Abstract
A previous pilot study suggested that rizatriptan reduces motion sickness induced by complex vestibular stimulation. In this double-blind, randomized, placebo-controlled study we measured motion sickness in response to a complex vestibular stimulus following pretreatment with either rizatriptan or a placebo. Subjects included 25 migraineurs with or without migraine-related dizziness (23 females) aged 21–45 years (31.0 ± 7.8 years). Motion sickness was induced by off-vertical axis rotation in darkness, which stimulates both the semicircular canals and otolith organs of the vestibular apparatus. Results indicated that of the 15 subjects who experienced vestibular-induced motion sickness when pretreated with placebo, 13 showed a decrease in motion sickness following pretreatment with rizatriptan as compared to pretreatment with placebo (P < 0.02). This significant effect was not seen when subjects were exposed to more provocative vestibular stimulation. We conclude that the serotonin agonist, rizatriptan, reduces vestibular-induced motion sickness by influencing serotonergic vestibular-autonomic projections.
Keywords: Migraine, Motion sickness, Triptans
Introduction
Motion sickness refers to a combination of autonomic and cognitive symptoms and signs induced by exposure to certain types of movement [1]. Although the definition of motion sickness has been expanded to include stimuli beyond pure vestibular stimulation, in the present study motion sickness was induced by a complex vestibular stimulation in darkness. The subjects in the present report include individuals with migraine headache. Of note is that some of the symptoms associated with migraine overlap with symptoms of motion sickness such as upper abdominal sensations, sleepiness, apathy, dizziness, cold sweating, increased salivation, pallor, and headache [2, 3]. It is also notable that motion sickness occurs in approximately 50% of patients with migraine [4]. In this study, we only included subjects who had both migraine and motion-sickness susceptibility. The pathophysiologic basis for the high incidence of motion sickness in migraine is unknown. This lack of understanding, in part, motivated the present study. Drummond [5] found that 30–40% of persons with migraine report motion sickness with exposure to vestibular stimulation and that motion-sickness susceptibility, like migraine itself, is found more often in females [6].
Motion sickness can have a significant negative impact on an individual’s quality of life especially if the motion sickness interferes with a person’s ability to get to and from work, interferes with business travel, or interferes with leisure activities.
The neurophysiologic basis of motion sickness is unknown; however, several neurotransmitters seem to be important including histamine, acetylcholine, noradrenaline, and serotonin. The importance of these neurotransmitters for motion sickness is based on classes of medication that can be effective for treating motion sickness including antihistamines, scopolamine, amphetamines, and serotonin agonists and antagonists [7–9]. Our recent pilot studies also suggest that serotonin may be an important neurotransmitter in the neural pathways that underlie motion sickness in that the serotonin 1B/1D agonist, rizatriptan, appears to interfere with the potentiation of visually induced motion sickness by cranial pain [10].
The purpose of this study was to test the hypothesis that serotonin agonists known to benefit migraine headache also reduce vestibular-induced motion sickness. For this study, we selected subjects who had both migraine and motion-sickness susceptibility. By addressing this hypothesis, we aimed to elucidate the neural mechanisms underlying motion sickness in humans. We also aimed to assess a potential remedy for motion sickness in migraineurs with high motion-sickness susceptibility.
Methods
Prior to initiating this double-blind, randomized, placebo-controlled study, the protocol and consent form were approved by the Institutional Review Board of the University of Pittsburgh. Advertisements were used to recruit healthy adult migraineurs with a history of motion-sickness susceptibility who had previously used triptans without adverse effects. An informed consent was obtained from all the subjects prior to enrollment.
Subjects
This study was designed to evaluate 25 subjects with migraine with or without an additional diagnosis of migraine-related dizziness [11, 12]. Eligible subjects included healthy adult migraineurs aged 21–45 years who had previously used and tolerated any triptan and who had reported a history of motion sickness with either actual motion or visually induced motion perception. A board-certified neurologist confirmed a diagnosis of migraine based on IHS criteria [13]. All enrolled subjects were also evaluated using the structured interview for migraine-related dizziness [14, 15] and a clinical assessment based on the criteria of Neuhauser et al. [11, 12]. Subjects were excluded from the study if they were pregnant, had a history of cardiac disease, hypercholesterolemia, complicated migraine, triptan intolerance, a family history of myocardial infarction before age 45 in a first-degree relative, a weight greater than 250 lb, corrected vision worse than 20/40 OU, an active otologic disorder such as Meniere’s disease, constant dizziness or vestibular symptoms, a previously identified vestibular abnormality such as benign positional vertigo or an allergy or intolerance to gelatin. Subjects using a beta-blocking agent or monamine oxidase inhibitor in the past 2 weeks or who were using other medications such as ergotamine were further excluded. Additional exclusion criteria included clinically significant abnormalities on ocular-motor and vestibulo-ocular testing, which was performed on a separate visit prior to randomization. Testing included video-oculographic recordings of saccades, pursuit, and optokinetic nystagmus, a search for positional, gaze-evoked, and spontaneous nystagmus, binaural bithermal caloric testing, and earth-vertical axis rotation. Audiometric testing was also performed. Standard techniques were used and subjects were excluded based on established normative data [16]. In order to enroll 26 subjects, 27 subjects underwent screening tests 1 of whom was unable to complete the two experimental visits described below because of elevated blood pressure while receiving placebo. The 25 subjects who completed the study consisted of 23 females and 2 males with an overall age range of 21–45 years with an average age of 31.0 ± 7.8 years. Of these 25 subjects, 12 subjects met the criteria for migraine-related dizziness (see Table 1).
Table 1.
Subject Demographics
| Subject number | Age | Gender | Aura/no aura | Migraine-related dizziness? | Motion-sickness type | Prior triptans used |
|---|---|---|---|---|---|---|
| 1* | 26 | Female | Aura | Yes | Vestibular, visual | Rizatriptan, sumatriptan |
| 2 | 33 | Female | Aura | No | Visual | Eletriptan |
| 3* | 26 | Female | Aura | Yes | Vestibular | Eletriptan, rizatriptan, sumatriptan |
| 4 | 26 | Male | No aura | Yes | Vestibular, visual | Rizatriptan, sumatriptan |
| 5* | 45 | Female | No aura | No | Vestibular | Sumatriptan |
| 6* | 25 | Female | Aura | Yes | Vestibular, visual | Sumatriptan |
| 7* | 40 | Female | No aura | No | Vestibular | Eletriptan, rizatriptan, sumatriptan |
| 8* | 21 | Female | Aura | Yes | Visual | Sumatriptan |
| 9 | 25 | Female | No aura | No | Vestibular, visual | Sumatriptan |
| 10* | 22 | Female | No aura | Yes | Vestibular, visual | Sumatriptan |
| 11* | 24 | Female | No aura | Yes | Vestibular | Sumatriptan |
| 12* | 28 | Female | Aura | Yes | Vestibular | Sumatriptan |
| 13* | 31 | Female | Aura | No | Vestibular | Naratriptan |
| 14 | 30 | Female | Aura | Yes | Vestibular, visual | Sumatriptan |
| 15 | 27 | Female | Aura | No | Vestibular | Rizatriptan, sumatriptan |
| 16* | 41 | Female | Aura | Yes | Vestibular, visual | Sumatriptan |
| 17 | 24 | Female | Aura | No | Vestibular | Sumatriptan |
| 18 | 25 | Female | Aura | Yes | Vestibular | Frovatriptan |
| 19* | 41 | Male | No aura | Yes | Vestibular | Sumatriptan |
| 20 | 25 | Female | No aura | No | Vestibular, visual | Sumatriptan |
| 21* | 42 | Female | No aura | No | Vestibular, visual | Sumatriptan |
| 22 | 26 | Female | No aura | No | Vestibular, visual | Eletriptan, sumatriptan |
| 23* | 40 | Female | No aura | No | Vestibular, visual | Rizatriptan, sumatriptan |
| 24 | 41 | Female | Aura | No | Vestibular | Rizatriptan, sumatriptan |
| 25* | 42 | Female | Aura | No | Visual | Rizatriptan, sumatriptan |
* Included for statistical analysis
Two experimental visits were scheduled for each subject. Each subject was randomized to receive a blinded dose of the study drug, rizatriptan 10 mg, on their first or second visit and a placebo for the other visit. The subjects, investigators, and technicians were all blinded to treatment type. The rizatriptan and placebo were blinded in identical capsules by a central pharmacy, which determined and maintained the randomization assignment and labeled each blinded medication with subject numbers. Subjects were required to be headache-free and triptan-free for at least 1 week prior to each experimental visit. After receiving the study drug or placebo, subjects were idle for 2 h during which their blood pressure was monitored and adverse events recorded. Then, each subject underwent a motion-sickness provocation and assessment protocol.
The motion-sickness provocation and assessment protocol began with a baseline assessment of motion sickness and subjective distress. Motion sickness was judged using the motion-sickness scale developed by Graybiel [3], which rates seven subjective and objective signs and symptoms of motion sickness. Subjective units of distress were rated 0–10 based on the method of Wolpe [17]. Subjects rated the severity of motion sickness and subjective units of distress before and after each rotation. If a subject reached a motion-sickness score of 16 or greater, the experimental visit was terminated. Following the baseline assessment, sinusoidal-earth–vertical earth axis rotation in darkness at 0.05 Hz was performed to assess post-drug/post-placebo vestibular-ocular reflex (VOR) function, i.e. VOR gain and phase. The complex vestibular stimulation to induce motion sickness consisted of off-vertical axis rotation in darkness either in the clockwise or counterclockwise direction at constant velocity. By using a “rotate, then tilt” paradigm [18] the stimulus primarily activated the otolith organs of the inner ear and produced motion sickness in susceptible subjects [19]. Off-vertical axis rotation was continued for approximately 50 s. If the subjects could tolerate additional stimulation, i.e. their motion-sickness score was <16, following a 2 min rest, they were then exposed to a second off-vertical axis rotation in the direction opposite to that of the first off-vertical axis rotation. This second stimulus, in general, provokes motion sickness in most subjects [18]. During both earth-vertical and off-vertical axis rotations, eye movements were recorded with electro-oculography.
Data analysis
Motion-sickness scores and subjective units of distress were analyzed by computing the differences between baseline (i.e. pre-earth-vertical axis rotation) and post-stimulus values. Eye-movement data were analyzed using standard techniques to compute gain and phase for earth-vertical axis rotations and modulation and bias components for off-vertical axis rotations [16]. At the completion of this analysis, the data were unblinded. It was determined that 10 of the 25 subjects developed negligible motion sickness induced by the first off-vertical axis rotation following pre-medication with placebo. The data from these subjects were not analyzed further. Statistical analysis of the change in motion-sickness scores was performed using a non-parametric paired samples sign test under the null hypothesis that the median baseline and post-stimulus scores are equal. The number of subjects displaying a subjective improvement in motion sickness was compared against the true value (i.e. 8 of 15 subjects with improvement) that would be expected if no rizatriptan treatment effect existed. A one-tailed type I error rate of 0.05 was used to judge the significance of improvement in motion-sickness scores.
Results
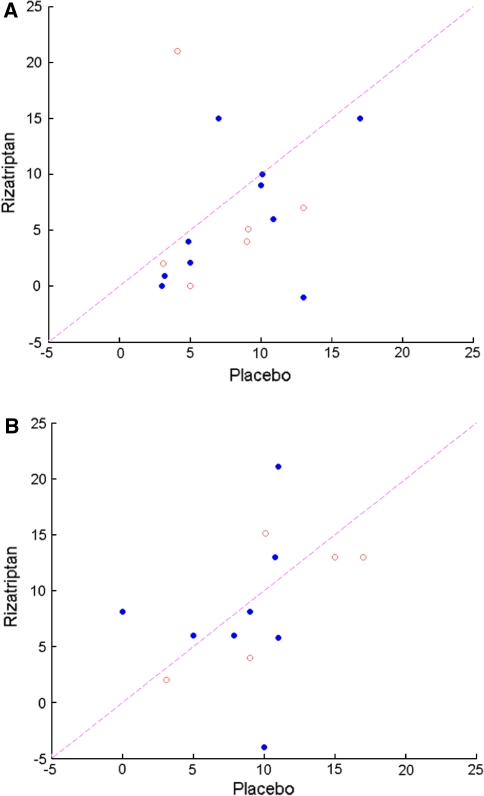
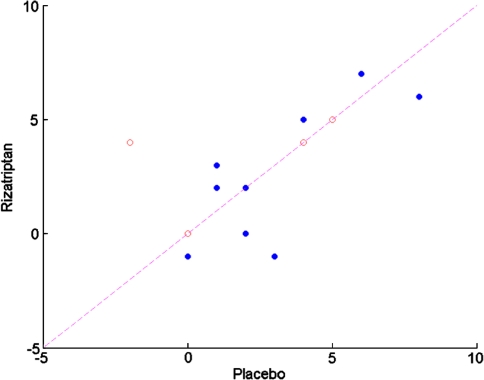
All 25 subjects who completed the study could undergo at least one off-vertical rotation at each of the two visits. Three subjects had motion-sickness scores of 16 or greater following the first off-vertical axis rotation during one or both visits and thus could not undergo a second off-vertical axis rotation on that visit. For one of these subjects, this occurred only during the placebo visit; for two of these subjects, this occurred only during the rizatriptan visit. Motion-sickness scores following the first off-vertical axis rotation referenced to baseline motion-sickness score are shown in Fig. 1a. Note that the data are shown only for 15 of the 25 subjects, namely, those subjects who experienced a motion-sickness score that was more than 3 points higher than baseline following their first off-vertical axis rotation on the experimental visit during which they received placebo. Note that 13 of these 15 subjects experienced decreased motion sickness with rizatriptan as compared to placebo, which is a statistically significant result (P < 0.02, sign test, one-tailed). Note further that the status of the patients regarding the presence or absence of a history of migraine-related dizziness did not appear to influence the effect of rizatriptan. When these same individuals were exposed to a second off-vertical axis rotation, there was no apparent benefit from rizatriptan regarding motion-sickness score referenced to baseline motion sickness (Fig. 1b). Although there was no significant effect of rizatriptan on motion-sickness score following the second off-vertical axis rotation, there was a trend for subjects without migraine-related dizziness to show a benefit with rizatriptan as compared to placebo (4 of 5 subjects). A further analysis of motion-sickness score was performed using the ten subjects who did not achieve significant motion sickness following the first off-vertical axis rotation on the experimental visit during which they received placebo. Of these ten individuals, three individuals experienced significant motion sickness following the second off-vertical axis rotation on the experimental visit during which they received placebo. Two of these individuals experienced more motion sickness following the second off-vertical axis rotation on the visit during which they received rizatriptan. An analysis of subjective units of distress indicated that there was no apparent difference between the effects of rizatriptan and placebo (See Fig. 2).
Fig. 1.
a Motion-sickness score following pretreatment with rizatriptan vs. motion-sickness score following pretreatment with placebo in response to a complex vestibular stimulus, i.e. off-vertical axis rotation. Scores represent the increase in motion sickness above pre-stimulus baseline. Note that data are shown only from those subjects whose vestibular-induced increase in motion-sickness score exceeded 3 after pretreatment with placebo. Data from subjects with migraine-related dizziness are shown as filled circles. Data from subjects without migraine-related dizziness are shown as open circles. Note that for 13 of 15 subjects that the increase in motion-sickness score above baseline was greater following pretreatment with placebo as compared to that seen following treatment with rizatriptan. b Motion-sickness scores following a second off-vertical axis rotation. Data are shown for those subjects who could complete a second off-vertical axis rotation following pretreatment with rizatriptan and following pretreatment with placebo. Note the lack of an obvious effect of rizatriptan
Fig. 2.
Subjective units of distress after off-vertical axis rotation following pretreatment with rizatriptan vs. placebo. Note that data are shown for those subjects whose data are illustrated in Fig. 1. Note the absence of a consistent influence of rizatriptan
Eye-movement parameters were analyzed for evidence of a consistent effect of rizatriptan on the VOR or a correlation between motion sickness and the VOR. No consistent effects were found.
Discussion
The results of this study corroborate the implications of our prior pilot study, which suggested that rizatriptan provided protection against motion sickness induced by complex vestibular stimulation in migraineurs [20]. In the present study, which also assessed migraineurs with a history of motion sickness, an additional diagnosis of migraine-related dizziness did not affect outcome aside from a trend toward a protective effect of rizatriptan in subjects without migraine-related dizziness following a second off-vertical axis rotation. Possibly, a protective effect was found in subjects with and without migraine-related dizziness because all subjects were individuals with motion-sickness susceptibility. The lack of an effect of pretreatment of rizatriptan on motion sickness in response to a second off-vertical axis rotation suggests a type of dose–response mechanism in that rizatriptan at a single dose provided protection for a lower level of stimulation but not a higher level of motion-sickness provocation. The trend for subjects without migraine-related dizziness to gain benefit from rizatriptan despite a high level of motion-sickness provocation suggests that subjects with migraine-related dizziness may be more susceptible to motion sickness [21, 22]. Our results suggest that rizatriptan may increase the threshold for producing motion sickness. The second off-vertical axis rotation may have been a suprathreshold stimulus despite rizatriptan, especially in subjects with migraine-related dizziness. This concept is consistent with the recent suggestion that motion-sickness susceptibility and migraine may be related to an innate vestibular hypersensitivity [23] and the expression of 5-HT1B and 5-HT1D receptor targets of triptans by most vestibular ganglion cells [24].
Our study also suggests that rizatriptan did not increase subjective units of discomfort. The side effects of rizatriptan can include fatigue, somnolence, head and neck pressure, nonspecific dizziness, and nausea, and thus might have been expected to increase subjective units of discomfort despite its benefit for reduction of motion sickness. Evidently, the level of distress caused by side effects of rizatriptan was offset by the reduction in motion sickness following pretreatment with rizatriptan. We speculate that the effect of rizatriptan was mediated via its effects on vestibulo-autonomic rather than vestibulo-ocular pathways.
Our prior study of rizatriptan suggested that the eye-movement response during off-vertical axis rotation was influenced by pretreatment with rizatriptan. Specifically, our study suggested that the bias component of the eye-movement response to off-vertical axis rotation was reduced following pretreatment with rizatriptan. The current study does not support this finding in a larger group of subjects. In that the bias component reflects central nervous system processing of peripheral vestibular activity, the absence of a reduction in the magnitude of the bias component suggests that rizatriptan does not interfere with the so called “velocity storage system” of central vestibulo-ocular processing. In addition, there were no consistent changes in VOR gain and phase following pretreatment with rizatriptan. This further suggests that the effect of rizatriptan on motion sickness is not mediated via changes in “velocity storage.”
The present study has implications for understanding the pathophysiology of migraine-related dizziness and motion sickness in migraineurs. Recent studies indicate a remarkable degree of neurochemical similarity between nociceptive trigeminal ganglion cells and vestibular ganglion cells. For example, most vestibular ganglion cells express functional biomarkers associated with nociception, such as TRPV1 receptors [25], substance P [26], P2X3 purinergic receptors [27], and both the 5-HT1B and 5-HT1D receptors [24]. Moreover, plasma extravasation occurs in the vestibular periphery at the same time as extravasation in the meninges in a murine model of neurogenic migraine [28]. Finally, functional imaging studies report that increased cerebral blood flow in a region of the dorsal and dorsolateral pons accompanies both spontaneous and glyceryl trinitrate induced migraine attacks [29–33]; the implicated region appears to include portions of the vestibular nuclei, medial parabrachial nucleus, locus coeruleus and dorsal raphe nuclei.
There is also a considerable overlap of symptoms between motion sickness and migraine. Motion sickness describes a progression of symptoms evoked by real or virtual motion. It begins with the ‘sopite syndrome’ [34], a form of motion sickness that is characterized by yawning, drowsiness, disinclination for either physical or mental work, and lack of participation in group activities. The symptoms can progress to vague abdominal discomfort, hyperventilation, salivation, pallor, profuse cold sweating, somnolence, and nausea. Vomiting and retching can also ensue. There is a striking overlap with symptoms of migraine and passive emotional coping responses to pain [35, 36]. Hence, the efficacy of rizatriptan against motion sickness is consistent with the hypothesis that engagement of migraine-related mechanisms, such as trigemino-vascular reflexes and central pain pathways, may contribute to the generation of motion sickness.
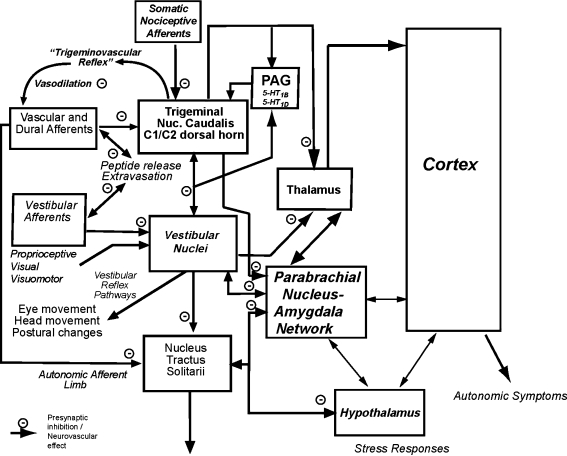
As summarized in Fig. 3, the enhanced motion-sickness susceptibility in migraineurs may be a function of the net vestibular nuclear and solitary nucleus effects of vestibular stimulation. Triptans have been shown to reduce nociceptive activation in animal studies through actions on somatic, vascular and dural afferents, and central trigeminal and solitary nucleus neurons [37–40]. These actions appear to be primarily by 5-HT1B and 5-HT1D-mediated presynaptic inhibition [40, 41]. Activation of 5-HT1B and 5-HT1D receptors in the ventrolateral periaqueductal gray inhibit trigeminal and solitary nucleus nociceptive responses [42] and affect passive coping responses to pain [36] that resemble the prodromal features of motion sickness. We propose that motion-sickness sensitivity is decreased by the additional actions of rizatriptan on 5-HT1B and 5-HT1D receptors on vestibular ganglion cells [24] and vestibular nuclear neurons [43], which would be expected to raise the threshold for eliciting symptoms. Rizatriptan can also have direct vascular effects via 5-HT1B and 5-HT1D receptors, attenuating vasodilatation and extravasation from cerebral and inner ear vasculature. Thus, these findings are consistent with the concept that parallel activation of vestibular and cranial nociceptive pathways may contribute to the otologic features of migraine-related dizziness. More generally, they provide a basis for an emerging view that motion sickness may be a form of interoceptive pain.
Fig. 3.
Schematic diagram to explain effects of rizatriptan on motion sickness in migraineurs. The enhanced motion-sickness susceptibility in migraineurs is hypothesized to reflect the net vestibular nuclear and solitary nucleus effects of vestibular stimulation. The trigeminal nociceptive pathway and vestibular nuclei also contribute ascending thalamic connections, which contribute to autonomic symptoms via projections to the cerebral cortex. Spinal trigeminal nuclear and vestibular nuclear neurons also project to the parabrachial nucleus. Stress responses and vasopressin release are mediated by interconnections of the parabrachial pathways with the hypothalamus. We propose that motion-sickness sensitivity is decreased by the actions of rizatriptan on 5-HT1B and 5-HT1D receptors on (1) vasculature (e.g. blocking vasodilatation), (2) vestibular ganglion cells, (3) trigeminal ganglion cells (for somatic, vascular and dural afferents), (4) several brainstem pathways and projections, and (5) neurons in the periaqueductal gray (PAG). The primary effect of rizatriptan is via presynaptic inhibition of terminals that can use either excitatory amino acids or GABA. These presynaptic effects can occur at primary afferent and in CNS pathways
At present, there are several symptomatic treatments for motion sickness including vestibular suppressant medications such as meclizine. Individuals especially prone to motion sickness often avoid provocative activities. Preventive treatments include scopolamine, promethazine, and antihistamines. These medications usually cause sedation or other unwanted effects.
The reduction in motion sickness following pretreatment with rizatriptan suggests that rizatriptan may provide benefit for migraineurs who suffer from motion sickness. As noted above, the mechanism whereby rizatriptan reduces motion sickness may be related to an alteration in the threshold for motion sickness based on serotonergic mechanisms. Of interest is that no changes in the VOR were found in response to rizatriptan despite effects on motion sickness. This finding does not support the current theories of motion sickness proffered by Dai et al. [44] and studied by Jeong et al. [23] that so called “velocity storage” in the VOR is fundamental to motion sickness. Possibly, migraineurs who cannot avoid motion sickness provoking environments could pre-medicate with rizatriptan with benefit. Further research would be required to establish this potential benefit in that the present study is laboratory-based and may not entirely reflect the level of symptoms produced in a natural environment.
Acknowledgments
The authors thank Anita Lieb and Susan Strelinski for technical assistance and Dr. Gregory Marchetti for statistical analysis. This work was supported by an investigator-initiated award from Merck & Co., Inc.
Conflict of interest None.
Contributor Information
Joseph M. Furman, Phone: +1-412-6472115, FAX: +1-412-6472080, Email: furmanjm@upmc.edu
Carey D. Balaban, Email: cbalaban@pitt.edu
References
- 1.Irwin JA. The pathology of sea-sickness. Lancet. 1881;2:907–909. doi: 10.1016/S0140-6736(02)38129-7. [DOI] [Google Scholar]
- 2.Marcus DA, Furman JM, Balaban CD. Motion sickness in migraine sufferers. Expert Opin Pharmacother. 2005;6(15):2691–2697. doi: 10.1517/14656566.6.15.2691. [DOI] [PubMed] [Google Scholar]
- 3.Graybiel A, Wood CD, Miller EF, Cramer DB. Diagnostic criteria for grading the severity of acute motion sickness. Aerosp Med. 1968;39(5):453–455. [PubMed] [Google Scholar]
- 4.Kayan A, Hood JD. Neuro-otological manifestations of migraine. Brain. 1984;107(Pt 4):1123–1142. doi: 10.1093/brain/107.4.1123. [DOI] [PubMed] [Google Scholar]
- 5.Drummond PD. Triggers of motion sickness in migraine sufferers. Headache. 2005;45(6):653–656. doi: 10.1111/j.1526-4610.2005.05132.x. [DOI] [PubMed] [Google Scholar]
- 6.Turner M, Griffin MJ, Holland I. Airsickness and aircraft motion during short-haul flights. Aviat Space Environ Med. 2000;71(12):1181–1189. [PubMed] [Google Scholar]
- 7.Yates BJ, Miller AD, Lucot JB. Physiological basis and pharmacology of motion sickness: an update. Brain Res Bull. 1998;47(5):395–406. doi: 10.1016/S0361-9230(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 8.Takeda N, Morita M, Horii A, Nishiike S, Kitahara T, Uno A. Neural mechanisms of motion sickness. J Med Invest. 2001;48(1–2):44–59. [PubMed] [Google Scholar]
- 9.Javid FA, Naylor RJ. The effect of serotonin and serotonin receptor antagonists on motion sickness in Suncus murinus. Pharmacol Biochem Behav. 2002;73(4):979–989. doi: 10.1016/S0091-3057(02)00955-3. [DOI] [PubMed] [Google Scholar]
- 10.Furman JM, Marcus DA. A pilot study of rizatriptan and visually-induced motion sickness in migraineurs. Int J Med Sci. 2009;6(4):212–217. doi: 10.7150/ijms.6.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neuhauser H, Leopold M, von Brevern M, Arnold G, Lempert T (2001) The interrelations of migraine, vertigo, and migrainous vertigo. Neurology 56(4):436–441 [DOI] [PubMed]
- 12.Abu-Arafeh I, Russell G. Paroxysmal vertigo as a migraine equivalent in children: a population-based study. Cephalalgia 15(1):22–5 (discussion 4) [DOI] [PubMed]
- 13.The International Classification of Headache Disorders (2004) 2nd edn. Cephalalgia 24(Suppl 1):9–160 [DOI] [PubMed]
- 14.Marcus DA, Kapelewski C, Rudy TE, Jacob JG, Furman JM (2004) Diagnosis of migrainous vertigo: validity of a structured interview. Med Sci Monit 10(5):CR197–201 [PubMed]
- 15.Marcus DA, Kapelewski C, Jacob RG, Rudy TE, Furman JM. Validation of a brief nurse-administered migraine assessment tool. Headache. 2004;44(4):328–332. doi: 10.1111/j.1526-4610.2004.04076.x. [DOI] [PubMed] [Google Scholar]
- 16.Furman JM, Wuyts F. Vestibular test techniques. In: Luxon LM, Martini A, Furman JM, Stephens D, editors. Textbook of audiological medicine. London: Martin Dunitz; 2003. pp. 735–746. [Google Scholar]
- 17.Wolpe J. The practice of behavior therapy. New York: Pergamon Press; 1982. [Google Scholar]
- 18.Furman JM, Schor RH, Schumann TL. Off-vertical axis rotation: a test of the otolith-ocular reflex. Ann Otol Rhinol Laryngol. 1992;101(8):643–650. doi: 10.1177/000348949210100803. [DOI] [PubMed] [Google Scholar]
- 19.Denise P, Etard O, Zupan L, Darlot C. Motion sickness during off-vertical axis rotation: prediction by a model of sensory interactions and correlation with other forms of motion sickness. Neurosci Lett. 1996;203(3):183–186. doi: 10.1016/0304-3940(96)12303-X. [DOI] [PubMed] [Google Scholar]
- 20.Marcus DA, Furman JM (2006) Prevention of motion sickness with rizatriptan: a double-blind, placebo-controlled pilot study. Med Sci Monit 12(1):PI1–7 [PubMed]
- 21.Evans RW, Marcus D, Furman JM. Motion sickness and migraine. Headache. 2007;47(4):607–610. doi: 10.1111/j.1526-4610.2007.00762.x. [DOI] [PubMed] [Google Scholar]
- 22.Neuhauser HK, Radtke A, Brevern M, Feldmann M, Lezius F, Ziese T, et al. Migrainous vertigo: prevalence and impact on quality of life. Neurology. 2006;67(6):1028–1033. doi: 10.1212/01.wnl.0000237539.09942.06. [DOI] [PubMed] [Google Scholar]
- 23.Jeong SH, Oh SY, Kim HJ, Koo JW, Kim JS. Vestibular dysfunction in migraine: effects of associated vertigo and motion sickness. J Neurol. 2009;257(6):905–912. doi: 10.1007/s00415-009-5435-5. [DOI] [PubMed] [Google Scholar]
- 24.Ahn S-K, Balaban CD (2010) Distribution of 5-HT1B and 5-HT1D receptors in the inner ear. Brain Res 1346:92–101 [DOI] [PMC free article] [PubMed]
- 25.Kitahara T, Li HS, Balaban CD. Changes in transient receptor potential cation channel superfamily V (TRPV) mRNA expression in the mouse inner ear ganglia after kanamycin challenge. Hear Res. 2005;201(1–2):132–144. doi: 10.1016/j.heares.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Kevetter GA, Leonard RB. Molecular probes of the vestibular nerve. II. Characterization of neurons in Scarpa’s ganglion to determine separate populations within the nerve. Brain Res. 2002;928(1–2):18–29. doi: 10.1016/S0006-8993(01)03264-4. [DOI] [PubMed] [Google Scholar]
- 27.Xiang Z, Bo X, Burnstock G. P2X receptor immunoreactivity in the rat cochlea, vestibular ganglion and cochlear nucleus. Hear Res. 1999;128(1–2):190–196. doi: 10.1016/S0378-5955(98)00208-1. [DOI] [PubMed] [Google Scholar]
- 28.Koo JW, Balaban CD. Serotonin-induced plasma extravasation in the murine inner ear: possible mechanism of migraine-associated inner ear dysfunction. Cephalalgia. 2006;26(11):1310–1319. doi: 10.1111/j.1468-2982.2006.01208.x. [DOI] [PubMed] [Google Scholar]
- 29.Afridi SK, Giffin NJ, Kaube H, Friston KJ, Ward NS, Frackowiak RS, et al. A positron emission tomographic study in spontaneous migraine. Arch Neurol. 2005;62(8):1270–1275. doi: 10.1001/archneur.62.8.1270. [DOI] [PubMed] [Google Scholar]
- 30.Afridi SK, Matharu MS, Lee L, Kaube H, Friston KJ, Frackowiak RS, et al. A PET study exploring the laterality of brainstem activation in migraine using glyceryl trinitrate. Brain. 2005;128(Pt 4):932–939. doi: 10.1093/brain/awh416. [DOI] [PubMed] [Google Scholar]
- 31.Afridi SK, Goadsby PJ. Neuroimaging of migraine. Curr Pain Headache Rep. 2006;10(3):221–224. doi: 10.1007/s11916-006-0049-4. [DOI] [PubMed] [Google Scholar]
- 32.May A, Matharu M. New insights into migraine: application of functional and structural imaging. Curr Opin Neurol. 2007;20(3):306–309. doi: 10.1097/WCO.0b013e328136c20b. [DOI] [PubMed] [Google Scholar]
- 33.May A. A review of diagnostic and functional imaging in headache. J Headache Pain. 2006;7(4):174–184. doi: 10.1007/s10194-006-0307-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graybiel A, Knepton J. Sopite syndrome: a sometimes sole manifestation of motion sickness. Aviat Space Environ Med. 1976;47(8):873–882. [PubMed] [Google Scholar]
- 35.Bernard JF, Bandler R. Parallel circuits for emotional coping behaviour: new pieces in the puzzle. J Comp Neurol. 1998;401(4):429–436. doi: 10.1002/(SICI)1096-9861(19981130)401:4<429::AID-CNE1>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 36.Bandler R, Price JL, Keay KA (2000) Brain mediation of active and passive emotional coping. Prog Brain Res (122):331–349 [DOI] [PubMed]
- 37.Donaldson C, Boers PM, Hoskin KL, Zagami AS, Lambert GA. The role of 5-HT1B and 5-HT1D receptors in the selective inhibitory effect of naratriptan on trigeminovascular neurons. Neuropharmacology. 2002;42(3):374–385. doi: 10.1016/S0028-3908(01)00190-3. [DOI] [PubMed] [Google Scholar]
- 38.Goadsby PJ, Classey JD. Evidence for serotonin (5-HT)1B, 5-HT1D and 5-HT1F receptor inhibitory effects on trigeminal neurons with craniovascular input. Neuroscience. 2003;122(2):491–498. doi: 10.1016/S0306-4522(03)00570-0. [DOI] [PubMed] [Google Scholar]
- 39.Jeggo RD, Wang Y, Jordan D, Ramage AG. Activation of 5-HT1B and 5-HT1D receptors in the rat nucleus tractus solitarius: opposing action on neurones that receive an excitatory vagal C-fibre afferent input. Br J Pharmacol. 2007;150(8):987–995. doi: 10.1038/sj.bjp.0707169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jennings EA, Ryan RM, Christie MJ. Effects of sumatriptan on rat medullary dorsal horn neurons. Pain. 2004;111:30–37. doi: 10.1016/j.pain.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 41.Matsuoka T, Hasuo H, Akasu T. 5-Hydroxytryptamine 1B receptors mediate presynaptic inhibition of monosynaptic IPSC in the rat dorsolateral septal nucleus. Neurosci Res. 2004;48(3):229–238. doi: 10.1016/j.neures.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 42.Bartsch T, Knight YE, Goadsby PJ. Activation of 5-HT(1B/1D) receptor in the periaqueductal gray inhibits nociception. Ann Neurol. 2004;56(3):371–381. doi: 10.1002/ana.20193. [DOI] [PubMed] [Google Scholar]
- 43.Ahn S-K, Balaban CD (2007) Regional distribution of 5-HT1A, 1B, and 1D receptors in rat vestibular nuclei (VN). Soc Neurosci Abstr 37(#180.5)
- 44.Dai M, Raphan T, Cohen B. Labyrinthine lesions and motion sickness susceptibility. Exp Brain Res. 2007;178(4):477–487. doi: 10.1007/s00221-006-0759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]