Abstract
Background
The incidence of right-sided colon cancers has been increasing in recent years. It is unclear whether patient prognosis varies by tumor location. In this study, we have compared the survival of right-and left-sided colon cancers in a longitudinal population-based database.
Methods
A retrospective survival analysis was performed using the Surveillance, Epidemiology, and End Results Program (SEER) database between 1988 and 2003 on subjects who underwent surgical resection for the a primary diagnosis of pathologically confirmed invasive colon adenocarcinoma. Cox proportional hazard regression analysis was used to assess long-term survival outcomes comparing right-sided (cecum to transverse colon, excluding appendix) versus left-sided (splenic flexure to sigmoid, excluding rectum) colon cancers.
Results
A total of 77,978 subjects were identified with adenocarcinoma of the colon. Overall median survival was 83 months. Median survival for right-sided cancers was 78 vs. 89 months for left-sided cancers (P<.001). By Cox proportional hazard regression analysis, controlling for statistically significant confounders, including age, sex, race, marital status, tumor stage, tumor size, histologic grade, number of lymph nodes examined, and year of diagnosis, right-sided colon cancers were associated with a 5% increased mortality risk compared with left-sided colon cancers (hazard ratio, 1.04; 95% confidence interval, 1.02–1.07). These findings were consistent across subsets of subjects.
Conclusion
On the basis of analysis of information from the SEER database, we found that right-sided colon cancers have a worse prognosis than left-sided colon cancers. The reason for this remains unclear but may be due to biological and/or environmental factors and may have particular bearing, given the rising incidence of right-sided colon cancers.
Keywords: Colon cancer, Survival, Left, Right
Colon cancer is the second leading cause of cancer death in men and the third leading cause in women in the United States, with an estimated 108,070 new cases per year, resulting in an estimated 49,960 deaths per year.1 Since the 1980s, there has been a persistent trend in the increasing percentage of right-sided colon cancers with an associated decreasing percentage of left-sided and sigmoid colon cancers. 2–4 Obrand and Gordon,2 who studied information from their institution’s database, reported an increase from 22% of colorectal cancers diagnosed in the right colon between 1979 and 1982 to 31% between 1991 and 1994.
Various hypotheses have been proposed to explain this change, including clinical presentation, genetic and environmental epidemiology, and sex distribution. 5,6 This may reflect growing use of colonoscopy and screening, and an increasing proportion of the elderly in the population. During embryologic development, the right colon arises from the midgut and the left colon from the hindgut. The right and left colons are exposed to different luminal content. In addition, analysis of large genetic databases from tumor specimens has revealed differential gene expression patterns in colon cancers originating in the right and left colons.7,8 However, analysis of different institutional databases has resulted in varying reports regarding differential prognosis of right-and left-sided colon cancer.9
Thus, it remains unclear whether the prognosis for right-sided colon cancers is different from left-sided colon cancers. We hypothesized that study of a large, longitudinal, nationwide, population-based database would elucidate a difference in the survival for right-versus left-sided colon cancers.
METHODS AND MATERIALS
Data Sources
A retrospective survival analysis was performed using the Surveillance, Epidemiology, and End Results Program (SEER) prospective cohort database. SEER is a large population-based cancer incidence and survival data registry covering 15 geographic areas in the United States, involving approximately 26% of the U.S. population.10 Data collected and followed by SEER include subject characteristics, primary tumor site, tumor morphology and stage at diagnosis, surgical treatment course, and subject survival follow-up. The SEER database includes tumors from January 1, 1973, through December 31, 2002, and captures 97% of the incident cancer cases.11 This study was approved by the Johns Hopkins Institutional Review Board, who exempted the need for subject consent.
Study Population
Data were collected from the SEER database; we limited diagnosis dates between January 1, 1988, and December 31, 2002, because detailed procedural and diagnostic codes were not available before 1988. In addition, these time points were chosen because of the completeness and homogeneity of encoded data specific to this period. All subjects included in this study were 18 years of age or older at the time of colon cancer diagnosis, and they underwent surgery with curative intent for pathologically confirmed adenocarcinoma of the colon. All patients who died within 60 days of surgery were excluded from the analysis to account for perioperative mortality. Tumor location was identified by International Classification of Diseases, Oncology, Third Revision, diagnosis codes (ICD-O-3) C18.0–C18.7. Tumors of the vermiform appendix (C18.1), rectum (C19.9), and rectosigmoid junction (C20.9) were excluded. Only subjects with their first malignant primary tumor being adenocarcinoma of the colon were included. Tumor locations coded as overlapping or large intestine, NOS (C18.8–C18.9, C26.0), which comprised 1.1% of the sample, were excluded from analysis, as were tumors in situ. Additional exclusion criteria were subjects who received pre- or postoperative radiotherapy.
Subject demographic variables examined included age, sex, race, year of diagnosis, marital status, and survival or censoring data. Tumor variables examined included location, diameter in millimeters, American Joint Commission on Cancer (AJCC) stage, histologic grade, and number of lymph nodes examined in the surgical specimen. Race was divided into three categories to facilitate interpretation: white, black, and other.
For comparison of left-versus right-sided colon cancer outcomes, the territory of the colon was divided, with tumors occurring from the cecum to the transverse colon considered right-sided tumors, and those occurring from the splenic flexure to the sigmoid colon as left-sided tumors. Tumors occurring within the appendix and rectum and those with unclear locations were excluded from analysis. Additionally, only tumors with pathology-confirmed diagnosis of adenocarcinoma were included in an effort to compare like entities and to minimize the inclusion of tumors of different and varied types. Tumor grade was separated into three categories: grade I (well differentiated), grade II (moderately differentiated), and grades III to IV (poorly differentiated or undifferentiated).
Statistical Analysis
Statistical analysis was performed by the statistical software package STATA 10.0 (StataCorp, College Station, TX). Bivariate analysis of categorical data was performed by the χ2 test. Analysis of continuous data was performed using Student’s t-test. Differences in continuous variables were compared by testing the difference in the medians by the Kruskal-Wallis test. Survival analysis was determined by the Kaplan-Meier method, and the log-rank test was used to compare statistical differences. Cox proportional hazard methods were used to assess the multivariate predictors of outcomes. The analysis was repeated in different subcohorts of subjects to determine consistency of findings across population. All statistical tests were two-sided, and a P value of ≤.05 was considered to be statistically significant.
RESULTS
Study Population
A total of 82,750 subjects who had undergone colon resections were identified in the SEER data set. The 60-day unadjusted postoperative mortality for subjects with left-sided colon cancer was 3.99% (95% confidence interval [95% CI], 3.78–4.20), compared with 5.28% for those with right-sided colon cancer (95% CI, 5.08–5.49). Therefore, the 4772 subjects who died within this period of surgery were excluded from the study. Demographics of the remaining 77,978 subjects included in the study are listed in Table 1. There were 37,050 men (47.5%) and 40,928 women (52.5%), with an overall median age of 71 years. Of 77,800 subjects with race data, 64,680 (83.1%) identified themselves as white, 7135 (9.2%) as black, and 5985 (7%) as belonging to other races. Of 75,242 subjects with marital status data, 43,520 (57.8%) were married. The median survival of all subjects studied was 83 months. AJCC stage was known for 77,481 subjects and was distributed among all four stages, with 21.5% of disease being stage I, 37.7% stage II, 27.7% stage III, and 13.1% stage IV. The median tumor size at time of surgical resection was 45 mm. The tumor grade distribution was primarily moderately differentiated (grade II, 69.6%), with 10.7% being grade I and 19.8% being grade III or IV. The overall median number of lymph nodes collected in surgical specimens was 10. Of 73,324 subjects with known lymph nodal status, most had N0 lymph node involvement (60.7%), with 25.1% having N1 and 14.2% having N2 disease.
TABLE 1.
Subject and tumor characteristics of colon cancer resections in the Surveillance, Epidemiology, and End Results Program database from 1988 to 2002
| Characteristic | Overall no. (%)(N = 77,978) | Left-sided colon cancer (N = 33,434; 42.9%) | Right-sided colon cancer (N = 44,544; 57.1%) | P value |
|---|---|---|---|---|
| Survival (mo), median (95% CI) | 83 (81–84) | 89 (87–91) | 78 (77–80) | < .001 |
| Age at diagnosis (y), median (IQR) | 71 (62–79) | 69 (60–77) | 73 (64–80) | < .001 |
| Male sex, N (%) | 37,050 (47.5%) | 17,365 (51.9%) | 19,685 (44.2%) | < .001 |
| Race, N (%) | N = 77,800 | N = 33,344 | N = 44,456 | < .001 |
| White | 64,680 (83.1%) | 27,211 (81.6%) | 37,469 (84.3%) | |
| Black | 7,135 (9.2%) | 2,881 (8.6%) | 4,254 (9.6%) | |
| Other | 5,985 (7.7%) | 3,252 (9.8%) | 2,733 (6.2%) | |
| Marital status, N (%) | N = 75,242 | N = 32,177 | N = 43,065 | < .001 |
| Single | 31,722 (42.2%) | 12,470 (38.8%) | 19,252 (44.7%) | |
| Married | 43,520 (57.8%) | 19,707 (61.2%) | 23,813 (55.3%) | |
| Mean year of diagnosis | September 1995 | June 1995 | October 1995 | < .001 |
| Tumor size (mm) | N = 67.914 | N = 27,525 | N = 40,389 | < .001 |
| Median (IQR) | 45 (30–60) | 40 (30–53) | 46 (35–62) | |
| AJCC tumor stage | N = 77,481 | N = 33,205 | N = 44,276 | < .001 |
| I | 16,688 (21.5%) | 8,610 (25.9%) | 8,078 (18.2%) | |
| II | 29,175 (37.7%) | 11,382 (34.3%) | 17,793 (40.2%) | |
| III | 21,475 (27.7%) | 8,727 (26.3%) | 12,748 (28.8%) | |
| IV | 10,143 (13.1%) | 4,486 (13.5%) | 5,657 (12.8%) | |
| Tumor grade | N = 77,978 | N = 33,434 | N = 44,544 | < .001 |
| Grade I (well differentiated) | 8,309 (10.7%) | 4,095 (12.3%) | 4,214 (9.5%) | |
| Grade II (moderately differentiated) | 54,251 (69.6%) | 24,829 (74.5%) | 29,422 (66.1%) | |
| Grade III–IV (poorly differentiated or undifferentiated) | 15,418 (19.8%) | 4,510 (13.5%) | 10,908 (24.5%) | |
| No. of lymph nodes examined, median (IQR) | 10 (6–16) | 8 (4–13) | 11 (7–17) | < .001 |
| Nodal status, N (%) | N = 73,324 | N = 30,259 | N = 43,065 | < .001 |
| N0 | 44,532 (60.7%) | 18,543 (61.3%) | 25,989 (60.4%) | |
| N1 | 18,397 (25.1%) | 7,946 (26.3%) | 10,451 (24.3%) | |
| N2 | 10,395 (14.2%) | 3,770 (12.5%) | 6,625 (15.4%) |
95% CI, 95% confidence interval; IQR, interquartile range; AJCC, American Joint Committee on Cancer. Sample size is presented for each variable when data are not available for all subjects.
Right-Versus Left-Sided Colon Cancer
Right-sided colon cancer significantly outnumbered left-sided colon cancer, with 44,544 (57.1%) of subjects having right-sided and 33,434 (42.9%) left-sided colon cancer (P<.001) (Table 1). Subjects with right-sided colon cancer were significantly older (median age of 73 vs. 69 years; P<.001) and had a significantly shorter survival time after diagnosis (78 vs. 89 months, P<.001) compared with those with left-sided colon cancer. Right-sided colon cancer subjects were less likely to be male (44.2% vs. 51.9%; P<.001) and married (55.3% vs. 61.2%; P<.001).
The majority of the right-and left-sided tumors were AJCC stage II (40.2% and 34.3% respectively). In addition, there were a far lower proportion of stage I right-sided tumors (18.2% vs. 25.9%) and a higher proportion of stage III right-sided tumors (28.8% vs. 26.3%) compared with left-sided tumors (P<.001 for χ2 test of all tumor stages). Overall, right-sided tumors were of higher grade than left-sided tumors, with 24.5% of right-sided tumors being grades III and IVvs. 13.5% of left-sided tumors (P < .001). Right-sided tumors were also larger at the time of surgery than left-sided tumors (median size 46 vs. 40 mm; P<.001).
The median number of lymph nodes examined in surgical specimens of right-sided cancers was 11 vs. 8 (P<.001) in left-sided cancer specimens. Most subjects in both cohorts had N0 lymph node involvement, 60.4% in right-sided vs. 61.3% in left-sided cancer cohorts. Overall, there was a higher percentage of right-sided cancer subjects with node-positive disease than left-sided cancer subject, a difference that was statistically significant (39.7% vs. 38.8%; P <.001). When compared by year of diagnosis, more right-sided tumors were diagnosed more recently in the study interval than left-sided tumors, as evidenced by the mean date of diagnosis of October 1995 for right-sided tumors versus June 1995 for left-sided tumors (P<.001).
Multivariate Association of Cancer Location with Mortality
The results of unadjusted and adjusted multivariate Cox survival regression analyses are listed in Table 2. The hazard ratios (HR) and 95% CIs were significant (P < .05) for all of the variables in the univariate analysis with the exception of white race, and in the multivariate analysis with the exception of subjects with grade II tumors.
TABLE 2.
Unadjusted and adjusted survival analysis of overall subject and tumor characteristics
| Characteristic | Unadjusted analysis |
Adjusted analysisa |
||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% confidence interval | P value | Hazard ratio | 95% confidence interval | P value | |
| Right colon | 1.150 | 1.126–1.174 | < .001 | 1.042 | 1.018–1.067 | .001 |
| Age (for every 1 additional year in age) | 1.032 | 1.031–1.033 | < .001 | 1.036 | 1.035–1.037 | < .001 |
| Male sex | 1.028 | 1.007–1.049 | .008 | 1.228 | 1.199–1.257 | < .001 |
| Race | ||||||
| White | 1.005 | .977–1.033 | NS | 1.0 | Referent | – |
| Black | 1.167 | 1.127–1.208 | < .001 | 1.183 | 1.138–1.230 | < .001 |
| Other | .816 | .783–.851 | < .001 | .898 | .858–.939 | < .001 |
| Married | .713 | .698–.728 | < .001 | .791 | .772–.810 | < .001 |
| AJCC tumor stage | ||||||
| I | .469 | .455–.483 | < .001 | 1.0 | Referent | – |
| II | .652 | .637–.666 | < .001 | 1.310 | 1.260–1.362 | < .001 |
| III | 1.215 | 1.188–1.242 | < .001 | 2.204 | 2.118–2.293 | < .001 |
| IV | 6.072 | 5.917–6.230 | < .001 | 9.998 | 9.581–10.434 | < .001 |
| Tumor grade | ||||||
| Grade I (well differentiated) | .729 | .704–.755 | < .001 | 1.0 | Referent | – |
| Grade II (moderately differentiated) | .853 | .835–.872 | < .001 | 1.038 | .996–1.081 | NS |
| Grade III–IV (poorly differentiated or undifferentiated) | 1.506 | 1.470–1.543 | < .001 | 1.305 | 1.247–1.365 | < .001 |
| No. of lymph nodes examined (for every 1 additional node examined) | .989 | .988–.991 | < .001 | .988 | .987–.990 | < .001 |
| Nodal status | ||||||
| N0 | .458 | .449–.469 | < .001 | –b | –b | |
| N1 | 1.374 | 1.342–1.407 | < .001 | –b | –b | |
| N2 | 2.866 | 2.792–2.943 | < .001 | –b | –b | |
| Tumor size (for every 1 mm increase) | 1.002 | 1.002–1.002 | < .001 | 1.001 | 1.001–1.001 | < .001 |
| Year of diagnosis (for every 1 year after 1988) | .987 | .984–.990 | < .001 | .987 | .984–.990 | < .001 |
AJCC, American Joint Committee on Cancer.
Multivariate Cox proportional hazard regression model includes the following variables: subject age, sex, race, marital status, tumor side, AJCC stage, tumor size, histologic grade, number of lymph nodes examined, year of diagnosis.
Nodal status was omitted from Cox proportional hazard regression analysis as this was already accounted for in the AJCC tumor staging algorithm.
Kaplan–Meier survival estimates for left- and right-sided colon cancer are shown in Fig. 1. Survival for left-sided colon cancers was 59.7% at 5 years (60 months), 41.9% at 10 years (120 months), and 29.5% at 15 years (180 months). For right-sided colon cancers, survival was 56.3% at 5 years, 37.8% at 10 years, and 24.5% at 15 years. Survival of left- and right-sided colon cancer subjects was significantly different at all three of these time points (P<.001).
FIG. 1.
Kaplan–Meier survival estimates for overall unadjusted survival of left-versus right-sided adenocarcinoma of the colon.
A Cox proportional hazard regression analysis of tumor side was performed including the variables of subject age, sex, race, marital status, AJCC stage, tumor size, histologic grade, number of lymph nodes examined, and year of diagnosis. These adjusted survival curves are shown in Fig. 2. Of note, nodal status was omitted because this was already accounted for in the AJCC tumor staging algorithm. Compared with subjects with left-sided colon cancer, those with right-sided cancer had a 4.2% increased mortality risk (adjusted HR = 1.042; 95% CI, 1.02–1.07; P = .001). For every increase in subject age by 1 year, mortality risk increased by 3.6%. Tumor stage was associated with the highest increase in mortality rate, with the increase in mortality over stage I being 31% for subjects with stage II cancer, 120% for those with stage III cancer, and 900% for those with stage IV cancer. Similarly, grade III and IV and N1 and N2 nodal disease (examined by univariate analysis only) were associated with worse prognosis. Being married was associated with a decreased risk of mortality, by 21%, when compared with unmarried subjects (P < .001). More recent diagnosis was associated with improved survival as well: a 1.3% reduction in mortality was experienced for every year diagnosis of colon cancer was made after 1988 (P < .001). Substratification and multivariate Cox proportional hazard regression analysis that used the above-listed variables was performed by tumor side and AJCC stage (Fig. 3 and Table 2).
FIG. 2.
Adjusted Kaplan–Meier survival estimates for overall survival of left-versus right-sided adenocarcinoma of the colon. Note magnified y-axis range. Multivariate Cox proportional hazard regression model includes the following variables: subject age, sex, race, marital status, tumor side, American Joint Committee on Cancer stage, tumor size, histologic grade, number of lymph nodes examined, and year of diagnosis.
FIG. 3.

Adjusted Kaplan–Meier survival estimates for overall survival of adenocarcinoma of the colon stratified by tumor stage for left- and right-sided tumors. Multivariate Cox proportional hazard regression model includes the following variables: subject age, sex, race, marital status, tumor side, American Joint Committee on Cancer stage, tumor size, histologic grade, number of lymph nodes examined, and year of diagnosis.
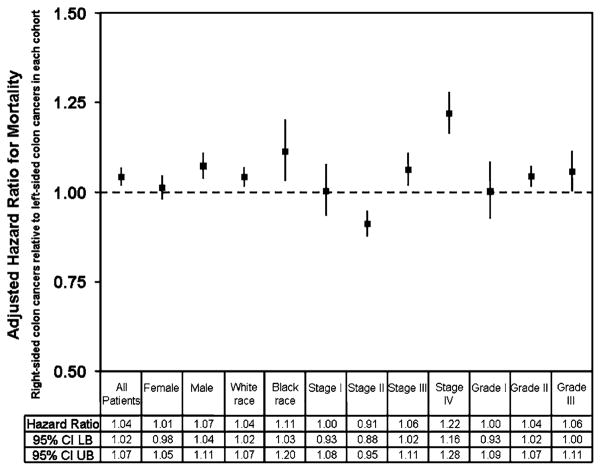
The multivariate Cox proportional hazard regression analyses were repeated in different cohorts of subjects (Fig. 4). At subset analysis, the results did not change qualitatively. Right-sided colon cancer was associated with a statistically significant increase in HR for mortality for most subgroups tested compared with left-sided colon cancer. However, women did not have a statistically significant difference in mortality between left- and right-sided colon cancer (HR = 1.01; P = .48). Similarly, no differences in survival between right-and left-sided colon cancer with stage I or grade I were observed (stage I: HR = 1.003; P = .93; grade I: HR = 1.002; P = .96). Of note, subjects with stage II right-sided colon cancers had lower HRs than those with left-sided colon cancers (HR = .91; P<.001).
FIG. 4.
Adjusted hazard ratio with 95% confidence intervals for mortality comparing right-sided colon cancers relative to left-sided colon cancers in the following cohorts: female subjects, male subjects, subjects of white race, subjects of black race, American Joint Committee on Cancer stage I, II, III and IV, and histology grades I, II, and III/IV. UB, upper bound; LB, lower bound.
Additional analysis was performed where right-sided colon cancer was further divided anatomically into two groups: cecum to ascending colon, and hepatic flexure to splenic flexure. These stratifications were compared with left-sided colon cancer (descending colon to sigmoid colon). There were 32,388 subjects with cancer between the cecum and ascending colon (41.8%), 15,055 subjects with cancer between the hepatic flexure and splenic flexure (19.4%), and 30,038 subjects with cancer between the descending colon and sigmoid colon (38.8%). Analysis of this anatomic stratification revealed that mortality risk was 8.0% greater for subjects with cancer between the hepatic flexure and splenic flexure compared with those with cancer between the descending colon and sigmoid colon (HR 1.08; 95% CI, 1.05–1.11; P < .001). Subjects with cancer between the cecum and ascending colon had a 3.7% greater mortality risk compared with those with cancer between the descending colon and sigmoid colon (HR 1.037; 95% CI, 1.01–1.06; P = .007).
DISCUSSION
Through examination of over 77,000 subjects with adenocarcinoma of the colon from the SEER database, we have shown that subjects with right-sided colon cancer have a decreased survival on average, and an increased mortality risk compared with those with left-sided colon cancer. This difference is statistically significant and persists when controlling for factors of subject age, sex, race, marital status, year of diagnosis, AJCC stage, tumor size, grade, lymph node disease, and number of lymph nodes on pathological examination. Consistent with reports from smaller database analyses, in more recent years, there has been an increase in the proportion of right-sided tumors diagnosed.2–4 However, this is associated with a statistically significant reduction in risk of death over time. In accordance with accepted data, outcome and prognosis are worse for subjects with higher stage or histologic grade tumors and presence of nodal disease.
The reason for the observed difference in survival for subjects with left- and right-sided adenocarcinoma of the colon remains unclear. It is speculated that the differences in embryologic origin and fecal exposure, as well as the difference in time to detection, play a role. To review, the embryonic midgut develops into the right-sided colon, extending from the cecum to the proximal two-thirds of the transverse colon. This is vascularized by the superior mesenteric artery. In contrast, the embryonic hindgut develops into the left-sided colon, extending from the distal third of the transverse colon to the upper anal canal. This is vascularized by the inferior mesenteric artery.12 To compare tumors of like morphology, tumors of the rectum were excluded. When we controlled for tumor stage, histological grade, and tumor size in addition to subject demographics, we found that the prognosis for subjects with right-sided colon cancer remains worse than that for left-sided disease. This suggests that the difference is more likely related to genetic and environmental factors. The differences in gene expression patterns between the normal right and left colons, as well as between cancers in these locations, have been studied.7,8
At subset analysis, the results for the mortality risk of different cohorts confirm the overall findings. It is interesting to note that among subjects with stage II tumors, those with right-sided tumors have a far better survival outcome compared with left-sided tumors, in contrast to findings from other subgroups. The reasons for this are unclear and warrant further study. However, tumors with microsatellite instability, which are often right-sided, have been associated with an improved prognosis in part as a result of decreased metastases.13–15 This may partially account for these observed findings. However, it is unlikely that tumors with microsatellite instability are solely limited to these right-sided stage II tumors. Therefore, the reasons for this difference in survival remain unclear and warrant further study.
The SEER database was chosen over other available databases because of the extensive nature of its records, which include longitudinal data and a large sample size that permits comparison of outcomes across the United States. That said, like all database-derived studies, the current analysis has several limitations. In examining the SEER database, the accuracy of the diagnostic, pathologic, and histologic coding was unable to be verified. Although the validity of the coding may be verified, the appropriateness of the coding used for diagnosis may not. However, it is assumed that this type of error would be equally distributed throughout the data. Data about subjects’ carcinoembryonic antigen levels and presence of vascular invasion in surgical specimens were unavailable, as were comorbidities and disease-specific survival. Similarly, whether or not subjects received chemotherapy for their cancer is not recorded in the SEER database. Finally, the models used are simplified, using available and accepted measures; they clearly do not adequately account for all variables associated with subject outcomes.
In recent years, the distribution of right-versus left-sided colon cancers has changed, with an increasing incident of right-sided colon cancer. The cause behind this is currently poorly understood and likely multifactorial. Our findings of worse survival for right-sided colon cancer bears further study to understand the cause. Moreover, understanding differences in tumor biology may ultimately affect the treatment modalities, specifically chemotherapy regimens, which are used for right-versus left-sided colon cancer.
Acknowledgments
R.A.M. was supported on the Ruth L. Kirschstein National Research Service Award (T32DK007713) while undertaking this study.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Obrand DI, Gordon PH. Continued change in the distribution of colorectal carcinoma. Br J Surg. 1998;85:246–8. doi: 10.1046/j.1365-2168.1998.00507.x. [DOI] [PubMed] [Google Scholar]
- 3.Levi F, Randimbison L, La Vecchia C. Trends in subsite distribution of colorectal cancers and polyps from the Vaud Cancer Registry. Cancer. 1993;72:46–50. doi: 10.1002/1097-0142(19930701)72:1<46::aid-cncr2820720111>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 4.Jass JR. Subsite distribution and incidence of colorectal cancer in New Zealand, 1974–1983. Dis Colon Rectum. 1991;34:56–9. doi: 10.1007/BF02050208. [DOI] [PubMed] [Google Scholar]
- 5.Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer. 2002;101:403–8. doi: 10.1002/ijc.10635. [DOI] [PubMed] [Google Scholar]
- 6.Distler P, Holt PR. Are right-and left-sided colon neoplasms distinct tumors? Dig Dis. 1997;15:302–11. doi: 10.1159/000171605. [DOI] [PubMed] [Google Scholar]
- 7.Glebov OK, Rodriguez LM, Nakahara K, et al. Distinguishing right from left colon by the pattern of gene expression. Cancer Epidemiol Biomarkers Prev. 2003;12:62–755. [PubMed] [Google Scholar]
- 8.Birkenkamp-Demtroder K, Olesen SH, Sorensen FB, et al. Differential gene expression in colon cancer of the caecum versus the sigmoid and rectosigmoid. Gut. 2005;54:374–84. doi: 10.1136/gut.2003.036848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reifferscheid M, Fass J, Hartung R, et al. Special aspects of right colon cancer. Langenbecks Arch Chir. 1987;371:193–200. doi: 10.1007/BF01259430. [DOI] [PubMed] [Google Scholar]
- 10.Surveillance, Epidemiology, and End Results Program (SEER) [Accessed 29 August 2006];Public-Use Data (1973–2002) released April 2005, based on November 2004 submission. Available at http://seer.cancer.gov/data/
- 11.Zippin C, Lum D, Hankey BF. Completeness of hospital cancer case reporting from the SEER Program of the National Cancer Institute. Cancer. 1995;76:2343–50. doi: 10.1002/1097-0142(19951201)76:11<2343::aid-cncr2820761124>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 12.Langman J. Medical Embryology. 4. Baltimore: Williams & Wilkins; 1981. [Google Scholar]
- 13.Gervaz P, Bucher P, Morel P. Two colons—two cancers: paradigm shift and clinical implications. J Surg Oncol. 2004;88:261–6. doi: 10.1002/jso.20156. [DOI] [PubMed] [Google Scholar]
- 14.Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23:609–18. doi: 10.1200/JCO.2005.01.086. [DOI] [PubMed] [Google Scholar]
- 15.Malesci A, Laghi L, Bianchi P, et al. Reduced likelihood of metastases in patients with microsatellite-unstable colorectal cancer. Clin Cancer Res. 2007;13:3831–9. doi: 10.1158/1078-0432.CCR-07-0366. [DOI] [PubMed] [Google Scholar]