Abstract
Ezrin is a membrane-cytoskeleton linker protein that can bind F-actin in its active conformation. Several means of regulation of ezrin's activity have been described including phosphorylation of Thr-567 and binding of L-α-phosphatidylinositol-4,5-bisphosphate (PIP2). However, the relative contributions of these events toward activation of the protein and their potential interdependence are not known. We developed an assay based on solid-supported membranes, to which different ezrin mutants (ezrin T567A (inactive mutant), wild-type, and T567D (active pseudophosphorylated mutant)) were bound, that enabled us to analyze the influence of phosphorylation and PIP2 binding on ezrin's activation state in vitro. The lipid bilayers employed contained either DOGS-NTA-Ni to bind the proteins via an N-terminal His-tag, or PIP2, to which ezrin binds via specific binding sites located in the N-terminal region of the protein. Quantitative analysis of the binding behavior of all three proteins to the two different receptor lipids revealed that all three bind with high affinity and specificity to the two receptor lipids. Fluorescence microscopy on ezrin-decorated solid-supported membranes showed that, dependent on the mode of binding and the phosphorylation state, ezrin is capable of binding actin filaments. A clear synergism between phosphorylation and the receptor lipid PIP2 was observed, suggesting a conformational switch from the dormant to the active, F-actin binding state by recognition of PIP2, which is enhanced by the phosphorylation.
Introduction
In eukaryotic cells, the organization and dynamic properties of the cell membrane architecture are regulated by an interplay between the lipid bilayer and the subjacent cortical actin cytoskeleton. Proteins of the ezrin, radixin, and moesin (ERM) family act as dynamic linkers between the actin cytoskeleton and the plasma membrane, and are therefore implicated in membrane organization in various cellular and developmental processes (1). It is established that ERM proteins can exist in an apparently dormant, closed conformation that localizes in the cytoplasm and an active form that interacts with plasma membrane proteins and actin filaments (2–4). In the dormant conformation, the N-terminal ERM association domain (N-ERMAD) and the C-terminal ERM association domain (C-ERMAD) are self-associated by a head-to-tail joining, thus masking the F-actin and membrane protein binding sites (2). Adoption of the open active conformation capable of binding F-actin requires disruption of the N-ERMAD/C-ERMAD interaction.
The first insights into the molecular mechanism of the regulation of ERM proteins came with the discovery that moesin is phosphorylated on Thr-558 located in the C-ERMAD during platelet activation (5). Highly conserved threonine residues in ezrin (Thr-567) and radixin (Thr-564) were then also found to be phosphorylated leading to activation. Förster resonance energy transfer analysis with GFP-tagged ezrin molecules indicated that phosphorylation of Thr-567 is sufficient to disrupt the closed conformation of ezrin (6). Phosphorylation turns the protein into the active form, which is able to bind F-actin (7). However, in addition to phosphorylation, evidence obtained from biochemical and cellular assays showed that ERM proteins directly bind to L-α-phosphatidylinositol-4,5-bisphosphate (PIP2). This also promotes their activation (5,8–10) and can be envisaged as the key player in the dynamics of ERM protein regulation.
Recent reports have demonstrated that dynamic changes in the PIP2 levels in cells can have an instructive role on ERM protein activity (11,12). Studies on mammalian ezrin revealed that PIP2 binding is a prerequisite for its subsequent phosphorylation (13). The major role of PIP2 is also manifested by experiments of Auvinen et al. (14), who showed that overexpression of PIPKα, an enzyme that catalyzes the production of PIP2, relocates endogenous ezrin partially to adherens junctions, suggesting opening of its dormant state. Hao et al. (15) demonstrated that a reduction of PIP2 levels by activation of phospholipase C promotes the release and dephosphorylation of moesin and ezrin in cellular systems. Based on these results, a two-step model of activation is envisioned (12): ERM proteins are recruited to membrane regions that are rich in PIP2, thus rendering the conserved threonine residue more accessible to phosphorylation.
Recently, we have developed an artificial membrane system, which gave us what we believe to be first insights into the influence of PIP2 binding on ezrin activation, i.e., F-actin binding, in a very well-defined in vitro system (16). We were able to demonstrate that ezrin bound via PIP2 to a solid-supported membrane (SSM) is capable of binding actin filaments, while ezrin bound via a His-tag to a lipid bilayer displaying nickel nitrilotriacetic acid (NTA-Ni) headgroups does not significantly interact with F-actin.
In this study, we aimed at elucidating the influence of PIP2 binding and phosphorylation and its possible synergism with respect to activation of ezrin's F-actin binding. We made use of ezrin mutants, namely ezrin T567D and ezrin T567A. In the T567D mutant, the threonine at position 567 is replaced by a negatively charged aspartate, which mimics a permanent phosphorylation. The T567A mutant has been used in cell experiments, where it is considered constitutively inactive because the phosphorylation site is removed (17,18). We compared the F-actin affinities of ezrin wild-type (wt) and the two mutants bound via an N-terminal His-tag to DOGS-NTA-Ni-bearing phospholipids with those of ezrin derivatives bound to PIP2-containing SSMs.
Experimental Procedures
All materials used can be found in the Supporting Material. All buffers were supplemented with 0.1 mM NaN3.
Protein purification
Ezrin wild-type (wt) and the mutants T567A and T567D were recombinantly expressed in Escherichia coli cells (strain BL21(DE3)pLysS) and purified as described in detail elsewhere (19). Briefly, transformed E. coli cells containing the bacterial expression vector pET28a+ (Novagen, Madison, WI) encoding ezrin wt, ezrin T567D, or ezrin T567A with an N-terminal histidine tag were grown to an OD600 of 0.6. Protein expression was induced by addition of isopropyl-β-D-thiogalactopyranoside (Sigma-Aldrich, St. Louis, MO) to a concentration of 1 mM. After 4 h, cells were harvested by centrifugation (3000 × g, 10 min, 4°C) and resuspended in lysis buffer (40 mM HEPES, 20 mM imidazole, 300 mM NaCl, 1 mM EDTA, 10 mM mercaptoethanol, and protease inhibitor mixtures (Complete; Roche Diagnostics, Basel, Switzerland) at pH = 7.4). Cell lysis was induced by sonication. Subsequently, the lysates were centrifuged (100,000 × g, 1 h, 4°C) and the supernatant was applied to a Ni-nitrilotriacetic acid (Ni-NTA) agarose column (Qiagen, Hilden, Germany) preequilibrated with lysis buffer. The column was washed twice with lysis buffer containing 25 mM imidazole/HCl at pH = 7.4 and 35 mM imidazole/HCl at pH = 7.4, respectively. Ezrin was eluted using 300 mM imidazole/HCl at pH = 7.4 and stored at 4°C until use. Protein concentration was determined by UV/Vis-spectroscopy using an extinction coefficient of ɛ280 = 66,900 M−1 cm−1.
Circular dichroism spectroscopy
Circular dichroism (CD) spectra were recorded on a model No. 810 spectrometer equipped with a model No. PTC432S temperature control unit (both by JASCO, Gross-Umstadt, Germany) using the Spectra Manager control (V 1.17.00) and application (V 1.53.00) software package (JASCO). CD spectra were recorded in 20 mM kh2po4/k2hpo4, and 0.1 mM rdta, pH = 8 in 1-mm quartz glass precision cells at 20°C in a wavelength range of 240–190 nm with 1.0 nm bandwidth, using the continuous-mode setting, with 1.0 s response and a scan speed of 50 nm/min. Five spectra were averaged. Spectra were background-corrected against pure buffer, smoothed (Savitzky-Golay), and expressed as mean residue ellipticity (10−3 deg⋅cm2⋅dmol−1). For normalization, the final protein concentrations were determined by UV/Vis-spectroscopy using pure buffer for background correction. Deconvolution of the data was performed using the program DichroWeb (http://dichroweb.cryst.bbk.ac.uk/html/home.shtml) with algorithm CDSSTR and reference set 7 (20).
Protein labeling
Ezrin wt was dissolved in 20 mM Tris/HCl, 50 mM KCl, 0.1 mM EDTA, pH = 7.4, at a concentration of 20 μM and mixed with dithiothreitol (DTT) in a 10-fold molar excess to reduce possible disulfide bonds. The solution was then dialyzed overnight against DTT-free, degassed buffer. AlexaFluor633-maleimide dissolved in dimethyl sulfoxide was added dropwise to the protein solution at a molar ratio of 20:1 (AlexaFluor633-maleimide:ezrin) and the mixture was stirred overnight. The reaction mixture was quenched by the addition of 2 mM mercaptoethanol, and the labeled protein was separated from free dye via size exclusion chromatography (Sephadex-NAP 25; GE Life Sciences, Piscataway, NJ). All steps were carried out at 4°C. The labeling efficiency was determined by UV/Vis-spectroscopy (ɛ632 = 91,300 M−1 cm−1), resulting in a dye/protein ratio of 0.7:1.
Actin polymerization
Nonmuscle G-actin was stored at a concentration of 10 mg/mL in G-buffer (5 mM Tris/HCl, 0.2 mM CaCl2, 0.2 mM ATP, 5% (w/v) sucrose, and 1% (w/v) dextran, pH = 8.0) at −80°C. Before use, G-actin was dissolved to a concentration of 0.4 mg/mL in G-buffer supplemented with 0.2 mM DTT, kept on ice for 1 h to dissolve actin oligomers, and then cleared by centrifugation (17,000 × g, 10 min, 4°C). Polymerization was induced by adding 1/10 of the total volume of polymerization solution (500 mM KCl, 20 mM MgCl2, and 20 mM ATP) to the actin solution. Phalloidin was added in an equimolar amount compared to monomeric actin to prevent depolymerization. For fluorescence microscopy, 0.01 mol % of AlexaFluor488-phalloidin was added to label the actin filaments.
Vesicle preparation
Mixed lipid films composed of POPC (2 mg/mL in chloroform) doped with 10 mol % of PIP2 (1 mg/mL in chloroform/methanol/water (20:5:1)) or DOPC (2 mg/mL in chloroform) doped with 10 mol % of DOGS-NTA-Ni (2 mg/mL in chloroform) were prepared at the bottom of glass test tubes by removing the solvent under a stream of nitrogen. This was followed by 3 h under vacuum at 35°C and storage at 4°C. A quantity of 1 mol % of the fluorophore perylene was added to the lipid mixture to allow us to observe the integrity of the formed planar SSMs by fluorescence microscopy. If defects are present in the membrane, they appear as dark spots in the fluorescence images. Multilamellar vesicles were obtained by swelling the lipid film in buffer solution (20 mM Tris/HCl, 50 mM KCl, 0.1 mM EDTA, pH = 7.4) for 30 min followed by rigorous vortexing of the suspension three times (30 s every 5 min). Multilamellar vesicles were transformed into large unilamellar vesicles (LUVs) by the extrusion method using a polycarbonate membrane with 100-nm pore diameter (LiposoFast; Avestin, Ottawa, Canada).
Preparation of solid-supported membranes on gold substrates
For quartz crystal microbalance (QCM)-experiments, hybrid bilayers were prepared on gold substrates. Immediately before use, the gold electrode of the quartz plate (0.255 cm2) was cleaned in argon plasma for 5 min (Plasma cleaner; Harrick Plasma, Ithaca, NY) and placed into the Teflon measuring cell. The surface was immersed in an ethanolic octanethiol solution (15 mM) for 1 h, rinsed first with ethanol and then with buffer (20 mM Tris/HCl, 50 mM KCl, and 0.1 mM EDTA, pH = 7.4). The capacitance of the resulting monolayer was monitored by impedance spectroscopy (Solartron Instruments, Farnborough, UK) to verify complete coverage of the gold electrode. A capacitance of (2.0 ± 0.2) μF/cm2 indicated the successful formation of an octanethiol monolayer. A second monolayer was generated by incubating the hydrophobic surface with a suspension of LUVs (0.5 mg/mL) for 2 h at room temperature. The quality of the resulting solid-supported bilayer was again analyzed by impedance spectroscopy. Successful formation of bilayers was confirmed by capacitance values of (1.2 ± 0.2) μF/cm2. Remaining vesicles were removed by rinsing the surface thoroughly with buffer solution.
Preparation of solid-supported lipid bilayers on silicon substrates
A silicon substrate (0.8 × 2.0 cm2) was rinsed thoroughly with isopropanol and water. Then it was immersed in an aqueous solution of NH3 and H2O2 (5:1:1 H2O/NH3/H2O2) for 20 min at 70°C to render the surface hydrophilic. The substrate was mounted into a flow cell chamber and immediately incubated with the LUV suspension for 1–2 h. Subsequent rinsing with buffer (20 mM Tris/HCl, 50 mM KCl, and 0.1 mM EDTA, pH = 7.4) removed remaining vesicles from the surface.
Quartz crystal microbalance
For the investigation of protein-lipid interactions, the quartz crystal microbalance (QCM 200; Stanford Research Systems, Sunnyvale, CA) was used in passive mode as described elsewhere (21). An AT-cut quartz plate with a 5 MHz fundamental resonance frequency was mounted in a fluid cell made of Teflon with the SSM facing the aqueous solution. Different protein concentrations were added from outside the fluid cell in buffer with a buffer flow of 0.35 mL/min to the SSM and the frequency shift was read-out in a time-dependent manner.
Fluorescence microscopy
An upright confocal laser scanning microscope (Zeiss LSM 710; Zeiss, Jena, Germany) equipped with a water immersion objective with 63× magnification (W Plan Apochromat, NA = 1.0, Zeiss) was used for fluorescence imaging. Perylene fluorescence of the SSMs was excited at 405 nm (diode laser) and detected at 426–485 nm. Proteins were bound to the membranes immobilized on silicon substrates by continuously flowing buffer (0.35 mL/min) across the sample for 30 min. The protein-bound fluorophore (AlexaFluor633) was excited with a 633-nm laser line and the fluorescence detected at 640–660 nm. For the excitation of phalloidin bound to filamentous actin (AlexaFluor488), an Ar-laser was used (488 nm). Emission was collected in the range of 515–552 nm.
Results
Recently, we presented what we believe to be first results on an in vitro system for the analysis of ezrin-mediated membrane-F-actin interactions (16). Our findings suggested that binding of ezrin wt to PIP2 embedded in a lipid bilayer partially activates the protein, which then becomes capable of binding F-actin. Based on this first evidence, we addressed the question whether phosphorylation of ezrin and the binding to PIP2 act synergistically toward this activation. To answer this question, we used two different ezrin mutants in comparison to the wild-type (wt) protein, which are frequently used in cell experiments (7,17). Ezrin T567A is a mutant, in which threonine is replaced by alanine, which prevents the phosphorylation of the protein in vivo. The wild-type protein is also nonphosphorylated, as all experiments were performed under nonphosphorylating conditions and it is highly unlikely that a phosphorylation occurs during recombinant expression in E. coli. In ezrin T567D, threonine is exchanged against the negatively charged amino acid aspartate, which mimics the negative charge of the phosphorylated state. Each protein was equipped with a His-tag at its N-terminus for purification purposes and for binding the protein to a lipid membrane containing the receptor lipid DOGS-NTA-Ni.
Before the binding experiments, the secondary structures of the three different proteins were analyzed by CD spectroscopy to prove their structural integrity and to investigate whether the mutations lead to structural changes of the protein (see the Supporting Material). Deconvolution of the CD spectra results in the secondary structure fractions as summarized in Table 1. For all three proteins, a rather large α-helical content in the range of 60–71% was found, while β-sheet contributions to the overall structures were rather small and in the range of only 4–7%. Interestingly, the α-helical content for ezrin T567A is ∼10% smaller than that of the wt protein, even though one would not expect a significant alteration of the protein's structure by this single point mutation. The change in secondary structure compared to the wt protein is less pronounced for ezrin T567D. In this case, a conformational change of the protein is discussed as a result of the addition of the negative charge (22).
Table 1.
Results of the deconvolution of CD spectra
| Ezrin T567A | Ezrin wt | Ezrin T567D | |
|---|---|---|---|
| α-Helix/% | 60 | 71 | 65 |
| β-Sheet/% | 7 | 4 | 4 |
| Turns/% | 8 | 7 | 9 |
| Random coil/% | 25 | 18 | 22 |
| NRMSD∗ | 0.019 | 0.007 | 0.016 |
CD spectra of ezrin T567A, ezrin wt, and ezrin T567D using the online server DichroWeb (http://dichroweb.cryst.bbk.ac.uk/html/home.shtml) employing the algorithm CDSSTR and reference data set 7.
Normalized root mean-squared deviation.
Binding of ezrin wt and the mutants to solid-supported membranes
Prerequisite for studying the capability of membrane-bound ezrin to interact with F-actin is its binding to a lipid membrane. In this study, we used planar bilayers attached to a solid substrate as they allow us not only to quantify ezrin binding to the membrane by surface-sensitive methods such as the quartz crystal microbalance (QCM) technique but also to visualize the interaction with F-actin. The QCM technique is a well-established quantitative method and allows the investigation of protein binding to a functionalized surface in a time-resolved and label-free fashion (21,23).
The binding of all three proteins to membranes composed of either DOPC/DOGS-NTA-Ni (9:1) or POPC/PIP2 (9:1) was quantified. A content of 10 mol % of the receptor lipid (DOGS-NTA-Ni or PIP2) in the membrane was chosen to ensure maximum protein coverage (see the Supporting Material) (24). Solid-supported hybrid membranes were prepared on 5 MHz quartz plates composed of a chemisorbed octanethiol monolayer and a second phospholipid monolayer obtained from spreading large unilamellar vesicles doped with the corresponding receptor lipid. Fig. 1, A and B, shows representative time courses of the frequency shifts of the quartz plate after several additions of ezrin wt to a DOGS-NTA-Ni-containing membrane (Fig. 1 A) and a PIP2-containing membrane (Fig. 1 B), respectively.
Figure 1.
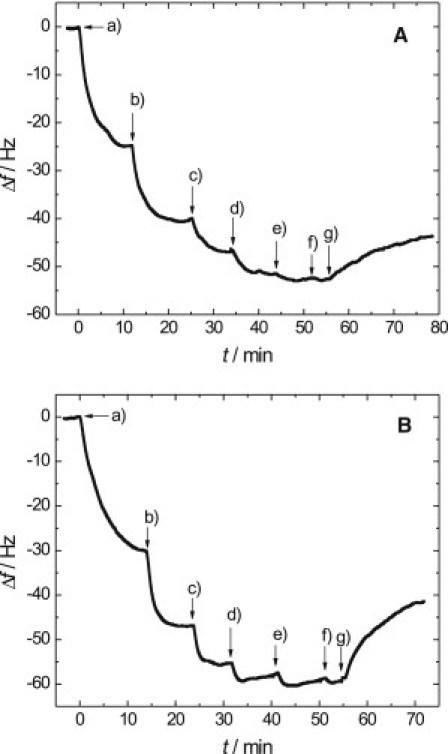
Representative time course of the frequency shift upon stepwise ezrin wt addition (a–f) to a solid-supported (A) OT-DOPC/DOGS-NTA-Ni (9:1) or (B) OT-POPC/PIP2 (9:1) membrane in 20 mM Tris/HCl, 50 mM KCl, 0.1 mM EDTA, pH = 7.4. (g) Rinsing with pure buffer. Concentrations in panel A: (a) 13 nM, (b) 39 nM, (c) 90 nM, (d) 161 nM, (e) 249 nM, and (f) 280 nM. Concentrations in panel B: (a) 19 nM, (b) 44 nM, (c) 91 nM, (d) 148 nM, (e) 233 nM, and (f) 282 nM.
Upon addition of the protein, a time-dependent decrease in resonance frequency of the quartz crystal is observed. The decrease in resonance frequency is proportional to the amount of ezrin adsorbed on the lipid layer. The value Δf is defined as the difference between the actual resonance frequency f(t) and f(t = 0). After several protein additions under constant flow conditions, the surface coverage reaches its maximum and no further decrease in resonance frequency is observed. From the time-dependent changes in resonance frequency, it can be deduced that equilibrium is reached within 15–20 min.
To obtain the protein concentration required for full membrane coverage the normalized frequency changes Δf/Δfmax at equilibrium obtained for each protein addition were plotted versus the ezrin concentrations in solution (Fig. 2). From the obtained adsorption isotherms, the protein concentration required for full surface coverage was read out. For all proteins, they were in the >100 nM regime. To ensure that the membrane surface is saturated for all binding scenarios, a protein concentration of c > 400 nM was used for all experiments except for those where the membranes were doped with only 0.5 mol % PIP2. The maximum frequency shifts varied up to 30% and were independent of the protein, in the range of 60 Hz. To analyze the reversibility of protein binding, the measuring chamber was rinsed with pure buffer. For all three proteins, 90% of the protein remains bound to the DOGS-NTA-Ni-containing membrane, while only ∼10% of the protein could be removed by rinsing within the observation time. For ezrin bound via PIP2, a slightly larger fraction desorbs from the surface upon rinsing (Table 2).
Figure 2.
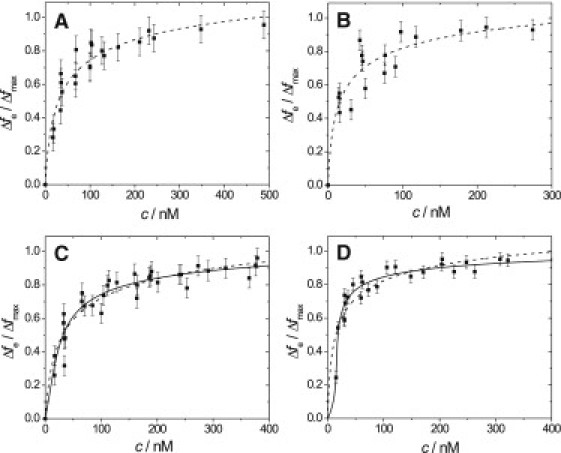
Adsorption isotherms of (A) ezrin T567A and (B) ezrin T567D binding to a DOGS-NTA-Ni-doped (10 mol %) SSM. Adsorption isotherms of (C) ezrin T567A and (D) ezrin T567D binding to a PIP2-doped (10 mol %) SSM. All experiments were performed in 20 mM Tris/HCl, 50 mM KCl, and 0.1 mM EDTA, pH = 7.4. (Dashed lines) Results of fitting Eq. 1 to the data. The results are summarized in Table 2. (Solid lines) Results of fitting Eq. 3 to the data. For further details, see text.
Table 2.
Parameters obtained from QCM-measurements on three different ezrin species bound to DOGS-NTA-Ni or PIP2-containing membranes
| Membrane protein | DOGS-NTA-Ni |
PIP2 |
||||
|---|---|---|---|---|---|---|
| T567A | wt | T567D | T567A | wt | T567D | |
| Δfmax/Hz | 58 ± 13(n = 5) | 67 ± 19(n = 3) | 64 ± 5(n = 5) | 66 ± 4(n = 6) | 46 ± 8(n = 3) | 49 ± 5(n = 6) |
| Reversibility/% | 9 ± 7(n = 6) | 7 ± 5(n = 7) | 8 ± 2(n = 6) | 16 ± 10(n = 5) | 19 ± 11(n = 4) | 14 ± 7(n = 10) |
| KD/nM | 47 ± 6(n = 5) | 14 ± 2(n = 3) | 21 ± 5(n = 5) | 45 ± 12(n = 6) | 52 ± 32(n = 3) | 18 ± 9(n = 6) |
Values are given as mean values ± SD. The term n gives the number of independent experiments.
To model the isotherms and extract dissociation constants KD of the protein-receptor interaction, we used:
| (1) |
The value Θ is the surface coverage and c is the protein concentration. The model takes into account that the protein area covering one binding site can expand over more than one lattice space, which is given by the available surface function Φ(Θ) (Eq. 2), which describes the available surface for deposition of particles from bulk solution as a function of coverage (scaled particle theory, SPT) (25):
| (2) |
The model assumes that particle adsorption occurs noncooperatively on a homogeneous surface in a monomolecular fashion, where all individual binding sites have the same adsorption energy for the adsorbate. The resulting dissociation constants are summarized in Table 2.
Visualization of membrane bound ezrin wt and its interaction with F-actin
To visualize the binding behavior of ezrin to PIP2-containing membranes by fluorescence microscopy and to elucidate the F-actin binding capability of membrane-bound ezrin, we labeled ezrin wt with an AlexaFluor633 fluorescence dye. SSMs composed of POPC doped with only 0.5 mol % PIP2 were prepared by spreading of large unilamellar vesicles on hydrophilic silicon substrates. Such low PIP2 concentration was chosen to ensure that only part of the membrane surface is covered with ezrin (see the Supporting Material), which can thus be readily distinguished from noncovered regions (24). This allows us not only to investigate the organization of the protein on the membrane surface but also to visualize how F-actin binds to partly ezrin-decorated membranes.
After 30 min of incubation with 200 nM of AlexaFluor633-labeled protein, the membrane surface was rinsed with buffer to remove nonbound protein, and fluorescence images were taken. The images show that ezrin forms protein clusters several tens of micrometers in size and of irregular shape. A characteristic protein cluster found on the membrane surface is depicted in Fig. 3 A (see also the Supporting Material). This result indicates that the protein not only binds to PIP2 in the membrane but is capable of laterally aggregating on the surface, which might be an indication of a positive cooperative binding behavior as discussed below.
Figure 3.
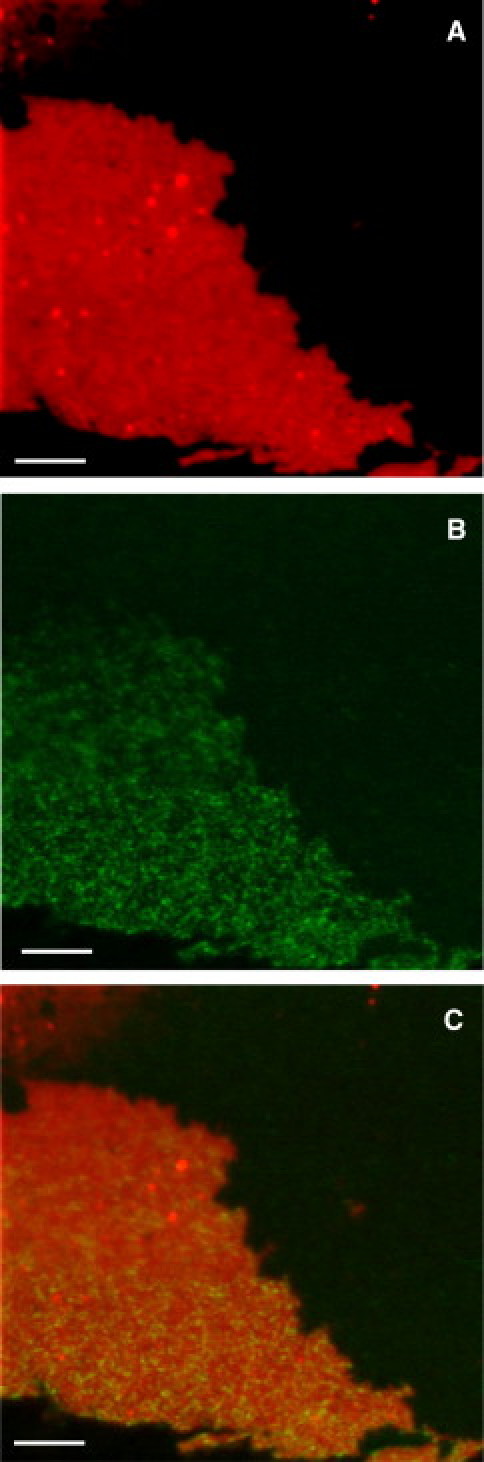
Confocal laser scanning fluorescence microscopy image of (A) AlexaFluor633-maleimide-labeled ezrin wt immobilized on a SSM containing 0.5% PIP2, 1% perylene, 98.5% POPC in F-buffer (20 mM Tris/HCl, 50 mM KCl, 2 mM MgCl2, and 2 mM ATP, pH = 7.4). (B) AlexaFluor488-phalloidin-labeled actin. (C) Overlay of the two images. Scale bars: 5 μm.
To elucidate whether ezrin wt bound via PIP2 specifically interacts with F-actin, we added an F-actin solution supplemented with phalloidin to stabilize the filaments and AlexaFluor488 phalloidin for fluorescence labeling. After 30 min of incubation and rinsing with buffer, the membrane surface was again inspected by fluorescence microscopy. Filamentous structures of actin became visible in the fluorescence images (Fig. 3 B). High concentrations of surface-bound F-actin were only found in those areas where ezrin wt was localized (Fig. 3 C). Only a small number of actin filaments were detected in areas where no ezrin was bound, which can be attributed to nonspecific adsorption of F-actin to the membrane surface. These results demonstrate that PIP2-bound ezrin wt is capable of specifically binding F-actin, suggesting that an exposure of the F-actin binding site in the C-ERMAD has taken place.
F-actin binding of ezrin mutants and ezrin wt
The QCM results have confirmed that ezrin wt as well as ezrin T567D and T567A adsorb specifically on DOPC/DOGS-NTA-Ni (9:1) and POPC/PIP2 (9:1) membranes, respectively, and that the main fraction remains bound even after rinsing with buffer. From the QCM results we also determined the required protein concentration in solution to obtain maximum surface coverage on membranes containing 10 mol % of the receptor lipid. These concentrations were, independent of the protein, in the >100-nM regime.
We also showed that F-actin is capable of binding with high specificity to ezrin wt, when it is bound to PIP2-containing membranes. To be able to elucidate and quantify the influence of the phosphorylation state of ezrin and its binding to PIP2 on the F-actin binding capability as well as a possible synergism, a combination of the different protein derivatives and the membrane-binding mode is required. Solid-supported lipid bilayers composed of DOPC/DOGS-NTA-Ni (9:1) and POPC/PIP2 (9:1), respectively, were prepared on hydrophilic silicon substrates by fusion of large unilamellar vesicles. Each membrane preparation was inspected by confocal laser scanning fluorescence microscopy using the membrane-confined fluorophore perylene.
A homogeneous fluorescence indicated a successful membrane formation (data not shown). Protein concentrations larger than 400 nM were used to obtain maximum protein coverage on the membrane. Each protein was incubated with the membrane under flow conditions for 30 min and the surface was rinsed afterwards with F-buffer. An F-actin solution stabilized with phalloidin and labeled with AlexaFluor 488 phalloidin was then added for 30 min to the membrane-bound ezrin derivatives. After rinsing the samples with pure buffer to remove nonbound F-actin, they were analyzed by confocal laser scanning fluorescence microscopy. These images allowed us to visualize directly the amount and structure of bound F-actin on the membrane.
Representative fluorescence images of the six possible different samples are depicted in Fig. 4 showing the green fluorescence of the phalloidin-labeled actin filaments that have an average length of ∼1–7 μm as determined from the fluorescence images by pixel analysis. While ezrin T567A bound via its His-tag recruits only few surface-bound individual actin filaments, we found an almost complete surface coverage of F-actin on surfaces, where ezrin T567D was immobilized via PIP2. To obtain quantitative data from the fluorescence images and be able to compare the results, we performed a detailed pixel analysis using the program ImageJ (National Institutes of Health, Bethesda, MD). The process of determining a threshold value in every individual picture is described in the Supporting Material.
Figure 4.
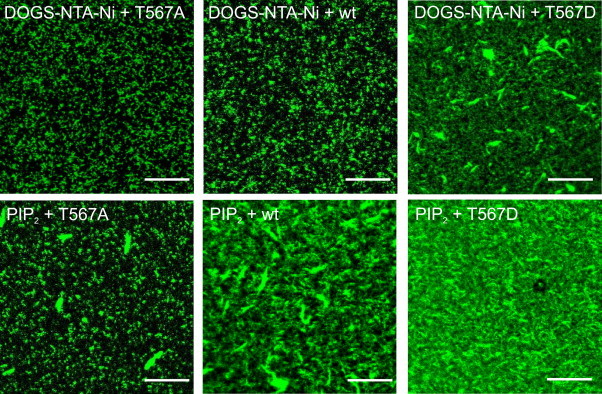
Confocal laser scanning fluorescence microscopy images of AlexaFluor488-phalloidin-labeled actin bound to ezrin on DOGS-NTA-Ni (10%) doped SSMs or PIP2 (10%) doped SSMs on silicon substrates using ezrin T567A, ezrin wt, and ezrin T567D as indicated. Scale bars: 10 μm.
Based on this approach, mean F-actin surface coverage values for DOGS-NTA-Ni-containing membranes of (15 ± 9)% (n = 8) for membrane-bound ezrin T567A, (21 ± 10)% (n = 13) for ezrin wt, and (58 ± 23)% (n = 10) for ezrin T567D were obtained (Fig. 5). The surface coverage obtained for ezrin T567A bound to DOGS-NTA-Ni is almost the same as that obtained for membranes incubated with F-actin without bound protein ((14 ± 6)%, n = 4). This result implies that F-actin binding to membrane-bound ezrin T567A cannot be safely attributed to a specific interaction. Only a slightly larger surface coverage was observed for ezrin wt bound via its His-tag to the membrane. It is expected that binding of ezrin wt and ezrin T567A via their N-terminal His-tags to a DOGS-NTA-Ni-containing membrane preserves their dormant conformation, i.e., that they do not expose the F-actin binding site, which in turn results in a rather low or even no affinity to F-actin. Ezrin T567D, however, mimics the phosphorylated (activated) state and it is thus supposed that this mutant is capable of binding F-actin, even if bound via its His-tag to DOGS-NTA-Ni-doped membranes. Indeed, a significant increase in surface coverage to almost 60% is observed.
Figure 5.
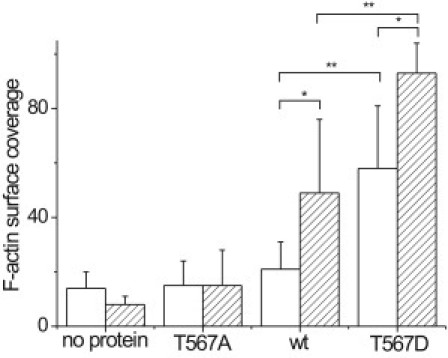
Quantification of the surface coverage via pixel analysis using the program ImageJ (National Institutes of Health). Results obtained for DOGS-NTA-Ni (10 mol %) (open bars) and PIP2 (10 mol %) (hatched bars) doped SSMs on silicon substrates using ezrin and the mutants. These data were generated from at least 2–3 independently prepared samples, from which 3–7 images were taken at different regions of the surface for coverage determination. The values are given as mean values ± SD. A Wilcoxon rank-sum test confirms that the difference in F-actin coverage is significantly different for ezrin wt bound to DOGS-NTA-Ni and PIP2 (P = 0.0007, ∗) and ezrin T567D bound to DOGS-NTA-Ni and PIP2 (P = 0.007, ∗). In addition, the differences in F-actin coverage comparing ezrin wt and ezrin T567D bound to DOGS-NTA-Ni (P = 0.00003, ∗∗) and ezrin wt and ezrin T567D bound to PIP2 (P = 0.0004, ∗∗) showed high significance.
In the second set of experiments, the influence of PIP2 binding in conjunction with the three different proteins was elucidated. The most striking results were obtained for ezrin wt and the pseudophosphorylated mutant T567D. If ezrin wt is bound via PIP2 to the SSM, the F-actin surface coverage increased by more than a factor of two to (49 ± 27)% (n = 14) compared to the value obtained for ezrin wt bound via its His-tag. This result suggests a partial activation of ezrin wt through PIP2 binding, as reported previously (16). The influence of PIP2 on the activation state could also be observed for the pseudophosphorylated mutant ezrin T567D. In this case, the F-actin surface coverage increased to (93 ± 11)% (n = 13) (Fig. 5), which is significantly larger than that obtained for ezrin T567D bound via its His-tag. This strongly implies that the synergistic action of PIP2 binding and the negative charge in position 567 results in the exposure of the F-actin binding site, which corresponds to full activation of ezrin.
Interestingly, an F-actin surface coverage of only (15 ± 13)% (n = 20) was found for ezrin T567A bound to PIP2-containing membranes, which is very similar to the value obtained for ezrin T567A bound to DOGS-NTA-Ni-doped membranes. Without bound protein, a nonspecific F-actin binding of (8 ± 3)% (n = 5) surface coverage was observed in the case of PIP2-containing membranes, which is lower than the adsorption of F-actin on DOGS-NTA-Ni-containing membranes ((14 ± 6)%). This difference can be attributed to the different net surface charges. While at pH 7.4, the PIP2-doped membrane is negatively charged (a charge of −3 to −5 per PIP2 (26)), the DOGS-NTA-Ni-doped membrane is only slightly negatively charged (a charge of −1 per DOGS-NTA-Ni (27)). Because actin filaments are also negatively charged (28), a larger electrostatic repulsion is expected on PIP2-containing membranes consistent with the observed lower F-actin adsorption on those membranes. Because the surface is considerably altered after adsorption of ezrin to either membrane type, we refrained from subtracting the nonspecific adsorption values from the specific ones.
Discussion
The activation of ezrin that results in F-actin binding is thought to occur through conformational changes, which can be triggered by events such as the binding of PIP2 or the Ca2+-regulated protein S100P (19) to the N-terminal ERM association domain (N-ERMAD) and the phosphorylation of the conserved Thr-567 in the actin binding C-ERMAD. While earlier studies of the mechanism of this activation revealed that phosphorylation is the key step (29,30), more recently a two-step model of activation has been favored, in which ERM proteins are recruited to membrane regions that are enriched in PIP2, thus rendering the conserved threonine residue more accessible to phosphorylation (12,13). To elucidate the specific interaction and adhesion of F-actin with membrane-bound ezrin in a quantitative manner, we performed in vitro studies on ezrin wt and two mutants mimicking the phosphorylated (T567D) state of the protein and one that cannot be phosphorylated in vivo (T567A).
By means of the QCM technique in conjunction with receptor-doped SSMs, we first demonstrated that all three proteins bind with high specificity to either DOGS-NTA-Ni via the N-terminal His-tag or to PIP2. PIP2 is known as a specific interaction partner of ezrin. Binding is based on the interaction between two lysine-rich, basic regions in the N-terminal region of the protein and the phosphate groups of PIP2. This structure resembles a PH-domain, which is the typical binding motif in PIP2-binding proteins. The QCM technique has been proven to be a versatile tool to monitor protein-lipid interactions in a label-free and time-resolved manner (21,31). Here, it allowed us to determine the protein concentrations required for maximum surface coverage, which were, independent of the protein, in the 100-nM range.
Previously, we demonstrated by atomic force microscopy and by analyzing the kinetics of ezrin binding that the protein laterally aggregates on PIP2-doped membranes (24). This result is corroborated by fluorescence images obtained for AlexaFluor-labeled ezrin bound to PIP2-containing membranes in this study (Fig. 3), showing that large protein aggregates are formed on PIP2-doped membranes. Several studies suggest that ezrin forms oligomers when bound to the membrane (6,32), while the protein in solution is mainly monomeric with some dimers (33). Such an interaction of adsorbed protein molecules on the surface has not been considered in the SPT-model used to evaluate the adsorption isotherms (Eq. 1). However, the Bragg-Williams approximation offers a simple solution to treat the adsorbate/adsorbate interaction in an average sense leading to an analytical expression for the adsorption isotherm (34):
| (3) |
The value ɛ is the average protein-protein interaction energy on the surface in units of kT. The results of fitting the Bragg-Williams model to the experimental isotherms are plotted together with the results obtained from the SPT model in Fig. 2, C and D. With the Bragg-Williams isotherm, a dissociation constant for ezrin T567A of KD = 104 nM is found with an average interaction energy of ɛ = −5 kT. The negative value indicates that the neighboring particles have a stabilizing effect. The dissociation constant KD describing only the protein-receptor interaction is increased compared to that without the assumption of positive cooperativity, as expected. A very similar result was obtained for the adsorption isotherm of ezrin wt to PIP2 with KD = 121 nM and ɛ = −6 kT. These values are in agreement with that used in a previous study (24). Of note, even though a stabilizing effect (i.e., positive cooperativity) is assumed, the modeled isotherms do not deviate significantly from those of the SPT-model (see Fig. 2 C). A slightly more pronounced deviation is only observed for the mutant ezrin T567D, where a KD = 235 nM and ɛ = −10 kT was obtained.
The results demonstrate that positive cooperativity of protein binding cannot be ruled out just from the fact that a model such as the scaled particle isotherm is in accordance with the measured isotherms. Additional experimental evidence is required such as fluorescence microscopy images, which demonstrated the formation of large protein clusters. The formation of lateral protein aggregates is also reflected in the finding that the majority of the protein remains bound on the surface within the observed time period, while only a minor fraction desorbs upon rinsing, which can be attributed to a reduced overall desorption—an effect arising from the lateral attraction of the proteins on the membrane surface.
By means of fluorescence microscopy, we were not only able to show the colocalization of F-actin and protein clusters but we could also quantify the amount of bound actin filaments, which turned out to be a function of the phosphorylation state of the protein and its binding mode. We suggest that an increased F-actin binding capacity of the protein is a direct measure of its activation state, i.e., the exposure of the F-actin binding site in the C-ERMAD. A very large surface coverage of >90% was observed for the pseudophosphorylated mutant T567D bound to PIP2, in full agreement with the two-step model, which suggests a fully activated state of the protein under conditions of phosphorylation and binding to the natural receptor lipid PIP2 at the same time.
A comparison of this F-actin surface coverage with the value (∼60%) obtained if the pseudophosphorylated mutant is bound via DOGS-NTA-Ni clearly demonstrates that even though the protein is still capable of binding actin filaments to a large extent, a significant increase in activation occurs as a result of its binding to PIP2. There is also some evidence from in vivo experiments that PIP2 contributes to the activation of ezrin. For example, Auvinen et al. (14) reported that an upregulation of PIP-kinase leads to a colocalization of activated ERM proteins and F-actin. A similar observation has been reported by Matsui et al. (35). They examined PI4P5K-overexpressing cells, in which ERM proteins were recruited to plasma membranes to form microvilli.
A comparison of the F-actin binding capacity of ezrin wt with that of ezrin T567D bound via PIP2 identifies a synergism of PIP2 binding and phosphorylation of ezrin. It is obvious that ezrin wt has a decreased F-actin binding capacity, i.e., it is less activated than the ezrin T567D mutant. However, it still binds considerably more F-actin (∼50% surface coverage) than if it is bound via DOGS-NTA-Ni (∼20% surface coverage). This is consistent with our previous results, where we were able to show that PIP2 not only serves as a membrane anchor, but is capable of partly activating ezrin in such a way that a conformational change unmasks the F-actin binding site in the C-ERMAD (16). Taking these results together, it can be concluded that the interaction of ezrin wt and ezrin T567D with PIP2 induces a conformational switch that renders the F-actin binding site accessible. The F-actin binding affinity is then further enhanced by the negative charge in position 567 mimicking the phosphorylated state in vivo.
The situation is different for ezrin T567A. Independent of the mode of binding to the membrane, an F-actin surface coverage of only roughly 15% is found, which is in the same range as F-actin adsorption/protein-free membranes (14% for DOGS-NTA-Ni- and 8% for PIP2-containing membranes). This result suggests that the mutant ezrin T567A is not partially activated by the binding to PIP2, as was observed for ezrin wt. This further supports the view that the amino acid at position 567 plays a crucial role in the conformational switch, which exposes the F-actin binding site. A reason for this behavior might be that alanine in position 567 locks the protein in its dormant conformation, which is not as easily transformed to the active state as in the native protein ezrin wt.
The results we obtained for the secondary structure elements of ezrin T567A and ezrin wt using CD spectroscopy further support our view that the structure, and as a consequence, the binding ability of the inactive mutant, might be affected by the mutation. That is, deviation in the α-helical as well as random coil contents from those obtained for ezrin wt suggest that a structural change is introduced by the replacement of threonine for alanine in this mutant.
Conclusions
In conclusion, our results are in good agreement with the model proposed recently for moesin activation (12). The authors found that, depending on the cellular context, PIP2 binding is essential for the first activation step, and at the same time often sufficient for the establishment of membrane-cortex connections. The second activation step, i.e., phosphorylation of Thr-567, is required for the formation and maintenance of specialized cellular structures. Based on their results, they introduced the idea that phosphorylation acts as fine-tuning on PIP2-bound ERM proteins.
An earlier study of Yonemura et al. (10) also reaches the conclusion that, at least in some cell types, activation of ERM proteins does not necessarily require phosphorylation, but is regulated by a local increase in the PIP2 concentration. Indeed, our in vitro results strongly support this model, proving that ezrin wt can be activated through PIP2 binding, which is demonstrated in an enhanced F-actin binding capability, and that C-terminal phosphorylation mimicked by ezrin T567D provides additional stability for the activated state.
Acknowledgments
We thank Jutta Gerber-Nolte for technical assistance and Andreas Janshoff for providing a MATLAB (The MathWorks, Natick, MA) program for the evaluation of the isotherms.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (No. GE514/8-1 and No. STE884/11-1) and the Interdisciplinary Center for Clinical Research of the Münster Medical School.
Supporting Material
References
- 1.Fehon R.G., McClatchey A.I., Bretscher A. Organizing the cell cortex: the role of ERM proteins. Nat. Rev. Mol. Cell Biol. 2010;11:276–287. doi: 10.1038/nrm2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gary R., Bretscher A. Ezrin self-association involves binding of an N-terminal domain to a normally masked C-terminal domain that includes the F-actin binding site. Mol. Biol. Cell. 1995;6:1061–1075. doi: 10.1091/mbc.6.8.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu L.X., Hatakeyama J., Forte J.G. Comparative study of ezrin phosphorylation among different tissues: more is good; too much is bad. Am. J. Physiol. Cell Physiol. 2008;295:C192–C202. doi: 10.1152/ajpcell.00159.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turunen O., Wahlström T., Vaheri A. Ezrin has a COOH-terminal actin-binding site that is conserved in the ezrin protein family. J. Cell Biol. 1994;126:1445–1453. doi: 10.1083/jcb.126.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakamura F., Huang L., Furthmayr H. Regulation of F-actin binding to platelet moesin in vitro by both phosphorylation of threonine 558 and polyphosphatidylinositides. Mol. Biol. Cell. 1999;10:2669–2685. doi: 10.1091/mbc.10.8.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu L., Liu Y., Forte J.G. Ezrin oligomers are the membrane-bound dormant form in gastric parietal cells. Am. J. Physiol. Cell Physiol. 2005;288:C1242–C1254. doi: 10.1152/ajpcell.00521.2004. [DOI] [PubMed] [Google Scholar]
- 7.Zhu L., Zhou R., Forte J.G. High turnover of ezrin T567 phosphorylation: conformation, activity, and cellular function. Am. J. Physiol. Cell Physiol. 2007;293:C874–C884. doi: 10.1152/ajpcell.00111.2007. [DOI] [PubMed] [Google Scholar]
- 8.Barret C., Roy C., Niggli V. Mutagenesis of the phosphatidylinositol 4,5-bisphosphate (PIP2) binding site in the NH2-terminal domain of ezrin correlates with its altered cellular distribution. J. Cell Biol. 2000;151:1067–1080. doi: 10.1083/jcb.151.5.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niggli V., Andréoli C., Mangeat P. Identification of a phosphatidylinositol-4,5-bisphosphate-binding domain in the N-terminal region of ezrin. FEBS Lett. 1995;376:172–176. doi: 10.1016/0014-5793(95)01270-1. [DOI] [PubMed] [Google Scholar]
- 10.Yonemura S., Matsui T., Tsukita S. Rho-dependent and -independent activation mechanisms of ezrin/radixin/moesin proteins: an essential role for polyphosphoinositides in vivo. J. Cell Sci. 2002;115:2569–2580. doi: 10.1242/jcs.115.12.2569. [DOI] [PubMed] [Google Scholar]
- 11.Rasmussen M., Alexander R.T., Pedersen S.F. Osmotic cell shrinkage activates ezrin/radixin/moesin (ERM) proteins: activation mechanisms and physiological implications. Am. J. Physiol. Cell Physiol. 2008;294:C197–C212. doi: 10.1152/ajpcell.00268.2007. [DOI] [PubMed] [Google Scholar]
- 12.Roch F., Polesello C., Payre F. Differential roles of PtdIns4,5P2 and phosphorylation in moesin activation during Drosophila development. J. Cell Sci. 2010;123:2058–2067. doi: 10.1242/jcs.064550. [DOI] [PubMed] [Google Scholar]
- 13.Fievet B.T., Gautreau A., Arpin M. Phosphoinositide binding and phosphorylation act sequentially in the activation mechanism of ezrin. J. Cell Biol. 2004;164:653–659. doi: 10.1083/jcb.200307032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Auvinen E., Kivi N., Vaheri A. Regulation of ezrin localization by Rac1 and PIPK in human epithelial cells. Exp. Cell Res. 2007;313:824–833. doi: 10.1016/j.yexcr.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Hao J.J., Liu Y., Shaw S. Phospholipase C-mediated hydrolysis of PIP2 releases ERM proteins from lymphocyte membrane. J. Cell Biol. 2009;184:451–462. doi: 10.1083/jcb.200807047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janke M., Herrig A., Janshoff A. Actin binding of ezrin is activated by specific recognition of PIP2-functionalized lipid bilayers. Biochemistry. 2008;47:3762–3769. doi: 10.1021/bi702542s. [DOI] [PubMed] [Google Scholar]
- 17.Gautreau A., Louvard D., Arpin M. Morphogenic effects of ezrin require a phosphorylation-induced transition from oligomers to monomers at the plasma membrane. J. Cell Biol. 2000;150:193–203. doi: 10.1083/jcb.150.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou R., Zhu L., Forte J.G. Phosphorylation of ezrin on threonine 567 produces a change in secretory phenotype and repolarizes the gastric parietal cell. J. Cell Sci. 2005;118:4381–4391. doi: 10.1242/jcs.02559. [DOI] [PubMed] [Google Scholar]
- 19.Koltzscher M., Neumann C., Gerke V. Ca2+-dependent binding and activation of dormant ezrin by dimeric S100P. Mol. Biol. Cell. 2003;14:2372–2384. doi: 10.1091/mbc.E02-09-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whitmore L., Wallace B.A. DICHROWEB, an online server for protein secondary structure analyses from circular dichroism spectroscopic data. Nucleic Acids Res. 2004;32(Web Server issue):W668–W673. doi: 10.1093/nar/gkh371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janshoff A., Galla H.J., Steinem C. Piezoelectric mass-sensing devices as biosensors—an alternative to optical biosensors? Angew. Chem. Int. Ed. Engl. 2000;39:4004–4032. doi: 10.1002/1521-3773(20001117)39:22<4004::aid-anie4004>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 22.Pearson M.A., Reczek D., Karplus P.A. Structure of the ERM protein moesin reveals the FERM domain fold masked by an extended actin binding tail domain. Cell. 2000;101:259–270. doi: 10.1016/s0092-8674(00)80836-3. [DOI] [PubMed] [Google Scholar]
- 23.Steinem C., Janshoff A. Springer Verlag; Berlin, Germany: 2007. Piezoelectric Sensors. [Google Scholar]
- 24.Herrig A., Janke M., Steinem C. Cooperative adsorption of ezrin on PIP2-containing membranes. Biochemistry. 2006;45:13025–13034. doi: 10.1021/bi061064a. [DOI] [PubMed] [Google Scholar]
- 25.Behn D., Bosk S., Steinem C. Quantifying the interaction of the C-terminal regions of polycystin-2 and polycystin-1 attached to a lipid bilayer by means of QCM. Biophys. Chem. 2010;150:47–53. doi: 10.1016/j.bpc.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 26.McLaughlin S., Wang J., Murray D. PIP2 and proteins: interactions, organization, and information flow. Annu. Rev. Biophys. Biomol. Struct. 2002;31:151–175. doi: 10.1146/annurev.biophys.31.082901.134259. [DOI] [PubMed] [Google Scholar]
- 27.Kremser L., Bilek G., Kenndler E. Capillary electrophoresis of viruses, subviral particles and virus complexes. J. Sep. Sci. 2007;30:1704–1713. doi: 10.1002/jssc.200700105. [DOI] [PubMed] [Google Scholar]
- 28.Holmes K.C., Popp D., Kabsch W. Atomic model of the actin filament. Nature. 1990;347:44–49. doi: 10.1038/347044a0. [DOI] [PubMed] [Google Scholar]
- 29.Matsui T., Maeda M., Tsukita S. Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J. Cell Biol. 1998;140:647–657. doi: 10.1083/jcb.140.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simons P.C., Pietromonaco S.F., Elias L. C-terminal threonine phosphorylation activates ERM proteins to link the cell's cortical lipid bilayer to the cytoskeleton. Biochem. Biophys. Res. Commun. 1998;253:561–565. doi: 10.1006/bbrc.1998.9823. [DOI] [PubMed] [Google Scholar]
- 31.Janshoff A., Steinem C. Quartz crystal microbalance for bioanalytical applications. Sensors Update. 2001;9:313–354. [Google Scholar]
- 32.Andréoli C., Martin M., Mangeat P. Ezrin has properties to self-associate at the plasma membrane. J. Cell Sci. 1994;107:2509–2521. doi: 10.1242/jcs.107.9.2509. [DOI] [PubMed] [Google Scholar]
- 33.Bretscher A., Gary R., Berryman M. Soluble ezrin purified from placenta exists as stable monomers and elongated dimers with masked C-terminal ezrin-radixin-moesin association domains. Biochemistry. 1995;34:16830–16837. doi: 10.1021/bi00051a034. [DOI] [PubMed] [Google Scholar]
- 34.Ceyrolles W.J., Viot P., Talbot J. Kinetics of heterogeneous adsorption: mean-field theory and simulations. Langmuir. 2002;18:1112–1118. [Google Scholar]
- 35.Matsui T., Yonemura S., Tsukita S. Activation of ERM proteins in vivo by Rho involves phosphatidyl-inositol 4-phosphate 5-kinase and not ROCK kinases. Curr. Biol. 1999;9:1259–1262. doi: 10.1016/s0960-9822(99)80508-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


