Abstract
In this paper, we report the clinical and molecular features of the distinct TGFBI (human transforming growth factor β-induced, OMIM No. 601692) gene-linked corneal dystrophy. Altogether, five pedigrees and ten unrelated individuals diagnosed as corneal dystrophy were recruited. Peripheral venous DNA was extracted, and then amplified by polymerase chain reaction (PCR) and scanned for mutation by single-stranded conformation polymorphism (SSCP). Direct DNA sequencing was used to analyze the mutations of the TGFBI gene. In our study, thirty patients from five pedigrees and ten sporadic patients were diagnosed as four TGFBI gene-linked corneal dystrophies of granular corneal dystrophy type I (GGCD I), Avellino corneal dystrophy (ACD), lattice corneal dystrophy type I (LCD I), and lattice corneal dystrophy type IIIA (LCD IIIA), and in total, seven disease-causing mutations, namely R555W, A546D, A546T, and T538P mutations in exon 12, R124H and R124C mutations in exon 4, and P501T mutation in exon 11, were identified, while four polymorphisms of V327V, L472L, F540F, and 1665–1666insC were screened in exons 8, 11, and 12. The study ascertained the tight genotype-phenotype relationship and confirmed the clinical and genetic features of four TGFBI gene-linked corneal dystrophies.
Keywords: TGFBI gene, Corneal dystrophy, Genotype, Phenotype, Mutation
1. Introduction
TGFBI (human transforming growth factor β-induced, OMIM No. 601692) gene-linked corneal dystrophy is one of the most common inherited corneal diseases. It is characterized by the extracellular deposition of insoluble protein in the cornea stroma (Biswell, 1999), which can present as amyloid, non-amyloid (granular), or both (Chakravarthi et al., 2005). Serious visual impairment can occur and a corneal transplantation may be required. The corneal lesion may recrudesce after the transplantation. In the past, the disease was usually classified based on the slit-lamp appearance, including the morphology of deposit, depth of corneal involvement, and the discoverers’ name. However, a misdiagnosis could be made if simply relying on clinical features.
Currently, genetics is a hot topic for both the ophthalmologist and geneticist. Corneal dystrophy caused by specific mutations in the human TGFBI gene has been categorized as granular corneal dystrophy type I (GGCD I), granular corneal dystrophy type II (GGCD II) or Avellino corneal dystrophy type (ACD), granular corneal dystrophy type III (GGCD III) or Reis-Bücklers corneal dystrophy type (RBCD), granular corneal dystrophy type IV (GGCD IV) or Thiel-Behnke corneal dystrophy type (TBCD), and lattice corneal dystrophy (LCD) types I, IIIA, I/IIIA, IIIB, and IVA (Clout and Hohenester, 2003; Zenteno et al., 2006). The gene includes 17 exon maps to 5q31.1 and exons 4, 11, 12, and 14 are the frequent sites of mutation (Korvatska et al., 1998). TGFBI gene-linked corneal dystrophies are autosomal dominant with high penetrance and show intra- or inter-familial variability of clinical phenotype (Klintworth, 1999). Generally, specific amino acid changes can result in specific phenotypes of corneal dystrophy as a genotype-phenotype correlation. Types GGCD I and LCD I are the most common phenotypes while R124 and R555 are the mutational hotspot genotypes in various populations. Many classic forms had been observed in patients belonging to different ethnic backgrounds: GGCD I/R555W, ACD/R124H, RBCD/R124L, TBCD/R555Q, LCD I/R124C, and so on. This study reports the clinical features, genetics, and gene mutations in patients with GGCD I, ACD, LCD I, and LCD IIIA.
2. Materials and methods
2.1. Subjects
The study was approved by the Institutional Review Board and Ethics Committee of Zhejiang University, China. Informed consent was obtained from all subjects before their participation. Altogether 30 patients from 5 pedigrees and 10 sporadic patients aged 19 to 71 years were recruited from the Eye Clinic of the First Affiliated Hospital, School of Medicine, Zhejiang University, China.
2.2. Clinical analysis
For the status of the corneas (affected or unaffected), slit-lamp examination was conducted to determine the corneal features for each subject, and the corneal lesions were photographed. The family history was collected and a pedigree chart was drawn. Fifty-three healthy subjects were used as controls.
2.3. Genetic analysis
Genomic DNA was obtained from peripheral venous blood using the salting-out method. Polymerase chain reaction (PCR) was performed to amplify the 17 exons of TGFBI gene as described previously (Korvatska et al., 1998; Munier et al., 2002). PCR products were analyzed in 2% (v/v) agarose gel from which the bands with the amplified templates were excised; then the PCR products were purified, denatured, and separated by electrophoresis on an 8% (v/v) single-stranded conformation polymorphism (SSCP) polyacrylamide gel containing 6% (v/v) glycerol or sucrose in 1× Tris-borate-EDTA (TBE) buffer at the correct temperature. The gels were silver stained and the PCR products with a mobility shift on each strand were purified and sequenced.
3. Results
3.1. Clinical results
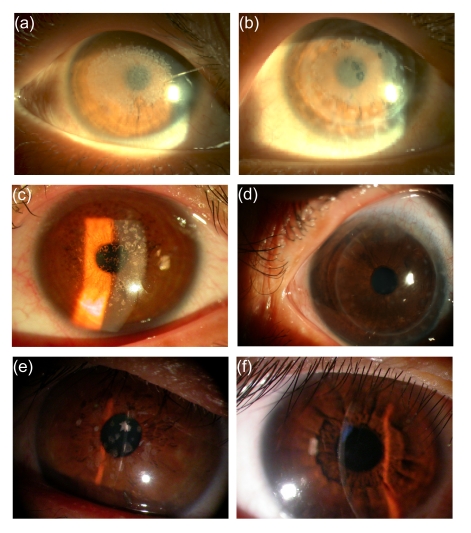
The 30 subjects from 5 pedigrees and 10 sporadic subjects were diagnosed as GGCD I (3 pedigrees and 4 sporadic subjects), ACD (1 pedigree and 2 sporadic subjects), LCD I (1 pedigree and 2 sporadic subjects), and LCD IIIA (2 sporadic subjects). No systemic diseases, extraocular congenital defects, ocular surgery history, trauma, or infection was found. After slit-lamp examination, 20 subjects showed bilateral gray-white granular opacities in the anterior stroma, diagnosed as GGCD I, while 4 subjects with ACD showed polymorphous opacities such as stellate and snowflake-like. Four subjects diagnosed as LCD I presented a network of linear opacities in the anterior stroma while two LCD IIIA subjects presented thicker and deeper linear opacities in the stroma (Table 1, Figs. 1 and 2).
Table 1.
Clinical information of 15 patients (probands) from 5 pedigrees and 10 sporadic subjects affected by GGCD I, ACD, LCD I/IIIA*
| Proband | Family history | Gender | Diagnosis | Age at diagnosis (year) | Keratoplasty/relapse |
| III2 (pedigree A) | Yes | Male | GGCD I | 19 | +/+ |
| III3 (pedigree B) | Yes | Male | GGCD I | 45 | +/+ |
| 3 (sporadic subject) | No | Female | GGCD I | 29 | −/− |
| 4 (sporadic subject) | No | Female | GGCD I | 23 | −/− |
| 5 (sporadic subject) | No | Male | GGCD I | 35 | −/− |
| I2 (pedigree C) | Yes | Female | GGCD I | 52 | −/− |
| 7 (sporadic subject) | No | Male | GGCD I | 36 | −/− |
| I1 (pedigree D) | Yes | Male | ACD | 62 | −/− |
| 9 (sporadic subject) | No | Male | ACD | 69 | −/− |
| 10 (sporadic subject) | No | Female | ACD | 33 | −/− |
| 11 (sporadic subject) | No | Female | LCD I | 24 | −/− |
| II2 (pedigree E) | Yes | Female | LCD I | 55 | +/+ |
| 13 (sporadic subject) | No | Female | LCD I | 26 | −/− |
| 14 (sporadic subject) | No | Female | LCD IIIA | 66 | −/− |
| 15 (sporadic subject) | No | Female | LCD IIIA | 71 | −/− |
The corneal phenotypes assessed by slit-lamp examination and the occurrence of keratoplasty and/or relapse of the CD are shown
Fig. 1.
Slit-lamp photographies of GGCD I, ACD, LCD I, and the relapse after the penetrating keratoplasty
(a) GGCD I from the right eye of the proband III2 in pedigree A with placoid opacities in subepithelial and anterior stromata of central cornea, and (b) relapse after keratoplasty of his left eye as he came to examine; (c) GGCD I from the right eye of the proband III3 in pedigree B with tiny “crumb-like” opacities in the anterior stroma, and (d) relapse after keratoplasty of the same eye; (e) ACD from the left eye of the proband I1 in pedigree D with thick macular, star-like opacities; (f) LCD I from the right eye of sporadic subject 11 with thin linear, branching deposits in the subepithelial and anterior stromata
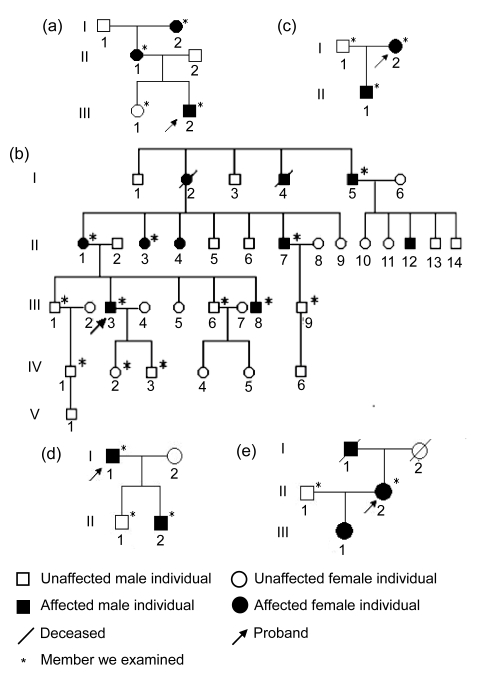
Fig. 2.
Generalogical charts of the five pedigrees
(a) Proband III2 and his mother, grandma affected by GGCD I; (b) Proband III3 and his relatives III8, II1, II3, II4, II7, II12, I2, I4, I5 affected by GGCD I; (c) Proband I2 and her son affected by GGCD I; (d) Proband I1 and his last son affected by ACD; (e) Proband II2 and her father, daughter affected by LCD I
3.2. Genotype-phenotype analysis
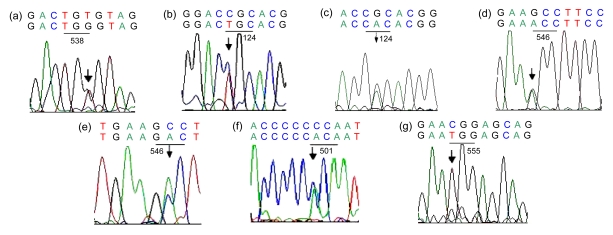
Direct sequencing in TGFBI exons 4, 8, 11, 12, 13, and 14 demonstrated seven heterozygous point mutations, including Arg124His (a G-to-A substitution at the second base position of codon 124 with ACD), Arg124Cys (a C-to-T substitution at the first base position of codon 124 with LCD I), Pro501Thr (a C-to-A substitution at the first base position of codon 501 with LCD IIIA), Thr538Pro (an A-to-C substitution at the first base position of codon 538, a novel mutation in pedigree E, with LCD I (Yu et al., 2006)), Gla546Asp (a C-to-A substitution at the second base position of codon 546; firstly reported to cause another phenotype as atypical GGCD I in pedigree B (Yu et al., 2008)), Gla546Thr (a G-to-A substitution at the first base position of codon 546 with LCD IIIA), and Arg555Trp (a C-to-T substitution at the first base position of codon 555 with GGCD I) (Table 2, Fig. 3). Meanwhile, four polymorphisms with isocoding mutations have been identified including GTA>GTG at codon 327 in exon 8 (V327V), CTC>CTT at codon 472 in exon 11 (L472L), TTT>TTC at codon 540 (F540F) in exon 12, and cytidine insertion between nucleotides 1665–1666 (1665–1666insC). No mutations were found in two sporadic patients. No additional pathogenetic nucleotide changes were detected in the remaining TGFBI exons analyzed.
Table 2.
Four types of corneal dystrophies and seven heterozygous point mutations
| Sporadic or pedigree | Phenotype | Genotype | Exon | Amino acid change |
| 1, 3–7 | GGCD I | R555W | 12 | CGG>TGG |
| 2 | GGCD I | A546D | 12 | GCC>GAC |
| 8–10 | ACD | R124H | 4 | CGC>CAC |
| 11, 13 | LCD I | R124C | 4 | CGC>TGC |
| 12 | LCD I | T538P | 12 | ACA>CCA |
| 14 | LCD IIIA | A546T | 12 | GCC>ACC |
| 15 | LCD IIIA | P501T | 11 | CCA>ACA |
Fig. 3.
Sequence chromatograms of the seven heterozygous point mutations
(a) TGT>TGG at codon 538 (exon 12) in pedigree E (II2) with LCD I (antisense strand), indicating an A-to-C substitution at the first nucleotide position (forward sequence); (b) CGC>TGC at codon 124 (exon 4) in sporadic subject 11 with LCD I; (c) CGC>CAC at codon 124 in pedigree D (I1) with ACD; (d) GCC>ACC at codon 546 (exon 12) in sporadic subject 14 with LCD IIIA; (e) GCC>GAC at codon 546 in pedigree B (III3) with GGCD I; (f) CCA>ACA at codon 501 (exon 11) in sporadic subject 15 with LCD IIIA; (g) CGG>TGG at codon 555 (exon 12) in pedigree A (III2) with CDGG I. The top lane is the normal sequence and the bottom lane is the mutated sequence. The arrows highlight the position of nucleotide substitution
4. Discussion
The TGFBI gene-linked corneal dystrophy is currently thought to be monogenic autosomal dominant with a high penetrance of 60% to 90%. The TGFBI gene was the first gene underlying corneal dystrophy reported by Munier et al. (1997). To date, about nine clinical types of CDs have been categorized and more than thirty-five CD-causing mutations in the TGFBI gene have been identified (Klintworth, 2003; Kannabiran and Klintworth, 2006). The TGFBI gene has 17 exons and exons 4, 11, 12, and 14 harbor the mutation hot spots (Korvatska et al., 1998). In our case, we have found seven missense mutations, namely R555W, A546D, R124H, R124C, T538P, P501T, and A546T, which were all located in these exons. Meanwhile, our study identified the definite relationships between three mutation types and the single nucleotide polymorphisms (SNPs), i.e., L472L (Yu et al., 2006) with P501T in exon 11, and F540F (Chen et al., 2005) with R555W and 1665–1666insC with A546D in exon 12. Finding more significant SNPs and the related monoploid type of the TGFBI gene mutation might be helpful in screening for corneal dystrophy. The TGFBI gene encodes the protein TGFBIp which contains a secretory signal, an N-terminal cysteine-rich (EMI) domain, four tandem repeats of fasciclin 1 (FAS1) domains homologous to fasciclin 1 protein, and a carboxy-terminal arginine-glycine-aspartic acid (RGD) sequence (Thapa et al., 2007). Most of the mutations were located in the amino acid R124 and FAS4 regions (Kim et al., 2002). For our cases, all mutations we found were located in the two regions, except the P501T of case 15 which was located in FAS3. In general, the particular mutation in TGFBI is linked to specific corneal dystrophy regardless of ethnic origin.
Our study demonstrated the close genotype-phenotype correlations among the four common corneal dystrophies, i.e., R555W and A546D with GGCD I, R124H with ACD, R124C and T538P with LCD I, A546T and P501T with LCD IIIA. In pedigree A, three patients from a three-generation pedigree appeared to have the typical clinical features of GGCD I in the first decade of life caused by R555W, and the opacities reoccurred in the proband three years after penetrating keratoplasty. In pedigree B, ten patients from five-generations were examined and found to be atypical GGCD I (Yu et al., 2008) with suffuse crumb-like and occasional linear opacities in the third decade of life caused by A546D, the proband’s son (IV3) of 16 years age showing no clinical symptom. We are following up this generation to learn more about the differences between the atypical phenotype and GGCD I and elucidate the underlying mechanism. In the ACD type caused by R124H, we found that the granular lesions usually appeared in the second decade of life and then were followed by the lattice lesions in advanced stages, causing the specific phenotypes different from those of GGCD I and LCD I. Clinically, the granular lesion was larger and less than that of GGCD I, while the lattice lesion was earlier and shallower than that of LCD I. The LCD I type lesions in our cases appeared early in the second decade of life with visual acuity changes and corneal erosions characterized by a fine network of linear opacities in the anterior stroma, whereas the LCD IIIA type lesions appeared in the sixth decade of life with thicker linear lesions in the deep stroma and without erosions. Dong et al. (2005) reported an atypical LCDi between the LCD I and LCD IIIA caused by the mutation H626R with asymmetric linear lesions. In our study, we also found that the same gene mutation might cause different corneal lesions. There were three kinds of mutations of R124 (R124C with LCD I, R124H with ACD, and R124L with RBCD). Case 9 with ACD in our study showed no irritation in the second stage and a late onset lesion of vision acuity, while case 11 with LCD I presented severe redness and pain, photophobia, and tearing symptoms due to the recurrent corneal erosions at an earlier age. Okada et al. (1998a) reported that the ocular irritation and decrease of visual acuity are least for ACD, less for LCD I, and greatest for RBCD. The clinical signs were also relevant to the homozygosis or heterozygosis of TGFBI, which was attributed to the dose effect of the mutant allele. In our cases, we provisionally had no relative discovery. Mashima et al. (1998) reported four severe cases of ACD with homozygous R124H, with onset in the first decade of life and corneal transplantation in the second decade of life. However, the disease recurred one year after the operation. Meanwhile, offspring of the consanguineous mating, who were homozygous for the mutation, showed a more severe phenotype than heterozygous relatives. Okada et al. (1998b) reported cases with the R555W mutation of GGCD I.
TGFBIp is found in TGFBI gene-linked corneal dystrophy as amyloid, non-amyloid (granular), or both. Although there have been some possible ideas being reported (Kim et al., 2000; Schmitt-Bernard et al., 2002; Thapa et al., 2007), the exact mechanisms of the corneal dystrophy are still unknown. In particular, an interesting phenomenon is that all the pathogenic TGFBI mutations reported are only linked to corneal dystrophy (Kochairi et al., 2006), although the TGFBIp is expressed in all organs other than the brain. Further research is needed to substantiate the cornea-specific physical or biochemical conditions, which might be responsible for the involvement of TGFBIp in the corneal lesion.
Over the past decades, corneal dystrophy has been classified based on the forms of corneal deposits or names of the finders. The TGFBI gene-linked corneal dystrophy lesions mainly include the granular and lattice types, as well as a lot of subtypes. Simply relying on the clinical features could lead to misdiagnosis. Understanding the relevant molecular genetics can be helpful to set up a new effective classification standard and to facilitate clinical diagnosis and treatment.
Footnotes
Project supported by the Ministry of Health Research Fund of China (No. WKJ2009-2-020) and the Science and Technology Specific Project of Zhejiang Province (No. 2009C03010-2), China
References
- 1.Biswell R. Cornea. In: Vaughan D, Asbury T, Riordan-Eva P, editors. General Ophthalmology. 15th Ed. Stamford: Appleton & Lange; 1999. pp. 119–141. [Google Scholar]
- 2.Chakravarthi S, Kannabiran C, Sridhar MS, Vemuganti GK. TGFBI gene mutations causing lattice and granular corneal dystrophies in Indian patients. Invest Ophthalmol Vis Sci. 2005;46(1):121–125. doi: 10.1167/iovs.04-0440. [DOI] [PubMed] [Google Scholar]
- 3.Chen LL, Yu P, Gu YS, Yang YH, Yan XY, Ge Z, Lv N, Guo L. Granular corneal dystrophy with Arg555Trp mutation in the BIGH3 gene. Chin Ophthal Res. 2005;23(1):60–62. (in Chinese) [Google Scholar]
- 4.Clout NJ, Hohenester E. A model of FAS1 domain 4 of the corneal protein β(ig)-h3 gives a clearer view on corneal dystrophies. Mol Vis. 2003;9(11):440–448. [PubMed] [Google Scholar]
- 5.Dong WL, Zou LH, Pang ZQ, Jin T, Yu J. Molecular-genetic analysis of Chinese patients with lattice corneal dystrophy in the BIGH3 gene. Chin J Ophthalmol. 2005;41(6):523–526. (in Chinese) [PubMed] [Google Scholar]
- 6.Kannabiran C, Klintworth GK. TGFBI gene mutations in corneal dystrophies. Hum Mutat. 2006;27(7):615–625. doi: 10.1002/humu.20334. [DOI] [PubMed] [Google Scholar]
- 7.Kim JE, Kim EH, Han EH, Park RW, Park IH, Jun SH, Kim JC, Young MF, Kim IS. A TGF-β-inducible cell adhesion molecule, β(ig)-h3, is downregulated in melorheostosis and involved in osteogenesis. J Cell Biochem. 2000;77(2):169–178. doi: 10.1002/(SICI)1097-4644(20000501)77:2<169::AID-JCB1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 8.Kim JE, Park RW, Cboi JY, Bae YC, Kim KS, Joo CK, Kim IS. Molecular properties of wild-type and mutant BIGH3 proteins. Invest Ophthalmol Vis Sci. 2002;43(3):656–661. [PubMed] [Google Scholar]
- 9.Klintworth GK. Advances in the molecular genetics of corneal dystrophies. Am J Ophthalmol. 1999;128(6):747–754. doi: 10.1016/S0002-9394(99)00358-X. [DOI] [PubMed] [Google Scholar]
- 10.Klintworth GK. The molecular genetics of corneal dystrophies—current status. Front Biosci. 2003;8(1-3):d687–d713. doi: 10.2741/1018. [DOI] [PubMed] [Google Scholar]
- 11.Kochairi IE, Letovanec I, Uffer S, Munier FL, Chaubert P, Schorderet DF. Systemic investigation of kerato-epithelin deposits in TGFBI/BIGH3-related corneal dystrophy. Mol Vis. 2006;12(10):461–466. [PubMed] [Google Scholar]
- 12.Korvatska E, Munier FL, Djemai A, Wang MX, Frueh B, Chiou AGY, Uffer S, Ballestrazzi E, Braunstein RE, Forster RK, et al. Mutation hot spots in 5q31-linked corneal dystrophies. Am J Hum Genet. 1998;62(2):320–324. doi: 10.1086/301720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mashima Y, Konishi M, Nakamura Y, Imamura Y, Yamada M, Ogata T, Kudoh J, Shimizu N. Severe form of juvenile corneal dystrophy with homozygous R124H mutation in the kerato-epithelin gene in five Japanese patients. Br J Ophthalmol. 1998;82(11):1280–1284. doi: 10.1136/bjo.82.11.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munier FL, Korvatska E, Djema A, le Paslier D, Zografos L, Pescia G, Schorderet DF. Kerato-epithelin mutations in four 5q31-linked corneal dystrophies. Nat Genet. 1997;15(3):247–251. doi: 10.1038/ng0397-247. [DOI] [PubMed] [Google Scholar]
- 15.Munier FL, Frueh BE, Othenin-Girard P, Uffer S, Cousin P, Wang MX, Héon E, Black GC, Blasi MA, Ballestrazzi E, et al. BIGH3 mutation spectrum in corneal dystrophies. Invest Ophthalmol Vis Sci. 2002;43(4):949–954. [PubMed] [Google Scholar]
- 16.Okada M, Yamamoto S, Tsujikawa M, Watanabe H, Inoue Y, Maeda N, Shimomura Y, Nishida K, Quantock AJ, Kinoshita S, et al. Two distinct kerato-epithelin mutations in Reis-Bucklers corneal dystrophy. Am J Ophthalmol. 1998;26(4):535–542. doi: 10.1016/S0002-9394(98)00135-4. [DOI] [PubMed] [Google Scholar]
- 17.Okada M, Yamamoto S, Watanabe H, Inoue Y, Tsujikawa M, Maeda N, Shimomura Y, Nishida K, Kinoshita S, Tano Y. Granular corneal dystrophy with homozygous mutations in the kerato-epithelin gene. Am J Ophthalmol. 1998;126(2):169–176. doi: 10.1016/S0002-9394(98)00075-0. [DOI] [PubMed] [Google Scholar]
- 18.Schmitt-Bernard CF, Chavanieu A, Herrada G, Subra G, Arnaud B, Demaille JG, Calas B, Argiles A. BIGH3 (TGFBI) Arg124 mutations influence the amyloid conversion of related peptides in vitro: implications in the BIGH3-linked corneal dystrophies. Eur J Biochem. 2002;269(21):5149–5156. doi: 10.1046/j.1432-1033.2002.03205.x. [DOI] [PubMed] [Google Scholar]
- 19.Thapa N, Lee BH, Kim IS. TGFBIp/βig-h3 protein: a versatile matrix molecular induced by TGF-β. Int J Biochem Cell Biol. 2007;39(12):2183–2194. doi: 10.1016/j.biocel.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Yu P, Gu YS, Yang YH, Yan XY, Chen LL, Ge Z, Qi M, Si JM, Guo L. A clinical and molecular-genetic analysis of Chinese patients with lattice corneal dystrophy and novel Thr538Pro mutation in the TGFBI (BIGH3) gene. J Genet. 2006;85(1):73–76. doi: 10.1007/BF02728974. [DOI] [PubMed] [Google Scholar]
- 21.Yu P, Gu YS, Jin F, Hu RR, Chen LL, Yan XY, Yang YH, Qi M. p.Ala546>Asp and Arg555>Trp mutations of TGFBI gene and their clinical manifestations in two large Chinese families with granular corneal dystrophy type I. Genetic Testing. 2008;12(3):421–426. doi: 10.1089/gte.2008.0005. [DOI] [PubMed] [Google Scholar]
- 22.Zenteno JC, Ramirez-Miranda A, Santacruz-Valdes C, Suarez-Sanchez R. Expanding the mutational spectrum in TGFBI-linked corneal dystrophies: identification of a novel and unusual mutation (Val113Ile) in a family with granular dystrophy. Mol Vis. 2006;12(10):331–335. [PubMed] [Google Scholar]