Abstract
Previous work done in our laboratory, using mouse models, showed that soluble Fas ligand (sFasL) can efficiently delete donor anti-host T cells during their activation against irradiated host cells in MLCs. In the mouse models, this ex vivo sFasL treatment abrogated graft-vs-host disease (GVHD) while sparing donor T cells with antitumor reactivity. The present work was performed with human cells, to extend our work toward reduction of clinical GVHD. PBMC responders from a given individual (first party) were stimulated in vitro with irradiated PBMC stimulators from a second person (second party), in the presence of sFasL. In control MLCs without sFasL, alloreacting T cells began to up-regulate Fas (CD95) detectably and became sensitive to Fas-mediated apoptosis by as early as day 1–2. In MLCs containing sFasL, there were greatly reduced numbers of alloreacting CD3+CFSElo cells, activation Ag-expressing CD4hi and CD8hi cells, IFN-γ-producing CD4+ and CD8+ cells, and CD8+CD107a+ CTLs. Furthermore, mice transplanted with the ex vivo sFasL/MLR-treated cells had prolonged time to fatal GVHD in an in vivo xenogeneic GVHD model. Responder cells harvested from primary MLCs containing sFasL had reduced proliferation in response to second party cells, but proliferated in response to CMV Ags, PHA, and third party cells. In addition, sFasL/MLR-treated cell populations contained influenza-specific T cells, CD4+FOXP3+ T cells, and CD4+CD25+ T cells. These data indicate that this ex vivo sFasL/MLR depletion of alloreacting human donor anti-host T cells was efficient and selective.
Allogeneic (Allo)3 hematopoietic stem cell transplantation is the only curative treatment for several malignant and nonmalignant hematopoietic disorders. The efficacy of this treatment is limited by graft-vs-host disease (GVHD), the major cause of morbidity and mortality in this setting. GVHD is a complex inflammatory disease mediated mainly by allo donor graft T cells that are stimulated by APCs to attack genetically disparate host tissues, especially skin, mucosa, and liver (1–3). Although pan-T cell depletion of the donor allograft can reduce GVHD, this approach has not translated into improved overall survival because of the following: 1) increased rate of graft failure, 2) increased frequency of leukemia relapse, 3) increased risk of posttransplantation EBV-associated lymphoproliferative disorders, and 4) delayed immune reconstitution with increased incidence of opportunistic infections (4). Although these observations support the overlapping role of donor alloreactive T cells in mediating both GVHD and graft vs leukemia (GVL), cases with complete remission of leukemia, but no clinical signs of GVHD after hematopoietic stem cell transplantation or donor leukocyte infusion support the presence of allo T cells with antileukemia activity that do not mediate (clinically detectable) GVHD, and suggest that GVHD and GVL activities can be separated, to a therapeutically useful extent (5–7). In addition, the existence of allo T cells with antileukemia activity, recognizing leukemia-specific Ags or minor histocompatibility Ags that are preferentially expressed on leukemia cells, has been reported by several groups (6, 8–11). These findings indicate the GVL potential of the donor allograft and support the search for a simple, clinically applicable method that would allow the transfer of allo donor T cells with reduced potential to mediate GVHD against the transplant host.
Several methods for depletion of donor anti-host GVHD-mediating T cells have been developed. These methods target ex vivo alloantigen-activated T cells (from a MLR culture with irradiated allo host cells) based on the expression of various T cell activation markers (such as CD25, CD69, CD71, HLA-DR, CD137, and CD134 (12–21)), the dilution of CFSE (22), or the uptake of the dye 4,5-dibromorhodamine 123 (TH9402) by activated cells, making them sensitive to light (23).
We recently reported a new approach for depletion of allo donor anti-host T cells that is based on the sensitivity of activated T cells to Fas (CD95)-mediated apoptosis (24). Using mouse models, we showed that recombinant human soluble Fas ligand (sFasL) can efficiently and selectively deplete donor antihost T cells during their activation against irradiated host cells in a MLC. This ex vivo sFasL treatment reduced GVHD while sparing antitumor reactivity and hematopoietic engrafting capacity in mouse models (24). The feasibility of our method is supported by a similar study in which an agonistic Ab to Fas (CD95) was used to reduce donor antihostalloreacting cells (25, 26). To move our work toward potential use for reduction of clinical GVHD, we extended these studies to a human model system.
Materials and Methods
Cell isolation for MLR cultures
Human PBMCs were isolated from heparinized normal human blood by density gradient centrifugation using Ficoll-Paque (Amersham/GE Healthcare Bio-Sciences), according to the manufacturer’s instructions. Signed informed consent was obtained for use of each human blood sample under an Institutional Review Board-approved protocol. Mouse splenocytes were isolated by mincing the spleens, and treating (30 min, room temperature) with liberase blendzymes and DNase I (Roche Applied Science; following manufacturer’s instructions), followed by EDTA (10 mM, 5 min, room temperature; Sigma-Aldrich). Light density cells were isolated after centrifugation in Nycodenz medium (Accurate Chemical & Scientific).
MLR cultures and proliferation assays
One-way allo MLRs were performed by culturing either of the following: 1) 2 × 105 responder PBMCs from one normal human donor (first party) with 4 × 105 irradiated (1,500 cGy, Gammacell 137Cs source; Atomic Energy) stimulator PBMCs of an unrelated normal human donor (second party); or 2) 105 responder PBMCs from a donor with 0.2 × 105 irradiated (10,000 cGy) allogeneic stimulator cells from a human B-lymphoblastoid cell line (LCL; established in the Levitsky laboratory by standard EBV transformation of peripheral blood B lymphocytes). One-way xenogeneic (xeno) MLRs were performed by culturing 2 × 105 responder PBMCs from a donor with 2 × 105 irradiated (5,000 cGy) mouse splenocytes as stimulators. Responder PBMCs cultured without stimulators were the background proliferation controls in all experiments. Cells were cultured in RPMI 1640 medium (Life Technologies) containing 10% human AB serum (GemCell; Gemini Bio-Products), 2 mM l-glutamine (Life Technologies), 10 mM HEPES (Life Technologies), and 100 µg/ml primocin (Amaxa Biosystems) in 96-well plates for 1–7 days in a humidified incubator (37°C, 5% CO2) with (0.5 µg/ml sFasL plus 2 µg/ml cross-linker; Alexis Biochemicals) or without (2 µg/ml cross-linker only; control) sFasL treatment. In the experiments of Fig. 7, 2 µg/ml super Fas ligand (FasL; Alexis Biochemicals; a more potent version of recombinant sFasL that trimerizes without cross-linker) was used instead of sFasL plus cross-linker. Proliferation of responder cells was analyzed by assessing [3H]thymidine incorporation or CFSE dilution.
FIGURE 7.
Mice transplanted with sFasL/MLR-treated cells had prolonged times to fatal GVHD. Three groups of NOD-scid Il2rg−/− mice were injected with 106, 2.5 × 106, or 7 × 106 human PBMCs: the first two groups received human PBMCs that had been stimulated with irradiated NOD-scid Il2rg−/− splenocytes for 7 days in the absence (treatment control; ■) or presence of 2 µg/ml super FasL (sFasL; —). The third group received freshly isolated PBMCs from the same donor (fresh control; □). In addition, a fourth group received no human cells (radiation control; data not shown). Total body weights (data not shown) and deaths were monitored. Survival curves of groups that received sFasL/MLR-treated cells are significantly different from both control groups (log-rank (Mantel-Cox) test p values shown). Mice that did not receive human cells (irradiation control) survived (100%), appeared clinically healthy, and gained weight progressively (data not shown).
In [3H]thymidine assays, MLCs (in 96-well round-bottom tissue culture plates) were pulsed for their last 18–24 h of cell culture with 1 µCi/well [3H]thymidine (Amersham/GE Healthcare Bio-Sciences). Then cells were harvested onto glass fiber filters (Printed Filtermat A; Wallac), and incorporated [3H]thymidine was measured (Wallac 1450 MicroBeta liquid scintillation counter; PerkinElmer Wallac). All samples were tested in triplicate, and values for individual experiments are reported as means ± SD. Combinations of results of ≥3 independent experiments are presented as mean ± SEM. Values of p were measured by Student’s t test.
In CFSE assays, responder PBMCs (107) were labeled with 1 µM CFSE (Molecular Probes) for 10 min at 37°C in 5 ml of staining buffer (PBS containing 0.1% (w/v) BSA; Sigma-Aldrich). CFSE was removed by three washes with RPMI 1640 medium containing 10% heat-inactivated FBS (BenchMark; Gemini Bio-Products). Combinations of results of ≥3 independent experiments are presented as mean ± SD.
In the experiments of supplemental Fig. 2, 4 CD4+CD25+ cells were isolated from fresh PBMCs using a MACS isolation kit (Miltenyi Biotec; kit 130-091-301), then labeled with CFSE and mixed with unlabeled PBMCs from the same donor.
For secondary challenges, day 2 sFasL/MLR-treated (and control) cells were washed twice with RPMI 1640 medium containing 10% human AB serum, and then cultured overnight in sFasL-free medium (to allow apoptosis to take place). On day 3, equal numbers of control- and sFasL-treated cells from primary MLCs were challenged with the same second party stimulators, third party stimulators, PHA (5 µg/ml; Sigma-Aldrich), and CMV Ags (1/50 final dilution; Microbix Biosystems). Background proliferation controls were control- and sFasL-treated cells from the primary MLR that were not challenged in the secondary MLRs. In CFSE dilution experiments, cells were relabeled with CFSE before the secondary challenge.
Flow cytometry
FACS analyses were performed using the following directly fluorochrome-labeled mAbs: CD3 (HIT3a), CD4 (RPA-T4), CD8 (RPA-T8), CD25 (MA251), CD38 (HIT2/HB7), HLA-DR (L243), CD45 (HI30 and 30-F11), CD95 (DX2), CD107a (H4A3) (BD Biosciences), FOXP3 (PCH101), and IFN-γ (4S.B3) (eBioscience). mAbs or isotype controls (mouse IgG1, IgG2a; BD Biosciences) were added to cells suspended in 100 µl of cold PBS containing 2.5% heat-inactivated FBS (PBS-FBS) for 30 min at 4°C. Cell suspensions were washed once, resuspended in 200 µl of PBS-FBS, and then analyzed using a FACSCalibur cytometer (BD Biosciences).
Influenza (Flu)-specific CD8+ cells were detected by incubating 106 PBMCs from a HLA-A*0201 Flu-positive donor (characterized by the manufacturer in an ELISPOT assay for ability to respond to the influenza A viral peptide GILGFVFTL; SeraCare catalog numbers 72000-020805 and 72000-031705-B) with 10 µl of PE-labeled HLA-A*0201/GILG FVFTL MHC class I Flu pentamer (ProImmune) for 10 min at room temperature, washing once with PBS-FBS, and then labeling with CD8-allophycocyanin and CD3-FITC. PE-labeled HLA-B*0702/RPPIFIRRL EBV pentamer (ProImmune) served as a control. Intracellular FOXP3 staining was performed using the anti-human FOXP3 staining set (eBioscience; catalog number 72-5776), according to manufacturer’s protocol. For intracellular IFN-γ staining, cells were first stimulated for 5 h with PMA (0.1 µg/ml; Sigma-Aldrich) and ionomycin (0.5 µg/ml; Sigma-Aldrich) in the presence of an inhibitor of intracellular protein transport (GolgiStop; BD Biosciences) at 37°C/5% CO2, and then immunostained using the BD Cytofix/Cytoperm plus fixation/permeabilization kit, according to manufacturer’s instructions (BD Biosciences; kit 554715). Flow cytometric data were analyzed using FlowJo software (Tree Star).
Apoptosis assay
Apoptosis was determined by FACS analysis of active cellular caspases 3 and 7 using the Vybrant FAM caspase 3/7 assay kit (Molecular Probes), according to manufacturer’s instructions.
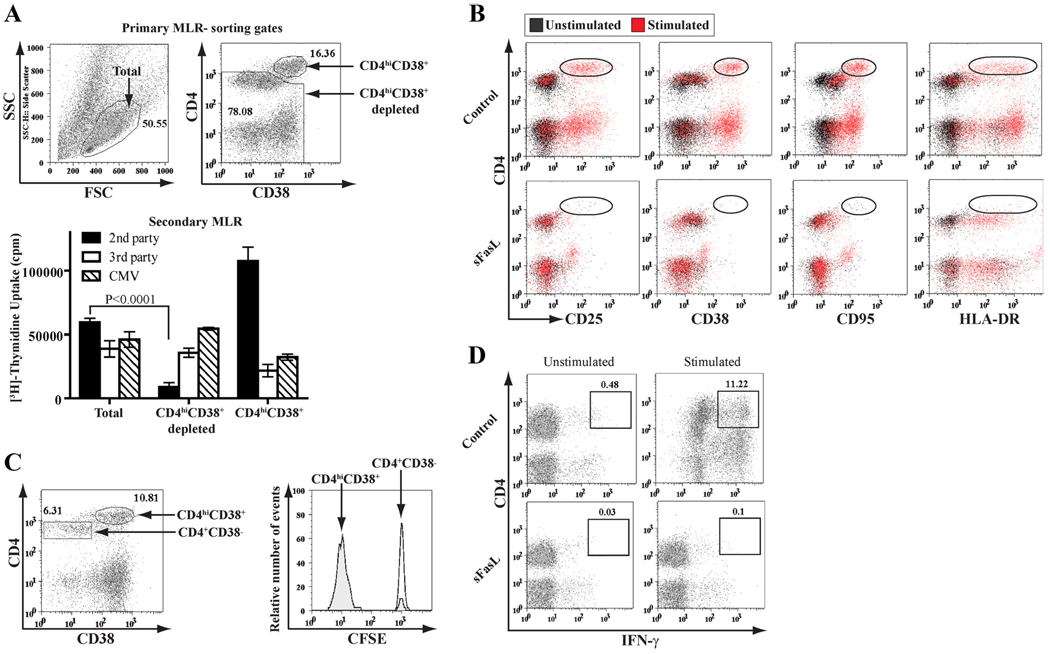
Depletion of alloreacting CD4hiCD38+ cells
Cells from a day 7 allo-MLC were harvested, dead cells were removed using the MACS dead cell removal kit (Miltenyi Biotec; kit 130-090-101), and viable cells were labeled with CD4-allophycocyanin and CD38-FITC (BD Biosciences). A high-speed MoFlow sorter (DakoCytomation) was used to FACS sort the CD4hiCD38+ cells and the complementary population depleted of these cells. Secondary responses of the FACS-sorted cell populations to the same second party stimulators, to unrelated third party cells, and to CMV Ags were assessed by [3H]thymidine incorporation.
Xeno-GVHD assay
Highly immunodeficient NOD.Cg-PrkdcscidIl2rgtmlWjl/SzJ (NOD-scid Il2rg−/−) mice (27, 28), purchased from The Jackson Laboratory, were bred and maintained in an immune-compromised mouse facility, under protocols approved by the Johns Hopkins Medical Institution Animal Use and Care Committee. Xeno GVHD was induced in NOD-scid Il2rg−/− mice following the protocol previously described by van Rijn et al. (29), with slight modifications. Briefly, 8-wk NOD-scid Il2rg−/− females received a single (sublethal) dose of 250 cGy of total body irradiation before injection (tail vein) of human cells on the same day. Radiation control mice did not receive human cells. Weight loss was monitored every 7 days. When mice in an experimental group developed clinical signs of severe GVHD (severe weight loss, hunched posture, ruffled fur, reduced mobility, tachypnea), one representative ill mouse from that group was sacrificed, and multiple organs/tissues (spleen, bone marrow, liver, lung, blood) were harvested for FACS analysis of human cell engraftment (using directly fluorochrome-labeled mAbs recognizing mouse CD45 and human CD45 (BD Biosciences)) and T cell activation. Blood was obtained via the retro-orbital sinus from the mice under brief anesthesia (Isoflurane USP; Hospira), then FACS analyzed, after RBC lysis (RBC lysis buffer; eBioscience). Histopathologic analysis of organs (skin, lungs, liver, gut, spleen) harvested postmortem from each mouse was done by an expert veterinary pathologist (C.B.).
Results
sFasL/MLR treatment reduced activated donor T cells
Alloreacting CD3+ T cells began to up-regulate Fas (CD95; Fig. 1A) as early as day 1–2 of the allo MLR. Alloreacting CD4+ and CD8+ T cell subsets exhibited similar kinetics of Fas up-regulation (Fig. 1, B and C), and T cells became sensitive to Fas-mediated apoptosis by day 2 (Fig. 1D). In preliminary experiments, we varied sFasL concentrations (with or without cross-linker), cell concentrations, and responder to stimulator ratios to determine the optimal conditions for efficient reduction of donor antihost T cell alloreactivity. In 7-day MLCs using PBMC responders taken from one person (first party) and irradiated PBMC or LCL stimulators from a second person (second party), at a responder to stimulator ratio of 1:2 or 5:1, respectively, treatment with 0.5 µg/ml sFasL plus 2 µg/ml cross-linker efficiently depleted anti-second party alloreactivity (data not shown). To shorten the ex vivo sFasL/MLR treatment to a minimum (potentially to reduce toxicity for future clinical application), we compared the efficacy of 2, 3, 4, and 5 days sFasL treatment. We found that a 2-day sFasL/MLR treatment was sufficient to eliminate most of the reactivity against the second party allo stimulators (data not shown). These conditions were used in the experiments presented in this study, unless otherwise indicated.
FIGURE 1.
Alloreacting T cells began to up-regulate Fas and became sensitive to Fas-mediated apoptosis by day 1–2 of allo MLCs. A–C, Responder PBMCs were cultured with (dotted lines) or without (solid lines) allo LCL stimulators for 1–7 days. Each day, cells from these MLCs were immunostained with CD25-PE, CD95-allophycocyanin, and either CD3-FITC, CD4-FITC, or CD8-FITC, and then analyzed for Fas (CD95) expression by FACS. A, Kinetics of CD95 expression (mean of median fluorescence intensities (MFI) from six experiments ± SD) on gated CD3+ cells in response to stimulator cells. B and C, CD95 expression on gated CD4+ and CD8+ T cells from a representative experiment; the mean MFIs of CD95 expression from three experiments on each selected day are indicated (±SD). T cells with up-regulated CD95 expression coexpressed CD25 (data not shown). D, Cells from allo MLC, cultured for 2 days with (dashed line; sFasL plus cross-linker) or without (filled; cross-linker only) sFasL treatment, were labeled with CD3-allophycocyanin and the fluorescent inhibitor of caspases (FLICA) 3/7 (which binds active caspases 3 and 7; Molecular Probes, Vybrant FAM caspase 3/7 assay kit), as per manufacturer’s instructions. A representative experiment is shown; in three repeat experiments, following sFasL/MLR treatment, 14% (mean ± 4% SD) of gated CD3+ cells expressed active caspase 3/7. A total of 5.4% (mean ± 1.1% SD) of the unstimulated CD3+ cells expressed active caspase 3/7 following the sFasL treatment.
sFasL/MLR-treated cells had reduced [3H]thymidine incorporation and CFSE dilution, compared with controls (Fig. 2, A and B); the pan-caspase inhibitor Z-VAD blocked the effect of sFasL treatment (Fig. 2A). CFSE-labeled responder cells were FACS analyzed for activated T cell subsets on each day of the 7-day allo MLR culture. Consistent with other reports (22), we found that, by day 5 of the allo MLC, three subpopulations of alloreacting CD3+ cells were evident: CFSEhiCD25+, CFSEloCD25+, and CFSEloCD25− (where “hi” represents “high” and “lo” represents “low”) (Fig. 2C). The sFasL/MLR treatment reduced the numbers of CFSEhiCD25+, CFSEloCD25+, and CFSEloCD25− CD3+ cells by 60–98% in five experiments, as compared with control cells cultured without sFasL (Fig. 2D).
FIGURE 2.
Alloreacting cells were eliminated by the sFasL/MLR treatment. Responder PBMCs were cultured, with or without (unstimulated background control) allo PBMC (A) or allo LCL stimulators (B and C), and with or without the sFasL treatment. A, On day 0, the general caspase inhibitor Z-VAD-fmk (50 µM final concentration; R&D Systems) or the vehicle control (0.25% DMSO final concentration) was added to control (■) or sFasL/MLRs ( ). On day 3, [3H]thymidine uptake was assessed; background control [3H]thymidine incorporation was subtracted. Background controls were <20% of experimentals in all cases. A representative experiment is shown. In three repeat experiments, proliferation was reduced by 85% (mean ± 4% SEM) in sFasL/MLRs, as compared with controls; 100% blockade of the sFasL effect was achieved by Z-VAD in all the experimental repeats (p = 0.0058; Student’s t test). B–D, Cells from 7-day allo MLCs done with CFSE-labeled PBMC responders, and control unstimulated responders, cultured for the first 2 days with or without sFasL treatment, were labeled with CD3-allophycocyanin and CD25-PE-Cy7. sFasL/MLR treatment reduced the numbers of gated large CD3+CFSElo cells by 97% (mean ± 2% SD; n = 5, a representative experiment is shown in B) and the numbers of the CD3+CFSEhiCD25+, CD3+CFSEloCD25+, and CD3+CFSEloCD25− cell subsets by 60 ± 27%, 98 ± 1%, and 93 ± 4% (mean ± SD; n = 5), respectively, as compared with control cells (a representative experiment is shown in C; combined data from five independent experimental repeats are in D).
). On day 3, [3H]thymidine uptake was assessed; background control [3H]thymidine incorporation was subtracted. Background controls were <20% of experimentals in all cases. A representative experiment is shown. In three repeat experiments, proliferation was reduced by 85% (mean ± 4% SEM) in sFasL/MLRs, as compared with controls; 100% blockade of the sFasL effect was achieved by Z-VAD in all the experimental repeats (p = 0.0058; Student’s t test). B–D, Cells from 7-day allo MLCs done with CFSE-labeled PBMC responders, and control unstimulated responders, cultured for the first 2 days with or without sFasL treatment, were labeled with CD3-allophycocyanin and CD25-PE-Cy7. sFasL/MLR treatment reduced the numbers of gated large CD3+CFSElo cells by 97% (mean ± 2% SD; n = 5, a representative experiment is shown in B) and the numbers of the CD3+CFSEhiCD25+, CD3+CFSEloCD25+, and CD3+CFSEloCD25− cell subsets by 60 ± 27%, 98 ± 1%, and 93 ± 4% (mean ± SD; n = 5), respectively, as compared with control cells (a representative experiment is shown in C; combined data from five independent experimental repeats are in D).
Alloreacting CD4+ and CD8+ T cells were reduced by sFasL
Immunostaining of responder cells from day 4–7 MLCs revealed CD4hi (Fig. 3) and CD8hi (supplemental Fig. 1) subpopulations. In three independent experiments, depletion of the CD4hiCD38+ subpopulation by FACS sorting reduced the secondary proliferative response against second party cells to 12% (mean ± 4% SEM) of the total cell control; however, the responses to third party and CMV Ags were 82% (mean ± 12% SEM) and 134% (mean ± 24% SEM) of the total cell control, respectively (Fig. 3A). The CD4hi and CD8hi cells from these MLCs coexpressed multiple T cell activation markers (Fig. 3B and supplemental Fig. 1A) and were actively dividing (CFSElo) (Fig. 3C and supplemental Fig. 1B). These CD4hi and CD8hi T cell subpopulations were >94% eliminated by the sFasL/MLR treatment (Fig. 3B and supplemental Fig. 1A). In preliminary analyses, we found that the anti-second party cytotoxic CD8+ T cells resided within the CD8hiCD38+ T cell subpopulation (O. Bohana-Kashtan et al., manuscript in preparation).
FIGURE 3.
Alloreacting CD4+ T cells were eliminated by the sFasL/MLR treatment. A, Cells from a 7-day allo MLC were FACS sorted for the following: total viable cells (left dot plot), CD4hiCD38+ cells, and total viable cells depleted of the CD4hiCD38+ subpopulation (right dot plot). On day 4, [3H]thymidine uptake in response to the same second party cells, to third party cells, and to CMV Ags was assessed; each sorted population had its own unstimulated background control [3H]thymidine incorporation (6361 cpm (mean ± 781 cpm SD) in wells containing total viable cells, 4914 cpm (mean ± 407 cpm SD) in wells containing total viable cells depleted of the CD4hiCD38+ subpopulation, and 2569 cpm (mean ± 347 cpm SD) in wells containing CD4hiCD38+ cells), which was subtracted (bar graph). The number of cells taken from each sorted population matched its actual frequency in the day 7 MLC, as assessed by FACS. B, Cells from a 7-day allo MLC, and control unstimulated responders, that were cultured for the first 2 days with or without sFasL treatment, were labeled with CD4-allophycocyanin and either CD25-FITC, CD38-FITC, CD95-FITC, or HLA-DR-FITC. The sFasL/MLR treatment reduced the numbers of CD4hiCD25+, CD4hiCD38+, CD4hiCD95+, and CD4hiHLA-DR+ cells by 96 ± 1.4%, 96 ± 3%, 96 ± 0.9%, and 98.5 ± 0.5% (mean ± SD; n = 3), respectively, as compared with control cells. In the representative experiment shown, the dot plots of PBMC responders stimulated with allo LCLs (red) were overlaid onto dot plots of unstimulated PBMC responders (black). C, Cells from a 7-day allo MLC using CFSE-labeled PBMC responders and allo LCL stimulators were labeled with CD4-allophycocyanin and either CD25-PE-Cy7, CD38-PE-Cy7, or HLA-DR-PE-Cy7; only the CD38-defined subsets from a representative experiment are shown (left panel). The CD4+CD38− and CD4hiCD38+ cell subsets were gated and analyzed for CFSE (right panel); similar results were obtained for the CD4hiCD25+ and CD4hiHLA-DR+ T cell subpopulations (data not shown). The experiment shown is representative of three repeats. D, Cells from a 7-day allo MLC that were cultured for the first 2 days with or without sFasL treatment were restimulated for 5 h with the same second party allo LCLs, PMA, and ionomycin, and then FACS analyzed after immunostaining with CD4-PE and IFN-γ-allophycocyanin. Day 7 unstimulated PBMC responders treated with PMA and ionomycin were controls.
In addition, sFasL/MLR treatment reduced the numbers of IFN-γ-secreting CD4+ and CD8+ cells by 99% (mean ± 1% SD; n = 3) and 89% (mean ± 5% SD; n = 3), respectively, compared with untreated controls (Fig. 3D and supplemental Fig. 1C). Moreover, the numbers of anti-second party allo-activated CTLs (expressing the degranulation marker, lysosome-associated membrane protein-1 (CD107a; a sensitive marker of CTL activity that is briefly exposed on the surface of CD8+ T cells during the act of killing) (30–32)) were markedly reduced by the sFasL/MLR treatment (91–94% reduction, compared with untreated controls; n = 2; supplemental Fig. 1D).
Retention of overall immune competence following sFasL/MLR treatment
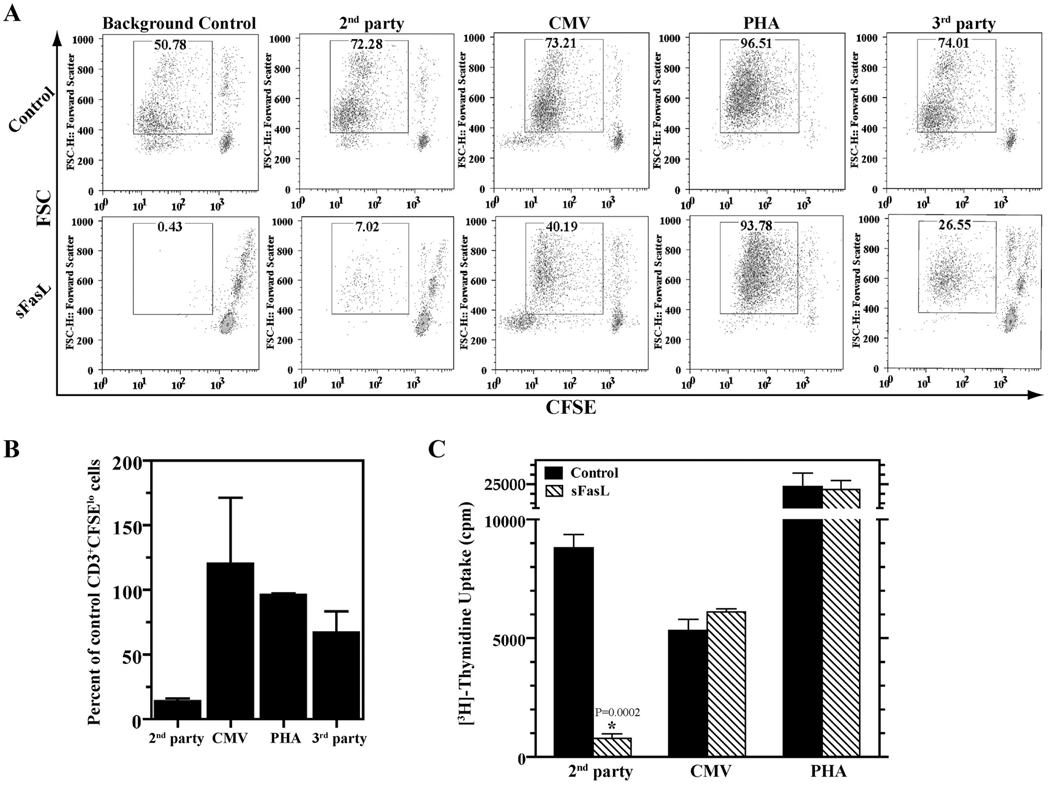
We tested the reactivity of equal numbers of cells from primary allo MLCs, with or without sFasL, in secondary MLCs. In three independent experiments, 87% (mean ± 3% SD) fewer CD3+ CFSElo cells were generated in response to second party cells, in the sFasL/MLR-treated group. However, there was no detectable decrease in the CD3+CFSElo cells generated in response to CMV Ags (as calculated after subtraction of the background control proliferation that represents residual anti-second party alloreactivity); the response to PHA was reduced by only 4% (mean ± 2% SD); and the response to third party cells (calculated after subtraction of the background control) was reduced by only 33% (mean ± 34% SD) (Fig. 4, A and B). Similar results were obtained in secondary proliferative responses assessed by [3H]thymidine incorporation (Fig. 4C).
FIGURE 4.
sFasL-mediated depletion of alloreacting anti-second party cells did not ablate immune responsiveness to CMV, PHA, and third party cells. Responder PBMCs were cultured for 2 days with allo LCL or allo second party PBMC stimulators with or without sFasL treatment. Secondary responses to the same second party cells, CMV Ags (added along with autologous PBMCs as APCs), PHA, and third party cells were assessed by CFSE dilution (A and B) and [3H]thymidine incorporation (C). Combined results of three experimental repeats of the representative experiment shown in A are shown in B. The response to CMV Ags and third party cells was calculated by subtraction of the background control (which represents residual anti-second party alloreactivity). Because PHA stimulates alloreacting and nonalloreacting T cells, proliferation with PHA is shown without subtraction of the background control. The experiment shown in C was done three times; a representative experiment is shown. [3H]Thymidine uptake is presented; background control [3H]thymidine incorporation (9535 cpm (mean ± 2042 cpm SD) in the control-treated wells and 1745 cpm (mean ± 345 cpm SD) in sFasL-treated wells) was subtracted.
There were no significant differences between sFasL/MLR and control-treated MLCs in the frequencies of Flu-specific CD3+ CD8+ cells (Fig. 5). In day 7 allo MLRs, there were no Flu-positive cells detected in the gated CD8hiCD25+ cell subpopulation (data not shown).
FIGURE 5.
Flu-specific T cells were present after the sFasL/MLR treatment. Day 0 PBMCs (A) and day 2 MLCs in the absence (unstimulated) or presence (stimulated) of allo LCL stimulators, with or without sFasL treatment (B), were FACS analyzed after immunostaining with CD8-allophycocyanin, CD3-FITC, and either PE-labeled Flu or EBV pentamer, as indicated. A, 0.21% of gated CD3+CD8+ PBMCs from a Flu-positive EBV-negative donor (SeraCare 031705-B; commercially characterized to contain Flu-specific T cells and lack EBV-specific T cells) bound the Flu pentamer, but only 0.01% bound the EBV pentamer. Only 0.01% of gated CD3+CD8+ PBMCs from a Flu-negative donor (SeraCare 041905-A) bound the Flu pentamer. B, The sFasL/MLR treatment did not change the numbers of Flu+CD3+CD8+ cells, as compared with control cells cultured without sFasL (n = 3, p > 0.5; Student’s t test). C, Combined data from three independent experimental repeats of B.
CD4+FOXP3+ and CD4+CD25+ T cells in sFasL-treated MLCs
We assessed the effect of sFasL on CD4+FOXP3+ cells in the populations of stimulated responders. We used LCLs (rather than PBMCs) as stimulators to avoid the presence of CD4+FOXP3+ cells in the stimulator cell population. Consistent with previous reports (33–35), all or most of the human alloreacting T cells (CD4hiCD38+ and CD4hiCD25+) had up-regulated FOXP3 expression (Fig. 6A). Indeed, in MLR cultures at day 3, a 41% (mean ± 10.5% SD; n = 3) increase in cells expressing FOXP3 was observed (Fig. 6B). In sFasL-treated MLRs, the frequency of FOXP3+ cells was similar to that seen in unstimulated controls (4.08 vs 5.14%; n = 3). All of the surviving CD4+FOXP3+ cells coexpressed CD25 (data not shown). Unstimulated sFasL-treated responders contained 56% (mean ± 9% SD; n = 3) of the numbers of CD4+FOXP3+ cells in the untreated (unstimulated) controls (Fig. 6B).
FIGURE 6.
CD4+FOXP3+ cells were present after the sFasL/MLR treatment. A, Cells from a 7-day allo MLC were immunostained with CD4-allophycocyanin, FOXP3-PE, and either CD38-FITC or CD25-FITC. FOXP3 expression of gated CD4+CD38−, CD4hiCD38+, CD4+CD25−, and CD4hiCD25+ cell populations is shown. B, PBMC responders were cultured for 2 days with (stimulated) or without (unstimulated) irradiated allo LCLs and with or without sFasL treatment. Cells were washed twice, cultured overnight in sFasL-free medium, and then immunostained with CD4-allophycocyanin, FOXP3-PE, and CD3-FITC for FACS analysis. FOXP3 expression of gated CD3+CD4+ cells is shown. In sFasL-treated MLRs, the frequency of FOXP3+ cells was 75% (mean ± 6%; n = 3), as compared with untreated, unstimulated controls.
As a second approach to address the relative sensitivity of T regulatory cells (Tregs) to sFasL, we isolated CD4+CD25+ cells from unstimulated PBMCs, labeled them with CFSE, and mixed them with unlabeled PBMCs from the same person (first party). These first party PBMCs containing CD4+CD25+ cell-enriched CFSE-labeled cells were used as responders, and irradiated allo PBMCs as second party stimulators. CFSE-labeled CD4+CD25+ cells persisted in the sFasL/MLR-treated cultures, actually at an increased frequency (121%, mean ± 18% SD, n = 3) relative to untreated (stimulated) controls, in part due to the elimination of activated unlabeled cells in the sFasL-treated group (supplemental Fig. 2).4 Unstimulated sFasL-treated responders contained 87% (mean ± 13% SD, n = 3) of the numbers of CFSE+CD4+CD25+ cells in the untreated (unstimulated) controls (supplemental Fig. 2).
Mice transplanted with sFasL/MLR-treated cells had prolonged times to severe GVHD
We compared the xeno-GVHD-mediating capacity of human PBMC responders that had been stimulated in vitro with irradiated NOD-scid Il2rg−/− splenocytes in the presence or absence of 2 µg/ml super FasL. In the first of two independent experiments, mice that received 7 × 106 sFasL/MLR-treated cells had a median survival time (MST) of 83 days, compared with 36 days for mice that received control cells from a MLR without super FasL ( p = 0.0018; data not shown). In the second experiment, done with a different PBMC donor, freshly isolated PBMCs provided an additional control group. Mice that received super FasL/MLR-treated cells had a significantly prolonged MST compared with both control groups, at each of the three human cell doses tested (Fig. 7).
Discussion
We developed a new method to selectively eliminate ex vivo activated first party (model donor) anti-second party (model host) alloreacting T cells based on their sensitivity to Fas-mediated apoptosis. We designed our strategy to be technically simple and robust, to facilitate potential translation to clinical use. In a haploidentical mouse model system, we previously demonstrated the ability of sFasL to efficiently and selectively reduce donor antihost T cells during their activation against irradiated host cells in a MLR culture. GVHD was potently reduced, whereas a graft-vs-tumor effect and the hematopoietic engrafting capacity of sFasL-treated bone marrow cells were retained (24).
The present study extends our work to a human model system and supports its potential clinical utility. The actively dividing CFSEloCD25+ and CFSEloCD25− subsets of alloreacting T cells were efficiently (>93%) reduced by the sFasL treatment during the allo MLR (Fig. 2). The identification of an activated proliferating CFSEloCD25− T cell subpopulation that no longer expressed the T cell activation marker CD25 demonstrates the importance of combined use of proliferation (CFSE) and activation markers to identify alloreacting T cells. The CFSEhiCD25+ T cell subpopulation that was partially, but not completely (mean 60%), eliminated by the sFasL/MLR treatment may be composed of early activated T cells (that are about to divide) and Tregs (22) (see text below; Fig. 6 and supplemental Fig. 2).
Prior reports showed, in a mouse model system, that the Ag-specific CD4+ T cells responding to an Ag challenge in vitro and in vivo were CD4hi (36, 37). As demonstrated by our data (Fig. 3A; graph) and as recently reported (38), sorting out the CD4hiCD38+ T cell subpopulation removed the antihost alloreactivity without ablation of overall immune responsiveness. The sFasL/MLR treatment deleted >96% of the CD4hi T cells (Fig. 3B). We found that, similar to activation of CD4+ T cells to become CD4hi, activated CD8+ T cells up-regulated CD8 and multiple T cell activation markers during in vitro and in vivo stimulation with allogeneic cells (supplemental Fig. 1 and O. Bohana-Kashtan et al., manuscript in preparation). The sFasL/MLR treatment deleted >94% of the CD8hi T cells (supplemental Fig. 1A). Furthermore, IFN-γ-producing CD8+ (and CD4+) T cells and actively killing CD8+CD107a+ CTLs were >89% reduced by the sFasL/MLR treatment (Fig. 3D and supplemental Fig. 1, C and D).
The efficacy of the sFasL/MLR treatment was further tested using a human anti- NOD-scid Il2rg−/− mouse xeno-GVHD model (27, 28). This model was at least as sensitive as the similar RAG2−/−γc−/− xeno-GVHD model (29), because as few as 1–5 × 106 freshly isolated human PBMCs induced lethal GVHD, as was assessed by the development of clinical signs (severe weight loss, hunched posture, ruffled fur, reduced mobility, tachypnea) and histopathological (pulmonary and hepatic vascular-perivascular mononuclear infiltrates) findings of acute GVHD (Fig. 7 and data not shown). Mice that received 7 × 106 sFasL/MLR-treated cells had a MST of 50 days, whereas mice that received 2.5 × 106 and 1 × 106 of the (two types of) control cells had a MST of 28–45 days and 37–58 days, respectively (Fig. 7). These and other comparisons suggest that the sFasL/MLR treatment reduced the numbers of GVHD-mediating cells by >70%. The differences seen in the MST of mice that received 7 × 106 cells in the two independent xeno-GVHD experiments can be partially explained by the fact that these experiments were done with different unrelated PBMC donors that may have differences in their human anti-mouse xeno-GVHD-mediating capacity.
The selectivity of this sFasL/MLR method is indicated by the detection of no depletion of the immune response to CMV, 4% depletion of the response to PHA, 33% depletion of the response to third party alloantigens, and no depletion of Flu-specific T cells (Figs. 4 and 5). The somewhat reduced response to third party alloantigens may be due to our use of PBMCs isolated from a third party blood donor who was unrelated, but not HLA typed, and thus might have HLA genes in common with the second party donor (as will often be the case in the clinical situation that we are modeling).
The ability of naturally occurring donor-derived Tregs to suppress allogeneic immune responses, in vitro and in vivo, has been reported by several groups (39–43). As shown by Edinger et al. (44) and others (45, 46), donor-derived CD4+CD25+ T cell-mediated reduction of GVHD did not reduce the ability of donor effector T cells to mediate antileukemia responses. The importance of donor-derived Tregs for control of GVHD is further suggested by clinical studies associating a high CD4+FOXP3+ T cell content in donor stem cell allogeneic transplant grafts with a low incidence of GVHD (47). In addition to their ability to inhibit GVHD, CD4+CD25+ cells have also been shown to support long-term allogeneic hematopoietic cell engraftment (48). A complicating factor is that, whereas FOXP3 expression is highly specific for Tregs in mice, our data confirmed previous reports (33–35) that most activated human T cells up-regulate FOXP3 (Fig. 6). We observed that 55% of the CD4+FOXP3+ T cells survived the sFasL/MLR treatment (Fig. 6), as compared with the untreated, stimulated cells; this represents ~75% of the numbers of CD4+FOXP3+ T cells in the untreated, unstimulated responder cell population. These surviving CD4+FOXP3+ T cells coexpressed CD25, and we speculate that Tregs are included in this subset.
In another approach to investigate the sensitivity of a Treg-enriched subset to the sFasL/MLR treatment, we isolated CD4+ CD25+ cells from fresh unstimulated PBMCs and labeled them with CFSE. These CFSE-labeled CD4+CD25+ cells were mixed with unlabeled whole PBMCs from the same donor, and this cell mixture was used as the first party PBMCs responders. We observed that the CFSE-labeled CD4+CD25+ cells survived the sFasL/MLR treatment (supplemental Fig. 2). Taken together, the survival of substantial fractions of CD4+FOXP3+ and CD4+ CD25+ cells suggests that some Tregs survived the sFasL treatment. These findings are consistent with other reports demonstrating lack of sensitivity of activated Tregs to Fas-mediated apoptosis. Murine Tregs were shown to be resistant to clonal deletion induced by viral superantigen in vivo and to Fas-mediated apoptosis following polyclonal activation in vitro (49, 50). Consistent with these observations, Fritzsching et al. (51) showed that Tregs freshly isolated from human PBMCs expressed high levels of Fas, but upon stimulation with anti-CD3 and anti-CD28, they became resistant to activation-induced cell death. Similar to Fritzsching et al. (51), we found that the CD4+FOXP3+ and CD4+CD25+ subsets of the unstimulated cells appeared to be more sensitive to Fas-mediated apoptosis (Fig. 6 and supplemental Fig. 2).
Several methods have been developed to ex vivo deplete donor antihost T cells during their activation in a MLC (12–20, 22, 23, 52). Allodepletion based on CD25 expression (14) has the disadvantage of deleting CD4+CD25+ Tregs. Compared with methods involving prelabeling (CFSE, TH9402) (22, 23), transduction (HSV-tk) (52), or FACS sorting (15–17), the sFasL/MLR approach is simple and rapid (2 days ex vivo incubation, which could be done in a closed system), and should be safe and nontoxic (e.g., cells can be washed to remove sFasL after the MLC). Pachnio and colleagues (53) recently reported that, similar to Godfrey et al. (22), nonproliferating CFSEhi T cells (FACS sorted following an allo MLC) have reduced ability to mediate GVHD. However, these nonproliferating CFSEhi T cells did not mediate GVL in vivo and did not support engraftment (53). The inability of CFSEhi T cells to mediate an in vivo immune response might be explained, in part, by CFSE toxicity (54, 55).
Removal of CD25+ cells has been tested clinically (12, 13, 56). Patients who received allografts that had been treated with the CD25 immunotoxin had a low incidence of GVHD, but had high rates of leukemia relapse. The high rates of leukemia relapse seen in patients receiving CD25-depleted grafts (12, 13) raise the possibility that the use of host PBMCs as stimulator cells may not be optimal; this may lead to the elimination of donor T cells that recognize minor hematopoietic-expressed histocompatibility Ags important in GVL. As demonstrated by van Dijk et al. (19), host keratinocytes can be used as alternative allo stimulators. In support of this idea, Jones et al. (57) demonstrated the inability of donor CD4+ T cells to induce GVHD in chimeric mice hosts that express only hematopoietic-derived alloantigens. Approaches to be considered in the future should also include attempts to selectively enhance the antileukemia immune response (6, 58, 59).
Supplementary Material
Acknowledgments
We gratefully acknowledge Dr. Ido Paz-Priel and Dr. Gitanjali I. Bechan for their assistance in blood drawing, and Amanda Blackford and Steven Goodman for their help in statistical analysis. We also thank Hao Zhang from the Bloomberg School of Hygiene and Public Health at Johns Hopkins University for his technical help in FACS sorting.
Footnotes
This work was supported in part by National Institutes of Health Grant CA70970 and a Fellow Award from the National Foundation for Cancer Research.
Abbreviations used in this paper: Allo, allogeneic; FasL, Fas ligand; Flu, influenza; GVHD, graft-vs-host disease; GVL, graft vs leukemia; LCL, lymphoblastoid cell line; MST, median survival time; sFasL, soluble FasL; Treg, T regulatory cell; xeno, xenogeneic; hi, high; lo, low.
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Couriel D, Caldera H, Champlin R, Komanduri K. Acute graft-versus-host disease: pathophysiology, clinical manifestations, and management. Cancer. 2004;101:1936–1946. doi: 10.1002/cncr.20613. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara JL, Reddy P. Pathophysiology of graft-versus-host disease. Semin. Hematol. 2006;43:3–10. doi: 10.1053/j.seminhematol.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Welniak LA, Blazar BR, Murphy WJ. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu. Rev. Immunol. 2007;25:139–170. doi: 10.1146/annurev.immunol.25.022106.141606. [DOI] [PubMed] [Google Scholar]
- 4.Ho VT, Soiffer RJ. The history and future of T-cell depletion as graft-versus-host disease prophylaxis for allogeneic hematopoietic stem cell transplantation. Blood. 2001;98:3192–3204. doi: 10.1182/blood.v98.12.3192. [DOI] [PubMed] [Google Scholar]
- 5.Chen BJ, Cui X, Sempowski GD, Liu C, Chao NJ. Transfer of allogeneic CD62L− memory T cells without graft-versus-host disease. Blood. 2004;103:1534–1541. doi: 10.1182/blood-2003-08-2987. [DOI] [PubMed] [Google Scholar]
- 6.Michalek J, Collins RH, Durrani HP, Vaclavkova P, Ruff LE, Douek DC, Vitetta ES. Definitive separation of graft-versus-leukemia- and graft-versus-host-specific CD4+ T cells by virtue of their receptor β loci sequences. Proc. Natl. Acad. Sci. USA. 2003;100:1180–1184. doi: 10.1073/pnas.0337543100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soiffer RJ, Alyea EP, Hochberg E, Wu C, Canning C, Parikh B, Zahrieh D, Webb I, Antin J, Ritz J. Randomized trial of CD8+ T-cell depletion in the prevention of graft-versus-host disease associated with donor lymphocyte infusion. Biol. Blood Marrow Transplant. 2002;8:625–632. doi: 10.1053/bbmt.2002.v8.abbmt080625. [DOI] [PubMed] [Google Scholar]
- 8.Datta AR, Barrett AJ, Jiang YZ, Guimaraes A, Mavroudis DA, van Rhee F, Gordon AA, Madrigal A. Distinct T cell populations distinguish chronic myeloid leukemia cells from lymphocytes in the same individual: a model for separating GVHD from GVL reactions. Bone Marrow Transplant. 1994;14:517–524. [PubMed] [Google Scholar]
- 9.Marijt WA, Heemskerk MH, Kloosterboer FM, Goulmy E, Kester MG, van der Hoorn MA, van Luxemburg-Heys SA, Hoogeboom M, Mutis T, Drijfhout JW, et al. Hematopoiesis-restricted minor histocompatibility antigens HA-1- or HA-2-specific T cells can induce complete remissions of relapsed leukemia. Proc. Natl. Acad. Sci. USA. 2003;100:2742–2747. doi: 10.1073/pnas.0530192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molldrem JJ, Lee PP, Wang C, Felio K, Kantarjian HM, Champlin RE, Davis MM. Evidence that specific T lymphocytes may participate in the elimination of chronic myelogenous leukemia. Nat. Med. 2000;6:1018–1023. doi: 10.1038/79526. [DOI] [PubMed] [Google Scholar]
- 11.Morita Y, Heike Y, Kawakami M, Miura O, Nakatsuka S, Ebisawa M, Mori S, Tanosaki R, Fukuda T, Kim SW, et al. Monitoring of WT1-specific cytotoxic T lymphocytes after allogeneic hematopoietic stem cell transplantation. Int. J. Cancer. 2006;119:1360–1367. doi: 10.1002/ijc.21960. [DOI] [PubMed] [Google Scholar]
- 12.Amrolia PJ, Muccioli-Casadei G, Huls H, Adams S, Durett A, Gee A, Yvon E, Weiss H, Cobbold M, Gaspar HB, et al. Adoptive immunotherapy with allodepleted donor T-cells improves immune reconstitution after haploidentical stem cell transplantation. Blood. 2006;108:1797–1808. doi: 10.1182/blood-2006-02-001909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andre-Schmutz I, Le Deist F, Hacein-Bey-Abina S, Vitetta E, Schindler J, Chedeville G, Vilmer E, Fischer A, Cavazzana-Calvo M. Immune reconstitution without graft-versus-host disease after haemopoietic stem-cell transplantation: a phase 1/2 study. Lancet. 2002;360:130–137. doi: 10.1016/S0140-6736(02)09413-8. [DOI] [PubMed] [Google Scholar]
- 14.Cavazzana-Calvo M, Fromont C, Le Deist F, Lusardi M, Coulombel L, Derocq JM, Gerota I, Griscelli C, Fischer A. Specific elimination of alloreactive T cells by an anti-interleukin-2 receptor B chain-specific immunotoxin. Transplantation. 1990;50:1–7. doi: 10.1097/00007890-199007000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Fehse B, Frerk O, Goldmann M, Bulduk M, Zander AR. Efficient depletion of alloreactive donor T lymphocytes based on expression of two activation-induced antigens (CD25 and CD69) Br. J. Haematol. 2000;109:644–651. doi: 10.1046/j.1365-2141.2000.02074.x. [DOI] [PubMed] [Google Scholar]
- 16.Garderet L, Snell V, Przepiorka D, Schenk T, Lu JG, Marini F, Gluckman E, Andreeff M, Champlin RE. Effective depletion of alloreactive lymphocytes from peripheral blood mononuclear cell preparations. Transplantation. 1999;67:124–130. doi: 10.1097/00007890-199901150-00021. [DOI] [PubMed] [Google Scholar]
- 17.Koh MB, Prentice HG, Lowdell MW. Selective removal of alloreactive cells from haematopoietic stem cell grafts: graft engineering for GVHD prophylaxis. Bone Marrow Transplant. 1999;23:1071–1079. doi: 10.1038/sj.bmt.1701749. [DOI] [PubMed] [Google Scholar]
- 18.Montagna D, Yvon E, Calcaterra V, Comoli P, Locatelli F, Maccario R, Fisher A, Cavazzana-Calvo M. Depletion of alloreactive T cells by a specific anti-interleukin-2 receptor p55 chain immunotoxin does not impair in vitro antileukemia and antiviral activity. Blood. 1999;93:3550–3557. [PubMed] [Google Scholar]
- 19.Van Dijk AM, Kessler FL, Stadhouders-Keet SA, Verdonck LF, de Gast GC, Otten HG. Selective depletion of major and minor histocompatibility antigen reactive T cells: towards prevention of acute graft-versus-host disease. Br. J. Haematol. 1999;107:169–175. doi: 10.1046/j.1365-2141.1999.01675.x. [DOI] [PubMed] [Google Scholar]
- 20.Wehler TC, Nonn M, Brandt B, Britten CM, Grone M, Todorova M, Link I, Khan SA, Meyer RG, Huber C, et al. Targeting the activation-induced antigen CD137 can selectively deplete alloreactive T cells from antileukemic and antitumor donor T-cell lines. Blood. 2007;109:365–373. doi: 10.1182/blood-2006-04-014100. [DOI] [PubMed] [Google Scholar]
- 21.Ge X, Brown J, Sykes M, Boussiotis VA. CD134− allodepletion allows selective elimination of alloreactive human T cells without loss of virus-specific and leukemia-specific effectors. Biol. Blood Marrow Transplant. 2008;14:518–530. doi: 10.1016/j.bbmt.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Godfrey WR, Krampf MR, Taylor PA, Blazar BR. Ex vivo depletion of alloreactive cells based on CFSE dye dilution, activation antigen selection, and dendritic cell stimulation. Blood. 2004;103:1158–1165. doi: 10.1182/blood-2003-04-1098. [DOI] [PubMed] [Google Scholar]
- 23.Chen BJ, Cui X, Liu C, Chao NJ. Prevention of graft-versus-host disease while preserving graft-versus-leukemia effect after selective depletion of host-reactive T cells by photodynamic cell purging process. Blood. 2002;99:3083–3088. doi: 10.1182/blood.v99.9.3083. [DOI] [PubMed] [Google Scholar]
- 24.Georgantas RW, III, Bohana-Kashtan O, Civin CI. Ex vivo soluble fas ligand treatment of donor cells to selectively reduce murine acute graft versus host disease. Transplantation. 2006;82:471–478. doi: 10.1097/01.tp.0000229435.58898.c5. [DOI] [PubMed] [Google Scholar]
- 25.Hartwig UF, Robbers M, Wickenhauser C, Huber C. Murine acute graft-versus-host disease can be prevented by depletion of alloreactive T lymphocytes using activation-induced cell death. Blood. 2002;99:3041–3049. doi: 10.1182/blood.v99.8.3041. [DOI] [PubMed] [Google Scholar]
- 26.Hartwig UF, Nonn M, Khan S, Link I, Huber C, Herr W. Depletion of alloreactive donor T lymphocytes by CD95-mediated activation-induced cell death retains antileukemic, antiviral, and immunoregulatory T cell immunity. Biol. Blood Marrow Transplant. 2008;14:99–109. doi: 10.1016/j.bbmt.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, Ueyama Y, Koyanagi Y, Sugamura K, Tsuji K, et al. NOD/SCID/γc (null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 28.Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Kotb M, Gillies SD, King M, Mangada J, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2Rγ null mice engrafted with mobilized human hemopoietic stem cells. J. Immunol. 2005;174:6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 29.Van Rijn RS, Simonetti ER, Hagenbeek A, Hogenes MC, de Weger RA, Canninga-van Dijk MR, Weijer K, Spits H, Storm G, van Bloois L, et al. A new xenograft model for graft-versus-host disease by intravenous transfer of human peripheral blood mononuclear cells in RAG2−/− γc−/− double-mutant mice. Blood. 2003;102:2522–2531. doi: 10.1182/blood-2002-10-3241. [DOI] [PubMed] [Google Scholar]
- 30.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 31.Mittendorf EA, Storrer CE, Shriver CD, Ponniah S, Peoples GE. Evaluation of the CD107 cytotoxicity assay for the detection of cytolytic CD8+ cells recognizing HER2/neu vaccine peptides. Breast Cancer Res. Treat. 2005;92:85–93. doi: 10.1007/s10549-005-0988-1. [DOI] [PubMed] [Google Scholar]
- 32.Aktas E, Kucuksezer UC, Bilgic S, Erten G, Deniz G. Relationship between CD107a expression and cytotoxic activity. Cell. Immunol. 2009;254:149–154. doi: 10.1016/j.cellimm.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Morgan ME, van Bilsen JH, Bakker AM, Heemskerk B, Schilham MW, Hartgers FC, Elferink BG, van der Zanden L, de Vries RR, Huizinga TW, et al. Expression of FOXP3 mRNA is not confined to CD4+CD25+ T regulatory cells in humans. Hum. Immunol. 2005;66:13–20. doi: 10.1016/j.humimm.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 34.Gavin MA, Torgerson TR, Houston E, DeRoos P, Ho WY, Stray-Pedersen A, Ocheltree EL, Greenberg PD, Ochs HD, Rudensky AY. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc. Natl. Acad. Sci. USA. 2006;103:6659–6664. doi: 10.1073/pnas.0509484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur. J. Immunol. 2007;37:129–138. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- 36.Krieger NR, Fathman CG, Shaw MK, Ridgway WM. Identification and characterization of the antigen-specific subpopulation of alloreactive CD4+ T cells in vitro and in vivo. Transplantation. 2000;69:605–609. doi: 10.1097/00007890-200002270-00023. [DOI] [PubMed] [Google Scholar]
- 37.Ridgway W, Fasso M, Fathman CG. Following antigen challenge, T cells up-regulate cell surface expression of CD4 in vitro and in vivo. J. Immunol. 1998;161:714–720. [PubMed] [Google Scholar]
- 38.Martins SL, St John LS, Champlin RE, Wieder ED, McMannis J, Molldrem JJ, Komanduri KV. Functional assessment and specific depletion of alloreactive human T cells using flow cytometry. Blood. 2004;104:3429–3436. doi: 10.1182/blood-2004-05-1918. [DOI] [PubMed] [Google Scholar]
- 39.Cohen JL, Trenado A, Vasey D, Klatzmann D, Salomon BL. CD4+CD25+ immunoregulatory T cells: new therapeutics for graft-versus-host disease. J. Exp. Med. 2002;196:401–406. doi: 10.1084/jem.20020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ermann J, Hoffmann P, Edinger M, Dutt S, Blankenberg FG, Higgins JP, Negrin RS, Fathman CG, Strober S. Only the CD62L+ subpopulation of CD4+CD25+ regulatory T cells protects from lethal acute GVHD. Blood. 2005;105:2220–2226. doi: 10.1182/blood-2004-05-2044. [DOI] [PubMed] [Google Scholar]
- 41.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4+CD25+ regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J. Exp. Med. 2002;196:389–399. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4+CD25+ immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99:3493–3499. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 43.Taylor PA, Noelle RJ, Blazar BR. CD4+CD25+ immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J. Exp. Med. 2001;193:1311–1318. doi: 10.1084/jem.193.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S, Negrin RS. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat. Med. 2003;9:1144–1150. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 45.Jones SC, Murphy GF, Korngold R. Post-hematopoietic cell transplantation control of graft-versus-host disease by donor CD425 T cells to allow an effective graft-versus-leukemia response. Biol. Blood Marrow Transplant. 2003;9:243–256. doi: 10.1053/bbmt.2003.50027. [DOI] [PubMed] [Google Scholar]
- 46.Trenado A, Charlotte F, Fisson S, Yagello M, Klatzmann D, Salomon BL, Cohen JL. Recipient-type specific CD4+CD25+ regulatory T cells favor immune reconstitution and control graft-versus-host disease while maintaining graft-versus-leukemia. J. Clin. Invest. 2003;112:1688–1696. doi: 10.1172/JCI17702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rezvani K, Mielke S, Ahmadzadeh M, Kilical Y, Savani BN, Zeilah J, Keyvanfar K, Montero A, Hensel N, Kurlander R, Barrett AJ. High donor FOXP3-positive regulatory T-cell (Treg) content is associated with a low risk of GVHD following HLA-matched allogeneic SCT. Blood. 2006;108:1291–1297. doi: 10.1182/blood-2006-02-003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hanash AM, Levy RB. Donor CD4+CD25+ T cells promote engraftment and tolerance following MHC-mismatched hematopoietic cell transplantation. Blood. 2005;105:1828–1836. doi: 10.1182/blood-2004-08-3213. [DOI] [PubMed] [Google Scholar]
- 49.Banz A, Pontoux C, Papiernik M. Modulation of Fas-dependent apoptosis: a dynamic process controlling both the persistence and death of CD4 regulatory T cells and effector T cells. J. Immunol. 2002;169:750–757. doi: 10.4049/jimmunol.169.2.750. [DOI] [PubMed] [Google Scholar]
- 50.Papiernik M, do Carmo Leite-de-Moraes M, Pontoux C, Joret AM, Rocha B, Penit C, Dy M. T cell deletion induced by chronic infection with mouse mammary tumor virus spares a CD25-positive, IL-10-producing T cell population with infectious capacity. J. Immunol. 1997;158:4642–4653. [PubMed] [Google Scholar]
- 51.Fritzsching B, Oberle N, Eberhardt N, Quick S, Haas J, Wildemann B, Krammer PH, Suri-Payer E. In contrast to effector T cells, CD4+CD25+FoxP3+ regulatory T cells are highly susceptible to CD95 ligand-but not to TCR-mediated cell death. J. Immunol. 2005;175:32–36. doi: 10.4049/jimmunol.175.1.32. [DOI] [PubMed] [Google Scholar]
- 52.Bonini C, Ferrari G, Verzeletti S, Servida P, Zappone E, Ruggieri L, Ponzoni M, Rossini S, Mavilio F, Traversari C, Bordignon C. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science. 1997;276:1719–1724. doi: 10.1126/science.276.5319.1719. [DOI] [PubMed] [Google Scholar]
- 53.Pachnio A, Dietrich S, Klapper W, Humpe A, Schwake M, Sedlacek R, Gramatzki M, Beck C. Proliferation-based T-cell selection for immunotherapy and graft-versus-host-disease prophylaxis in the context of bone marrow transplantation. Bone Marrow Transplant. 2006;38:157–167. doi: 10.1038/sj.bmt.1705411. [DOI] [PubMed] [Google Scholar]
- 54.Leon B, Martinez del Hoyo G, Parrillas V, Vargas HH, Sanchez-Mateos P, Longo N, Lopez-Bravo M, Ardavin C. Dendritic cell differentiation potential of mouse monocytes: monocytes represent immediate precursors of CD8− and CD8+ splenic dendritic cells. Blood. 2004;103:2668–2676. doi: 10.1182/blood-2003-01-0286. [DOI] [PubMed] [Google Scholar]
- 55.Givan AL, Fisher JL, Waugh MG, Bercovici N, Wallace PK. Use of cell-tracking dyes to determine proliferation precursor frequencies of antigen-specific T cells. In: Hawley TS, Hawley RG, editors. Flow Cytometry Protocols. 2nd Ed. Totowa: Human Press; 2004. pp. 109–124. [DOI] [PubMed] [Google Scholar]
- 56.Solomon SR, Mielke S, Savani BN, Montero A, Wisch L, Childs R, Hensel N, Schindler J, Ghetie V, Leitman SF, et al. Selective depletion of alloreactive donor lymphocytes: a novel method to reduce the severity of graft-versus-host disease in older patients undergoing matched sibling donor stem cell transplantation. Blood. 2005;106:1123–1129. doi: 10.1182/blood-2005-01-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jones SC, Murphy GF, Friedman TM, Korngold R. Importance of minor histocompatibility antigen expression by nonhematopoietic tissues in a CD4+ T cell-mediated graft-versus-host disease model. J. Clin. Invest. 2003;112:1880–1886. doi: 10.1172/JCI19427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cathcart K, Pinilla-Ibarz J, Korontsvit T, Schwartz J, Zakhaleva V, Papadopoulos EB, Scheinberg DA. A multivalent bcr-abl fusion peptide vaccination trial in patients with chronic myeloid leukemia. Blood. 2004;103:1037–1042. doi: 10.1182/blood-2003-03-0954. [DOI] [PubMed] [Google Scholar]
- 59.Falkenburg JH, Wafelman AR, Joosten P, Smit WM, van Bergen CA, Bongaerts R, Lurvink E, van der Hoorn M, Kluck P, Landegent JE, et al. Complete remission of accelerated phase chronic myeloid leukemia by treatment with leukemia-reactive cytotoxic T lymphocytes. Blood. 1999;94:1201–1208. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.