Abstract
Rationale
γ-Aminobutyric acid (GABA) is the major inhibitory neurotransmitter in the brain and is implicated in the modulation of central reward processes. Acute or chronic administration of GABAB receptor agonists or positive modulators decreased self-administration of various drugs of abuse. Furthermore, GABAB receptor agonists inhibited cue-induced reinstatement of nicotine- and cocaine-seeking behavior. Because of their fewer adverse side effects compared with GABAB receptor agonists, GABAB receptor positive modulators are potentially improved therapeutic compounds for the treatment of drug dependence compared with agonists.
Objectives and methods
We examined whether the acute effects of the GABAB receptor positive modulator N-[(1R,2R,4S)-bicyclo[2.2.1]hept-2-yl]-2-methyl-5-[4-(trifluoromethyl)phenyl]-4-pyrimidinamine (BHF177) on nicotine self-administration and food-maintained responding under a fixed-ratio 5 schedule of reinforcement were maintained after repeated administration. The effects of acute BHF177 administration on cue-induced nicotine- and food-seeking behavior, a putative animal model of relapse, were also examined.
Results
Repeated administration of BHF177 for 14 days decreased nicotine self-administration, with small tolerance observed during the last 7 days of treatment, whereas BHF177 minimally affected food-maintained responding. Acute BHF177 administration dose-dependently blocked cue-induced reinstatement of nicotine-, but not food-, seeking behavior after a 10-day extinction period.
Conclusions
These results showed that BHF177 selectively blocked nicotine self-administration and prevented cue-induced reinstatement of nicotine seeking, with minimal effects on responding for food and no effect on cue-induced reinstatement of food seeking. Thus, GABAB receptor positive modulators could be useful therapeutics for the treatment of different aspects of nicotine dependence by facilitating smoking cessation by decreasing nicotine intake and preventing relapse to smoking in humans.
Keywords: Relapse prevention, Smoking cessation, Addictive drugs, Nicotine dependence, Treatment, Medication
Introduction
Tobacco addiction, which is partly attributed to the addictive properties of nicotine (Rose 2007), is among the most prevalent worldwide health problems (Proctor 2004). Dependence on tobacco smoking is characterized by unsuccessful attempts to quit smoking and high rates of relapse after a period of abstinence (Hughes et al. 2004; Nides 2008). Currently available smoking cessation therapies include different forms of nicotine replacement therapy, antidepressants (i.e., bupropion, nortryptiline), nicotine vaccines, and relatively selective nicotinic α4β2 receptor partial agonists, such as cytisine and the cytisine derivative varenicline (Zaniewska et al. 2009; Laniado-Laborin 2010). The abstinence success rates 1 year after treatment with any of the above therapies is low (Jorenby et al. 2006; Perkins et al. 2010) and only reaches 24% with combined pharmacotherapy and behavioral therapy. Novel targets and mechanisms of action are currently being investigated to discover new anti-smoking medications, including compounds that act as positive modulators at γ-aminobutyric acid B (GABAB) receptors.
Stimulation of GABAB receptors by acute and/or repeated administration of GABAB receptor agonists blocked the reinforcing and motivational effects of various drugs of abuse, such as cocaine, amphetamine, nicotine, opiates, and ethanol, in rodents (for review, Vlachou and Markou 2010), as assessed by the self-administration, cue-, drug-, or stress-induced reinstatement of drug seeking, intracranial self-stimulation, and conditioned place preference procedures. Most relevant to the present studies, acute and/or repeated administration of GABAB receptor agonists decreased self-administration of nicotine (Dewey et al. 1999; Corrigall et al. 2000; Fattore et al. 2002; Paterson and Markou 2002; Paterson et al. 2004, 2005b, 2008). Furthermore, the GABAB receptor agonist (3-amino-2[S]-hydroxypropyl)-methylphosphinic acid (CGP44532) blocked cue-induced reinstatement of nicotine seeking (Paterson et al. 2005b). Moreover, administration of (R,S)-4-amino-3-(4-chlorophenyl)butanoic acid (baclofen) or other GABAB receptor agonists, such as (R,S)-4-aminohex-5-enoic acid (GVG; inhibits breakdown of GABA) and CGP44532, blocked the expression and acquisition of drug-induced conditioned place preference (CPP) when administered either systemically or directly into different brain regions, such as the nucleus accumbens and ventral tegmental area (VTA) (Dewey et al. 1999; Le Foll et al. 2008; Fattore et al. 2009).
Consistent with the results from animal studies, clinical evidence showed that baclofen negatively impacted subjective cigarette pleasure and increased feelings of relaxation, indicating a possible effect of baclofen on smoking abstinence and relapse prevention (Cousins et al. 2001). Most recently, it was shown that baclofen reduced the number of cigarettes smoked per day and craving, a major precipitant of relapse (Franklin et al. 2009). Importantly, baclofen was also more effective than placebo in reducing cocaine use in cocaine-abstinent humans (Ling et al. 1998; Shoptaw et al. 2003) and prevented cue-induced craving and concurrent activation of relevant brain areas, such as the anterior cingulate and amygdala, in cocaine-abstinent humans (Brebner et al. 2002). Other preliminary clinical studies demonstrated that baclofen reduced alcohol consumption and craving, led to abstinence from alcohol drinking, and treated the alcohol withdrawal syndrome after either acute or chronic treatment (e.g., Addolorato et al. 2000, 2002; Flannery et al. 2004; Bucknam 2007; Leggio et al. 2008). Thus, GABAB receptor agonists appear to be effective in reversing several drug dependence-related behaviors.
However, GABAB receptor agonists also have adverse side effects, such as muscle relaxation, memory impairment, sedation, and tolerance, which restrict their therapeutic potential (Ong and Kerr 2005). On the other hand, GABAB receptor positive allosteric modulators are devoid of substantial intrinsic agonistic activity in the absence of GABA; they do not perturb receptor signaling on their own, but potentiate the effect of GABA only where and when GABA is endogenously released to activate GABAB receptors (Urwyler et al. 2003). Thus, these compounds have fewer adverse side effects than GABAB receptor agonists. Acute administration of a GABAB receptor positive modulator, such as 2,6-di-tert-butyl-4-(3-hydroxy-2,2-dimethyl-propyl)-phenol (CGP7930) (Urwyler et al. 2001), N,N'-dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4,6-diamine (GS39783) (Urwyler et al. 2003), (R,S)-5,7-di-tert-butyl-3-hydroxy-3-trifluoromethyl-3H-benzofuran-2-one (rac-BHFF) (Malherbe et al. 2008), and N-[(1R,2R,4S)-bicyclo[2.2.1]hept-2-yl]-2-methyl-5-[4-(trifluoromethyl)phenyl]-4-pyrimidinamine (BHF177) (Guery et al. 2007), blocked cocaine, nicotine, and ethanol self-administration under either fixed- or progressive-ratio schedules of reinforcement (Smith et al. 2004; Liang et al. 2006; Maccioni et al. 2007, 2008b, 2009; Paterson et al. 2008). BHF177 also blocked the nicotine-induced enhancement of brain stimulation reward (Paterson et al. 2008).
All of the aforementioned studies evaluated the effects of the GABAB receptor positive modulators only after acute administration. However, humans attempting to quit tobacco smoking would receive medications daily and for long periods of time to treat their drug dependence. Thus, the present study assessed the effects of repeated daily administration of the GABAB receptor positive modulator BHF177 on nicotine self-administration in rats.
Furthermore, the effects of acute BHF177 administration on cue-induced reinstatement of nicotine seeking were assessed. Cue-induced reinstatement is a widely used animal procedure for studying the incentive motivational properties of stimuli previously associated with the primary rewarding properties of drugs of abuse. This procedure has been postulated to be relevant to relapse in humans (Epstein et al. 2006; Kalivas et al. 2006; however see, Katz and Higgins 2003). Parallel studies on the effects of BHF177 administration on food-maintained responding and cue-induced reinstatement of food seeking using similar procedures as those used for the nicotine-motivated behaviors were also conducted to evaluate the possible selectivity of the effects of BHF177 on only the rewarding effects of nicotine and not on the reinforcing effects of a natural reinforcer such as food.
Methods
Subjects
Male Wistar rats (Charles River, Raleigh, NC, USA), weighing 250–300 g upon arrival in the laboratory, were group housed in a temperature- and humidity-controlled vivarium on a 12 h/12 h reverse light/dark cycle with unrestricted access to water except during testing. Rats were food-restricted to 12–20 g/day throughout the experiments (see below), with the exception of the surgical recovery and initial habituation periods during which rats had ad libitum access to food and water. Behavioral testing occurred during the dark phase of the light/dark cycle. All subjects, animal facilities, and experimental protocols were in accordance with National Institutes of Health and Association for the Assessment and Accreditation of Laboratory Animal Care guidelines and were approved by the Institutional Animal Care and Use Committee.
Drugs
(−)Nicotine hydrogen tartrate (Sigma, St. Louis, MO, USA) was dissolved in saline (pH adjusted to 7.0 ± 0.5 with sodium hydroxide). The solution was then filtered through a 0.22-μm syringe filter (Fisher Scientific, Pittsburgh, PA, USA) for sterilization purposes. Nicotine doses are reported as freebase concentrations, whereas BHF177 doses are reported as the salt concentration. BHF177 (compound #27 in Guery et al. 2007) was synthesized and provided by The Scripps Research Institute (La Jolla, CA, USA; experiment 1) and Novartis Pharma AG (Basel, Switzerland; experiment 2). In GTPy35S assays on CHO-K1 membranes from GABA-B(1b/2) co-expressing cells, the potency of BHF177 was 1.7 μM (measured at 1 μM GABA; Guery et al. 2007), similar to the previously published positive modulators CGP7930 and GS39783 (Urwyler et al. 2001, 2003), although BHF177 is structurally different than CGP7930 and GS39783. BHF177 crosses the blood–brain barrier (Paterson et al. 2008). BHF177 was suspended in 0.5% methylcellulose and administered orally (2 ml/kg, 1 h pretreatment).
Apparati
Intravenous nicotine self-administration and food-maintained responding were conducted in 22 Plexiglas experimental chambers (25 × 31 × 24 cm; Med Associates, St. Albans, VT, USA), each housed in a sound-attenuated box. One wall of the chamber contained two levers, measuring ∼3 cm in width and located ∼3 cm above the metal grid floor of the chamber.
Intravenous catheterization surgery
Rats were anesthetized with an isoflurane/oxygen mixture (1–1.5% isoflurane) and prepared with a catheter inserted into the right jugular vein. Catheters were constructed as previously described (Paterson et al. 2005b, 2008). Animals were given 7 days to recover from surgery prior to being trained to lever press for 45-mg food pellets (described below). All rats were administered 20 mg/day of a drug combination consisting of the semi-synthetic antibiotic ticarcillin disodium and the β-lactamase inhibitor clavulanate potassium (Timentin) for 7 days after surgery. Additionally, animals received a 0.1-ml infusion of heparinized saline (33.3 U/ml) before and after each self-administration session.
Behavioral procedures
Food training and testing
The food training and testing procedures were identical to those previously described (Liechti et al. 2007; Paterson et al. 2005b, 2008). Rats were food-restricted (5 g/day) for 48 h prior to starting food training and received 12 g of rat chow per day, at least 1 h after the end of the 1 h food training session. Rats were initially required to press a lever to receive one food pellet (45 mg Noyes food pellet) on a fixed-ratio 1 timeout 1 s (FR1 TO 1 s) schedule of reinforcement. Animals progressed through the sequence FR1 TO 1 s, FR2 TO 10 s, FR5 TO 20 s only after the successful acquisition of the previous schedule (>80 pellets/1 h session). After successful acquisition of food-maintained responding, all rats were maintained on 20 g rat chow per day.
An identical training procedure was implemented for the subjects used to assess the effects of the test compounds on food-maintained responding. These rats were allowed to continue responding for food on an FR5 TO 20 s schedule (1 h sessions/5 days per week). This testing procedure was identical in all parameters to the nicotine self-administration procedure (see below). Although levels of responding for nicotine and food self-administration are quite different, food-maintained responding is used as a measure of selectivity of a manipulation for the reinforcing effects of drug and non-drug reinforcers as well as for assessing potentially non-specific effects of the drug manipulation on the ability of the animal to perform the task, such as learning and memory impairments, and the inability to physically perform the task. Used for these purposes, food-maintained responding provides highly valuable information.
Nicotine self-administration under a fixed-ratio schedule of reinforcement
After the successful completion of food training, rats were allowed to self-administer nicotine (0.03 mg/kg/infusion; base) by switching the delivery of a food pellet with the delivery of a nicotine infusion on an FR5 TO 20 s schedule (1 h sessions/5 days per week). Two levers were present in the operant testing chamber, only one of which (the active lever previously paired with food delivery) was paired with the delivery of a nicotine infusion (0.1 ml over 1 s; 0.03 mg/kg/inj base; Razel model A syringe pump, Razel Scientific Instruments, Stamford, CT, USA). The delivery of an infusion was paired with a cue light located above the active lever that was lit simultaneously with the initiation of the nicotine infusion and remained illuminated throughout the 20-s timeout period, during which responding was recorded but not reinforced. Responses on the inactive lever were recorded but had no consequences.
Cue-induced reinstatement of nicotine-seeking and food-maintained responding
This procedure is described in detail in experiment 2 below.
Experimental designs
Experiment 1: effects of repeated administration of BHF177 on nicotine- and food-maintained responding under a fixed-ratio schedule of reinforcement
After successful acquisition of stable nicotine- (n = 19) or food- (n = 14) maintained responding, the effects of repeated administration of BHF177 (0 and 20 mg/kg, PO) were assessed. BHF177 or its vehicle (0.5% methylcellulose in saline) were administered orally 1 h before daily test sessions for 14 consecutive days, after rats exhibited stable nicotine- or food-maintained responding (<20% variation in responding for three consecutive days). Nicotine- and food-responding groups were semi-randomly split into two groups each (vehicle or 20 mg/kg), so that baseline responding was equal for the groups (within reinforcer) assigned to the vehicle or BHF177 treatment conditions. Previous studies showed that acute administration of 10, 20, and 40 mg/kg BHF177 selectively and dose-dependently decreased the reinforcing and motivational properties of nicotine, whereas none of these doses affected responding for food in a fixed-ratio schedule of reinforcement, and only the highest dose of BHF177 (40 mg/kg) significantly decreased breakpoints for both nicotine and food in a progressive-ratio schedule (Paterson et al. 2008). Thus, we selected the 20 mg/kg dose, which was devoid of any adverse or nonspecific effects, for repeated administration in the present study. Rats were allowed to respond for nicotine or food for 7 days after BHF177/vehicle administration ceased. Body weights were recorded daily from the beginning of the repeated BHF177/vehicle administration until the completion of the experiment.
Experiment 2: effects of acute BHF177 administration on cue-induced reinstatement of nicotine-seeking behavior
After successful acquisition of the FR5 TO 20 s schedule (less than 20% variation in responding during the previous three baseline sessions), naïve rats were tested under extinction conditions (withholding of nicotine/food and its associated cue light) during ten consecutive daily 1-h extinction sessions. Animals were not attached to the tubing during that time, and pumps were not running or making sound. Responses at both the inactive and active levers were recorded but had no consequences (i.e., no cue light illumination, no syringe pump activation, no pump sound, and no infusion). The reinstatement test phase began 1 day after the last extinction session. During the reinstatement test sessions, responses at the active lever resulted in contingent presentation of the previously nicotine-associated cue light and the delivery of a saline infusion instead of nicotine in the nicotine group under an FR5 TO 20 s schedule of reinforcement. Additionally, the cue light previously associated with nicotine self-administration or pellet delivery was non-contingently presented for 20 s at the beginning of the reinstatement session. For the nicotine group (n = 15), the very first reinstatement session was conducted after vehicle administration (1 h pretreatment, PO) to ensure that all subjects show reliable reinstatement after vehicle administration. Then, the effects of BHF177 (0, 2.5, 5, 10, 20, and 40 mg/kg, PO; 1 h pretreatment) were investigated using a within-subjects Latin square design that included the assessment of the effects of five doses of the compound and one vehicle administration. After the completion of the Latin square design, one final reinstatement session was conducted after vehicle administration (1 h pretreatment, PO). Thus, there were eight assessments of cue-induced reinstatement (six reinstatement test sessions as part of the Latin square design and two reinstatement test sessions after vehicle administration, one before and one after the Latin square design) that spanned the entire duration of this experiment and provided an assessment of the stability of this behavior with repeated testing and across time. Two of the animals were excluded from the experiment due to catheter patency issues (i.e., the number of animals completing the experiment was 13). Reinstatement sessions were conducted every fourth day and were separated by three extinction sessions (cue and nicotine absent) to re-extinguish responding.
For the food group, a between-subjects design was used because cue-induced food-seeking behavior exhibits rapid extinction with repeated reinstatement testing, prohibiting the use of a within-subjects design (Bespalov et al. 2005; Liechti et al. 2007). Thus, six independent groups of rats (n = 8–9/group) that responded for food were tested, with each group treated with a different BHF177 dose (0, 2.5, 5, 10, 20, and 40 mg/kg, PO; 1 h pretreatment) in a single reinstatement session after the 10-day extinction period. Cue-induced responding included the total number of responses emitted on the active lever, including the responses emitted during the 20-s timeout period when the cue light was illuminated. The cue condition used was 20 s illumination of the cue light located above the active lever.
Statistical analysis
Self-administration data are expressed as the mean number of infusions/food pellets earned during each daily nicotine self-administration session throughout the BHF177/vehicle treatment period. Baseline was defined as the mean number of rewards earned over the 3 days prior to the initiation of BHF177/vehicle administration (experiment 1) or the number of lever presses during the last of the three extinction sessions preceding each reinstatement session (experiment 2). Additionally, all data and body weights obtained from the intravenous self-administration and food-maintained responding groups were analyzed as the absolute number of responses and body weights, respectively. Data were analyzed using appropriate two-way mixed-factor analyses of variance (ANOVAs). In experiment 1, BHF177/Vehicle Treatment was the between-subjects factor, and Time was the within-subjects factor. In experiment 2, BHF177 Treatment was the between-subjects factor, and Session (last day of extinction vs. reinstatement sessions) was the within-subjects factor. Significant main or interaction effects were followed by one- or two-way ANOVAs or the Newman–Keuls post hoc test. The level of significance was set at 0.05. Statistical analyses were conducted using Prism v.5.0 (GraphPad, San Diego, CA, USA) and the Statistical Package for the Social Sciences v.16.0 (SPSS, Chicago, IL, USA).
Results
Experiment 1: Effects of repeated administration of BHF177 on nicotine- and food-maintained responding under a fixed-ratio schedule of reinforcement
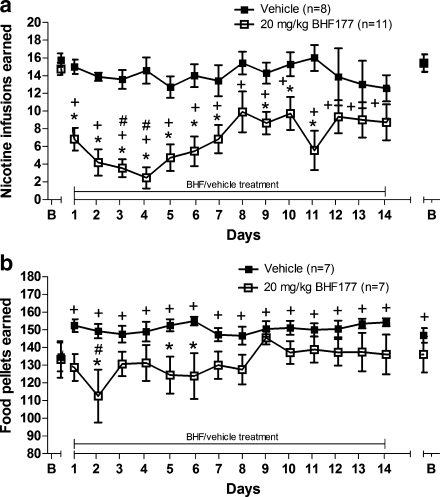
For the nicotine group (Fig. 1a), there were significant main effects of Treatment (F 1,255 = 22.07, p < 0.001) and Time (F 15,255 = 5.903, p < 0.0001) and a significant Time × Treatment interaction (F 15,255 = 3.041, p < 0.001). Post hoc tests revealed no difference between the vehicle- and 20 mg/kg BHF177-treated groups during the baseline days prior to treatment, whereas a significant BHF177-induced decrease in nicotine self-administration was found on days 1–7 and days 9–11 of chronic BHF177 administration compared with the vehicle-treated group (p < 0.05). No differences were found between groups on the days after the termination of BHF/vehicle administration. Furthermore, post hoc tests comparing responding on different days for the BHF177 group indicated a significant decrease in responding on all days of BHF177 administration compared with the 3-day baseline before the initiation and after the termination of treatment (p < 0.05). Post hoc tests also indicated a significant decrease in responding on day 3 of BHF177 administration compared with days 8 and 10 and on day 4 of BHF177 administration compared to days 8–10 and days 12–14 (p < 0.05). Post hoc tests comparing responding on different days for the vehicle group indicated no statistically significant effect within the vehicle group across sessions.
Fig. 1.
Effects of repeated BHF177/vehicle treatment for 14 days on a nicotine self-administration and b food-maintained responding. Data are expressed as nicotine infusions or food pellets earned under a fixed-ratio 5 schedule of reinforcement during 1-h test sessions. The abscissa shows the pre-treatment baseline (B), the 14 days of BHF177/vehicle treatment, and the post-treatment baseline after discontinuing BHF177/vehicle treatment (B; the two time-points overlap on the graph for the nicotine group). Closed squares (black) represent the groups receiving vehicle. Open squares (white) represent the groups receiving 20 mg/kg BHF177. *p < 0.05, significant differences between the vehicle and 20 mg/kg of BHF177 treatment groups on the same days of testing; + p < 0.05, significant differences between reinforcers earned on the various days of BHF177/vehicle treatment compared with the pretreatment baseline within each group; # p < 0.05, significant differences between reinforcers earned on day 3 compared with days 8 and 10 and day 4 compared with days 8–10 and days 12–14 (a), and significant differences between reinforcers earned on day 2 compared with days 9 and 13 (b)
Mean (±SEM) body weights during the three baseline days prior to repeated administration of vehicle/BHF177 were 364.75 ± 8.39 and 374.09 ± 7.21, respectively. Mean (±SEM) body weights increased to 380.09 ± 7.23 and 383.44 ± 8.22 during the 3 days following vehicle/BHF177 administration, respectively. An ANOVA on body weights showed no main effect of Treatment, but a main effect of Time (F 15,255 = 19.59, p < 0.0001) and a Time × Treatment interaction (F 15,255 = 5.404, p < 0.0001). However, post hoc analyses indicated no significant differences in body weights between the two groups at any time point. These ANOVA results reflect the gradual increase in weight over time for both the BHF177- and vehicle-treated groups and a difference in the weight increase over time between the two groups.
Responding on the inactive lever was very low. The number of inactive lever presses (mean ± SEM) during the last three nicotine self-administration sessions prior to initiating repeated BHF177 or vehicle administration was 1.08 ± 0.5 and 1.75 ± 1.19 for the two groups, respectively. Analysis of inactive lever responses showed no statistically significant effects on inactive lever responses (data not shown).
For the food-responding groups (Fig. 1b), there were significant main effects of Treatment (F 15,180 = 4.987, p < 0.05) and Time (F 15,180 = 2.271, p < 0.01) and a significant Time × Treatment interaction (F 15,180 = 2.345, p < 0.01). Post hoc tests revealed no difference between the vehicle- and 20 mg/kg BHF177-treated groups during the baseline days prior to treatment, whereas a significant BHF177-induced decrease in food-maintained responding was found on day 2 and days 5–6 of chronic BHF177 administration compared with the vehicle-treated group (p < 0.05). Furthermore, post hoc tests comparing responding on different days for the BHF177 group indicated a significant decrease in responding on day 2 of BHF177 administration compared with days 9 and 13 (p < 0.05). There were no statistically significant differences among days for the BHF177 group, compared with the pretreatment baseline. Post hoc tests comparing responding across days for the vehicle group indicated significantly increased responding on all days of vehicle treatment and during the baseline after the termination of treatment compared with baseline responding before the initiation of treatment (p < 0.05).
Mean (±SEM) body weights during the baseline days prior to repeated administration of BHF177/vehicle were 386.57 ± 9.42 and 389.056 ± 6.82, respectively. Mean (±SEM) body weights increased to 428.35 ± 11.21 and 449.21 ± 12.07 during the 3 days after vehicle/BHF177 administration, respectively. An ANOVA on body weights showed a significant main effect of Time (F 15,180 = 139.70, p < 0.0001), but no effect of Treatment and no Time × Treatment interaction.
Responding on the inactive lever was very low. The number of inactive lever presses (mean ± SEM) during the baseline period prior to initiating repeated BHF177 or vehicle administration was 0.76 ± 0.3 and 0.57 ± 0.37 for the two groups, respectively. Analysis of the inactive lever responses showed no statistically significant effects (data not shown).
Experiment 2: Effects of acute BHF177 administration on cue-induced reinstatement of nicotine-seeking behavior
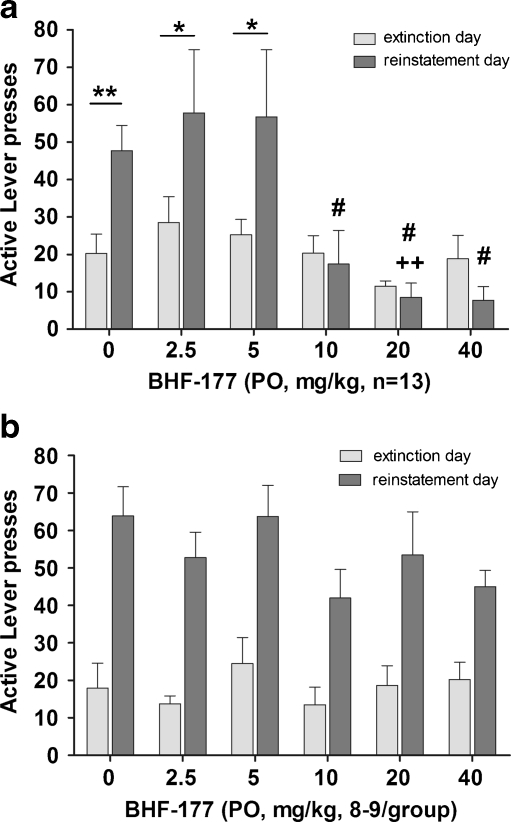
For the nicotine group (Fig. 2a), BHF177 blocked cue-induced reinstatement of nicotine seeking, reflected in a main effect of Treatment (F 5,144 = 6.471, p < 0.0001), a main effect of Session (extinction session preceding the reinstatement session vs. the reinstatement session—F 1,144 = 6.519, p < 0.05), and a statistically significant Treatment × Session interaction (F 5,144 = 2.926, p < 0.05). Post hoc comparisons indicated that the reinstatement sessions preceded by administration of vehicle or any of the three lower BHF177 doses were characterized by a significantly increased number of responses compared with the extinction sessions preceding these reinstatement sessions (vehicle, p < 0.01; 2.5 and 5 mg/kg, p < 0.05), but not when the highest doses of BHF177 (10, 20, and 40 mg/kg) were administered. Confirming the above results, a follow-up one-way ANOVA on only the cue-induced reinstatement sessions, with Treatment as the one within-subject factor, showed a significant effect of BHF177 administration on cue-induced reinstatement of nicotine seeking (F 5,72 = 5.368, p < 0.0001). A follow-up one-way ANOVA on the extinction sessions, with Treatment as the one within-subject factor, showed no difference between the different extinction sessions preceding the reinstatement sessions, demonstrating the stability of responding during the extinction periods.
Fig. 2.
Effects of the GABAB receptor positive modulator BHF177 on cue-induced reinstatement of a nicotine-seeking and b food-seeking behavior. Data are expressed as the number of active lever presses during the extinction session (last extinction session out of the three conducted between reinstatement sessions) preceding each reinstatement session (light gray bars) and the reinstatement session (dark gray bars). The abscissa shows BHF177 doses (0, 2.5, 5, 10, 20, and 40 mg/kg, PO) administered before the reinstatement session. *p < 0.05, **p < 0.01, significant differences in responding between the reinstatement day and the preceding extinction day. ### p < 0.001, significant differences in responding between the 10, 20, and 40 mg/kg doses compared with vehicle and the 2.5 and 5 mg/kg doses. ++ p < 0.01, significant difference between the 5 and 20 mg/kg doses
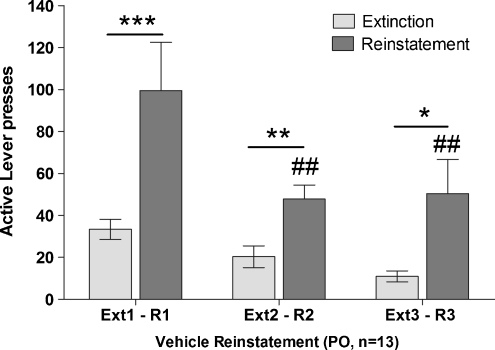
Additionally, an ANOVA on the three different vehicle reinstatement sessions (i.e., one immediately after the 10-day extinction phase, one as part of the Latin square design, and one after the completion of the Latin square design) revealed a main effect of Treatment (F 2,72 = 9.86, p < 0.001), a main effect of Session (F 1,72 = 24.86, p < 0.001), and a Treatment × Session interaction (F 2,72 = 3.279, p < 0.05). Post hoc tests indicated that reinstatement session 1 (p < 0.001), reinstatement session 2 (p < 0.01), and reinstatement session 3 (p < 0.05) were characterized by higher response rates than the extinction sessions that preceded each reinstatement session. Furthermore, post hoc tests indicated a significant difference between reinstatement session 1 and reinstatement sessions 2 and 3 (p < 0.05), whereas there was no statistically significant difference between extinction sessions (Fig. 3).
Fig. 3.
Effect of vehicle administration on cue-induced reinstatement of nicotine over time and with repeated cue-induced reinstatement testing. Three cue-induced reinstatement sessions (R1, R2, R3) were conducted as part of the cue-induced reinstatement experiment. Extinction sessions (light gray bars) are the last extinction sessions (out of three sessions conducted in-between reinstatement sessions) preceding the reinstatement sessions (dark gray bars). The abscissa shows the extinction and reinstatement sessions. The ordinate shows the number of active lever presses per session. A vehicle injection was administered before each of these reinstatement sessions. The first reinstatement session was conducted immediately after the 10-day extinction phase (R1), before the initiation of the within-subjects Latin square design of experiment 2. The second reinstatement session was conducted as part of the within-subjects Latin square design of experiment 2 and corresponds to the vehicle condition presented in Fig. 2a (R2). The third reinstatement session was conducted after completing the Latin square design of experiment 2 (R3). Responses during the first vehicle reinstatement session are higher than responses during the two subsequent reinstatement sessions. The subsequent two reinstatement sessions were characterized by similar numbers of responses. *p < 0.05, **p < 0.01, ***p < 0.001, significant differences between responding during the reinstatement sessions and responding on the preceding extinction session. # p < 0.05, ## p < 0.01, significant differences in responding between R1 and R3 or between R1 and R2
An ANOVA on the number of inactive lever presses during the extinction and reinstatement sessions showed no effect of Treatment or Session and no Treatment × Session interaction (Table 1). Mean (±SEM) body weights at the time of the first reinstatement of nicotine seeking were 434.62 ± 7.40 g.
Table 1.
Mean (±SEM) responses on the inactive lever (including timeout responses) during the third extinction session (out of the three extinction sessions that took place in-between reinstatement sessions) prior to each reinstatement day, and during the reinstatement sessions
| Experimental group | Group size | Reinforcer | Responding on the inactive lever during extinction | Responding on the inactive lever during reinstatement |
|---|---|---|---|---|
| Vehicle | n = 13 | Nicotine | 3.15 ± 0.99 | 4.77 ± 1.04 |
| 2.5 mg/kg BHF177 | n = 13 | Nicotine | 4.51 ± 1.29 | 3.67 ± 0.90 |
| 5 mg/kg BHF177 | n = 13 | Nicotine | 4.45 ± 2.31 | 5.11 ± 2.57 |
| 10 mg/kg BHF177 | n = 13 | Nicotine | 4.15 ± 1.77 | 2.93 ± 1.21 |
| 20 mg/kg BHF177 | n = 13 | Nicotine | 2.84 ± 0.67 | 1.85 ± 0.76 |
| 40 mg/kg BHF177 | n = 13 | Nicotine | 2.38 ± 0.73 | 2.46 ± 1.44 |
| Vehicle | n = 9 | Food | 3.55 ± 1.01 | 1.67 ± 0.40 |
| 2.5 mg/kg BHF177 | n = 9 | Food | 3.00 ± 0.76 | 1.44 ± 0.50 |
| 5 mg/kg BHF177 | n = 8 | Food | 2.13 ± 0.58 | 0.88 ± 0.22 |
| 10 mg/kg BHF177 | n = 8 | Food | 3.25 ± 1.32 | 3.12 ± 1.76 |
| 20 mg/kg BHF177 | n = 8 | Food | 7.00 ± 2.35 | 3.63 ± 0.91 |
| 40 mg/kg BHF177 | n = 8 | Food | 3.75 ± 2.22 | 5.50 ± 2.85 |
For the food group (Fig. 2b), there was a statistically significant increase in responding during the reinstatement sessions (main effect of Session—F 1,88 = 56.54, p < 0.0001), but BHF177 did not block cue-induced reinstatement of food-seeking behavior (no main effect of Treatment and no significant Treatment × Session interaction).
An ANOVA on the inactive lever presses during the extinction and reinstatement sessions showed a small effect of Session (F 1,88 = 4.360, p < 0.05), but no effect of Treatment and no Treatment × Session interaction (Table 1). Mean (±SEM) body weights at the time of the first reinstatement of food seeking were 374.33 ± 5.67 g.
Discussion
The results indicate that repeated administration of 20 mg/kg of the newly synthesized GABAB receptor positive modulator BHF177 significantly decreased nicotine self-administration, with small tolerance development on day 8 and during the last 3 days of chronic treatment. After termination of repeated BHF177 treatment, nicotine self-administration returned to the same levels of responding as before BHF177 treatment. Repeated administration of 20 mg/kg BHF177 only minimally decreased food-maintained responding on three of the 14 days of treatment (day 2 and days 5–6). Furthermore, the results showed that cue-induced reinstatement of nicotine seeking dose-dependently decreased after acute administration of BHF177 at the three highest doses tested (10, 20, and 40 mg/kg), whereas the two lowest doses (2.5 and 5 mg/kg) were ineffective. Cue-induced reinstatement of food-maintained responding was not affected by BHF177 administration at any of the doses tested. Additionally, BHF177 only decreased responding on the active lever and not on the inactive lever. Altogether, the latter two findings indicate that the effects of BHF177 are likely attributable to a selective effect on the motivational properties of stimuli predictive of nicotine availability and not on the motivational properties of stimuli associated with natural reinforcers, such as food.
Tolerance development to the effects of BHF177 was seen at the same time point (day 8) as after administration of the GABAB receptor agonist CGP44532 (Paterson et al. 2005b). However, there was still a strong effect of BHF177 on nicotine self-administration on days 9–11 when there appeared to be small tolerance to the effects of the GABAB receptor agonist. Importantly, the magnitude of effect of repeated BHF177 treatment was larger (∼50–90%) than the effect observed with CGP44532 (∼40%) at doses at which both compounds did not affect responding for food. Overall, the results show an improved pharmacological profile of GABAB receptor positive modulators in terms of less tolerance development and a greater magnitude of effect on nicotine self-administration compared with a GABAB receptor agonist.
The effects of repeated BHF177 administration on nicotine self-administration are consistent with the dose-dependent decrease in acquisition of ethanol intake in Sardinian alcohol-preferring rats after 5 days of administration of either of two GABAB receptor positive modulators, CGP7930 or GS39783 (Orru et al. 2005). Similar effects were observed after acute administration of the GABAB receptor positive modulators CGP7930, GS39783, BHF177, and rac-BHFF on alcohol self-administration under fixed- (Liang et al. 2006; Maccioni et al. 2007, 2009, 2010) or progressive-ratio schedules of reinforcement (Maccioni et al. 2008b), whereas none of these compounds affected responding for natural reinforcers, such as water or sucrose (Liang et al. 2006; Maccioni et al. 2007).
The effects of BHF177 on cue-induced reinstatement of nicotine seeking are consistent with the effects of GABAB receptor agonists on cue-induced reinstatement of seeking for other drugs of abuse. The GABAB receptor agonists baclofen, CGP44532, and SKF97541 blocked reinstatement of nicotine, cocaine, heroin, and alcohol seeking induced either by a priming injection of the drug or by presentation of a cue previously associated with drug delivery (Campbell et al. 1999; Paterson et al. 2005b; Filip and Frankowska 2007; Spano et al. 2007; Weerts et al. 2007; Maccioni et al. 2008a); in some of these studies, the compounds also affected cue-induced reinstatement of food- and sucrose-seeking behavior (Paterson et al. 2005b; Filip and Frankowska 2007), However, consistent with our results, the GABAB receptor positive modulator CGP7930 did not affect reinstatement of food seeking (Filip and Frankowska 2007).
To assess the stability of cue-induced reinstatement of nicotine-seeking behavior, three different reinstatement sessions after vehicle administration were conducted during the cue-induced reinstatement experiment. Responses during the first vehicle reinstatement session, which was conducted immediately after the 10-day extinction phase, were higher than responses during the two subsequent reinstatement sessions, which were conducted as part of the within-subjects Latin square design or after the completion of the Latin square design. Notably, the two subsequent reinstatement sessions were characterized by a similar number of responses. Thus, cue-induced reinstatement of nicotine-seeking behavior is not rapidly extinguished, and a within-subjects design for assessing the effects of various doses of a test compound is suitable for these studies after an initial reinstatement session is conducted under vehicle conditions.
GABAB receptors are widely distributed in several areas of the brain reward circuitry where they negatively modulate reward, reinforcement, and reinstatement of drug seeking (Laviolette and van der Kooy 2003; Sagara et al. 2008; Xi et al. 2009; Yang et al. 2009). VTA dopaminergic neurons receive descending GABAergic inputs from the ventral pallidum and nucleus accumbens (Walaas and Fonnum 1979, 1980; Sugita et al. 1992) that inhibit dopaminergic tone (Klitenick et al. 1992; Engberg et al. 1993). GABAB receptors are co-localized with nicotinic acetylcholine receptors (nAchR), mainly α4β2 and α7 nAchR subtypes, on inhibitory GABAergic neurons and interneurons in the VTA (Mansvelder and McGehee 2002). Nicotine exerts its effects on GABAergic neurotransmission through nicotinic receptors located on these neurons. BHF177 administration likely induced its behavioral effects on nicotine self-administration by augmenting the effects of endogenously released GABA on GABAB receptor-mediated inhibition of dopaminergic and glutamatergic neuronal activity in areas enriched with GABAB receptors, such as the VTA, nucleus accumbens, ventral pallidum, hippocampus, and extended amygdala. These brain sites have been implicated in the effects of GABAB receptor agonists on the rewarding effects of drugs of abuse (Panagis and Kastellakis 2002; Fadda et al. 2003; Zarrindast et al. 2006; Rogers and See 2007).
Interestingly, decreased GABAB1 and/or GABAB2 mRNA receptor expression was found in the rat hippocampus and/or areas of the prefrontal cortex immediately after discontinuation of chronic nicotine administration or passive cigarette smoke inhalation (Li et al. 2002, 2004). However, Paterson and colleagues (2005a) showed that chronic subcutaneous nicotine infusion for 7–24 days did not induce significant changes in GABAB receptor-mediated regulation of brain reward function. In the Paterson study, systemic administration of either the GABAB receptor agonist GVG or CGP44532, and intra-VTA administration of CGP44532 did not produce differential effects on brain reward function, assessed by intracranial self-stimulation, between saline- and nicotine-treated rats, with nicotine/saline onboard at the time of testing. The present studies were also conducted while subjects were exposed to nicotine. Although the Li and colleagues findings suggest decreased GABAB receptor expression after chronic nicotine exposure and withdrawal from nicotine, the findings by Paterson and colleagues indicate a lack of functional adaptation of GABAB receptors with nicotine on board. Thus, chronic exposure to nicotine during the 6 weeks of self-administration (4 weeks before the initiation of BHF177 treatment and 2 weeks during BHF177 treatment) may have altered GABAB receptor expression (consistent with the Li findings), but the net function of GABAB receptors may not have been altered (consistent with the Paterson findings).
Importantly, less tolerance developed during the last 7 days of the repeated BHF177 treatment than the tolerance seen after repeated administration of the GABAB receptor agonist CGP44532 (Paterson et al. 2005b). Accordingly, repeated administration of the GABAB receptor agonist baclofen reduced electrophysiological and pharmacological responsiveness to baclofen on both pre- and postsynaptic GABAB receptors in the prefrontal cortex, spinal cord, and hippocampus in rats (Malcangio et al. 1995; Wetherington and Lambert 2002). Thus, the tolerance seen with GABAB receptor agonists is likely attributable to the repeated agonist action on the receptor, whereas the less tolerance development with the positive modulator is likely attributable to the fact that such compounds only enhance the activity of endogenous GABA on the receptor.
In summary, the present studies showed that repeated administration of the GABAB receptor positive modulator BHF177 decreased the reinforcing effects of nicotine with small tolerance development during the last 7 days of the chronic treatment compared with previously observed tolerance during chronic treatment with the GABAB receptor agonist CGP44532 (Paterson et al. 2005b). Repeated BHF177 administration minimally decreased food-maintained responding. Moreover, acute administration of BHF177 selectively and dose-dependently blocked cue-induced reinstatement of nicotine-, but not food-, seeking behavior. These findings provide clear evidence that GABAB receptors play a significant role in the regulation of reinstatement processes and that activation of these receptors blocks the motivational effects of stimuli associated with nicotine that lead to reinstatement. Thus, GABAB receptor positive modulators may be used therapeutically for the treatment of different aspects of nicotine dependence by reducing nicotine intake and preventing relapse in humans.
Acknowledgments
This work was supported by U.S. National Institutes of Health grant U19DA026838 to AM. SV was supported by Individual Postdoctoral Research Fellowship 18FT-0048 from the Tobacco-Related Disease Research Program from the State of California, USA. The authors would like to thank Dr. Klemens Kaupmann for his contributions to the discovery and investigation of BHF177 and Mr. Michael Arends for editorial assistance.
Conflict of interest
The authors declare no conflicts of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- GABA
γ-Aminobutyric acid
- Baclofen
(R,S)-4-amino-3-(4-chlorophenyl)butanoic acid
- GVG
(R,S)-4-aminohex-5-enoic acid
- CGP44532
(3-amino-2[S]-hydroxypropyl)-methylphosphinic acid
- CPP
Conditioned place preference
- CGP7930
2,6-di-tert-butyl-4-(3-hydroxy-2,2-dimethyl-propyl)-phenol
- GS39783
N,N′-dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4,6-diamine
- BHF177
N-[(1R,2R,4S)-bicyclo[2.2.1]hept-2-yl]-2-methyl-5-[4-(trifluoromethyl)phenyl]-4-pyrimidinamine
- rac-BHFF
(R,S)-5,7-di-tert-butyl-3-hydroxy-3-trifluoromethyl-3H-benzofuran-2-one
- FR
Fixed ratio
- PO
Per os
- ANOVA
Analysis of variance
- nAchR
Nicotinic acetylcholine receptor
- VTA
Ventral tegmental area
References
- Addolorato G, Caputo F, Capristo E, Colombo G, Gessa GL, Gasbarrini G. Ability of baclofen in reducing alcohol craving and intake: II. Preliminary clinical evidence. Alcohol Clin Exp Res. 2000;24:67–71. [PubMed] [Google Scholar]
- Addolorato G, Caputo F, Capristo E, Janiri L, Bernardi M, Agabio R, Colombo G, Gessa GL, Gasbarrini G. Rapid suppression of alcohol withdrawal syndrome by baclofen. Am J Med. 2002;112:226–229. doi: 10.1016/S0002-9343(01)01088-9. [DOI] [PubMed] [Google Scholar]
- Bespalov AY, Dravolina OA, Sukhanov I, Zakharova E, Blokhina E, Zvartau E, Danysz W, van Heeke G, Markou A. Metabotropic glutamate receptor (mGluR5) antagonist MPEP attenuated cue- and schedule-induced reinstatement of nicotine self-administration behavior in rats. Neuropharmacology. 2005;49(Suppl 1):167–178. doi: 10.1016/j.neuropharm.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Brebner K, Childress AR, Roberts DCS. A potential role for GABAB agonists in the treatment of psychostimulant addiction. Alcohol Alcohol. 2002;37:478–484. doi: 10.1093/alcalc/37.5.478. [DOI] [PubMed] [Google Scholar]
- Bucknam W. Suppression of symptoms of alcohol dependence and craving using high-dose baclofen. Alcohol Alcohol. 2007;42:158–160. doi: 10.1093/alcalc/agl091. [DOI] [PubMed] [Google Scholar]
- Campbell UC, Lac ST, Carroll ME. Effects of baclofen on maintenance and reinstatement of intravenous cocaine self-administration in rats. Psychopharmacol Berl. 1999;143:209–214. doi: 10.1007/s002130050937. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL, Chow BL, Zhang J. Response of nicotine self-administration in the rat to manipulations of mu-opioid and γ-aminobutyric acid receptors in the ventral tegmental area. Psychopharmacol Berl. 2000;149:107–114. doi: 10.1007/s002139900355. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Stamat HM, de Wit H. Effects of a single dose of baclofen on self-reported subjective effects and tobacco smoking. Nicotine Tob Res. 2001;3:123–129. doi: 10.1080/14622200110042624. [DOI] [PubMed] [Google Scholar]
- Dewey SL, Brodie JD, Gerasimov M, Horan B, Gardner EL, Ashby CR., Jr A pharmacologic strategy for the treatment of nicotine addiction. Synapse. 1999;31:76–86. doi: 10.1002/(SICI)1098-2396(199901)31:1<76::AID-SYN10>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Engberg G, Kling-Petersen T, Nissbrandt H. GABAB-receptor activation alters the firing pattern of dopamine neurons in the rat substantia nigra. Synapse. 1993;15:229–238. doi: 10.1002/syn.890150308. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacol Berl. 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadda P, Scherma M, Fresu A, Collu M, Fratta W. Baclofen antagonizes nicotine-, cocaine-, and morphine-induced dopamine release in the nucleus accumbens of rat. Synapse. 2003;50:1–6. doi: 10.1002/syn.10238. [DOI] [PubMed] [Google Scholar]
- Fattore L, Cossu G, Martellotta MC, Fratta W. Baclofen antagonizes intravenous self-administration of nicotine in mice and rats. Alcohol Alcohol. 2002;37:495–498. doi: 10.1093/alcalc/37.5.495. [DOI] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Cossu G, Scherma M, Fratta W, Fadda P. Baclofen prevents drug-induced reinstatement of extinguished nicotine-seeking behaviour and nicotine place preference in rodents. Eur Neuropsychopharmacol. 2009;19:487–498. doi: 10.1016/j.euroneuro.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Filip M, Frankowska M. Effects of GABAB receptor agents on cocaine priming, discrete contextual cue and food induced relapses. Eur J Pharmacol. 2007;571:166–173. doi: 10.1016/j.ejphar.2007.05.069. [DOI] [PubMed] [Google Scholar]
- Flannery BA, Garbutt JJ, Cody MW, Renn W, Grace K, Osborne M, Crosby K, Morreale M, Trivette A. Baclofen for alcohol dependence: a preliminary open-label study. Alcohol Clin Exp Res. 2004;28:1517–1523. doi: 10.1097/01.ALC.0000141640.48924.14. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Harper D, Kampman K, Kildea-McCrea S, Jens W, Lynch KG, O'Brien CP, Childress AR. The GABA B agonist baclofen reduces cigarette consumption in a preliminary double-blind placebo-controlled smoking reduction study. Drug Alcohol Depend. 2009;103:30–36. doi: 10.1016/j.drugalcdep.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guery S, Floersheim P, Kaupmann K, Froestl W. Syntheses and optimization of new GS39783 analogues as positive allosteric modulators of GABAB receptors. Bioorg Med Chem Lett. 2007;17:6206–6211. doi: 10.1016/j.bmcl.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99:29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR. Efficacy of varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Peters J, Knackstedt L. Animal models and brain circuits in drug addiction. Mol Interv. 2006;6:339–344. doi: 10.1124/mi.6.6.7. [DOI] [PubMed] [Google Scholar]
- Katz JL, Higgins ST. The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacol Berl. 2003;168:21–30. doi: 10.1007/s00213-003-1441-y. [DOI] [PubMed] [Google Scholar]
- Klitenick MA, Deutch AY, Churchill L, Kalivas PW. Topography and functional role of dopaminergic projections from the ventral mesencephalic tegmentum to the ventral pallidum. Neuroscience. 1992;50:371–386. doi: 10.1016/0306-4522(92)90430-A. [DOI] [PubMed] [Google Scholar]
- Laniado-Laborin R. Smoking cessation intervention: an evidence-based approach. Postgrad Med. 2010;122:74–82. doi: 10.3810/pgm.2010.03.2124. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, van der Kooy D. The motivational valence of nicotine in the rat ventral tegmental area is switched from rewarding to aversive following blockade of the α7-subunit-containing nicotinic acetylcholine receptor. Psychopharmacol Berl. 2003;166:306–313. doi: 10.1007/s00213-002-1317-6. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Wertheim CE, Goldberg SR. Effects of baclofen on conditioned rewarding and discriminative stimulus effects of nicotine in rats. Neurosci Lett. 2008;443:236–240. doi: 10.1016/j.neulet.2008.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio L, Ferrulli A, Cardone S, Malandrino N, Mirijello A, D’Angelo C, Vonghia L, Miceli A, Capristo E, Kenna GA, Gasbarrini G, Swift RM, Addolorato G. Relationship between the hypothalamic–pituitary–thyroid axis and alcohol craving in alcohol-dependent patients: a longitudinal study. Alcohol Clin Exp Res. 2008;32:2047–2053. doi: 10.1111/j.1530-0277.2008.00792.x. [DOI] [PubMed] [Google Scholar]
- Li SP, Park MS, Bahk JY, Kim MO. Chronic nicotine and smoking exposure decreases GABAB1 receptor expression in the rat hippocampus. Neurosci Lett. 2002;334:135–139. doi: 10.1016/S0304-3940(02)01065-0. [DOI] [PubMed] [Google Scholar]
- Li SP, Park MS, Kim JH, Kim MO. Chronic nicotine and smoke treatment modulate dopaminergic activities in ventral tegmental area and nucleus accumbens and the γ-aminobutyric acid type B receptor expression of the rat prefrontal cortex. J Neurosci Res. 2004;78:868–879. doi: 10.1002/jnr.20329. [DOI] [PubMed] [Google Scholar]
- Liang JH, Chen F, Krstew E, Cowen MS, Carroll FY, Crawford D, Beart PM, Lawrence AJ. The GABAB receptor allosteric modulator CGP7930, like baclofen, reduces operant self-administration of ethanol in alcohol-preferring rats. Neuropharmacology. 2006;50:632–639. doi: 10.1016/j.neuropharm.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Lhuillier L, Kaupmann K, Markou A. Metabotropic glutamate 2/3 receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence. J Neurosci. 2007;27:9077–9085. doi: 10.1523/JNEUROSCI.1766-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling W, Shoptaw S, Majewska D. Baclofen as a cocaine anti-craving medication: a preliminary clinical study. Neuropsychopharmacology. 1998;18:403–404. doi: 10.1016/S0893-133X(97)00128-0. [DOI] [PubMed] [Google Scholar]
- Maccioni P, Pes D, Orru A, Froestl W, Gessa GL, Carai MA, Colombo G. Reducing effect of the positive allosteric modulator of the GABAB receptor, GS39, 783, on alcohol self-administration in alcohol-preferring rats. Psychopharmacol Berl. 2007;193:171–178. doi: 10.1007/s00213-007-0776-1. [DOI] [PubMed] [Google Scholar]
- Maccioni P, Bienkowski P, Carai MA, Gessa GL, Colombo G. Baclofen attenuates cue-induced reinstatement of alcohol-seeking behavior in Sardinian alcohol-preferring (sP) rats. Drug Alcohol Depend. 2008;95:284–287. doi: 10.1016/j.drugalcdep.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Maccioni P, Fantini N, Froestl W, Carai MA, Gessa GL, Colombo G. Specific reduction of alcohol’s motivational properties by the positive allosteric modulator of the GABAB receptor, GS39783: comparison with the effect of the GABAB receptor direct agonist, baclofen. Alcohol Clin Exp Res. 2008;32:1558–1564. doi: 10.1111/j.1530-0277.2008.00725.x. [DOI] [PubMed] [Google Scholar]
- Maccioni P, Carai MA, Kaupmann K, Guery S, Froestl W, Leite-Morris KA, Gessa GL, Colombo G. Reduction of alcohol's reinforcing and motivational properties by the positive allosteric modulator of the GABAB receptor, BHF177, in alcohol-preferring rats. Alcohol Clin Exp Res. 2009;33:1749–1756. doi: 10.1111/j.1530-0277.2009.01012.x. [DOI] [PubMed] [Google Scholar]
- Maccioni P, Thomas AW, Carai MA, Gessa GL, Malherbe P, Colombo G. The positive allosteric modulator of the GABAB receptor, rac-BHFF, suppresses alcohol self-administration. Drug Alcohol Depend. 2010;109:96–103. doi: 10.1016/j.drugalcdep.2009.12.019. [DOI] [PubMed] [Google Scholar]
- Malcangio M, Libri V, Teoh H, Constanti A, Bowery NG. Chronic (−)baclofen or CGP 36742 alters GABAB receptor sensitivity in rat brain and spinal cord. NeuroReport. 1995;6:399–403. doi: 10.1097/00001756-199501000-00042. [DOI] [PubMed] [Google Scholar]
- Malherbe P, Masciadri R, Norcross RD, Knoflach F, Kratzeisen C, Zenner MT, Kolb Y, Marcuz A, Huwyler J, Nakagawa T, Porter RH, Thomas AW, Wettstein JG, Sleight AJ, Spooren W, Prinssen EP. Characterization of (R, S)-5, 7-di-tert-butyl-3-hydroxy-3-trifluoromethyl-3H-benzofuran-2-one as a positive allosteric modulator of GABAB receptors. Br J Pharmacol. 2008;154:797–811. doi: 10.1038/bjp.2008.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Cellular and synaptic mechanisms of nicotine addiction. J Neurobiol. 2002;53:606–617. doi: 10.1002/neu.10148. [DOI] [PubMed] [Google Scholar]
- Nides M. Update on pharmacologic options for smoking cessation treatment. Am J Med. 2008;121(4 Suppl 1):S20–S31. doi: 10.1016/j.amjmed.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Ong J, Kerr DI. Clinical potential of GABAB receptor modulators. CNS Drug Rev. 2005;11:317–334. doi: 10.1111/j.1527-3458.2005.tb00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orru A, Lai P, Lobina C, Maccioni P, Piras P, Scanu L, Froestl W, Gessa GL, Carai MA, Colombo G. Reducing effect of the positive allosteric modulators of the GABAB receptor, CGP7930 and GS39783, on alcohol intake in alcohol-preferring rats. Eur J Pharmacol. 2005;525:105–111. doi: 10.1016/j.ejphar.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Panagis G, Kastellakis A. The effects of ventral tegmental administration of GABAA, GABAB, NMDA and AMPA receptor agonists on ventral pallidum self-stimulation. Behav Brain Res. 2002;131:115–123. doi: 10.1016/S0166-4328(01)00353-9. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Markou A. Increased GABA neurotransmission via administration of gamma-vinyl GABA decreased nicotine self-administration in the rat. Synapse. 2002;44:252–253. doi: 10.1002/syn.10073. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Froestl W, Markou A. The GABAB receptor agonists baclofen and CGP44532 decreased nicotine self-administration in the rat. Psychopharmacol Berl. 2004;172:179–186. doi: 10.1007/s00213-003-1637-1. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Bruijnzeel AW, Kenny PJ, Wright CD, Froestl W, Markou A. Prolonged nicotine exposure does not alter GABAB receptor-mediated regulation of brain reward function. Neuropharmacology. 2005;49:953–962. doi: 10.1016/j.neuropharm.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Froestl W, Markou A. Repeated administration of the GABAB receptor agonist CGP44532 decreased nicotine self-administration, and acute administration decreased cue-induced reinstatement of nicotine-seeking in rats. Neuropsychopharmacology. 2005;30:119–128. doi: 10.1038/sj.npp.1300524. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Vlachou S, Guery S, Kaupmann K, Froestl W, Markou A. Positive modulation of GABAB receptors decreased nicotine self-administration and counteracted nicotine-induced enhancement of brain reward function in rats. J Pharmacol Exp Ther. 2008;326:306–314. doi: 10.1124/jpet.108.139204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Mercincavage M, Fonte CA, Lerman C. Varenicline’s effects on acute smoking behavior and reward and their association with subsequent abstinence. Psychopharmacol Berl. 2010;210:45–51. doi: 10.1007/s00213-010-1816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor RN. The global smoking epidemic: a history and status report. Clin Lung Cancer. 2004;5:371–376. doi: 10.3816/CLC.2004.n.016. [DOI] [PubMed] [Google Scholar]
- Rogers JL, See RE. Selective inactivation of the ventral hippocampus attenuates cue-induced and cocaine-primed reinstatement of drug-seeking in rats. Neurobiol Learn Mem. 2007;87:688–692. doi: 10.1016/j.nlm.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE. Multiple brain pathways and receptors underlying tobacco addiction. Biochem Pharmacol. 2007;74:1263–1270. doi: 10.1016/j.bcp.2007.07.039. [DOI] [PubMed] [Google Scholar]
- Sagara H, Kitamura Y, Yae T, Shibata K, Suemaru K, Sendo T, Araki H, Gomita Y. Nicotinic acetylcholine α4β2 receptor regulates the motivational effect of intracranial self stimulation behavior in the runway method. J Pharmacol Sci. 2008;108:455–461. doi: 10.1254/jphs.08168FP. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Yang X, Rotheram-Fuller EJ, Hsieh YC, Kintaudi PC, Charuvastra VC, Ling W. Randomized placebo-controlled trial of baclofen for cocaine dependence: preliminary effects for individuals with chronic patterns of cocaine use. J Clin Psychiatry. 2003;64:1440–1448. doi: 10.4088/JCP.v64n1207. [DOI] [PubMed] [Google Scholar]
- Smith MA, Yancey DL, Morgan D, Liu Y, Froestl W, Roberts DCS. Effects of positive allosteric modulators of the GABAB receptor on cocaine self-administration in rats. Psychopharmacol Berl. 2004;173:105–111. doi: 10.1007/s00213-003-1706-5. [DOI] [PubMed] [Google Scholar]
- Spano MS, Fattore L, Fratta W, Fadda P. The GABAB receptor agonist baclofen prevents heroin-induced reinstatement of heroin-seeking behavior in rats. Neuropharmacology. 2007;52:1555–1562. doi: 10.1016/j.neuropharm.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Sugita S, Johnson SW, North RA. Synaptic inputs to GABAA and GABAB receptors originate from discrete afferent neurons. Neurosci Lett. 1992;134:207–211. doi: 10.1016/0304-3940(92)90518-C. [DOI] [PubMed] [Google Scholar]
- Urwyler S, Mosbacher J, Lingenhoehl K, Heid J, Hofstetter K, Froestl W, Bettler B, Kaupmann K. Positive allosteric modulation of native and recombinant γ-aminobutyric acidB receptors by 2, 6-Di-tert-butyl-4-(3-hydroxy-2, 2-dimethyl-propyl)-phenol (CGP7930) and its aldehyde analog CGP13501. Mol Pharmacol. 2001;60:963–971. [PubMed] [Google Scholar]
- Urwyler S, Pozza MF, Lingenhoehl K, Mosbacher J, Lampert C, Froestl W, Koller M, Kaupmann K. N, N’-dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4, 6-diamine (GS39783) and structurally related compounds: novel allosteric enhancers of γ-aminobutyric acidB receptor function. J Pharmacol Exp Ther. 2003;307:322–330. doi: 10.1124/jpet.103.053074. [DOI] [PubMed] [Google Scholar]
- Vlachou S, Markou A. GABAB receptors in reward processes. Adv Pharmacol. 2010;58:315–371. doi: 10.1016/S1054-3589(10)58013-X. [DOI] [PubMed] [Google Scholar]
- Walaas I, Fonnum F. The distribution and origin of glutamate decarboxylase and choline acetyltransferase in ventral pallidum and other basal forebrain regions. Brain Res. 1979;177:325–336. doi: 10.1016/0006-8993(79)90783-2. [DOI] [PubMed] [Google Scholar]
- Walaas I, Fonnum F. Biochemical evidence for γ-aminobutyrate containing fibres from the nucleus accumbens to the substantia nigra and ventral tegmental area in the rat. Neuroscience. 1980;5:63–72. doi: 10.1016/0306-4522(80)90071-8. [DOI] [PubMed] [Google Scholar]
- Weerts EM, Froestl W, Kaminski BJ, Griffiths RR. Attenuation of cocaine-seeking by GABAB receptor agonists baclofen and CGP44532 but not the GABA reuptake inhibitor tiagabine in baboons. Drug Alcohol Depend. 2007;89:206–213. doi: 10.1016/j.drugalcdep.2006.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherington JP, Lambert NA. GABAB receptor activation desensitizes postsynaptic GABAB and A1 adenosine responses in rat hippocampal neurones. J Physiol. 2002;544:459–467. doi: 10.1113/jphysiol.2002.023093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Spiller K, Gardner EL. Mechanism-based medication development for the treatment of nicotine dependence. Acta Pharmacol Sin. 2009;30:723–739. doi: 10.1038/aps.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Hu J, Lucero L, Liu Q, Zheng C, Zhen X, Jin G, Lukas RJ, Wu J. Distinctive nicotinic acetylcholine receptor functional phenotypes of rat ventral tegmental area dopaminergic neurons. J Physiol. 2009;587:345–361. doi: 10.1113/jphysiol.2008.162743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaniewska M, Przegalinski E, Filip M. Nicotine dependence: human and animal studies, current pharmacotherapies and future perspectives. Pharmacol Rep. 2009;61:957–965. doi: 10.1016/s1734-1140(09)70157-4. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Massoudi R, Sepehri H, Rezayof A. Involvement of GABAB receptors of the dorsal hippocampus on the acquisition and expression of morphine-induced place preference in rats. Physiol Behav. 2006;87:31–38. doi: 10.1016/j.physbeh.2005.08.041. [DOI] [PubMed] [Google Scholar]