Abstract
The authors present a 21-year-old woman who has been receiving rapamycin for 5 months for bilateral subependymal giant cell astrocytomas. The patient was started at a dose of 0.2 mg/kg/day. Levels were maintained between 11 and 13 ng/mL. Magnetic resonance imaging of the brain 2½ months after initiating rapamycin demonstrated a decrease in size of both astrocytomas (11 to 7.5 mm on the right and 8 to 5 mm on the left). Further studies are needed with prolonged observation to confirm these findings, determine the length of necessary treatment, and evaluate recurrence risk after discontinuation of rapamycin.
Keywords: subependymal giant cell astrocytoma, tuberous sclerosis complex, rapamycin, astrocytoma
Rapamycin is postulated to be beneficial in many tumor types found in tuberous sclerosis secondary to its inhibitory effect on the mammalian target of rapamycin. Inhibition of the mammalian target of rapamycin should block its constitutive overexpression, preventing cell overgrowth and tumor formation. Preclinical trials have shown favorable efficacy of rapamycin in tuberous sclerosis rat and mouse models.1–2 Preliminary clinical trials have supported these results in pulmonary, renal, and central nervous system tumors in tuberous sclerosis.3–4 Franz and colleagues reported 4 patients with tuberous sclerosis that demonstrated regression of their subependymal giant cell astrocytomas with systemic administration of rapamycin.4 At the University of Texas Tuberous Sclerosis Center, we follow a 21-year-old woman who has been receiving rapamycin for 5 months for bilateral subependymal giant cell astrocytomas.
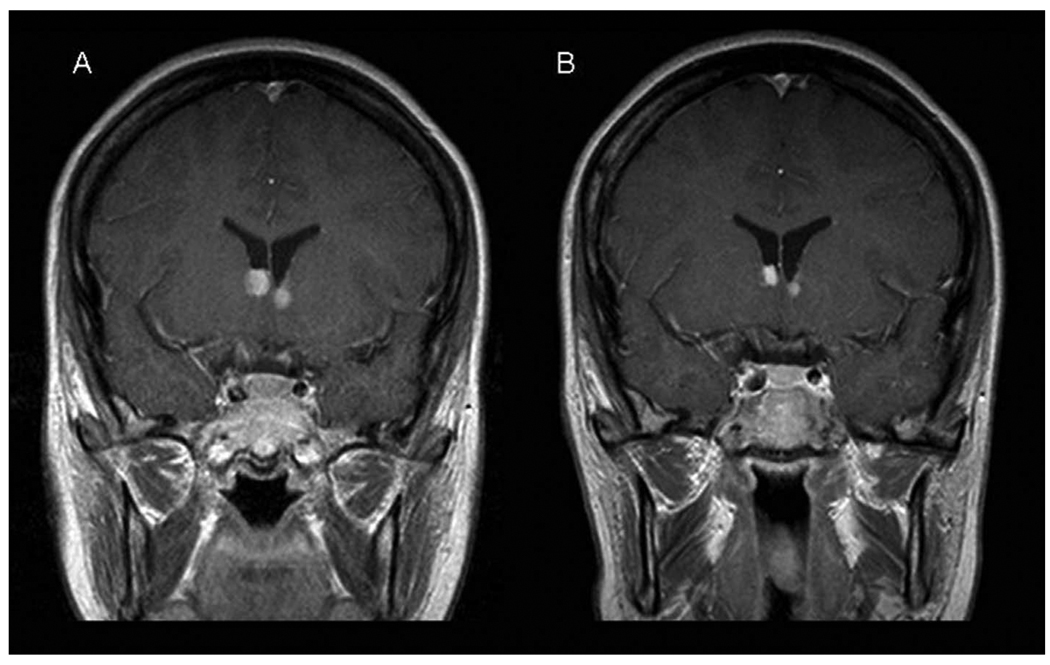
Our patient was clinically diagnosed with tuberous sclerosis at age 11 years. Other family members were assessed and found to have no signs of disease. All exons of the TSC1 and TSC2 genes were sequenced, and the TSC2 gene was assessed for large deletions/insertions with no mutation identified. Initial imaging studies demonstrated bilateral, calcified, subependymal nodules without enhancement or mass effect. Magnetic resonance imaging of the brain at age 14 years demonstrated enhancement around the subependymal nodules at the foramen of Monro. By age 16 years, it was apparent that these lesions were growing, and they had the appearance of subependymal giant cell astrocytomas. The bilateral astrocytomas continued to grow over the next several years. At age 21 years, she complained of intermittent headaches and blurred vision associated with balance difficulties. Symptoms were brief (less than 15 min) and always resolved spontaneously. Magnetic resonance imaging demonstrated growth of the bilateral astrocytomas measuring 11 mm on the right and 8 mm on the left. Symptoms were attributed to intermittent obstruction of the foramen of Monro with reversible hydrocephalus. Following the report by Franz and colleagues,4 the patient and her family were offered a trial of oral rapamycin. The patient was started at a dose of 0.2 mg/kg/day. Levels were maintained between 11 and 13 ng/mL. The patient experienced no adverse effects. Symptoms improved and repeat brain imaging 2½ months after initiating rapamycin demonstrated a decrease in size of both astrocytomas (11 to 7.5 mm on the right and 8 to 5 mm on the left; Figure 1). These results support the work by Franz and colleagues by demonstrating a reduction in size of bilateral subependymal giant cell astrocytomas in a patient with tuberous sclerosis following administration of systemic rapamycin. Further studies are needed with prolonged observation to confirm these findings, determine the length of necessary treatment, and evaluate recurrence risk after discontinuation of rapamycin.
Figure 1.
Magnetic resonance imaging of the brain, coronal, T1, postcontrast. A, Pretreatment image with right-sided astrocytoma measuring 11 mm and left-sided astrocytoma measuring 8 mm. B, Posttreatment image with right-sided astrocytoma measuring 8 mm and left-sided astrocytoma measuring 5 mm.
Acknowledgment
All work was performed at the University of Texas Health Science Center in Houston.
Footnotes
References
- 1.Kenerson H, Dundon TA, Yeung RS. Effects of rapamycin in the Eker rat model of tuberous sclerosis complex. Pediatr Res. 2005;57:67–75. doi: 10.1203/01.PDR.0000147727.78571.07. [DOI] [PubMed] [Google Scholar]
- 2.Rauktys A, Lee N, Lee L, Dabora SL. Topical rapamycin inhibits tuberous sclerosis tumor growth in a nude mouse model. BMC Dermatol. 2008;8:1–9. doi: 10.1186/1471-5945-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bissler JJ, McCormack FX, Young LR, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008;358:140–151. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franz DN, Leonard J, Tudor C, et al. Rapamycin causes regression of astrocytomas in tuberous sclerosis complex. Ann Neurol. 2006;59:490–498. doi: 10.1002/ana.20784. [DOI] [PubMed] [Google Scholar]