Abstract
AIM: To investigate the indication, feasibility, safety, and clinical utility of endoscopic submucosal dissection (ESD) in the management of various gastrointestinal pathologies.
METHODS: The medical records of 60 consecutive patients (34 female, 26 male) who underwent ESD at the gastroenterology department of Kocaeli University from 2006-2010 were examined. Patients selected for ESD had premalignant lesions or non-invasive early cancers of the gastrointestinal tract and had endoscopic and histological diagnoses. Early cancers were considered to be confined to the submucosa, with no lymph node involvement by means of computed tomography and endosonography.
RESULTS: Sixty ESD procedures were performed. The indications were epithelial lesions (n = 39) (33/39 adenoma with high grade dysplasia, 6/39 adenoma with low grade dysplasia), neuroendocrine tumor (n = 7), cancer (n = 7) (5/7 early colorectal cancer, 2/7 early gastric cancer), granular cell tumor (n = 3), gastrointestinal stromal tumor (n = 2), and leiomyoma (n = 2). En bloc and piecemeal resection rates were 91.6% (55/60) and 8.3% (5/60), respectively. Complete and incomplete resection rates were 96.6% (58/60) and 3.3% (2/60), respectively. Complications were major bleeding [n = 3 (5%)] and perforations [n = 5 (8.3%)] (4 colon, 1 stomach). Two patients with colonic perforations and two patients with submucosal lymphatic and microvasculature invasion (1 gastric carcinoid tumor, 1 colonic adenocarcinoma) were referred to surgery. During a mean follow-up of 12 mo, 1 patient with adenoma with high grade dysplasia underwent a second ESD procedure to resect a local recurrence.
CONCLUSION: ESD is a feasible and safe method for treatment of premalignant lesions and early malignant gastrointestinal epithelial and subepithelial lesions. Successful en bloc and complete resection of lesions yield high cure rates with low recurrence.
Keywords: Endoscopic submucosal dissection, Premalignant gastrointestinal lesion, Noninvasive early gastrointestinal cancer, Neuroendocrine tumor, Gastrointestinal stromal tumor
INTRODUCTION
New developments in the optical technology of endoscopes allow early detection of mucosal abnormalities that are amenable to endoscopic therapies. Endoscopic therapies are used for premalignant lesions and noninvasive early cancers with low risk of lymph node metastasis. Endoscopic therapies include ablation and resection-based modalities. Resection-based modalities consist of endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD). The major advantage of resection-based modalities is the recovery of the specimen for histopathological analysis. A recent study has shown the long-term prognosis of complete en bloc EMR to be comparable to surgery for differentiated early gastric cancer with 10-year survival rates of 99%[1]. Although lesions with a diameter of less than 20 mm can be resected in an en bloc fashion with EMR, larger lesions can only be removed in a piecemeal fashion. It is difficult to have an accurate histopathological evaluation of a lesion that is removed in a piecemeal fashion. Furthermore, the risk of local recurrence after piecemeal resection is higher than that of en bloc resection[1-3].
ESD is a newly developed technique that allows en bloc resection of larger (usually more than 20 mm) mucosal as well as subepithelial gastrointestinal lesions above the muscularis propria with the use of cutting devices. En bloc resection rate of ESD ranges between 83%-98%, which is significantly higher than EMR. Local recurrence rates range between 0%-3%[4]. However, compared to EMR, ESD is technically more challenging, requires higher endoscopic skills, is time consuming and has a prolonged learning curve[5-7]. Although ESD has been accepted in the armamentarium of endoscopic management of premalignant and noninvasive early gastrointestinal cancers in Japan and Asia, the Western experience with this new modality has been quite limited. This may be related to differences in the epidemiology of certain gastrointestinal diseases (e.g. gastric cancer), differences in technical expertise, due to its prolonged procedure time, due to its long learning curve, or possibly due to legal concerns, as well as procedural reimbursement. Our study is the first series of ESD cases from Turkey and among the few studies performed in Europe. The aim of this study is to describe indications, feasibility, safety, complications, and recurrence rate of the mucosal and subepithelial ESD cases in the upper and lower gastrointestinal tract.
MATERIALS AND METHODS
Patients
From September 2006 to June 2010, a consecutive series of patients who underwent an ESD at a tertiary referral center (Kocaeli University Hospital) were reviewed. Premalignant lesions larger than 15 mm in size and noninvasive early cancers with low risk for lymph node metastasis larger than 10 mm were included in the study. The inclusion criteria for carcinoma were histological well differentiation, diameter of ulceration ≤ 30 mm, lack of submucosal invasion and lymph node involvement detected with computed tomography (CT) or endoscopic ultrasound (EUS). Prior to an ESD attempt, each case was reviewed by a team consisting of an oncologist, a general surgeon, and an anesthesiologist. ESD indications, procedural information (instruments, sedation, procedure duration, findings, interventions, outcome, and complications) were retrospectively collected and analyzed. The research protocol was approved by the local ethics committee. Both oral and written informed consents were obtained from the patients. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki and in compliance with good clinical practice.
Equipment and procedure
All ESD procedures were performed by a single operator (S.H.) who had studied ESD in 2006 for three months (under the supervision of Hironori Yamamoto, MD; Jichi Medical School, Tochigi, Japan). During his study as a visiting professor he studied the indication, techniques and other basic knowledge regarding ESD for the esophagus, stomach and colon in the endoscopy unit of Jichi Medical University. The author also joined ESD courses in porcine stomach models organized by the gastrointestinal endoscopy society during national gastroenterology weeks. In these courses he achieved more experience, but also contributed to the education of physicians interested in this area.
Prior to an ESD attempt, all lesions were examined with an optical magnifying endoscope (EG-450 ZW5; Fujinon) and colonoscope (-EC-590 Z/WL; Fujinon), with 1% Indigo Carmine used as an adjunct to magnification. The lesion size was determined upon the comparison of standard open biopsy forceps with the lesion. The invasion depth of the lesions was examined either with high frequency ultrasound mini probes (P1912-MB, P1915-MB, P2012-M, 12-20 MHZ; Fujinon) or echo-endoscopes (GF-UE 160-AL5; Olympus) depending on the size and location of the lesion. Superficial lesions were classified according to the Paris classification system as type I (protruding), type II (flat) a, b, c and type III (excavated)[8]. Kudo classification was used for characterization of colonic pit patterns[9].
ESD procedures were performed in a standardized way. The margins of the lesion were marked with an electrocautery (30 W soft coagulation) to determine the resection border (except in the colon). Then submucosal injection was performed to lift the lesion. For the injection, a special mixture (1 unit of 2% sodium hyaluronic acid, 3 units of saline, 0.5 mL of epinephrine (1/10 000) and 0.5% of indigo carmine) was used. After sufficient lifting, a flush knife (DK2618JN 20; Fujinon), insulated-tip knife (KD-610L; Olympus) or needle knife (KD-11Q-1) was used to create a circumferential incision around the lesion extending into the submucosa. After circumferential incision, a submucosal dissection was performed to remove the lesion in an en bloc fashion.
A high frequency generator with an automatically controlled system (Endo-cut mode; ERBE ICC 200, Elektromedizin GmbH, Germany) was used for dissection and coagulation. A specialized cap (EMR ST Hood DH 15CR, Fujinon) was placed on the tip of the endoscope to make the dissection easier by increasing stability. Initially marked lesions were dissected with a diathermic knife (Olympus or Fujinon) circumferentially using endo-cut mode (2-3/80 W). An insulated-tip (IT) knife (KD-610L; Olympus) was used to dissect the borders of the lesions with a high perforation risk (wide based lesions and colonic lesions between haustral folds) using endo-cut mode (3/120 W). Submucosal dissection was performed with spray coagulation (45 W). Small vessels were coagulated with spray coagulation. Larger vessels or arteries with high bleeding risk were coagulated with hemostatic forceps (Fujinon).
Circumferential incision was completed in all cases. In colonic lesions, semi-circumferential incision was performed initially. After submucosal dissection, circumferential incision was completed. A few cases were finished with snare resection, but only after 80% of ESDs were completed. Standard or therapeutic gastroscopes (Fujinon EG-530 D) were used for lesions located in the rectum and sigmoid colon. Lesions close to the anus were treated in the retroflexion position. Colonoscopes were used for lesions proximal to the splenic flexure.
The first gastric lesions treated by ESD were located at the antrum in our series, as most gastric lesions were. Later on, cardiac lesions were treated by ESD in the retroflexion position.
All of the ESD procedures were performed under deep sedation. A combination of propofol and fentanyl was provided by an anesthesiologist. Patients were continuously monitored with an electrocardiogram, and blood pressure and oxygen saturation were monitored. The position of the patient could be easily changed whenever required with the help of medical attendants under the control of the anesthesiologist.
Definitions and follow-up strategy
All the specimens were examined by a single pathologist who is specialized in gastrointestinal pathology. En bloc resection was defined as the removal of a lesion in a single piece. Piecemeal resection was defined as the removal of a lesion in more than one piece.
A recurrent disease was defined as the reappearance of neoplastic tissue at the site of initial ESD at the 6th mo follow-up endoscopy. In the case of a perforation, hemoclips were used. Bleeding that could be managed with endoscopic intervention was considered as minor bleeding. Bleeding with hemodynamic instability and blood transfusion requirement with or without the need for surgical intervention was considered as major bleeding.
A lesion was considered to be completely removed (R0 resection), when the vertical and lateral surgical margins were 2 mm away from the lesion. When neoplastic cells were present at surgical margins, this was considered as an incomplete resection (R1). Patients found to have undifferentiated or signet cell adenocarcinoma and submucosal/lymphovascular invasion on histopathological evaluation were referred to surgery. Patients were hospitalized for observation after the procedure and underwent a control endoscopy within 2 d of the ESD procedure. Patients underwent follow-up endoscopies at 3 and 6 mo. After a normal endoscopy at the 6th mo, annual follow-up was offered.
Statistical analysis
A median of continuous variables was used to present data. The Kruskal-Wallis test was used to compare median procedure time of ESD groups. When Kruskal-Wallis test results were statistically significant (P < 0.05), a Mann-Whitney test using Bonferroni correction was used to compare median procedure time between ESD groups. P < 0.01 was accepted as statistically significant. Statistical Packages for Social Sciences version 16.0 for Windows (SPSS, Chicago, IL, USA) was used for statistical analysis.
RESULTS
Over the 46-mo period, 60 ESD procedures were performed by a single operator (S.H.). There were 34 female (56.6%) and 26 male (43.3%) patients. The mean age (via standard deviation) of patients was 54.6 (± 14.1) years.
The majority of ESD procedures (65%) were performed for intraepithelial lesions (n = 39) (33/39 adenoma with high grade dysplasia, 6/39 adenoma with low grade dysplasia). The indication of the remaining ESD procedures were as following: neuroendocrine tumor (NET) (n = 7), cancer (n = 7) [5/7 early colorectal cancer (ECC), 2/7 early gastric cancer (EGC)], granular cell tumor (n = 3), gastrointestinal stromal tumor (GIST) (n = 2), and leiomyoma (n = 2). Microscopic types of the lesions in the different locations according to Paris classification is given in Table 1. En bloc and piecemeal resection rates were 91.6% (55/60) and 8.3% (5/60), complete and incomplete resection rates were 96.6% (58/60) and 3.3% (2/60), respectively.
Table 1.
Paris classification according to the endoscopic imaging of lesions
Two cases are submucosal;
Nine granular type lateral spreading tumor(LST);
Seven pseudo-depressed type LST.
An adenoma (piecemeal resection is done on purpose, Figure 1), early gastric cancer located in the antrum, and three flat adenomas with lateral invasion in the colon were resected in piecemeal fashion. A patient with a NET that was incompletely resected was referred to surgery due to vascular invasion noted on histopathologic evaluation. A patient with an incompletely resected early colon cancer (ECC) due to lateral spreading was referred to surgery. Invasion into the muscularis propria was seen in the surgical specimen of the patient with gastric NET referred for surgery. But there was no invasion in the surgical specimen of the patient with ECC (this patient was referred for surgery because histology revealed neoplastic cells at the surgical margins of the specimen resected in en bloc fashion). En bloc, piecemeal, complete, and incomplete resection rates are shown in Table 2.
Figure 1.
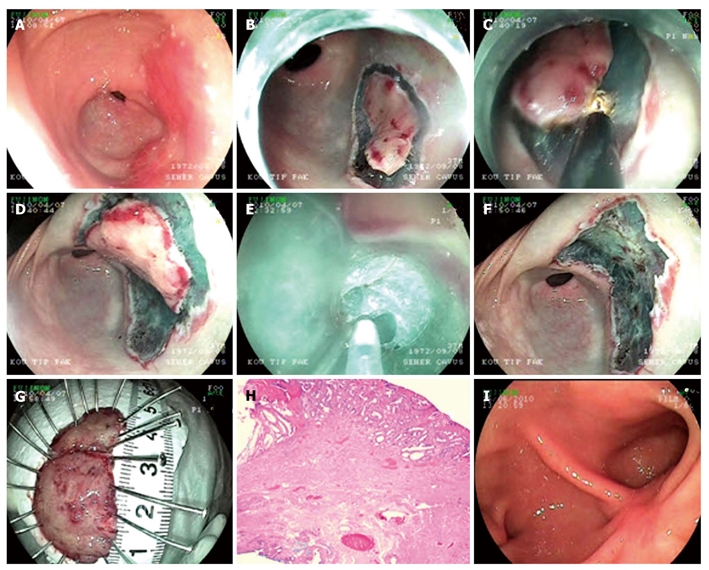
Endoscopic submucosal dissection procedure for adenoma with high grade dysplasia at antrum. A: Endoscopic view flat adenoma at antrum; B: Cutting of the first piece of the lesion which was decided to be extracted in two pieces; C: Submucosal dissection of the first piece; D: Cutting of the second piece of the lesion; E: Submucosal dissection of the second piece; F: Endoscopic view after the lesion is being extracted; G: Microscopic view of the lesion; H: Histology; Adenoma including fields of marked glandular atypia and distortion (HE × 20); I: Endoscopic view ten weeks after the procedure.
Table 2.
En bloc, piecemeal, complete, incomplete resection rates, median follow up and recurrence rates
| En bloc res. rate | Piecemeal res. rate | Complete res. rate | Incomplete res. rate | Median follow-up (mo) | Local recurrence | |
| Esophagus | 7/7 (100%) | 0 | 7/7 (100%) | 0 | 15 | 0 |
| Stomach | 22/24 (91.7%) | 2/24 (8.3%) | 23/24 (95.8%) | 1/24 (4.1%) | 11.8 | 1 |
| Small intestine and colon | 26/29 (89.7%) | 3/29 (10.3%) | 28/29 (96.5%) | 1/29 (3.4%) | 12.5 | 0 |
| Total | 55/60 (91.6%) | 5/60 (8.3%) | 58/60 (96.6%) | 2/60 (3.3%) | 12.51 | 1/58 (1.7%)1 |
Two patients who underwent surgery were excluded. Res: Resection.
The duration of ESD procedures per cm2 were as follows; 27.8, 21.8, and 18.3 min for stomach, esophagus, and colon, respectively. However, the mean procedure durations were as follows; 158, 90.4 and 50.5 min for colon, stomach, and esophagus, respectively. The mean procedure time of colonic and gastric lesions was significantly longer than esophageal lesions [Kruskal-Wallis test (P < 0.01)]. This is due to the differences in size of the lesions removed in different anatomic locations; colon (8.61 cm2) > stomach (3.25 cm2) > esophagus (2.34 cm2). Technical challenges related to the anatomic location of lesions as well as nature of lesions contribute to the procedure time (endoscopic resection should be done in the retroflexed position for lesions located in the cardia and proximal corpus, neuroendocrine tumors (NET) with rich vascularization have more bleeding).
Esophageal lesions
Seven esophageal lesions consisting of 3 granular cell tumors, 2 adenomas with high grade dysplasia, 1 gastrointestinal stromal tumor (submucosal), and 1 leiomyoma (submucosal) were treated with ESD (Table 3). No complications occurred in patients with esophageal lesions that were removed with ESD. No recurrences were noted in the 7 patients that had follow-up data. The mean follow-up period for these 7 patients was 15 mo.
Table 3.
Esophageal endoscopic submucosal dissection cases
| Location | Number | Histology (n) |
| Proximal esophagus | 1 | Granular cell tumor (1) |
| Middle esophagus | 2 | Granular cell tumor (2) |
| Distal esophagus | 4 | GIST (1) |
| HGD-A (2) | ||
| Leiomyoma (1) | ||
| Specimen size (median) | 2.34 cm² (1.5-3 cm2) | |
| Procedure time (median) | 50.5 min (21.8 min/cm²) |
GIST: Gastrointestinal stromal tumor; HGD-A: Adenoma with high grade dysplasia.
Gastric lesions
Twenty four gastric lesions, consisting of 16 adenomas (12 adenomas with HGD, 4 adenomas with LGD), 4 carcinoid tumors, 2 early gastric cancers, 1 GIST (submucosal), and 1 leiomyoma (submucosal) were treated with ESD (Table 4) (Figures 1 and 2). One lesion was located in the cardia (adenoma with HGD), 3 lesions were located in the gastric corpus (3 carcinoid tumors), and 20 lesions were located in the antrum (11 adenomas with HGD, 4 adenomas with LGD, 2 EGC, 1 GIST, 1 carcinoid tumor, 1 leiomyoma). A patient with a gastric adenoma with HGD had recurrence at the site of prior resection. This patient had a second ESD, 12 mo after the first ESD.
Table 4.
Gastric endoscopic submucosal dissection cases
| Location | Number | Histology (n) |
| Cardia | 1 | HGD (TVA) (1) |
| Corpus | 3 | NET (3) |
| Antrum | 20 | HGD-A (11) |
| LGD-A (4) | ||
| NET (1) | ||
| EGC (2) | ||
| GIST (1) | ||
| Leiomyoma (1) | ||
| Specimen size (median) | 3.25 cm² (1.5-12 cm²) | |
| Procedure time (median) | 90.4 min (27.8 min/cm²) |
HGD: High grade dysplasia; NET: Neuroendocrine tumor; HGD-A: Adenoma with high grade dysplasia; LGD-A: Adenoma with low grade dysplasia; EGC: Early gastric cancer; LGD: Low grade dysplasia; GIST: Gastrointestinal stromal tumor; TVA: Tubulovillous adenoma.
Figure 2.
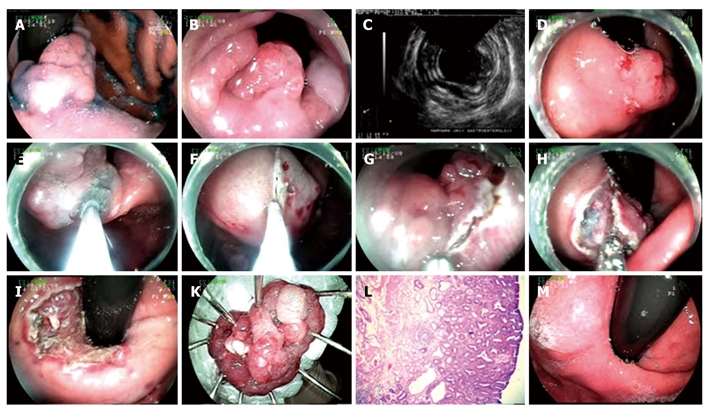
Endoscopic submucosal dissection procedure for adenoma with high grade dysplasia at cardia. A: Adenoma at cardia; B: View of the lesion from esophageal aspect; C: Endosonographic image of the lesion; D, E: Marking the borders of the lesion with needle knife and lifting it; F, G: Cutting the lesion circumferentially with endo-cut above Z line, in retroflexion; H: Dissection of the submucosal area; I: Appearance of the mucosa after the lesion being extracted; K: Microscopic view of the lesion; L: Histology: mucosa, muscularis mucosa and superficial submucosa of stomach (HE × 20). Adenoma structure including adenomatous epithelium formed by irregular glands at mucosa; M: Endoscopic view six months after the procedure.
Colonic and small intestinal lesions
Twenty-nine lesions including 21 adenomas (2 tubulovillous adenoma with HGD in duodenal bulb, 1 tubulovillous adenoma with HGD in cecum, 2 tubulovillous adenoma with HGD in transverse colon, 3 tubulovillous adenoma with HGD in sigmoid colon, 1 tubular adenoma in sigmoid colon, 9 tubulovillous adenoma with HGD in rectum, and 3 tubular adenoma in rectum), 3 carcinoid tumors (1 in duodenal bulb, 1 in terminal ileum and 1 in rectum), and 5 early colon cancers (4 in rectum and 1 transverse colon) were treated with ESD (Table 5) (Figures 3 and 4). All early colon cancers were classified as IIa + IIc according to the Paris classification.
Table 5.
Colonic and intestinal endoscopic submucosal dissection cases
| Location | Number | Histology (n) |
| Duodenal bulb | 3 | HGD (TVA) (2) |
| NET (1) | ||
| Terminal ileum | 1 | NET (1) |
| Cecum | 1 | HGD (TVA) (1) |
| Transverse colon | 3 | HGD (TVA) (2) |
| ECC (1) | ||
| Sigmoid colon | 4 | HGD (TVA) (3) |
| LGD-TA (1) | ||
| Rectum | 17 | ECC (4) |
| HGD (9 TVA, 2 TA) | ||
| LGD-TA (1) | ||
| NET (1) | ||
| Specimen size (median) | 8.61 cm2 (1.5-25 cm²) | |
| Procedure time (median) | 158 min (18.3 min/cm²) |
HGD: High grade dysplasia; NET: Neuroendocrine tumor; TA: Tubular adenoma; ECC: Early colonic cancer; LGD: Low grade dysplasia; TVA: Tubulovillous adenoma.
Figure 3.
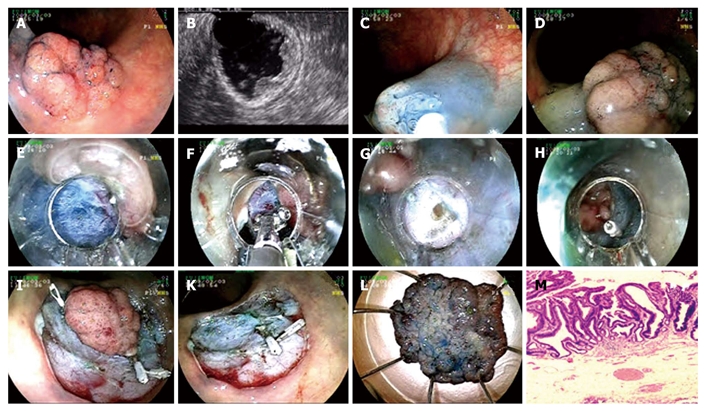
Endoscopic submucosal dissection procedure for tubulovillous adenoma with high grade dysplasia at sigmoid colon. A: Adenoma at sigmoid colon; B: Endosonographic image of tubulovillous adenoma; C-D: Lifting the lesion; E: Cutting with endo-cut; F: Coagulation of submucosal vein with hemostatic forceps; G: Mini perforation during the procedure; H: Fixing perforation with hemoclip; I: Hemoclip application to control bleeding that occured after cutting the lesion circumferentially with endo-cut; K: Appearance of the mucosa after the lesion being extracted; L: Microscopic view of the lesion; M: Histology; tubulovillous adenoma with high grade dysplasia (HE × 40).
Figure 4.
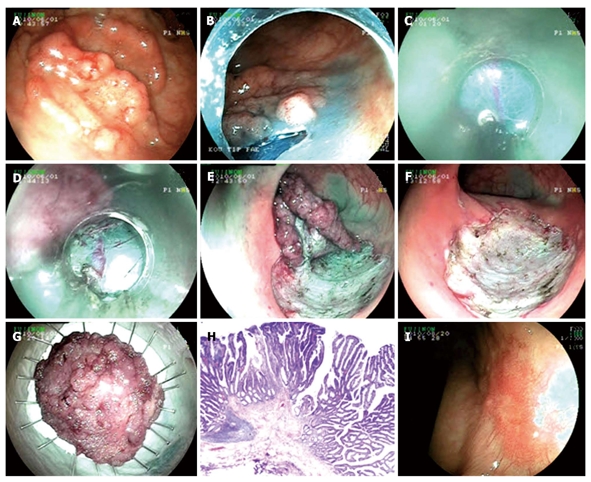
Endoscopic submucosal dissection procedure for pseudo-depressed type lateral spreading tumor with high grade dysplasia at rectum. A: Pseudo-depressed type lateral spreading tumor at rectum; B: Cutting the lesion circumferentially with endo-cut; C, D: Submucosal dissection with semipermeable cap; E: Endoscopic view just before completing submucosal dissection; F: Appearance of the mucosa after the lesion being extracted; G: Microscopic view of the lesion; H: Histology; tubulovillous adenoma including fields of focal pattern loss and dysplasia (HE × 20); I: Endoscopic view ten weeks after the procedure.
Safety
Among 5 perforations (8.3%), four were located in the colon (1 early colon cancer, 2 laterally spreading lesions and 1 large tubulovillous adenoma located on a colonic haustra) and one (carcinoid tumor) was located in the stomach. Two colonic perforations were managed with surgical intervention and the other three were managed with hemoclip application. Those managed with hemoclip application were smaller than 5 mm in size; hence it was easy to treat them. The patients with perforation were hospitalized for an average of ten days. There was no association of perforation with scarring and fibrosis from previous procedures. No post-ESD stenosis was noted. Major bleeding occurred in three patients (5%). One of these patients had a lesion located in the colon and two of them had lesions located in the stomach. Contributing factors to major bleeding may possibly be the nature as well as the location of the lesion, possibly due to the rich vascularization of the lesion. Both of the bleeding lesions in the stomach were NETs and located in the lesser curvature. Bleeding from gastric lesions was delayed and required blood transfusion. The colonic case that had a major bleed was an early colonic cancer. Minor bleeding occurred in 13 cases (7 lesions located in the colon and 6 lesions located in the stomach) (21.7%). Table 6 shows complications in detail.
Table 6.
Complications with regard to location and diagnosis
| n | Diagnosis | Minor bleeding | Major bleeding | Perforation | |
| Esophagus | 7 | 3 granular cell tumor | |||
| 1 GIST | |||||
| 2 Premalignant lesions | |||||
| 1 Leiomyoma | |||||
| Stomach | 24 | 4 NETs | 4 | 2 | 1 |
| 2 EGC | 2 | ||||
| 18 Premalignant lesions | |||||
| Small intestine | 4 | 2 Premalignant lesions | |||
| 2 NETs | |||||
| Colon | 25 | 5 ECCs | 3 | 1 | 1 |
| 19 Premalignant lesions | 4 | 3 | |||
| 1 NETs | |||||
| Total | 60 | 13 (21.7%) | 3 (5%) | 5 (8.3%) |
GIST: Gastrointestinal stromal tumor; NET: Neuroendocrine tumor; EGC: Early gastric cancer; ECC: Early colorectal cancer.
DISCUSSION
Although endoscopic resection-based therapeutic modalities (EMR and ESD) are considered to be the treatment of choice for premalignant and early gastrointestinal neoplasias in Japan, they are not widely practiced by Western endoscopists[10]. This is the first study from Turkey and among the few studies from outside of Japan and Asia on the application of ESD in the management of premalignant and noninvasive early gastrointestinal cancers from various anatomic locations in the gastrointestinal tract.
It is difficult or impossible to remove large lesions with EMR technique in one fragment. Removal of a lesion in one piece is very important to accurately diagnose the tumor depth as well as decreasing the risk of local recurrence. A recent study has illustrated that this problem can be solved with the use of ESD for larger lesions[11]. In early esophageal cancers with a diameter of less than 20 mm, en bloc and curative resection rate of ESD (97%) was found to be significantly higher than that of EMR using a transparent cap (71%) and 2-channel EMR (46%)[11]. However, no significant difference was found between ESD and EMR using a transparent cap in en bloc and curative resection rate of lesions less than 15 mm in diameter. Therefore, ESD would be a better therapeutic modality than EMR for esophageal lesions with a diameter of greater than 15 mm. Given the size (median size of esophageal/gastric/colonic lesions 2.34 cm²/3.25 cm²/8.61 cm2 respectively), various anatomic location of lesions and subepithelial nature of some lesions, ESD would be the treatment of choice for our cases.
There are few studies from Europe that were published in full manuscript format. Dinis-Ribeiro et al[12]. evaluated feasibility and effectiveness of ESD in 19 gastric superficial lesions with HGD, LGD and noninvasive epithelial neoplasias. Probst et al[13]. evaluated ESD in 71 flat adenomas, early cancers and submucosal tumors located in various locations of the gastrointestinal tract (51 gastric, 17 rectal, 2 esophageal and 1 duodenal). In the study of Dinis-Ribeiro et al, complete and en bloc resection rates were 89% and 79%, respectively. Major bleeding occurred in 1 case (5%). There were no perforations. Recurrence of a lesion (5%) was noted within a mean follow-up of 10 mo. In order to evaluate ESD learning curve, Probst et al[13]. compared various aspects of ESD procedures performed in the first and second halves of the study.
A statistically significant increase in specimen size and decrease in procedural duration were noted between the two groups. En bloc resection rates and R0 en bloc resection rates in the first half of the study (77.1% and 65.7%, respectively) increased when compared with the second half of the study (86.1% and 72.2%, respectively), however this difference did not reach statistical significance. No recurrence occurred after R0 en bloc resection; however 38% recurrence occurred after piecemeal resection. Complications in the study of Probst included 2 perforations (gastric submucosal tumors) that required surgery (2.7%), 2 other perforations (large flat rectal lesions) (2.7%), 8 minor bleedings (10.9%) and 3 pyloric stenosis (4.1%) that were endoscopically managed.
ESD complication rates among the published studies have been variable depending on the size of the study as well as the experience of the operator. In our study, a patient (1.7%) was found to have a recurrence of an adenoma with HGD at the site of prior ESD on 12-mo follow-up endoscopy. The recurrent lesion was treated with a repeat ESD. The patient was free of disease at the 6-mo follow-up. No recurrences were noted with esophageal and colonic lesions. Compared to other studies, lower recurrence rate in our study may be related to the relatively shorter follow up (median = 12.5 mo) of the patients after ESD. Our bleeding rate is consistent with other studies. Our perforation rate is higher than the study of Probst et al[13]. However, all of our perforations occurred at the first half of the study, which is a reflection of the impact of the operator’s experience with the success of ESD procedures. Eighty percent of perforations occurred with colonic cases, which may be related to the relatively thinner colonic wall thickness and larger size of colonic lesions. Most perforations took place in initial cases. In those cases needle knives were used. We believe that the use of these knives also contributed to this relatively high number of perforations. After providing IT-knives we did not encounter any perforations. As stated above we could not refuse the patients and it is true that we had to perform colorectal cases with insufficient experience in gastric ESD, resulting in relatively high perforation rates.
In a review article from Japan, the en bloc resection rate of early gastric cancers was reported to be 79%-100%, with local recurrence, bleeding and perforation rates of 0%-1%, 1.7%-38%, 0%-5%, respectively[14]. Another study from Japan evaluating ESD in colorectal epithelial neoplasms revealed the rate of en bloc resection and en bloc resection with tumor free margins to be 91.5% and 70.5%[15]. In this study, perforation and local recurrence rates were found to be 5% and 1.7%, respectively. The sample size of studies coming from outside of Japan and Asia is quite modest; therefore it is premature to compare Western experience with the Asian one.
Ideally one should begin with gastric cases located in the antrum. After getting sufficient experience in gastric cases they can proceed with esophageal and colorectal cases, which are more risky. Our practice seems incompatible with this idea. But ESD is only performed in our institute in Turkey and patients are referred to our hospital from the entire country. So we did not have the chance to refuse the patients and performed esophageal and colorectal cases before having sufficient experience in gastric cases.
We believe that, besides EMR and endoscopic piece-meal mucosal resection, ESD will be a good alternative in the treatment of non-epithelial esophageal lesions. We observed that neuroendocrine tumors which have rich vascularization are more likely to bleed, so more attention should be paid when operating on them. During the ESD procedures in the colon and esophagus, a needle knife should be avoided in endo-cutting because of the perforation risk. Perforation risk is even higher in laterally spreading colonic lesions so IT-knife is a better choice for those lesions.
In summary, ESD, which originates in Japan, has been gaining popularity in other parts of the world as well. Comparable outcomes of ESD to surgery play an important role in the rapid propagation of this therapeutic endoscopic modality. Although no procedure related mortality has been reported, there is considerable morbidity with this technique. There is a significant learning curve to achieve proficiency in order to acquire skills to perform ESD safely and effectively. Therefore, importance of training can not be overemphasized. Further studies from outside of Japan and Asia are needed to better determine the global role of ESD in the management of premalignant and early malignant epithelial, as well as subepithelial lesions.
COMMENTS
Background
Advances in endoscopic diagnosis techniques allowed premalignant lesions and non-invasive cancers of the gastrointestinal system to be detected early and be treated effectively and safely by endoscopic methods. Among these methods, endoscopic submucosal dissection (ESD) is being used more and more commonly and has pleasing results.
Research frontiers
ESD is a safe and effective modality for the treatment of premalignant lesions and early non-invasive cancers of the gastrointestinal system and when compared to surgery it has the advantage of preserving the gastrointestinal system and its functions. This is the first study on ESD reported from Turkey.
Innovations and breakthroughs
Complications related to ESD are similar to other studies in the literature except for the complication rate. Recurrence rates are lower compared to other studies. In this study we observed that neuroendocrine tumors had higher bleeding rates due to hypervascularization and the use of a needle knife in colonic and esophageal lesions increased the risk of perforation.
Applications
When performed by experienced endoscopists ESD has pleasing results and can be safely performed for the treatment of premalignant lesions and early cancers of gastrointestinal system.
Terminology
ESD is being used for the treatment of premalignant and lesions (with no lymph node involvement) of the gastrointestinal tractus confined to mucosa and submucosa. After lifting the lesion by injecting the specially prepared solution (Na - Hyaluronate + Adrenalin + Saline + Indigo carmine), the basement of the lesion, along with the surrounding area, are cut with special knives and the lesion is extracted.
Peer review
This retrospective study sets out to evaluate the feasibility, safety and clinical outcomes of ESD for premalignant lesions and early gastrointestinal cancers. It establishes that the rates of en bloc and complete resection of these lesions were good, and comparable those of previous studies.
Footnotes
Peer reviewers: Marcela Kopacova, Associate Professor, MD, PhD, 2nd Department of Internal Medicine, Charles University Teaching Hospital, Sokolska 581, 500 05 Hradec Kralove, Czech Republic; Dr. László Czakó, MD, PhD, Associate Professor, First Department of Medicine, University of Szeged, PO Box 427, Szeged, H-6701, Hungary
S- Editor Tian L L- Editor Rutherford A E- Editor Ma WH
References
- 1.Uedo N, Iishi H, Tatsuta M, Ishihara R, Higashino K, Takeuchi Y, Imanaka K, Yamada T, Yamamoto S, Yamamoto S, et al. Longterm outcomes after endoscopic mucosal resection for early gastric cancer. Gastric Cancer. 2006;9:88–92. doi: 10.1007/s10120-005-0357-0. [DOI] [PubMed] [Google Scholar]
- 2.Muto M, Miyamoto S, Hosokawa A, Doi T, Ohtsu A, Yoshida S, Endo Y, Hosokawa K, Saito D, Shim CS, et al. Endoscopic mucosal resection in the stomach using the insulated-tip needle-knife. Endoscopy. 2005;37:178–182. doi: 10.1055/s-2004-826194. [DOI] [PubMed] [Google Scholar]
- 3.Kim JJ, Lee JH, Jung HY, Lee GH, Cho JY, Ryu CB, Chun HJ, Park JJ, Lee WS, Kim HS, et al. EMR for early gastric cancer in Korea: a multicenter retrospective study. Gastrointest Endosc. 2007;66:693–700. doi: 10.1016/j.gie.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Hyatt BJ, Paull PE, Wassef W. Gastric oncology: an update. Curr Opin Gastroenterol. 2009;25:570–578. doi: 10.1097/MOG.0b013e328331b5c9. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto H, Kita H. Endoscopic therapy of early gastric cancer. Best Pract Res Clin Gastroenterol. 2005;19:909–926. doi: 10.1016/j.bpg.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Soetikno RM, Gotoda T, Nakanishi Y, Soehendra N. Endoscopic mucosal resection. Gastrointest Endosc. 2003;57:567–579. doi: 10.1067/mge.2003.130. [DOI] [PubMed] [Google Scholar]
- 7.Gotoda T. Endoscopic resection for premalignant and malignant lesions of the gastrointestinal tract from the esophagus to the colon. Gastrointest Endosc Clin N Am. 2008;18:435–450, VIII. doi: 10.1016/j.giec.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy. 2005;37:570–578. doi: 10.1055/s-2005-861352. [DOI] [PubMed] [Google Scholar]
- 9.Kudo S, Hirota S, Nakajima T, Hosobe S, Kusaka H, Kobayashi T, Himori M, Yagyuu A. Colorectal tumours and pit pattern. J Clin Pathol. 1994;47:880–885. doi: 10.1136/jcp.47.10.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergman JJ. How to justify endoscopic submucosal dissection in the Western world. Endoscopy. 2009;41:988–990. doi: 10.1055/s-0029-1215247. [DOI] [PubMed] [Google Scholar]
- 11.Ishihara R, Iishi H, Uedo N, Takeuchi Y, Yamamoto S, Yamada T, Masuda E, Higashino K, Kato M, Narahara H, et al. Comparison of EMR and endoscopic submucosal dissection for en bloc resection of early esophageal cancers in Japan. Gastrointest Endosc. 2008;68:1066–1072. doi: 10.1016/j.gie.2008.03.1114. [DOI] [PubMed] [Google Scholar]
- 12.Dinis-Ribeiro M, Pimentel-Nunes P, Afonso M, Costa N, Lopes C, Moreira-Dias L. A European case series of endoscopic submucosal dissection for gastric superficial lesions. Gastrointest Endosc. 2009;69:350–355. doi: 10.1016/j.gie.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 13.Probst A, Golger D, Arnholdt H, Messmann H. Endoscopic submucosal dissection of early cancers, flat adenomas, and submucosal tumors in the gastrointestinal tract. Clin Gastroenterol Hepatol. 2009;7:149–155. doi: 10.1016/j.cgh.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929–942. doi: 10.1007/s00535-006-1954-3. [DOI] [PubMed] [Google Scholar]
- 15.Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S, Yamamichi N, Tateishi A, Oka M, Ogura K, et al. Outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms in 200 consecutive cases. Clin Gastroenterol Hepatol. 2007;5:678–683; quiz 645. doi: 10.1016/j.cgh.2007.01.006. [DOI] [PubMed] [Google Scholar]


