Abstract
Objective
To evaluate the performance of a novel biomarker, a disintegrin and metalloprotease -12 (ADAM-12), to differentiate an ectopic pregnancy (EP) from normal intrauterine pregnancies (IUP).
Design
Case-control study
Setting
Three urban academic centers
Patients
Women who presented to the emergency room with pain or bleeding in the first trimester of pregnancy
Intervention
Sera from women with diagnosed EP or intrauterine pregnancy (IUP) were evaluated via proteomics and an ADAM-12 dissociation-enhanced lanthanide fluoroimmunoassay
Main outcome measures
Differences between groups, area under the receiver operating curve, sensitivity and specificity
Results
Via a proteomics evaluation, we found a statistically significant decrease in ADAM-12 in the sera of patients with EP, which we confirmed in a larger group of 199 patients (median IUP 18.6ng/ml versus median EP 2.5ng/ml, p <0.0001) with good discrimination between the groups as assessed by receiver operating characteristics (AUC=0.82). At a low cut-point, the sensitivity was 70% and specificity 84%, but at a higher cut-point optimizing sensitivity, the ADAM-12 test demonstrated a sensitivity of 97%.
Conclusions
ADAM-12 is a promising marker for the diagnosis of EP in symptomatic first trimester women, validating the proteomics findings. Further studies on additional patient populations and in combination with other biomarkers are needed.
Key terms: ADAM-12, ectopic pregnancy, proteomics
INTRODUCTION
Ectopic pregnancy (EP) is major cause of maternal morbidity and is responsible for 6% of pregnancy deaths.(1) There is no single test for its early diagnosis and treatment, and current diagnosis relies on serial hCG levels and ultrasound when the location of the pregnancy is unclear on initial presentation.(2) A biomarker is a molecule produced by an affected individual that signals a specific exposure or disease state. A biomarker can be used for early diagnosis of a disease (3–4). The identification and development of a biomarker has distinct phases. (3, 5–7) The first phase is that of a preclinical exploration to identify promising markers. The second phase is the establishment of a clinical assay to be used on a larger scale. Phase III is testing the utility of the biomarker often with a longitudinal or retrospective cohort.(3, 5–6) Multiple biomarkers have been examined as tools for differentiating EP from IUP with limited clinical utility (8).
Although hypothesis-driven testing for differences between normal and abnormal pregnancy has been the traditional method to search for new markers, more recently researchers have begun to use genomics (9–10) and proteomics (6) for unbiased biomarker discovery. In this study, we selected a novel marker, a disintegrin and metalloprotease -12 (ADAM-12), from a proteomics evaluation of serum from women with EP and IUPs and evaluated its performance to differentiate EP from IUP in a large group of women.
MATERIALS AND METHODS
The study was approved by the institutional review boards of the University of Pennsylvania, University of Southern California and University of Miami.
Subjects
Cases and controls were retrospectively selected from amongst serum collected as part of the Ectopic Pregnancy Biomarkers Bank protocol between July, 2000 and October, 2005 for the proteomics evaluation and between September, 2000 and April, 2009 for the ADAM-12 evaluation. Subjects were women who presented to emergency rooms at one of the three participating sites with pain and/or bleeding and a positive pregnancy test. All the serum in the study was collected at the same time the patient presented for clinical care, and per the IRB protocol, informed consent was obtained by all patients whom the study staff could reach prior to the clinical venipuncture. Patients ultimately diagnosed with live IUP (less than 12 weeks) by ultrasound with fetal heart motion or diagnosed EP was considered eligible for the study. A diagnosis of EP was confirmed by visualization of an ectopic gestation using ultrasound or laparoscopy or with an increase in hCG after uterine evacuation when a ectopic gestation was not visualized.
Serum samples were collected at the initial visit before treatment by peripheral venous puncture, often before a definitive diagnosis was made. The serum was separated into aliquot tubes and stored at −70° to −80°C until assays were performed. If the clinician was unable to make a diagnosis on this first visit, the patient was followed until a diagnosis of a viable IUP or EP was confirmed. Information on last menstrual period, race, ethnicity, and maternal age were collected at the time of initial visit. Serum samples from the University of Southern California and University of Miami were shipped to the University of Pennsylvania for storage.
Cases and controls were selected that had enough volume to compete panned assays, had a minimum of missing clinical information and were similar in gestational age. This was not a prospective study.
Proteomics
An in-depth, label-free quantitative serum proteomics comparison of pooled samples from EP and IUP patients was conducted, as described in detail in a separate manuscript (Beer et al, submitted to Journal of Proteomics Research), was conducted to find novel biomarkers for ectopic pregnancy. Briefly, three pools of sera from three patients in each group (IUP and EP) were immunodepleted of the 20 most abundant serum proteins, the depleted fraction was separated on 1-D SDS gels, each lane was sliced into 20 fractions, and each fraction was digested with trypsin. All tryptic digests were analyzed by liquid chromatography and tandem mass spectrometry (LC-MS/MS) on an LTQ Orbitrap XL (Thermo) mass spectrometer, and data were quantitatively compared using the Rosetta Elucidator® label-free pattern analysis software to identify significant differences between EP patients and IUP controls. A number of candidate biomarkers were identified, including previously reported possible EP biomarkers and novel candidate biomarkers. The levels of selected candidate biomarkers of interest in serum pools and individual patient samples were verified using multiple reaction monitoring (MRM), which is an independent targeted mass spectrometry-based, quantitative assay, where levels of multiple peptides from the protein of interest serve as surrogates of the protein amount. After discovery of a potential biomarker, the next step in development is to assess it ability to distinguish disease state in a separate population.
ADAM-12 Assay
For assessment of validation of the novel biomarker, assays Immunoassays were conducted at the Penn Clinical and Translational Research Center. ADAM-12 concentrations were determined by a dissociation-enhanced lanthanide fluoroimmunoassay (DELFIA) platform DELFIA/AutoDELFIA ADAM-12 research kit (PerkinElmer, Turku, Finland). The lower limit of detection for ADAM-12 was 2.5 ng/mL, and the interassay coefficients of variation were 6.11% and 7.70% for low and high controls, respectively. Values below detection thresholds were given the detection threshold value in analyses.
Statistical Analyses
Baseline characteristics were compared using 2 sample t-test for continuous variables and Pearson chi-square analysis or Fisher’s exact test for categorical variables. Intensity values for individual peptides from the MRM assay were normalized and averaged to determine relative amounts of the protein of interest in different serum samples. hCG and biomarker levels were analyzed nonparametrically by the Wilcoxon Rank Sum test and are presented as median and range. Correlations were presented as Spearman’s rank correlation coefficients. 'Area under the receiver operating characteristic' curves (AUC) were calculated to assess the discrimination for each biomarker. Performance of the biomarkers was examined both individually and in combination using Classification and regression tree (CART). CART is a nonparametric statistical analysis based on recursive partitioning with a binary split of the biomarkers at a cut-point determined by the software. The choice of biomarker for each node is dependent on the variable that minimizes misclassification, given user specified costs for each type of error, for that node prior to splitting the dataset and then reanalyzing the subgroups to assess for the next best split. The performance of the trees in CART was assessed via 10-fold cross validations. Trees optimizing sensitivity were created by adjusting the cost of misclassifying an EP relative to an IUP, while trees optimizing specificity were created by adjusting the cost of misclassifying an IUP as an EP prior to tree development. Sensitivity and specificity were calculated at the optimal cut-point, where misclassification of the groups was minimized. Sensitivity and specificity values were compared via Pearson chi-square analysis or Fisher’s exact test, where appropriate. Two-sided 95% confidence intervals were calculated except where values were 100%, in which case a one-sided 97.5% confidence interval was presented.
All statistics were performed using either CART 6.0 (Salford Systems, San Diego, CA) or STATA 11.0 (StataCorp, College Station, TX). Statistical significance was defined as a p-value less than 0.05.
RESULTS
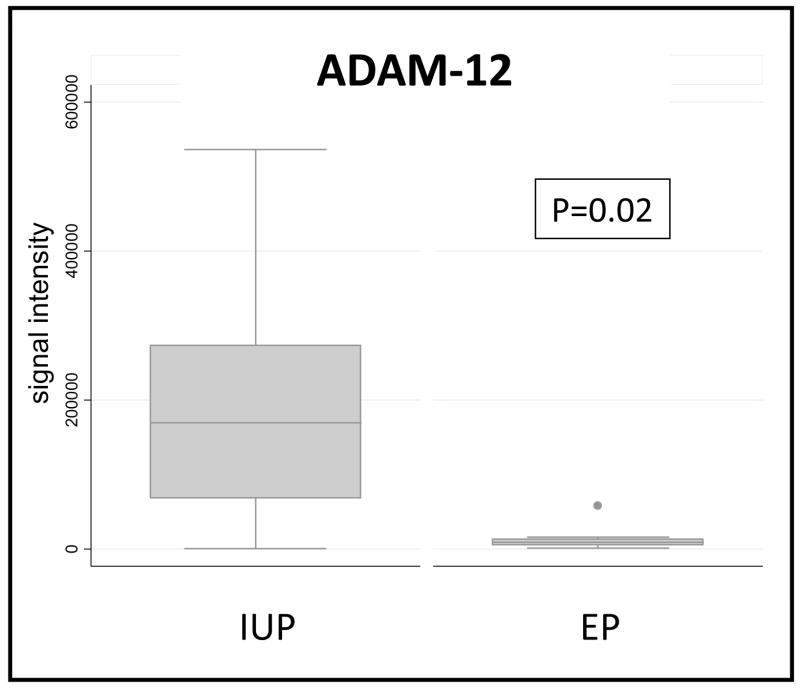
For the initial proteomics study, serum from 9 women with IUP and 9 women with EP collected at the University of Pennsylvania were studied. There were no significant differences in maternal age. A promising novel candidate biomarker for EP that emerged from this discovery study was ADAM-12. The quantitative MRM data for three ADAM-12 peptides in individualized serum samples that comprised the pools used for discovery are shown in Figure 1. The AUC from this initial dataset was 0.81 for ADAM-12. Picking a cut-point that minimizes misclassification between the groups, the specificity was 78% for ADAM-12, with a sensitivity of 100%. Combining the ADAM-12 results with values of two known biomarkers (PAEP and CHS-1) using CART, we were unable to achieve better discrimination. ADAM-12 was highly correlated with CSH1 (rho=0.90, p<0.0001), although PAEP was not significantly correlated with either CSH1 (rho=0.41, p=0.09) nor ADAM-12 (rho=0.33, p=0.19).
Figure 1.
Box plot of ADAM-12 peptides from proteomics analysis using MRM quantification and statistical significance determined by Wilcoxon-Rank sum test.
Based upon these results, ADAM-12 was selected for further evaluation in serum from 99 women with EP and 100 women with IUP using a dissociation-enhanced lanthanide fluoroimmunoassay (DELFIA). Subject characteristics for the much larger independent cohort are shown in Table 1. There were no significant differences in maternal age, gestational age, race, ethnicity, site, or time frame of collection between the cases and controls. Gestational age was missing in 19/99 women in the EP group due to an unknown last menstrual period. The level of hCG was higher in IUP group (7,586mIU/ml) compared to the EP group (1,150mIU/ml, p<0.0001). (Table 1)
TABLE 1.
SUBJECT CHARACTERISTICS FOR ADAM-12 ASSAY
| Intrauterine Pregnancy (n) | Intrauterine Pregnancy value | Ectopic Pregnancy (n) | Ectopic Pregnancy value | P | |
|---|---|---|---|---|---|
| Age (years)a | 98 | 27.51±6.70 | 98 | 29.02±6.11 | 0.101c |
| GA (days)a | 100 | 48.80±12.34 | 80 | 45.14±19.07 | 0.140c |
| Beta-HCG (mIU/ml)b | 98 | 7,586 (47–36,589) | 99 | 1,150 (22–29,323) | <0.0001d |
| Race | 97 | 96 | 0.888e | ||
| White | 63 | 65% | 63 | 65% | |
| Black | 27 | 28% | 28 | 29% | |
| Other | 7 | 7% | 5 | 5% | |
| Ethnicity- Hispanic | 58/97 | 50% | 57/96 | 50% | 0.953f |
| Site | 100 | 99 | 0.744f | ||
| Penn | 22 | 22% | 18 | 18% | |
| Miami | 42 | 42% | 46 | 46% | |
| USC | 36 | 36% | 35 | 35% | |
| Year | 100 | 99 | 0.400e | ||
| 2000–2003 | 13 | 13% | 7 | 7% | |
| 2004–2006 | 29 | 29% | 30 | 30% | |
| 2007–2009 | 58 | 58% | 62 | 63% |
Mean±SD
Median (range)
Two-sample t-test
Wilcoxon-Rank sum test
Fisher’s exact test
Pearson Chi-square test
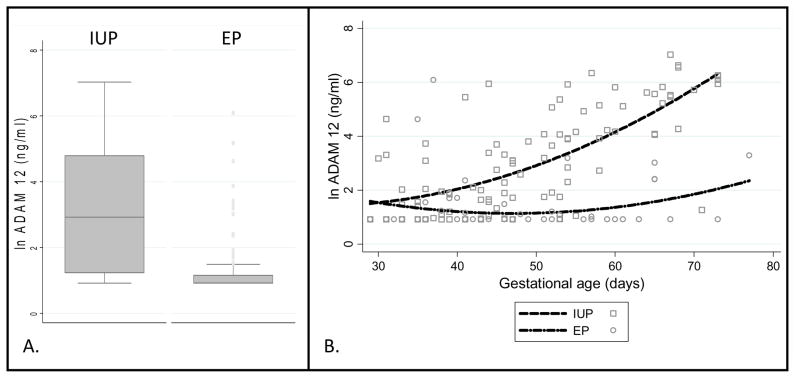
We again found a statistically significant decrease in ADAM-12 in the EP group [mean 11.7ng/ml±48.2; median 2.5ng/ml (range 2.5–440)] compared to the IUP group [mean 115.4ng/ml±214.1; median 18.6ng/ml (range 2.5–1131), p <0.0001)] (Figure 2). There was good discrimination between the groups as assessed by receiver operating characteristics (AUC=0.82). Whereas only 16/100 IUPs were below the minimum detectable limit, the majority of the patients with an EP (68/99) were below the sensitivity for the assay.
Figure 2.
A. Box plot of natural log-transformed ADAM-12 assay in 99 women with EP and 100 women with IUP.
B. Graph of natural log-transformed ADAM-12 levels between 4 and 12 weeks gestational age.
We examined the sensitivity and specificity of the test at 3 cut-points, for the entire group and for subgroups stratified by gestational age and stratified by hCG level (Table 2). For all comparisons, specificity was maximized at the lowest cut-point and sensitivity was maximized at higher cut-points. For the group as a whole, as the cut-point was elevated from 2.53 to 48.49, the sensitivity increased (70% vs. 97%: p < 0.001) while the specificity decreased (84% vs. 37%: p <0.001). The same change in cut point resulted in a decrease in accuracy (77% vs. 67%: p=0.03).
TABLE 2.
SENSTIVITY AND SPECIFICTY OF ADAM-12 TEST
| Group | EP | IUP | Cut-point 2.53 | Cut-point 6.81 | Cut-point 48.49 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | n | Sensitivity % (CI)a | Specificity % (CI)a | Accuracy % (CI)a | Sensitivity % (CI)a | Specificity % (CI)a | Accuracy % (CI)a | Sensitivity % (CI)a | Specificity % (CI)a | Accuracy % (CI)a | |
| Whole group | 99 | 100 | 70 (60–79) | 84 (75–91) | 77 (70–83) | 89 (81–94) | 62 (52–72) | 75 (69–81) | 97 (91–99) | 37 (28–47) | 67 (60–73) |
| < 7 weeks | 53 | 54 | 75 (62–86) | 70 (56–82) | 73 (63–81) | 92 (82–98) | 41 (28–55) | 66 (57–75) | 96 (87–100) | 7 (2–18) | 52 (42–62) |
| ≥ 7 weeks | 27 | 46 | 59 (39–78) | 100 (93–100) | 85 (75–92) | 78 (58–91) | 87 (74–95) | 84 (73–91) | 100 (87–100) | 72 (57–84) | 82 (71–90) |
| < 2000 | 59 | 19 | 83 (71–92) | 53 (29–76) | 76 (65–85) | 98 (91–100) | 32 (13–57) | 82 (72–90) | 100 (94–100) | 21 (6–46) | 81 (70–89) |
| ≥ 2000 | 40 | 79 | 50 (34–66) | 91 (83–96) | 77 (69–84) | 75 (59–87) | 68 (57–78) | 71 (62–79) | 93 (80–98) | 39 (28–51) | 57 (48–66) |
Two-sided 95% confidence interval (CI) are presented ,except where values equal 100%, in which case a one-sided 97.5% CI is presented
Dichotomizing the samples by gestational age at 7 weeks demonstrated that the specificity of the test is greater at a gestational age of ≥7 weeks than <7 weeks for all three cut-points (100% vs. 70%, p<0.001 for cut-point 2.53, 87% vs. 41%, p<0.001 for cut-point 6.81; and 72% vs. 7%, p<0.001 for cut-point 48.49). There was no statistically significant difference in the sensitivity between the higher and lower gestational age groups (59% vs. 75%, p=0.14 for cut-point 2.53, 78% vs. 92%, p=0.08 for cut-point 6.81 and 100% vs. 96%, p=0.55 for cut-point 48.49). Accuracy was not significantly different between the high and low gestational ages at a lowest cut-point (85% vs. 73%, p=0.06), but was significantly higher in gestational age ≥ 7 weeks as compared to <7 weeks at a cut-point of 48.49 (82% vs. 52%, p<0.001).
Dichotomized at an hCG level of 2000, ADAM-12 demonstrated higher specificity with higher hCG levels. The specificity was higher for hCG ≥2000 than hCG<2000 at cut-point 2.53 and 6.81 (91% vs. 53%, p<0.001 and 68% vs. 32%, p=0.004, respectively). The sensitivity, however, was higher at hCG <2000 compared with ≥2000 at cut-points of 2.53 and 6.81 (83% vs. 50%, p=0.001 and 98% vs. 75%, p<0.001, respectively). The extreme cut-point of 48.49, which optimized sensitivity did not demonstrate significant differences between either sensitivity or specificity between the groups (100% for hCG <2000 versus 93% > 2000, p=0.06 and 21% vs. 39%, p=0.19, respectively. Accuracy was not different at the low cut-point (76% for hcg <2000 vs. 77% for hCG ≥2000, p=0.79), but was significantly higher at hCG levels <2000 vs. ≥2000 at the highest cut-point (81% vs. 57%, p=0.001)
Correlation between ADAM-12 levels and both gestational age and hCG levels were performed in the overall IUP and EP groups. ADAM-12 was significantly correlated with gestational age in the IUP group (rho=0.66, p<0.0001), but not in the EP group (rho=0.20, p=0.07). Graphing the 2 groups from four to 12 weeks, ADAM-12 levels rise in the IUP group as EP levels remain suppressed with increasing gestational age (Figure 2). ADAM-12 was more weakly, but significantly correlated with hCG in both the IUP group (rho=0.53, p<0.0001) and the EP group (0.50, p<0.0001).
DISCUSSION
There is a great need for biomarkers of important clinical conditions such as ectopic pregnancy. Via an unbiased proteomics discovery of potential novel biomarkers, we identified multiple proteins in a small group of patients which may be useful in diagnosing patients with EP. One of the most promising, novel candidate biomarkers was ADAM-12, which was selected for further evaluation in a larger group of women and found to be significantly decreased in EP over IUP. Because a DELFIA assay is available for ADAM-12 we were able to validate our discovery in a separate case control study. Our data confirm the value of ADAM-12 as a potential biomarker as we demonstrated that it can discriminate an EP from an IUP with a sensitivity of 70% and specificity of 84%. Choosing a higher cut-point, we optimized sensitivity to 97% (with a lower specificity). This marker performed better in women ≥ 7 weeks gestational age, with 100% specificity and 59% sensitivity at a low-cut-point, and 100% sensitivity and 72% specificity at a higher cut-point.
ADAM-12, which has both an adhesion and protease domain, plays a role in myoblast fusion (12-14) as well as giant cell macrophage and osteoclast formation in bone (15). In humans, ADAM-12 has a secreted form which is expressed in placenta, and potently provokes myogenesis.(12) In first-trimester placentas, ADAM-12 is localized to the cytotrophoblasts as well as the apical side of the synctiotrophoblasts.(16) Given its localization and role in cell-fusion in other tissues, it has been postulated to play a role in syncytial fusion in the trophoblast.(16) ADAM-12 has been studied as a first-trimester marker for prediction of small-for-gestational-age fetuses (17–19), aneuploidy (20–26), and preeclampsia. (17, 27–28) If ADAM-12 is involved in the normal implantation of pregnancy, and decreased levels are a harbinger of an abnormal pregnancy or the abnormal implantation of pregnancy, then decreased levels in ectopic pregnancy may be biologically plausible.
Prior studies have demonstrated that maternal serum ADAM-12 levels increase with gestational age (21–22, 29), but there is a paucity of information on normal pregnancies prior to 6–7 weeks, and no information on levels in EP. In this study, we also found that ADAM-12 levels positively correlated with gestational age in the IUP group, but not the EP group. The increase in specificity at higher gestational age and hCG levels is likely due to the rise of ADAM-12 levels in the IUP group without a corresponding rise in EP with increasing gestational age. The increased sensitivity levels at lower hCG levels in all but the group with near-perfect sensitivity (cut-point 48.49) may be a reflection of the weak, but significant correlation of EPs with hCG. Therefore, the ADAM-12 test would be more sensitive in the group of EPs with lower hCG levels, irrespective of gestational age.
Strengths of this study include the use of proteomics to select novel biomarkers, the large sample size from multiple centers, and the study population including only symptomatic women at risk for EP. Limitations of this study include the length of time over which specimens were collected and stored. Although there were no differences between groups in terms of timeframe of collection, it is not yet known how storage can affect the levels of ADAM-12 over the course of several years. Previous studies on ADAM-12 stability have not looked at prolonged storage at −80°C directly, but it is reassuring that ADAM-12 is stable in serum after six months of storage at −20°C (21) and after multiple freeze-thaw cycles ( 21, 30), despite its considerably increased degradation at room and refrigerator temperatures.(21, 30) Further, our study did not include women with miscarriages, and how ADAM-12 will perform in differentiating EPs from miscarriages cannot be assessed in the current study.
The diagnosis of EP in early pregnancy requires both excellent sensitivity and specificity given that a false negative could lead to serious morbidity and mortality and a false positive could result in interruption of a potentially desired normal pregnancy. As no single marker has been consistently demonstrated to have both superior sensitivity and specificity, combining markers into one test is a possible solution. Algorithms combining multiple markers into a screening test with enhanced accuracy have been described for other diseases, such as endometriosis.(31–32) Further, combining markers into one test has been suggested as a possible solution for the diagnosis of ectopic pregnancy.(11, 33) Hence, Even if ADAM-12 does not prove to be a useful marker for ectopic pregnancy in isolation, it may be a useful component in a multiple-marker test.
In conclusion, this study demonstrates validation of a serum proteomic discovery and verification study by, identifying ADAM-12 as a potential novel marker for evaluating women with symptomatic first trimester pregnancies. Although currently being investigated as a predictor for pregnancy complications and aneuploidy, it has never been proposed in the literature as a diagnostic marker for ectopic pregnancy. Further evaluation of early pregnancy with ADAM-12, both singly and in combination with other diagnostic markers, may prove useful in developing an accurate test for the diagnosis of ectopic pregnancy.
Acknowledgments
We would like to thank Dr Shiv Kapoor for assistance with the ADAM-12 assay.
Funding/Support: This work was supported by the following grants: R01-HD036455 (KB, MDS), K24HD060687 (KB) and T32-HD007440 (MR).
Footnotes
Financial Disclosures: All of the authors report no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ectopic pregnancy--United States, 1990-1992. MMWR Morb Mortal Wkly Rep. 1995;44:46–48. [PubMed] [Google Scholar]
- 2.Barnhart KT. Clinical practice. Ectopic pregnancy. N Engl J Med. 2009;361:379–387. doi: 10.1056/NEJMcp0810384. [DOI] [PubMed] [Google Scholar]
- 3.Cartwright J, Duncan WC, Critchley HO, Horne AW. Serum biomarkers of tubal ectopic pregnancy: current candidates and future possibilities. Reproduction. 2009;138:9–22. doi: 10.1530/REP-09-0060. [DOI] [PubMed] [Google Scholar]
- 4.Horne AW, Duncan WC, King AE, Burgess S, Lourenco PC, Cornes P, et al. Endometrial cysteine-rich secretory protein 3 is inhibited by human chorionic gonadotrophin, and is increased in the decidua of tubal ectopic pregnancy. Mol Hum Reprod. 2009;15:287–294. doi: 10.1093/molehr/gap019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horne AW, van den Driesche S, King AE, Burgess S, Myers M, Ludlow H, et al. Endometrial inhibin/activin beta-B subunit expression is related to decidualization and is reduced in tubal ectopic pregnancy. J Clin Endocrinol Metab. 2008;93:2375–2382. doi: 10.1210/jc.2008-0136. [DOI] [PubMed] [Google Scholar]
- 6.Gerton GL, Fan XJ, Chittams J, Sammel M, Hummel A, Strauss JF, et al. A serum proteomics approach to the diagnosis of ectopic pregnancy. Ann N Y Acad Sci. 2004;1022:306–316. doi: 10.1196/annals.1318.046. [DOI] [PubMed] [Google Scholar]
- 7.Bonassi S, Neri M, Puntoni R. Validation of biomarkers as early predictors of disease. Mutat Res. 2001;480–481:349–358. doi: 10.1016/s0027-5107(01)00194-4. [DOI] [PubMed] [Google Scholar]
- 8.McMichael AJHA. The use of biological markers as predictive early-outcome measures in epidemiological research. In: Toniolo PBP, Shuker DEG, Rothman N, Hulka B, Pearce N, editors. Application of biomarkers in cancer epidemiology. Carey; NC Lyon: 1997. pp. 281–289. [PubMed] [Google Scholar]
- 9.Hall JA, Brown R, Paul J. An exploration into study design for biomarker identification: issues and recommendations. Cancer Genomics Proteomics. 2007;4:111–119. [PubMed] [Google Scholar]
- 10.Pepe MS, Etzioni R, Feng Z, Potter JD, Thompson ML, Thornquist M, et al. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst. 2001;93:1054–1061. doi: 10.1093/jnci/93.14.1054. [DOI] [PubMed] [Google Scholar]
- 11.Rothman N, Stewart WF, Schulte PA. Incorporating biomarkers into cancer epidemiology: a matrix of biomarker and study design categories. Cancer Epidemiol Biomarkers Prev. 1995;4:301–311. [PubMed] [Google Scholar]
- 12.Gilpin BJ, Loechel F, Mattei MG, Engvall E, Albrechtsen R, Wewer UM. A novel, secreted form of human ADAM 12 (meltrin alpha) provokes myogenesis in vivo. J Biol Chem. 1998;273:157–166. doi: 10.1074/jbc.273.1.157. [DOI] [PubMed] [Google Scholar]
- 13.Galliano MF, Huet C, Frygelius J, Polgren A, Wewer UM, Engvall E. Binding of ADAM12, a marker of skeletal muscle regeneration, to the muscle-specific actin-binding protein, alpha -actinin-2, is required for myoblast fusion. J Biol Chem. 2000;275:13933–13939. doi: 10.1074/jbc.275.18.13933. [DOI] [PubMed] [Google Scholar]
- 14.Yagami-Hiromasa T, Sato T, Kurisaki T, Kamijo K, Nabeshima Y, Fujisawa-Sehara A. A metalloprotease-disintegrin participating in myoblast fusion. Nature. 1995;377:652–656. doi: 10.1038/377652a0. [DOI] [PubMed] [Google Scholar]
- 15.Abe E, Mocharla H, Yamate T, Taguchi Y, Manolagas SC. Meltrin-alpha, a fusion protein involved in multinucleated giant cell and osteoclast formation. Calcif Tissue Int. 1999;64:508–515. doi: 10.1007/s002239900641. [DOI] [PubMed] [Google Scholar]
- 16.Huppertz B, Bartz C, Kokozidou M. Trophoblast fusion: fusogenic proteins, syncytins and ADAMs, and other prerequisites for syncytial fusion. Micron. 2006;37:509–517. doi: 10.1016/j.micron.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 17.Poon LC, Chelemen T, Granvillano O, Pandeva I, Nicolaides KH. First-trimester maternal serum a disintegrin and metalloprotease 12 (ADAM12) and adverse pregnancy outcome. Obstet Gynecol. 2008;112:1082–1090. doi: 10.1097/AOG.0b013e318188d6f9. [DOI] [PubMed] [Google Scholar]
- 18.Pihl K, Larsen T, Krebs L, Christiansen M. First trimester maternal serum PAPP-A, beta-hCG and ADAM12 in prediction of small-for-gestational-age fetuses. Prenat Diagn. 2008;28:1131–1135. doi: 10.1002/pd.2141. [DOI] [PubMed] [Google Scholar]
- 19.Cowans NJ, Spencer K. First-trimester ADAM12 and PAPP-A as markers for intrauterine fetal growth restriction through their roles in the insulin-like growth factor system. Prenat Diagn. 2007;27:264–271. doi: 10.1002/pd.1665. [DOI] [PubMed] [Google Scholar]
- 20.Laigaard J, Cuckle H, Wewer UM, Christiansen M. Maternal serum ADAM12 levels in Down and Edwards' syndrome pregnancies at 9-12 weeks' gestation. Prenat Diagn. 2006;26:689–691. doi: 10.1002/pd.1480. [DOI] [PubMed] [Google Scholar]
- 21.Laigaard J, Sorensen T, Frohlich C, Pedersen BN, Christiansen M, Schiott K, et al. ADAM12: a novel first-trimester maternal serum marker for Down syndrome. Prenat Diagn. 2003;23:1086–1091. doi: 10.1002/pd.762. [DOI] [PubMed] [Google Scholar]
- 22.Spencer K, Cowans NJ, Uldbjerg N, Torring N. First-trimester ADAM12s as early markers of trisomy 21: a promise still unfulfilled? Prenat Diagn. 2008;28:338–342. doi: 10.1002/pd.1978. [DOI] [PubMed] [Google Scholar]
- 23.Laigaard J, Christiansen M, Frohlich C, Pedersen BN, Ottesen B, Wewer UM. The level of ADAM12-S in maternal serum is an early first-trimester marker of fetal trisomy 18. Prenat Diagn. 2005;25:45–46. doi: 10.1002/pd.1029. [DOI] [PubMed] [Google Scholar]
- 24.Spencer K, Cowans NJ. ADAM12 as a marker of trisomy 18 in the first and second trimester of pregnancy. J Matern Fetal Neonatal Med. 2007;20:645–650. doi: 10.1080/14767050701483389. [DOI] [PubMed] [Google Scholar]
- 25.Poon LC, Chelemen T, Minekawa R, Frisova V, Nicolaides KH. Maternal serum ADAM12 (A disintegrin and metalloprotease) in chromosomally abnormal pregnancy at 11–13 weeks. Am J Obstet Gynecol. 2009;200:508, e501–506. doi: 10.1016/j.ajog.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 26.Christiansen M, Pihl K, Hedley PL, Gjerris AC, Lind PO, Larsen SO, et al. ADAM 12 may be used to reduce the false positive rate of first trimester combined screening for Down syndrome. Prenat Diagn. 2010;30:110–114. doi: 10.1002/pd.2405. [DOI] [PubMed] [Google Scholar]
- 27.Laigaard J, Sorensen T, Placing S, Holck P, Frohlich C, Wojdemann KR, et al. Reduction of the disintegrin and metalloprotease ADAM12 in preeclampsia. Obstet Gynecol. 2005;106:144–149. doi: 10.1097/01.AOG.0000165829.65319.65. [DOI] [PubMed] [Google Scholar]
- 28.Spencer K, Cowans NJ, Stamatopoulou A. ADAM12s in maternal serum as a potential marker of pre-eclampsia. Prenat Diagn. 2008;28:212–216. doi: 10.1002/pd.1957. [DOI] [PubMed] [Google Scholar]
- 29.Makrydimas G, Sotiriadis A, Spencer K, Cowans NJ, Nicolaides KH. ADAM12-s in coelomic fluid and maternal serum in early pregnancy. Prenat Diagn. 2006;26:1197–1200. doi: 10.1002/pd.1581. [DOI] [PubMed] [Google Scholar]
- 30.Cowans NJ, Stamatopoulou A, Jaakohuhta S, Spencer K. ADAM-12 stability in first trimester maternal serum. Prenat Diagn. 2010;30:555–560. doi: 10.1002/pd.2522. [DOI] [PubMed] [Google Scholar]
- 31.Seeber B, Sammel MD, Fan X, Gerton GL, Shaunik A, Chittams J, et al. Proteomic analysis of serum yields six candidate proteins that are differentially regulated in a subset of women with endometriosis. Fertil Steril. 2010;93:2137–2144. doi: 10.1016/j.fertnstert.2008.12.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seeber B, Sammel MD, Fan X, Gerton GL, Shaunik A, Chittams J, et al. Panel of markers can accurately predict endometriosis in a subset of patients. Fertil Steril. 2008;89:1073–1081. doi: 10.1016/j.fertnstert.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 33.Horne AW, Duncan WC, Critchley HO. The need for serum biomarker development for diagnosing and excluding tubal ectopic pregnancy. Acta Obstet Gynecol Scand. 2010;89:299–301. doi: 10.3109/00016340903568191. [DOI] [PMC free article] [PubMed] [Google Scholar]