Abstract
Deterioration of the hippocampus occurs in elderly individuals with and without dementia, yet individual variation exists in the degree and rate of hippocampal decay. Determining the factors that influence individual variation in the magnitude and rate of hippocampal decay may help promote lifestyle changes that prevent such deterioration from taking place. Aerobic fitness and exercise are effective at preventing cortical decay and cognitive impairment in older adults and epidemiological studies suggest that physical activity can reduce the risk for developing dementia. However, the relationship between aerobic fitness and hippocampal volume in elderly humans is unknown. In this study, we investigated whether individuals with higher levels of aerobic fitness displayed greater volume of the hippocampus and better spatial memory performance than individuals with lower fitness levels. Furthermore, in exploratory analyses, we assessed whether hippocampal volume mediated the relationship between fitness and spatial memory. Using a region-of-interest analysis on magnetic resonance images in 165 nondemented older adults, we found a triple association such that higher fitness levels were associated with larger left and right hippocampi after controlling for age, sex, and years of education, and larger hippocampi and higher fitness levels were correlated with better spatial memory performance. Furthermore, we demonstrated that hippocampal volume partially mediated the relationship between higher fitness levels and enhanced spatial memory. Our results clearly indicate that higher levels of aerobic fitness are associated with increased hippocampal volume in older humans, which translates to better memory function.
Keywords: aging, MRI, spatial memory, cognition, brain
INTRODUCTION
Severe hippocampal and entorhinal cortex deterioration occurs in Alzheimer’s disease (AD) and is also observed to a lesser degree in nondemented older adults (Raz et al., 2004a; 2005; Kramer et al., 2007; Kennedy et al., 2009). Longitudinal studies report nonlinear rates of atrophy with relative sparing of hippocampal volume until the fifth decade of life, followed by between a 1 and 2% annual decline in later years for nondemented older adults (Raz et al., 2004a; 2005; Mungas et al., 2005) and between a 3 and 5% annual decline in hippocampal volume for individuals with mild cognitive impairment (MCI) and AD (Jack et al., 1998; Mungas et al., 2005). Change in hippocampal volume is correlated with decline in memory performance, suggesting that decline in volume translates to decline in function (Peterson et al., 2000; Kramer et al., 2007). Hippocampal volume, however, is moderated by a number of lifestyle and health related factors such as depression (O’Brien et al., 2004), hypertension (Raz et al., 2005), chronic heavy drinking (Beresford et al., 2006), the use of hormone therapy (Erickson et al., 2005), and reports of chronic stress (McEwen, 2006; Gianaros et al., 2007). Such individual variation in hippocampal volume and atrophy suggests that decay is not necessarily an inevitable phenomenon and that modifying one’s lifestyle might be effective at preventing or reversing deterioration of the hippocampus.
Given the increasing aging population throughout the industrialized world (Administration on Aging, 2005) and a recent report from the Institute of Medicine (2008) that the workforce to treat and care for the geriatric population is both “too small and critically unprepared” to handle the escalating number of older adults, it is both socially and scientifically paramount to assess methods that could prevent or reverse cognitive and neural decay in the older adult population. Participation in physical and aerobic activities is one method that might prevent neural and cognitive decline among older adults (Kramer and Erickson, 2007). Clinical randomized trials suggest that participating in aerobic exercise regimens can begin to restore neural and cognitive decay already manifest in nondemented individuals (Kramer et al., 1999; Colcombe et al., 2004, 2006) and recent meta-analyses demonstrate that exercise interventions can improve cognitive function in people with AD and MCI (Heyn et al., 2004, 2008). These results are generally consistent with a large epidemiological literature that reports that physical inactivity is a significant risk factor for the development of MCI and AD (Barnes et al., 2003; Podewils et al., 2005; Larson et al., 2006; Andel et al., 2008).
Aerobic exercise interventions have been found to reverse decay in prefrontal and lateral temporal regions (Colcombe et al., 2006), and cardiorespiratory fitness levels moderate age-related declines in volume of the prefrontal and parietal regions (Colcombe et al., 2003). Consistent with these findings, higher aerobic fitness levels have been found to be positively associated with whole-brain volume in early-stage AD (Burns et al., 2008). However, whether higher levels of aerobic fitness levels are associated with the preservation of hippocampal volume is unknown.
There is reason to think that higher fitness levels would prevent hippocampal atrophy in older adults. First, nonhuman rodent research has unequivocally demonstrated that voluntary exercise affects the hippocampus. For example, wheel-running induces an increase in cell proliferation and survival in the dentate gyrus (Van Praag et al., 1999; 2005), increases mRNA and protein levels of brain-derived neurotrophic factor (BDNF) in the hippocampus (Neeper et al., 1995; Cotman and Berchtold, 2002; Vaynman et al., 2004; Berchtold et al., 2005), is therapeutic after hippocampal damage (Griesbach et al., 2007), enhances long-term potentiation and induces synaptic plasticity (Farmer et al., 2004; Christie et al., 2008), and enhances hippocampal-dependent learning and memory processes as assessed by Morris water maze and radial arm maze performance (Fordyce and Wehner, 1993; Anderson et al., 2000; Vaynman et al., 2004; Van Praag et al., 2005). Although the histological and chemical basis for volumetric change in humans is unknown, the broad effects that are observed with exercise training on the hippocampus in rodents suggest that higher fitness levels may preserve hippocampal volume in aged humans.
Second, as was discussed previously, a large epidemiological literature argues that physical activity is associated with a reduced risk for developing AD or MCI. Because hippocampal atrophy occurs in individuals with AD and MCI (Jack et al., 1998), and to a lesser degree in nondemented individuals (Raz et al., 2005), increased physical activity may reduce the risk for developing cognitive impairment or dementia through the preservation of hippocampal volume. Furthermore, exercise training appears to improve cognitive function in individuals with cognitive impairment and dementia to the same degree as nondemented individuals (Heyn et al., 2004, 2008) suggesting that any restorative effects of aerobic exercise on the hippocampus observed in healthy older adults might also extend to individuals with more severe hippocampal atrophy.
Finally, cerebral blood volume, considered an in vivo correlate of neurogenesis, is increased in the adult hippocampus and is correlated with improved memory after 3-months of an exercise intervention (Pereira et al., 2007). If aerobic exercise increases cerebral blood volume in the adult hippocampus then aerobic exercise might also be associated with increased tissue volume in the hippocampus.
Previous studies have established that higher levels of fitness are associated with enhanced cognitive function (Kramer et al., 1999; Colcombe et al., 2004). Spatial memory has been associated with hippocampal function in both rodents (e.g., O’Keefe and Nadel, 1978; Kesner, 2007) and humans (Kelley et al., 1998; Maguire et al., 1997). In humans, the magnitude of hippocampal activation is related to the magnitude of relational binding necessary for successful retrieval in both nonspatial and spatial memory tasks (Henke et al., 1997; Maguire et al., 1997; Rombouts et al., 1999; Brassen et al., 2006), indicating that conditions which require little relational binding might be more reliant on prefrontal or parahippocampal circuits than hippocampal. Therefore, the magnitude of fitness effects on spatial memory performance may vary as a function of the degree of relational memory needed to perform the task.
In this study, we examined (1) whether aerobic fitness levels were related to hippocampal volume and spatial memory in a large sample of high functioning nondemented older adults, and (2) whether hippocampal volume mediates the association between fitness and spatial memory performance. Other studies have investigated mediator variables such as executive functioning constructs and the integrity of white matter tracts, cerebral blood flow, and diet in the context of aging and cognitive impairment (Salthouse et al., 2003; Scarmeas et al., 2006; Madden et al., 2007; Vaidya et al., 2007; Madden et al., 2009). A mediator variable is a third explanatory variable that seeks to explain completely, or in part, the relationship between an independent variable and a dependent variable through multiple regression analyses (see Materials and Methods). Using this analytical technique we could test whether hippocampal volume mediates the association between fitness and spatial memory.
We predicted that individuals with higher levels of cardiorespiratory fitness would have greater hippocampal volume even after statistically adjusting for potentially confounding variables including age, sex, and years of education. We also predicted that greater hippocampal volume would mediate the fitness effects on spatial memory for the most challenging memory condition (Maguire et al., 1997; Rombouts et al., 1999; Brassen et al., 2006).
MATERIALS AND METHODS
Participants
One-hundred and sixty-five older adults (109 female; 56 male) between 59 and 81 yr of age participated in the study (mean age = 66.55; SD = 5.6). All participants were screened for dementia by the revised and modified Mini-Mental Status Examination (Stern et al., 1987) and were excluded from participation if they did not reach the required cut-off of 51 (high score of 57). All participants met or surpassed all criteria for participating in a magnetic resonance imaging study including no previous head trauma, no previous head or neck surgery, no diagnosis of diabetes, no neuropsychiatric or neurological condition including brain tumors, and no metallic implants that could interfere with or cause injury due to the magnetic field. Additionally, all participants received a physician’s clearance to engage in a maximal graded exercise test. Finally, all participants signed an informed consent approved by the University of Illinois.
Cardiorespiratory Fitness Assessment
All participants were required to obtain consent from their personal physician before cardiorespiratory fitness testing. Aerobic fitness (VO2 peak) was assessed by maximal graded exercise testing on a motor-driven treadmill with continuous monitoring of respiration, heart rate, and blood pressure by a cardiologist and nurse.
MR Imaging Protocol and Image Processing
For all participants, high resolution (1.3 × 1.3 × 1.3 mm) T1-weighted brain images were acquired using a 3D MPRAGE (Magnetization Prepared Rapid Gradient Echo Imaging) protocol with 144 contiguous slices collected in an ascending fashion. All images were collected on a 3T Siemens Allegra scanner with an echo time (TE) = 3.87 ms, repetition time (TR) = 1,800 ms, field of view (FOV) = 256 mm, an acquisition matrix of 192 × 192 mm, and a flip angle of 8 degrees.
For segmentation and volumetric analysis of the left and right hippocampus we employed FMRIB’s Integrated Registration and Segmentation Tool (FIRST) in FMRIB’s Software Library (FSL) version 4.0. FIRST is a semiautomated model-based subcortical segmentation tool utilizing a Bayesian framework from shape and appearance models obtained from manually segmented images from the Center for Morphometric Analysis, Massachusetts General Hospital, Boston. Structural and landmark information were obtained from 317 manually segmented and labeled T1 weighted images of the brain from normal children, adults and pathological populations (including schizophrenia and Alzheimer’s disease) and were modeled as a point distribution model in which the geometry and variation of the shape of the structure are submitted as priors. Volumetric labels are parameterized by a 3D deformation of a surface model based on multivariate Gaussian assumptions. FIRST then searches through linear combinations of shape modes of variation for the most probable shape given the intensity distribution in the T1 weighted image (see Patenaude et al., 2007a,b for further description of this method).
This method first runs a two-stage affine registration to a standard space template (MNI space) with 1 mm resolution using 12-degrees of freedom and a subcortical mask to exclude voxels outside the subcortical regions. Second, the left and right hippocampi are segmented with 30 modes of variation. The modes of variation are optimized based on leave-one-out cross-validation on the training set and increases the robustness and reliability of the results (Patenaude et al., 2007b). Finally, boundary correction takes place for each structure that classifies the boundary voxels as belonging to the structure or not based on a statistical probability (z-score >3.00; P < 0.001). The volume of each structure was measured as mm3 and then converted to the more common cm3 to describe cortical volume. The hippocampus volume comprised the dentate gyrus, the ammonic subfields (CA1–4), the prosubiculum, and the subiculum and did not include the fimbria/fornix behind the posterior commissure. Segmentations from each participant were visibly checked for any significant error that could have occurred during the segmentation process. No errors were noted.
Intracranial volume (ICV) is frequently used to adjust the regional volumes for sex and height (e.g., Raz et al., 2005). Here, we calculated ICV as the sum of gray, white, and cerebrospinal fluid and adjusted the hippocampal regions by this measure using FMRIB’s automated segmentation tool in FSL version 4.0. (Zhang et al., 2001; Smith et al., 2004). In accordance with other volumetric analyses, adjustment was performed for each region by an analysis of covariance approach: adjusted volume = raw volume–b × (ICV–mean ICV), where b is the slope of a regression of an ROI volume on ICV (Raz et al., 2004b, 2005; Head et al., 2008; Kennedy et al., 2009). Adjusted volume was used as a dependent variable and mediator variable for all analyses described in this article.
Previous studies have successfully used similar semiautomated subcortical segmentation routines to reliably and accurately discriminate between hippocampal volumes in individuals with AD, MCI, and normal aging (Colliot et al., 2008).
Spatial Memory Task
We used a task in which performance has been found to vary as a function of aging and a genetic predisposition for AD, which is associated with increased hippocampal atrophy (Greenwood et al., 2005). First, a fixation crosshair appeared for 1 s and participants were instructed to keep their eyes on the crosshair. After the fixation, either one, two, or three black dots appeared at random locations on the screen for a duration of 500 ms. The dots were removed from the display and the fixation cross re-appeared on the screen for a period of 3 s. During this time, participants were instructed to try and remember the locations of the previously presented black dots. At the end of the 3-s delay, a red dot appeared on the screen in either one of the same locations as the target dots (match condition) or at a different location (nonmatch condition). Participants had 2 s to respond to the red dot by pressing one of two keys on a standard keyboard—the ‘x’ key for a nonmatch trial, and the ‘m’ key for a match trial. Forty trials were presented for each set size (1, 2, or 3 locations), with 20 trials as match trials and 20 trials as nonmatch trials. Participants were instructed to respond as quickly and accurately as possible. Several practice trials were performed before the task began to acquaint the participants with the task instructions and responses.
Statistical Analyses
We examined whether aerobic fitness measures (VO2 peak) were associated with adjusted left or right hippocampal volume by a hierarchical linear regression (HLR) analysis with age, sex, years of education, and VO2 peak as predictors for left and right hippocampal volume. Age, years of education, and VO2 peak were entered as continuous variables, and sex as a categorical variable. We entered age, sex, and years of education in the model first followed by VO2 peak values to quantify the unique contribution of fitness on hippocampal volume after the variance associated with age, sex, and years of education were explained (see Results section for Pearson correlations between the independent variables). T-scores, and standardized betas (β) are presented along with change in explained variance. Performance measures (accuracy rates (% correct) and response times (RT)) were analyzed by a series of repeated measures ANOVAs with set-size (1-item, 2-item, 3-item) as a within-subjects factor.
Mediation is a hypothesis about a causal relation among variables (Fig. 1), but the conclusions of such a relation are only valid if the causal assumptions are valid (Judd and Kenny, 1981; Baron and Kenny, 1986; MacKinnon et al., 2007). There are four conditions that need to be met to establish whether hippocampal volume mediates a fitness-cognition relationship (Judd and Kenny, 1981; Baron and Kenny, 1986; MacKinnon et al., 2007). First, the independent variable (fitness level) must be associated with the dependent variable (spatial memory performance). Second, the independent variable (fitness level) must be associated with the mediator (hippocampal volume). Third, the mediator variable (hippocampal volume) must be associated with the dependent variable (spatial memory). Finally, hippocampal volume significantly mediates the relationship between fitness and spatial memory if controlling for hippocampal volume reliably reduces the variance in spatial memory explained by fitness levels (Fig. 1). To test these relationships we conducted a series of HLR analyses. First, relationships between VO2 peak and memory performance (accuracy and RT) for each set size of the spatial memory task was conducted in a HLR analysis with age, sex, and years of education entered first followed by VO2 peak. The results from this analysis allowed us to quantify how much of the variance in cognitive function could be explained by variation in fitness levels. We next analyzed how much of the variance in spatial memory performance could be explained by left and right hippocampal volume after including age, sex, and years of education as covariates in the model. Next, a HLR analysis was conducted with age, sex, years of education, and hippocampal volume entered first followed by fitness level with spatial memory entered as the dependent variable. If the magnitude of the association between fitness and spatial memory is significantly reduced when hippocampal volume is controlled, then it can be concluded that hippocampal volume is a mediator in the relationship between fitness level and spatial memory. To test whether the effect of mediation was significant, we used a version of the Sobel test (Sobel, 1982) popularized by Baron and Kenny (1986). The Sobel test analyzes if the effect of the mediator on the dependent variable is significantly different from zero using a two-tailed z-test with ± 1.96 as the critical values in a unit normal distribution. Simulations suggest that the modified Sobel test is preferable when sample sizes are larger than 50 (MacKinnon et al., 1995). This analysis was performed separately for the left and right hippocampal volumes, and was only performed on the 3-item spatial memory (accuracy) condition, as it was the only memory condition to meet the criteria for conducting a mediation analysis (Baron and Kenny, 1986). However, we also conducted a mediation analysis on the response times for the 1-item and 2-item condition with the right hippocampal volume as these were the only conditions to meet the criteria for mediation using RT as a dependent variable.
FIGURE 1.
Figural representation of the mediation model presented in this article. Aerobic fitness (IV) influences spatial memory performance (DV). Hippocampal volume is predicted to mediate this relationship. The proposed model hypothesizes that aerobic fitness causes an increase in hippocampal volume, which in turn causes an improvement in spatial memory.
RESULTS
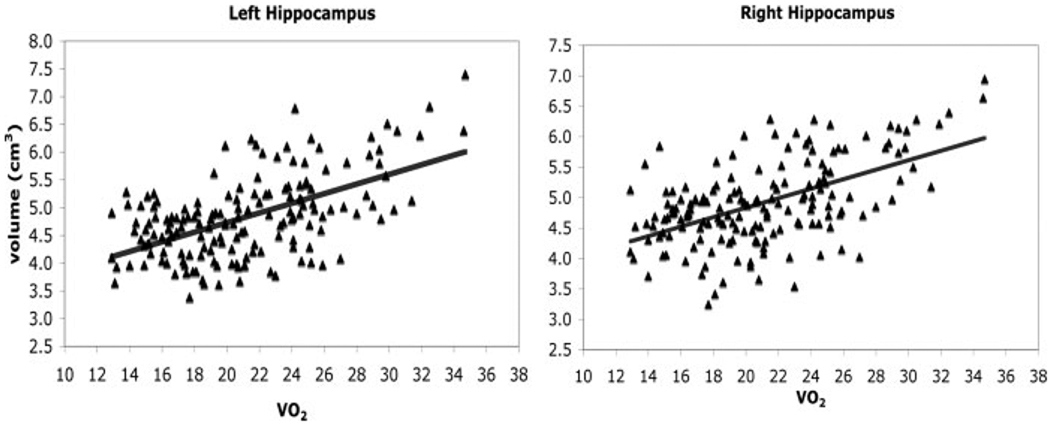
There were significant correlations between VO2 peak and age (r = −0.41; P < 0.001), sex (r = −0.50; P < 0.001), and years of education (r = 0.23; P < 0.003), thereby highlighting the necessity to control for these confounding factors to isolate effects associated with fitness. In the HLR analysis, the variables age, sex, and years of education (M = 15.65; SD = 2.91) were entered first as covariates to isolate the influence of VO2 peak (M = 21.11; SD = 4.80) on hippocampal volume. With all four factors entered the overall model was significant for the right hemisphere (F (4,163) = 21.43; P < 0.001) with all four variables accounting for 35.0% of the total variance in adjusted right hippocampal volume. Consistent with our hypotheses, VO2 peak significantly predicted adjusted right hippocampal volume (t = 4.38; β = 0.36; P < 0.001) with higher fitness levels associated with greater volume (see Fig. 2). In this analysis, 17.1% of the variation in right hippocampal volume was accounted for by age (F(1,162) = 33.34; P < 0.001) with older individuals having smaller hippocampal volumes. Sex explained additional variance in right hippocampal volume with a R2 change of 9.9% (F(1,161) = 21.89; P < 0.001) with women having larger adjusted hippocampal volumes than men. Years of education explained a nonsignificant 0.02% of additional variance in volume (F(1,160) = 0.40; P < 0.53). Fitness measurements, however, explained 7.8% of the variance in the right hippocampus volume (F(1,159) = 19.18; P < 0.001) after accounting for the variance from age, sex, and years of education. The age × fitness interaction did not account for any significant variation in right hippocampal volume (F(1,158) = 0.762; P < 0.38, R2 = 0.03).
FIGURE 2.
Scatterplots showing that with an increase in fitness (VO2 peak) there is an increase in hippocampal volume (cm3). Correlations for both the left and right hippocampus with fitness remained significant even after including age, sex, and years of education as covariates.
We performed the same analysis on the left hemisphere hippocampus. The overall model was significant with all four factors entered (F(4,163) = 21.22; P < 0.001) and explained 34.8% of the total variance in adjusted left hippocampal volume. Similar to the right hippocampus, aerobic fitness levels were positively associated with left adjusted hippocampal volume (t = 5.45; β = 0.45; P < 0.001). Age explained 15% of the variation in volume (F(1,162) = 28.58; P < 0.001). The addition of sex to the model explained an additional 7.5%, a significant increase in explained variance (F(1,161) = 15.69; P < 0.001), but years of education only explained 0.01% of additional variance after age and sex (F(1,160) = 0.20; P < 0.65). Like the right hippocampus, fitness measurements explained 12.2% of additional variance in hippocampal volume after variance associated with age, sex, and years of education were accounted for (F(1,159) = 29.65; P < 0.001). Similar to the right hemisphere, the age × fitness interaction was not significant (F(1,158) = 0.16; P < 0.69) and only accounted for 0.01% of the variation in left hippocampal volume. These analyses clearly identify aerobic fitness levels, along with age and sex, as an important factor in explaining both left and right hippocampal volume in older adults (Fig. 2).
For the spatial memory task, we found comparable results to other studies using this paradigm and other similar spatial memory paradigms (Greenwood et al., 2005; Beigneux et al., 2007). Computer errors resulted in lost data for four individuals leaving a total of 161 individuals for all analyses with memory performance (see Table 1). A repeated-measures analysis revealed that accuracy rates significantly decreased from the 1-item set size to the 3-item set size (F(2,320) = 74.62; P < 0.001) and response times significantly increased from the 1-item to 3-item set size (F(2,320) = 487.60; P < 0.001). Planned comparisons demonstrated that both accuracy rates and response times were reliably different between each of the three set sizes (all P < 0.001).
TABLE 1.
Mean Scores and Standard Deviations for the Spatial Memory Task Along With Correlation Coefficients and Partial Correlation Coefficients (Controlling for Age, Sex, and Years of Education) for Each Independent Variable
| Condition | Mean (SD) | Age | Sex | Education | Fitness (partial) |
L. Hippo. (partial) |
R. Hippo. (partial) |
|---|---|---|---|---|---|---|---|
| 1-item RT | 847.27 (170.20) | 0.18* | 0.24** | 0.00 | −0.15* | −0.31*** | −0.21** |
| 2-item RT | 958.34 (180.86) | 0.22** | 0.29*** | −0.03 | −0.15* | −0.20** | −0.13 |
| 3-item RT | 1046.49 (185.90) | 0.19** | 0.26*** | −0.04 | −0.12 | −0.16 | −0.09 |
| 1-item ACC | 0.87 (0.13) | −0.23** | 0.01 | 0.03 | 0.07 | 0.21** | 0.15 |
| 2-item ACC | 0.81 (0.14) | −0.20** | −0.14 | 0.03 | 0.12 | 0.18* | 0.13 |
| 3-item ACC | 0.77 (0.14) | −0.35*** | −0.11 | 0.10 | 0.24** | 0.25** | 0.20** |
Values for age, sex (positive correlations indicate that females tend to perform better and have greater hippocampal volume than males), and education are results from a Pearson correlation and represent the correlation coefficients (r). Values for fitness, left hippocampus, and right hippocampus are partial correlation results from a hierarchical linear regression analysis with age, sex, and years of education as covariates. Marked values met significance at P < 0.05 (*), P < 0.01 (**), and P < 0.001 (***).
RT, response time; ACC, percent correct; L. Hippo, left hippocampus; R.Hippo, right hippocampus.
In a HLR analysis with age, sex, and years of education entered as covariates, we found that fitness levels were reliably associated with accuracy rates for the 3-item memory condition, with 5.0% of the variance (t = 3.09; β = 0.29; P < 0.002) in accuracy accounted for by fitness levels (F(1,155) = 9.58; P < 0.002). Fitness was not related to 1-item or 2-item accuracy rates (see Table 1 for a list of partial correlations associated with fitness and hippocampal volume). Furthermore, with age, sex, and years of education entered first in the HLR, a significant amount of variance in accuracy rates for the 3-item memory condition were accounted for by right hippocampal volume (F(1,155) = 6.73; P < 0.01) with 3.6% of the variation explained. Right hippocampal volume, however, did not account for variance in either 1-item or 2-item accuracy rates (both P > 0.05). The left hemisphere accounted for 5.3% of the variance in 3-item accuracy rates after accounting for age, sex, and years of education (F(1,155) = 10.09; P < 0.002), 3.1% of the variation in 2-item accuracy rates (F(1,155) = 5.32; P < 0.02), and 4.4% of the variance in 1-item accuracy rates (F(1,155) = 7.56; P < 0.007). Therefore, the right hippocampal volume was significantly related to spatial memory performance for the 3-item set-size and the left hemisphere hippocampal volume was related to memory performance regardless of the set-size (see Table 1 for the partial correlations). Because higher levels of fitness were associated with enhanced performance in the 3-item memory condition and not in the 1-item or 2-item conditions, only the 3-item memory condition could be assessed in a mediation analysis.
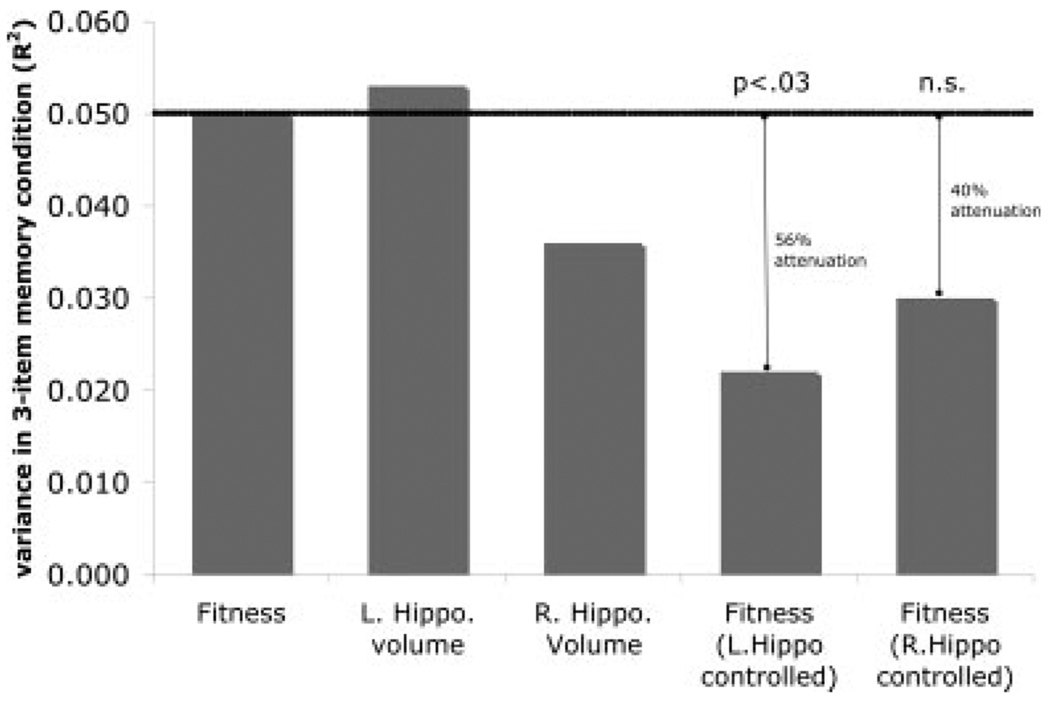
In the exploratory mediation analysis, we found that the effect of fitness on 3-item spatial memory (accuracy) was attenuated, but still significant when age, sex, years of education, and right hippocampal volume were entered into the model (F(1,154) = 5.81; P < 0.01) with fitness level explaining 3.0% (t = 2.41; β = 0.23) of the variance in 3-item memory performance (Fig. 3). As noted earlier, without including the right hippocampus as a mediator, fitness levels explained 5% of spatial memory performance. This amounted to a drop in 2% of explained variance when right hippocampal volume was entered into the model, or an attenuation of 40% of the fitness-spatial memory relationship. However, using a modified Sobel test (see Materials and Methods) we found that the right hippocampal volume was not a significant mediator in the fitness-spatial memory relationship (z = 1.57; P < 0.116).
FIGURE 3.
In this bar graph, we represent on the Y-axis the variance (R2) in accuracy on the 3-item spatial memory task explained by each factor or combination of factors on the X-axis. A test of mediation examines whether controlling for the variance associated with hippocampal volume significantly reduces the variance in 3-item spatial memory associated with fitness. It is clear that the magnitude of the relationship between 3-item spatial memory performance and fitness is significantly reduced when left hemisphere volume was entered into the regression model thereby indicating a mediating relationship for the left hippocampus on the fitness-spatial memory relationship (P < 0.03).
For the left hippocampus volume, we found that the effect of fitness on 3-item spatial memory (accuracy) was attenuated, but still significant when age, sex, years of education, and left hippocampal volume were entered into the model (F(1,154) = 4.25; P < 0.04) with fitness level explaining 2.2% (t = 2.06; β = 0.20) of the variance in 3-item memory performance (Fig. 3). This amounted to a drop in 2.8% of explained variance when left hippocampal volume was entered into the model, or in other words, an attenuation of 56% of the fitness-spatial memory relationship. Using a Sobel test of significance, we found that the left hippocampus volume was a significant partial mediator between fitness and spatial memory (z = 2.056; P < 0.03), suggesting that the volume of the left hippocampus contributes to the memory benefits associated with higher fitness levels.
We conducted similar analyses with the RT data. In the HLR analysis, fitness was marginally related to the 1-item RT (t = −1.96; β = −0.19; P < 0.05) and 2-item RT (t = −1.95; β = −0.19; P < 0.052) conditions with 2.2 and 2.1% of the variance explained respectively after age, sex, and years of education were entered into the model. There was also a trend for RT for the 3-item condition to be negatively related to fitness levels (t = −1.56; β = −0.15; P < 0.12). Furthermore, we found that 4.2% of the variance in 1-item RT (F(1,155) = 7.38; P < 0.007) was accounted for by the right hippocampus volume after age, sex, and years of education were entered into the model. The right hippocampus volume did not explain a significant amount of variance in 2-item RT or 3-item RT scores (both P > 0.05). The left hippocampus volume explained 8.5% of the variance in 1-item RT scores (F(1,155) = 15.97; P < 0.001), 3.6% of the variance in 2-item RT scores (F(1,155) = 6.74; P < 0.01), and 2.1% of the variance in 3-item RT scores (F(1,155) = 3.74; P < 0.055) after age, sex, and years of education were entered into the model. Because of the constraints of the mediation criteria, only the 1-item and 2-item RT scores were subjected to a mediation analysis with the left and right hippocampus volumes.
In an exploratory mediation analysis we found that the right hippocampal volume completely mediated the effect of fitness on 1-item RT scores. Specifically, the fitness-memory relationship dropped from 2.2% to a nonsignificant 0.08% for the 1-item RT scores indicating that the fitness-RT relationship for the 1-item memory condition was completely mediated by right hippocampal volume (z = −2.71; P < 0.006). The right hippocampus also reduced the relationship between fitness and RT for the 2-item memory condition from 2.1% to a nonsignificant 1.2%, yet this failed to reach significance using a modified Sobel test (z = −1.81; P < 0.06). The left hippocampus volume also partially mediated the fitness-RT relationship for the 1-item memory condition. Specifically, fitness explained 8.5% of the 1-item RT scores, but this dropped to 2.2% when the left hippocampus volume was included in the regression (z = −2.04; P < 0.04). The left hippocampus did not significantly mediate the 2-item RT scores (P > 0.05). These results indicate that the volume of the left and right hippocampus mediates the fitness-RT relationship for the easier 1-item memory condition, but not for the more challenging 2-item condition.
DISCUSSION
We have demonstrated that higher aerobic fitness levels are associated with the preservation of left and right hippocampal volume in a sample of nondemented older adults even after adjusting for potentially confounding factors including age, sex, and years of education. Higher fitness levels were also associated with better performance on the spatial memory task. The left hippocampal volume partially mediated the fitness effects on memory performance for the 3-item condition, whereas both the left and right hippocampal volume mediated the fitness effects on response times for the 1-item condition. Therefore, these results demonstrate a clear triple association between fitness, spatial memory performance, and left and right hippocampal volume.
The scientific search for lifestyle factors that moderate neural and cognitive decay in old age is imperative given the increasing aging population throughout the world (Administration on Aging, 2005). Aerobic fitness and exercise have the capability of reversing and preventing cognitive and cortical decay in individuals with and without dementia (Colcombe et al., 2004, 2006; Burns et al., 2008; Heyn et al., 2008). Aerobic exercise also increases cerebral blood volume in the dentate gyrus of adults, which is related to enhanced memory function and neurogenesis (Pereira et al., 2007). Our results add to this literature by clearly demonstrating that higher aerobic fitness levels are associated with greater hippocampal volumes in elderly humans and that larger hippocampal volumes translate to better spatial memory function.
Our results are also consistent with a large literature demonstrating marked benefits of aerobic activity on hippocampal structure and function in rodents (e.g., Cotman and Berchtold, 2002). Although there is not yet evidence indicating which aspects of histology or neurochemistry the volumetric measures most closely represent, our results are in line with evidence that aerobic activity induces cell proliferation and survival (Van Praag et al., 2005), promotes synaptic plasticity (Christie et al., 2008), and provides protection from insult (Griesbach et al., 2007)—all effects which could conceivably increase hippocampal volume measurements in older adults. Whatever combination of cellular and neurochemical factors represent the volume measures, the volumetric measures and differences appear to be cognitively meaningful given the relationship with memory function. It will be important for future research to define which combinations of cellular components are most associated with the volumetric measurements as examined in this study.
Deterioration of the hippocampus leads to memory impairment and dementia. Longitudinal studies report a one percent annual decline in hippocampal volume in nondemented older individuals with a more rapid decline in those with MCI and AD (Raz et al., 2004a; Jack et al., 1998). Furthermore, individuals with larger hippocampal volumes at initial baseline measurements show a reduced rate of conversion from MCI to AD over a 2-yr period (Grundman et al., 2002). Therefore, larger hippocampal volumes in higher-fit individuals might be directly related to a reduced risk for developing AD. This is consistent with epidemiological studies that report that higher physical activity levels are associated with a reduced risk of AD (e.g., Andel et al., 2008). Our results suggest the intriguing possibility that age-related atrophy of the medial temporal lobe is not an inevitable phenomenon and that increased physical or aerobic activity may act to reduce age-related deterioration of the hippocampal formation.
Importantly, we found that higher fitness levels did not moderate the age-related reduction in hippocampal volume in this sample, indicating that the rate of hippocampal decay with advancing age was not reduced by higher fitness levels like that reported in previous studies for prefrontal and parietal regions (Colcombe et al., 2003). Instead, a main effect of fitness on hippocampal volume suggests that higher fitness levels may moderate hippocampal decay earlier in life before deterioration begins. Thus, an activity-induced increase in hippocampal volume earlier in life would protect from decay in old age by affecting baseline hippocampal volume and not the rate of decay in advanced age. There is evidence, that aerobic exercise moderates the trajectory of cognitive impairment over a 5-yr period (Barnes et al., 2007), which would presumably be reflected by the sparing of medial temporal lobe volume with higher levels of fitness. It will be important for future studies to investigate the moderating effects of aerobic activity and fitness on cognitive impairment and hippocampal atrophy further in both nondemented and demented populations.
We predicted that fitness and hippocampal volume would only be associated with memory conditions in which there was a heightened demand for relational binding. Partially consistent with this hypothesis we found that higher fitness levels were associated with higher accuracy rates in the spatial memory paradigm only for the 3-item memory condition. However, higher fitness levels were also associated with faster response times in the 1-item memory condition suggesting that fitness improves processing speed functions in this task. We did not predict that hippocampal volume would be related to reaction times, yet we found that the right and left hippocampus volume significantly mediated the fitness-response speed relationship in the easiest of the three memory conditions. This suggests that the hippocampus might play a role in processing speed for low-load memory conditions, like the 1-item spatial memory task employed in this paradigm. This particular result was surprising given the lack of literature on hippocampal volume and processing speed or reaction times in this population (Bucur et al., 2008; Colcombe et al., 2005; Park et al., 2003). Future studies should further assess the mediating role that the hippocampus might play in processing speed in low-load memory conditions.
The exploratory mediator analysis suggested that the volume of the left hippocampus might play an important role in mediating the fitness-spatial memory relationship (Fig. 3) and both the left and right hippocampus in mediating the fitness–response speed relationship for low-load memory conditions. However, like all mediational analyses, interpretations of the causal relationship should be performed cautiously and only within the context of an entire program of research (MacKinnon et al., 2007). Although findings from clinical randomized trials, epidemiological studies, and experiments on rodents all converge and lend credibility to the model we tested, it is quite possible that other unmeasured factors (e.g., diet) that covary with fitness levels and hippocampal volume could explain the mediated effects observed here. In short, our results provide intriguing evidence that hippocampal volume has an important causal role in the fitness-cognition relationship. Yet, it is critical for future studies that use longitudinal designs, clinical populations, and other measuring techniques to replicate, validate, and characterize the causal relationships between aerobic fitness, hippocampal volume, and cognition.
It is important to remember that although our results suggest that aerobic fitness is associated with the preservation of hippocampal volume, randomized clinical trials are needed to analyze whether fitness improvements brought about by exercise training can reliably treat and reverse hippocampal decay. Furthermore, although there is meta-analytic evidence that individuals with cognitive impairment and dementia benefit from an exercise intervention to the same degree as nondemented individuals (Heyn et al., 2008), we cannot conclude that aerobic fitness and exercise would preserve hippocampal volume in populations with pathological disturbances of the hippocampus. More research on the influence that aerobic fitness has on these populations is warranted. Finally, although we found a positive relationship between hippocampal volume and aerobic fitness levels, the participants in our study were still relatively sedentary. However, even within a restricted range, aerobic fitness level was found to be associated with hippocampal volume and related memory functions. It will be important for future studies to examine whether hippocampal volume continues to increase with higher levels of aerobic fitness in older adults.
Acknowledgments
We thank the following people for their assistance during data collection: Susan Herrel, Nancy Dodge, Holly Tracy, Dawn Epstein, Zuha Warraich, Jennifer Kim, Maritza Alvarado, Heloisa Alves, Edward Malkowski, Susie Heo, and Jason Lewis.
Grant sponsor: National Institute on Aging; Grant numbers: RO1 AG25667 and RO1 AG25302.
REFERENCES
- Administration on Aging, US Department of Health and Human Services. A profile of Older Americans. 2005 Available at: http://www.aoa.gov/PROF/Statistics/profile/2005/profiles2005.asp.
- Andel R, Crowe M, Pederson NL, Fratiglioni L, Johansson B, Gatz M. Physical exercise at midlife and risk of dementia three decades later: a population-based study of Swedish twins. J Gerontol A Biol Sci Med Sci. 2008;63:62–66. doi: 10.1093/gerona/63.1.62. [DOI] [PubMed] [Google Scholar]
- Anderson BJ, Rapp DN, Baek DH, McCloskey DP, Coburn-Litvak PS, Robinson JK. Exercise influences spatial learning in the radial arm maze. Physiol Behav. 2000;70:425–429. doi: 10.1016/s0031-9384(00)00282-1. [DOI] [PubMed] [Google Scholar]
- Barnes DE, Yaffe K, Satariano WA, Tager IB. A longitudinal study of cardiorespiratory fitness and cognitive function in healthy older adults. J Am Geriatr Soc. 2003;51:459–465. doi: 10.1046/j.1532-5415.2003.51153.x. [DOI] [PubMed] [Google Scholar]
- Barnes DE, Whitmer RA, Yaffe K. Physical activity and dementia: the need for prevention trials. Exerc Sport Sci Rev. 2007;35:24–29. doi: 10.1097/JES.0b013e31802d6bc2. [DOI] [PubMed] [Google Scholar]
- Barron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Beigneux K, Plaie T, Isingrini M. Aging effect on visual and spatial components of working memory. Int J Aging Hum Dev. 2007;65:301–314. doi: 10.2190/AG.65.4.b. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133:853–861. doi: 10.1016/j.neuroscience.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Beresford TP, Arciniegas DB, Alfers J, Clapp L, Martin B, Du Y, Liu D, Shen D, Davatzikos C. Hippocampus volume loss due to chronic heavy drinking. Alcohol Clin Exp Res. 2006;30:1866–1870. doi: 10.1111/j.1530-0277.2006.00223.x. [DOI] [PubMed] [Google Scholar]
- Brassen S, Weber-Fahr W, Sommer T, Lehmbeck JT, Braus DF. Hippocampal-prefrontal encoding activation predicts whether words can be successfully recalled or only recognized. Behav Brain Res. 2006;171:271–278. doi: 10.1016/j.bbr.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Bucur B, Madden DJ, Spaniol J, Provenzale JM, Cabeza R, White LE, Huettel SA. Age-related slowing of memory retrieval: contributions of perceptual speed and cerebral white matter integrity. Neurobiol Aging. 2008;29:1070–1079. doi: 10.1016/j.neurobiolaging.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JM, Cronk BB, Anderson HS, Donnelly JE, Thomas GP, Harsha A, Brooks WM, Swerdlow RH. Cardiorespiratory fitness and brain atrophy in early Alzheimer disease. Neurology. 2008;71:210–216. doi: 10.1212/01.wnl.0000317094.86209.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie BR, Eadie BD, Kannangara TS, Robillard JM, Shin J, Titterness AK. Exercising our brains: How physical activity impacts synaptic plasticity in the dentate gyrus. Neuromolecular Med. 2008;10:47–58. doi: 10.1007/s12017-008-8033-2. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, Kramer AF. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci. 2003;58:176–180. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S. Cardiovascular fitness, cortical plasticyt, and aging. Proc Natl Acad Sci USA. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P. The implications of cortical recruitment and brain morphology for individual differences in inhibitory function in aging humans. Psychol Aging. 2005;20:363–375. doi: 10.1037/0882-7974.20.3.363. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- Colliot O, Chetalat G, Chupin M, Desgranges B, Magnin B, Benali H, Dubois B, Garnero L, Eustache F, Lehericy S. Discrimination between Alzheimer disease, mild cognitive impairment, and normal aging by using automated segmentation of the hippocampus. Radiology. 2008;248:194–201. doi: 10.1148/radiol.2481070876. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: A behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Colcombe SJ, Raz N, Korol DL, Scalf P, Webb A, Cohen NJ, McAuley E, Kramer AF. Selective sparing of brain tissue in postmenopausal women receiving hormone replacement therapy. Neurobiol Aging. 2005;26:1205–1213. doi: 10.1016/j.neurobiolaging.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124:71–79. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Fordyce DE, Wehner JM. Physical activity enhances spatial learning performance with an associated alteration in hippocampal protein kinase C activity in C57BL/6 and DBA/2 mice. Brain Res. 1993;619:111–119. doi: 10.1016/0006-8993(93)91602-o. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Jennings JR, Sheu LK, Greer PJ, Kuller LH, Matthews KA. Prospective reports of chronic life stress predict decreased grey matter volume in the hippocampus. Neuroimage. 2007;35:795–803. doi: 10.1016/j.neuroimage.2006.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood PM, Lambert C, Sunderland T, Parasuraman R. Effects of apolipoprotein E genotype on spatial attention, working memory, and their interaction in healthy, middle-aged adults: Results from the National Institute of Mental Health’s BIOCARD study. Neuropsychology. 2005;19:199–211. doi: 10.1037/0894-4105.19.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesbach GS, Gomez-Pinilla F, Hovda DA. Time window for voluntary exercise-induced increases in hippocampal neuroplasticity molecules after traumatic brain injury is severity dependent. J Neurotrauma. 2007;24:1161–1171. doi: 10.1089/neu.2006.0255. [DOI] [PubMed] [Google Scholar]
- Grundman M, Sencakova D, Jack CR, Petersen RC, Kim HT, Schultz A, Weiner MF, DeCarli C, DeKosky ST, van Dyck C, Thomas RG, Thal LJ Alzheimer’s Disease Cooperative Study. Brain MRI hippocampal volume and prediction of clinical status in a mild cognitive impairment trial. J Mol Neurosci. 2002;19:23–27. doi: 10.1007/s12031-002-0006-6. [DOI] [PubMed] [Google Scholar]
- Head D, Rodrigue KM, Kennedy KM, Raz N. Neuroanatomical and cognitive mediators of age-related differences in episodic memory. Neuropsychology. 2008;22:491–507. doi: 10.1037/0894-4105.22.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke K, Buck A, Weber B, Wieser HG. Human hippocampus establishes associations in memory. Hippocampus. 1997;7:249–256. doi: 10.1002/(SICI)1098-1063(1997)7:3<249::AID-HIPO1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Heyn PC, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta-analysis. Arch Phys Med Rehabil. 2004;85:1694–1704. doi: 10.1016/j.apmr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Heyn PC, Johnson KE, Kramer AF. Endurance and strength training outcomes on cognitively impaired and cognitively intact older adults: a meta-analysis. J Nutr Health Aging. 2008;12:401–409. doi: 10.1007/BF02982674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine of the National Academies. Retooling for an Aging America: Building the health care workforce. Committee on the Future Health Care Workforce for Older Americans. Washington D.C.: The National Academic Press; 2008. [PubMed] [Google Scholar]
- Jack CR, Jr, Peterson RC, Xu Y, O’Brien PC, Smith GE, Ivnik RJ, Tangalos EG, Kokmen E. Rate of medial temporal lobe atrophy in typical aging and Alzheimer’s disease. Neurology. 1998;51:993–999. doi: 10.1212/wnl.51.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd CM, Kenny DA. Process analysis: estimating mediation in treatment evaluations. Eval Rev. 1981;5:602–619. [Google Scholar]
- Kelley WM, Miezen FM, McDermott KB, Buckner RL, Raichle ME, Cohen NJ, Ollinger JM, Akbudak E, Conturo TE, Snyder AZ, Peterson SE. Hemispheric specialization in human dorsal frontal cortex and medial temporal lobe for verbal and nonverbal memory encoding. Neuron. 1998;20:927–936. doi: 10.1016/s0896-6273(00)80474-2. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Erickson KI, Rodrigue KM, Voss MW, Colcombe SJ, Kramer AF, Acker JD, Raz N. Age-related differences in regional brain volumes: A comparison of optimized voxel-based morphometry to manual volumetry. Neurobiol Aging. 2009;30:1657–1676. doi: 10.1016/j.neurobiolaging.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP. A behavioral analysis of dentate gyrus function. Prog Brain Res. 2007;163:567–576. doi: 10.1016/S0079-6123(07)63030-1. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Erickson KI. Capitalizing on cortical plasticity: Influence of physical activity on cognition and brain function. Trends Cogn Sci. 2007;11:342–348. doi: 10.1016/j.tics.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, Chason J, Vakil E, Bardell L, Bioleau RA, Colcombe A. Ageing, fitness, and neurocognitive function. Nature. 1999;400:418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Mungas D, Reed BR, Wetzel ME, Burnett MM, Miller BL, Weiner MW, Chui HC. Longitudinal MRI and cognitive change in healthy elderly. Neuropsychology. 2007;21:412–418. doi: 10.1037/0894-4105.21.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson EB, Wang L, Bowen JD, McCormick WC, Teri L, Crane P, Kukull W. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Warsi G, Dwyer JH. A simulation study of mediated effect measures. Multivariate Behav Res. 1995;30:41–62. doi: 10.1207/s15327906mbr3001_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Ann Rev Psychol. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Whiting WL, Bucur B, Provenzale JM, Cabeza R, White LE, Huettel SA. Adult age differences in the functional neuroanatomy of visual attention: A combined fMRI and DTI study. Neurobiol Aging. 2007;28:459–476. doi: 10.1016/j.neurobiolaging.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Costello MC, Bucur B, White LE, Cabeza R, Davis SW, Dennis NA, Provenzale JM, Huettel SA. Cerebral white matter integrity mediates adult age differences in cognitive performance. J Cogn Neurosci. 2009;21:289–302. doi: 10.1162/jocn.2009.21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Frackowiak RSJ, Frith CD. Recalling routes around London: activation of the right hippocampus in taxi drivers. J Neurosci. 1997;17:7103–7110. doi: 10.1523/JNEUROSCI.17-18-07103.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators: Central role of the brain. Dialogues Clin Neurosci. 2006;8:367–381. doi: 10.31887/DCNS.2006.8.4/bmcewen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungas D, Harvey D, Reed BR, Jagust WJ, DeCarli C, Beckett L, Mack WJ, Kramer JH, Weiner MW, Schuff N, Chui HC. Longitudinal volumetric MRI change and rate of cognitive decline. Neurology. 2005;65:565–571. doi: 10.1212/01.wnl.0000172913.88973.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- O’Brien JT, Lloyd A, McKeith I, Gholkar A, Ferrier N. A longitudinal study of hippocampal volume, cortisol levels, and cognition in older depressed subjects. Am J Psychiatry. 2004;161:2081–2090. doi: 10.1176/appi.ajp.161.11.2081. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The Hippocampus as a Cognitive Map. New York: Oxford University Press; 1978. [Google Scholar]
- Park DC, Welsh RC, Marshuetz C, Gutchess AH, Mikels J, Polk TA, Noll DC, Taylor SF. Working memory for complex scenes: Age differences in frontal and hippocampal activations. J Cogn Neurosci. 2003;15:1122–1134. doi: 10.1162/089892903322598094. [DOI] [PubMed] [Google Scholar]
- Patenaude B, Smith SM, Kennedy D, Jenkinson M. FIRST-FMRIB’s integrated registration and segmentation tool; Human Brain Mapping Conference, 2007; 2007a. [Google Scholar]
- Patenaude B, Smith SM, Kennedy D, Jenkinson M. Technical report TR07BP1, FMRIB Center. University of Oxford; 2007b. Bayesian shape and appearance models. [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci USA. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RC, Jack CR, Jr, Xu YC, Waring SC, O’Brien PC, Smith GE, Ivnik RJ, Tangalos EG, Boeve BF, Kokmen E. Memory and MRI-based hippocampal volumes in aging and AD. Neurology. 2000;54:581–587. doi: 10.1212/wnl.54.3.581. [DOI] [PubMed] [Google Scholar]
- Podewils LJ, Guallar E, Kuller LH, Fried LP, Lopez OL, Carlson M, Lyketsos CG. Physical activity, APOE genotype, and dementia risk: Findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005;161:639–651. doi: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Head D, Kennedy KM, Acker JD. Differential aging of the medial temporal lobe: A study of a five-year change. Neurology. 2004a;62:433–438. doi: 10.1212/01.wnl.0000106466.09835.46. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Rodrigue KM, Williamson A, Acker JD. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: Replicability of regional differences in volume. Neurobiol Aging. 2004b;25:377–396. doi: 10.1016/S0197-4580(03)00118-0. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Rombouts SA, Scheltens P, Machielson WC, Barkhof F, Hoogenraad FG, Veltman DJ, Valk J, Witter MP. Parametric fMRI analysis of visual encoding in the human medial temporal lobe. Hippocampus. 1999;9:637–643. doi: 10.1002/(SICI)1098-1063(1999)9:6<637::AID-HIPO4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Atkinson TM, Berish DE. Executive functioning as a potential mediator of age-related cognitive decline in normal adults. J Exp Psychol Gen. 2003;132:566–594. doi: 10.1037/0096-3445.132.4.566. [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Stern Y, Mayeau R, Luchsinger JA. Mediterranean diet, Alzheimer disease, and vascular mediation. Arch Neurol. 2006;63:1709–1717. doi: 10.1001/archneur.63.12.noc60109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy R, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Mathews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. Sociol Methodol. 1982;13:290–312. [Google Scholar]
- Stern Y, Sano M, Paulson J, Mayeau R. Modified mini-mental state examination: Validity and reliability. Neurology. 1987;37:179. [Google Scholar]
- Vaidya JG, Paradiso S, Boles Ponto LL, McCormick LM, Robinson RG. Aging, grey matter, and blood flow in the anterior cingulate. Neuroimage. 2007;37:1346–1353. doi: 10.1016/j.neuroimage.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neuroscience. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and expectation maximization algorithm. IEEE Trans on Medical Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]