Abstract
The objective of this study was to determine how parents of preverbal children determine whether their child is having otalgia. We constructed 8 cases describing a 1 year old child with acute otitis media (AOM) using various combinations of the following 6 observable symptoms: fussiness, ear tugging, eating less, fever, sleeping difficulty and playing less. Parents of children with a history of AOM presenting for well or sick appointments to an ambulatory clinic were asked to assign a pain level to each case on a visual analog scale. 69 parents participated in the study. Each of the 6 behaviors was associated with increased pain levels (P < 0.0001). Ear tugging and fussiness had the highest impact on the assigned pain levels. Higher level of parental education and private insurance were associated with higher reported pain levels (P = 0.007 and P = 0.001, respectively). Because interpretation of symptoms appears to be influenced by socioeconomic status, we question the utility of using an overall pain score from a 1-item parent scale as an outcome measure in clinical trials that include preverbal children.
Keywords: Otalgia, otitis media, ear pain
Background
Otalgia (i.e., ear pain) is the most prevalent and important symptom of AOM.10, 1, 6 Concern regarding otalgia prompts parents to bring their child for medical evaluation. Clinicians often prescribe antimicrobials or analgesics with the hope of shortening the duration of otalgia. If antimicrobial therapy is not prescribed initially, as in the watchful waiting approach, persistence of otalgia is an indication for starting antimicrobials. Furthermore, otalgia is frequently used as a criteria for inclusion of children in AOM studies.5, 8 A previous study by Heikkinen found that the positive and negative predictive value of otalgia for the diagnosis of AOM was 83% and 78%, respectively.3 A systematic review of the signs and symptoms of AOM concluded that otalgia was the most useful symptom in the diagnosis of AOM (Likelihood ratio 3.0–7.3).12 In addition, otalgia is often used in the assessment of outcome in clinical trials.2, 13 The Cochrane review on the efficacy of antimicrobials for AOM focused on otalgia as the most relevant outcome in AOM trials. Accordingly, the methods used to measure severity of otalgia are of considerable importance.
Yet, in preverbal children, who constitute the majority of children with AOM, it is not clear how parents determine whether their child is having otalgia. Specifically, it is unclear which of the symptoms of AOM impacts parental pain assessment the most. Information regarding specific symptoms with high levels of association with otalgia can help clinicians focus their history taking.
To our knowledge, no study to date has investigated what information parents of preverbal children use to determine the severity of otalgia. By creating scenarios of children with various constellations of symptoms, we aimed to provide a preliminary data to inform this question.
Methods
We constructed cases that described typical symptoms of a 1 year old child with AOM. We used combinations of the following 6 observable symptoms (fussiness, ear tugging, eating less, fever, sleeping difficulty, playing less) to construct the cases. We chose these 6 symptoms because in a previous study,14 these symptoms 1) were associated with the otoscopic diagnosis, 2) were identified by parents as an important, and 3) could be manifestations of otalgia.
For each case, certain symptoms were present and others were absent. The order in which the cases were presented was chosen at random and is shown in Table 1. We used an SPSS package (ORTHOPLAN) to generate the minimum number of orthogonal cases and to ensure the absence of multicollinearity between them.11 This resulted in a total of 8 cases; one case described an asymptomatic child.
Table 1.
Description of symptoms used in each case
| Symptom | Case | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Fussy | + | − | − | + | − | + | − | + |
| Trouble sleeping | + | − | + | − | + | + | − | − |
| Playing less | + | − | + | + | − | − | + | − |
| Fever | − | − | − | + | + | + | + | − |
| Ear tugging | − | − | + | − | − | + | + | + |
| Eating less | − | − | + | + | + | − | − | + |
The University of Pittsburgh Institutional Review Board approved the study. We enrolled parents of children 3 months to 2 years old with a history of AOM in the past 12 months presenting for well or sick appointments to an outpatient ambulatory clinic. Parents were asked for permission to be interviewed while waiting for their child to be evaluated by a physician. Interviews were conducted in a private setting. The instructions stated: “We are trying to find out how parents can tell when their child is in pain. Below are 8 descriptions of what a 1-year-old child with an ear infection may have. If you were the parent of this child, how you would rate the child’s pain level?” For each case, we provided parents with list of all symptoms that were present or absent in the hypothetical child (see Table 1). Aside from this list, no further descriptive or qualifying information was provided regarding the child or their symptoms.
After reviewing the symptoms that were present or absent in each case, we asked parents to slide a pointer to the most appropriate “pain level” on a 55 cm vertical visual analog scale numerically scaled in units from 0 to 100 (“No pain =0” and “Worst imaginable pain=100”). From each respondent, we also collected information regarding race, education level, and health insurance status.
Parents who assigned a pain score of >10 out of a possible 100 to the case with the asymptomatic child were excluded from all analyses. Exclusion of parents who do not appear to understand the instructions is standard practice for behavioral studies that use rating scales.11 A multivariate linear mixed effects model with a random intercept was used to test the association between the predictor variables (demographic characteristics, symptoms) with the outcome (assigned pain level). We used a mixed effects model because of the multiple observations (8 case scores) per respondent. We used the log-transformed pain level because pain scores were heavily skewed (Table 3). To describe the independent association between each symptom and pain levels in the mixed model, we report the slope of the regression line (β), and a p value. To determine the magnitude of variance in level of pain explained by each symptom, we calculated a pseudo-R2 using the univariate correlations.15
Table 3.
Mean level of pain and standard deviation (SD) according to family characteristics
| Number | Mean pain level (SD) |
P | |
|---|---|---|---|
| Gender of own child | |||
| Female | 23 | 22.2 (18.4) | 0.49 |
| Male | 36 | 28.9 (26.0) | |
| Race | |||
| African American | 41 | 25.0 (23.7) | 0.19 |
| Bi-racial | 4 | 28.8 (27.2) | |
| Caucasian | 13 | 31.0 (21.7) | |
| Education | |||
| College graduate | 13 | 39.8 (26.4) | 0.007 |
| HS or GED | 21 | 21.5 (21.0) | |
| Less than HS | 5 | 21.5 (21.6) | |
| Some college/tech school | 19 | 24.6 (21.0) | |
| Insurance | |||
| Private | 13 | 37.0 (25.3) | 0.001 |
| Public | 44 | 22.9 (22.2) | |
Results
69 parents participated in the study. 10 were excluded because they assigned a high level of pain to the case describing an asymptomatic child. Most parents were African American (71%), had public insurance (77%) and were not college graduates (79%).
Parents assigned higher pain scores to the cases with more symptoms: higher pain scores were assigned to the cases with 4 symptoms than to the cases with 3 symptoms (P<0.001). The case describing the asymptomatic child received the lowest pain scores.
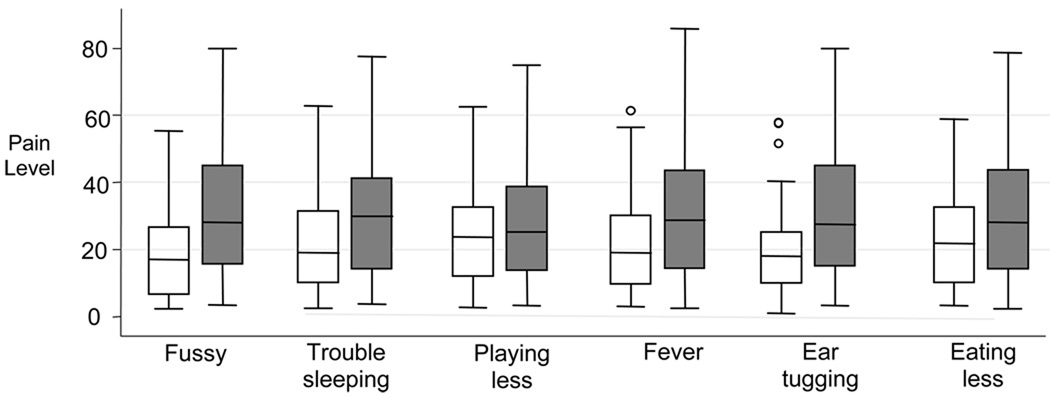
In univariate analysis, each of the 6 behaviors was associated with increased levels of pain (Figure 1, P < 0.0001 for each). On multivariate analysis, ear tugging and fussiness had the highest impact, whereas eating less and playing less had the least impact on perceived levels of pain (Table 2). Overall, the 6 symptoms explained 47% of the variance of the assigned level of pain.
Figure 1.
Effect of each symptom on the assigned pain level*
*Box plots comparing pain score in children with (gray boxes) and without (white boxes) the symptom in question. The median (horizontal line dividing each box) and the minimum and maximum values (represented by the edges of the box) are also shown.
Table 2.
Association between each symptom and assigned level of pain on multivariate analysis
| Symptom | β* | se(β)† | p-value | pseudo-R2‡ |
|---|---|---|---|---|
| Ear tugging | 0.948 | 0.111 | <0.001 | 0.115 |
| Fussiness | 0.879 | 0.113 | <0.001 | 0.098 |
| Sleeping difficulty | 0.767 | 0.115 | <0.001 | 0.075 |
| Fever | 0.749 | 0.115 | <0.001 | 0.072 |
| Eating less | 0.674 | 0.116 | <0.001 | 0.058 |
| Playing less | 0.642 | 0.117 | <0.001 | 0.053 |
β = Slope oF the regression line, symptoms with higher β coefficients have a larger impact on pain levels
se(β) = standard error of the β coefficients
Pseudo-R2 assesses the proportion of variation in outcome “explained” by the model.
Parent education and private insurance were associated with slightly higher reported levels of pain (see Table 3; P = 0.007 and P = 0.001, respectively). Adjusting for these variables in the multivariable model did not appreciably modify the magnitude or significance of the associations between each symptom and the assigned level of pain; ear tugging and fussiness remained the two most important factors regardless of level of parent education or insurance status. Race was not significantly associated with the level of pain (P = 0.19).
Discussion
Our results support the hypothesis that parents of children with AOM use information from child’s observable behaviors to determine their child’s level of pain. Although no symptom by itself dominated parental assessment, ear tugging and fussiness seem to be the most important symptoms in influencing perception of pain by parents.
Our findings also suggest that reporting of overall pain level by parents may depend not only on the symptoms present in the child, but also on a host of other child and parental factors. Maternal education and insurance status, characteristics that are frequently used as proxies for socioeconomic status appear to influence pain assessment. This is not entirely surprising because pain is ultimately a subjective construct which is therefore likely to be influenced by biological, psychological and social factors. Although the biopsychosocial model of pain is well accepted, there is little data in the pediatric literature linking socioeconomic status and the level of acute pain. Most of the studies examining the influence of psychosocial factors on pain levels were conducted among adults with chronic pain or among subjects who were exposed to experimentally induced pain.
This study has important implications for pain measurement in clinical trials that include preverbal children. Although it is attractive to simply ask parents to rate their child’s pain level on a 1-item severity scale, there has been evidence questioning the validity of this approach.16 Because of the myriad of behavioral and non-behavioral factors go into parental assessment of the overall pain level in preverbal children, it is unclear what such a global question is actually measuring. In this study, we have shown that observable behaviors only account for approximately 50% of the pain level. We have also shown that overall pain levels are associated with socioeconomic status. These findings, if corroborated by other studies, suggest that in the context of research, where the emphasis is on validity and reliability, the use of 1-item assessment of pain may not be appropriate. An alternative approach may be to focus on the measurement of the behaviors that are can be clearly observed and measured. Previous studies in adults have shown that direct observation of pain behaviors and self-reports of pain intensity are strongly correlated, especially in individuals with acute pain.7, 4 In a previous, and much larger study, we did not find any association between the reporting of symptom severity by parents from different socioeconomic classes or educational backgrounds.14 This suggests that reporting of the individual symptoms and pain behaviors is less prone to variability than reporting of a global pain level. In the most recent guidelines on the measurement of pain in pediatric clinical trials, all pain measures recommended were multi-item scales; use of global single item scales for the measurement of pain in preverbal children was not endorsed.9
In a clinical setting, we feel that asking parents about specific observable behaviors, such ear tugging and fussiness would nicely complement a general question about ear pain. This would ensure that clinicians are aware of the specific symptoms present and at the same time fully understand and address parental concerns about their child’s condition.
Our study was limited by the relatively small sample size and the homogeneous study population. In addition, parent’s response to scenarios about a hypothetical child may differ from their real life responses regarding their own child. Yet, we feel that we were able to convincingly show that parental assessment of the degree of otalgia is based on both behavioral cues as well as parental interpretation of these cues. The association between socioeconomic status and ear pain has not been previously reported and further research is warranted to confirm these findings and explore the reasons for these differences.
Acknowledgements
Dr. Shaikh had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Feng and Lin were supported in part by Grant Number UL1 RR024153 from the National Center for Research Resources (NCRR).
Footnotes
Conflict of Interest: The authors have no conflicts of interest to disclose.
Contributor Information
Diana H. Kearney, Email: diana.kearney@chp.edu.
D. Kathleen Colborn, Email: colbdk@chp.edu.
Tracy Balentine, Email: tracy.balentine@chp.edu.
Wentao Feng, Email: wentao.feng@novartis.com.
Yan Lin, Email: yal14@pitt.edu.
Alejandro Hoberman, Email: hoberman@chp.edu.
References
- 1.Del Castillo F, Corretger JM, Medina J, Rosell J, Cruz M. Acute otitis media in childhood: a study of 20,532 cases. Infection. 1995;23 Suppl 2:S70–S73. doi: 10.1007/BF01742987. [DOI] [PubMed] [Google Scholar]
- 2.Glasziou PP, Del Mar CB, Sanders SL, Hayem M. Antibiotics for acute otitis media in children. Cochrane Database of Systematic Reviews. 2004 doi: 10.1002/14651858.CD000219.pub2. CD000219. [DOI] [PubMed] [Google Scholar]
- 3.Heikkinen T, Ruuskanen O. Signs and symptoms predicting acute otitis media. Arch Pediatr Adolesc Med. 1995;149:26–29. doi: 10.1001/archpedi.1995.02170130028006. [DOI] [PubMed] [Google Scholar]
- 4.Horgas AL, Elliott AF, Marsiske M. Pain assessment in persons with dementia: relationship between self-report and behavioral observation. J Am Geriatr Soc. 2009;57:126–132. doi: 10.1111/j.1532-5415.2008.02071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaleida PH, Casselbrant ML, Rockette HE, Paradise JL, Bluestone CD, Blatter MM, Reisinger KS, Wald ER, Supance JS. Amoxicillin or myringotomy or both for acute otitis media: results of a randomized clinical trial. Pediatrics. 1991;87:466–474. [PubMed] [Google Scholar]
- 6.Kontiokari T, Koivunen P, Niemela M, Pokka T, Uhari M. Symptoms of acute otitis media. Pediatr Infect Dis J. 1998;17:676–679. doi: 10.1097/00006454-199808000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Labus JS, Keefe FJ, Jensen MP. Self-reports of pain intensity and direct observations of pain behavior: when are they correlated? Pain. 2003;102:109–124. doi: 10.1016/s0304-3959(02)00354-8. [DOI] [PubMed] [Google Scholar]
- 8.McCormick DP, Chonmaitree T, Pittman C, Saeed K, Friedman NR, Uchida T, Baldwin CD. Nonsevere acute otitis media: a clinical trial comparing outcomes of watchful waiting versus immediate antibiotic treatment. Pediatrics. 2005;115:1455–1465. doi: 10.1542/peds.2004-1665. [DOI] [PubMed] [Google Scholar]
- 9.McGrath PJ, Walco GA, Turk DC, Dworkin RH, Brown MT, Davidson K, Eccleston C, Finley GA, Goldschneider K, Haverkos L, Hertz SH, Ljungman G, Palermo T, Rappaport BA, Rhodes T, Schechter N, Scott J, Sethna N, Svensson OK, Stinson J, von Baeyer CL, Walker L, Weisman S, White RE, Zajicek A, Zeltzer L. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT recommendations. J Pain. 2008;9:771–783. doi: 10.1016/j.jpain.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Niemela M, Uhari M, Jounio-Ervasti K, Luotonen J, Alho OP, Vierimaa E. Lack of specific symptomatology in children with acute otitis media. Pediatr Infect Dis J. 1994;13:765–768. doi: 10.1097/00006454-199409000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Osman LM, McKenzie L, Cairns J, Friend JA, Godden DJ, Legge JS, Douglas JG. Patient weighting of importance of asthma symptoms. Thorax. 2001;56:138–142. doi: 10.1136/thorax.56.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rothman R, Owens T, Simel DL. Does this child have acute otitis media? JAMA. 2003;290:1633–1640. doi: 10.1001/jama.290.12.1633. [DOI] [PubMed] [Google Scholar]
- 13.Rovers MM, Glasziou P, Appelman CL, Burke P, McCormick DP, Damoiseaux RA, Little P, Le Saux N, Hoes AW. Predictors of pain and/or fever at 3 to 7 days for children with acute otitis media not treated initially with antibiotics: a meta-analysis of individual patient data. Pediatrics. 2007;119:579–585. doi: 10.1542/peds.2006-2092. [DOI] [PubMed] [Google Scholar]
- 14.Shaikh N, Hoberman A, Paradise JL, Wald ER, Switze GE, Kurs-Lasky M, Colborn DK, Kearney DH, Zoffel LM. Development and preliminary evaluation of a parent-reported outcome instrument for clinical trials in acute otitis media. Pediatr Infect Dis J. 2009;28:5–8. doi: 10.1097/INF.0b013e318185a387. [DOI] [PubMed] [Google Scholar]
- 15.Singer J, Willett J. Applied Longitudinal Data Analysis. Oxford University Press; 2003. [Google Scholar]
- 16.van Dijk M, Koot HM, Saad HH, Tibboel D, Passchier J. Observational visual analog scale in pediatric pain assessment: useful tool or good riddance? Clin J Pain. 2002;18:310–316. doi: 10.1097/00002508-200209000-00006. [DOI] [PubMed] [Google Scholar]