Slimani N, Ferrari P, Norat T, Fahey M, et al. European prospective investigation into cancer and nutrition (EPIC): study populations and data collection. Public Health Nutr 2002;5:1113-24. [DOI] [PubMed] [Google Scholar]
- 8.Riboli E, Kaaks R. The EPIC project: rationale and study design. European Prospective Investigation into Cancer and Nutrition. Int J Epidemiol 1997;26(suppl 1):S6-14. [DOI] [PubMed] [Google Scholar]
- 9.Kaaks R, Slimani N, Riboli E. Pilot phase studies on the accuracy of dietary intake measurements in the EPIC project: overall evaluation of results. European Prospective Investigation into Cancer and Nutrition. Int J Epidemiol 1997;26(suppl 1):S26-36. [DOI] [PubMed] [Google Scholar]
- 10.Sieri S, Agudo A, Kesse E, Klipstein-Grobusch K, San-Jose B, Welch AA, et al. Alcohol consumption in EPIC cohorts from ten European countries. IARC Sci Publ 2002;156:173-6. [PubMed] [Google Scholar]
- 11.Slimani N, Ferrari P, Ocke M, Welch A, Boeing H, Liere M, et al. Standardization of the 24-hour diet recall calibration method used in the European Prospective Investigation into Cancer and Nutrition (EPIC): general concepts and preliminary results. Eur J Clin Nutr 2000;54:900-17. [DOI] [PubMed] [Google Scholar]
- 12.Boffetta P, Couto E, Wichmann J, Ferrari P, Trichopoulos D, Bueno-de-Mesquita HB, et al. Fruit and vegetable intake and overall cancer risk in the European Prospective Investigation Into Cancer and Nutrition (EPIC). J Natl Cancer Inst 2010;102:529-37. [DOI] [PubMed] [Google Scholar]
- 13.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008: cancer incidence and mortality worldwide. 2008. www-dep.iarc.fr/.
- 14.Symons MJ, Moore DT. Hazard rate ratio and prospective epidemiological studies. J Clin Epidemiol 2002;55:893-9. [DOI] [PubMed] [Google Scholar]
- 15.Weikert C, Dietrich T, Boeing H, Bergmann M, Boutron-Ruault MC, Clavel-Chapelon F, et al. Lifetime and baseline alcohol intake and risk of cancer of the upper aero-digestive tract in the European Investigation into Cancer and Nutrition (EPIC) study. Int J Cancer 2009;125:406-12. [DOI] [PubMed] [Google Scholar]
- 16.Tjonneland A, Christensen J, Olsen A, Stripp C, Thomsen BL, Overvad K, et al. Alcohol intake and breast cancer risk: the European Prospective Investigation into Cancer and Nutrition (EPIC). Cancer Causes Control 2007;18:361-73. [DOI] [PubMed] [Google Scholar]
- 17.Rohrmann S, Linseisen J, Vrieling A, Boffetta P, Stolzenberg-Solomon RZ, Lowenfels AB, et al. Ethanol intake and the risk of pancreatic cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC). Cancer Causes Control 2009;20:785-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rohrmann S, Linseisen J, Key TJ, Jensen MK, Overvad K, Johnsen NF, et al. Alcohol consumption and the risk for prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev 2008;17:1282-7. [DOI] [PubMed] [Google Scholar]
- 19.Rohrmann S, Linseisen J, Boshuizen HC, Whittaker J, Agudo A, Vineis P, et al. Ethanol intake and risk of lung cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC). Am J Epidemiol 2006;164:1103-14. [DOI] [PubMed] [Google Scholar]
- 20.Ferrari P, Jenab M, Norat T, Moskal A, Slimani N, Olsen A, et al. Lifetime and baseline alcohol intake and risk of colon and rectal cancers in the European Prospective Investigation into Cancer and Nutrition (EPIC). Int J Cancer 2007;121:2065-72. [DOI] [PubMed] [Google Scholar]
- 21.Wareham NJ, Jakes RW, Rennie KL, Schuit J, Mitchell J, Hennings S, et al. Validity and repeatability of a simple index derived from the short physical activity questionnaire used in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Public Health Nutr 2003;6:407-13. [DOI] [PubMed] [Google Scholar]
- 22.Hardy RJ, Thompson SG. Detecting and describing heterogeneity in meta-analysis. Stat Med 1998;17:841-56. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bagnardi V, Blangiardo M, La Vecchia C, Corrao G. A meta-analysis of alcohol drinking and cancer risk. Br J Cancer 2001;85:1700-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arico S, Corrao G, Torchio P, Galatola G, Tabone M, Valenti M, et al. A strong negative association between alcohol consumption and the risk of hepatocellular carcinoma in cirrhotic patients. A case-control study. Eur J Epidemiol 1994;10:251-7. [DOI] [PubMed] [Google Scholar]
- 26.Rehm J, Klotsche J, Patra J. Comparative quantification of alcohol exposure as risk factor for global burden of disease. Int J Methods Psychiatr Res 2007;16:66-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. Global status report. Country profiles. WHO, 2011. www.who.int/substance_abuse/publications/global_alcohol_report/en/index.html.
- 28.World Health Organization. Global information system on alcohol and health (GISAH). 2007. http://apps.who.int/globalatlas/default.asp.
- 29.Rehm J, Kehoe T, Gmel G, Stinson F, Grant B. Statistical modeling of volume of alcohol exposure for epidemiological studies of population health: the US example. Popul Health Metr 2010;8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kehoe T, Gmel G, Rehm J. Fitting different distributions to alcohol consumption among drinkers. CAMH, 2009.
- 31.Kokkevi A, Loukadakis M, Plagianakou S, Politikou K, Stefanis C. Sharp increase in illicit drug use in Greece: trends from a general population survey on licit and illicit drug use. Eur Addict Res 2000;6:42-9. [DOI] [PubMed] [Google Scholar]
- 32.Flegal KM, Williamson DF, Graubard BI. Fraction of premature deaths in the Canadian population that were attributable to overweight and obesity. Can J Public Health 2004;95:235. [PubMed] [Google Scholar]
- 33.Flegal KM, Williamson DF, Graubard BI. Using adjusted relative risks to calculate attributable fractions. Am J Public Health 2006;96:398-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greenland S, Robins JM. Conceptual problems in the definition and interpretation of attributable fractions. Am J Epidemiol 1988;128:1185-97. [DOI] [PubMed] [Google Scholar]
- 35.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health 1998;88:15-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferlay J, Bray F, Pisani P, Parkin DM. GLOBOCAN 2002: cancer incidence, mortality and prevalence worldwide. 2004. www-dep.iarc.fr/.
- 37.Rehm J, Sulkowska U, Manczuk M, Boffetta P, Powles J, Popova S, et al. Alcohol accounts for a high proportion of premature mortality in central and eastern Europe. Int J Epidemiol 2007;36:458-67. [DOI] [PubMed] [Google Scholar]
- 38.Boffetta P, Hashibe M, La Vecchia C, Zatonski W, Rehm J. The burden of cancer attributable to alcohol drinking. Int J Cancer 2006;119:884-7. [DOI] [PubMed] [Google Scholar]
- 39.Boffetta P, Tubiana M, Hill C, Boniol M, Aurengo A, Masse R, et al. The causes of cancer in France. Ann Oncol 2009;20:550-5. [DOI] [PubMed] [Google Scholar]
- 40.Corrao G, Rubbiati L, Zambon A, Arico S. Alcohol-attributable and alcohol-preventable mortality in Italy. A balance in 1983 and 1996. Eur J Public Health 2002;12:214-23. [DOI] [PubMed] [Google Scholar]
- 41.Danaei G, Vander Hoorn S, Lopez AD, Murray CJ, Ezzati M. Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet 2005;366:1784-93. [DOI] [PubMed] [Google Scholar]
- 42.Grant I, Springbett A, Graham L. Alcohol attributable mortality and morbidity: alcohol population attributable fractions for Scotland. Scotland NHS, 2009. www.isdscotland.org/isd/5964.html.
- 43.Jones L, Bellis MA, Dedman D, Sumnall H, Tocque K. Alcohol-attributable fractions for England—alcohol-attributable mortality and hospital admissions. Centre for Public Health, 2008.
- 44.WHO IARC. Attributable causes of cancer in France in the year 2000. IARC Working Group Reports, Vol 3, 2007.
- 45.World Cancer Research Fund/American Institute for Cancer Research. Policy and action for cancer prevention. Food, nutrition, and physical activity: a global perspective. WCRF/AICR, 2007.
- 46.Corrao G, Bagnardi V, Zambon A, La Vecchia C. A meta-analysis of alcohol consumption and the risk of 15 diseases. Prev Med 2004;38:613-9. [DOI] [PubMed] [Google Scholar]
- 47.Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, de Gaetano G. Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch Intern Med 2006;166:2437-45. [DOI] [PubMed] [Google Scholar]
- 48.Mukamal KJ, Chen CM, Rao SR, Breslow RA. Alcohol consumption and cardiovascular mortality among US adults, 1987 to 2002. J Am Coll Cardiol 2010;55:1328-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klatsky AL. Alcohol and cardiovascular health. Physiol Behav 2010;100:76-81. [DOI] [PubMed] [Google Scholar]
- 50.Di Castelnuovo A, Costanzo S, Donati MB, Iacoviello L, de Gaetano G. Prevention of cardiovascular risk by moderate alcohol consumption: epidemiologic evidence and plausible mechanisms. Intern Emerg Med 2010;5:291-7. [DOI] [PubMed] [Google Scholar]
- 51.Corrao G, Bagnardi V, Zambon A, Arico S. Exploring the dose-response relationship between alcohol consumption and the risk of several alcohol-related conditions: a meta-analysis. Addiction 1999;94:1551-73. [DOI] [PubMed] [Google Scholar]
- 52.Taylor B, Rehm J. When risk factors combine: the interaction between alcohol and smoking for aerodigestive cancer, coronary heart disease, and traffic and fire injury. Addict Behav 2006;31:1522-35. [DOI] [PubMed] [Google Scholar]
- 53.Hashibe M, Brennan P, Benhamou S, Castellsague X, Chen C, Curado MP, et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J Natl Cancer Inst 2007;99:777-89. [DOI] [PubMed] [Google Scholar]
- 54.Bagnardi V, Blangiardo M, La Vecchia C, Corrao G. Alcohol consumption and the risk of cancer: a meta-analysis. Alcohol Res Health 2001;25:263-70. [PMC free article] [PubMed] [Google Scholar]
- 55.Boeing H, Korfmann A, Bergmann MM. Recruitment procedures of EPIC-Germany. European Investigation into Cancer and Nutrition. Ann Nutr Metab 1999;43:205-15. [DOI] [PubMed] [Google Scholar]
- 56.World Health Organization. European alcohol action plan. WHO, 1993.