Abstract
Although glucocorticoid (GC) is widely used for treating hematopoietic malignancies including adult T-cell leukemia (ATL), the mechanism by which leukemic cells become resistant to GC in the clinical course remains unclear. Using a series of T-cell lines infected with human T lymphotropic virus type-I (HTLV-I), the causative virus of ATL, we have dissected the transformation from interleukin (IL)-2-dependent to -independent growth stage. The transformation associates the loss of thioredoxin-binding protein-2 (TBP-2), a tumor suppressor and regulator of lipid metabolism. Here we show that TBP-2 is responsible for GC-induced apoptosis in ATL cells. In the IL-2-dependent stage, dexamethasone induced TBP-2 expression and apoptosis, both of which were blocked by GC receptor (GR) antagonist RU486. Knockdown of TBP-2 consistently reduced the amount of GC-induced apoptosis. In IL-2-independent stage, however, expression of GR and TBP-2 was suppressed and GC failed to induce apoptosis. Forced expression of GR led the cells to mild sensitivity to GC, which was also accomplished by treatment with suberoylanilide hydroxamic acid, a TBP-2 inducer. A transfection experiment showed that TBP-2 expression induced apoptosis in IL-2-independent ATL cells. Thus, TBP-2 is likely to be one of the key molecules for GC-induced apoptosis and a potential target for treating the advanced stage of ATL.
Keywords: HTLV-I, ATL, glucocorticoid receptor, glucocorticoid, thioredoxin-binding protein-2/thioredoxin-interacting protein, apoptosis
Introduction
Since the first reports of adult T-cell leukemia (ATL) in the 1970s,1, 2 decades of studies have focused on this hematological malignancy because of its unique late-onset clinical manifestations, the dysregulation of the interleukin 2 (IL-2) receptor and the causative agent, human T-cell leukemia virus type I (HTLV-I).3, 4, 5 The pathogenesis of ATL is a multistep process because overt leukemia develops in some HTLV-I carriers after a long latent period.6 It is thought that in the initial stage of leukemogenesis, HTLV-I-infected CD4+ T cells grow on the condition that IL-2 is available, whereas in the terminal and acute stage of ATL, these cells expand independently of growth factors in vivo.7, 8 Many HTLV-I-infected T-cell lines derived from ATL patients need to be maintained initially in the presence of IL-2 (IL-2-dependent growth stage (D stage)), although the same cell lines spontaneously acquire independence of exogenous IL-2 supplementation later during the long-term culture (IL-2-independent growth stage (I stage)). On the basis of the parallelism between in vivo and in vitro growth of ATL cells, the in vitro transition from IL-2-dependent to -independent growth has been a useful model for investigating the mechanism by which ATL develops in vivo, as well as for exploring possible strategies to treat this leukemia.9, 10, 11
Glucocorticoid (GC) has been included in the standard therapy of hematological malignancies.12, 13 However, GC is of limited use in the treatment of ATL owing to the frequent occurrence of GC resistance and exacerbation of the immunosuppressive status inherently associated with this leukemia.14 The mechanism for the lack of GC efficacy seen in ATL cases remains unclear. GC receptor (GR), represented by the functional α isoform (GRα), mediates most of the pleiotropic GC activities. Upon ligand binding, GR translocates from the cytoplasm to the nucleus and regulates the expression of target genes through trans-activation or trans-repression mechanism.15, 16 Extensive studies have been conducted to clarify the mechanism by which HTLV-I affects GR signaling, revealing that the oncoprotein Tax1 encoded by HTLV-I plays a critical role in repressing nuclear receptor-dependent transcription.17, 18 Tax1 also affects nuclear factor-κB and signal transducer and activator of transcription 5 signaling, both of which are major downstream targets of GR signaling.19, 20 Taken together, there is a unique alteration in the responsiveness to GC in ATL.
Thioredoxin is a 12-kDa protein that harbors the CXXC motif and plays a critical role in redox regulation. After reporting that thioredoxin was expressed in ATL cells at an extremely high level,21 our studies have focused on the role of thioredoxin in the leukemogenesis by HTLV-I. A yeast two-hybrid assay allowed us to identify thioredoxin-binding protein-2 (TBP-2/VDUP-1/TXNIP) as an endogenous binding partner and antagonist of thioredoxin.22 Thioredoxin and TBP-2 are involved in the ASK1-dependent apoptosis pathway.23, 24 Blocking thioredoxin of cancer cells was suggested to be a useful approach to cancer therapy.25, 26 TBP-2 is induced by GC in murine normal thymocytes and the T-cell lymphoma line WEHI7.2.27 Intriguingly, TBP-2 expression is lost during the progression of HTLV-I-induced transformation.28, 29 On the basis of these observations, we hypothesized that TBP-2 plays a critical role in GC-induced ATL cell death. If this is the case, it is possible that the diversity in the GC efficacy in treating ATL patients is explained by the expression of TBP-2.
In this study, we used a series of HTLV-I-infected T-cell lines independently established from ATL patients and found that the GR–TBP-2 signaling axis plays a key role in mediating GC-induced cell death.
Materials and methods
Cell culture
HTLV-I-infected human T-cell lines (ED40515, ATL43, ATL2 and Sez627) were cultured in RPMI 1640 medium (Sigma-Aldrich, St Louis, MO, USA) containing 10% heat-inactivated fetal calf serum (Invitrogen, Carlsbad, CA, USA) and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin; Nacalai Tesque, Kyoto, Japan) at 37 °C in a humid atmosphere of 5% CO2 in air. To maintain the growth of the IL-2-dependent T cells, recombinant human IL-2 (7.5 ng/ml; Shionogi Co., Osaka, Japan) was added to the culture medium. Each set of IL-2-dependent and -independent cells had the same clonal origin as confirmed by the T-cell receptor-β gene rearrangement and the HTLV-I proviral integration sites.9, 10, 11 D-ED40515, D-ATL43, D-Sez627 or D-ATL2T cell lines are IL-2-dependent HTLV-I-transformed T cells that grew solely in the presence of IL-2, whereas I-ED40515, I-ATL43, I-Sez627 or I-ATL2T cell lines are IL-2-independent derivatives of the above cells that grew independent of IL-2. Exogenous IL-2 is indispensable for the continued cell expansion in the D stage. In I stage, however, exogenous IL-2 is no longer required for maintaining cell growth, and neither is cell growth affected by IL-2 supplementation (data not shown).
Plasmids
The full-length cDNA of human GRα was cloned in-frame into the pCMV-tag3B vector (Stratagene, La Jolla, CA, USA). Briefly, the cDNA was first amplified by PCR using primers (forward: 5′-GGATCCATGGACTCCAAAGAATCATT-3′ and reverse: 5′-CTCGAGTCACTTTTGATGAAACAGAAG-3′). The PCR product was then subcloned into pCR-BluntII-TOPO vector (Invitrogen) to obtain pCR-BluntII-TOPO-GR, which was further digested with BamHI and XhoI restriction enzymes (Toyobo, Tokyo, Japan). The fragment was finally cloned into the pCMV-tag3B vector to obtain the pCMV-tag3B-GRα plasmid. With regard to the pCMV-tag2A-TBP-2, full-length cDNA of human TBP-2 was cloned in-frame into the pCMV-tag2A vector (Stratagene), as described previously.28 Each plasmid was verified by DNA sequencing.
Transient transfection and RNA interference assay
Plasmids were transfected into cells (1 × 106 cells) using the Nucleofector II and AMAXA cell line kit V (Lonza Cologne, Cologne, Germany), according to the manufacturer's instructions. Unless otherwise mentioned, 5 μg of DNA was used for each transfection. Expression of the target protein was verified by western blotting. In RNAi assays, we employed 100 pmol of duplex oligonucleotides selectively silencing the TBP-2 (RNA interference (RNAi) no. 1: UUUCAGGGUCGUUAAAGACCACCUC; RNAi no. 2: UCAAUUCGAGCAGAGACAGACACCC or RNAi no. 3: AUCCAAAGCACUUUAGCCACUCCGC) (Invitrogen) or control oligonucleotides (Stealth RNAi-negative control) (Invitrogen). siRNAs were transfected into cells (1 × 106 cells) using the Nucleofector II and AMAXA cell line kit V. IL-2 was added at 7.5 ng/ml at least 24 h after the transfection to maintain the growth of D-ED40515 and D-ATL43 cells.29
Treatment of ATL cells with GC or other reagents
IL-2-dependent HTLV-I-infected T cells were first deprived of IL-2 for at least 24 h to exclude the carry-over effect of IL-2 on cell growth. Then, viable cells were enriched using LSM Lymphocyte Separation Medium according to the manufacturer's instruction (MP Biomedicals, Aurora, OH, USA). Dexamethasone (Dex) (Nacalai Tesque) or GC antagonist RU486 (Sigma-Aldrich, San Diego, CA, USA) was added to the culture medium, whereas ethanol (0.004% v/v) was added as a vehicle control. In experiments using IL-2-independent HTLV-I-infected cells, Dex or suberoylanilide hydroxamic acid (SAHA) (Qbiogene, San Diego, CA, USA) was added directly to the cells.
RNA extraction and real-time quantitative RT-PCR
Total RNA was extracted using TRIzol reagent according to the manufacturer's instructions (Invitrogen). Total RNA (1 μg) was used as a template for the cDNA synthesis using the PrimeScript RT reagent kit according to the manufacturer's instruction (Takara, Otsu, Japan). A 1:20 fraction of each reverse transcriptase reaction mixture was further used as a template for real-time quantitative PCR using the SYBR Premix TaqII kit (Takara) with TBP-2 primers (forward: 5′-GCCACACTTACCTTGCCAAT-3′ and reverse: 5′-GGAGGAGCTTCTGGGGTATC-3′), GR-α-specific primers (forward: 5′-CCATTGTCAAGAGGGAAGGA-3′ and reverse: 5′-CAGCTAACATCTCGGGGAAT-3′) or glyceraldehyde 3-phosphate dehydrogenase primers (forward: 5′-ACCCACTCCTCCACCTTTG-3′ and reverse: 5′-CTCTTGTGCTCTTGCTGGG-3′). Fluorescent detection and data analysis were performed on an ABI Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA, USA).
Immunoblotting
Cells were washed twice in cold phosphate-buffered saline and resuspended in lysis buffer (0.5% NP-40, 50 m Tris-Cl (pH 7.2), 150 m NaCl) supplemented with 1 × protease inhibitor cocktail (Roche, Tokyo, Japan) and 1 m phenylmethylsulfonyl fluoride (Nacalai Tesque). The cell lysates were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis using 4–12% NuPage Bis–Tris pre-cast gel (Invitrogen) and transferred to a polyvinylidene fluoride membrane (GE Lifesciences, Pittsburgh, PA, USA). The membranes were first incubated with anti-TBP-2 antibody (JY2, 1:1000; MBL, Nagoya, Japan), anti-GR-α (P-20, 1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-β-actin (1:2000; Sigma-Aldrich or sc-81178, 1:200; Santa Cruz), anti-cleaved caspase-3 (1:1000; Cell Signaling, Danvers, MA, USA), anti-poly (ADP-ribose) polymerase (PARP) (1:1000; Cell Signaling), anti-Flag (1:2000; Sigma-Aldrich) or anti-myc (9E10, 1:200; Santa Cruz), washed and incubated with horse radish peroxidase-conjugated secondary anti-mouse-immunoglobulin G or anti-rabbit-immunoglobulin G (GE Lifesciences). Washing and incubation were performed using SNAP i.d. Protein Detection System (Millipore, Billerica, MA, USA). Finally, chemiluminescence was detected using Chemi-Lumi One L kit (Nacalai Tesque). For densitometry analysis, luminescence images were analyzed by the ImageJ software (NIH, Bethesda, MD, USA).
Flow cytometry
Cells were washed twice in phosphate-buffered saline and resuspended in 1 × Annexin V binding buffer (BD Pharmingen, San Diego, CA, USA). For apoptosis analysis, cells were immediately stained using an Annexin V-FITC apoptosis detection kit (BD Pharmingen) and analyzed using a BD FACSCanto II Flow Cytometry System (BD Biosciences, San Jose, CA, USA). Cell debris was first excluded by gating on the forward and the side scatters. The annexin V-positive and propidium iodide-negative quadrant represents the population undergoing early apoptosis, but not late apoptosis/necrosis. Data were analyzed using the FlowJo software (Treestar, Ashland, OR, USA).
Cell proliferation assay
Cells (0.5–1 × 104 cells) were seeded in 96-well flat-bottom microtiter culture plates with low evaporation lids (Corning Inc., Corning, NY, USA). Cell growth was examined using SF cell count reagent according to the manufacturer's instruction (Nacalai Tesque). The optical density value after the addition of SF reagent was measured using a Microplate Reader (Molecular Devices, Sunnyvale, CA, USA) at optical density 450–650 nm wavelengths. The mean optical density value of control group was arbitrarily set to 100%.
Statistical analysis
Data are expressed as mean±s.d. Statistical analysis was performed using the unpaired Student's t-test (two tailed). Results were considered statistically significant when P<0.05.
Results
Loss of GC sensitivity during transformation of HTLV-I-infected T cells
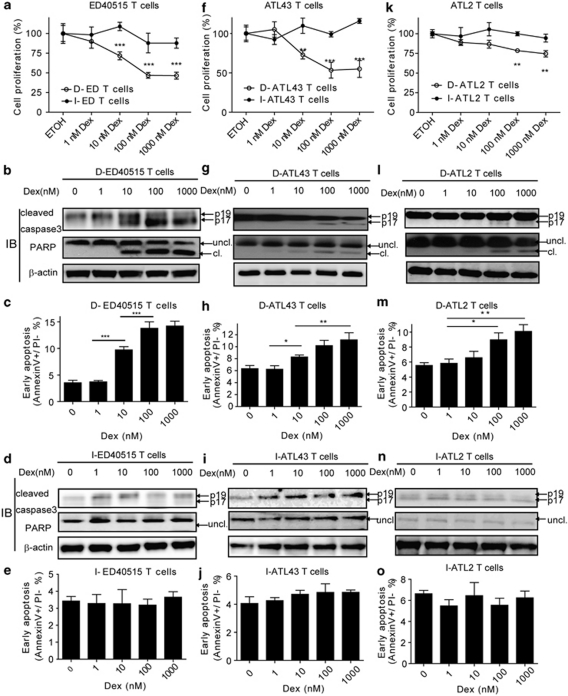
HTLV-I-infected T cells tend to undergo a transition from D stage to I stage. On the other hand, ATL has been postulated to develop in vivo in a multistep manner.6 Several lines of evidence have indicated that the D stage cells represent the early transformed leukemic cells, whereas the I stage cells represent the fully transformed leukemic cells.8, 30 To investigate the responsiveness of ATL cells to GC, we took advantage of HTLV-I-infected T-cell lines (ED40515, ATL43 or ATL2) established from three distinct ATL patients. The IL-2-dependent lines (D-ED40515, D-ATL43 or D-ATL2T cells) spontaneously gave rise to subclones that grew in the absence of IL-2 (I-ED40515, I-ATL43 or I-ATL2T cells, respectively) (Supplementary Figure S1).11 Dex inhibited the growth of IL-2-dependent cells, but not of IL-2-independent cells in all three cell lines examined (Figures 1a, f and k and Supplementary Figure S2). We next addressed apoptosis induced by Dex. In the IL-2-dependent cells, Dex increased the active form (p17) of caspase 3 (Figures 1b, g and l, upper) and the cleaved form of its target, nuclear PARP (Figures 1b, g and l, middle) in a dose-dependent manner. In contrast, IL-2-independent T cells did not respond to Dex in caspase activation (Figures 1d, i and n). Consistently, Dex increased the early apoptotic population in the D stage (Figures 1c, h and m), but not in the I stage cells (Figures 1e, j and o) as revealed by flow cytometry.
Figure 1.
Dexamethasone inhibits cell growth in the early, but not the late stage of HTLV-I-induced transformation. IL-2-dependent HTLV-I-infected T cells (D-ED40515, D-ATL43, D-ATL2T cells), representing the early transformation, and their IL-2-independent derivatives (I-ED40515, I-ATL43, I-ATL2T cells), representing the late stage of transformation, were treated with Dex or ethyl alcohol. (a) Cell proliferations of D-ED40515/I-ED40515T cells were examined by SF cell count reagent at 72 h after treatments. Data were shown as mean±s.d. (n=6). *P<0.05; **P<0.01; ***P<0.001. (b) At 48 h after Dex treatment, D-ED40515T cells were collected and used for immunoblotting to detect cleaved caspase-3 and PARP. β-Actin served as a loading control. Uncl, uncleaved; cl, cleaved. (c) At 48 h of culture, D-ED40515 (upper panel) and I-ED40515 (lower panel) T cells were collected and used for flow cytometry to assess the proportion of early apoptosis. Data are expressed as mean±s.d. (n=3). (d and e) At 48 h after Dex treatment, I-ED40515T cells were collected and immunoblotting and flow cytometry were carried out, respectively, as in (b) and (c). (f) Cell proliferations of D-ATL43/I-ATL43T cells were as in (a). (g–j) D-ATL43/I-ATL43 cells after Dex treatment were used for immunoblotting and flow cytometry analysis as in (b–d). (k) Cell proliferations of D-ATL2/I-ATL2T cells were as in (a). (l–o) D-ATL2/I-ATL2 cells after Dex treatment were used for immunoblotting and flow cytometry analysis as in (b–d).
GR and TBP-2 expression are diminished during transformation of HTLV-I-infected T cells
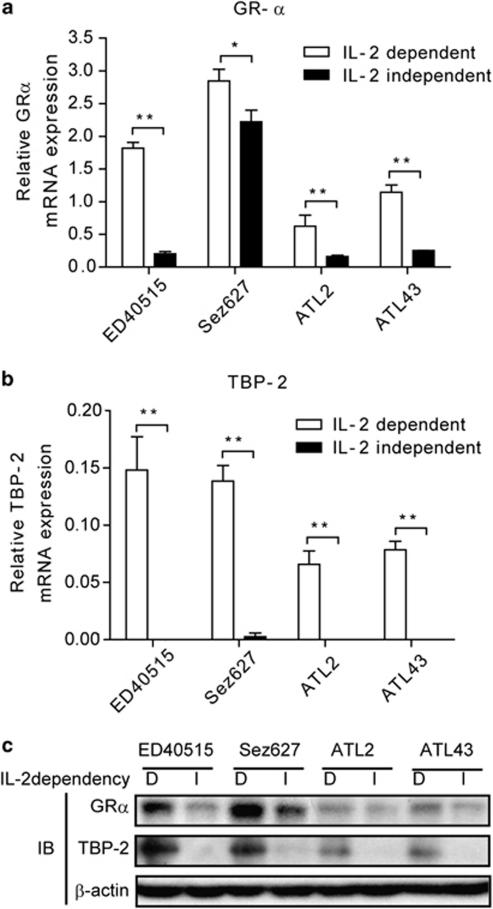
To dissect the mechanism of the differential sensitivities to Dex seen during the transformation of HTLV-I-infected T cells, we examined the expression of GR. Quantitative real-time PCR (qRT-PCR) revealed that I-ED40515 cells expressed approximately one-eighth as much the functional isoform of GR (GRα) as D-ED40515 cells (Figure 2a). Likewise, three other pairs of I and D stage T-cell lines showed a tendency of losing GRα expression when they proceeded from the early to the late phase of transformation. Consistent with our previous findings,28, 29 the expression of TBP-2 declined when HTLV-I-infected T cells went through the transition (Figure 2b). Of note, the decline of TBP-2 mRNA was more pronounced than that of GR in the course of the transition. In line with the mRNA levels, immunoblotting indicates that the protein amounts of GRα and, more markedly, those of TBP-2 diminished in the transition from the D stage to I stage of HTLV-I-infected T cells (Figure 2c).
Figure 2.
Expression of GR and TBP-2 was diminished during HTLV-I-induced transformation. The amounts of GRα (a) and TBP-2 mRNA (b) were determined by real-time quantitative RT-PCR. The relative mRNA expressions normalized to the glyceraldehyde 3-phosphate dehydrogenase expressions are shown and expressed as mean±s.d. (n=3). *P<0.05; **P<0.01. (c) Immunoblotting was performed to detect GRα and TBP-2 proteins. β-Actin served as a loading control. Representative results of three independent experiments were shown.
GR mediates upregulation of TBP-2, growth arrest and apoptosis induced by GC in the early stage of transformation by HTLV-I
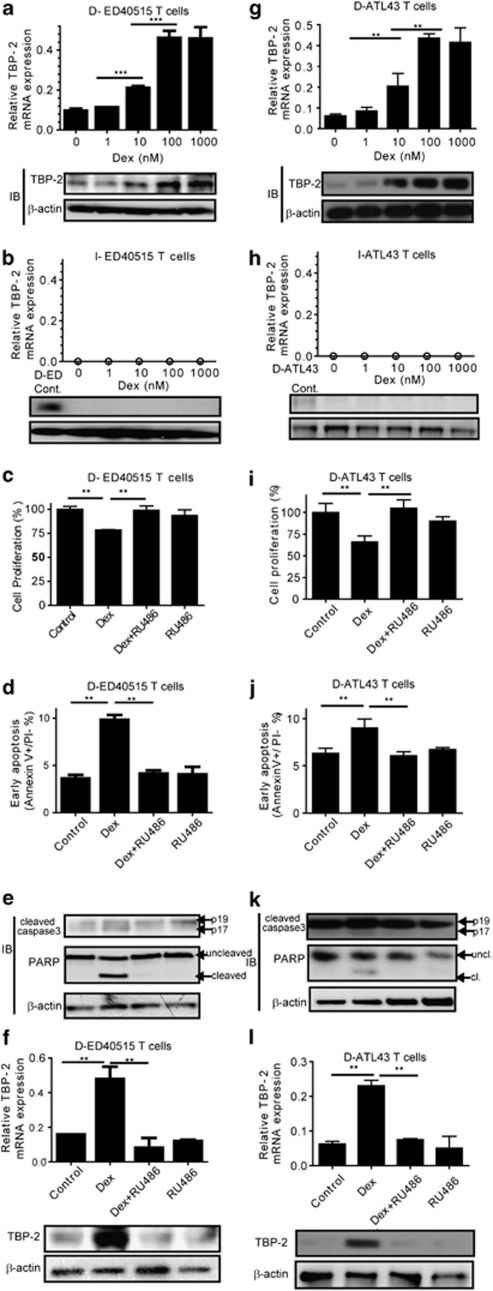
Dex induced TBP-2 mRNA and protein in a dose-dependent manner in the D-ED40515, D-ATL43 or D-ATL2T cells (Figures 3a and g and Supplementary Figure S3), whereas it failed to do so in the I-ED40515, I-ATL43 or I-ATL2T cells (Figures 3b and h and Supplementary Figure S3). To determine whether TBP-2 is involved in the signals downstream of GR in the D stage of T cells, we included a GR antagonist (RU486) in the culture. RU486 abolished growth inhibition, early apoptosis and caspase activation induced by Dex in D-ED40515 (Figures 3c, d, and e) or D-ATL43 T cells (Figures 3i, j and k). RU486 also abrogated the increment of TBP-2 induced by Dex in both cell lines (Figures 3f and l). These data indicate that the signal through GR upregulates TBP-2 in the D stage of HTLV-I-infected cells, but not in the I stage of the cells. Furthermore, the expression levels of TBP-2 paralleled GR-mediated growth inhibition, cell death and caspase activation.
Figure 3.
GR mediates TBP-2 induction, apoptosis and caspase activation in the early stage of HTLV-I-induced transformation. (a) At 48 h after treatment with doses of Dex, D-ED40515T cells were collected. TBP-2 mRNA levels were measured by quantitative RT-PCR (upper panel). Data are shown as mean±s.d. (n=4). ***P<0.001. Immunoblotting was also performed to detect TBP-2 protein (lower panel). β-Actin served as a loading control. (b) At 48 h after treatment with doses of Dex, I-ED40515T cells were collected. TBP-2 mRNA (upper panel) and protein (lower panel) were measured as in (a). The ‘0' indicated no detection within the PCR cycles (n=40). (c–f) D-ED40515T cells were treated, respectively, with either 10 n Dex or 100 n RU486, or both, for 48 h. Samples treated with ethanol vehicle (0.004%, v/v) were used as a negative control. (c) Cell growth was examined using SF cell count reagent. (d) Early apoptosis was determined by flow cytometry. (e) Immunoblotting was performed to detect cleaved caspase-3 and PARP. Uncl, uncleaved; cl, cleaved. (f) TBP-2 mRNA (upper panel) and protein (lower panel) were measured by real-time quantitative RT-PCR and immunoblotting, respectively. **P<0.01. Representative results of three independent experiments were shown. (g–l) ATL43T cells (D-ATL43/I-ATL43) were used as in (a–f).
Knockdown of TBP-2 in the early stage of HTLV-I-induced transformation abrogates GC effects
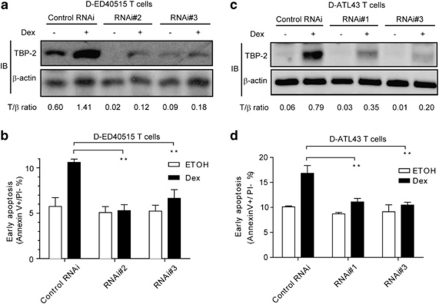
As TBP-2 can mediate apoptosis in several cell types,31, 32 we hypothesized that this protein plays an important role when GC inhibits the expansion of the D stage of ATL cells. To test this, we performed an RNAi assay to reduce the expression of TBP-2 in D-ED40515 and D-ATL43 cells. We transfected the cells with siRNA, treated them with Dex for 24 h and determined cell growth and apoptosis. Two independent sets of siRNA specific for TBP-2 likewise diminished the protein expression by more than 80%, compared with scrambled control siRNA in both of the D stage T cells (Figures 4a and c). Gene silencing of TBP-2 abolished apoptosis induced by Dex (Figures 4b and d). These data indicate that TBP-2 is an essential intermediate for cell death induced by GC in the early stage of T-cell transformation.
Figure 4.
Knockdown of TBP-2 in the early stage of HTLV-I-induced transformation abolishes GC-induced growth inhibition and apoptosis. (a and b) D-ED40515T cells were transfected with control oligonucleotides (Control RNAi) or two sets of TBP-2 antisense oligonucleotides (RNAi no. 2, RNAi no. 3) as described in the ‘Materials and methods' section. At 24 h after the transfection, cells were treated with 1 μ Dex or the ethanol (0.1%, v/v) for another 24 h. (a) Immunoblotting was performed to detect TBP-2 protein. β-Actin was used as a loading control. Densitometric ratios of TBP-2/β-actin (T/β) are listed below. ‘+', Dex added; ‘−', ethanol added. (b) Flow cytometry assay for detecting the early apoptotic proportion was performed. Data from three independent experiments are shown as mean±s.d., *P<0.05. (c and d) D-ATL43T cells were used for the TBP-2 knockdown assays as in (a and b).
Forced expression of GR and treatment with SAHA in the late stage of HTLV-I-induced transformation restores GC sensitivity
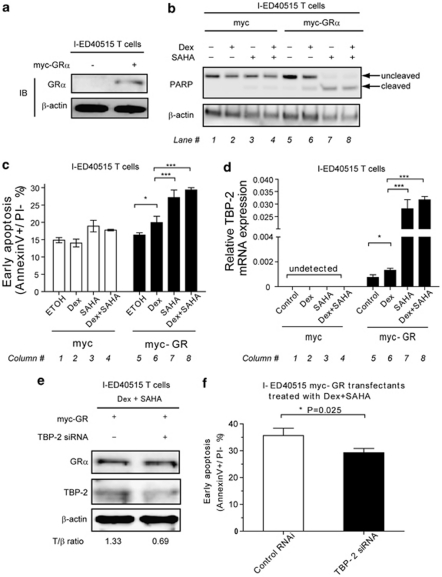
One possible strategy to control the growth of advanced ATL cells is to restore the responsiveness to GC. To test this in vitro, we used I-ED40515T cells. As these I stage T cells lacked the expression of both GR and TBP-2, we replenished the cells with the expression of either or both of the molecules and tested whether they were rendered responsive to GC. I-ED40515T cells were transfected with a high dose of GRα-encoding plasmids (Figure 5a). Transfection with GRα partially rendered the cells responsive to Dex and induced a moderate activation of caspase 3 as determined by the cleavage of PARP (Figures 5b, compare lanes 5 and 6) and early apoptosis (Figure 5c, compare columns 5 and 6). Furthermore, the cells transfected with a high-dose GRα-encoding plasmid slightly increased the expression of TBP-2 in response to Dex (Figure 5d, column 6).
Figure 5.
Forced expression of GR and treatment with SAHA in the late stage of HTLV-I-induced transformation restores GC sensitivity. I-ED40515T cells were transfected with 5 μg of pCMV-tag3B-GRα or pCMV-tag3B control plasmids. (a) At 24 h after transfection, immunoblotting was performed to verify the expression of GRα. (b–d) Then, ethanol vehicle (0.1%, v/v), dexamethasone (1 μ), SAHA (2.5 μ) or dexamethasone (1 μ)/SAHA (2.5 μ) combinations were added, respectively, into cells. (b) Immunoblotting for PARP was performed. β-Actin was used as a loading control. Each lane number was labeled below the blot. (c) Cell pellets were collected and flow cytometry analysis was performed. Each column number was labeled below the graph. (d) Cell pellets were collected to measure TBP-2 mRNA expression by real-time quantitative RT-PCR. Data of relative TBP-2 expression normalized to the glyceraldehyde 3-phosphate dehydrogenase expression are shown. **P<0.01; ***P<0.001. Each column number was labeled below the graph. (e and f) I-ED40515T cells were transfected with 5 μg of pCMV-tag3B-GRα plus TBP-2 antisense oligonucleotides (RNAi no. 2) or control oligonucleotides. At 24 h after transfection, dexamethasone (1 μ)/SAHA (2.5 μ) combinations were added into cells. (e) Immunoblotting for myc-tagged GRα and TBP-2 was performed to verify the transfection and knockdown efficiency. ‘+', RNAi no. 2 added; ‘−‘, control RNAi added. Densitometric ratios of TBP-2/β-actin (T/β) are listed below. Representative data of two independent experiments were shown. (f) Cell pellets were collected and flow cytometry analysis was performed. Data were shown as mean±s.d. in triplicate.
SAHA, an inhibitor for histone deacetylase, strongly enhances the expression of mutiple genes including TBP-2.33 Unexpectedly, treatment with SAHA in the absence of Dex robustly induced TBP-2 mRNA in myc-GR transfectants (Figure 5d, column 7), but not in the control transfectants (Figure 5d, column 3). The treatment with SAHA alone also induced caspase activation (Figure 5b, lane 7) and early apoptosis (Figure 5c, column 7) only in myc-GR transfectants. The addition of Dex to SAHA did not significantly augment cell death or TBP-2 expression as compared with SAHA alone in either myc-GR or control transfectants. Collectively, induction of TBP-2 in the I stage T cells by various reagents associated with caspase activation and enhancement of early apoptosis.
To address the role of TBP-2 in GR-mediated apoptosis, TBP-2 expression was diminished by TBP-2 siRNA in I-ED40515 cells transfected with myc-GR and treated with SAHA and Dex. The knockdown modestly but significantly reduced both TBP-2 mRNA (Figure 5e) and apoptosis (Figure 5f). These results suggest that TBP-2 expression is a crucial step in GR-mediated apoptosis in HTLV-I-transformed cells.
Ectopic expression of TBP-2 alone induces cell death in the late stage of HTLV-I-transformation
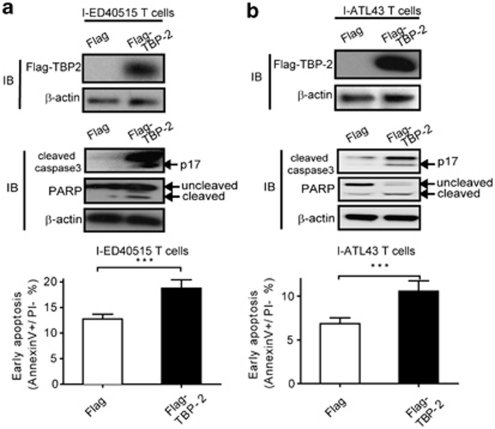
Although SAHA strongly enhanced the effect of Dex on growth suppression and caspase activation, this reagent could affect expression of other molecules than TBP-2.33 Therefore, we determined whether direct expression of TBP-2 in the advanced stage of HTLV-I-infected cells regulates cell growth and apoptosis. We transfected I-ED40515T cells (Figure 6a) and I-ATL43T cells (Figure 6b) with TBP-2 and determined cell death. The expression of TBP-2 alone significantly increased active caspase-3 and cleavage of PARP with enhanced apoptosis 48 h after the transfection regardless of GC treatment (Supplementary Figure S4). These data indicate that TBP-2, when expressed in the late stage of HTLV-I-infected T-cell line cells, can control cell growth and apoptosis.
Figure 6.
Ectopic expression of TBP-2 in the late stage of HTLV-I-transformed T cells inhibits cell growth and causes apoptosis. I-ED40515T cells (a) and I-ATL43T cells (b) were transfected with 2 μg pCMV-tag2A-TBP2 or empty vector pCMV-tag2A, respectively. After 48 h, immunoblotting was performed using an anti-Flag antibody to detect Flag-tagged TBP-2 expression (upper panel) and other corresponding antibodies to detect cleaved caspase-3, PARP, β-actin, respectively (middle panel). Flow cytometry assay was performed to detect early apoptotic proportion (lower panel). Data are shown as mean±s.d. (n=3). ***P<0.001.
Discussion
After infection with HTLV-I, most patients remain asymptomatic, although a minority of them go through multistep tumorigenesis, ending up with acute leukemia resistant to any therapeutic agents. The in vivo development of aggressive leukemia is emulated in vitro by the transition of HTLV-I infected T-cell line cells. In the initial stage of tissue culture, they grow on the condition of the existence of IL-2 and are prone to apoptosis upon exposure to GC. As they are kept in culture for a long time, they start to grow independently of IL-2 and become insensitive to GC. Herein, we show that TBP-2 is critically involved in the transition of HTLV-I-infected T cells. The significance of TBP-2 in regulating GC-induced growth suppression and cell death is supported by the following findings: (i) knocking down of TBP-2 in the early stage of HTLV-I-infected T cells rendered them unresponsive to GC and (ii) forced expression of TBP-2 induced caspase activation and cell death without engagement of GR.
Cell lines are useful when dissecting molecular mechanisms of cell growth and death, although there is a caveat that the experimental results obtained by using cell lines represent artifact in vitro. Therefore, we used several HTLV-I-infected T-cell lines that we had established from distinct ATL patients. All of the cell lines made a transition from the status where they grow solely in the presence of IL-2 and undergo apoptosis in response to GC to that in which their growth is independent of IL-2 and resistant to GC. All cell lines at an early transformation stage expressed TBP-2, which was further enhanced in response to GC, although after the transition into the advanced stage they consistently lost the expression of GR and, more strikingly, TBP-2. Thus, the findings in this study suggest the important role of GR/TBP-2 axis in the apoptosis of leukemic cells in vivo. Clinical relevance of TBP-2 is currently under investigation. We collected fresh leukemic cells so far from four ATL patients that had received chemotherapy including GC (unpublished observation). Three of them were found to express TBP-2 in their leukemic cells at the levels as high as normal CD4 cells from health volunteers and have survived for at least 6 months. In contrast, one patient died within a month after TBP-2 expression in the tumor cells turned out to be markedly suppressed. These results are consistent with the notion that the loss of TBP-2 expression reflects the clinical progression of ATL.
TBP-2 has been implicated in multiple functions, including regulation of cell growth, lipid metabolism and non-classical secretion of cytokines.34, 35 However, the role of this molecule in mediating GC signaling has not been widely recognized. Apoptosis assays revealed that T cells purified from TBP-2-deficient mice36 were less sensitive to GC than wild-type T cells (our unpublished observation), suggesting the physiological relevance of TBP-2 to GC-induced cell death.
The expression of TBP-2 strikingly declined after HTLV-I-infected T-cell line cells became IL-2 independent, that is, more advanced in transformation. Beyond this point, the T cells failed to express TBP-2 even when they were treated with GC. After forced expression of GR, SAHA, an inhibitor for histone deacetylase, strongly induced TBP-2. The mechanism by which SAHA induced TBP-2 without the addition of Dex remains unknown. The induction of TBP-2 associated the inhibition of cell growth and the activation of caspase 3. Furthermore, forced expression of TBP-2 resulted in caspase activation and cell death in the advanced stage of HTLV-I-infected T cells. Thus, enhancement of the expression of TBP-2 in leukemic cells could provide a promising therapeutic strategy. SAHA is known to regulate the expression of multiple genes and is currently widely used in hematological malignancies.13, 33, 37 Owing to the heavy CpG DNA methylation in the advanced stage of ATL,29, 38 it may be necessary to combine SAHA with other demethylating agents, including 5-aza-deoxycytidine for better efficacy. One mechanism of action for these drugs might be the induction of TBP-2 and restoration of the GR/TBP-2 axis, leading to enhanced apoptosis of leukemic cells. In addition, our preliminary analyses with a limited number of ATL patients suggested the correlation of TBP-2 expression and prognosis. Although it is obviously necessary to study much more cases, these findings suggest that TBP-2 can be a useful measure to predict the prognosis of HTLV-I-infected patients as well as a target of drug development to treat advanced ATL patients.
In conclusion, our results show that TBP-2 plays an important role in mediating GC-induced cell death in HTLV-I-infected T-cell lines. Our results also raise the therapeutic prospect of inducing TBP-2 expression in improving the efficacy of the current GC-based chemotherapy.
Acknowledgments
We would sincerely like to thank Dr Y Satou and Dr M Matsuoka for their helpful discussions and critical reading of the manuscript. We would also like to thank Dr T Hori and Dr K Ishizaka for their constructive advice. This study was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and by the Program for Promotion of Fundamental Studies in Health Sciences of National Institute of Biomedical Innovation (NIBIO).
Author contributions
ZC, KS, MM and JY designed research; ZC, DAL-R, EY and MM performed research; ZC and KS analyzed the results and made the figures; YM collected fresh blood samples from patients; and ZC, KS, MM, YM and JY wrote the paper.
Glossary
- ATL
adult T-cell leukemia
- HTLV-I
human T-cell leukemia virus type I
- TBP-2
thioredoxin-binding protein-2
- GC
glucocorticoid
- GR
glucocorticoid receptor
- Dex
dexamethasone
- SAHA
suberoylanilide hydroxamic acid
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu)
Supplementary Material
References
- Yodoi J, Takatsuki K, Masuda T. Letter: two cases of T-cell chronic lymphocytic leukemia in Japan. N Engl J Med. 1974;290:572–573. doi: 10.1056/NEJM197403072901018. [DOI] [PubMed] [Google Scholar]
- Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50:481–492. [PubMed] [Google Scholar]
- Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinuma Y, Nagata K, Hanaoka M, Nakai M, Matsumoto T, Kinoshita KI, et al. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci USA. 1981;78:6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic M, Reitz MS, Jr, Sarngadharan MG, Robert-Guroff M, Kalyanaraman VS, Nakao Y, et al. The virus of Japanese adult T-cell leukaemia is a member of the human T-cell leukaemia virus group. Nature. 1982;300:63–66. doi: 10.1038/300063a0. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Ohno Y, Tsugane S, Watanabe S, Shimoyama M, Tajima K, et al. Multi-step carcinogenesis model for adult T-cell leukemia. Jpn J Cancer Res. 1989;80:191–195. doi: 10.1111/j.1349-7006.1989.tb02289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford RN, Battiata AP, Burton JD, Sharma H, Waldmann TA. Interleukin (IL) 15/IL-T production by the adult T-cell leukemia cell line HuT-102 is associated with a human T-cell lymphotrophic virus type I region /IL-15 fusion message that lacks many upstream AUGs that normally attenuates IL-15 mRNA translation. Proc Natl Acad Sci USA. 1996;93:2897–2902. doi: 10.1073/pnas.93.7.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migone TS, Lin JX, Cereseto A, Mulloy JC, O'Shea JJ, Franchini G, et al. Constitutively activated Jak-STAT pathway in T cells transformed with HTLV-I. Science. 1995;269:79–81. doi: 10.1126/science.7604283. [DOI] [PubMed] [Google Scholar]
- Maeda M, Arima N, Daitoku Y, Kashihara M, Okamoto H, Uchiyama T, et al. Evidence for the interleukin-2 dependent expansion of leukemic cells in adult T cell leukemia. Blood. 1987;70:1407–1411. [PubMed] [Google Scholar]
- Maeda M, Shimizu A, Ikuta K, Okamoto H, Kashihara M, Uchiyama T, et al. Origin of human T-lymphotrophic virus I-positive T cell lines in adult T cell leukemia. Analysis of T cell receptor gene rearrangement. J Exp Med. 1985;162:2169–2174. doi: 10.1084/jem.162.6.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda M. Human T lymphotropic virus type-I (HTLV-I) immortalizes human T cells in vitro--its implication in the pathogenesis of adult T cell leukemia (ATL) Hum Cell. 1992;5:70–78. [PubMed] [Google Scholar]
- Sionov RV, Spokoini R, Kfir-Erenfeld S, Cohen O, Yefenof E. Mechanisms regulating the susceptibility of hematopoietic malignancies to glucocorticoid-induced apoptosis. Adv Cancer Res. 2008;101:127–248. doi: 10.1016/S0065-230X(08)00406-5. [DOI] [PubMed] [Google Scholar]
- Tsukasaki K, Hermine O, Bazarbachi A, Ratner L, Ramos JC, Harrington W, Jr, et al. Definition, prognostic factors, treatment, and response criteria of adult T-cell leukemia-lymphoma: a proposal from an international consensus meeting. J Clin Oncol. 2009;27:453–459. doi: 10.1200/JCO.2008.18.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor GP, Matsuoka M. Natural history of adult T-cell leukemia/lymphoma and approaches to therapy. Oncogene. 2005;24:6047–6057. doi: 10.1038/sj.onc.1208979. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Rainer J, Ploner C, Presul E, Riml S, Kofler R. Glucocorticoid-induced apoptosis and glucocorticoid resistance: molecular mechanisms and clinical relevance. Cell Death Differ. 2004;11 (Suppl 1:S45–S55. doi: 10.1038/sj.cdd.4401456. [DOI] [PubMed] [Google Scholar]
- Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354:166–178. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- Chin KT, Chun AC, Ching YP, Jeang KT, Jin DY. Human T-cell leukemia virus oncoprotein tax represses nuclear receptor-dependent transcription by targeting coactivator TAX1BP1. Cancer Res. 2007;67:1072–1081. doi: 10.1158/0008-5472.CAN-06-3053. [DOI] [PubMed] [Google Scholar]
- Doucas V, Evans RM. Human T-cell leukemia retrovirus-Tax protein is a repressor of nuclear receptor signaling. Proc Natl Acad Sci USA. 1999;96:2633–2638. doi: 10.1073/pnas.96.6.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung MM, Chu YL, Fink JL, Wallace A, McGuire KL. IL-2- and STAT5-regulated cytokine gene expression in cells expressing the Tax protein of HTLV-1. Oncogene. 2005;24:4624–4633. doi: 10.1038/sj.onc.1208507. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Tsubata C, Kondo R, Yoshida S, Takahashi M, Oie M, et al. Cooperation of NF-kappaB2/p100 activation and the PDZ domain binding motif signal in human T-cell leukemia virus type 1 (HTLV-1) Tax1 but not HTLV-2 Tax2 is crucial for interleukin-2-independent growth transformation of a T-cell line. J Virol. 2007;81:11900–11907. doi: 10.1128/JVI.00532-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teshigawara K, Maeda M, Nishino K, Nikaido T, Uchiyama T, Tsudo M, et al. Adult T leukemia cells produce a lymphokine that augments interleukin 2 receptor expression. J Mol Cell Immunol. 1985;2:17–26. [PubMed] [Google Scholar]
- Nishiyama A, Matsui M, Iwata S, Hirota K, Masutani H, Nakamura H, et al. Identification of thioredoxin-binding protein-2/vitamin D(3) up-regulated protein 1 as a negative regulator of thioredoxin function and expression. J Biol Chem. 1999;274:21645–21650. doi: 10.1074/jbc.274.31.21645. [DOI] [PubMed] [Google Scholar]
- Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, et al. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CL, Lin CF, Chang WT, Huang WC, Teng CF, Lin YS. Ceramide induces p38 MAPK and JNK activation through a mechanism involving a thioredoxin-interacting protein-mediated pathway. Blood. 2008;111:4365–4374. doi: 10.1182/blood-2007-08-106336. [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Martin SG. The thioredoxin system: a key target in tumour and endothelial cells. Br J Radiol. 2008;81 (Spec No. 1:S57–S68. doi: 10.1259/bjr/34180435. [DOI] [PubMed] [Google Scholar]
- Watanabe R, Nakamura H, Masutani H, Yodoi J. Anti-oxidative, anti-cancer and anti-inflammatory actions by thioredoxin 1 and thioredoxin-binding protein-2. Pharmacol Ther. 2010;127:261–270. doi: 10.1016/j.pharmthera.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Wang Z, Rong YP, Malone MH, Davis MC, Zhong F, Distelhorst CW. Thioredoxin-interacting protein (txnip) is a glucocorticoid-regulated primary response gene involved in mediating glucocorticoid-induced apoptosis. Oncogene. 2006;25:1903–1913. doi: 10.1038/sj.onc.1209218. [DOI] [PubMed] [Google Scholar]
- Nishinaka Y, Nishiyama A, Masutani H, Oka S, Ahsan KM, Nakayama Y, et al. Loss of thioredoxin-binding protein-2/vitamin D3 up-regulated protein 1 in human T-cell leukemia virus type I-dependent T-cell transformation: implications for adult T-cell leukemia leukemogenesis. Cancer Res. 2004;64:1287–1292. doi: 10.1158/0008-5472.can-03-0908. [DOI] [PubMed] [Google Scholar]
- Ahsan MK, Masutani H, Yamaguchi Y, Kim YC, Nosaka K, Matsuoka M, et al. Loss of interleukin-2-dependency in HTLV-I-infected T cells on gene silencing of thioredoxin-binding protein-2. Oncogene. 2006;25:2181–2191. doi: 10.1038/sj.onc.1209256. [DOI] [PubMed] [Google Scholar]
- Takemoto S, Mulloy JC, Cereseto A, Migone TS, Patel BK, Matsuoka M, et al. Proliferation of adult T cell leukemia/lymphoma cells is associated with the constitutive activation of JAK/STAT proteins. Proc Natl Acad Sci USA. 1997;94:13897–13902. doi: 10.1073/pnas.94.25.13897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junn E, Han SH, Im JY, Yang Y, Cho EW, Um HD, et al. Vitamin D3 up-regulated protein 1 mediates oxidative stress via suppressing the thioredoxin function. J Immunol. 2000;164:6287–6295. doi: 10.4049/jimmunol.164.12.6287. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Masutani H, Nakamura H, Nishinaka Y, Yodoi J. Redox regulation by thioredoxin and thioredoxin-binding proteins. IUBMB Life. 2001;52:29–33. doi: 10.1080/15216540252774739. [DOI] [PubMed] [Google Scholar]
- Butler LM, Zhou X, Xu WS, Scher HI, Rifkind RA, Marks PA, et al. The histone deacetylase inhibitor SAHA arrests cancer cell growth, up-regulates thioredoxin-binding protein-2, and down-regulates thioredoxin. Proc Natl Acad Sci USA. 2002;99:11700–11705. doi: 10.1073/pnas.182372299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaimul AM, Nakamura H, Masutani H, Yodoi J. Thioredoxin and thioredoxin-binding protein-2 in cancer and metabolic syndrome. Free Radic Biol Med. 2007;43:861–868. doi: 10.1016/j.freeradbiomed.2007.05.032. [DOI] [PubMed] [Google Scholar]
- Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- Oka S, Liu W, Masutani H, Hirata H, Shinkai Y, Yamada S, et al. Impaired fatty acid utilization in thioredoxin binding protein-2 (TBP-2)-deficient mice: a unique animal model of Reye syndrome. FASEB J. 2006;20:121–123. doi: 10.1096/fj.05-4439fje. [DOI] [PubMed] [Google Scholar]
- Richon VM, Sandhoff TW, Rifkind RA, Marks PA. Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation. Proc Natl Acad Sci USA. 2000;97:10014–10019. doi: 10.1073/pnas.180316197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosaka K, Maeda M, Tamiya S, Sakai T, Mitsuya H, Matsuoka M. Increasing methylation of the CDKN2A gene is associated with the progression of adult T-cell leukemia. Cancer Res. 2000;60:1043–1048. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.