Abstract
Purpose
The present study evaluated whether oral supplementation with a branched-chain amino acid (BCAA) improves the biochemical and amino acid profiles of liver tumor patients undergoing radiotherapy.
Materials and Methods
Patients were randomly assigned to one of 2 groups: a group given oral supplementation with BCAA granules (LIVACT granules; Samil Pharm Co., Korea, each granule containing L-isoleucine 952 mg, L-leucine 1,904 mg, and L-valine 1,144 mg) during radiotherapy, or a placebo group. Physical and biochemical examinations and measurements, including subjective symptoms, Child-Pugh class, body mass index, plasma albumin concentration, and plasma amino acid profiles were monitored.
Results
Fifty were enrolled between November 2005 and November 2006. We also analyzed data from 37 hepatocellular carcinoma (HCC) patients in order to evaluate a more homogenous group. The two groups of patients were comparable in terms of age, gender, Child-Pugh score, and underlying hepatitis virus type. Serum albumin, total protein, liver enzymes, and cholesterol showed a tendency to increase in the BCAA group. In this group, the percentage of cases that reverted to normal serum albumin levels between 3 and 10 weeks after administration of BCAA was significantly higher (41.18%) than in the placebo group (p=0.043).
Conclusion
Oral supplementation with a BCAA preparation seems to help HCC patients undergoing radiotherapy by increasing the BCAA concentration.
Keywords: Branched-chain amino acids, Hepatocellular carcinoma, Radiotherapy
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies and is the third leading cause of cancer mortality in the world. It is the second leading cause of cancer death in Korea [1,2]. The first choice in the management of HCC is surgical treatment with partial hepatectomy for localized solitary resectable HCC. Localized unresectable cases of HCC may be candidates for chemoembolization, intra-arterial infusion chemotherapy, cryosurgery, percutaneous ethanol injection, radioimmunotherapy, radiotherapy, or radiofrequency ablation [3]. Radiotherapy of HCC, however, has long been overlooked, because the entire liver cannot tolerate a high dose of radiation [4,5]. Nevertheless, recent reports reveal that development of 3-dimensional conformal radiotherapy has been increasingly applied to HCC management with substantial success [6-12].
Most patients referred for radiation therapy have chronic liver disease, and their lesions are at an advanced stage that is frequently associated with a state of protein-calorie malnutrition. In addition, radiation induces toxicities such as abnormal liver function tests. General fatigue, nausea and vomiting remain a challenge to achieving effective radiotherapy [13-15]. These adverse effects may further aggravate the patients' nutritional status.
Branched-chain amino acid (BCAA) supplementation has previously been shown to increase serum albumin levels and the Fischer's ratio in cirrhotic patients [16]. Moreover, randomized controlled studies have demonstrated that parenteral or oral nutritional support with BCAA-enriched preparations in patients undergoing hepatic resection for HCC significantly improves their post-operative nutritional status and reduces morbidity and length of hospital stay [17,18].
Patients with unresectable HCC who have either advanced tumors or severe cirrhosis are more likely to suffer from malnutrition. Therefore, oral BCAA support may play an important role in the management of patients who receive radiation therapy for HCC. However, there have been no studies on nutritional support in patients undergoing radiotherapy for HCC. We hypothesized that BCAA support is helpful for maintaining liver function and nutritional build-up. Therefore, we designed a double-blind, randomized, placebo-controlled study to evaluate whether BCAA improves biochemical and amino acid profiles of HCC patients undergoing radiotherapy.
Materials and Methods
1. Patients
Patients older than 16 years but younger than 75 years with less than a 30% indocyanin green retention rate at 15 min score were eligible for this study. Patients with alcohol-induced liver cirrhosis, hepatic encephalopathy, hepatic failure higher than grade III, serum total bilirubin higher than 5 mg/dL and obstruction of the extrahepatic bile duct, or showing reactivation of hepatitis, were excluded. After providing written informed consent, all subjects received a package of drugs, which consisted of either BCAA or a placebo, in a double-blind fashion. This study was approved by our Institutional Review Board, and was registered under number 4-2005-0112. Primary endpoints were: serum biochemical and amino acid profiles as a result of receiving the BCAA supplement; the number of patients that reverted to normal serum albumin values. Secondary end points included: subjective symptoms and nutritional parameters.
Assumptions for the sample-size calculation were based on the primary result being a significant increase of biochemical profiles and amino acid profiles from baseline profiles, or, a significant recovery from baseline to normal values. We assumed that 50% of patients treated with BCAA will show a significant increase or recovery of biochemical profiles and amino acid profiles, and that patients treated with placebo will improve in 10% of cases. A sample size of 20 patients per group was needed for the statistical verification of such a result (significance level α=0.05; power, 90%). After allowing for a drop-out and withdrawal rate of up to 20%, recruitment of 50 patients was planned.
2. Methods
1) Random oral administration of BCAA and placebo
Patients were randomized (ratio 1 : 1) to receive double-blind treatment with either BCCA, or placebo during radiotherapy. Patient randomization was performed using a blindly labeled sachet from Samil Pharm Co. (Seoul, Korea). Both the BCCA and placebo were packaged in the sachet. Both were the same shape and contained a code that was known only to the principal investigator. The contents were known neither to the participants nor to the physician. Once a patient signed the study consent, we chose the code number in a regular sequence. Therefore, patients were randomly administered the placebo or BCAA in a double-blind fashion. The BCAA group was given a sachet (4.74 g) of LIVACT (Samil Pharm Co.), which contained 952 mg of L-isoleucine, 1,904 mg of L-leucine, and 1,144 mg of L-valine, orally three times a day after meals during radiotherapy (5-6 weeks). The placebo group was given placebo on the same schedule.
General blood tests (complete blood count and platelets) and plasma biochemical tests (blood urea nitrogen/creatinine, aspartate aminotransferase/alanine aminotransferase, total protein, total bilirubin, albumin, and cholesterol) were done during the double-blind treatment period and one month after termination of radiation therapy. Serum amino acids (isoleucine, leucine, valine, phenylalanine, tyrosine, and methionine) were measured twice, before and after radiation therapy. The Fischer ratio (isoleucine+leucine+valine/phenylalanine+tyrosine) was also calculated.
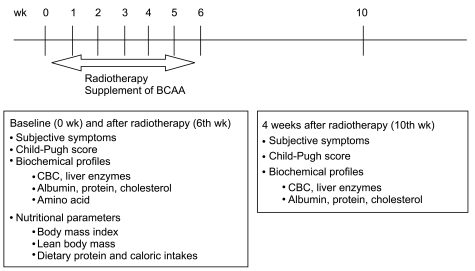
Subjective symptoms such as ascites, edema, jaundice, anorexia, abdominal distension, fatigue, itching sensation, and hepatic encephalopathy were scored before and at the end of radiation therapy, and one month after the termination of radiation therapy (Fig. 1). We scored each subjective symptom, according to Muto et al.'s research [19] from 0 (normal) to 3 (severe). Nutritional evaluation was performed at baseline and post-radiotherapy by interview with professional nutrition teams. Nutritional parameters included dietary nutrient intake, and anthropometric measures such as body weight, body mass index (BMI), lean body mass, calorie intake, and protein intake, etc. Body fat measurement was done using a body fat analyzer (body fat analyzer model TBF-105, Tanita Co., Tokyo, Japan) based on the principle of biomedical impedance.
Fig. 1.
Trial design. Subjects were randomized into branched-chain amino acid (BCAA) and placebo groups. Subjective symptoms, Child-Pugh score, biochemical profiles, and nutritional states were evaluated. CBC, complete blood count.
2) Radiotherapy planning and treatment
For radiotherapy planning and treatment, patients were positioned supine with their arms above their head. The patients had been trained to breathe as shallowly as possible and had planning computed tomography (CT) and treatment with free set-up. Helical CT with a 5 mm slice was used for this planning (Picker PQ 5000, Marconi Medical Systems Inc., Cleveland, OH). All gross tumor volumes (GTV) were contoured on intravenous contrast-enhanced lesions. For a better delineation of the tumor volume, a hepatic angiographic image was used as a reference. A minimum of 5 mm around the GTV was included in the clinical target volume. In designing the planning target volume (PTV), the margins were individualized by observing the position of the liver as well as liver movement at the time of simulation. To verify cranio-caudal motion, we checked diaphragm movement by fluoroscopy before treatment, and radiation therapy was administered using a 6 or 10 MV linear accelerator (Varian Medical Systems, Palo Alto, CA). The radiation dose to the target volume was determined depending on the functional reserve of the liver and chosen to be in the range of dose prescription guidelines at each V50% category. For the guidelines from Yonsei University, if the percentage of non-tumor liver volume receiving 50% of the isocenter dose was <25%, the total dose was increased to 59.4 Gy; if 25-50%, the dose was 45-54 Gy; if 50-75%, the dose was 30.6-41.4 Gy; and if >75%, no treatment was administered [20]. A total of 45 Gy was usually prescribed in 25 fractions of 1.8 Gy over 5 weeks. We intended to deliver 95% of the prescribed dose encompassing the PTV around the GTV. A multiport combination of two or more ports was adopted, depending on the tumor location, and the median total dose was 46.8±9.7 Gy.
3. Statistical analysis
All data are expressed as mean±standard deviation, unless otherwise indicated. Differences were considered significant when a two-sided p-value was less than 0.05. The placebo and BCAA groups were compared using the Student's t-test, and the change in measured values for each group was analyzed using a paired t-test.
Results
1. Patient characteristics
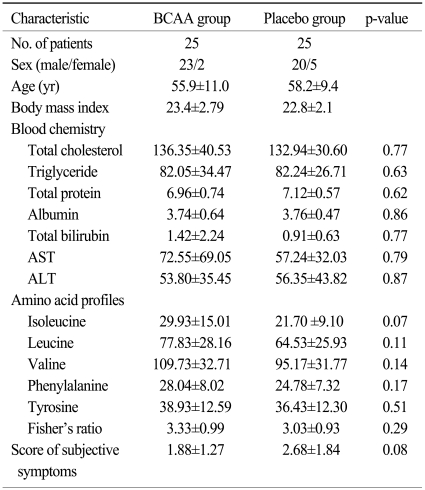
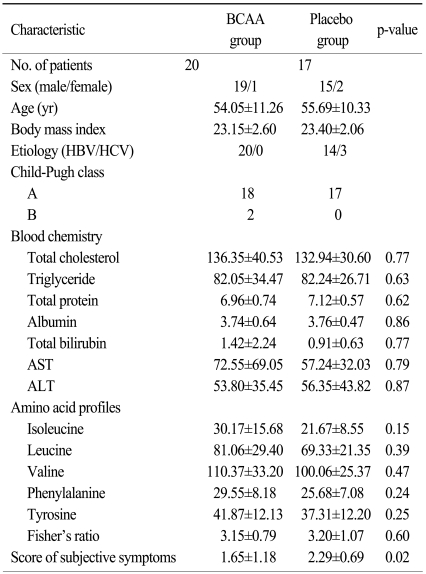
We tried to enroll patients who had received radiation treatment to the liver, and fifty patients were enrolled between November 2005 and November 2006. The characteristics and results of all patients are described in Table 1. However, HCC patients with liver cirrhosis and other bile ductal cancer patients without cirrhosis are quite different with regards to the volume of the liver irradiated, dose of radiation received, and liver function, and we analyzed 37 HCC patients in order to conduct an evaluation with a more homogenous group. Of these, 20 patients were assigned to the BCAA group, and 17 to the placebo group (Table 2). During supplementation with BCAA, one patient died of hepatic failure and 1 patient in the BCAA group was lost to followup. In the placebo group, 2 patients died of hepatic failure, and 2 were lost to follow-up. Oral BCAA supplementation was terminated in one patient due to severe anorexia and nausea. Therefore, 30 patients completed the entire protocol. In the analysis of biochemical profiles, it was not possible to evaluate one patient in the placebo group due to lack of serum sampling. A total of 7 patients (4 patients in the BCAA group and 3 patients in the placebo group) had missing serum levels in their amino acid profiles. We enrolled all patients with liver failure and evaluated them using the intention-to-treat (ITT) and per-protocol (PP) analysis.
Table 1.
Characteristics of subjects enrolled in the trial by randomization status
BCAA, branched-chain amino acid; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Table 2.
Characteristics of subjects enrolled in the trial by randomization status for HCC patients
HCC, hepatocellular carcinoma; BCAA, branched-chain amino acid; HBV, hepatitis B virus; HCV, hepatitis C virus; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
We studied 34 males and 3 females. The mean age was 55.2 years. Except for 2 patients, hepatic function received a Child-Pugh class A score. None had hepatic encephalopathy before enrollment in this study. All had hepatitis B virus infection except for three cases (3 cases of hepatitis C virus infection in the placebo group). The age and BMI of the two groups were comparable and HCC patient characteristics are shown in Table 2. Baseline nutritional status and biochemical profiles were similar between BCAA and placebo groups, except for subjective symptom scores.
2. Effect of BCAA on subjective symptoms, Child-Pugh score, and anthropometric measurements
Subjective symptoms and Child-Pugh scores showed no significant differences between BCAA and placebo groups after ITT and PP analyses. No patients developed flapping tremor or hepatic encephalopathy. There were no significant differences in subjective symptoms such as ascites, edema, jaundice, anorexia, abdominal distension, fatigue, and itching.
BMI, lean body mass, calorie intake, and protein intake were measured as nutritional parameters. There was no significant difference in these parameters between the two groups. Furthermore, there was no trend towards improvement in these parameters after the administration of BCAA.
3. Effect of BCAA on biochemical profiles
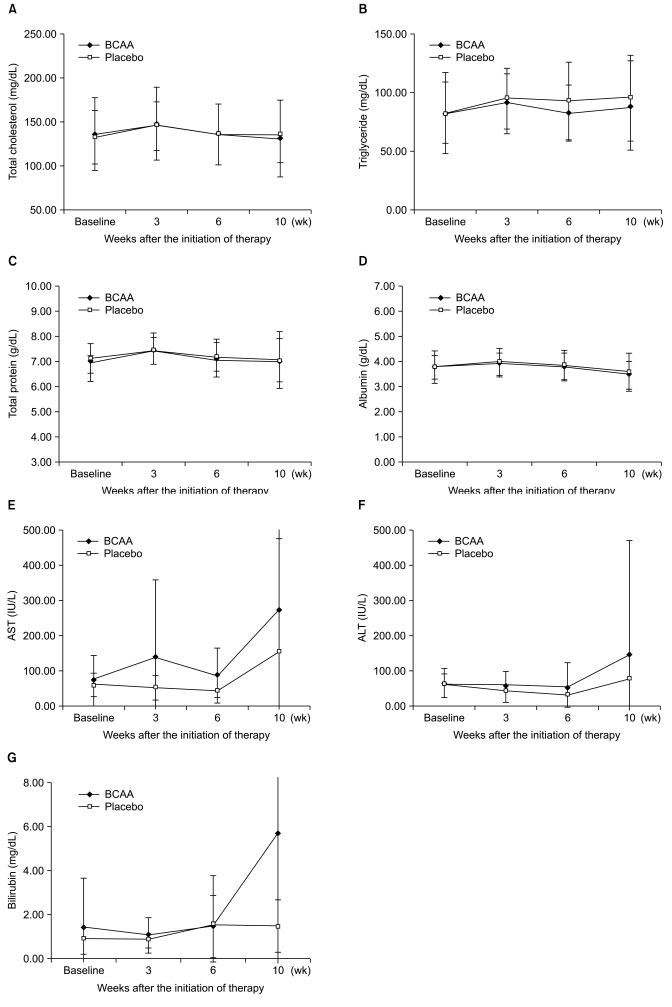
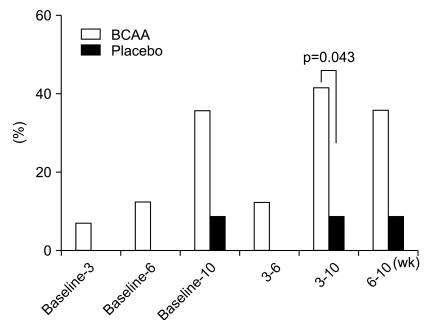
Biochemical profiles, such as mean values of serum total cholesterol, triglycerides, total protein, and bilirubin were not significantly different between the two groups for ITT or PP analyses (Fig. 2). In addition, there were no substantial differences in hemoglobin concentration and platelet counts. However, there was a slightly higher tendency in the BCAA group for the cases to return to normal serum albumin levels. In particular, between the period of 3 and 10 weeks after the administration of BCAA, the serum albumin levels of 7 patients (of 17 patients; 41.18%) returned to normal values, whereas only 1 (of 12 patients; 8.33%) in the placebo group returned to normal. This difference was statistically significant (p=0.043) (Fig. 3).
Fig. 2.
Changes in biochemical profiles showing no significant differences between patients treated with branched-chain amino acid (BCAA) and placebo. (A) Total cholesterol. (B) Serum triglyceride. (C) Serum total protein. (D) Serum albumin. (E) Serum aspartate aminotransferase (AST). (F) Serum alanine aminotransferase (ALT). (G) Serum bilirubin.
Fig. 3.
Percentage of patients whose serum albumin returned to a normal level. Serum albumin levels recovered from 3 to 10 wk in the branched-chain amino acid (BCAA) treatment group (p= 0.043).
4. Effect of BCAA on profiles of amino acids
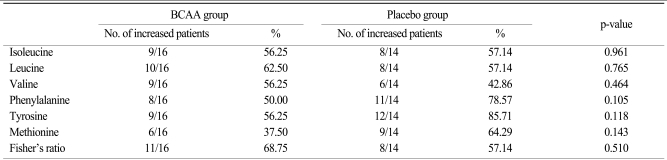
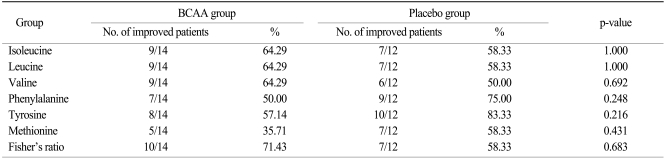
As seen in ITT and PP analyses, there was a slight tendency towards an increase of amino acids' serum value and Fisher's ratio in the BCAA group (Tables 3 and 4). The number of patients showing improved leucine and valine values increased by 5.36% and 13.39%, whereas the number of patients in the placebo group showing improved isoleucine values increased by 19.64% compared with the BCAA group.
Table 3.
Changes in the mean serum value of amino acid and Fisher's ratio for HCC patients (ITT analysis)
HCC, hepatocellular carcinoma; ITT, intension to treat; BCAA, branched-chain amino acid.
Table 4.
Changes in the mean serum value of amino acid and Fisher's ratio for HCC patients (PP analysis)
HCC, hepatocellular carcinoma; PP, per protocol; BCAA, branched-chain amino acid.
Values for aromatic amino acids such as phenylalanine and tyrosine showed no substantial differences between the two groups. More patients in the BCAA group returned to a normal Fisher's ratio than in the placebo group, although the difference did not reach statistical significance.
Discussion
BCAA constitutes approximately 40% of the essential amino acids present in the human body. In contrast to other amino acids that are metabolized in the liver, most BCAA are utilized in muscles and peripheral tissues. The major clinical effects of BCAA that have been reported include awakening effect on hepatic encephalopathy and nutritional supplementation on liver toxicity, and chronic renal failure. It has generally been considered that the effect of BCAA on hepatic encephalopathy is caused by the impairment of amino acid transport to the brain through the blood-brain barrier, reduction of ammonia consumption in the brain and muscles, and the acceleration of epinephrine synthesis in the brain. BCAA has been considered beneficial for nutritional improvement because BCAA can be used as a calorie source or accelerate protein synthesis in muscles as well as suppress protein degradation and accelerate protein synthesis in the liver.
Marchesini et al. [21] did a multicenter randomized study comparing 1 year of nutritional supplementation with BCAA in 174 patients with advanced cirrhosis. The average time of hospital admission was lower in the BCAA arm compared with the control treatments (p=0.006 and p=0.003, respectively). In addition, nutritional parameters, liver function tests, and the Child-Pugh scores improved in patients treated with BCAA (p=0.013). However, long-term compliance with taking BCAA was poor. Our previous prospective comparative study was conducted to investigate the efficacy of orally administered BCAA in cirrhotic patients. We found that there was a significant increase in plasma levels of BCAA in the BCAA group (p<0.001). Fischer's ratio also increased significantly after the administration of oral BCAA (p<0.001) [22].
A decrease in Fisher's ratio after resection of liver tumors correlates with the duration of post-resection hepatic failure [23]. For patients who had liver cancer complicated by liver cirrhosis and who underwent surgery, serum albumin levels recovered earlier in the BCAA group [24]. Nutritional support may also be important in patients undergoing chemoembolization. Poon et al. showed that nutritional supplementation using BCAA is effective in reducing morbidity after transarterial chemoembolization [25].
To the best of our knowledge, this is the first randomized double blind study to evaluate the efficacy of BCAA supplementation during radiotherapy. In our study, changes in plasma amino-acid profiles showed no substantial differences between the BCAA and placebo groups. However, BCAA had a tendency to increase in the BCAA group. Changes in Fisher's ratio also increased slightly after oral supplementation with BCAA. The proportion of patients in the BCAA group whose serum albumin recovered to normal levels significantly increased from 3 to 10 weeks.
However, our trial had some limitations. In most reports, BCAA was administered for approximately 2 months to 2 years [21,22,24], but in our study it was administered only during the period of radiation therapy (5-6 weeks). Furthermore, the number of patients recruited was small. Therefore, the change in albumin values or other parameters might not be significant. Our study was conducted using advanced HCC patients with liver cirrhosis. It would have been difficult to conduct a long-term double-blind randomized study of LIVACT and enroll enough patients. Therefore, more studies should be conducted to further elucidate the role of nutritional support in radiotherapy for HCC.
A supplementation study with more cases and a longer intervention period might be necessary to determine the effect of BCAA on patients with underlying liver cirrhosis during radiotherapy. In our study, although BCAA was administered for only a short period, it appears that BCAA supplementation is helpful in restoring albumin levels as well as Fisher's ratio in HCC patients receiving radiotherapy.
Conclusion
Oral supplementation with a BCAA preparation seems to help HCC patients undergoing radiotherapy by increasing BCAA concentrations. BCAA supplementation may provide further hope for HCC patients with low biochemical profiles during radiotherapy.
Acknowledgments
This work was supported by the national R & D program grant for cancer control, the Ministry of Health and Welfare (0620390).
Footnotes
This study was selected for presentation at the 17th annual meeting of the Asian Pacific Association for the Study of the Liver (APASL) in Kyoto, Japan, March 27-30. 2007.
Conflict of interest relevant to this article was not reported.
References
- 1.el-Serag HB. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2001;5:87–107. vi. doi: 10.1016/s1089-3261(05)70155-0. [DOI] [PubMed] [Google Scholar]
- 2.Annual report on the cause of death statistics [Internet] Daejeon: Korea National Statistical Office; 2009. [cited 2010 Mar 4]. Available from: http://www.kosis.kr. [Google Scholar]
- 3.Poon RT, Fan ST, Tsang FH, Wong J. Locoregional therapies for hepatocellular carcinoma: a critical review from the surgeon's perspective. Ann Surg. 2002;235:466–486. doi: 10.1097/00000658-200204000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cochrane AM, Murray-Lyon IM, Brinkley DM, Williams R. Quadruple chemotherapy versus radiotherapy in treatment of primary hepatocellular carcinoma. Cancer. 1977;40:609–614. doi: 10.1002/1097-0142(197708)40:2<609::aid-cncr2820400203>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 5.Ingold JA, Reed GB, Kaplan HS, Bagshaw MA. Radiation hepatitis. Am J Roentgenol Radium Ther Nucl Med. 1965;93:200–208. [PubMed] [Google Scholar]
- 6.Lawrence TS, Tesser RJ, ten Haken RK. An application of dose volume histograms to the treatment of intrahepatic malignancies with radiation therapy. Int J Radiat Oncol Biol Phys. 1990;19:1041–1047. doi: 10.1016/0360-3016(90)90031-e. [DOI] [PubMed] [Google Scholar]
- 7.Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 8.Lawrence TS, Ten Haken RK, Kessler ML, Robertson JM, Lyman JT, Lavigne ML, et al. The use of 3-D dose volume analysis to predict radiation hepatitis. Int J Radiat Oncol Biol Phys. 1992;23:781–788. doi: 10.1016/0360-3016(92)90651-w. [DOI] [PubMed] [Google Scholar]
- 9.Robertson JM, McGinn CJ, Walker S, Marx MV, Kessler ML, Ensminger WD, et al. A phase I trial of hepatic arterial bromodeoxyuridine and conformal radiation therapy for patients with primary hepatobiliary cancers or colorectal liver metastases. Int J Radiat Oncol Biol Phys. 1997;39:1087–1092. doi: 10.1016/s0360-3016(97)00550-6. [DOI] [PubMed] [Google Scholar]
- 10.Seong J, Keum KC, Han KH, Lee DY, Lee JT, Chon CY, et al. Combined transcatheter arterial chemoembolization and local radiotherapy of unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 1999;43:393–397. doi: 10.1016/s0360-3016(98)00415-5. [DOI] [PubMed] [Google Scholar]
- 11.Park W, Lim DH, Paik SW, Koh KC, Choi MS, Park CK, et al. Local radiotherapy for patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2005;61:1143–1150. doi: 10.1016/j.ijrobp.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 12.Shim SJ, Seong J, Han KH, Chon CY, Suh CO, Lee JT. Local radiotherapy as a complement to incomplete transcatheter arterial chemoembolization in locally advanced hepatocellular carcinoma. Liver Int. 2005;25:1189–1196. doi: 10.1111/j.1478-3231.2005.01170.x. [DOI] [PubMed] [Google Scholar]
- 13.Lee IJ, Seong J, Shim SJ, Han KH, Chon CY. Reappraisal of risk factors predicting liver complications from radiotherapy for hepatocellular carcinoma. Korean J Hepatol. 2006;12:420–428. [PubMed] [Google Scholar]
- 14.Cheng JC, Wu JK, Huang CM, Huang DY, Cheng SH, Lin YM, et al. Radiation-induced liver disease after radiotherapy for hepatocellular carcinoma: clinical manifestation and dosimetric description. Radiother Oncol. 2002;63:41–45. doi: 10.1016/s0167-8140(02)00061-0. [DOI] [PubMed] [Google Scholar]
- 15.Liang SX, Zhu XD, Xu ZY, Zhu J, Zhao JD, Lu HJ, et al. Radiation-induced liver disease in three-dimensional conformal radiation therapy for primary liver carcinoma: the risk factors and hepatic radiation tolerance. Int J Radiat Oncol Biol Phys. 2006;65:426–434. doi: 10.1016/j.ijrobp.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 16.Nompleggi DJ, Bonkovsky HL. Nutritional supplementation in chronic liver disease: an analytical review. Hepatology. 1994;19:518–533. [PubMed] [Google Scholar]
- 17.Fan ST, Lo CM, Lai EC, Chu KM, Liu CL, Wong J. Perioperative nutritional support in patients undergoing hepatectomy for hepatocellular carcinoma. N Engl J Med. 1994;331:1547–1552. doi: 10.1056/NEJM199412083312303. [DOI] [PubMed] [Google Scholar]
- 18.Meng WC, Leung KL, Ho RL, Leung TW, Lau WY. Prospective randomized control study on the effect of branched-chain amino acids in patients with liver resection for hepatocellular carcinoma. Aust N Z J Surg. 1999;69:811–815. doi: 10.1046/j.1440-1622.1999.01701.x. [DOI] [PubMed] [Google Scholar]
- 19.Muto Y, Yoshida T, Sato S, Suzuki K, Okada H, Hayashi S, et al. Effect of oral administration with branched-chain amino acid granules (BCAA-G) in patients with liver cirrhosis: a clinical study. JJPEN. 1989;11:1119–1134. [Google Scholar]
- 20.Lee IJ, Seong J, Shim SJ, Han KH. Radiotherapeutic parameters predictive of liver complications induced by liver tumor radiotherapy. Int J Radiat Oncol Biol Phys. 2009;73:154–158. doi: 10.1016/j.ijrobp.2008.04.035. [DOI] [PubMed] [Google Scholar]
- 21.Marchesini G, Bianchi G, Merli M, Amodio P, Panella C, Loguercio C, et al. Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: a double-blind, randomized trial. Gastroenterology. 2003;124:1792–1801. doi: 10.1016/s0016-5085(03)00323-8. [DOI] [PubMed] [Google Scholar]
- 22.Song KH, Kim MS, Han KH, Lee KS, Chon CY, Moon YM, et al. Prospective study on efficacy of oral supplement of branched-chain amino acid granules on the nutritional status of the cirrhotics. Korean J Hepatol. 2001;7:432–438. [Google Scholar]
- 23.Nijveldt RJ, Wiezer MJ, Meijer C, Prins HA, Statius Muller MG, Gouma DJ, et al. Major liver resection results in a changed plasma amino acid pattern as reflected by a decreased Fischer ratio which improves by bactericidal/permeability increasing protein. Liver. 2001;21:56–63. doi: 10.1034/j.1600-0676.2001.210109.x. [DOI] [PubMed] [Google Scholar]
- 24.Togo S, Tanaka K, Morioka D, Sugita M, Ueda M, Miura Y, et al. Usefulness of granular BCAA after hepatectomy for liver cancer complicated with liver cirrhosis. Nutrition. 2005;21:480–486. doi: 10.1016/j.nut.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 25.Poon RT, Yu WC, Fan ST, Wong J. Long-term oral branched chain amino acids in patients undergoing chemoembolization for hepatocellular carcinoma: a randomized trial. Aliment Pharmacol Ther. 2004;19:779–788. doi: 10.1111/j.1365-2036.2004.01920.x. [DOI] [PubMed] [Google Scholar]