Abstract
AIM: To review the application of nutrition support in patients after surgery for colorectal cancer, and to propose appropriate nutrition strategies.
METHODS: A total of 202 consecutive surgical patients admitted to our hospital with a diagnosis of colon cancer or rectal cancer from January 2010 to July 2010, meeting the requirements of Nutrition Risk Screening 2002, were enrolled in our study. Laboratory tests were performed to analyze the nutrition status of each patient, and the clinical outcome variables, including postoperative complications, hospital stay, cost of hospitalization and postoperative outcome, were analyzed.
RESULTS: The “non-risk” patients who did not receive postoperative nutrition support had a higher rate of postoperative complications than patients who received postoperative nutrition support (2.40 ± 1.51 vs 1.23 ± 0.60, P = 0.000), and had a longer postoperative hospital stay (23.00 ± 15.84 d vs 15.27 ± 5.89 d, P = 0.009). There was higher cost of hospitalization for patients who received preoperative total parenteral nutrition (TPN) than for patients who did not receive preoperative TPN (62 713.50 ± 5070.66 RMB Yuan vs 43178.00 ± 3596.68 RMB Yuan, P = 0.014). Applying postoperative enteral nutrition significantly shortened postoperative fasting time (5.16 ± 1.21 d vs 6.40 ± 1.84 d, P = 0.001) and postoperative hospital stay (11.92 ± 4.34 d vs 15.77 ± 6.03 d, P = 0.002). The patients who received postoperative TPN for no less than 7 d had increased serum glucose levels (7.59 ± 3.57 mmol/L vs 6.48 ± 1.32 mmol/L, P = 0.006) and cost of hospitalization (47 724.14 ± 16 945.17 Yuan vs 38 598.73 ± 8349.79 Yuan, P = 0.000). The patients who received postoperative omega-3 fatty acids had a higher rate of postoperative complications than the patients who did not (1.33 ± 0.64 vs 1.13 ± 0.49, P = 0.041). High level of serum glucose was associated with a high risk of postoperative complications of infection.
CONCLUSION: Appropriate and moderate nutritional intervention can improve the postoperative outcome of colorectal cancer patients.
Keywords: Nutritional support, Nutrition assessment, Colorectal cancer, Surgery, Prognosis
INTRODUCTION
Colorectal cancer is the fourth most common cancer in men and the third most common cancer in women worldwide[1]. It is also a significant cause of morbidity and mortality throughout the world[2]. Malnutrition is common in patients presenting for surgical management of colorectal cancer, and multiple factors, such as tumor location, tumor type, tumor stage, and preoperative radiation or chemotherapy, may predispose the patients to malnutrition[3]. Postoperative outcomes, including incidence of complications, morbidity and survival, are usually better in the patients who are in a good nutritional condition[4]. Comprehensive clinical application of nutrition support in colorectal cancer patients appears to be necessary.
Unfortunately, malnutrition has remained a troublesome problem because of lack of nutrition support routines and a discrepancy between clinical practice and guidelines regarding nutrition support [5].
Currently, international guidelines on nutrition support have been established, such as the European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines and the American Society for Parenteral and Enteral Nutrition (ASPEN) guidelines. Both are the authoritative guidelines at present, and should be followed and used in clinical practice as appropriate to the specific medical condition. However, since fewer than one sixth of the recommendations in the current guidelines are Grade A, and more than 50% are Grade C[6], more and better controlled trials are needed in the specific fields.
We carried out a retrospective study to evaluate the nutritional risk of colorectal cancer patients who underwent elective surgery, and assessed the nutrition support process by analyzing the postoperative clinical outcomes and comparing with the international recommendations or guidelines. In particular, we investigated the current status of nutrition support for patients undergoing surgery for colorectal cancer, and determined the requirements of feasible and appropriate nutrition support strategies for such patients.
MATERIALS AND METHODS
Case selection
We reviewed a total of 220 consecutive patients admitted to our hospital with a diagnosis of colon cancer or rectal cancer from January 2010 to July 2010, and excluded 18 patients, including one with hydroperitoneum according to the exclusion criteria of the Nutrition Risk Screening (NRS) 2002[7], and 17 who had received non-surgical treatment. The remaining 202 patients were enrolled in this study.
Methods
In order to evaluate the clinical effect of different nutritional strategies in colorectal cancer patients with different nutritional status, we stratified the patients into five groups.
In Group A, to evaluate the effect of NRS score, we excluded the patients who received preoperative nutritional support, and divided the remaining 199 patients into a “non-risk” group (n = 148) whose NRS score was 0-2, and an “at-risk” group (n = 51) whose NRS score was ≥ 3. We further divided the two groups into two subgroups, a nutrition support group (NS) who received postoperative nutrition support and a non-nutrition support group (NNS) who did not receive postoperative nutrition support (Table 1). Diagnosis and tumor stage were used to illustrate preoperative health status. The tumor stage was determined according to the 7th edition of the AJCC cancer staging manual (American Joint Committee on Cancer)[8]. Complications, postoperative hospital stay, cost of hospitalization and postoperative outcomes were used to assess the clinical effect of postoperative nutritional intervention. In addition, we graded complications as none = 1, infection = 2, fistula = 3, others = 4, and postoperative outcome as recovery = 1, no recovery = 2, death = 3, for statistical evaluation of the results.
Table 1.
Nutrition risk screening
|
Non-risk |
P |
At-risk |
P | |||
| NS | NNS | NS | NNS | |||
| Patient number | 143 | 5 | 49 | 2 | ||
| Diagnosis (colon / rectal cancer) | 50/93 | 1/4 | 24/25 | 2/0 | ||
| Gender (male/female) | 63/80 | 2/3 | 0.893 | 30/19 | 2/0 | 0.088 |
| Tumor stage1 | 0.066 | 0.358 | ||||
| Complications2 | 1.23 ± 0.60 | 2.40 ± 1.51 | 0.000 | 1.20 ± 0.45 | 1.50 ± 0.70 | 0.348 |
| None (= 1) | ||||||
| Infection (= 2) | ||||||
| Fistula (= 3) | ||||||
| Others (= 4) | ||||||
| Postoperative hospital stay (d) | 15.27 ± 5.89 | 23.00 ± 15.84 | 0.009 | 14.55 ± 4.11 | 14.50 ± 2.12 | 0.986 |
| Cost of hospitalization (RMB Yuan) | 43 469.88 ± 9961.67 | 35 825.00 ± 16 271.94 | 0.301 | 41 802.97 ± 13 300. 99 | 33 845.80 ± 8374.80 | 0.187 |
| Postoperative outcome2 | 1.02 ± 0.16 | 1.00 ± 0.00 | 0.707 | 1.10 ± 0.30 | 1.50 ± 0.70 | 0.090 |
The tumor stage of the patients was judged according to the 7th edition of American Joint Committee on Cancer staging manual[8];
Complications are defined as none = 1, infection = 2, fistula = 3, others = 4, and postoperative outcome as recovery = 1, no recovery = 2, death = 3, for easier statistical presentation of the results. NS: Nutrition support; NNS: Non-nutrition.
Group B consisted of five patients whose NRS score was > 4, the clinical effect of preoperative TPN in patients with severe malnutrition was evaluated. They were divided into two groups: group 1 (n = 2) who received preoperative TPN and group 2 (n = 3) who did not (Table 2). Diagnosis, tumor stage, and preoperative albumin, potassium, and sodium levels reflected the preoperative nutrition status. Postoperative enteral nutrition (EN), postoperative TPN and postoperative TPN duration were indicative of the postoperative nutritional intervention. Postoperative serum glucose level was fluctuated according to the proportion of insulin in TPN. Postoperative day 1 (POD1) albumin, potassium and sodium, and POD5 albumin, potassium and sodium levels reflected the postoperative nutritional status. Complications, postoperative hospital stay, cost of hospitalization, and postoperative outcomes were used to assess clinical outcome.
Table 2.
Preoperative total parenteral nutrition in malnourished patients
| Group 1 | Group 2 | P | |
| Patient number | 2 | 3 | |
| Diagnosis (colon/rectal cancer) | 2/0 | 1/2 | |
| Gender (male/female) | 2/0 | 1/2 | 0.219 |
| Tumor stage | 1 | 2 | |
| Preoperative albumin (g/L) | 36.30 ± 5.65 | 32.83 ± 9.00 | 0.669 |
| Preoperative potassium (mmol/L) | 4.53 ± 0.36 | 3.75 ± 0.92 | 0.352 |
| Preoperative sodium (mmol/L) | 138.45 ± 2.05 | 142.13 ± 3.40 | 0.274 |
| Postoperative EN | 2 | 0 | |
| Postoperative TPN | 2 | 2 | |
| Postoperative TPN duration (d) | 7.00 ± 1.41 | 3.66 ± 3.2 | 0.276 |
| POD1a serum glucose (mmol/L) | 6.91 ± 1.11 | 9.05 ± 3.65 | 0.498 |
| POD1 albumin (g/L) | 27.80 ± 3.11 | 26.13 ± 3.19 | 0.605 |
| POD1 potassium (mmol/L) | 4.49 ± 0.36 | 4.45 ± 0.78 | 0.953 |
| POD1 sodium (mmol/L) | 135.8 ± 3.11 | 137.66 ± 2.51 | 0.508 |
| POD5a serum glucose (mmol/L) | 6.29 ± 0.24 | 6.39 ± 2.88 | 0.968 |
| POD5 albumin (g/L) | 28.65 ± 5.16 | 32.33 ± 4.67 | 0.466 |
| POD5 potassium (mmol/L) | 4.67 ± 0.11 | 4.42 ± 0.12 | 0.115 |
| POD5 sodium (mmol/L) | 135.95 ± 1.34 | 135.93 ± 2.72 | 0.994 |
| Complications | 0.445 | ||
| Postoperative hospital stay(d) | 10.50 ± 4.94 | 13.00 ± 2.64 | 0.500 |
| Cost of hospitalization (RMB) | 62713.50 ± 5070.66 | 43178.00 ± 3596.68 | 0.014 |
| Postoperative outcome | 0.495 |
POD1: Postoperative day 1; POD5: Postoperative day 5; EN: Enteral nutrition; TPN: Total parenteral nutrition.
In Group C, the application of postoperative EN in colorectal cancer patients was assessed. Patients who received preoperative nutrition support were excluded, and the remaining 199 patients were divided into two groups: group 1 (n = 25) who received postoperative EN and group 2 (n = 174) who did not (Table 3). Diagnosis, tumor stage and NRS 2002 score reflected the preoperative nutrition status. As an interferential factor in this group, postoperative TPN was used in the statistical analysis to identify the effect of postoperative EN. Postoperative fasting time, occurrence of complications, postoperative hospital stay, cost of hospitalization, and postoperative outcome indicated clinical outcome.
Table 3.
Postoperative enteral nutrition and clinical outcome
| Group 1 | Group 2 | P | |
| Patient number | 25 | 174 | |
| Diagnosis (colon /rectal cancer) | 8/17 | 66/108 | 0.568 |
| Gender (male/female) | 9/16 | 91/83 | 0.129 |
| Tumor stage | 0.777 | ||
| NRS 2002 score | 1.88 ± 0.88 | 1.96 ± 1.01 | 0.689 |
| Postoperative fasting time (d) | 5.16 ± 1.21 | 6.40 ± 1.84 | 0.001 |
| Postoperative TPN | 27 | 167 | 0.996 |
| Complications | 1.04 ± 0.20 | 1.29 ± 0.66 | 0.060 |
| None (= 1) | |||
| Infection (= 2) | |||
| Fistula (= 3) | |||
| Others (= 4) | |||
| Postoperative hospital stay (d) | 11.92 ± 4.34 | 15.77 ± 6.03 | 0.002 |
| Cost of hospitalization (RMB) | 44210.88 ± 7635.85 | 42060.09 ± 13066.15 | 0.752 |
| Postoperative outcome | 1.00 ± 0.00 | 1.06 ± 0.27 | 0.214 |
| Recovery (= 1) | |||
| Unrecovery (= 2) | |||
| Dead (= 3) |
Group 1: Patients who received enteral nutrition postoperatively; Group 2: Patients who did not receive enteral nutrition postoperatively. TPN: Total parenteral nutrition; NRS: Nutrition risk screening.
In Group D, to determine the effect of postoperative TPN duration, we excluded the patients who received preoperative nutrition support and postoperative EN, and included the remaining 174 patients who received postoperative TPN only, dividing them into two groups: group 1 (n = 66) with postoperative TPN duration of no less than 7 d, and group 2 (n = 108) with a duration of less than 7 d (Table 4). Diagnosis, tumor stage, NRS 2002 score, preoperative albumin, potassium and sodium levels gave an indication of preoperative nutrition status. POD1 albumin, potassium and sodium, and POD5 albumin, potassium and sodium reflected postoperative nutritional status. Complications, postoperative hospital stay, cost of hospitalization and postoperative outcome were used to assess the clinical outcome. In addition, the comparison of preoperative serum glucose, POD1 serum glucose, and POD5 serum glucose reflected the contribution of postoperative TPN duration to postoperative serum glucose.
Table 4.
Postoperative total parenteral nutrition duration
| Group 1 | Group 2 | P | |
| Patient number | 66 | 108 | |
| Diagnosis (colon/rectal cancer) | 31/35 | 35/73 | |
| Gender (male/female) | 32/34 | 59/49 | 0.434 |
| Tumor stage | 0.493 | ||
| NRS 2002 score | 1.92 ± 0.94 | 1.99 ± 1.05 | 0.676 |
| Preoperative serum glucose (mmol/L) | 6.10 ± 1.86 | 5.75 ± 1.17 | 0.134 |
| Preoperative albumin (g/L) | 38.47 ± 4.44 | 39.51 ± 6.37 | 0.249 |
| Preoperative potassium (mmol/L) | 3.98 ± 0.38 | 6.57 ± 1.84 | 0.270 |
| Preoperative sodium (mmol/L) | 140.98 ± 3.23 | 139.58 ± 14.1 | 0.435 |
| POD1 serum glucose (mmol/L) | 8.59 ± 3.39 | 7.37 ± 2.06 | 0.100 |
| POD1 albumin (g/L) | 32.24 ± 3.65 | 35.49 ± 4.11 | 0.725 |
| POD1 potassium (mmol/L) | 4.05 ± 0.44 | 3.99 ± 0.45 | 0.424 |
| POD1 sodium (mmol/L) | 136.35 ± 3.59 | 135.72 ± 14.50 | 0.763 |
| POD5 serum glucose (mmol/L) | 7.59 ± 3.57 | 6.48 ± 1.32 | 0.006 |
| POD5 albumin (g/L) | 31.79 ± 3.53 | 39.91 ± 3.66 | 0.063 |
| POD5 potassium (mmol/L) | 4.21 ± 0.50 | 4.16 ± 0.53 | 0.553 |
| POD5 sodium (mmol/L) | 136.5 ± 18.60 | 137.84 ± 2.81 | 0.348 |
| Complications | 0.533 | ||
| Postoperative hospital stay (d) | 15.59 ± 5.32 | 15.87 ± 6.45 | 0.761 |
| Cost of hospitalization (RMB) | 47724.14 ± 16945.17 | 38598.73 ± 8349.79 | 0.000 |
| Postoperative outcome | 0.166 |
Group 1: The duration of postoperative total parenteral nutrition (TPN) was not less than 7 d; Group 2: The duration of postoperative TPN was less than 7 d. NRS: Nutrition Risk Screening.
Group E excluded the patients who received preoperative nutrition support or postoperative EN, and included the remaining 167 patients who received postoperative TPN. This group was subdivided into two groups: group 1 (n = 102), those who received postoperative application of omega-3 fatty acids, and group 2 (n = 65), those who did not, as shown in Table 5. Diagnosis, tumor stage, and NRS 2002 score were indicative of preoperative nutrition status. The total lymphocyte count reflected the immune status. Complications, postoperative hospital stay, cost of hospitalization, and postoperative outcome were used to assess the clinical outcome.
Table 5.
Postoperative administration of omega-3 fatty acids
| Group 1 | Group 2 | P | |
| Patient number | 102 | 65 | |
| Diagnosis (colon /rectal cancer) | 42/60 | 40/25 | |
| Gender (male/female) | 49/53 | 45/38 | 0.089 |
| Tumor stage | 0.317 | ||
| NRS 2002 score | 1.94 ± 0.87 | 2.00 ± 1.15 | 0.710 |
| Preoperative total lymphocyte count | 1.80 ± 0.63 | 1.93 ± 0.59 | 0.186 |
| POD1 total lymphocyte count | 0.99 ± 0.34 | 1.11 ± 0.40 | 0.067 |
| POD5 total lymphocyte count | 1.26 ± 0.59 | 1.29 ± 0.35 | 0.660 |
| Complications | 1.33 ± 0.64 | 1.13 ± 0.49 | 0.041 |
| None (= 1) | |||
| Infection (= 2) | |||
| Fistula (= 3) | |||
| Others (= 4) | |||
| Postoperative hospital stay (d) | 16.04 ± 5.81 | 14.81 ± 4.29 | 0.159 |
| Cost of hospitalization (RMB) | 43936.75 ± 14260.31 | 39938.89 ± 10741.40 | 0.055 |
| Postoperative outcome | 1.09 ± 0.33 | 1.06 ± 0.27 | 0.055 |
| Recovery (= 1) | |||
| Unrecovery (= 2) | |||
| Dead (= 3) |
Group 1: Patients who received omega-3 fatty acids; Group 2: Patients who did not receive omega-3 fatty acids.
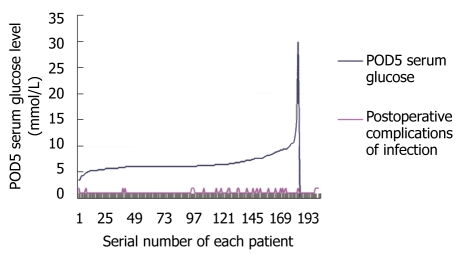
We also analyzed the relationship between postoperative day 5 serum glucose levels and postoperative complications of infection (Figure 1).
Figure 1.
Relationship between postoperative serum glucose level and complications of infection. Abscissa: the serial number of each patient, arranged according to the Postoperative day 5 serum glucose level; Ordinate: numerical value; The red line shows the incidence of postoperative complications of infection.
Statistical analysis
Analyses were performed using SPSS statistical software (SPSS for Windows Ver. 11.5). Results of different groups were compared using descriptive statistics (mean ± SD). P ≤ 0.05 was considered statistically significant.
RESULTS
Nutrition risk screening is a necessary and effective tool to identify the nutritional status of colorectal cancer patients, and to aid in providing the appropriate nutrition intervention. As Table 1 shows, the “non-risk” patients who did not receive postoperative nutrition support had a higher rate of postoperative complications than those who received postoperative nutrition support (2.40 ± 1.51 vs 1.23 ± 0.60, P = 0.000), and also had a longer postoperative hospital stay (23.00 ± 15.84 vs 15.27 ± 5.89, P = 0.009), which indicated that postoperative nutrition support may be necessary for “non-risk” patients. Postoperative nutrition support or not did not show a significant difference in the outcome of “at-risk” patients, though postoperative nutrition support tended to improve the postoperative outcome (1.10 ± 0.30 vs 1.50 ± 0.70, P = 0.090), thus moderate nutrition support is allowable for “at-risk” patients.
Table 2 shows that the cost of hospitalization for malnourished patients who received preoperative TPN was significantly higher than in patients who did not (62 713.50 ± 5070.66 RMB Yuan vs 43 178.00 ± 3596.68 RMB Yuan, P = 0.014) with no significant difference in the outcome.
Postoperative EN markedly improved postoperative recovery course, including a reduction in postoperative fasting time (5.16 ± 1.21 d vs 6.40 ± 1.84 d, P = 0.001) and postoperative hospital stay (11.92 ± 4.34 d vs 15.77 ± 6.03 d, P = 0.002), as shown in Table 3.
Longer postoperative TPN was not associated with better clinical outcome (Table 4). The patients who received postoperative TPN for no less than 7 d had increased POD5 serum glucose (7.59 ± 3.57 mmol/L vs 6.48 ± 1.32 mmol/L, P = 0.006) and cost of hospitalization (47 724.14 ± 16 945.17 Yuan vs 38598.73 ± 8349.79 Yuan, P = 0.000), compared to those with less than 7 d postoperative TPN, suggesting that less than 7 d nutrition support for postoperative colorectal cancer patients is adequate.
More postoperative complications occurred in the patients with postoperative administration of omega-3 fatty acid (1.33 ± 0.64 vs 1.13 ± 0.49, P = 0.041) than in patients who did not receive the fatty acid (Table 5).
Postoperative complications were positively correlated with the postoperative serum glucose level, a high postoperative serum glucose level being associated with a higher risk of complications of infection (Figure 1).
DISCUSSION
Malnutrition is common in patients with colorectal cancer, and in our study, 52 (25.7%) of the 202 cases had a NRS score of more than 3, and had a high nutrition risk[7]. Poor nutrition status impacts on the recovery of physical performance status in cancer patients after treatment[9]. It was reported that about 20% of cancer patients died of malnutrition or related complications rather than the malignant disease itself[10]. Malnutrition is often neglected in our daily clinical practice, and can also induce many clinical problems, including impaired wound-healing, immunocompromization, diminished cardiac and respiratory function, and a host of other complications that can lead to longer hospitalization and a higher mortality rate[11]. Although provision of nutrition support to cancer patients may cause tumors to grow more quickly, nutrition support is recommended when the nutrition status is so compromised that patients are at a high risk of complications, or cannot comply with the oncologic therapy as reported in the clinical practice ESPEN guidelines[12]. Thus perioperative nutrition support is beneficial for moderately or severely malnourished gastrointestinal cancer patients[13]. The implementation of nutrition support guidelines has facilitated many appropriate nutritional support procedures for colorectal cancer patients[4,6,14-16].
Preoperative nutrition risk screening can identify nutritional risks
Patients with cancer are at a risk of malnutrition, and nutrition screening should be performed to identify those who require nutrition support[17]. When a patient is admitted to our ward, knowledge of the nutritional status, which is a clinical predictor of postoperative mortality and morbidity in surgery for colorectal cancer[18-20], is essential, not only for screening malnourished or non-malnourished patients, but also for multimodal oncological treatment[21]. There are various kinds of screening methods, including NRS 2002, which is a rapid screening tool recommended by ESPEN[7], and has been proven to be an appropriate scoring system for predicting unfavorable clinical outcomes[22]. ASPEN suggested using a subjective global assessment (SGA) as a screening tool[23], and was shown to be a reliable assessment tool which could predict hospital stay and medical expenditure of surgical gastrointestinal cancer patients[24,25]. We believe that the assessment of nutritional status requires a multidimensional approach, which includes different clinical indices and various nutritional parameters, so it is better to use both SGA and NRS 2002 to predict the clinical outcome[26]. Our study indicated that, for “non-risk” colorectal patients, postoperative nutrition support is necessary to avoid postoperative complications and shorten postoperative hospital stay. Although postoperative nutrition support to “at-risk” colorectal patients showed no significant advantage, in our opinion, moderate nutrition support is allowable, as no harm or economic burden was incurred. Further prospective studies are necessary to confirm this.
Preoperative TPN is not always necessary
The goal of preoperative nutrition support is to minimize negative protein balance by avoiding starvation, to maintain muscle, immune and cognitive functions, and to enhance postoperative recovery, as the ESPEN guidelines indicated[27]. Preoperative parenteral nutrition is indicated in severely undernourished patients in whom enteral nutrition cannot be adequately administered either orally or enterally. Conversely, its use in well-nourished patients has no benefit but increased morbidity. In our study, preoperative nutrition support in severely malnourished colorectal cancer patients only increased the economic burden, with little beneficial effect. This is in agreement with the ASPEN guidelines, which recently recommended that nutrition support should not be used routinely in patients undergoing major cancer surgeries[17]. Because of the limited sample size, further prospective studies with a larger sample size should be carried out.
Gunerhan’s study[28] recently showed that preoperative immunonutrition resulted in a significant increase in serum prealbumin levels, but it did not significantly alter the T lymphocyte subpopulation count, the rate of postoperative complications and the hospitalization duration, thus preoperative immunonutrition should not be provided routinely. None of our patients received preoperative immunonutrition.
Postoperative EN can shorten the fasting time and hospital stay
Previously, many colorectal doctors believed that nutrients in the gut disrupted anastomoses, so they preferred delaying the EN postoperatively, and administered TPN instead to avoid anastomotic leak, which requires substantial use of hospital resources[29]. However, Seidner[11] emphasized that there were no significant differences in morbidity and mortality between patients who received EN or TPN, and recommended the guideline: if the gut works, use it. The available evidence lends support to the use of enteral over parenteral feeding in inpatients with functioning gastrointestinal tracts[30]. The application of EN can reverse the loss of gut mucosal integrity resulting from surgical trauma[31], and early nutrition support (EEN) is associated with a decreased infection risk, a decreased mortality, a reduced hospital stay, an increase in collagen deposition at anastomosis and wound strength, and a clear trend of a reduction in anastomotic breakdown[32,33]. In addition, EEN can reduce the use of nasogastric tubes, which may delay the return of bowel function and increase pulmonary complications[34,35]. Osland[3] even suggested adopting EEN as a standard of care in cancer patients undergoing gastrointestinal resections. As Table 3 shows, postoperative EN in colorectal cancer patients can significantly shorten the postoperative fasting time and postoperative hospital stay, and there is a tendency to reduce postoperative complications (P = 0.060). Although it remains to be determined how much should be provided initially, underfeeding with a small amount of nutrients, which “bathe” the gut mucosa, makes EEN necessary or desirable.
The risk of overfeeding should not be neglected, as it can overwhelm the digestive and absorptive capacity of the gastrointestinal tract, and lead to occurrence of some clinical complications, such as gastric distention, nausea, and diarrhea[32].
Postoperative TPN can offer a smooth postoperative recovery
Parenteral nutrition (PN) has been widely used in clinical practice, and a safe PN system must be developed which minimizes procedural incidents and maximizes the ability to meet individual patient requirements[36]. Thus, it is desirable to provide, devise, or make available customized PN formulations for individuals who have complex requirements secondary to disease or underlying illness, or when otherwise warranted by routine monitoring of electrolytes, organ function, growth, and development. Not only fat and carbohydrates, but also a full range of vitamins and trace elements should be important components of the TPN bag, and optimal nitrogen-sparing can be achieved when all components of the PN mix are administered simultaneously over 24 h. However, when early oral food intake or EN is combined with PN, intravenous supplementation with vitamins appears to be unnecessary[27].
Should colorectal cancer patients be administered postoperative TPN? Planas[4] recommended that such patients having elective surgery should not be given postoperative PN routinely. Seidner[11] implied that administering PN in disregard of the patient’s nutritional status could do more harm than good, and suggested that postoperative TPN should be reserved for patients who have a prolonged postoperative ileus, generally more than 7-10 d, and for those who are severely malnourished and whose feeding cannot be started within 3-5 d. According to the ESPEN guidelines[27], postoperative PN is recommended in patients who cannot meet their caloric requirements within 7-10 d both orally or enterally, and in patients who require postoperative artificial nutrition, enteral feeding or a combination of enteral and supplementary parenteral feeding.
In our study, most colorectal cancer patients could resume feeding 5-8 d postoperatively (Table 3), so postoperative TPN may be beneficial during the period of postoperative fasting. Should we give the patients TPN for 7 d or more, or is less than 7 d adequate? The results in Table 4 indicate that a longer duration of TPN incurs high hospitalization costs and induces hyperglycemia, which is associated with a higher rate of postoperative complications (Figure 1), thus less than 7 d’ postoperative TPN appears to be appropriate.
PN can be delivered through short-term, non-tunneled central venous catheters, and the appropriate choice, insertion, and monitoring of the venous access are of paramount importance to avoid a catheter-related bloodstream infection, an important and still very common complication of PN[37]. Such infections can be reduced by adopting cost-effective, evidence-based interventions, including specific training of staff, an adequate handwashing, the correct type of device and site of insertion, the use of maximal barrier protection during insertion, and removal of central lines as soon as they are no longer necessary.
Postoperative application of omega-3 fatty acids
There is controversy as to whether visceral proteins should be used to assess nutrient status in hospitalized patients. Seidner[11] suggested that visceral proteins can be used in the hospital setting, because they can identify patients at risk of a poor outcome who may benefit from nutrition support. In addition, the total lymphocyte count can be used to assess a patient’s immune function, which has been shown to correlate with the degree of visceral protein depletion and clinical outcome. Therefore, total lymphocyte counts were used in our study to assess the effect of the omega-3 fatty acids.
Postoperative supplementation of omega-3 fatty acids by TPN has been reported to have a favorable effect in the outcomes of colorectal cancer patients undergoing radical resection, by lowering the magnitude of the inflammatory response and modulating the immune response[38,39]. In contrast, the application of omega-3 fatty acids showed no significant benefit in our study, and indeed there was a trend of an increased risk of postoperative complications, an increased economic burden, and a poorer postoperative outcome. Further prospective research is necessary with a larger sample to assess the functional benefit or otherwise of omega-3 fatty acids in the postoperative setting.
Currently, many barriers, including low priority of nutritional support, no routine or established procedures in many medical centers, insufficient knowledge of nutritional support, lack of qualified and optional nutritional menus for the patients, and lack of leadership support from the medical team, make the nutritional therapy difficult to carry out in many hospitals[40]. A greater effort should be made in the nutritional assessment of patients.
In conclusion, nutrition support is an important therapy for colorectal cancer patients, and appropriate and moderate nutritional intervention can significantly improve the postoperative recovery course, relieve the patient’s suffering, and reduce the medical cost of the patients. Clinicians must be aware of nutrition support principles and methods in order to administer appropriate nutrition support and avoid blind nutrition administration.
COMMENTS
Backgrounds
Nutrition support has been widely used in the area of surgery, where the benefit on patients’ prognosis is evident. Colorectal cancer is the fourth most common cancer in men and the third most common cancer in women worldwide, and is also a significant cause of morbidity and mortality throughout the world, thus an appropriate and feasible nutrition support strategy is necessary and beneficial for patients’ prognosis.
Research frontiers
Nutritional support is widely used in postoperative colorectal cancer patients, but the role of nutrients has not been clearly defined. This study investigated the effect of nutrition support on the outcomes of patients with different nutritional status.
Innovations and breakthroughs
The authors found that appropriate and moderate nutritional intervention can significantly improve the postoperative outcome of the patients with colorectal cancer.
Applications
The study provides a reference for daily clinical practice and future research. A prospective, multicenter, randomized, controlled trial with a larger sample is necessary to validate the statistical results and diminish bias.
Peer review
Although this is a retrospective review, I believe it will be of interest to the readers. And, it does add something to the literature.
Acknowledgments
The authors thank Sangeeta Sharma for the help with manuscript preparation.
Footnotes
Supported by the Postgraduate Scientific Research Fund of Shengjing Hospital, China Medical University
Peer reviewers: Laura E Matarese, PhD, RD, LDN, FADA, CNSD, Assistant Professor of Surgery, University of Pittsburgh Medical Center, Director of Nutrition, Intestinal Rehabilitation and Transplantation Center, Thomas E. Starzl Transplantation Institute, UPMC Montefiore, 7 South, 3459 Fifth Avenue, Pittsburgh, PA 15213, United States; Dr. Jose Perea, Department of Surgery, 12 De Octubre University Hospital, Rosas De Aravaca, 82a, Madrid 28023, Spain
S- Editor Sun H L- Editor Ma JY E- Editor Ma WH
References
- 1.Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA Cancer J Clin. 2009;59:366–378. doi: 10.3322/caac.20038. [DOI] [PubMed] [Google Scholar]
- 2.Gellad ZF, Provenzale D. Colorectal cancer: national and international perspective on the burden of disease and public health impact. Gastroenterology. 2010;138:2177–2190. doi: 10.1053/j.gastro.2010.01.056. [DOI] [PubMed] [Google Scholar]
- 3.Osland EJ, Memon MA. Early postoperative feeding in resectional gastrointestinal surgical cancer patients. World J Gastrointest Oncol. 2010;2:187–191. doi: 10.4251/wjgo.v2.i4.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Planas M, Peñalva A, Burgos R, Puiggrós C, Pérez-Portabella C, Espín E, Armengol M, Rosselló J. Guidelines for colorectal cancer: effects on nutritional intervention. Clin Nutr. 2007;26:691–697. doi: 10.1016/j.clnu.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Johansson U, Rasmussen HH, Mowe M, Staun M. Clinical nutrition in medical gastroenterology: room for improvement. Clin Nutr. 2009;28:129–133. doi: 10.1016/j.clnu.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Bozzetti F, Forbes A. The ESPEN clinical practice Guidelines on Parenteral Nutrition: present status and perspectives for future research. Clin Nutr. 2009;28:359–364. doi: 10.1016/j.clnu.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Kondrup J, Allison SP, Elia M, Vellas B, Plauth M. ESPEN guidelines for nutrition screening 2002. Clin Nutr. 2003;22:415–421. doi: 10.1016/s0261-5614(03)00098-0. [DOI] [PubMed] [Google Scholar]
- 8.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York: Springer; 2010. pp. 143–464. [Google Scholar]
- 9.Tian J, Chen ZC, Hang LF. Effects of nutritional and psychological status in gastrointestinal cancer patients on tolerance of treatment. World J Gastroenterol. 2007;13:4136–4140. doi: 10.3748/wjg.v13.i30.4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ottery FD. Cancer cachexia: prevention, early diagnosis, and management. Cancer Pract. 1994;2:123–131. [PubMed] [Google Scholar]
- 11.Seidner DL. Nutritional issues in the surgical patient. Cleve Clin J Med. 2006;73 Suppl 1:S77–81. doi: 10.3949/ccjm.73.suppl_1.s77. [DOI] [PubMed] [Google Scholar]
- 12.Bozzetti F, Mori V. Nutritional support and tumour growth in humans: a narrative review of the literature. Clin Nutr. 2009;28:226–230. doi: 10.1016/j.clnu.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Wu GH, Liu ZH, Wu ZH, Wu ZG. Perioperative artificial nutrition in malnourished gastrointestinal cancer patients. World J Gastroenterol. 2006;12:2441–2444. doi: 10.3748/wjg.v12.i15.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McClave SA, Martindale RG, Vanek VW, McCarthy M, Roberts P, Taylor B, Ochoa JB, Napolitano L, Cresci G. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) JPEN J Parenter Enteral Nutr. 2009;33:277–316. doi: 10.1177/0148607109335234. [DOI] [PubMed] [Google Scholar]
- 15.Clinical Guidelines for the Use of Parenteral and Enteral Nutrition in Adult and Pediatric Patients, 2009. JPEN J Parenter Enteral Nutr. 2009;33:255–259. doi: 10.1177/0148607109333115. [DOI] [PubMed] [Google Scholar]
- 16.Braga M, Ljungqvist O, Soeters P, Fearon K, Weimann A, Bozzetti F. ESPEN Guidelines on Parenteral Nutrition: surgery. Clin Nutr. 2009;28:378–386. doi: 10.1016/j.clnu.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 17.August DA, Huhmann MB. A.S.P.E.N. clinical guidelines: nutrition support therapy during adult anticancer treatment and in hematopoietic cell transplantation. JPEN J Parenter Enteral Nutr. 2009;33:472–500. doi: 10.1177/0148607109341804. [DOI] [PubMed] [Google Scholar]
- 18.Schwegler I, von Holzen A, Gutzwiller JP, Schlumpf R, Mühlebach S, Stanga Z. Nutritional risk is a clinical predictor of postoperative mortality and morbidity in surgery for colorectal cancer. Br J Surg. 2010;97:92–97. doi: 10.1002/bjs.6805. [DOI] [PubMed] [Google Scholar]
- 19.Salvino RM, Dechicco RS, Seidner DL. Perioperative nutrition support: who and how. Cleve Clin J Med. 2004;71:345–351. doi: 10.3949/ccjm.71.4.345. [DOI] [PubMed] [Google Scholar]
- 20.Karlsson S, Andersson L, Berglund B. Early assessment of nutritional status in patients scheduled for colorectal cancer surgery. Gastroenterol Nurs. 2009;32:265–270. doi: 10.1097/SGA.0b013e3181aead68. [DOI] [PubMed] [Google Scholar]
- 21.Senesse P, Assenat E, Schneider S, Chargari C, Magné N, Azria D, Hébuterne X. Nutritional support during oncologic treatment of patients with gastrointestinal cancer: who could benefit? Cancer Treat Rev. 2008;34:568–575. doi: 10.1016/j.ctrv.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Raslan M, Gonzalez MC, Dias MC, Nascimento M, Castro M, Marques P, Segatto S, Torrinhas RS, Cecconello I, Waitzberg DL. Comparison of nutritional risk screening tools for predicting clinical outcomes in hospitalized patients. Nutrition. 2010;26:721–726. doi: 10.1016/j.nut.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 23.Detsky AS, McLaughlin JR, Baker JP, Johnston N, Whittaker S, Mendelson RA, Jeejeebhoy KN. What is subjective global assessment of nutritional status? JPEN J Parenter Enteral Nutr. 1987;11:8–13. doi: 10.1177/014860718701100108. [DOI] [PubMed] [Google Scholar]
- 24.Wu BW, Yin T, Cao WX, Gu ZD, Wang XJ, Yan M, Liu BY. Clinical application of subjective global assessment in Chinese patients with gastrointestinal cancer. World J Gastroenterol. 2009;15:3542–3549. doi: 10.3748/wjg.15.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Filipović BF, Gajić M, Milinić N, Milovanović B, Filipović BR, Cvetković M, Sibalić N. Comparison of two nutritional assessment methods in gastroenterology patients. World J Gastroenterol. 2010;16:1999–2004. doi: 10.3748/wjg.v16.i16.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raslan M, Gonzalez MC, Torrinhas RS, Ravacci GR, Pereira JC, Waitzberg DL. Complementarity of Subjective Global Assessment (SGA) and Nutritional Risk Screening 2002 (NRS 2002) for predicting poor clinical outcomes in hospitalized patients. Clin Nutr. 2011;30:49–53. doi: 10.1016/j.clnu.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Braga M, Ljungqvist O, Soeters P, Fearon K, Weimann A, Bozzetti F. ESPEN Guidelines on Parenteral Nutrition: surgery. Clin Nutr. 2009;28:378–386. doi: 10.1016/j.clnu.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Gunerhan Y, Koksal N, Sahin UY, Uzun MA, Ekşioglu-Demiralp E. Effect of preoperative immunonutrition and other nutrition models on cellular immune parameters. World J Gastroenterol. 2009;15:467–472. doi: 10.3748/wjg.15.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frye J, Bokey EL, Chapuis PH, Sinclair G, Dent OF. Anastomotic leakage after resection of colorectal cancer generates prodigious use of hospital resources. Colorectal Dis. 2009;11:917–920. doi: 10.1111/j.1463-1318.2008.01728.x. [DOI] [PubMed] [Google Scholar]
- 30.Zaloga GP. Parenteral nutrition in adult inpatients with functioning gastrointestinal tracts: assessment of outcomes. Lancet. 2006;367:1101–1111. doi: 10.1016/S0140-6736(06)68307-4. [DOI] [PubMed] [Google Scholar]
- 31.Jiang XH, Li N, Li JS. Intestinal permeability in patients after surgical trauma and effect of enteral nutrition versus parenteral nutrition. World J Gastroenterol. 2003;9:1878–1880. doi: 10.3748/wjg.v9.i8.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ochoa JB, Caba D. Advances in surgical nutrition. Surg Clin North Am. 2006;86:1483–1493. doi: 10.1016/j.suc.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Fearon KC, Ljungqvist O, Von Meyenfeldt M, Revhaug A, Dejong CH, Lassen K, Nygren J, Hausel J, Soop M, Andersen J, et al. Enhanced recovery after surgery: a consensus review of clinical care for patients undergoing colonic resection. Clin Nutr. 2005;24:466–4`77. doi: 10.1016/j.clnu.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Nelson R, Tse B, Edwards S. Systematic review of prophylactic nasogastric decompression after abdominal operations. Br J Surg. 2005;92:673–680. doi: 10.1002/bjs.5090. [DOI] [PubMed] [Google Scholar]
- 35.Zhou T, Wu XT, Zhou YJ, Huang X, Fan W, Li YC. Early removing gastrointestinal decompression and early oral feeding improve patients’ rehabilitation after colorectostomy. World J Gastroenterol. 2006;12:2459–2463. doi: 10.3748/wjg.v12.i15.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kochevar M, Guenter P, Holcombe B, Malone A, Mirtallo J. ASPEN statement on parenteral nutrition standardization. JPEN J Parenter Enteral Nutr. 2007;31:441–448. doi: 10.1177/0148607107031005441. [DOI] [PubMed] [Google Scholar]
- 37.Pittiruti M, Hamilton H, Biffi R, MacFie J, Pertkiewicz M. ESPEN Guidelines on Parenteral Nutrition: central venous catheters (access, care, diagnosis and therapy of complications) Clin Nutr. 2009;28:365–377. doi: 10.1016/j.clnu.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 38.Liang B, Wang S, Ye YJ, Yang XD, Wang YL, Qu J, Xie QW, Yin MJ. Impact of postoperative omega-3 fatty acid-supplemented parenteral nutrition on clinical outcomes and immunomodulations in colorectal cancer patients. World J Gastroenterol. 2008;14:2434–2439. doi: 10.3748/wjg.14.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang ZM, Wilmore DW, Wang XR, Wei JM, Zhang ZT, Gu ZY, Wang S, Han SM, Jiang H, Yu K. Randomized clinical trial of intravenous soybean oil alone versus soybean oil plus fish oil emulsion after gastrointestinal cancer surgery. Br J Surg. 2010;97:804–809. doi: 10.1002/bjs.6999. [DOI] [PubMed] [Google Scholar]
- 40.Rasmussen HH, Kondrup J, Staun M, Ladefoged K, Lindorff K, Jørgensen LM, Jakobsen J, Kristensen H, Wengler A. A method for implementation of nutritional therapy in hospitals. Clin Nutr. 2006;25:515–523. doi: 10.1016/j.clnu.2006.01.003. [DOI] [PubMed] [Google Scholar]