Abstract
High hydrostatic pressure (HHP) present in natural environments impacts on cell membrane biophysical properties and protein quaternary structure. We have investigated the effect of high hydrostatic pressure on G22E-MscL, a spontaneously opening mutant of Escherichia coli MscL, the bacterial mechanosensitive channel of large conductance. Patch-clamp technique combined with a flying-patch device and hydraulic setup allowed the study of the effects of HHP up to 90 MPa (as near the bottom of the Marianas Trench) on the MscL mutant channel reconstituted into liposome membranes, in addition to recording in situ from the mutant channels expressed in E. coli giant spheroplasts. In general, against thermodynamic predictions, hydrostatic pressure in the range of 0.1–90 MPa increased channel open probability by favoring the open state of the channel. Furthermore, hydrostatic pressure affected the channel kinetics, as manifested by the propensity of the channel to gate at subconducting levels with an increase in pressure. We propose that the presence of water molecules around the hydrophobic gate of the G22E MscL channel induce hydration of the hydrophobic lock under HHP causing frequent channel openings and preventing the channel closure in the absence of membrane tension. Furthermore, our study indicates that HHP can be used as a valuable experimental approach toward better understanding of the gating mechanism in complex channels such as MscL.
Introduction
Throughout the course of evolution, deep-sea organisms developed a set of mechanisms protecting them from the effects of high hydrostatic pressure (HHP), a major determinant of their habitat. Physically, HHP is scalar quantity acting in any direction on these organisms at macroscopic as well as molecular structural levels. Because in vitro studies have shown that pressure of approximately tens of MPa (1 MPa = 10 atm) can impair the quaternary structure of proteins and modify the biophysical properties of cell membranes (1,2), it is of particular interest to understand the effects of HHP at a molecular level.
Ion channels, transporters, and ion exchangers serve as transducers of environmental stimuli acting on a biological cell. High pressure has previously been used as a means to study the functioning of a number of ion channels in excitable cells (3–9). No doubt, their structure, function, and regulation can be disturbed or damaged by HHP (1). The detailed mechanism of action is unclear and can vary depending on the particular protein considered. Hydrostatic pressure is a scalar physical entity that targets living matter at every level of its organization, bringing the difficulty to understand its mechanism of action. In the case of membrane integral proteins, it is reasonable to assume that in addition to HHP acting directly on a protein itself it may also act indirectly by changing the physico-chemical properties of phospholipids, the major constituents of cellular membranes (8,10). Consequently, an approach to study the effects of HHP on membrane proteins is by using a reductionist method by reconstituting the protein of interest in an artificial phospholipid bilayer in isolation from the whole organism. Among the most elegant examples of studying membrane proteins by reconstitution into liposomes has been the bacterial mechanosensitive channel of large conductance (MscL) (11,12–16).
In this study, we have investigated the effect of HHP, using a development of Heinemann's flying-patch patch-clamp technique (4,8,17). We applied it on the spontaneously active gain-of-function (GOF) G22E mutant (18) of MscL in situ in giant spheroplasts of Escherichia coli (19), as well as in vitro by reconstitution into azolectin liposomes (11,20). The functioning of MscL is intimately related to its boundary lipids, and therefore it is reasonable to expect that HHP would affect its gating by perturbing the protein-bilayer interaction. G22, the glycine residue at position 22 in the first transmembrane (TM1) helix of MscL, plays a pivotal role in the channel gating (18,21).
Together with A20, V21, V23, I24, I25, G26, and A27 residues, G22 residues of five adjacent TM1 helices of the channel form a hydrophobic-lock, void of water molecules (22). The hydrophobic lock can be deactivated by tension from the membrane (18,22,23). If no tension is applied, the lock keeps the channel in the closed state. Single substitution of the glycine (hydropathy index −0.4) at position 22 to more hydrophilic glutamate (hydropathy index −3.5) is sufficient to produce a channel that spontaneously gates (18). The choice of the spontaneously active GOF mutant channel for HHP studies reported here results from the fact that membrane tension, which would otherwise activate wild-type MscL, cannot be applied in the high-pressure chamber.
Among mechanosensitive ion channels, high-pressure effects have previously been only reported for MscS, the bacterial mechanosensitive channel of small conductance (8). To our knowledge, this report presents the first high-pressure study for MscL.
Although homologs of MscL have (to our knowledge) thus far not been found in deep-sea organisms, the finding of the bacterial type MS channels has been reported. As an example, methanogenic archaeon Methanococcus jannaschii has two mechanosensitive channels: MscMJ and MScMJLR (24). Both MscMJ and MscMJLR show a high degree of sequence and secondary structure conservation with MscS and its other bacterial homologs. The alignment of sequences of MscL, MscS, and MscMJ homologs further revealed that bacterial and archaeal channels may share a common ancestral origin, suggesting the evolution of prokaryotic MS channels via gene duplication of an MscL-like progenitor gene followed by divergence—indicating that the common ancestor of the prokaryotic MS channels could have resembled MscL (25). Given that mechanosensitive channels are also present in some deep-sea organisms, such as those that live naturally at pressures of up to 100 MPa near the bottom of the Marianas Trench (24,26,27), this study may contribute to our understanding of the adaptations required for the function of mechanosensitive channels in high-pressure environments.
Materials and Methods
Cell-free expression construct
MscL was subcloned by PCR (employing the primers gacctgcagctggttccgcgtgga and attaagctttcaggcgcttgtta) from the MscL pGEX1.1 expression vector (11) into vector pQE-31 (Qiagen, Germantown, MD) PstI and HindIII sites. The resulting clone, MscL 2.1, was constructed to give higher expression yields in E. coli, while maintaining an identical protein after thrombin cleavage from a fusion tag containing six histidines rather than glutathione S-transferase. G22E MscL site-directed mutagenesis was performed on the MscL 2.1 construct using a QuikChange II (Stratagene, La Jolla, CA) strategy employing the primers gtggatttggcggtggaggtcattatcggtgcgg and ccgcaccgataatgacctccaccgccaaatccac (from Invitrogen, Carlsbad, CA).
G22E MscL cell-free expression
Due to cellular toxicity and poor G22E spheroplast patch yield, MscL G22E was produced in a cell-free expression system essentially as described previously (28) scaled to a 2 mL reaction mixture with a 20 mL outer buffer, incubated for 13 h at 200 rpm (3/4 inch stroke) at 37°C. Because the MscL G22E expression vector was under control of a T5 promoter, a T7 RNA polymerase expression vector was not included. The expected lower yields of a T5 promoter system was adequate for patch-clamp recordings. After incubation, MscL was solubilized with the addition of DDM detergent (Anatrace D310; Affymetrix, Santa Clara, CA) to 8 mM for 3.5 h. A quantity of 100 μL suspension of washed Talon metal affinity resin (cat. No. 635502; Clontech, Mountain View, CA) was added to the solubilization to allow binding of the hex-histidine fusion-tagged MscL. After 2 h, the resin was pelleted and washed (via pelleting) with 1 mM DDM PBS pH 7.5, and resuspended to a final 100 μL of wash buffer. MscL was released into solution from the histidine fusion tag by cleavage with 5 U thrombin (T6634-1KU; Sigma, St. Louis, MO) at 4°C over a period of a weekend.
Liposome reconstitution of G22E MscL
The purified G22E MscL protein was reconstituted into azolectin liposomes using dehydration/rehydration (D/R) technique (modified from Häse et al. (11) and Delcour et al. (29)): 20 mg of dry azolectin was placed in glass centrifuge tube and dissolved in 1–2 mL of chloroform. Chloroform was evaporated by nitrogen flow with constant rotating of the tube to form homogenous lipid layer without lumps, then with an extra 20 min drying with nitrogen flow. A quantity of 2 mL of D/R buffer (200 mM KCl, 5 mM HEPES, pH 7.2 with KOH) was added to the dried azolectin and thoroughly resuspended using a small brush. Next, the tube (bottom part) was put into an ultrasound bath for 10 min. After sonication, the emulsion appeared transparent and opalescent. A quantity of 200 μL of the emulsion was mixed with the G22E MscL protein to achieve a protein/lipid mass ratio of 1:1000.
The obtained mixture was diluted with D/R buffer to reach a total volume of 3 mL. The sample was mixed by a platform rocker for 1 h, then 20–30 mg of Biobeads (BioRad, Hercules, CA) were added for a further 3 h. After that, the beads were allowed to settle, and the supernatant was centrifuged at 440,000 × g for 30 min. The pellet was resuspended with 40 μL D/R buffer. A quantity of 20 μL aliquots were put as spots on clean pieces of cover glass and dehydrated overnight in a desiccator. Next morning spots were rehydrated by placing 15–20 μL of D/R buffer on each spot and left for 24 h at 4°C. A small amount (1–2 μL) of a liposome spot was placed in the recording chamber containing the recording solution. Blisters started to appear after 10–20 min as thin-walled bubbles on sides of lipid lumps.
Preparation of giant spheroplasts
Patch-clamp recordings from expressed MscL G22E channels were initially measured within native membranes of giant spheroplasts preparations made from the triple knockout E. coli strain MJF465 (mscL−/mscS−/mscK−) devoid of inherent activities of the MscL, MscS, and MscK channels (30), using a previously described MscL G22E expression vector (18). Spheroplasts were prepared as previously described (19) with the following modifications: After culturing with 60 μg/mL cephalexin antibiotic (to obtain elongated cells), expression was induced with 1 mM IPTG for <1 h. The following formation of spheroplasts with 6 mM EDTA buffer, was stopped with 5 mM MgCl2 buffer with 15 mM HEPES pH 7.6 rather than 10 mM Tris pH 7.2, as this allowed for better cell integrity.
Patch-clamp and HHP setup
An Axopatch 1D patch-clamp amplifier (Axon Instruments, Molecular Devices, Sunnyvale, CA) combined with a homemade HHP setup was used for recording. The HHP setup consisted of an oil pump, manometer, HHP chamber, oil reservoir, and a flying-patch device, as detailed previously (17). Each experiment consisted of the following steps:
-
1.
Forming an inside-out excised patch from a spheroplast or azolectin membrane.
-
2.
Testing the mechanosensitivity and spontaneous activity of G22E MscL.
-
3.
Transferring the flying-patch device from the patch-clamp setup into the HHP chamber.
-
4.
Applying depolarizing (negative pipette) voltage to a membrane patch until MscL activity was observed.
-
5.
Recording the channel spontaneous activity under HHP.
Each HHP increase from a given level to the next level was done slowly, within 20–30 s, to minimize heating of the preparation.
Patch pipettes were pulled from borosilicate glass capillaries (Drummond Scientific, Broomall, PA) on a P87 pipette puller (Sutter Instruments, Novato, CA) and had a resistance of 3–5 MΩ. Both pipette and bath solution contained 200 mM KCl, 40 mM MgCl2, and 5 mM HEPES (pH 7.2 adjusted with KOH). When spheroplasts were used, gigaOhm seals were formed by catching a spheroplast floating in the bath solution on the tip of a pipette and applying gentle suction to the pipette. When azolectin was used, gigaOhm seals were formed by touching the giant unilamellar blister with pipette tip. Inside-out excised patch recording configuration was achieved by air exposure of the patch pipette with a spheroplast or azolectin vesicle on its tip. Presence of the G22E MscL channels in a patch was tested by applying suction and voltage (+30 mV and −30 mV) to the patch pipette.
To test the mechanosensitivity of G22E MscL, opening steps of negative pressure were applied to the patch pipette and converted to voltage by a piezoelectric pressure transducer (Omega Engineering, Stamford, CT) with monitoring by an oscilloscope (TDS 210; Tektronix, Beaverton, OR). Acquisition and data analysis software used was pClamp 9.2 (Axon Instruments, Molecular Devices) and Origin 6.1 (OriginLab, Northampton, MA).
Open probability of N channels, NPo was calculated using two different methods:
-
1.
By dividing the total current integrated over the time of recording Δt by the single channel current integrated over the same time Δt, which gives NPo as the open probability of unknown number of channels in a patch, or
-
2.
By using the built-in utility in Clampfit 9.2 (Axon Instruments) software: for each continuous recording, separate NPo values were calculated for time periods when the steady-state voltage step was applied at a given HHP level.
Because NPo at 0.1 MPa (1 atm) varied from patch to patch, all NPo values were normalized to the maximal value obtained during each recording. Statistical data analysis was done using Student t-test. Voltage was applied to each single patch by applying negative pipette voltage in 10 mV steps, starting from −10 mV. Patches with a voltage threshold of −80 mV or greater were excluded because of a risk of loosing a gigaOhm seal during application of HHP.
All chemicals were analytical grade and were purchased from Sigma.
Results and Discussion
The technical design of the flying-patch device does not allow applying stretch to the membrane patch to activate mechanosensitive channels because the holder with the patch electrode is inside the high pressure chamber. Nevertheless, there are a few approaches to obtain spontaneously active mechanosensitive channel at atmospheric (0.1 MPa) pressure. One is to reconstitute MscL into a thinner bilayer (made of DPPC, for example) where channels require very low membrane tension for activation or are spontaneously active due to hydrophobic mismatch (13). Another approach is to apply conical lipids such as lysophosphatidylcholine(LPC) to one leaflet of membrane to deform it chemically with subsequent activation of MscL (14,31). Note that neither approach was used in this study due to the fragility of liposome membrane patches either made of DPPC lipids or exposed to LPC under the experimental conditions used.
Previous electrophysiological studies have shown similar behavior of G22E MscL and G22K, another spontaneously active MscL mutant (compare Fig. 1 and Fig. 3 A in Yoshimura et al. (18)). In our hands, G22E MscL has exhibited stable spontaneous activity for tens of minutes, which indicated that this mutant would be amenable for flying-patch recordings under HHP. No difference could be observed in G22E channel gating behavior in recordings from giant spheroplasts or proteoliposomes, except that spheroplast patches were more stable under HHP. Importantly, in giant liposomes MscL channels are oriented uniformly and right-side-out such that in excised liposome patches the extracellular periplasmic loops of the channel pentamer are facing the interior of the patch pipette, whereas the intracellularly located N- and C-terminal domains are facing the bath (32). This channel orientation is the same as in the native membrane of giant bacterial spheroplasts. At apparent 0 mmHg, channels were spontaneously active and mechanosensitive (Fig. 1).
Figure 1.
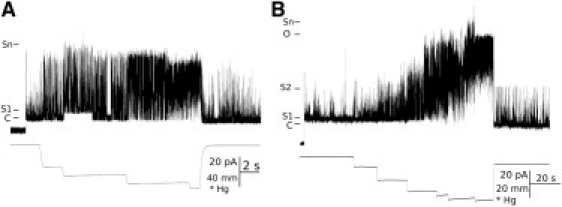
Patch-clamp recordings of G22E GOF MscL. Spontaneous and mechanosensitive activity of G22E MscL in an E. coli spheroplast patch (A) and in a liposome membrane patch (B). C, S1, S2, O, and Sn denote closed, first open substate, second open substate, open state, and n-open substates (from unknown number of channels in a patch), accordingly. Scales for recorded current, negative pressure, and time are shown below the pressure line.
Figure 3.
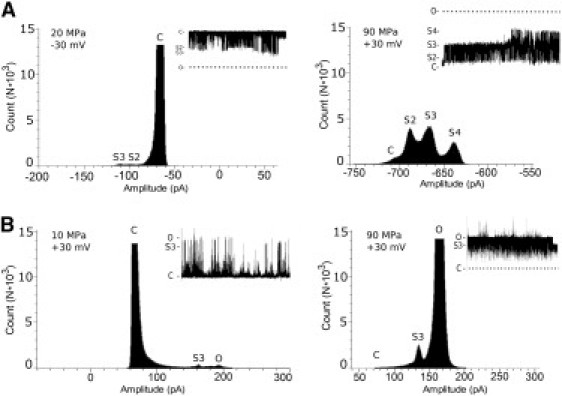
Effect of HHP on substate distribution of G22E MscL. Amplitude histograms of G22E MscL currents recorded from the channels reconstituted in azolectin liposomes (A) or expressed E. coli spheroplasts (B). At modest hydrostatic pressure, channels stay mostly closed but also gate at an open level (bottom left) or at subconducting levels (upper left). If HHP was applied, channels become open (bottom right) or spend more time at subconducting levels (upper right). Furthermore, channels also gate between different substate levels, usually transiting toward the higher open state. (Dashed lines) Closed and fully open levels. Applied voltages and HHP were as indicated.
If, after applying the suction to the membrane patch, the G22E channels retained their spontaneous activity, then the patch was moved into the high-pressure chamber, pipette voltage of +30 mV or −30 mV was applied, and channel activity was recorded for 3–5 min to test the stability of the seal. HHP was applied in 20 MPa steps, starting from 10 MPa and increasing it up to the maximum of 90 MPa. Pressure at each step was held for 1–2 min and after that released back to a lower, usually atmospheric pressure (0.1 MPa). The G22E channels were spontaneously active regardless of sign of voltage across the membrane. Given that MscL gates independently of voltage and its activity depends only on the applied pressure in this study, we have applied occasionally negative rather than positive voltages in our experiments because we observed that some patches became leakier at positive than at negative pipette voltages. In such cases, recordings have been obtained at negative pipette voltages. No difference was observed in channel activity, whether the negative or positive voltage was applied to a patch (data not shown). In every recorded patch, NPo was measured at each level of applied high pressure. The NPo values were normalized against the maximal open probability measured in a particular patch. NPo values determined for each particular HHP level were averaged together with other data from other patches examined at the same HHP step. The necessity to use the normalized NPo data was because the number of channels and their activity largely varied from patch to patch. At all HHP levels, we have observed fully open states of G22E MscL (Figs. 2 and 3). This indicates that the channel structure was intact and the channel was fully functional under HHP. This notion is supported by the fact that, upon return to lower pressures, the channels exhibited similar activity as before the application of HHP. In other words, the high-pressure effects on the channel gating were reversible—indicating that the channel proteins were not denatured by HHP.
Figure 2.
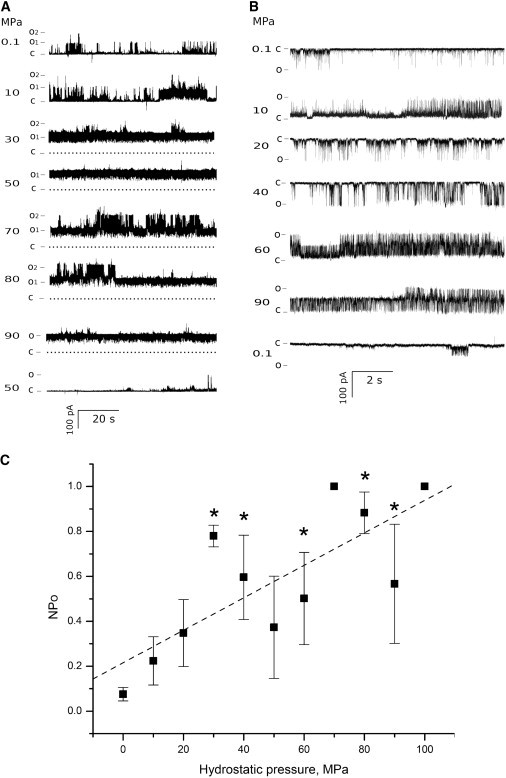
Effect of high hydrostatic pressure on G22E GOF MscL activity. Recordings from a single spheroplast patch (A) and from a liposome patch (B) are shown. Segments of channel activity are shown at different levels of HHP, starting at 0.1 MPa (atmospheric pressure). The term “C” indicates closed state, and “O1” and “O2” indicate open states for two channels. (Dashed lines) Closed state for all channels in the patch. The currents were recorded at +30 mV, except for 20 and 40 MPa for the liposome patch. (C) Normalized open probabilities (NPo) for n recordings. Each data point represents mean ± SE (n ≥ 3). (Asterisks) Significantly different from mean at 0.1 MPa (p ≤ 0.05). (Dashed line) Overall NPo change upon increase of hydrostatic pressure.
The overall results of our study show that increasing the pressure increased the open probability NPo of G22E MscL channels (Fig. 2 C). Examination of currents recorded under different HHP indicates that the higher the hydrostatic pressure, the longer the channel stayed in an open state, being either subconducting or a fully open state. This has been observed both for channels reconstituted in liposomes and those expressed in E. coli spheroplasts. Thermodynamically, it is expected that HHP would affect the conformation of the channel favoring the state of smaller volume (9), which in the case of MscL corresponds to the closed channel configuration. Recent published structures of the open MscL channel allow an estimate of ∼100 nm3 for the volume of the transmembrane portion of the open channel compared to ∼70 nm3 for the volume of the closed channel, which were calculated by approximating the transmembrane portion of the channel to a cylinder of 50 Å in diameter and 35 Å in height for the closed, and 70 Å in diameter and 25 Å in height for the open channels, respectively (14,33,34).
In addition, the increase in the thickness of the lipid bilayer caused by the HHP (9,35) due to the hydrophobic mismatch would also favor the closed channel state (13). Contrary to these predictions, which could previously be confirmed for MscS studied under HHP (8), in the case of G22E MscL, HHP favored the open state—i.e., the state of apparently bigger volume. In the volume estimate for the open channel, the void volume of the open channel pore was not subtracted from the overall volume. If the void volume of the open pore of ∼20 nm3 estimated by approximating it to a cylinder of 30 Å in diameter (36) and 25 Å in height (14), is subtracted, the volume of the open channel, excluding the void volume, would be ∼80 nm3, which is still larger than the volume of the closed channel. The apparent discrepancy between the G22E channel behavior and general applicability of Le Chatelier principle to any thermodynamic system becomes resolved when the following experimental results are taken into consideration:
-
1.
The significant increase in the channel gating at subconducting levels observed in G22E MscL with increasing HHP is an indication of the channel behaving in accordance with thermodynamics. At subconducting levels, the channel pore is only partially open—corresponding to the smaller volumes of partially open channels, which at high hydrostatic pressures is thermodynamically more favorable.
-
2.
The fact that the channel opens more frequently at high pressures is consistent with the increase in electrostatic repulsion of glutamic acid residues as the channel is forced to remain closed with increase in HHP.
Based on these results, we hypothesize that electrostatic repulsion between glutamic acid residues is counteracting the thermodynamic effect of HHP in the 100 MPa range of pressures we could apply in our experiments. Consequently, the observed increase in open probability of G22E MscL with increasing HHP is largely due to electrostatic repulsive forces between glutamic acid residues in the hydrophobic lock of the channel with a consequence that the entire channel gate becomes energetically unstable and more accessible to water molecules.
It has been estimated that in the closed conformation MscL could be filled with water molecules to a point 2.4 nm below the periplasmic surface of the channel (37). The second cavity at the cytoplasmic face of the channel was also found to be water-accessible, resulting in most of the interior of the closed MscL being filled with water molecules except for a hydrophobic stretch of 0.8 nm forming a watertight occlusion (i.e., the hydrophobic lock). In the open state, the hydrophobic gate of the channel becomes exposed to water, as originally proposed (18,22,36). Under HHP, water molecules can penetrate more easily into voids near the gate and thus unlock the gate by impairing the contact between the pore-forming TM1 subunits within the channel pentamer (38). Furthermore, molecular-dynamics simulations of the GOF mutant G22N having the pore size comparable to the wild-type MscL, showed that, in the G22N mutant, water molecules permeated without membrane tension applied to the lipid bilayer. The spontaneous water permeation was mediated by hydrogen bonds between Asn22 and water molecules (39). Similarly, hydrogen bonds between Glu22 and water molecules are likely to mediate water permeation in the G22E mutant.
In contrast to the G22E MscL mutant channel, HHP generally favored closing of the MscS channel—resulting in a reduction of the channel open probability with increase in hydrostatic pressure. However, similar to the G22E channel, MscS conductance remained unaffected and the channel exhibited frequent gating at subconducting levels as the pressure was increased, in agreement with results of other studies of ion channels in which pressure was found to manifest a variety of kinetic effects but no change in conductance (6,7). Using the classical thermodynamic relationship
where K is the equilibrium constant, ΔV is the volume change across the equilibrium, p is pressure, and R and T are the gas constant and temperature, respectively, the change in volume per MscS channel molecule ΔV of ∼−155 Å3 was calculated, with the negative sign of ΔV indicating that the closed state of the channel is thermodynamically favored at high pressures (8). This volume change, which is very small compared to the volume of the MscS channel heptamer (∼6.0 × 105 Å5), was interpreted to reflect a change in the volume of the MscS channel-bilayer complex as it adopts its open and closed configuration, with the closed state occupying less volume due to the bilayer component (8). Similar to the G22E MscL behavior described in this study, the tendency of the MscS channel to gate more frequently at subconducting levels is consistent with the overall reduction in volume of the channel-bilayer complex under HHP.
As discussed above, with the increase in pressure there was an increased tendency of the G22E channel to gate at subconducting levels. MscL has previously been reported to reach the fully open state through a number of short-lived subconducting states (S1, S2, S3, …) (40,41). A prominent substate near the closed state (S1, see Fig. 1) at 0.1 MPa became a low occupancy state under HHP, whereas other substate levels (S2, S3, and S4) became clearly visible because of high occupancy of these sublevels (Fig. 3). In addition, the channels were gating over extended periods at the fully open state. Hidden Markov analysis (41) and modeling of the MscL gating process (40) gave a conservative estimate of 5–7 conductance levels consisting of 3–5 subconducting levels in addition to a closed and fully open level. The reported subconductance levels were ∼9%, 20–25%, 51–56%, ∼74%, and ∼90% of the fully open state. In our experiments, S1 was 7% of the fully open state, whereas S2, S3, and S4 were 55%, 72%, and 87% of the open state, respectively.
The small differences between our and the reported substates may be due to variation in the biological preparation used in our and other studies. Here, we used the recombinant GOF MscL mutant protein purified for liposome reconstitution as described in Materials and Methods. In other studies (40,41), the MscL proteins were either obtained by thrombin digestion of the glutathione-S-transferase-MscL fusion protein and had an N-terminal part 9-amino-acids longer than the native wild-type MscL (11) or they had a 6×His-tag attached to the N-terminus of each subunit (42). Nevertheless, based on our data, this study shows reasonable correspondence to the previously reported subconductance levels, suggesting that studying gating of ion channels under HHP may be a useful experimental approach to uncover short-lived subconducting levels, which are usually difficult to observe under normal recording conditions at 0.1 MPa. Furthermore, we believe that, combined with hidden Markov analysis, HHP studies of ion channels could provide a potent experimental strategy to study the question of the number and amplitude of subconducting levels in complex channels such as MscL, which is relevant to a conceptual understanding of ion channel gating.
Overall, our results suggest that the HHP applied in this study (10–90 MPa) was sufficient to destabilize the hydrophobic lock by forcing an increased number of water molecules into the gate region. This would cause the G22E channel to open more frequently by gating predominantly between subconducting levels and the fully open state with increasing HHP (Figs. 2 and 3), as conceptualized in Fig. 4. The contribution of other structural domains of MscL to the effects of HHP remains unclear at the present moment and requires further investigation.
Figure 4.
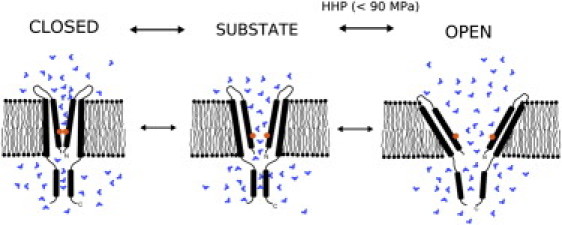
Hypothetical mechanism of the pressure effect on G22E MscL gating under high hydrostatic pressure. Hydrophilic substitution (glycine to glutamate) at the narrowest part of the MscL channel gate impairs the hydrophobic lock and causes the excess hydration. Under the influence of HHP, the water molecules enter the voids of TM1 pore-forming helices, interfering with their intimate contact within the channel pentamer and channel closing. At pressures above atmospheric pressure (0.1 MPa), the channel is gating predominantly at the fully open and different subconducting states. (Orange circle) G22E mutation. (Blue shapes) Water molecules. For clarity, only two MscL monomers are shown.
Conclusion
In this study, we have shown that the gating mechanism of G22E MscL is affected by HHP. Thermodynamically, an increase in HHP should favor the channel to remain predominantly in a smaller volume that would enforce a closed state. However, our results demonstrate that the impairment of the hydrophobic lock by substitution of the critical glycine 22 residue to glutamic acid, results in a predominantly open channel under HHP. Most likely this is due to increased water permeation mediated by hydrogen bonds between the Glu22 residue and water molecules. Furthermore, the results of the study indicate that HHP can be employed as a valuable experimental approach, which could help to resolve complexity in estimation of the number and amplitude of subconductance levels characteristic for the gating process in complex ion channels.
Acknowledgments
We thank Dr. Kenjiro Yoshimura for kindly donating the G22E expression vector.
This project was supported by grants from the Australian Research Council to B. M.
Contributor Information
Evgeny Petrov, Email: petrov67@gmail.com.
Boris Martinac, Email: b.martinac@victorchang.edu.au.
References
- 1.Macdonald A.G. The effects of pressure on the molecular structure and physiological functions of cell membranes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1984;304:47–68. doi: 10.1098/rstb.1984.0008. [DOI] [PubMed] [Google Scholar]
- 2.Mozhaev V.V., Heremans K., Balny C. High pressure effects on protein structure and function. Proteins. 1996;24:81–91. doi: 10.1002/(SICI)1097-0134(199601)24:1<81::AID-PROT6>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 3.Conti F., Inoue I., Stühmer W. Pressure dependence of sodium gating currents in the squid giant axon. Eur. Biophys. J. 1984;11:137–147. doi: 10.1007/BF00276629. [DOI] [PubMed] [Google Scholar]
- 4.Heinemann S.H., Conti F., Neher E. Effects of hydrostatic pressure on membrane processes. Sodium channels, calcium channels, and exocytosis. J. Gen. Physiol. 1987;90:765–778. doi: 10.1085/jgp.90.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyer R., Heinemann S.H. Temperature and pressure dependence of Shaker K+ channel N- and C-type inactivation. Eur. Biophys. J. 1997;26:433–445. doi: 10.1007/s002490050098. [DOI] [PubMed] [Google Scholar]
- 6.Macdonald A.G. Experiments on ion channels at high pressure. Biochim. Biophys. Acta. 2002;1595:387–389. doi: 10.1016/s0167-4838(01)00359-4. [DOI] [PubMed] [Google Scholar]
- 7.Macdonald A.G. Ion channels under high pressure. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002;131:587–593. doi: 10.1016/s1095-6433(01)00510-4. [DOI] [PubMed] [Google Scholar]
- 8.Macdonald A.G., Martinac B. Effect of high hydrostatic pressure on the bacterial mechanosensitive channel MscS. Eur. Biophys. J. 2005;34:434–441. doi: 10.1007/s00249-005-0478-8. [DOI] [PubMed] [Google Scholar]
- 9.Wann K.T., Macdonald A.G. The effects of pressure on excitable cells. Comp. Biochem. Phys. A Phys. 1980;66:1–12. [Google Scholar]
- 10.Petrov, E., P. R. Rohde, …, B. Martinac. 2007. Effect of high hydrostatic pressure and voltage on gating of the bacterial mechanosensitive channel of small conductance. In Proceedings of the 4th International Conference on High Pressure Bioscience and Biotechnology. F. Abe and A. Suzuki, editors. Tsukuba, Japan. 20–27.
- 11.Häse C.C., Le Dain A.C., Martinac B. Purification and functional reconstitution of the recombinant large mechanosensitive ion channel (MscL) of Escherichia coli. J. Biol. Chem. 1995;270:18329–18334. doi: 10.1074/jbc.270.31.18329. [DOI] [PubMed] [Google Scholar]
- 12.Kloda A., Petrov E., Martinac B. Mechanosensitive channel of large conductance. Int. J. Biochem. Cell Biol. 2008;40:164–169. doi: 10.1016/j.biocel.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Perozo E., Kloda A., Martinac B. Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nat. Struct. Biol. 2002;9:696–703. doi: 10.1038/nsb827. [DOI] [PubMed] [Google Scholar]
- 14.Perozo E., Cortes D.M., Martinac B. Open channel structure of MscL and the gating mechanism of mechanosensitive channels. Nature. 2002;418:942–948. doi: 10.1038/nature00992. [DOI] [PubMed] [Google Scholar]
- 15.Moe P., Blount P. Assessment of potential stimuli for mechano-dependent gating of MscL: effects of pressure, tension, and lipid headgroups. Biochemistry. 2005;44:12239–12244. doi: 10.1021/bi0509649. [DOI] [PubMed] [Google Scholar]
- 16.Sukharev S.I., Blount P., Kung C. Methods: A Companion to Methods in Enzymology. Academic Press; New York: 1994. Functional reconstitution as an assay for biochemical isolation of channel proteins: application to the molecular identification of a bacterial mechanosensitive channel; pp. 51–59. [Google Scholar]
- 17.Macdonald A.G., Martinac B. Effect of high hydrostatic pressure on the porin OmpC from Escherichia coli. FEMS Microbiol. Lett. 1999;173:327–334. doi: 10.1111/j.1574-6968.1999.tb13521.x. [DOI] [PubMed] [Google Scholar]
- 18.Yoshimura K., Batiza A., Kung C. Hydrophilicity of a single residue within MscL correlates with increased channel mechanosensitivity. Biophys. J. 1999;77:1960–1972. doi: 10.1016/S0006-3495(99)77037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinac B., Buechner M., Kung C. Pressure-sensitive ion channel in Escherichia coli. Proc. Natl. Acad. Sci. USA. 1987;84:2297–2301. doi: 10.1073/pnas.84.8.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Battle A.R., Petrov E., Martinac B. Rapid and improved reconstitution of bacterial mechanosensitive ion channel proteins MscS and MscL into liposomes using a modified sucrose method. FEBS Lett. 2009;583:407–412. doi: 10.1016/j.febslet.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 21.Hamill O.P., Martinac B. Molecular basis of mechanotransduction in living cells. Physiol. Rev. 2001;81:685–740. doi: 10.1152/physrev.2001.81.2.685. [DOI] [PubMed] [Google Scholar]
- 22.Moe P.C., Levin G., Blount P. Correlating a protein structure with function of a bacterial mechanosensitive channel. J. Biol. Chem. 2000;275:31121–31127. doi: 10.1074/jbc.M002971200. [DOI] [PubMed] [Google Scholar]
- 23.Colombo G., Marrink S.J., Mark A.E. Simulation of MscL gating in a bilayer under stress. Biophys. J. 2003;84:2331–2337. doi: 10.1016/S0006-3495(03)75038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kloda A., Martinac B. Structural and functional differences between two homologous mechanosensitive channels of Methanococcus jannaschii. EMBO J. 2001;20:1888–1896. doi: 10.1093/emboj/20.8.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinac B., Kloda A. Evolutionary origins of mechano-sensitive ion channels. Progress Biophys. Mol. Biol. 2003;82:11–24. doi: 10.1016/s0079-6107(03)00002-6. [DOI] [PubMed] [Google Scholar]
- 26.Kloda A., Martinac B. Molecular identification of a mechanosensitive channel in archaea. Biophys. J. 2001;80:229–240. doi: 10.1016/S0006-3495(01)76009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashcroft F.M. The Science of Survival. Harper Collins; London: 2000. Life at the extremes; pp. 47–99. [Google Scholar]
- 28.Apponyi M.A., Ozawa K., Otting G. Cell-free protein synthesis for analysis by NMR spectroscopy. Methods Mol. Biol. 2008;426:257–268. doi: 10.1007/978-1-60327-058-8_16. [DOI] [PubMed] [Google Scholar]
- 29.Delcour A.H., Martinac B., Kung C. Modified reconstitution method used in patch-clamp studies of Escherichia coli ion channels. Biophys. J. 1989;56:631–636. doi: 10.1016/S0006-3495(89)82710-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levina N., Tötemeyer S., Booth I.R. Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. EMBO J. 1999;18:1730–1737. doi: 10.1093/emboj/18.7.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maingret F., Patel A.J., Honoré E. Lysophospholipids open the two-pore domain mechano-gated K+ channels TREK-1 and TRAAK. J. Biol. Chem. 2000;275:10128–10133. doi: 10.1074/jbc.275.14.10128. [DOI] [PubMed] [Google Scholar]
- 32.Ajouz B., Berrier C., Ghazi A. Contributions of the different extramembranous domains of the mechanosensitive ion channel MscL to its response to membrane tension. J. Biol. Chem. 2000;275:1015–1022. doi: 10.1074/jbc.275.2.1015. [DOI] [PubMed] [Google Scholar]
- 33.Corry B., Rigby P., Martinac B. Conformational changes involved in MscL channel gating measured using FRET spectroscopy. Biophys. J. 2005;89:L49–L51. doi: 10.1529/biophysj.105.072009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corry B., Hurst A.C., Martinac B. An improved open-channel structure of MscL determined from FRET confocal microscopy and simulation. J. Gen. Physiol. 2010;136:483–494. doi: 10.1085/jgp.200910376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braganza L.F., Worcester D.L. Structural changes in lipid bilayers and biological membranes caused hydrostatic pressure. Biochemistry. 1986;25:7484–7488. doi: 10.1021/bi00371a034. [DOI] [PubMed] [Google Scholar]
- 36.Cruickshank C.C., Minchin R.F., Martinac B. Estimation of the pore size of the large-conductance mechanosensitive ion channel of Escherichia coli. Biophys. J. 1997;73:1925–1931. doi: 10.1016/S0006-3495(97)78223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oakley A.J., Martinac B., Wilce M.C. Structure and function of the bacterial mechanosensitive channel of large conductance. Protein Sci. 1999;8:1915–1921. doi: 10.1110/ps.8.10.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balny C., Masson P., Heremans K. High pressure effects on biological macromolecules: from structural changes to alteration of cellular processes. Biochim. Biophys. Acta. 2002;1595:3–10. doi: 10.1016/s0167-4838(01)00331-4. [DOI] [PubMed] [Google Scholar]
- 39.Sawada Y., Sokabe M. Coupling of water permeation with mechano-gating in the E. coli mechanosensitive channel MscL. Biophys. J. 2010;98 A1700-Pos. [Google Scholar]
- 40.Sukharev S., Betanzos M., Guy H.R. The gating mechanism of the large mechanosensitive channel MscL. Nature. 2001;409:720–724. doi: 10.1038/35055559. [DOI] [PubMed] [Google Scholar]
- 41.Khan R.N., Martinac B., Edeson R.O. Hidden Markov analysis of mechanosensitive ion channel gating. Math. Biosci. 2005;193:139–158. doi: 10.1016/j.mbs.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Sukharev S.I., Sigurdson W.J., Sachs F. Energetic and spatial parameters for gating of the bacterial large conductance mechanosensitive channel, MscL. J. Gen. Physiol. 1999;113:525–540. doi: 10.1085/jgp.113.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]


