Abstract
Objective
To determine the effect of an endothelin type A receptor antagonist (ETA) on uterine artery resistive index (UARI) and mean arterial pressure (MAP) in a placental ischemia rat model of pre-eclampsia produced by Reductions in Uterine Perfusion Pressure (RUPP).
Study Design
UARI was assessed by Doppler velocimetry in the RUPP and normal pregnant controls (NP) on gestation days (GD) 12, 15 and 18. UARI was also determined on GD 18 in NP and RUPP pregnant dams after pretreatment with ETA. MAP was recorded on GD 19.
Results
The RUPP group had a higher MAP and UARI on GD 15 and 18 than the NP group. Pretreatment with ETA attenuated both the MAP and GD 18 UARI in the RUPP group without affecting these parameters in the NP group.
Conclusion
The improvement in UARI could be one potential mechanism for the reduction in MAP in response to ETA in pregnant dams with ischemic placentas.
Keywords: Doppler velocimetry, Uterine artery resistive index, Endothelin type A receptor antagonist, hypertension, preeclampsia
INTRODUCTION
Preeclampsia complicates about 5–8% of pregnancies and is a significant cause of maternal-fetal morbidity1, 2. The pathophysiology of this condition is not fully understood and remains a subject under active investigation3–5. There is evidence that suggests that endothelin 1 may cause hypertension during pregnancy by the activation of endothelin type A receptor in response to several vascular mediators of preeclampsia including tumor necrosis factor (TNF) -α, agonistic autoantibodies to the angiotensin II type I receptor and soluble fms-like tyrosine kinase 1 (sFlt-1)6–8. Endothelin is elevated in preeclamptic women and it increases uterine vascular resistance9–12. A rise in endothelin 1 associated with hypertension was noted following a reduction of utero-placental perfusion (RUPP) in pregnant dams13. Therefore, we tested the hypothesis that pretreatment with an endothelin type A receptor antagonist (ETA) would reverse chronic placental ischemia-induced hypertension by lowering the uterine vascular resistance in pregnant dams. In order to test this hypothesis, the changes in uterine artery resistive index (UARI) with gestational age in RUPP treated pregnant dams and untreated controls were first studied.
MATERIALS AND METHODS
Pregnant Sprague-Dawley rats purchased from Harlan Sprague Dawley Inc. (Indianapolis, IN) were used in the study. Animals were housed in a temperature-controlled room (23° C) with a 12:12-hour light/dark cycle. All experimental procedures executed in this study were in accordance with the National Institute of Health guidelines for use and care of animals. The study protocol was approved by the Institutional Animal Care and Use Committee at the University of Mississippi Medical Center.
Experiments were performed in the following four groups of rats: pregnant controls (NP; n=12), RUPP treated pregnant dams (n=17), ETA treated pregnant dams (NP/ETA; n=8), and RUPP and ETA treated pregnant dams (RUPP/ETA; n=9). Pregnant dams that underwent surgical procedures and ultrasonography were anesthetized with 2% isoflurane (WA Butler Co) delivered by an anesthesia apparatus (Vaporizer for Forane Anesthetic, Ohio Medical Products).
Pregnant dams in the RUPP groups underwent laparotomy on gestation day 14, the lower abdominal aorta was isolated, and a silver clip (0.203 mm ID) was placed around the aorta above the iliac bifurcation. The right and left ovarian arteries were also constricted using a silver clip (0.100 mm ID), as described previously14. Pregnant dams treated with ETA, ABT 627, received the agent in their drinking water (5mg/Kg/day)8, 15 on gestation days 12–19. The concentration of the ETA in the drinking water was 0.11mM.
Power Doppler velocimetry measurements were performed on anesthetized pregnant dams at an imaging station with a Vevo 770 unit (Visual sonics) using a 30 Hz transducer and an insonating angle <30°. The peak systolic flow velocity (PSV) and end diastolic flow velocity (EDV) were recorded using the uterine artery Doppler waveform. The UARI was calculated using the following formula: UARI= (PSV−EDV)/PSV. UARI was determined for the uterine artery bilaterally at three levels and the mean UARI was calculated. UARI was measured in the RUPP and normal pregnant controls (NP) on gestation days 12, 15 and 18. UARI was also determined on gestation day 18 in NP and RUPP pregnant dams after pretreatment with ETA. Rats were also surgically instrumented with a carotid catheter on gestation day 18 for subsequent mean arterial pressure measurement (MAP) on gestation day 19. Pup weight, placenta weight, litter size (live and reabsorbed pups) and proportion of live pups in the litter were recorded at harvest on gestation day 19.
All data are expressed as mean ± standard error of the mean. Difference between control and experimental groups were analyzed using ANOVA with Tukey-Kramer multiple comparison tests. The t-test was used when comparing two groups of dams. Data was considered statistically different at P values < 0.05.
RESULTS
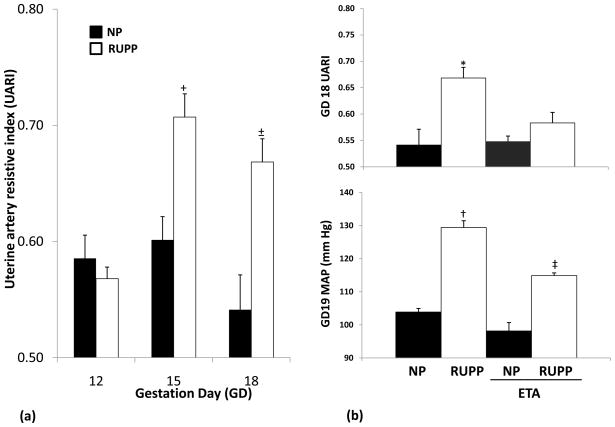
The UARI in NP dams was relatively unchanged up to gestation day 15 and was noted to be lower by gestation day 18. UARI for the RUPP dams was similar to the untreated controls on gestation day 12 and rose significantly by the day following treatment and continued to remain higher than the controls on gestation day 18 (Figure I(a)). The mean UARI in NP and RUPP groups were 0.59+/−0.02 vs. 0.57+/−0.01 (P = 0.423), 0.60+/−0.02 vs. 0.71+/−0.02 (P = <0.001) and 0.54+/−0.03 vs. 0.67+/−0.02 (P = <0.001) on gestation days 12, 15 and 18 respectively.
Figure I.
(a): Uterine artery resistive index changes by gestation day (GD) in NP and RUPP dams. All data are expressed as mean ±SEM. +P <0.05 vs. NP GD 12, NP GD 15, NP GD18 and RUPP GD 12. ±P <0.05 vs. NP GD 12, NP GD 18 and RUPP GD12.
(b): Uterine artery resistive index and mean arterial blood pressure in NP and RUPP dams with and without ETA pretreatment. All data are expressed as mean ±SEM. *P <0.05 vs. NP, NP/ETA and RUPP/ETA. †P <0.05 vs. NP, NP/ETA, RUPP/ETA. ‡P <0.05 vs. NP, NP/ETA and RUPP.
When compared to NP controls, the MAP was significantly elevated in RUPP dams. The MAP in the NP and RUPP groups were 104+/−1 and 129+/−2 mm Hg, respectively (P = <0.001).
Figure I (b) illustrates that pretreatment with ETA attenuated both the gestation day 19 MAP and gestation day 18 UARI in the RUPP group (115+/−1 mm Hg (P = <0.001); 0.58+/−0.02 (P = <0.001)) without affecting these parameters in the NP group (98+/−2 mm Hg (P = 0.054); 0.55+/−0.02 (P = 0.150)).
The effect of ETA pretreatment on selected pregnancy outcomes such as pup weight, placenta weight, litter size and proportion of live pups in the litter is summarized in Table I. A lower proportion of pups were alive in the litter from RUPP dams than NP dams (57% vs. 92%; P <0.05) and this was improved by ETA pretreatment (82% vs. 99%). ETA pretreatment did not however, result in any significant changes in RUPP dams’ litter size, pup and placenta weights when compared to NP dams. ETA pretreated NP dams had significantly higher placenta weight (gm) than the other 3 groups (0.67±0.03 vs 0.54±0.03 (NP), 0.51±0.02 (RUPP) and 0.48±0.03 (RUPP/ETA); P < 0.05).
Table I.
Effect of endothelin type A receptor antagonist (ETA) pretreatment on selected pregnancy outcomes.
| Variable | NP | RUPP | ETA pretreatment |
|
|---|---|---|---|---|
| NP | RUPP | |||
| Pup weight (gm) | 2.19 ± 0.04 | 1.99 ± 0.06 | 2.34*± 0.05 | 2.07 ± 0.12 |
| Placenta weight (gm) | 0.54 ±0.03 | 0.51 ±0.02 | 0.67+± 0.03 | 0.48 ± 0.03 |
| Litter size | 14 ± 1 | 13 ± 1 | 12 ± 1 | 12 ± 1 |
| Proportion of live pups (%) | 92 ± 3 | 57† ± 4 | 99 ± 1 | 82 ± 8 |
All data are expressed as mean ± SEM.
P<0.05 vs. RUPP dams.
P <0.05 vs. RUPP dams and RUPP/ETA dams.
P <0.05 vs. NP dams, NP/ETA dams and RUPP/ETA dams.
COMMENT
The reduction of UARI with increasing gestation age in pregnant dams is similar to that seen in pregnant women16 and perhaps reflects changes in the uterine circulation to accommodate the increasing metabolic demand of the utero-placental-fetal unit. The rise in UARI in response to induction of chronic placental ischemia associated with the RUPP procedure17, 18 appears to be immediate and sustained over time. Acute cardiovascular changes, including increases in peripheral vascular resistance and blood pressure, have been reported within 24-hours of a reduction in uterine perfusion pressure in other mammals19. The acute rise in UARI following the RUPP procedure may reflect a lack of autoregulation of uteroplacental blood flow as observed in other mammals subjected to a similar procedure20. In the case of Sprague Dawley dams, the greater reduction in uteroplacental blood flow (60%)17 than in perfusion pressure (30–35%)21 achieved by the RUPP procedure is consistent with the increased UARI. In addition to the clipping procedure, it is possible that release of vascular mediators, including endothelin, associated with endothelial dysfunction following chronic placental ischemia may also be responsible for this increased UARI22, 23. The increased UARI in RUPP dams also corroborates previous reports of impaired relaxation of uterine artery in such dams24.
The higher MAP in RUPP dams may be the direct result of increased peripheral vascular resistance triggered by vascular mediators released in response to the chronic placental ischemia. The attenuation of the higher UARI and MAP in RUPP dams and the lack of any significant effect on these parameters in NP controls by ETA pretreatment are noteworthy. This observation suggests that endothelin 1 may cause hypertension during pregnancy and this effect may be mediated through the endothelin type A receptor. While we have previously reported that preproendothelin 1 levels are elevated in RUPP dams15, an investigation into the distribution and effects of endothelin receptors in the placenta and vascular tissues may provide insight into the paracrine and autocrine mechanisms by which endothelin modulates utero-placental circulation. The hypertensive effect of endothelin 1 may occur in response to several vascular mediators of preeclampsia such as tumor necrosis factor (TNF) -α, agonistic autoantibodies to the angiotensin II type I receptor and soluble fms-like tyrosine kinase 1 (sFlt-1) all of which are also increased in RUPP dams6–8,25–27. The degree of placental ischemia and the expression of vascular mediators leading to increased MAP in RUPP dams were perhaps attenuated by ETA pretreatment. ETA pretreatment may have achieved this effect through improved utero-placental perfusion as evidenced by a reduction in UARI. A reduction in UARI could be one potential mechanism by which ETA attenuated the rise in MAP and improved pregnancy outcomes (e.g. proportion of live pups) in dams with ischemic placentas.
Acknowledgments
SPONSOR OR GRANT INFORMATION: This work was supported by National Institute of Health Grant HL-51971
Footnotes
Accepted for an oral presentation at the 31st Annual Meeting of the Society for Maternal Fetal Medicine, San Francisco, California, February 7–12, 2011.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 2.Roberts JM, Pearson G, Cutler J, Lindheimer M. Summary of the NHLBI Working Group on Research on Hypertension During Pregnancy. Hypertension. 2003;41:437–445. doi: 10.1161/01.HYP.0000054981.03589.E9. [DOI] [PubMed] [Google Scholar]
- 3.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989;161:1200–1204. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- 4.Redman CW. Current topic: pre-eclampsia and the placenta. Placenta. 1991;12:301–308. doi: 10.1016/0143-4004(91)90339-h. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2008;294:H541–H550. doi: 10.1152/ajpheart.01113.2007. [DOI] [PubMed] [Google Scholar]
- 6.LaMarca BB, Cockrell K, Sullivan E, Bennett W, Granger JP. Role of endothelin in mediating tumor necrosis factor-induced hypertension in pregnant rats. Hypertension. 2005 Jul;46(1):82–6. doi: 10.1161/01.HYP.0000169152.59854.36. Epub 2005 May 31. [DOI] [PubMed] [Google Scholar]
- 7.LaMarca B, Parrish M, Ray LF, Murphy SR, Roberts L, Glover P, Wallukat G, Wenzel K, Cockrell K, Martin JN, Jr, Ryan MJ, Dechend R. Hypertension in response to autoantibodies to the angiotensin II type I receptor (AT1-AA) in pregnant rats: role of endothelin–1. Hypertension. 2009 Oct;54(4):905–9. doi: 10.1161/HYPERTENSIONAHA.109.137935. Epub 2009 Aug 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy SR, LaMarca BB, Cockrell K, Granger JP. Role of endothelin in mediating soluble fms-like tyrosine kinase 1-induced hypertension in pregnant rats. Hypertension. 2010 Feb;55(2):394–8. doi: 10.1161/HYPERTENSIONAHA.109.141473. Epub 2009 Dec 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shinagawa T, Suzuki S, Sawa R, Yoneyama Y, Asakura H, Araki T. Maternal plasma adenosine and endothelin-1 levels in twin gestation complicated by preeclampsia. Arch Gynecol Obstet. 2002 Dec;267(2):72–5. doi: 10.1007/s00404-001-0283-2. [DOI] [PubMed] [Google Scholar]
- 10.Dittrich R, Sinduwinatha C, Maltaris T, Mueller A, Hoffmann I, Beckmann MW, Oppelt PG. The intrauterine to intra-arterial pressure ratio: a new parameter for the study of uterine contractility physiology. Reprod Biomed Online. 2010 Mar;20(3):430–6. doi: 10.1016/j.rbmo.2009.11.023. Epub 2009 Dec 11. [DOI] [PubMed] [Google Scholar]
- 11.Fried G, Samuelson U. Endothelin and neuropeptide Y are vasoconstrictors in human uterine blood vessels. Am J Obstet Gynecol. 1991 May;164(5 Pt 1):1330–6. doi: 10.1016/0002-9378(91)90709-z. [DOI] [PubMed] [Google Scholar]
- 12.Yang D, Clark KE. Effect of endothelin-1 on the uterine vasculature of the pregnant and estrogen-treated nonpregnant sheep. Am J Obstet Gynecol. 1992 Dec;167(6):1642–50. doi: 10.1016/0002-9378(92)91755-y. [DOI] [PubMed] [Google Scholar]
- 13.Veillon EW, Jr, Keiser SD, Parrish MR, Bennett W, Cockrell K, Ray LF, Granger JP, Martin JN, Jr, LaMarca B. 17-Hydroxyprogesterone blunts the hypertensive response associated with reductions in uterine perfusion pressure in pregnant rats. Am J Obstet Gynecol. 2009 Sep;201(3):324.e1–6. doi: 10.1016/j.ajog.2009.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Granger JP, LaMarca BB, Cockrell K, Sedeek M, Balzi C, Chandler D, Bennett W. Reduced uterine perfusion pressure (RUPP) model for studying cardiovascular-renal dysfunction in response to placental ischemia. Methods Mol Med. 2006;122:383–392. doi: 10.1385/1-59259-989-3:381. [DOI] [PubMed] [Google Scholar]
- 15.Alexander BT, Rinewalt AN, Cockrell KL, Massey MB, Bennett WA, Granger JP. Endothelin type a receptor blockade attenuates the hypertension in response to chronic reductions in uterine perfusion pressure. Hypertension. 2001 Feb;37(2 Part 2):485–9. doi: 10.1161/01.hyp.37.2.485. [DOI] [PubMed] [Google Scholar]
- 16.Thaler I, Manor D, Itskovitz J, Rottem S, Levit N, Timor-Tritsch I, Brandes JM. Changes in uterine blood flow during human pregnancy. Am J Obstet Gynecol. 1990 Jan;162(1):121–5. doi: 10.1016/0002-9378(90)90834-t. [DOI] [PubMed] [Google Scholar]
- 17.Sholook MM, Gilbert JS, Sedeek MH, Huang M, Hester RL, Granger JP. Systemic hemodynamic and regional blood flow changes in response to chronic reductions in uterine perfusion pressure in pregnant rats. Am J Physiol Heart Circ Physiol. 2007 Oct;293(4):H2080–4. doi: 10.1152/ajpheart.00667.2007. Epub 2007 Jul 20. [DOI] [PubMed] [Google Scholar]
- 18.Gilbert JS, Gilbert SA, Arany M, Granger JP. Hypertension produced by placental ischemia in pregnant rats is associated with increased soluble endoglin expression. Hypertension. 2009 Feb;53(2):399–403. doi: 10.1161/HYPERTENSIONAHA.108.123513. Epub 2008 Dec 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Losonczy G, Brown G, Venuto RC. Increased peripheral resistance during reduced uterine perfusion pressure hypertension in pregnant rabbits. Am J Med Sci. 1992 Apr;303(4):233–40. doi: 10.1097/00000441-199204000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Laird MR, Faber JJ, Binder ND. Maternal placental blood flow is reduced in proportion to reduction in uterine driving pressure. Pediatr Res. 1994 Jul;36(1 Pt 1):102–10. doi: 10.1203/00006450-199407001-00019. [DOI] [PubMed] [Google Scholar]
- 21.Eder DJ, McDonald MT. A Role for Brain Angiotensin II in Experimental Pregnancy-Induced Hypertension in Laboratory Rats. Hypertension in Pregnancy. 1987;b6(3):431–451. [Google Scholar]
- 22.Roberts L, LaMarca BB, Fournier L, Bain J, Cockrell K, Granger JP. Enhanced endothelin synthesis by endothelial cells exposed to sera from pregnant rats with decreased uterine perfusion. Hypertension. 2006 Mar;47(3):615–8. doi: 10.1161/01.HYP.0000197950.42301.dd. Epub 2006 Jan 3. [DOI] [PubMed] [Google Scholar]
- 23.Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2008 Feb;294(2):H541–50. doi: 10.1152/ajpheart.01113.2007. Epub 2007 Nov 30. [DOI] [PubMed] [Google Scholar]
- 24.Anderson CM, Lopez F, Zhang HY, Pavlish K, Benoit JN. Reduced uteroplacental perfusion alters uterine arcuate artery function in the pregnant Sprague-Dawley rat. Biol Reprod. 2005 Mar;72(3):762–6. doi: 10.1095/biolreprod.104.036715. Epub 2004 Nov 24. [DOI] [PubMed] [Google Scholar]
- 25.LaMarca B, Speed J, Fournier L, Babcock SA, Berry H, Cockrell K, Granger JP. Hypertension in response to chronic reductions in uterine perfusion in pregnant rats: effect of tumor necrosis factor-alpha blockade. Hypertension. 2008 Dec;52(6):1161–7. doi: 10.1161/HYPERTENSIONAHA.108.120881. Epub 2008 Nov 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LaMarca B, Wallukat G, Llinas M, Herse F, Dechend R, Granger JP. Autoantibodies to the angiotensin type I receptor in response to placental ischemia and tumor necrosis factor alpha in pregnant rats. Hypertension. 2008 Dec;52(6):1168–72. doi: 10.1161/HYPERTENSIONAHA.108.120576. Epub 2008 Oct 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilbert JS, Babcock SA, Granger JP. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension. 2007 Dec;50(6):1142–7. doi: 10.1161/HYPERTENSIONAHA.107.096594. Epub 2007 Oct 8. [DOI] [PubMed] [Google Scholar]