Abstract
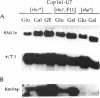
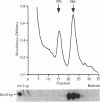
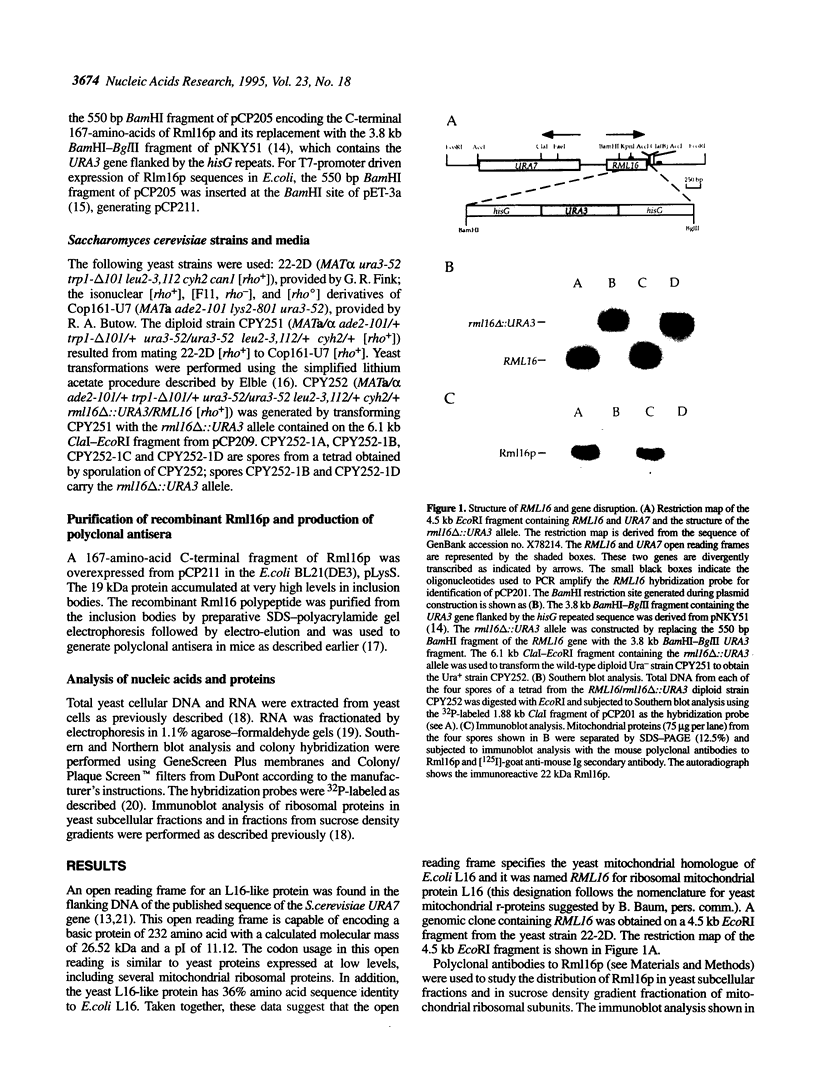
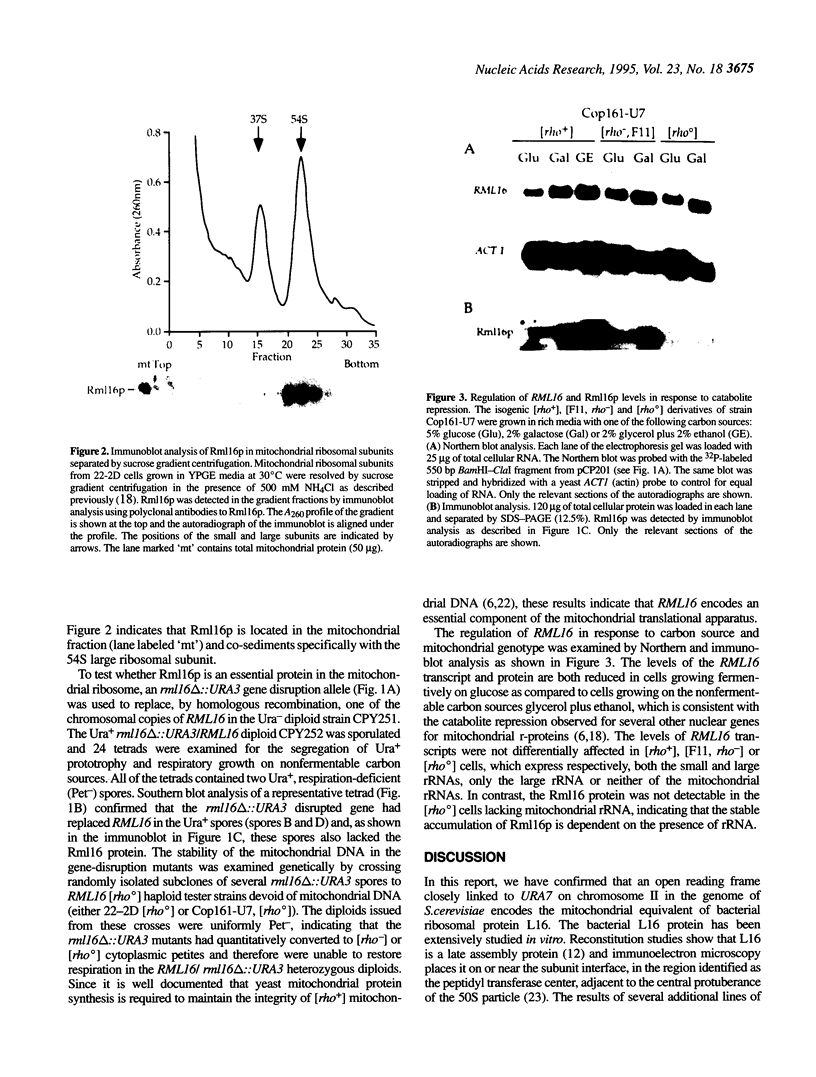
An open reading frame encoding a member of the L16 family of ribosomal proteins is adjacent to the URA7 gene on the left arm of chromosome II in Saccharomyces cerevisiae. The predicted L16-like polypeptide is basic (pl 11.12), contains 232 amino acids (26.52 kDa) and has 36% amino acid sequence identity to E. coli L16. Immunoblot analysis with polyclonal antibodies to the L16-like polypeptide showed specific cross-reaction with a 22,000 Mr mitochondrial polypeptide that co-sediments with the large subunit of the mitochondrial ribosome in sucrose density gradients. The levels of the L16 mRNA and protein varied in response to carbon source. In [rho degree] cells lacking mitochondrial rRNA, the L16 mRNA accumulated at normal levels, but the protein was barely detectable, indicating RNA-dependent accumulation of the L16 protein. Gene disruption experiments demonstrated that the yeast mitochondrial L16 is an essential ribosomal protein in vivo.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alani E., Cao L., Kleckner N. A method for gene disruption that allows repeated use of URA3 selection in the construction of multiply disrupted yeast strains. Genetics. 1987 Aug;116(4):541–545. doi: 10.1534/genetics.112.541.test. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock H., Brennicke A., Schuster W. Rps3 and rpl16 genes do not overlap in Oenothera mitochondria: GTG as a potential translation initiation codon in plant mitochondria? Plant Mol Biol. 1994 Mar;24(5):811–818. doi: 10.1007/BF00029863. [DOI] [PubMed] [Google Scholar]
- Brosius J., Chen R. The primary structure of protein L16 located at the peptidyltransferase center of Escherichia coli ribosomes. FEBS Lett. 1976 Sep 15;68(1):105–109. doi: 10.1016/0014-5793(76)80415-2. [DOI] [PubMed] [Google Scholar]
- Burger G., Plante I., Lonergan K. M., Gray M. W. The mitochondrial DNA of the amoeboid protozoon, Acanthamoeba castellanii: complete sequence, gene content and genome organization. J Mol Biol. 1995 Feb 3;245(5):522–537. doi: 10.1006/jmbi.1994.0043. [DOI] [PubMed] [Google Scholar]
- Chiu M. I., Mason T. L., Fink G. R. HTS1 encodes both the cytoplasmic and mitochondrial histidyl-tRNA synthetase of Saccharomyces cerevisiae: mutations alter the specificity of compartmentation. Genetics. 1992 Dec;132(4):987–1001. doi: 10.1093/genetics/132.4.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo M. C., Fox T. D. Control of mitochondrial gene expression in Saccharomyces cerevisiae. Annu Rev Genet. 1990;24:91–113. doi: 10.1146/annurev.ge.24.120190.000515. [DOI] [PubMed] [Google Scholar]
- Dabbs E. R., Hasenbank R., Kastner B., Rak K. H., Wartusch B., Stöffler G. Immunological studies of Escherichia coli mutants lacking one or two ribosomal proteins. Mol Gen Genet. 1983;192(3):301–308. doi: 10.1007/BF00392166. [DOI] [PubMed] [Google Scholar]
- Dabbs E. R. Mutants lacking individual ribosomal proteins as a tool to investigate ribosomal properties. Biochimie. 1991 Jun;73(6):639–645. doi: 10.1016/0300-9084(91)90043-z. [DOI] [PubMed] [Google Scholar]
- De Wergifosse P., Jacques B., Jonniaux J. L., Purnelle B., Skala J., Goffeau A. The sequence of a 22.4 kb DNA fragment from the left arm of yeast chromosome II reveals homologues to bacterial proline synthetase and murine alpha-adaptin, as well as a new permease and a DNA-binding protein. Yeast. 1994 Nov;10(11):1489–1496. doi: 10.1002/yea.320101113. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elble R. A simple and efficient procedure for transformation of yeasts. Biotechniques. 1992 Jul;13(1):18–20. [PubMed] [Google Scholar]
- Fearon K., Mason T. L. Structure and regulation of a nuclear gene in Saccharomyces cerevisiae that specifies MRP7, a protein of the large subunit of the mitochondrial ribosome. Mol Cell Biol. 1988 Sep;8(9):3636–3646. doi: 10.1128/mcb.8.9.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fukuzawa H., Kohchi T., Sano T., Shirai H., Umesono K., Inokuchi H., Ozeki H., Ohyama K. Structure and organization of Marchantia polymorpha chloroplast genome. III. Gene organization of the large single copy region from rbcL to trnI(CAU). J Mol Biol. 1988 Sep 20;203(2):333–351. doi: 10.1016/0022-2836(88)90003-4. [DOI] [PubMed] [Google Scholar]
- Graack H. R., Grohmann L., Kitakawa M., Schäfer K. L., Kruft V. YmL9, a nucleus-encoded mitochondrial ribosomal protein of yeast, is homologous to L3 ribosomal proteins from all natural kingdoms and photosynthetic organelles. Eur J Biochem. 1992 Jun 1;206(2):373–380. doi: 10.1111/j.1432-1033.1992.tb16937.x. [DOI] [PubMed] [Google Scholar]
- Grivell L. A. Nucleo-mitochondrial interactions in yeast mitochondrial biogenesis. Eur J Biochem. 1989 Jul 1;182(3):477–493. doi: 10.1111/j.1432-1033.1989.tb14854.x. [DOI] [PubMed] [Google Scholar]
- Hampl H., Schulze H., Nierhaus K. H. Ribosomal components from Escherichia coli 50 S subunits involved in the reconstitution of peptidyltransferase activity. J Biol Chem. 1981 Mar 10;256(5):2284–2288. [PubMed] [Google Scholar]
- Henkin T. M., Moon S. H., Mattheakis L. C., Nomura M. Cloning and analysis of the spc ribosomal protein operon of Bacillus subtilis: comparison with the spc operon of Escherichia coli. Nucleic Acids Res. 1989 Sep 25;17(18):7469–7486. doi: 10.1093/nar/17.18.7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt M. D., Newton K. J. The NCS3 mutation: genetic evidence for the expression of ribosomal protein genes in Zea mays mitochondria. EMBO J. 1991 May;10(5):1045–1052. doi: 10.1002/j.1460-2075.1991.tb08043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang W., Matsushita Y., Grohmann L., Graack H. R., Kitakawa M., Isono K. Cloning and analysis of the nuclear gene for YmL33, a protein of the large subunit of the mitochondrial ribosome in Saccharomyces cerevisiae. J Bacteriol. 1991 Jul;173(13):4013–4020. doi: 10.1128/jb.173.13.4013-4020.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitakawa M., Isono K. The mitochondrial ribosomes. Biochimie. 1991 Jun;73(6):813–825. doi: 10.1016/0300-9084(91)90061-5. [DOI] [PubMed] [Google Scholar]
- Lou J. K., Wu M., Chang C. H., Cuticchia A. J. Localization of a r-protein gene within the chloroplast DNA replication origin of Chlamydomonas. Curr Genet. 1987;11(6-7):537–541. doi: 10.1007/BF00384617. [DOI] [PubMed] [Google Scholar]
- Michalowski C. B., Pfanzagl B., Löffelhardt W., Bohnert H. J. The cyanelle S10 spc ribosomal protein gene operon from Cyanophora paradoxa. Mol Gen Genet. 1990 Nov;224(2):222–231. doi: 10.1007/BF00271555. [DOI] [PubMed] [Google Scholar]
- Myers A. M., Pape L. K., Tzagoloff A. Mitochondrial protein synthesis is required for maintenance of intact mitochondrial genomes in Saccharomyces cerevisiae. EMBO J. 1985 Aug;4(8):2087–2092. doi: 10.1002/j.1460-2075.1985.tb03896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda K., Yamato K., Ohta E., Nakamura Y., Takemura M., Nozato N., Akashi K., Kanegae T., Ogura Y., Kohchi T. Gene organization deduced from the complete sequence of liverwort Marchantia polymorpha mitochondrial DNA. A primitive form of plant mitochondrial genome. J Mol Biol. 1992 Jan 5;223(1):1–7. doi: 10.1016/0022-2836(92)90708-r. [DOI] [PubMed] [Google Scholar]
- Ozier-Kalogeropoulos O., Fasiolo F., Adeline M. T., Collin J., Lacroute F. Cloning, sequencing and characterization of the Saccharomyces cerevisiae URA7 gene encoding CTP synthetase. Mol Gen Genet. 1991 Dec;231(1):7–16. doi: 10.1007/BF00293815. [DOI] [PubMed] [Google Scholar]
- Partaledis J. A., Mason T. L. Structure and regulation of a nuclear gene in Saccharomyces cerevisiae that specifies MRP13, a protein of the small subunit of the mitochondrial ribosome. Mol Cell Biol. 1988 Sep;8(9):3647–3660. doi: 10.1128/mcb.8.9.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth H. E., Nierhaus K. H. Structural and functional studies of ribonucleoprotein fragments isolated from Escherichia coli 50 S ribosomal subunits. J Mol Biol. 1975 May 5;94(1):111–121. doi: 10.1016/0022-2836(75)90408-8. [DOI] [PubMed] [Google Scholar]
- Schulze H., Nierhaus K. H. Minimal set of ribosomal components for reconstitution of the peptidyltransferase activity. EMBO J. 1982;1(5):609–613. doi: 10.1002/j.1460-2075.1982.tb01216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Sutton C. A., Conklin P. L., Pruitt K. D., Calfee A. J., Cobb A. G., Hanson M. R. Editing of rps3/rpl16 transcripts creates a premature truncation of the rpl16 open reading frame. Curr Genet. 1993 May-Jun;23(5-6):472–476. doi: 10.1007/BF00312637. [DOI] [PubMed] [Google Scholar]
- Takemura M., Oda K., Yamato K., Ohta E., Nakamura Y., Nozato N., Akashi K., Ohyama K. Gene clusters for ribosomal proteins in the mitochondrial genome of a liverwort, Marchantia polymorpha. Nucleic Acids Res. 1992 Jun 25;20(12):3199–3205. doi: 10.1093/nar/20.12.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teraoka H., Nierhaus K. H. Proteins fro Escherichia coli ribosomes involved in the binding of erythromycin. J Mol Biol. 1978 Dec 5;126(2):185–193. doi: 10.1016/0022-2836(78)90358-3. [DOI] [PubMed] [Google Scholar]
- Tzagoloff A., Dieckmann C. L. PET genes of Saccharomyces cerevisiae. Microbiol Rev. 1990 Sep;54(3):211–225. doi: 10.1128/mr.54.3.211-225.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff G., Plante I., Lang B. F., Kück U., Burger G. Complete sequence of the mitochondrial DNA of the chlorophyte alga Prototheca wickerhamii. Gene content and genome organization. J Mol Biol. 1994 Mar 18;237(1):75–86. doi: 10.1006/jmbi.1994.1210. [DOI] [PubMed] [Google Scholar]
- Wower J., Hixson S. S., Zimmermann R. A. Labeling the peptidyltransferase center of the Escherichia coli ribosome with photoreactive tRNA(Phe) derivatives containing azidoadenosine at the 3' end of the acceptor arm: a model of the tRNA-ribosome complex. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5232–5236. doi: 10.1073/pnas.86.14.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Zurawski S. M. Structure of the Escherichia coli S10 ribosomal protein operon. Nucleic Acids Res. 1985 Jun 25;13(12):4521–4526. doi: 10.1093/nar/13.12.4521. [DOI] [PMC free article] [PubMed] [Google Scholar]